Abstract
The hypothalamus is an important center for coordinating mammalian physiology and maintenance of homeostasis. Accordingly, the hypothalamus regulates feeding, body temperature, energy expenditure, glucose metabolism, thirst, blood pressure, reproductive axis, and other metabolic functions associated with the overall metabolism. At the central level, the hypothalamus is the primary component of the nervous system in interpreting adiposity and nutrient-related inputs; it delivers hormonal and behavioral responses with the ultimate purpose of regulating body weight, food intake, and energy consumption (Williams et al. 2001; Wardlaw 2011; Toorie and Nillni 2014). Among the hormonal inputs that feed into the hypothalamic circuitries are adipose tissue-derived hormone leptin and adiponectin, pancreatic hormone insulin, and several hormones secreted by the gastrointestinal tract, such as ghrelin. The activity of the hypothalamic feeding centers is also responsive to basic nutrients including glucose, amino acids, and fatty acids besides other metabolites, such as ketone bodies. Much like the hypothalamus acting at the organismal level to regulate homeostasis by integrating such hormonal and nutritional signals, there are evolutionarily conserved proteins and protein complexes that act at the cellular level as “nutrient sensors” that couple cellular energetics to downstream pathways to regulate various cellular functions. The activity of these nutrient sensors in key hypothalamic feeding centers plays a major role in the regulation of energy balance and glucose metabolism.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
1 Introduction
The hypothalamus is an important center for coordinating mammalian physiology and maintenance of homeostasis. Accordingly, the hypothalamus regulates feeding, body temperature, energy expenditure, glucose metabolism, thirst, blood pressure, reproductive axis, and other metabolic functions associated with the overall metabolism. At the central level, the hypothalamus is the primary component of the nervous system in interpreting adiposity and nutrient-related inputs; it delivers hormonal and behavioral responses with the ultimate purpose of regulating body weight, food intake, and energy consumption (Williams et al. 2001; Wardlaw 2011; Toorie and Nillni 2014). Among the hormonal inputs that feed into the hypothalamic circuitries are adipose tissue-derived hormone leptin and adiponectin, pancreatic hormone insulin, and several hormones secreted by the gastrointestinal tract, such as ghrelin. The activity of the hypothalamic feeding centers is also responsive to basic nutrients including glucose, amino acids, and fatty acids besides other metabolites, such as ketone bodies. Much like the hypothalamus acting at the organismal level to regulate homeostasis by integrating such hormonal and nutritional signals, there are evolutionarily conserved proteins and protein complexes that act at the cellular level as “nutrient sensors” that couple cellular energetics to downstream pathways to regulate various cellular functions. The activity of these nutrient sensors in key hypothalamic feeding centers plays a major role in the regulation of energy balance and glucose metabolism.
Among the most prominent regulators within the hypothalamus, neurons in the arcuate nucleus (ARC) of the hypothalamus located in the mediobasal hypothalamus, anteriorly juxtaposing the median eminence (ME), are of critical importance for the regulation of energy balance. The ME is one of the secretory circumventricular organs (Kaur and Ling 2017) (lies outside the blood-brain barrier), which in turn confers the ARC the advantage to have access to the factors in the systemic circulation over other nuclei in the hypothalamus. The ARC receives circulating adiposity and nutritional signals and transmits responses to “second-order” neurons within and outside the hypothalamus. The ARC is sensitive to peripheral signals such as postprandial fluctuations in peripheral and central hormones as well as the nutrients such as amino acids, lipids, and glucose. The ARC neurons express the receptors for majority of the peripherally derived hormones including the adipostatic factor leptin, insulin from pancreas, ghrelin from the gut, hormones of the noradrenergic system, and thyroid hormone, which are among the major regulators of feeding and energy expenditure (Hahn et al. 1998; Morton et al. 2006). The inputs from the periphery provoke a response from these neurons by releasing neuropeptide hormones and neurotransmitters to extra-ARC hypothalamic sites such as the paraventricular nucleus (PVN) of the hypothalamus (Lu et al. 2003; Wittmann et al. 2005; Füzesi et al. 2007; Cyr et al. 2013). Within the ARC, the anorexigenic alpha-melanocyte stimulating hormone (a-MSH), derived from the posttranslational processing of its pro-opiomelanocortin (POMC) precursor, increases its levels after feeding and decreases upon fasting, whereas the orexigenic neuropeptide Y (NPY) and Agouti-related peptide (AgRP) follow an opposite pattern. AgRP expression is restricted to the ARC in the entire nervous system, and about 90% of AgRP neurons also express NPY. POMC is expressed in the ARC as well as the NTS in adult rodents, while NPY is one of the most ubiquitously expressed and abundant neuropeptides. AgRP neurons are widely distributed throughout the rostro-caudal axis of the ARC, while POMC neurons are more restricted to anterior and medial hypothalamus (Anderson et al. 2016). While the AgRP neurons are GABAergic (inhibitory), most POMC neurons are glutamatergic (excitatory). a-MSH increases energy expenditure through its action on melanocortin 3/4 receptors (MC3/4R) found in hypothalamic and extra-hypothalamic nuclei reported to bind a-MSH (Cummings and Schwartz 2000; Adan et al. 2006; Perello et al. 2007; Anderson et al. 2016). AgRP acts as an endogenous antagonist on MC3/4R receptors to block a-MSH action, while NPY acts independently through its own set of receptors (NPY1-5R) expressed throughout the nervous system. POMC and AgRP neurons and the melanocortin receptors (MC3/4R) constitute the central melanocortin signaling (Cone 2005). Anorexigenic peripheral hormones including leptin and insulin positively regulate POMC expression and suppress AgRP and NPY expressions (Cowley et al. 2001; Benoit et al. 2002; Heuer et al. 2005).
Two of the major intracellular signaling pathways regulated by leptin are JAK2-STAT3 pathway and phosphatidylinositol-4,5-bisphosphate 3-kinase (PI3K) signaling. PI3K is required for the anorectic responses to leptin as well as insulin (11677594). Insulin exerts its effects through activation of the insulin receptor substrate (IRS) proteins. IRS phosphorylation activates a series of signaling events in the PI3K-Akt pathway resulting in the phosphorylation of Akt (also called protein kinase B), which in turn phosphorylates FoxO1. Phosphorylated FoxO1 is recruited from the nucleus to the cytosol, which leads to its degradation through the proteasome system. In the hypothalamus, FoxO1 acts as an inhibitor of POMC and activator of NPY/AgRP expressions (Kitamura et al. 2006; Kim et al. 2006; Yang et al. 2009), and STAT3-induced POMC expression appears to require release of FoxO1 repression (Ernst et al. 2009). POMC neurons are glucose responsive and express insulin receptors (Ibrahim et al. 2003), and an intact PI3K signaling in POMC neurons is required for maintenance of glucose homeostasis (Hill et al. 2009). POMC-specific ablation of PI3K through deletion of its regulatory subunits blocks acute anorectic response to central leptin infusion; however these mice exhibit normal body weight in the long term (Hill et al. 2008). Deletion of the p110β, but not p110α, catalytic subunit of PI3K has a more sustained effect in body weight with POMC-specific ablation resulting in obesity and AgRP-specific ablation conferring protection against weight gain (Al-Qassab et al. 2009). PI3K signaling also mediates leptin and insulin-induced changes neuronal activation status in other hypothalamic nuclei including the hypothalamic ventral premammillary nucleus (Williams et al. 2011) and the ventromedial hypothalamic nucleus (Sohn et al. 2016). PI3K-mediated neuronal depolarization or hyperpolarization seems to be mediated by transient receptor potential C (TRPC) channels or ATP-sensitive K+ (KATP) channels, respectively (Sohn et al. 2016). Compared to the PI3K signaling, JAK2-STAT3 signaling is indispensable for leptin’s effect on energy homeostasis. Neuron-specific STAT3 KO mice mimic most of the defects observed in ob/ob and db/db mice including hyperphagic obesity and infertility (Gao et al. 2004). Mutation of Tyr 1138 on LepRb, the critical residue for leptin-induced JAK2-STAT3 activation, also results in hyperphagic obesity with intact fertility (Bates et al. 2003).
Although it is not going to be discussed in detail here, the hormonal regulation of the hypothalamic feeding circuitries is inarguably more complex than summarized above and involves other components, whose regulation is spatially and temporarily distinct. The signaling cascades regulated by leptin involve additional signaling components such as STAT5, ERK/MAPK, mTOR, and AMPK pathways (Park and Ahima 2014). Furthermore, peripherally derived hormones regulate the activity of their target neurons not only by directly acting on them but also indirectly through synaptic inputs from other neurons. For example, while leptin can directly regulate AgRP neurons, these cells are subject to additional regulation by innervation through a population of leptin-responsive, GABAergic neurons in the dorsomedial nucleus of the hypothalamus (Garfield et al. 2016). This is at least one of the culprits of conditional knockout strategies heavily used in the past two decades to understand the contribution of individual hormones and their role in chemically defined neuronal populations.
Below, we start with an introduction to the biology of nutrient sensors. Next, we summarize the findings in the literature on the role of nutrient sensors in the hypothalamus and their role in the regulation of energy homeostasis. We conclude this chapter with explaining how our knowledge on metabolism is integrated at the level of nutrient sensors and prohormone processing. For a full description of the hypothalamus and hypothalamic neuropeptides controlling energy balance, we refer the readers to Chaps. 2 and 5.
2 Cellular Nutrient Sensors
Nutrient sensors have the ability to sense and respond to fluctuations in environmental nutrient levels, which represent a key requisite for life. There are diverse nutrient-sensing pathways detecting intracellular and extracellular levels of sugars, amino acids, lipids, and other metabolites while also integrating hormonal and stress signals to coordinate homeostasis at the organismal level. Nutrients are simple organic compounds involved in biochemical reactions to produce energy and act as components of cellular biomass or signaling molecules. During periods of food richness, nutrient-sensing pathways engage in anabolism and energy storage. On the contrary, nutrient deprivation triggers homeostatic mechanisms including mobilization of internal stores through pathways such as autophagy to globally shut down anabolic pathways while supplying energy for vital cellular processes. Besides responding hormonal inputs, the hypothalamus is directly sensitive to nutritional changes. For example, POMC neurons are glucose responsive (Ibrahim et al. 2003), and their firing rate changes with the glucose concentration. Amino acids and lipids can also be directly sensed by hypothalamic centers; central infusion of branch chain amino acid leucine (Cota et al. 2006) or oleic acid (Obici et al. 2002) inhibits food intake and weight gain. The integration of the nutritional signals in the CNS is accomplished by a set of evolutionally conserved proteins and protein complexes called nutrient sensors. The activity of the nutrient sensors in the hypothalamus is coupled to the regulation of energy homeostasis. While leucine-mediated suppression of food intake involves hypothalamic mechanistic target of rapamycin signaling (mTOR), fatty acids act through modulating hypothalamic malonyl-CoA levels, which is regulated by AMPK. mTOR and AMPK are nutrient/energy sensors whose activity is coupled to the cellular energy status and levels of certain nutrients. The mTOR kinase, when part of mTOR complex 1 (mTORC1), plays a role in cellular energetics by inducing numerous anabolic protein processes and lipid synthesis (Laplante and Sabatini 2012). AMPK, on the other hand, is typically activated by energy deficit and acts to suppress the anabolic pathways while activating the catabolic reactions to in general antagonize mTOR action. Another energy sensor, sirtuin 1 (SIRT1), a class III histone deacetylase, is also activated by low-energy status and acts in parallel with AMPK. Below we briefly describe the biochemistry of nutrient sensing, an overall view of the nutrient sensors in metabolic pathways, and how nutrient sensors regulate energy balance in the hypothalamus, giving a special emphasis to the connection between these enzymes and neuropeptide biosynthesis.
2.1 mTOR
mTOR (mechanistic target of rapamycin, or formerly called mammalian target of rapamycin) is an evolutionarily conserved serine/threonine kinase in the phosphatidylinositol 3-kinase-related kinase family. mTOR is typically activated by energy surplus and promotes anabolic processes including protein and lipid synthesis to induce cell growth and proliferation while suppressing catabolic pathways such as autophagy. Coordination of these processes involves two mTOR protein complexes: mTOR complex 1 (mTORC1) and mTOR complex 2 (mTORC2). mTOR activity is regulated by hormones and growth factors (such as insulin), as well as by nutrients including branch chain amino acids. mTORC1 kinase activity is stimulated by interaction with Ras homolog enriched in brain (Rheb), which is a ubiquitously expressed GTP-binding protein. Rheb GTPase activity is stimulated by a tumor suppressor complex formed between two proteins, tuberous sclerosis complex 1 and 2 (TSC1 and TSC2). Therefore, TSCs act as upstream inhibitors of mTORC1 signaling. Anabolic factors such as insulin and IGFs result in activation of Akt, which phosphorylates and inhibits TSC. Another negative regulator of mTORC1, PRAS40 (proline-rich Akt substrate 40 kDa), is phosphorylated by Akt, which prevents PRAS40-mTORC1 interaction (Vander Haar et al. 2007; Sancak et al. 2007; Wang et al. 2007). These PI3K-Akt-dependent phosphorylation events couple insulin and IGF signaling to mTORC1 activation. Active mTORC1, in turn, triggers the major anabolic pathways: It stimulates protein synthesis in part through phosphorylation of S6 K1 (S6 kinase 1), which phosphorylates ribosomal protein S6, and through phosphorylation of translational regulator eukaryotic translation initiation factor 4E binding protein 1 (4E-BP1), which results in the dissociation of 4E-BP1 from eukaryotic translation initiation factor 4E (eIF4E) and ribosomal assembly and activation of translation. mTORC1 is also activated by amino acids. It is worth to note that amino acids serve as major nitrogen source as well as the building blocks for proteins. For example, in fasting, protein breakdown mostly in the muscle provides glutamine into the circulation, where the glutamine is delivered to various tissues to be used as a major nitrogen source. Activation of mTORC1 by amino acids and amino acid sensing in general involve a set of protein complexes that are characterized only recently. Amino acid sensing involves the heterodimer of a family of four small GTPases called Rag proteins (Rag A-D). Amino acids stimulate the GTP-bound state of Rags (Sancak et al. 2008; Kim et al. 2008a). A protein complex composed of at least five distinct proteins called the Ragulator acts as the guanidine exchange factor for Rags and recruits Rags and mTORC1 to the lysosomal membrane, where Rheb is also localized (Sancak et al. 2010; Bar-Peled et al. 2012). mTORC1 senses cytosolic as well as lysosomal amino acid levels through distinct pathways that involve other protein complexes, which ultimately merge on their regulation of mTORC1 activity through Rags. Cytosolic amino acid sensing works by dissociating upstream inhibitors from an mTORC1 activating protein complex called GATOR2: Cytosolic leucine is thought to be directly sensed by Sestrin2, which acts as an upstream inhibitor of GATOR2. Leucine binding releases the Sestrin2-GATOR2 complex and results in mTORC1 activation. Likewise, cytosolic arginine binds another protein called CASTOR1, which in the absence of arginine inhibits GATOR2 (Chantranupong et al. 2016). The 11-pass lysosomal transmembrane protein, SLC38A9, interacts with the Ragulator, Rag GTPases, and vacuolar H(+)-ATPase complex in an amino acid-sensitive manner and therefore acts as the lysosomal arginine sensor (Zoncu et al. 2011; Wang et al. 2015; Rebsamen et al. 2015; Jung et al. 2015). Accordingly, upon genetic ablation of either Sestrin2, CASTOR1, or SLC38A9, amino acid-mediated activation of mTORC1 is significantly compromised.
mTORC1 acts a major metabolic hub that responds to various forms of cellular stress such as hypoxia and DNA damage, ATP level, amino acids, and growth factors to regulate glycolysis, mitochondrial and lysosome biogenesis, lipid and protein biosynthesis, proteasome formation, and autophagy (Saxton and Sabatini 2017). For example, mTORC1 activation couples cellular nutrient levels and hormonal inputs to lipid and cholesterol synthesis through activation of SREBPs (sterol regulatory element-binding proteins), which are basic helix-loop-helix (bHLH) transcription factors (Lewis et al. 2011). Accordingly, inhibition of mTORC1 activity impairs adipogenesis (Lamming and Sabatini 2013). mTORC1 also stimulates purine and pyrimidine synthesis (Ben-Sahra et al. 2013, 2016), promotes mitochondrial oxidative capacity (Cunningham et al. 2007) in skeletal muscle, and induces genes for the enzymes involved in glycolysis and pentose phosphate pathway (Düvel et al. 2010). While activating anabolism, mTORC1 activation results in suppression of major catabolic pathways. Nutrient deprivation promotes autophagy by AMPK-mediated phosphorylation and activation of ULK1, a kinase that drives autophagosome formation (Kim et al. 2011). However, in conditions of nutrient surplus, activated mTORC1 phosphorylates ULK1 on a residue different than targeted by AMPK and blocks the interaction between ULK1 and AMPK (Kim et al. 2011).
2.2 AMPK
AMPK is a Ser/Thr kinase composed of three subunits: catalytic α subunit, one scaffold β, and the regulatory γ subunit, which binds adenine nucleotides. Mammals have two catalytic subunits α and β and three γ isoforms. Unlike mTOR, which senses amino acid levels indirectly through its interaction partners (discussed in the section on mTORC1), AMPK activity is directly affected by cellular AMP/ATP and ADP/ATP ratios. This regulation is accomplished through four tandem repeats of cystathionine β-synthase motifs located in the γ subunit, which form the nucleotide-binding pockets of the enzyme. AMP binding to the γ subunit triggers the phosphorylation of the activation loop at Thr172 in the kinase domain of the α subunit by the upstream activating kinase LKB1. AMP and ADP binding protects AMPK against dephosphorylation; however only AMP triggers an allosteric activation of the enzyme (Suter et al. 2006; Sanders et al. 2007; Xiao et al. 2011). These activatory effects of AMP and ADP are antagonized by ATP. Therefore, AMPK activity is coupled to fluctuations in AMP/ATP and ADP/ATP ratios, which typically rise in conditions of nutrient deprivation.
There are two well-characterized AMPK kinases: LKB1 (liver kinase B 1, also known as serine/threonine kinase 11) (Hawley et al. 2003) and CAMKKβ (the Ca2+/calmodulin-dependent kinase kinase β) (Hawley et al. 2005; Woods et al. 2005). LKB1 requires heterodimerization with two other proteins, sterile20-related adaptor (STRAD) and mouse protein 25 (MO25), for full activation (Hawley et al. 2003). LKB1 phosphorylates most other kinases in the AMPK-related kinase family (Lizcano et al. 2004). CAMKK activity is coupled to Ca2+ levels, and its activation of AMPK is AMP-independent. Apart from LKB1 and CAMKKβ, there are other kinases proposed to phosphorylate and activate AMPK; however whether they act as endogenous AMPK kinases in vivo has yet to be established (Momcilovic et al. 2006). AMPK activation by LKB1 involves the recruitment of AMPK by Ragulator to the cytosolic surface of the lysosomal membrane under nutrient deprivation conditions. Explained in more detail in the mTOR section, Ragulator is a protein complex involved in the amino acid sensing of mTORC1 through a process that also requires mTORC1 recruitment to the lysosomal membrane. Activation of AMPK results in the phosphorylation of TSC1/TSC2 complex, which inhibits mTORC1 activity, thereby coupling the low-energy state-dependent AMPK activation to mTOR activation. A reciprocal regulation between AMPK and mTORC1 was also described, where activated S6 K1 phosphorylates and inhibits AMPK activity (Dagon et al. 2012).
Activation of AMPK triggers catabolic pathways aimed to restore cellular energy status. Therefore AMPK activity is negatively regulated by anabolic signals. For example, insulin-/IGF-mediated Akt activation results in the phosphorylation of a serine residue in AMPK α subunit, which in turn blocks the activatory Thr172 phosphorylation (Hawley et al. 2014). Other inhibitory phosphorylation sites on the α subunit were also reported including regulation by Fyn kinase (Yamada et al. 2016), a Src tyrosine kinase family member that also phosphorylates and inhibits LKB1 (Yamada et al. 2010), by protein kinase C (PKC) (Heathcote et al. 2016), and by Unc-51-like kinase 1 (Ulk1) (Löffler et al. 2011). AMPK plays a major role in starvation-induced autophagy induction through at least two distinct pathways: As described above, AMPK inhibits mTORC1 activity through direct phosphorylation of TSC2 and regulatory-associated protein of mTOR (Raptor) (Inoki et al. 2003; Gwinn et al. 2008), a component of the mTORC1 protein complex. In addition, AMPK directly phosphorylates and activates Unc-51-like kinase 1 (ULK1) (Lee et al. 2010; Egan et al. 2011; Kim et al. 2011; Zhao and Klionsky 2011), which triggers autophagy and mitophagy. ULK1 in turn phosphorylates and inhibits AMPK function, forming a negative feedback loop evolved to probably block excess autophagic flux (Löffler et al. 2011). Besides autophagy, AMPK induces glucose and fatty acid update, through increased membrane localization of glucose transporters, and fatty acid transporter CD36, respectively (Kurth-Kraczek et al. 1999; Fryer et al. 2002; Wu et al. 2013a). Catabolic pathways including glycolysis, mitochondrial biogenesis, and fatty acid oxidation are also activated by AMPK (Witters et al. 1991; Winder and Hardie 1996; Merrill et al. 1997; Marsin et al. 2000, 2002; Jäger et al. 2007; Cantó et al. 2009). On the other hand, synthesis of fatty acids, proteins, and glycogen is suppressed by AMPK. These are accomplished either through direct phosphorylation of AMPK targets involved in these individual processes or indirectly through inhibition of mTORC1 and its downstream targets. Inhibition of fatty acid synthesis and promotion of fatty acid oxidation by AMPK largely depend on phosphorylation and inhibition of acetyl-CoA carboxylase (ACC) (Hardie and Pan 2002). ACC catalyzes the conversion of acetyl-CoA to malonyl-Coa, which is the precursor for fatty acid synthesis. Malonyl-CoA is also an inhibitor of carnitine palmitoyltransferase I (CPT1), an outer mitochondrial membrane protein that transports fatty acyl CoAs from cytosol to mitochondria for their oxidation. In summary, AMPK-mediated inhibition of ACC results in a decrease in cellular malonyl-CoA pool, which in turn blocks fatty acid synthesis and triggers fatty acid oxidation. It is worth to note that manipulations altering hypothalamic malonyl-CoA levels also affect the food intake and body weight (Lane et al. 2008). For example, fatty acid synthase inhibitors C75 or cerulenin administered by intraperitoneal or intracerebroventricular route result in suppression of food intake and reduced body weight (Loftus et al. 2000). ICV C75 rapidly increases the hypothalamic malonyl-CoA levels and suppresses food intake; however, prior ICV administration of an ACC inhibitor prevents the malonyl-CoA accumulation and the decreased food intake induced by ICV C75 (Hu et al. 2003).
2.3 Histone Deacetylases: Sirtuins and Classical HDACs
Acetylation is a major posttranslational modification regulating protein function and contributes to metabolic homeostasis (Iyer et al. 2012; Menzies et al. 2016). Protein acetylation is regulated by acetyltransferases and deacetylases. Apart from the N-terminal acetylation (Starheim et al. 2012), which is thought to be irreversible, the coordinated action of acetyltransferases and deacetylases makes protein acetylation a reversible process. Acetyltransferase reaction involves the transfer of an acetyl group from acetyl coenzyme A (acetyl-CoA) to an amino acid residue on the substrate protein. N-terminal acetyltransferases acetylate the first amino acid in the nascent polypeptide chain following the initial methionine residue, whereas the acceptor residue is usually a lysine in other acetylation reactions catalyzed by lysine acetyltransferases. The cytosolic enzyme adenosine triphosphate (ATP)-citrate lyase converts the glucose-derived citrate into acetyl-CoA, thereby regulating a critical step that links glucose availability to histone acetylation and regulation of gene expression (Wellen et al. 2009). Histone acetyltransferases (HATs) are broadly grouped into two categories, nuclear A-type HATs and the cytoplasmic B-type HATs, which are further categorized into several subfamilies (Roth et al. 2001). The B-type HATs generally regulate the shuttling of the newly synthesized histones from the cytosol to the nucleus, while the nuclear HATs coordinate transcription-related processes.
The histone deacetylase family is grouped into four classes based on sequence homology. Class I, II, and IV are comprised of the classical HDACs (HDAC 1–11), while the sirtuin family (SIRT1–7) constitutes the class III. Classical HDACs have one deacetylase domain except HDAC6 and HDAC10, which have two catalytic domains, although one of these domains in HDAC10 is inactive (de Ruijter et al. 2003; Seto and Yoshida 2014). Classical HDACs are Zn + −dependent enzymes that deacetylate histones and other nuclear and cytosolic targets. They are subject to posttranslational modifications themselves, such as phosphorylation, which couple hormonal and nutritional inputs to their subcellular localization and therefore to their histone deacetylase activity [REF]. HDACs also have nonenzymatic functions: For example, HDAC6, which is a class IIb HDAC and the largest member of the HDAC family, has a C-terminal ubiquitin-binding domain, which renders this protein a central component of protein homeostasis. Likewise, SIRT1 has been proposed to confer, e.g., neuroprotection, independently of its deacetylase activity (Pfister et al. 2008).
Sirtuins (silent mating type information regulation 2 homolog, from here on abbreviated as SIRTs) are evolutionarily conserved enzymes that are involved in biological processes including cellular differentiation, apoptosis, metabolism, and aging. Unlike other members of the HDAC family, the activity of class III HDACs is coupled to cellular concentration of the oxidized form of nicotinamide adenine dinucleotide (NAD+). Sirtuin-mediated deacetylation (and ADP-ribosylation) reaction utilizes NAD+ as a coenzyme and generates nicotinamide and O-acetyl-ribose as by-products (Tong and Denu 2010). Sirtuins are insensitive to the inhibitors of classical HDACs such as trichostatin A, whereas nicotinamide inhibits the sirtuin enzymatic activity [REF].
NAD+/NADH ratio typically rises in conditions of energy deficit. At the organismal level, fasting and calorie restriction result in elevated NAD+ levels in several tissues and lead to increased sirtuin activity. Mammals have seven members of the sirtuin family of deacetylases (SIRT 1–7), which differ in substrate specificity, subcellular localization, and tissue distribution (Houtkooper et al. 2012). While SIRT1 is both nuclear and cytosolic, SIRT2 is predominantly localized to the cytosol. SIRT3 is both mitochondrial and nuclear, SIRT4 and SIRT5 are mitochondrial, and SIRT6 and SIRT7 are exclusively nuclear proteins. SIRT4 and SIRT6 both have deacylase (Jiang et al. 2013; Laurent et al. 2013; Anderson et al. 2017) and ADP-ribosyltransferase activity [16,959,573, 10,381,378]. For example, SIRT6 catalyzes mono-ADP-ribosylation of PARP (poly[adenosine diphosphate (ADP)-ribose] polymerase 1) under oxidative stress to promote DNA repair (Mao et al. 2011). SIRT5 is unique in the sense that it is the only enzyme identified in mammals that can desuccinylate, demalonylate, and deglutarylate substrate proteins (Du et al. 2011; Tan et al. 2014), and the role of these additional modifications on protein function has not been completely uncovered.
SIRT1 has been the most extensively studied member of the sirtuin family. SIRT1 has the closest homology to yeast Sir2, and unlike other sirtuin knockout (KO) mice, global deletion of SIRT1 is mostly lethal at embryonic or early postnatal period [REF]. Inducible deletion of SIRT1 in adult mice does not lead to lethality, suggesting a fundamental role for SIRT1 in development (Price et al. 2012). SIRT1 is ubiquitously expressed in mammals, with the highest expression detected in the heart and nervous system in the developing embryo and in the lung, testis, and ovaries in adult rodents (Sakamoto et al. 2004).
The activity of SIRT1 is also subject to indirect regulation by a series of enzymes involved in the synthesis and degradation of the cellular NAD+. Nicotinamide phosphoribosyltransferase (NAMPT) and nicotinamide mononucleotide adenylyltransferase 1 (NMNAT1) synthesize NAD+ from nicotinamide in a two-step reaction called the NAD-salvage pathway (Revollo et al. 2004; Zhang et al. 2009). NMNAT1-mediated NAD+ biosynthesis and the resulting SIRT1 activation help prevent neurodegeneration in mice. Hydrolysis of NAD+ is predominantly regulated by a cyclic ADP-ribose hydrolase called CD38 (Aksoy et al. 2006b). CD38 knockout mice have 10–20 times elevated NAD+ levels across various tissues. This in turn results in increased SIRT1 activity in the absence of CD38 (Aksoy et al. 2006a). The role of NAD+-dependent regulation of SIRT1 in overall physiology is further emphasized by that the CD38 knockout mice are protected from high fat diet-induced obesity, and this phenotype is dependent of SIRT1 deacetylase activity (Barbosa et al. 2007). The expression of SIRT1, NAMPT, and the cellular NAD+ levels are all subject to a circadian regulation, where SIRT1 directly controls activity of key factors involved in the regulation of circadian rhythm, such as BMAL1, CLOCK, and PER2 as well as NAMPT, thereby regulating the formation of its own coenzyme (NAD+) and forming a transcriptional feedback loop (Asher et al. 2008; Nakahata et al. 2008, 2009; Ramsey et al. 2009). Regulation of the circadian clock by SIRT1 extends to the central pacemaker, the hypothalamic suprachiasmatic nucleus, where loss of SIRT1 activity drives the dysfunctional circadian rhythm observed in aging (Chang and Guarente 2013).
SIRT1 activity is subject to an indirect regulation by AMPK. Activation of AMPK by AICAR in the skeletal muscle leads to increased cellular NAD+/NADH ratio and leads to SIRT1 activation and increased deacetylation of SIRT1 targets such as PGC1α (Cantó et al. 2009). Increased SIRT1 activity then promotes mitochondrial biogenesis and oxygen consumption (Cantó et al. 2009). AMPK can directly phosphorylate and regulate SIRT1. Furthermore, increased SIRT1 activity leads to AMPK activation (Price et al. 2012) probably though deacetylation of LKB1 (Hou et al. 2008; Lan et al. 2008). Deleted in breast cancer 1 (DBC1), a tumor suppressor, acts as an endogenous inhibitor of SIRT1, while (Kim et al. 2008b) another nuclear protein called ribosomal protein S19 binding protein 1 (also called active regulator of SIRT1 – AROS) increases SIRT1 activity (Kim et al. 2007b). SIRT1 activity is also regulated by posttranscriptional modifications including phosphorylation by kinases such as AMPK, casein kinase 2 (CK2), and c-Jun N-terminal kinase 1 (JNK1) (Sasaki et al. 2008; Kang et al. 2009; Nasrin et al. 2009).
Because of its NAD+ dependence for activity, having a large spectrum of nuclear and cytosolic targets, and its expression being regulated by changes in the nutritional status, SIRT1 functions as a major nutrient and redox sensor, which regulates metabolism at different levels (Fulco et al. 2003). High expression of SIRT1 is shown in the periphery and in neurons of the central nervous system including the hypothalamic nuclei ARC, VMH, and PVN (Ramadori et al. 2008; Zakhary et al. 2010; Toorie and Nillni 2014). SIRT1’s subcellular localization is predominantly nuclear (Zakhary et al. 2010), but SIRT1 can translocate to the cytoplasm in a cell-specific and cell-autonomous manner, in response to various physiological stimuli and disease states (Tanno et al. 2007). SIRT1 regulates cellular survival and metabolism at several steps largely through deacetylation of protein targets (Brooks and Gu 2009; Peek et al. 2012; Orozco-Solis and Sassone-Corsi 2014), although there are reports on the deacetylase-independent function of SIRT1 (Pfister et al. 2008; Shah et al. 2012). During the past decade, the metabolic role of SIRT1 in mammals has been studied by tissue-specific transgenic models and pharmacological tools targeting its deacetylase activity (Blander and Guarente 2004; Chang and Guarente 2014). In the liver, SIRT1 deacetylates and activates the transcriptional coactivator PGC1-alpha and the FoxO1 to promote gluconeogenesis (Rodgers et al. 2005; Frescas et al. 2005). In adipose tissue, SIRT1 promotes fat mobilization by inhibiting peroxisome proliferator-activated receptor (PPAR-gamma) (Picard et al. 2004) and therefore functions to decrease fat storage, promote lipolysis, and protect against obesity-induced inflammation (Picard et al. 2004; Gillum et al. 2011; Chalkiadaki and Guarente 2012). SIRT1 improves glucose tolerance and positively regulates insulin secretion in pancreatic beta cells by repressing uncoupling protein 2 (UCP2) promoter (Moynihan et al. 2005; Bordone et al. 2006). In the liver SIRT1 protein levels increase upon fasting (Rodgers et al. 2005). SIRT1 deacetylates and activates PGC1α and FoxO1 and represses CREB-regulated transcription coactivator 2 (CRTC2, also known as TORC2) to regulate hepatic glucose output (Liu et al. 2008). Exposure to high-fat diet results in decreased liver SIRT1 levels (Deng et al. 2007). Compounds that lead to SIRT1 activation, such as resveratrol, have been shown to alleviate several adverse effects of high-fat diet, which otherwise results in the development of obesity and associated disorders (Baur et al. 2006; Lagouge et al. 2006). An increase in the activity and/or the expression level of SIRT1 is thought to mediate the physiology induced by calorie restriction (Cohen et al. 2004; Bordone and Guarente 2005; Bordone et al. 2007a, b). While the role of SIRT1 in the periphery is not going to be discussed further, it is important to note that by deacetylating a spectrum of metabolic regulators, SIRT1 links tissue energy status to the coordination of lipid, cholesterol, amino acid, and glucose metabolisms (Baur et al. 2006; Lagouge et al. 2006; Haigis and Sinclair 2010). Contrary to its role in the periphery, recent evidence shows that hypothalamic SIRT1 induces positive energy balance, and this activity is directly related to the regulation of hypothalamic peptide hormones by SIRT1. Therefore, this chapter will further focus on the role of central SIRT1 under different nutritional conditions on body weight, prohormone maturation, and the pro-converting enzymes involved in the processing of prohormones.
3 Regulation of Energy Homeostasis by the Hypothalamic Nutrient Sensors
3.1 mTOR
The initial studies on the role of hypothalamic mTOR signaling on energy homeostasis were conducted on rats (Cota et al. 2006; Blouet et al. 2008). mTOR is expressed in AgRP and POMC neurons (Cota et al. 2006), and fasting decreases mTOR activity in the ARC (Cota et al. 2006; Blouet et al. 2008; Cakir et al. 2009). Central administration of leucine activates central mTORC1, suppresses hypothalamic NPY expression, and inhibits fast-induced refeeding and weight gain in a rapamycin-sensitive manner (Cota et al. 2006). Rapamycin alone was also reported to activate ARC NPY expression (Shimizu et al. 2010). Furthermore, leptin activates hypothalamic mTOR signaling, likely through a PI3K-dependent pathway (Harlan et al. 2013), and this activation is required for leptin-mediated suppression of food intake (Cota et al. 2006; Ropelle et al. 2008). Accordingly, S6 K1 knockout (S6 K1 KO) mice are resistant to the anorectic action of exogenous leptin (Cota et al. 2008). Furthermore, acute and chronic exposure to high-fat diet (HFD) suppresses hypothalamic mTORC1 signaling, and leptin cannot activate hypothalamic mTORC1 in HFD-exposed animals (Cota et al. 2008). Hypothalamic mTORC1 activity is also sensitive, although indirectly, to the activity of fatty acid synthase (FASN), whose central inhibition induces potent weight loss and suppresses food intake (Loftus et al. 2000; Kumar et al. 2002). The anorectic action of FASN inhibitors is compromised upon mTORC1 inhibition (Proulx et al. 2008). These findings were supported by genetic data where activation of S6 K1 in the mediobasal hypothalamus (MBH) of rats suppressed food intake and weight gain while overexpression of a dominant negative S6 K1 increased both parameters and blunted central leptin action (Blouet et al. 2008). Leptin-induced sympathetic outflow to the periphery also depends on hypothalamic mTORC1 function (Harlan et al. 2013). mTORC1-mediated control of food intake could also, at least in part, depend on a S6K-dependent inhibitory phosphorylation of AMPK (Dagon et al. 2012). Contrary to these findings, deletion of TSC1 from rat insulin promoter (RIP)-positive cells (by RIP1-Cre), which cover pancreatic beta cells and a subpopulation of hypothalamic neurons, results in hyperphagic obesity (Mori et al. 2009). While this study did not report a problem with neuronal viability in the absence of TSC1, neuronal TSC1 knockout mice suffer from reduced myelination and neuronal survival (Meikle et al. 2007). POMC and AgRP/NPY neurons are RIP-Cre negative; however POMC-specific TSC1 deletion mimics the obese RIP1-Cre TSC1 knockout phenotype (Mori et al. 2009; Yang et al. 2012). Importantly, rapamycin treatment could reverse the effect of TSC1 deletion in these mice (Mori et al. 2009), as well as in old WT mice with elevated mTORC1 activity in POMC neurons (22884327). MBH-specific overexpression of DEPTOR, a negative regulator of mTORC1, also protected mice from HFD-induced weight gain (Caron et al. 2016a). This effect seems to be independent of mTOR activity in POMC neurons (Caron et al. 2016b). These seemingly contradictory findings listed above are likely due to the differential regulation of hypothalamic mTORC1 signaling in response to nutrient deprivation and hormonal inputs in different subhypothalamic nuclei. For example, fasting or lack of leptin action (ob/ob mice) suppresses mTORC1 signaling in the VMH but activates it in the ARC of mice (19628573). Furthermore, ghrelin and insulin, two orexigenic and anorexigenic hormones, respectively, both resulted in elevated ARC mTORC1 activity (Villanueva et al. 2009; Muta et al. 2015). The positive effect of ghrelin on hypothalamic mTORC1 signaling is supported by findings from other groups, which showed that S6 K1 KO mice were not responsive to ghrelin-induced weight gain (Stevanovic et al. 2013), and the ghrelin-induced food intake was reduced upon inhibition of mTORC1 activity (Martins et al. 2012). Furthermore, rapamycin reversed the hyperphagic phenotype of hyperthyroid rats, which had had elevated hypothalamic mTOR activity, resulting in weight loss (Varela et al. 2012), and blocked the anorectic effect of central insulin administration (Muta et al. 2015). Finally, cholecystokinin (CCK), which inhibits food intake by activating the responsive neurons in the NTS and the hypothalamus, also increases mTORC1 activity in the PVN (Lembke et al. 2011).
mTORC2 function has been less studied compared to that of mTORC1. The best-characterized role of mTORC2 is phosphorylation of Akt on Ser473, which facilitates Thr308 phosphorylation by PDK1 downstream of PI3K (Sarbassov et al. 2005). By phosphorylating PKCα (protein kinase C alpha) (Sarbassov et al. 2004; Jacinto et al. 2004), and other PKC family members (Gan et al. 2012; Li and Gao 2014), mTORC2 regulates the actin cytoskeleton and cell migration. It also activates glucose and lipid metabolism in liver and adipose tissue (Kumar et al. 2010; Hagiwara et al. 2012; Yuan et al. 2012). A recent study suggested that mTORC2 also acts as nutrient sensor: While acute glutamine deprivation activates mTORC2, prolonged lack of glutamine diminished mTORC2 activity (Moloughney et al. 2016). mTORC2 regulates the expression of GFAT1 in a glutamine-dependent manner (Moloughney et al. 2016). GFAT1 is the rate-limiting enzyme in the hexosamine biosynthetic pathway (HBP), which is involved in the hypothalamic regulation of energy balance (discussed below). The role of mTORC2 signaling in the brain and specifically in POMC and AgRP neurons was studied by targeted deletion of Rictor to inactivate mTORC2 signaling (Kocalis et al. 2014). Neuronal deletion of Rictor did not alter the food intake significantly despite increased hypothalamic NPY expression. The neuronal KO mice had higher fat mass and lower lean mass than WT counterparts (Kocalis et al. 2014). Accordingly, the mice had increased leptin levels, and resistance to the anorectic action of leptin, and glucose intolerance. While AgRP-specific Rictor KOs did not have a major phenotype, POMC-specific ablation resulted in heavier mice with increased food intake and adiposity even more than the neuronal KOs (Kocalis et al. 2014). The exact mechanism of mTORC2-mediated regulation of energy balance is not clear. While mTORC2 positively regulating Akt phosphorylation is important in the insulin action, ablation of insulin signaling in POMC neurons by targeted deletion of IRS2 (Choudhury et al. 2005) or PDK1 (Belgardt et al. 2008) does not recapitulate the effects of POMC-specific deletion of Rictor, suggesting that the effect of hypothalamic mTORC2 is mediated through an PI3K-Akt independent pathway.
3.2 AMPK
In the hypothalamus, AMPK acts a positive regulator of energy balance. Hypothalamic AMPK activity increases by fasting and decreases by refeeding (Minokoshi et al. 2004; Taleux et al. 2008), an effect not observed in an obesity-resistant rat strain (Taleux et al. 2008). Activation of central AMPK, either by ICV infusion of a synthetic AMP analog and AMPK activator (AICAR) (Sullivan et al. 1994) or MBH-specific overexpression of constitutively active AMPK (CA-AMPK), results in increased food intake and body weight (Andersson et al. 2004; Minokoshi et al. 2004). Conversely, overexpression of dominant negative AMPK decreases food intake and weight gain. Accordingly, hypothalamic AMPK activity is suppressed by anorectic factors including insulin, leptin, MTII (an αMSH analog), CNTF, and estradiol but induced by ghrelin, adiponectin, and orexin-A (Andersson et al. 2004; Minokoshi et al. 2004; Steinberg et al. 2006; Kubota et al. 2007; López et al. 2008; Wu et al. 2013b; Martínez de Morentin et al. 2014). Furthermore, suppression of central AMPK activity is required for leptin’s anorectic effect, and hypothalamic overexpression of CA-AMPK can block central leptin action (Minokoshi et al. 2004; Gao et al. 2007). Consequently, leptin activates hypothalamic ACC activity and results in elevated central malonyl-CoA level, which is required for leptin’s anorectic effect (Gao et al. 2007). Additionally, the sympathetic outflow regulated by leptin to kidney and adipose tissue (Tanida et al. 2013) but not to liver (Tanida et al. 2015) also depends on an intact hypothalamic AMPK signaling. It is worth noting that the hormonal regulation of AMPK activity in the periphery vs. the CNS is different. For example, leptin activates AMPK in the skeletal muscle and induces fatty acid oxidation (Hardie and Pan 2002), while inhibition of hypothalamic AMPK activity by central action of leptin is essential for leptin’s anorectic effect (Andersson et al. 2004; Minokoshi et al. 2004).
Central infusion of glucose inhibits food intake in an AMPK-dependent manner, whereas a non-oxidizable form of glucose, 2-deoyxglucose, activates hypothalamic AMPK and induces food intake (Kim et al. 2004). This is likely due to the effect of glucose on cellular AMP/ATP ratio, which in turn alters AMPK activity. Consistent with its positive role in energy balance, hypothalamic AMPK activity positively correlates with NPY and AgRP expressions (Minokoshi et al. 2004; Lee et al. 2005). Leptin-mediated suppression of hypothalamic AMPK activity is compromised in DIO mice. Furthermore, in the medial hypothalamus, leptin activates AMPK selectively in DIO mice contrary to its effect in lean mice, which might contribute the leptin desensitization observed in obesity (Martin et al. 2006). Deletion of the α subunit of AMPK from POMC neurons results in induced weight gain and food intake and suppresses energy expenditure, whereas AgRP-specific AMPKα knockout mice were protected from weight gain (Claret et al. 2007). Central inhibition of CaMKK results in suppression of food intake and induces weight loss, and lack of CaMKKβ (CaMKK2 KO mice) confers protection from diet-induced obesity (Anderson et al. 2008). CaMKK2 knockout mice have reduced expression of AgRP and NPY and resistance to fasting-induced upregulation of NPY (Anderson et al. 2008). Furthermore, central glucagon-mediated suppression of food intake, in part, depends of suppression of hypothalamic CamKK2-AMPK signaling by glucagon (Quiñones et al. 2015).
Hypothalamic actions of AMPK extend beyond the regulation of energy balance and involve other homeostatic processes including glucose metabolism, sleep homeostasis, thyroid axis, and possibly the reproductive axis (Rougon et al. 1990; Chikahisa et al. 2009; Yang et al. 2010; Cheng et al. 2011; Claret et al. 2011; Kinote et al. 2012). BAT-mediated thermogenesis, for example, in response to agents such as GLP-1 agonists, estradiol, BMP-8B (bone morphogenetic protein 8B), and triiodothyronine (T3) also involves regulation of hypothalamic AMPK activity (Martínez de Morentin et al. 2014; Beiroa et al. 2014; Martins et al. 2016; Martínez-Sánchez et al. 2017).
As summarized above, AMPK and mTORC1 are master regulators of autophagy, which seems to play a role in the AMPK- and mTOR-mediated regulation of energy homeostasis. Inhibition of autophagy attenuates the AMPK- and rapamycin-mediated activation of NPY and suppression of POMC expressions in hypothalamic cell lines (Oh et al. 2016). Additionally, knockdown of AMPKα subunits in the ARC reduces autophagy and results in reduced food intake and weight gain (Oh et al. 2016). The role of central autophagy in the regulation of energy balance is cell type specific. Inhibition of autophagy in AgRP neurons blocks the fasting-induced AgRP upregulation and results in elevated αMSH levels (Kaushik et al. 2011). However, selective loss of autophagy in POMC neurons decreases αMSH levels and results in increased adiposity (Coupé et al. 2012; Kaushik et al. 2012; Quan et al. 2012).
3.3 Histone Deacetylases: Sirtuins and Classical HDACs with Special Emphasis on SIRT1
The hypothalamic role of the histone deacetylase family of proteins has recently begun to be uncovered. As noted below, the most extensively characterized HDAC member in regard to its central role has been SIRT1. We will begin this section with a brief summary of our current knowledge on the hypothalamic activity of classical HDACs and then focus most of the remainder of the chapter on SIRT1.
The expression pattern and regulation of the hypothalamic HDACs in response to nutritional challenges are not uniform. Hypothalamic histone acetylation and the expression of classical HDACs have been reported to be nutrient-sensitive. In lean mice, fasting decreases the global histone acetylation only in the ventrolateral subdivision of the ventromedial hypothalamus (Funato et al. 2011). Among the classical HDACs, HDAC10 and HDAC11 are the most highly expressed genes in the hypothalamus (Funato et al. 2011). Upon fasting, hypothalamic expressions of HDAC3 and HDAC4 increase, while that of HDAC10 and HDAC11 decline (Funato et al. 2011), while another study suggested a fasting-induced decrease in HDAC5 expression (Kabra et al. 2016). Hypothalamic HDAC8 expression was reported to be restricted to the paraventricular nucleus, where its expression is upregulated by fasting and high-fat diet. While one study reported increased hypothalamic HDAC5 expression upon 4-week high-fat diet feeding (Funato et al. 2011), another study suggested that chronic high-fat diet decreased total hypothalamic HDAC5 mRNA levels (Kabra et al. 2016). There was no detectible change in the hypothalamic expression of other classical HDACs under high-fat diet feeding (Funato et al. 2011). HDAC5 is the only classical HDAC reported to be involved in the regulation of leptin signaling. Hypothalamic HDAC5 levels are positively regulated by leptin, and ablation of HDAC5 potentiates weight gain on high-fat diet (Kabra et al. 2016). Mechanistically, HDAC5 was suggested to deacetylate and increase the transcriptional activity of STAT3 on POMC promoter (Kabra et al. 2016). It is important to note, however, that several other studies indicated that acetylation positively regulates STAT3 phosphorylation, dimerization, and transcriptional activity (Yuan et al. 2005; Wang et al. 2005; Nie et al. 2009; Dasgupta et al. 2014; Chen et al. 2015). For example, SIRT1 inhibits hepatic STAT3 activity in the liver by deacetylation of multiple lysine residues (Nie et al. 2009), although hypothalamic SIRT1 inhibition does not alter STAT3 acetylation at least on Lys 685 (Cakir et al. 2009). The precise role of STAT3 acetylation on individual residues and their contribution to energy homeostasis remain to be further characterized.
Neuronal SIRT1 expression is predominantly nuclear in rodent and human brain (Zakhary et al. 2010). Brain nuclei associated with neurodegeneration (such as the prefrontal cortex, hippocampus, and basal ganglia) as well as the homeostatic centers including the hypothalamus and the brain stem have detectable SIRT1 expression (Ramadori et al. 2008; Zakhary et al. 2010). Activation of neuronal SIRT1 has neuroprotective effects and phenocopies some of the benefits of caloric restriction on neurodegeneration, such as the attenuation of amyloid plaques observed in Alzheimer disease (Qin et al. 2006; Kim et al. 2007a). Developmentally, neuronal SIRT1 plays a fundamental role in neural differentiation and survival. In neuronal progenitor cells (NPCs), SIRT1 couples oxidative stress to inhibition of neurogenesis and directs the progenitor cells toward an astrocyte lineage (Prozorovski et al. 2008). However, under normal conditions, SIRT1 blocks Notch signaling, which normally suppresses proneural gene expression and confers neural progenitor cell maintenance. Therefore, neuronal SIRT1 action promotes neurogenesis (Hisahara et al. 2008). Furthermore, deletion of SIRT1 in adult NPCs in rodents leads to increased progenitor oligodendrocyte population (Rafalski et al. 2013). The role of SIRT1 in neuroprotection is also supported by findings obtained from Wallerian degeneration slow (Wlds) mice where a mutation encompassing the NAMPT gene results in increased NAD+ production and elevated SIRT1 activity, which in turn delays the axonal degeneration (Araki et al. 2004).
The role of hypothalamic SIRT1 on metabolism in rodents has been studied by several groups both genetically and pharmacologically. SIRT1 expression is sensitive to cellular energy status such that increased SIRT1 expression and nuclear localization have been reported by many studies supporting its role as an energy sensor in the periphery and in the brain (Kanfi et al. 2008; Cantó et al. 2009; Cakir et al. 2009; Dietrich et al. 2010). SIRT1 protein levels in rodents increase by calorie restriction in several metabolic tissues including the brain (Cohen et al. 2004). Upon fasting, SIRT1 activity increases specifically in the hypothalamus, but not in the hindbrain (Ramadori et al. 2008; Cakir et al. 2009); this is due to the fasting-induced increase in hypothalamic SIRT1 protein level and NAD+ concentration. Inhibition of central SIRT1 activity leads to cessation of feeding and results in weight loss in lean as well as obese rodents, while its acute activation by resveratrol appears to have beneficial effects in glucose homeostasis (Knight et al. 2011). Neuronal depletion of SIRT1 promotes systemic insulin sensitivity and counters diet- and age-induced weight gain, while forebrain-specific overexpression of SIRT1 results in decreased glucose tolerance, energy expenditure, and increased adiposity. In contrast, in older mice neuronal overexpression of SIRT1 seems to counteract the aging-induced gene expression profile (Oberdoerffer et al. 2008). Therefore, the specific role of SIRT1 in mammalian physiology especially in the context of its role in the regulation of neuroendocrine circuits should be evaluated based on specific tissue/cell population in consideration of the nutritional status and stress factors.
One well-known substrate of SIRT1 is the transcription factor, FoxO1, whose deacetylation augments its activity (Huang and Tindall 2007; Xia et al. 2013). FoxO1 is also a metabolic sensor in that it integrates both leptin and insulin signaling (Kitamura et al. 2006; Kim et al. 2006) pathways. It is abundantly expressed in metabolically relevant hypothalamus nuclei, including neurons of the ARC, DMH, and VMH (Toorie et al. 2016). Among its metabolic functions, FoxO1 transcriptionally regulates AgRP and NPY expression in a positive manner, while transcriptionally repressing POMC, carboxypeptidase E (CPE), and steroidogenic factor 1 SF1 expressions (Kitamura et al. 2006; Kim et al. 2006; Plum et al. 2009). In addition, FoxO1 positively regulates SIRT1 expression at the transcriptional level (Xiong et al. 2011). Physiologically, SIRT1’s role as energy sensor is determined by its dependency on NAD+ (Ramadori et al. 2008; Cantó et al. 2012). New studies have uncovered the involvement of hypothalamic SIRT1 in nutrient sensing (Ramadori et al. 2008; Cakir et al. 2009). As mentioned in the introduction and further developed in the following sections, SIRT1 in the ARC induces positive energy balance by affecting POMC and CPE gene expression through FoxO1. The role of hypothalamic SIRT1 and the consequences of genetic ablation of SIRT1 from distinct hypothalamic neuronal populations are discussed extensively in the following sections. SIRT1 also affects proCRH processing either through a pathway of FoxO1, preproPC2/proPC2, or directly through preproPC2/proPC2. Lastly, the potential effect of SIRT1 action on the transcription factor nescient helix-loop-helix 2 protein (Nhlh2) will also be considered.
3.4 Other Players
Apart from AMPK, mTOR, and sirtuins, our knowledge on the role of hypothalamic nutrient sensors on energy homeostasis has been limited. Two recent studies addressed the role of the hypothalamic hexosamine biosynthetic pathway (HBP) in energy homeostasis. A small percentage of the cellular glucose is shuttled through the HBP, where glucose is metabolized to UDP-N-acetylglucosamine (UDP-GlcNAc) (Yang and Qian 2017). Besides the N-linked and O-linked glycosylation reactions, UDP-GlcNAc is the major sugar donor in O-GlcNAcylation, which is the posttranslational modification of proteins with O-linked N-acetylglucosamine on serine and threonine residues. This process involves at least two enzymes, O-GlcNAc transferase (OGT) and O-GlcNAcase (OGA), which collectively regulate the cellular pool of O-GlcNAcylated substrates in a nutrient-sensitive manner. For example, increased glucose concentration stimulates O-GlcNAcylation in hyperglycemia (Liu et al. 2000). Cellular O-GlcNAcylation increases upon increased HBP flux typically observed upon hyperglycemia, unfolded protein response, and nutrient deprivation-induced OGT expression.
OGT is expressed in the AgRP neurons, and its expression is induced by fasting and ghrelin [25303527]. Deletion of OGT from AgRP neurons blocks their excitability and confers resistance to diet-induced weight gain (Ruan et al. 2014), at least in part by modulating the O-GlcNAcylation and thus activity of the voltage-dependent potassium channel Kcnq3. Driven by earlier genome-wide association studies (GWAS) indicating that glucosamine-6-phosphate deaminase (GNPDA2), a regulator of HBP, correlates with obesity, another group characterized the role of OGT in the forebrain and PVN in mice (Lagerlöf et al. 2016). Using a tamoxifen-inducible CaMKII-Cre model, Lagerlöf and colleagues showed that deletion of OGT from the forebrain (including the hypothalamus) results in hyperphagic obesity in adult mice (Lagerlöf et al. 2016). This phenotype was reproduced by deletion of OGT specifically in the PVN. O-GlcNAc level was positively regulated by glucose in the PVN, while fasting decreased O-GlcNAc levels. Furthermore, these changes were restricted to CaMKII-positive neurons (Lagerlöf et al. 2016). These findings also point to the heterogeneous role of the nutrient sensors in the hypothalamus as described for SIRT1 and mTORC1 above.
Another conserved cellular nutrient sensor is a family of proteins called Per-Arnt-Sim (PAS) kinases (DeMille and Grose 2013). PASK is typically activated by high glucose concentrations and regulates glucose partitioning in yeast (Smith and Rutter 2007) and glucagon secretion in mammals (da Silva Xavier et al. 2011). PASK knockout mice are protected from HFD-induced obesity and insulin resistance (Hao et al. 2007). The role of central PASK in the regulation of energy balance has not been directly addressed; however, hypothalamic PASK signaling is sensitive to glucose concentrations and GLP-1 at least in the LH and VMH (Hurtado-Carneiro et al. 2013). Hypothalamic PASK signaling appears to regulate AMPK and mTORC1 pathways such that the response of AMPK and mTORC1 signaling to fasting and refeeding is dysregulated in PASK-deficient mice (Hurtado-Carneiro et al. 2014).
4 Prohormone Processing and Prohormone Convertases
The biosynthesis of mammalian neuropeptide hormones follows the principles of the prohormone theory, which begins with a messenger RNA (mRNA) translation into a large, inactive precursor polypeptide, followed by a limited posttranslational proteolysis to release different products of processing (Steiner 1998; Nillni - Endocrinology and 2007 2007). In rodents and humans, abnormalities in prohormone processing result in deleterious health consequences, including metabolic dysfunctions (Naggert et al. 1995; Jackson et al. 1997; Challis et al. 2002; Nillni et al. 2002; Jing et al. 2004; Lloyd et al. 2006). To produce an active hormone, the prohormone is subjected to differential processing by the action of different members of the family of PCs, which results in biological and functional diversity within the central nervous system (CNS) as well as in endocrine cells in the periphery. The PCs comprise a family composed of seven subtilisin−/kexin-like endoproteases, and relevant to the neuroendocrine tissues are PC1 and PC2 (Seidah et al. 1990, 1991, 1992, 1996; Smeekens and Steiner 1990; Smeekens et al. 1991; Constam et al. 1996). These enzymes cleave at the C-terminal side of single, paired, or tetra basic amino acid residue motifs (Rouillé et al. 1995), followed by removal of remaining basic residue(s) by carboxypeptidase E and D (CPE, CPD) (Fricker et al. 1996; Xin et al. 1997; Nillni et al. 2002). The selective expression of PC1 and PC2 in endocrine and neuroendocrine cells demonstrated to be important in prohormone tissue-specific processing (Seidah et al. 1990, 1991, 1994; Schafer et al. 1993). PC1 and PC2 have been shown to process proTRH (Friedman et al. 1995; Nillni et al. 1995; Pu et al. 1996; Schaner et al. 1997; Nillni 2010), proinsulin (Smeekens et al. 1992; Steiner et al. 1992; Rouillé et al. 1995), proenkephalin (Breslin et al. 1993), prosomatostatin (Galanopoulou et al. 1993; Brakch et al. 1995), progrowth hormone-releasing hormone (proGHRH) (Posner et al. 2004; Dey et al. 2004), POMC (Thomas et al. 1991; Benjannet et al. 1991), pro-corticotropin-releasing hormone (proCRH) (Vale et al. 1981a; Rivier et al. 1983; Castro et al. 1991; Brar et al. 1997; Perone et al. 1998), proNPY (Paquet et al. 1996), proCART (Dey et al. 2003), and proneurotensin (Villeneuve et al. 2000) to various intermediates and end products.
Within the hypothalamus, PC1 and PC2 display an extensive overlapping pattern of expression (Schafer et al. 1993; Winsky-Sommerer et al. 2000). The critical role of PC1 and PC2 in prohormone processing is underscored by studies from animals lacking the genes encoding PC1 (Zhu et al. 2002) and PC2 (Furuta et al. 1997) as well as 7B2 (Westphal et al. 1999; Laurent et al. 2002) a neuropeptide essential for the maturation of PC2. Disruption of the gene-encoding mouse PC1 results in a syndrome of severe postnatal growth impairment and multiple defects in the processing of many hormone precursors, including dysfunctional processing of the hypothalamic proGHRH to mature GHRH, pituitary POMC to adrenocorticotropic hormone, islet proinsulin to insulin, and intestinal proglucagon to glucagon-like peptide-1 and peptide-2. A mouse model of PC1 deficiency generated by random mutagenesis (Lloyd et al. 2006) with a missense mutation in the PC1 catalytic domain (N222D) leads to obesity with abnormal proinsulin processing and multiple endocrine deficiencies. Although there was defective proinsulin processing leading to glucose intolerance, neither insulin resistance nor diabetes had developed despite obesity. The apparent key factor in the induction of obesity was impaired autocatalytic activation of mature PC1 causing reduced production of hypothalamic α-MSH (Lloyd et al. 2006). A patient with a compound heterozygous mutation in the PC1 gene resulting in production of nonfunctional PC1 had severe childhood obesity (Jackson et al. 1997). An analogous obese condition was found in a patient with a defect in POMC processing (Challis et al. 2002). As PC1 and PC2 are essential for the processing of a variety of proneuropeptides, alterations in the expression and protein biosynthesis of PC1 and PC2 also have profound effects on neuropeptide homeostasis.
4.1 SIRT1 Regulates POMC and AgRP Peptides
The interplay between POMC biosynthesis/processing to its derived peptides regulated by SIRT1 was first demonstrated in our laboratory using the Sprague-Dawley rat model (Cakir et al. 2009). This model shares with humans many characteristics of obesity physiology (Challis et al. 2002), and rats are considered to be an excellent model for this type of studies (Levin et al. 1997; Nillni et al. 2002; Cottrell and Ozanne 2008). Early studies using this rat model showed that POMC biosynthesis and processing is nutrition dependent (Perello et al. 2007). Indeed, the biosynthesis of POMC and its derived peptides including ACTH and α-MSH decrease by fasting and increase when fasted animals were treated with leptin that mimics response to refeeding. Similar correlation was observed for PC1 and PC2. These findings demonstrated that the production of POMC-derived peptides and processing enzymes in the ARC are nutrient-sensitive (Perello et al. 2007).
SIRT1 protein levels in the hypothalamus change in response to diet and appear to mediate several aspects of hypothalamic control; therefore, understanding its role in regulating the melanocortin system is timely. Indeed, we recently showed that SIRT1 expressed in the ARC participates in the regulation of AgRP and POMC neurons in an orexigenic capacity to control feeding behavior. In that study, central inhibition of SIRT1 decreased body weight and food intake as a result of a FoxO1-mediated increase in the POMC and decrease in the orexigenic AgRP expressions in the ARC (Cakir et al. 2009; Cyr et al. 2014; Toorie and Nillni 2014). This conclusion was supported by an array of different studies from our laboratory and others. For example, siRNA-mediated knockdown of ARC SIRT1 expression resulted in reduced food intake and body weight gain (Cakir et al. 2009). In addition, pharmacological inhibition of SIRT1 activity using the chemical compound EX-527 via intracerebroventricular route (ICV) suppressed fasting-induced hyperphagia and weight gain in lean rodents (Cakir et al. 2009; Cyr et al. 2014). This reduction in body weight was associated with elevated ARC POMC (Cakir et al. 2009; Cyr et al. 2014) and reduced AgRP expressions (Cakir et al. 2009). Conversely, ICV infusion of a SIRT1 activator (Sinclair and Guarente 2014) resulted in an immediate (and short-lived) increase in food intake in refed rats (Cakir et al. 2009). Co-administration of a MC3/4R antagonist, SHU9119, with the SIRT1 inhibitor (EX-527) reversed the anorectic effect of EX-527, suggesting the involvement of the central melanocortin system in mediating the effect of hypothalamic SIRT1 on energy homeostasis. Central SIRT1 inhibition resulted in reversal of fasting-induced suppression of hypothalamic mTORC1 pathway (Cakir et al. 2009), which is a negative regulator of energy balance (Cota et al. 2006), suggesting that SIRT1 acted as an upstream negative regulator of mTOR, possibly acting through a TSC2-dependent pathway (Ghosh et al. 2010). Overall, these results point to an orexigenic role of ARC SIRT1 in the lean animals (Cakir et al. 2009; Cyr et al. 2014).
Hypothalamic SIRT1 action also regulates the central effects of ghrelin, a potent orexigenic factor secreted mainly from the stomach. Dietrich and colleagues demonstrated that peripheral as well as central administration of EX-527 was sufficient to reduce food intake during the dark cycle and suppress the ghrelin-induced hyperphagia. This effect on food intake was not associated with concomitant changes in energy expenditure; rather, it was mediated through reduced melanocortin tone and was dependent on the action of uncoupling protein 2 (UCP2) (Dietrich et al. 2010). It was also demonstrated that the orexigenic action of ghrelin is mediated via a SIRT1-p53 pathway, proposed to be acting upstream of hypothalamic AMPK: Central SIRT1 inhibition blocked the ghrelin-induced hyperphagia and hypothalamic fatty acid metabolism, reducing the ghrelin’s effect on FoxO1, NPY, and AgRP expressions (Velásquez et al. 2011). SIRT1 also increases the expression of the type 2 orexin receptor in the dorsomedial and lateral hypothalamic nuclei (DMH and LH, respectively) by deacetylating the homeodomain transcription factor Nkx2–1 and augmenting the response to ghrelin (Satoh et al. 2010, 2013).
Further support for the orexigenic role of neuronal SIRT1 came from a study on a neuron-specific SIRT1 knockout (SINKO) model that was generated by crossing SIRT1-floxed mice to mice expressing the Cre transgene under the control of neuron-specific synapsin I promoter (Syn1-Cre) (Lu et al. 2013). SINKO mice had a reduced neuronal SIRT1 mRNA and protein content and significantly elevated acetylation of hypothalamic p53 and STAT3. Neuronal SIRT1 deficiency resulted in increased central insulin signaling and protection against weight gain, systemic insulin resistance, and inflammation in the white adipose tissue in mice fed a high-fat diet (Lu et al. 2013). These effects were at least in part mediated by lack of an inhibitory deacetylation of IRS-1 by SIRT1 in SINKO mice (Lu et al. 2013). Additionally, AgRP-specific SIRT1 depletion phenocopies the neuronal SIRT1 KO mice in regard to their lower body weight (Dietrich et al. 2010). Another neuronal SIRT1 KO model was generated using Nestin-Cre mice: Resulting mice were smaller than WT counterparts. This was in part due to a downregulation of the growth hormone levels in the KO mice, which also had smaller pituitary mass (Cohen et al. 2009). Brain SIRT1 expression was reported to decrease by aging in the anteroventral thalamic nucleus (AV) and the ARC in mice (Lafontaine-Lacasse et al. 2010). Using mouse prion promoter, a brain-specific SIRT1 transgenic mouse was developed (Satoh et al. 2010). The mice had normal food intake and body weight compared to the WT mice at 5 and 20 months of age. One of the transgenic lines that expressed SIRT1 at higher levels in the DMH and LH compared to other hypothalamic nuclei had an extended life span. Surprisingly, another transgenic line that had uniformly higher SIRT1 expression throughout the hypothalamus did not mimic these phenotypes, suggesting a differential role of SIRT1 in different subhypothalamic nuclei at least in the context of aging. Overexpression of SIRT1 under the CaMKIIα promoter to achieve forebrain-specific expression resulted in decreased glucose tolerance and energy expenditure, decreased plasma T3 and T4 levels, and increased adiposity (Wu et al. 2011). Overall, these studies suggest that hypothalamic SIRT1 is a positive regulator of energy balance, and pharmacological or genetic inhibition of central SIRT1 function results in decreased weight gain and food intake.
In contrast to the findings summarized above, some studies suggested a negative regulation of energy balance by hypothalamic SIRT1. Hypothalamic SIRT1 expression was proposed to be increased by short-term refeeding of fasted mice, and overexpression of SIRT1 in the mediobasal hypothalamus resulted in reduction of FoxO1-induced hyperphagia (Sasaki et al. 2010). SIRT1 overexpression rescued the obese phenotype induced by constitutively active POMC-specific FoxO1 (Susanti et al. 2014). Furthermore, POMC- or AgRP-specific SIRT1 overexpression prevented age-induced weight gain (Sasaki et al. 2014). In line with this finding, another study showed that SIRT1 and FoxO1 form a complex with necdin, a nuclear protein expressed in postmitotic neurons and located in the Prader-Willi locus (MacDonald and Wevrick 1997; Jay et al. 1997). The heterotrimeric complex promoted the SIRT1-mediated FoxO1 deacetylation (Hasegawa et al. 2012). Necdin KO mice had decreased serum levels of TSH, T3, and T4, decreased hypothalamic proTRH expression, and increased NPY and AgRP levels; however the body weight, food intake, or the energy expenditure of the mice was unaltered compared to the wild-type mice (Hasegawa et al. 2012). We would like to emphasize that the acute and chronic effects of factors that regulate energy balance could be dramatically different, and combined with the plasticity of the neuronal circuitry (Elson and Simerly 2015), these factors might account for some discrepancies in the literature. For example, PI3K is the main signaling node utilized by insulin receptor signaling; however, inhibition of PI3K pathway in rodents and primates has proven to induce weight loss and improve overall metabolism (Ortega-Molina et al. 2015), and reduced insulin/IGF-I signaling extends life span in metazoans. Orexins acutely stimulate food intake and weight gain (Sakurai et al. 1998). However, chronic orexin deficiency is associated with obesity in both rodents and humans, and transgenic overexpression of orexin confers resistance to diet-induced obesity (Funato et al. 2009). Likewise, acute inhibition of hypothalamic mTOR signaling has been associated with decreased feeding (Cota et al. 2006); however, mice with Rip2/Cre-mediated hypothalamic deletion of mTOR inhibitor Tsc1 (Rip-Tsc1cKO mice) develop hyperphagia and obesity (Mori et al. 2009).
Genetic ablation of SIRT1 from chemically distinct hypothalamic neurons resulted in phenotypes depending on the identity of the neuronal population. POMC-specific SIRT1 ablation resulted in increased high-fat diet-induced weight gain specifically in female mice but not in male mice (Ramadori et al. 2010). POMC neuronal survival, POMC mRNA expression, or hypothalamic ACTH or αMSH levels were not affected by depletion of SIRT1. These mice were reported to have decreased energy expenditure and reduced sympathetic tone to the perigonadal adipose tissue (Ramadori et al. 2010). However, central infusion of the SIRT1 inhibitor EX-527 resulted in increased POMC mitochondrial density and decreased inhibitory inputs onto POMC neurons (Dietrich et al. 2010). AgRP-specific SIRT1 KO mice showed an opposite phenotype to POMC-specific SIRT1 KOs, resulting in leaner mice with decreased food consumption (Dietrich et al. 2010). AgRP-specific ablation of PGC1α, a transcriptional coactivator positively regulated by SIRT1, results in decreased food intake, unaltered body weight with increased adiposity, but decreased lean mass (Gill et al. 2016). Deletion of SIRT1 from SF1 neurons, a subpopulation of VMH neurons, results in mice with increased body weight, normal food intake, and decreased energy expenditure (Ramadori et al. 2011). Furthermore, these mice had an altered circadian rhythm under food restriction (Orozco-Solis et al. 2015).
A full description and debate about different views on orexigenic vs. anorexigenic role of SIRT1 in the brain was previously described in a separate review (Toorie and Nillni 2014). It was concluded then that in lean animals, central SIRT1 functions as a nutrient sensor, and it is elevated during fasting state in neurons of the melanocortin system. Activated SIRT1 deacetylates and activates FoxO1, which in turn alters the biosynthesis and processing of POMC. Inhibition of central SIRT1 is sufficient to promote a negative energy balance. In the PVN, SIRT1 has also an effect on the CRH peptide that in turn also affects energy balance (see the next section). Table 6.1 depicts the impact that SIRT1 has in ARC and PVN under different nutritional conditions.
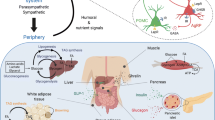
The next question is then, how does SIRT1 regulate POMC processing as well as downstream changes in body weight and energy expenditure in diet-induced obesity (DIO) state? Indeed, central inhibition of SIRT1 in DIO decreased body weight and increased energy expenditure at higher rate than in the lean state suggesting that a different mechanism is triggered in the obese state (Cyr et al. 2014). DIO and fasted lean rodents displayed elevated SIRT1 levels in their ARC (Cyr et al. 2014), and DIO animals exhibited weight loss due to acute central SIRT1 inhibition (Table 6.1). This is consistent with a prior study in mice where SIRT1 was reported to be elevated in the hypothalamus of db/db mice, which are obese because of a mutation in the leptin receptor (Sasaki et al. 2010). Interestingly, whereas reduced SIRT1 activity reduced food consumption in lean animals (Cakir et al. 2009; Dietrich et al. 2010; Cyr et al. 2014), DIO animals subjected to central SIRT1 inhibition remained normophagic (Table 6.1) (Cyr et al. 2014). Results from our group and others (Dietrich et al. 2010) show that lean rodents subjected to central SIRT1 inhibition lost weight because of a decrease in food intake, without any changes in energy expenditure. However, DIO animals subjected to SIRT1 inhibition by ICV infusion of EX-527 lost significant body weight not because of decreased in food intake but instead because of an increase in oxygen consumption (elevated energy expenditure). Brain inhibition of SIRT1 in DIO increased acetylated FoxO1, which in turn increased phosphorylated FoxO1 via improved insulin/pAKT signaling (Cyr et al. 2014). Elevated acetylation and phosphorylation of FoxO1 resulted in increased POMC levels along with an increase in the expression of the a-MSH maturation enzyme CPE, which resulted in more of the bioactive POMC product α-MSH released into PVN (Fig. 6.1). Increased in a-MSH led to augmented thyrotropin-releasing hormone (TRH) levels and circulating T3 levels (triiodothyronine, thyroid hormone). These results indicate that inhibiting hypothalamic SIRT1 in DIO enhances the activity of the hypothalamic-pituitary-thyroid (HPT) axis, which stimulates energy expenditure. Therefore, pharmacological inhibition of SIRT1 in DIO state causes negative energy balance by increasing energy expenditure and HPT axis activity (Cyr et al. 2014). What remains to be determined is whether SIRT1 also regulates proTRH in the PVN and POMC in the nucleus of the solitary track (Fig. 6.1).
Model depicting the regulation of hypothalamic POMC by SIRT1: In the ARC, SIRT1 deacetylates FoxO1, which decreases production of POMC and the POMC-processing enzyme CPE causing less α-MSH to reach MC3/MC4 receptors on target tissues. Lower α-MSH reduces TRH, T3, and thus energy expenditure. Inhibiting SIRT1 sensitizes Akt signaling, which increases pFox01. pFox01 promotes Fox01 nuclear exclusion, thereby increasing POMC and CPE, which increases α-MSH. SIRT1-mediated changes in POMC could affect target tissues such as the PVN and NTS that regulate energy expenditure. In the lean state, leptin and insulin signal through their respective receptors to ultimately increase Akt phosphorylation. pAKT translocates to the nucleus and phosphorylates the transcription factor FoxO1, thereby facilitating its inactivation and nuclear exclusion. Consequentially, POMC and CPE transcription is enhanced resulting in increased α-MSH, while AgRP transcription is repressed resulting in reduced AgRP. In both DIO and fasted conditions, insulin and leptin signaling are impaired via distinct mechanisms resulting in reduced pAKT levels, which promotes FoxO1 nuclear retention. In addition, SIRT1 protein content is increased within the ARC of both fasted and DIO individuals. SIRT1 via its deacetylation of FoxO1 positively regulates AgRP transcription while negatively regulating POMC and CPE transcription. Reduced POMC and CPE expression results in diminished levels of α-MSH, which signals through MC3/MC4 receptors to exert its potent anorectic effect; AgRP functions as an endogenous antagonist of the MC3/4R. SIRT1 also increases the number of GABAergic synapses onto POMC neurons by AgRP neurons, resulting in hyperpolarization of POMC neurons and reduced melanocortinergic tone. SIRT1 could deacetylate Nhlh2 in POMC neurons causing its activation of Nhlh2 that in turn has a direct role for transcriptional control of PC1. JAK, Janus-activated kinase; IRS, insulin receptor substrate; PIP2, phosphatidylinositol-4,5-bisphosphate; PIP3, phosphatidylinositol-3,4,5-trisphosphate; PI3K, phosphoinositide 3-kinase; STAT3, signal transducer and activator of transcription 3; Akt, protein kinase B; ac, acetyl; p, phosphate; GABA, gamma-aminobutyric acid. (This figure is adopted from our prior publication on the Molecular and Cellular Endocrinology (Mol Cell Endocrinol. 2016 Dec 15;438:77–88))
Collaborative studies conducted between the Good Laboratory and our group (Jing et al. 2004) revealed that targeted deletion of the neuronal basic helix-loop-helix (bHLH) transcription factor (Nhlh2) in mice results in adult-onset obesity. Nhlh2 expression can be found in rostral POMC neurons and TRH neurons of the paraventricular nucleus (PVN). We also demonstrated that in the absence of Nhlh2 in these neurons, the expression of the PC1 and PC2 mRNA and a-MSH peptides was reduced (Jing et al. 2004). In a further study, it was shown that Nhlh2 is part of the Janus kinase/STAT signaling pathway and is involved in the control of PC1 transcription by STAT3 after leptin stimulation (Fox and Good 2008). Interestingly, recent studies proposed that Nhlh2 is also involved in motivation behavior for exercise (Good et al. 2015) and it is also directly regulated by SIRT1 (Libert et al. 2011). SIRT1 deacetylates and activates Nhlh2, which in turn has a direct role for transcriptional control of PC1. Since food abundance suppresses SIRT1 and food scarcity increases SIRT1 in the ARC, it is possible that during fasting SIRT1 activation of Nhlh2 may affect PC1 in POMC neurons, an area worth exploring.
DIO results in increased hypothalamic SIRT1 expression. Central SIRT1 inhibition in DIO increases the α-MSH peptide released into PVN to stimulate TRH neurons, but these effects were not seen in lean rats (Table 6.1). In obese rats, TRH peptide levels were elevated in the PVN along with circulating levels of the active thyroid hormone T3, a generally accepted indicator of energy expenditure (Nillni 2010), yet no changes in TRH or T3 were detected in lean rats treated with EX-527 under the same conditions. However, EX-527 treatment resulted in a significant increase in plasma T3 levels in lean rats upon prolonged fasting (Cakir et al. 2009). These results suggest that regulation of POMC by SIRT1 is nutrition and stress dependent. In view of these findings, it seems that physiological changes or metabolic stressors affect prohormone processing. For example, we now know that cold stress (Nillni et al. 2002) and endoplasmic reticulum stress (Cakir et al. 2013) produce alterations in the processing of proTRH and POMC, respectively. Central SIRT1 inhibition further increases ARC POMC and CPE biosynthesis in DIO rats. This change in CPE was correlative with increases in a-MSH and TRH in the PVN of DIO rats as well as circulating T3 levels and oxygen consumption. These results collectively support the hypothesis that central SIRT1 promotes positive energy balance, as shown earlier (Cakir et al. 2009), and blocking SIRT1’s hypothalamic activity can promote a negative energy balance and weight loss. Supporting the notion of SIRT1 acting on the HPT axis, a previous study showed that SIRT1 positively regulates TSH exocytosis from pituitary thyrotropes (Akieda-Asai et al. 2010). Together these results suggest that SIRT1 regulates the HPT axis at various levels and that regulation depends on the nutritional status. Since a-MSH can affect energy expenditure through several mechanisms (Xu et al. 2011), future studies should explore alternative pathways underlying the increase in energy expenditure with central SIRT1 inhibition especially in the context of metabolic syndrome and obesity.
4.2 SIRT1 Regulates proCRH
The major role of CRH neurons present in the PVN is to regulate the adrenal axis. Stimulation of the adrenal axis by CRH produces an increase of circulating glucocorticoids (GC) from the adrenal gland affecting energy metabolism as well as glucose metabolism. Chronic increases of basal GC are associated with increased food drive and enhanced abdominal adiposity. Early studies by Vale and others showed a role for CRH (Vale et al. 1981b) in mediating the stress response (Kovács 2013). However, CRH also regulates metabolic, immunologic, and homeostatic changes under normal and pathologic conditions (Chrousos 1995; Seimon et al. 2013; Sominsky and Spencer 2014). CRH exerts an anorexigenic effect in through its direct action within the hypothalamus (Heinrichs et al. 1993). However, glucocorticoids, whose level rises in response to CRH action in the pituitary, act as orexigenic agents. Accordingly, ob/ob and db/db mice have elevated glucocorticoid levels when compared to DIO wild-type mice, and the response of hypothalamic CRH levels to nutritional challenges in wt vs. the ob/ob mice is different (Jang and Romsos 1998).
Although CRH is expressed in mast cells (Cao et al. 2005), amygdala, locus, and hippocampus, the PVN-derived CRH has a major role in metabolism (Raadsheer et al. 1993; Ziegler et al. 2007; Korosi and Baram 2008). CRH is produced in the medial parvocellular division of the PVN and functions as the central regulator of the HPA axis (Aguilera et al. 2008). The levels of bioactive CRH secreted to the circulation are dependent on different peripheral and brain inputs and on the ability of the cell in performing an effective posttranslational processing from its precursor proCRH by the PCs. Like most hypophysiotropic neurons, CRH is released from nerve terminals anteriorly juxtaposed to the median eminence where fenestrated capillaries in the hypophyseal portal system facilitate a rapid exchange between the hypothalamus and the pituitary (Rho and Swanson 1987). Upon binding to its corticotropin-releasing hormone receptor 1 (CRHR1) (Lovejoy et al. 2014) in corticotropic cells, CRH stimulates the synthesis and secretion of adrenocorticotropic hormone (ACTH) derived from POMC as well as other bioactive molecules such as β-endorphin (Solomon 1999). ACTH engages the melanocortin 2 receptor expressed by cells of the adrenal cortex and stimulates the production and secretion of steroid hormones such as cortisol (Veo et al. 2011). Both ACTH and GC function to regulate HPA axis activity via long and short negative feedback loops that signal at the level of the hypothalamus, extra-hypothalamic brain sites, and the adenohypophysis.
Among the brain circuitries involved in the control of food intake and energy expenditure capable of integrating peripheral signals is the CRH system, which has many clusters of brain neurons and the closely related peptides including urocortin (Henckens et al. 2016). The CRH system showed to have a certain degree of plasticity in obesity and in starvation. Based on those observations, it is possible that obesity can block or activate the expression of the CRH type 2 alpha receptor in the ventromedial hypothalamic nucleus and induce the expression of the CRH-binding protein in brain areas involved in the anorectic and thermogenic actions of CRH (Richard et al. 2000; Mastorakos and Zapanti 2004; Toriya et al. 2010). On the other hand, CRH acting in the adrenal axis stimulates the production GC that promotes positive energy balance partly by affecting glucose metabolism and lipid homeostasis and increasing appetite drive (Tataranni et al. 1996). Although a consensus on the exact role of adrenal activity in relation to energy dysfunction has yet to be reached, increased and sustained basal GC is implicated in the development of visceral obesity, insulin resistance, and metabolic disease (Spencer and Tilbrook 2011; Laryea et al. 2013; Kong et al. 2015).
The first evidence showing that SIRT1 could regulate CRH came from recent studies conducted in our laboratory (Toorie et al. 2016). We showed that PVN SIRT1 activates the HPA axis and basal corticoid levels by enhancing the biosynthesis of CRH through an increase in the biochemical processing of proCRH by PC2, a key enzyme in the maturation of CRH from proCRH (Fig. 6.2), also demonstrated in an in vivo model (Dong et al. 1997). Moreover, in the DIO state, PVN SIRT1 increases basal (not stress induced) circulating corticoids in a manner independent of preproCRH transcriptional changes. Instead, SIRT1’s effects on adrenal activity are mediated via a posttranslational processing mechanism in concert with an increase in PC2. Increased hypophysiotropic CRH release from the PVN stimulates pituitary ACTH synthesis and release and in turn increased circulating basal corticoid concentrations (Toorie et al. 2016). Cumulative findings from this study suggest that increasing PVN SIRT1 activity results in an increase in the amount of CRH targeted to the anterior pituitary, thereby enhancing ACTH signaling and basal corticoid concentration. Furthermore, our results demonstrate that inhibiting SIRT1 specifically in the PVN of obese rodents caused a reduction in PVN proPC2 and the active form of PC2, but not PC1, protein. This decrease in PC2 was associated with a decrease in CRH in the ME, as well as reduced circulating corticoids, effects that were not observed in lean individuals (Toorie et al. 2016) (Fig. 6.2). It is possible that the SIRT1-CRH axis in the PVN is responsible, at least in part, for the improved glucose homeostasis observed in neuronal SIRT1 KO mice (23457303).
Model depicting SIRT1’s regulation on proCRH. During high-fat feeding, an increase of PVN SIRT1 is recorded and in turn promotes the increase proPC2 and its active form PC2. The effect of SIRT1 on preproPC2 appears to be mediated by FoxO1 or through preproPC2 directly. Since FoxO1 is a positive transcriptional regulator of SIRT1, FoxO1 could also act as upstream of SIRT1 and, in turn, affect PC2. The increased in PC2 production enhances proCRH processing, thus increasing CRH output to the hypophyseal portal system. The increase in CRH release to the circulation in turn increases POMC biosynthesis and processing in the anterior pituitary leading to more production of ACTH by corticotroph cells of the anterior pituitary. ACTH then acts on the adrenal cortex, stimulating the production and release of cortisol, thereby promoting a positive energy balance that may ultimately lead to excessive weight gain if unresolved. AC, acetyl; black bars in the proCRH molecule indicate the site of pair of basic residues; HFD, high-fat diet; PVN, paraventricular nucleus of the hypothalamus. Arrows directed to PC2 from SIRT1 and FoxO1 indicate the suggested possible actions on preproPC2 gene and proPC2 protein posttranslational processing. (This figure is adopted from our prior publication on the Molecular and Cellular Endocrinology (Mol Cell Endocrinol. 2016 Dec 15;438:77–88))
In the same line of studies, we identified that the transcription factor FoxO1 is responsible for the upregulation of PC2 (Toorie et al. 2016). Since FoxO1 is a positive transcriptional regulator of SIRT1 (Xiong et al. 2011), FoxO1 could also act as upstream of SIRT1 and, in turn, affect PC2 (Fig. 6.2). In summary, SIRT1 deacetylates Fox01 to upregulate PC2 but may also have a direct action on proPC2 to enhance the processing of proPC2 to PC2 (Toorie et al. 2016). Another observation from these studies was that inhibition of PVN SIRT1 activity did not significantly alter sated glucose levels; however, there was a trend for decreased glucose in DIO animals. Nevertheless, it resulted in a significant increase in sated serum insulin in obese rats. These results were consistent with another study showing that loss of functional SIRT1 in neurons of the central nervous system enhanced both brain and peripheral insulin sensitivity and reversed the hyperglycemia associated with obesity (Lu et al. 2013). On the other hand, an increase in the activity of SIRT1 in the medial ARC of lean rats was sufficient to improve glucose homeostasis and increase insulin sensitivity (Knight et al. 2011). This apparent contradiction shows that, as seen for many enzymes, that the role of SIRT1 in metabolism is tissue-specific. However, we can say that brain SIRT1 is involved in regulating insulin and glucose homeostasis. Interestingly, in addition to the regulatory role of SIRT1 in the thyroid and adrenal axes, it was also shown that SIRT1 regulates the gonadal axis in mediating Leydig and Sertoli cell maturity by regulating steroidogenic gene expression and, at the central level, by increasing the expression of gonadotropin-releasing hormone, thereby resulting in increased circulating luteinizing hormone and intratesticular testosterone levels (Kolthur-Seetharam et al. 2009), which accounts for the fact that the survivals of embryonic SIRT1 deletion are sterile. Finally, it is possible that other hypothalamic nutrient sensors might participate in the regulation of the CRH axis. For example, central administration of citrate suppressed the activity of hypothalamic AMPK and resulted in increased expression of CRH and POMC, but inhibited NPY expression (Stoppa et al. 2008).
In summary, SIRT1 regulates the CRH peptide by regulating the processing of PC2 and FoxO1 adding a novel regulatory link between PVN SIRT1 and HPA axis activity. Together, these results suggest that PVN SIRT1 has the capacity to activate the adrenal axis and increase basal GC levels via its positive regulation of PC2- (and possibly PC1)-mediated processing of proCRH into bioactive CRH.
5 Concluding Remarks
The last two decades have witnessed an enormous leap in our understanding of the central regulation of whole-body energy metabolism. With the help of recent advances in mouse genetics, electrophysiology, and optogenetic techniques, it has been possible to identify the role of peripheral hormone receptors and neuronal circuits involved in the action of these hormones on behavior and peripheral tissue functions at spatial resolution. A key component in maintaining energy balance is the hypothalamus, and the discovery of an increasing number of peptide hormones within the hypothalamus that contribute to the process of energy homeostasis has been paramount to this progress. Furthermore, since the discovery of the PCs in the late 1980s, a new frontier on the propeptide hormone biosynthesis and processing research had come to surface.
Another major progress was done with the discovery of nutrient sensors in their ability to sense and respond to fluctuations in environmental and intracellular nutrient levels. The last nutrient sensor that came to surface as an important energy balance regulator is SIRT1. The function of SIRT1 on overall metabolism depends on the tissue of interest: While peripheral SIRT1 activity positively correlates with overall health and physiology, central SIRT1’s role in energy balance showed convincing evidence that points to promoting positive energy balance. One of SIRT1’s effector targets, FoxO1, plays a crucial role in determining whether SIRT1 will function to promote negative or positive energy balance. A fascinating aspect of this regulation is that SIRT1 directly regulates the biosynthesis of POMC and proCRH in the ARC and PVN, respectively, while at the same time coordinating prohormone processing, at least through the regulation of PC2.
As described above, due to the cell-specific nature of SIRT1’s action in promoting either positive or negative energy balance in response to dietary excess, a more targeted therapy approach against cell-specific downstream mediators of SIRT1 signaling may prove beneficial in combating obesity and the physiological outcomes that predispose to the development of metabolic disorders. Indeed, as demonstrated by Ren and colleagues, ablation of GRP17, a G-coupled protein receptor and a downstream target of FoxO1 that is prominently expressed in AgRP neurons, results in enhanced anabolic activity and decreased food intake (Ren et al. 2012). Targeted therapeutic approaches may help to circumvent the potentially deleterious and counterproductive mechanisms of global SIRT1 activation/inactivation. Coordinating the activity of other master nutrient sensors, namely, AMPK and mTOR, SIRT1 emerges as a master regulator of energy homeostasis in the CNS. Collectively, evidence points to brain SIRT1 in mediating the adaptive responses to nutritional stress via its broad regulation of mechanisms involved in energy homeostasis. It is notable, however, that central SIRT1 inhibition or neuronal SIRT1 ablation is sufficient to promote negative energy balance. Therefore, approaches aimed at enhancing peripheral SIRT1 activity, while reducing central SIRT1 activity, may prove most beneficial in the treatment of obesity.
Questions
-
1.
How do nutrient sensors detect the cellular energy status? What are the similarities and differences between AMPK, SIRT1, and mTOR in this regard? What are some of the inputs recognized by them?
-
2.
What is the relationship between the anatomical location of the ARC and its sensing the circulating nutritional and hormonal signals?
-
3.
Discuss the potential problems associated with the Cre-LoxP technology in regard to its use in the metabolic studies.
-
4.
What are some of the possible reasons for different phenotypes reported in the literature on the role of hypothalamic nutrient sensors on energy metabolism? Discuss the advantages and disadvantages of genetic and pharmacological approaches utilized.
-
5.
The regulation of gene expression of the hypothalamic neuropeptides is governed at various levels, including their transcription and posttranscriptional processing/modifications. At what stages do nutrient sensors interfere with the gene expression of hypothalamic neuropeptides?
References
Adan, R. A. H., Tiesjema, B., Hillebrand, J. J. G., la Fleur, S. E., Kas, M. J. H., & de Krom, M. (2006). The MC4 receptor and control of appetite. British Journal of Pharmacology, 149(7), 815–827.
Aguilera, G., Subburaju, S., Young, S., & Chen, J. (2008). The parvocellular vasopressinergic system and responsiveness of the hypothalamic pituitary adrenal axis during chronic stress. Progress in Brain Research, 170, 29–39.
Akieda-Asai, S., Zaima, N., Ikegami, K., Kahyo, T., & Yao, I. SIRT1 Regulates Thyroid-Stimulating Hormone Release by Enhancing PIP5Kγ [subscript gamma] Activity through Deacetylation of Specific Lysine Residues in …. 2010. Available at: https://dspace.mit.edu/handle/1721.1/60353.
Aksoy, P., White, T. A., Thompson, M., & Chini, E. N. (2006a). Regulation of intracellular levels of NAD: A novel role for CD38. Biochemical and Biophysical Research Communications, 345(4), 1386–1392.
Aksoy, P., Escande, C., White, T. A., Thompson, M., Soares, S., Benech, J. C., & Chini, E. N. (2006b). Regulation of SIRT 1 mediated NAD dependent deacetylation: A novel role for the multifunctional enzyme CD38. Biochemical and Biophysical Research Communications, 349(1), 353–359.
Al-Qassab, H., Smith, M. A., Irvine, E. E., Guillermet-Guibert, J., Claret, M., Choudhury, A. I., Selman, C., Piipari, K., Clements, M., Lingard, S., Chandarana, K., Bell, J. D., Barsh, G. S., Smith, A. J. H., Batterham, R. L., Ashford, M. L. J., Vanhaesebroeck, B., & Withers, D. J. (2009). Dominant role of the p110beta isoform of PI3K over p110alpha in energy homeostasis regulation by POMC and AgRP neurons. Cell Metabolism, 10(5), 343–354.
Anderson, K. A., Ribar, T. J., Lin, F., Noeldner, P. K., Green, M. F., Muehlbauer, M. J., Witters, L. A., Kemp, B. E., & Means, A. R. (2008). Hypothalamic CaMKK2 contributes to the regulation of energy balance. Cell Metabolism, 7(5), 377–388.
Anderson, E. J. P., Çakir, I., Carrington, S. J., Cone, R. D., Ghamari-Langroudi, M., Gillyard, T., Gimenez, L. E., & Litt, M. J. (2016). 60 YEARS OF POMC: Regulation of feeding and energy homeostasis by α-MSH. Journal of Molecular Endocrinology, 56(4), T157–T174.
Anderson, K. A., Huynh, F. K., Fisher-Wellman, K., Stuart, J. D., Peterson, B. S., Douros, J. D., Wagner, G. R., Thompson, J. W., Madsen, A. S., Green, M. F., Sivley, R. M., Ilkayeva, O. R., Stevens, R. D., Backos, D. S., Capra, J. A., Olsen, C. A., Campbell, J. E., Muoio, D. M., Grimsrud, P. A., & Hirschey, M. D. (2017). SIRT4 is a lysine Deacylase that controls leucine metabolism and insulin secretion. Cell Metabolism, 25(4), 838–855.e15.
Andersson, U., Filipsson, K., Abbott, C. R., Woods, A., Smith, K., Bloom, S. R., Carling, D., & Small, C. J. (2004). AMP-activated protein kinase plays a role in the control of food intake. The Journal of Biological Chemistry, 279(13), 12005–12008.
Araki, T., Sasaki, Y., & Milbrandt, J. (2004). Increased nuclear NAD biosynthesis and SIRT1 activation prevent axonal degeneration. Science, 305(5686), 1010–1013.
Asher, G., Gatfield, D., Stratmann, M., Reinke, H., Dibner, C., Kreppel, F., Mostoslavsky, R., Alt, F. W., & Schibler, U. (2008). SIRT1 regulates circadian clock gene expression through PER2 deacetylation. Cell, 134(2), 317–328.
Barbosa, M. T. P., Soares, S. M., Novak, C. M., Sinclair, D., Levine, J. A., Aksoy, P., & Chini, E. N. (2007). The enzyme CD38 (a NAD glycohydrolase, EC 3.2.2.5) is necessary for the development of diet-induced obesity. The FASEB Journal, 21(13), 3629–3639.
Bar-Peled, L., Schweitzer, L. D., Zoncu, R., & Sabatini, D. M. (2012). Ragulator is a GEF for the rag GTPases that signal amino acid levels to mTORC1. Cell, 150(6), 1196–1208.
Bates, S. H., Stearns, W. H., Dundon, T. A., Schubert, M., Tso, A. W. K., Wang, Y., Banks, A. S., Lavery, H. J., Haq, A. K., Maratos-Flier, E., Neel, B. G., Schwartz, M. W., & Myers, M. G., Jr. (2003). STAT3 signalling is required for leptin regulation of energy balance but not reproduction. Nature, 421(6925), 856–859.
Baur, J. A., Pearson, K. J., Price, N. L., Jamieson, H. A., Lerin, C., Kalra, A., Prabhu, V. V., Allard, J. S., Lopez-Lluch, G., Lewis, K., Pistell, P. J., Poosala, S., Becker, K. G., Boss, O., Gwinn, D., Wang, M., Ramaswamy, S., Fishbein, K. W., Spencer, R. G., Lakatta, E. G., Le Couteur, D., Shaw, R. J., Navas, P., Puigserver, P., Ingram, D. K., de Cabo, R., & Sinclair, D. A. (2006). Resveratrol improves health and survival of mice on a high-calorie diet. Nature, 444(7117), 337–342.
Beiroa, D., Imbernon, M., Gallego, R., Senra, A., Herranz, D., Villarroya, F., Serrano, M., Fernø, J., Salvador, J., Escalada, J., Dieguez, C., Lopez, M., Frühbeck, G., & Nogueiras, R. (2014). GLP-1 agonism stimulates brown adipose tissue thermogenesis and browning through hypothalamic AMPK. Diabetes, 63(10), 3346–3358.
Belgardt, B. F., Husch, A., Rother, E., Ernst, M. B., Wunderlich, F. T., Hampel, B., Klöckener, T., Alessi, D., Kloppenburg, P., & Brüning, J. C. (2008). PDK1 deficiency in POMC-expressing cells reveals FOXO1-dependent and -independent pathways in control of energy homeostasis and stress response. Cell Metabolism, 7(4), 291–301.
Benjannet, S., Rondeau, N., Day, R., Chrétien, M., & Seidah, N. G. (1991). PC1 and PC2 are proprotein convertases capable of cleaving proopiomelanocortin at distinct pairs of basic residues. Proceedings of the National Academy of Sciences of the United States of America, 88(9), 3564–3568.
Benoit, S. C., Air, E. L., Coolen, L. M., Strauss, R., Jackman, A., Clegg, D. J., Seeley, R. J., & Woods, S. C. (2002). The catabolic action of insulin in the brain is mediated by melanocortins. The Journal of Neuroscience, 22(20), 9048–9052.
Ben-Sahra, I., Howell, J. J., Asara, J. M., & Manning, B. D. (2013). Stimulation of de novo pyrimidine synthesis by growth signaling through mTOR and S6K1. Science, 339(6125), 1323–1328.
Ben-Sahra, I., Hoxhaj, G., Ricoult, S. J. H., Asara, J. M., & Manning, B. D. (2016). mTORC1 induces purine synthesis through control of the mitochondrial tetrahydrofolate cycle. Science, 351(6274), 728–733.
Blander, G., & Guarente, L. (2004). The Sir2 family of protein deacetylases. Annual Review of Biochemistry, 73, 417–435.
Blouet, C., Ono, H., & Schwartz, G. J. (2008). Mediobasal hypothalamic p70 S6 kinase 1 modulates the control of energy homeostasis. Cell Metabolism, 8(6), 459–467.
Bordone, L., & Guarente, L. (2005). Calorie restriction, SIRT1 and metabolism: Understanding longevity. Nature Reviews. Molecular Cell Biology, 6(4), 298–305.
Bordone, L., Motta, M. C., Picard, F., Robinson, A., Jhala, U. S., Apfeld, J., McDonagh, T., Lemieux, M., McBurney, M., Szilvasi, A., Easlon, E. J., Lin, S.-J., & Guarente, L. (2006). Sirt1 regulates insulin secretion by repressing UCP2 in pancreatic beta cells. PLoS Biology, 4(2), e31.
Bordone, L., Cohen, D., Robinson, A., Motta, M. C., van Veen, E., Czopik, A., Steele, A. D., Crowe, H., Marmor, S., Luo, J., Gu, W., & Guarente, L. (2007a). SIRT1 transgenic mice show phenotypes resembling calorie restriction. Aging Cell, 6(6), 759–767.
Bordone, L., Guarente - Diabetes L., & Metabolism O. (2007b). Sirtuins and β‐cell function. Wiley Online Library 2007. Available at: http://onlinelibrary.wiley.com/doi/10.1111/j.1463-1326.2007.00769.x/full.
Brakch, N., Galanopoulou, A. S., Patel, Y. C., Boileau, G., & Seidah, N. G. (1995). Comparative proteolytic processing of rat prosomatostatin by the convertases PC1, PC2, furin, PACE4 and PC5 in constitutive and regulated secretory pathways. FEBS Letters, 362(2), 143–146.
Brar, B., Sanderson, T., Wang, N., & Lowry, P. J. (1997). Post-translational processing of human procorticotrophin-releasing factor in transfected mouse neuroblastoma and Chinese hamster ovary cell lines. The Journal of Endocrinology, 154(3), 431–440.
Breslin, M. B., Lindberg, I., Benjannet, S., & Mathis - Journal of Biological … JP. (1993). Differential processing of proenkephalin by prohormone convertases 1 (3) and 2 and furin. ASBMB 1993. Available at: http://www.jbc.org/content/268/36/27084.short.
Brooks, C. L., & Gu, W. (2009). How does SIRT1 affect metabolism, senescence and cancer? Nature Reviews. Cancer, 9(2), 123–128.
Cakir, I., Perello, M., Lansari, O., Messier, N. J., Vaslet, C. A., & Nillni, E. A. (2009). Hypothalamic Sirt1 regulates food intake in a rodent model system. PLoS One, 4(12), e8322.
Cakir, I., Cyr, N. E., Perello, M., Litvinov, B. P., Romero, A., Stuart, R. C., & Nillni, E. A. (2013). Obesity induces hypothalamic endoplasmic reticulum stress and impairs proopiomelanocortin (POMC) post-translational processing. The Journal of Biological Chemistry, 288(24), 17675–17688.
Cantó, C., Gerhart-Hines, Z., Feige, J. N., Lagouge, M., Noriega, L., Milne, J. C., Elliott, P. J., Puigserver, P., & Auwerx, J. (2009). AMPK regulates energy expenditure by modulating NAD+ metabolism and SIRT1 activity. Nature, 458(7241), 1056–1060.
Cantó, C., Houtkooper, R. H., Pirinen, E., Youn, D. Y., Oosterveer, M. H., Cen, Y., Fernandez-Marcos, P. J., Yamamoto, H., Andreux, P. A., Cettour-Rose, P., Gademann, K., Rinsch, C., Schoonjans, K., Sauve, A. A., & Auwerx, J. (2012). The NAD(+) precursor nicotinamide riboside enhances oxidative metabolism and protects against high-fat diet-induced obesity. Cell Metabolism, 15(6), 838–847.
Cao, J., Papadopoulou, N., Kempuraj, D., Boucher, W. S., Sugimoto, K., Cetrulo, C. L., & Theoharides, T. C. (2005). Human mast cells express corticotropin-releasing hormone (CRH) receptors and CRH leads to selective secretion of vascular endothelial growth factor. Journal of Immunology, 174(12), 7665–7675.
Caron, A., Labbé, S. M., Lanfray, D., Blanchard, P.-G., Villot, R., Roy, C., Sabatini, D. M., Richard, D., & Laplante, M. (2016a). Mediobasal hypothalamic overexpression of DEPTOR protects against high-fat diet-induced obesity. Molecular Metabolism, 5(2), 102–112.
Caron, A., Labbé, S. M., Mouchiroud, M., Huard, R., Lanfray, D., Richard, D., & Laplante, M. (2016b). DEPTOR in POMC neurons affects liver metabolism but is dispensable for the regulation of energy balance. American Journal of Physiology. Regulatory, Integrative and Comparative Physiology, 310(11), R1322–R1331.
Castro, M., Lowenstein, P., Glynn, B., Hannah, M., Linton, E., & Lowry, P. (1991). Post-translational processing and regulated release of corticotropin-releasing hormone (CRH) in AtT20 cells expressing the human proCRH gene. Biochemical Society Transactions, 19(3), 246S.
Chalkiadaki, A., & Guarente, L. (2012). High-fat diet triggers inflammation-induced cleavage of SIRT1 in adipose tissue to promote metabolic dysfunction. Cell Metabolism, 16(2), 180–188.
Challis, B. G., Pritchard, L. E., & Creemers - Human molecular … J. (2002). A missense mutation disrupting a dibasic prohormone processing site in pro-opiomelanocortin (POMC) increases susceptibility to early-onset obesity through a novel …. academic.oup.com 2002. Available at: https://academic.oup.com/hmg/article-abstract/11/17/1997/589952.
Chang, H.-C., & Guarente, L. (2013). SIRT1 mediates central circadian control in the SCN by a mechanism that decays with aging. Cell, 153(7), 1448–1460.
Chang, H.-C., & Guarente, L. (2014). SIRT1 and other sirtuins in metabolism. Trends in Endocrinology and Metabolism, 25(3), 138–145.
Chantranupong, L., Scaria, S. M., Saxton, R. A., Gygi, M. P., Shen, K., Wyant, G. A., Wang, T., Harper, J. W., Gygi, S. P., & Sabatini, D. M. (2016). The CASTOR proteins are arginine sensors for the mTORC1 pathway. Cell, 165(1), 153–164.
Chen, Y., Wu, R., Chen, H.-Z., Xiao, Q., Wang, W.-J., He, J.-P., Li, X.-X., Yu, X.-W., Li, L., Wang, P., Wan, X.-C., Tian, X.-H., Li, S.-J., Yu, X., & Wu, Q. (2015). Enhancement of hypothalamic STAT3 acetylation by nuclear receptor Nur77 dictates leptin sensitivity. Diabetes, 64(6), 2069–2081.
Cheng, X.-B., Wen, J.-P., Yang, J., Yang, Y., Ning, G., & Li, X.-Y. (2011). GnRH secretion is inhibited by adiponectin through activation of AMP-activated protein kinase and extracellular signal-regulated kinase. Endocrine, 39(1), 6–12.
Chikahisa, S., Fujiki, N., Kitaoka, K., Shimizu, N., & Séi, H. (2009). Central AMPK contributes to sleep homeostasis in mice. Neuropharmacology, 57(4), 369–374.
Choudhury, A. I., Heffron, H., Smith, M. A., Al-Qassab, H., Xu, A. W., Selman, C., Simmgen, M., Clements, M., Claret, M., Maccoll, G., Bedford, D. C., Hisadome, K., Diakonov, I., Moosajee, V., Bell, J. D., Speakman, J. R., Batterham, R. L., Barsh, G. S., Ashford, M. L. J., & Withers, D. J. (2005). The role of insulin receptor substrate 2 in hypothalamic and beta cell function. The Journal of Clinical Investigation, 115(4), 940–950.
Chrousos, G. P. (1995). The hypothalamic-pituitary-adrenal axis and immune-mediated inflammation. The New England Journal of Medicine, 332(20), 1351–1362.
Claret, M., Smith, M. A., Batterham, R. L., Selman, C., Choudhury, A. I., Fryer, L. G. D., Clements, M., Al-Qassab, H., Heffron, H., Xu, A. W., Speakman, J. R., Barsh, G. S., Viollet, B., Vaulont, S., Ashford, M. L. J., Carling, D., & Withers, D. J. (2007). AMPK is essential for energy homeostasis regulation and glucose sensing by POMC and AgRP neurons. The Journal of Clinical Investigation, 117(8), 2325–2336.
Claret, M., Smith, M. A., Knauf, C., Al-Qassab, H., Woods, A., Heslegrave, A., Piipari, K., Emmanuel, J. J., Colom, A., Valet, P., Cani, P. D., Begum, G., White, A., Mucket, P., Peters, M., Mizuno, K., Batterham, R. L., Giese, K. P., Ashworth, A., Burcelin, R., Ashford, M. L., Carling, D., & Withers, D. J. (2011). Deletion of Lkb1 in pro-opiomelanocortin neurons impairs peripheral glucose homeostasis in mice. Diabetes, 60(3), 735–745.
Cohen, H. Y., Miller, C., Bitterman, K. J., Wall, N. R., Hekking, B., Kessler, B., Howitz, K. T., Gorospe, M., de Cabo, R., & Sinclair, D. A. (2004). Calorie restriction promotes mammalian cell survival by inducing the SIRT1 deacetylase. Science, 305(5682), 390–392.
Cohen, D. E., Supinski, A. M., Bonkowski, M. S., Donmez, G., & Guarente, L. P. (2009). Neuronal SIRT1 regulates endocrine and behavioral responses to calorie restriction. Genes & Development, 23(24), 2812–2817.
Cone, R. D. (2005). Anatomy and regulation of the central melanocortin system. Nature Neuroscience, 8(5), 571–578.
Constam, D. B., Calfon, M., & Robertson, E. J. (1996). SPC4, SPC6, and the novel protease SPC7 are coexpressed with bone morphogenetic proteins at distinct sites during embryogenesis. The Journal of Cell Biology, 134(1), 181–191.
Cota, D., Proulx, K., Smith, K. A. B., Kozma, S. C., Thomas, G., Woods, S. C., & Seeley, R. J. (2006). Hypothalamic mTOR signaling regulates food intake. Science, 312(5775), 927–930.
Cota, D., Matter, E. K., Woods, S. C., & Seeley, R. J. (2008). The role of hypothalamic mammalian target of rapamycin complex 1 signaling in diet-induced obesity. The Journal of Neuroscience, 28(28), 7202–7208.
Cottrell, E. C., & Ozanne, S. E. (2008). Early life programming of obesity and metabolic disease. Physiology & Behavior, 94(1), 17–28.
Coupé, B., Ishii, Y., Dietrich, M. O., Komatsu, M., Horvath, T. L., & Bouret, S. G. (2012). Loss of autophagy in pro-opiomelanocortin neurons perturbs axon growth and causes metabolic dysregulation. Cell Metabolism, 15(2), 247–255.
Cowley, M. A., Smart, J. L., Rubinstein, M., Cerdán, M. G., Diano, S., Horvath, T. L., Cone, R. D., & Low, M. J. (2001). Leptin activates anorexigenic POMC neurons through a neural network in the arcuate nucleus. Nature, 411(6836), 480–484.
Cummings, D. E., & Schwartz, M. W. (2000). Melanocortins and body weight: A tale of two receptors. Nature Genetics, 26(1), 8–9.
Cunningham, J. T., Rodgers, J. T., Arlow, D. H., Vazquez, F., Mootha, V. K., & Puigserver, P. (2007). mTOR controls mitochondrial oxidative function through a YY1-PGC-1alpha transcriptional complex. Nature, 450(7170), 736–740.
Cyr, N. E., Toorie, A. M., Steger, J. S., Sochat, M. M., Hyner, S., Perello, M., Stuart, R., & Nillni, E. A. (2013). Mechanisms by which the orexigen NPY regulates anorexigenic α-MSH and TRH. American Journal of Physiology. Endocrinology and Metabolism, 304(6), E640–E650.
Cyr, N. E., Steger, J. S., Toorie, A. M., Yang, J. Z., & Stuart, R. (2014). Central Sirt1 regulates body weight and energy expenditure along with the POMC-derived peptide α-MSH and the processing enzyme CPE production in diet- …. press.endocrine.org 2014. Available at: http://press.endocrine.org/doi/pdf/10.1210/en.2013-1998.
da Silva Xavier, G., Farhan, H., Kim, H., Caxaria, S., Johnson, P., Hughes, S., Bugliani, M., Marselli, L., Marchetti, P., Birzele, F., Sun, G., Scharfmann, R., Rutter, J., Siniakowicz, K., Weir, G., Parker, H., Reimann, F., Gribble, F. M., & Rutter, G. A. (2011). Per-arnt-Sim (PAS) domain-containing protein kinase is downregulated in human islets in type 2 diabetes and regulates glucagon secretion. Diabetologia, 54(4), 819–827.
Dagon, Y., Hur, E., Zheng, B., Wellenstein, K., Cantley, L. C., & Kahn, B. B. (2012). p70S6 kinase phosphorylates AMPK on serine 491 to mediate leptin’s effect on food intake. Cell Metabolism, 16(1), 104–112.
Dasgupta, M., Unal, H., Willard, B., Yang, J., Karnik, S. S., & Stark, G. R. (2014). Critical role for lysine 685 in gene expression mediated by transcription factor unphosphorylated STAT3. The Journal of Biological Chemistry, 289(44), 30763–30771.
de Ruijter, A. J. M., van Gennip, A. H., Caron, H. N., Kemp, S., & van Kuilenburg, A. B. P. (2003). Histone deacetylases (HDACs): Characterization of the classical HDAC family. The Biochemical Journal, 370(Pt 3), 737–749.
DeMille, D., & Grose, J. H. (2013). PAS kinase: A nutrient sensing regulator of glucose homeostasis. IUBMB Life, 65(11), 921–929.
Deng, X.-Q., Chen, L.-L., & Li, N.-X. (2007). The expression of SIRT1 in nonalcoholic fatty liver disease induced by high-fat diet in rats. Liver International, 27(5), 708–715.
Dey, A., Xhu, X., Carroll, R., Turck, C. W., Stein, J., & Steiner, D. F. (2003). Biological processing of the cocaine and amphetamine-regulated transcript precursors by prohormone convertases, PC2 and PC1/3. The Journal of Biological Chemistry, 278(17), 15007–15014.
Dey, A., Norrbom, C., Zhu, X., Stein, J., Zhang, C., Ueda, K., & Steiner, D. F. (2004). Furin and prohormone convertase 1/3 are major convertases in the processing of mouse pro-growth hormone-releasing hormone. Endocrinology, 145(4), 1961–1971.
Dietrich, M. O., Antunes, C., Geliang, G., Liu, Z.-W., Borok, E., Nie, Y., Xu, A. W., Souza, D. O., Gao, Q., Diano, S., Gao, X.-B., & Horvath, T. L. (2010). Agrp neurons mediate Sirt1’s action on the melanocortin system and energy balance: Roles for Sirt1 in neuronal firing and synaptic plasticity. The Journal of Neuroscience, 30(35), 11815–11825.
Du, J., Zhou, Y., Su, X., Yu, J. J., Khan, S., Jiang, H., Kim, J., Woo, J., Kim, J. H., Choi, B. H., He, B., Chen, W., Zhang, S., Cerione, R. A., Auwerx, J., Hao, Q., & Lin, H. (2011). Sirt5 is a NAD-dependent protein lysine demalonylase and desuccinylase. Science, 334(6057), 806–809.
Dong W1., Seidel B., Marcinkiewicz M., Chrétien M., Seidah NG., Day R. (1997). Cellular localization of the prohormone convertases in the hypothalamic paraventricular and supraoptic nuclei: selective regulation of PC1 in corticotrophin-releasing hormone parvocellular neurons mediated by glucocorticoids. J Neurosci. 15;17(2):563–75.
Düvel, K., Yecies, J. L., Menon, S., Raman, P., Lipovsky, A. I., Souza, A. L., Triantafellow, E., Ma, Q., Gorski, R., Cleaver, S., Vander Heiden, M. G., MacKeigan, J. P., Finan, P. M., Clish, C. B., Murphy, L. O., & Manning, B. D. (2010). Activation of a metabolic gene regulatory network downstream of mTOR complex 1. Molecular Cell, 39(2), 171–183.
Egan, D. F., Shackelford, D. B., Mihaylova, M. M., Gelino, S., Kohnz, R. A., Mair, W., Vasquez, D. S., Joshi, A., Gwinn, D. M., Taylor, R., Asara, J. M., Fitzpatrick, J., Dillin, A., Viollet, B., Kundu, M., Hansen, M., & Shaw, R. J. (2011). Phosphorylation of ULK1 (hATG1) by AMP-activated protein kinase connects energy sensing to mitophagy. Science, 331(6016), 456–461.
Elson, A. E., & Simerly, R. B. (2015). Developmental specification of metabolic circuitry. Frontiers in Neuroendocrinology, 39, 38–51.
Ernst, M. B., Wunderlich, C. M., Hess, S., Paehler, M., Mesaros, A., Koralov, S. B., Kleinridders, A., Husch, A., Münzberg, H., Hampel, B., Alber, J., Kloppenburg, P., Brüning, J. C., & Wunderlich, F. T. (2009). Enhanced Stat3 activation in POMC neurons provokes negative feedback inhibition of leptin and insulin signaling in obesity. The Journal of Neuroscience, 29(37), 11582–11593.
Fox, D. L., & Good, D. J. (2008). Nescient helix-loop-helix 2 interacts with signal transducer and activator of transcription 3 to regulate transcription of prohormone convertase 1/3. Molecular Endocrinology, 22(6), 1438–1448.
Frescas, D., Valenti, L., & Accili, D. (2005). Nuclear trapping of the forkhead transcription factor FoxO1 via Sirt-dependent deacetylation promotes expression of glucogenetic genes. The Journal of Biological Chemistry, 280(21), 20589–20595.
Fricker, L. D., Berman, Y. L., Leiter, E. H., & Devi, L. A. (1996). Carboxypeptidase E activity is deficient in mice with the fat mutation: EFFECT ON PEPTIDE PROCESSING. The Journal of Biological Chemistry, 271(48), 30619–30624.
Friedman, T. C., Loh, Y. P., Cawley, N. X., Birch, N. P., Huang, S. S., Jackson, I. M., & Nillni, E. A. (1995). Processing of prothyrotropin-releasing hormone (pro-TRH) by bovine intermediate lobe secretory vesicle membrane PC1 and PC2 enzymes. Endocrinology, 136(10), 4462–4472.
Fryer, L. G. D., Foufelle, F., Barnes, K., Baldwin, S. A., Woods, A., & Carling, D. (2002). Characterization of the role of the AMP-activated protein kinase in the stimulation of glucose transport in skeletal muscle cells. The Biochemical Journal, 363(Pt 1), 167–174.
Fulco, M., Schiltz, R. L., Iezzi, S., King, M. T., Zhao, P., Kashiwaya, Y., Hoffman, E., Veech, R. L., & Sartorelli, V. (2003). Sir2 regulates skeletal muscle differentiation as a potential sensor of the redox state. Molecular Cell, 12(1), 51–62.
Funato, H., Tsai, A. L., Willie, J. T., Kisanuki, Y., Williams, S. C., Sakurai, T., & Yanagisawa, M. (2009). Enhanced orexin receptor-2 signaling prevents diet-induced obesity and improves leptin sensitivity. Cell Metabolism, 9(1), 64–76.
Funato, H., Oda, S., Yokofujita, J., Igarashi, H., & Kuroda, M. (2011). Fasting and high-fat diet alter histone deacetylase expression in the medial hypothalamus. PLoS One, 6(4), e18950.
Furuta, M., Yano, H., Zhou, A., Rouillé, Y., Holst, J. J., Carroll, R., Ravazzola, M., Orci, L., Furuta, H., & Steiner, D. F. (1997). Defective prohormone processing and altered pancreatic islet morphology in mice lacking active SPC2. Proceedings of the National Academy of Sciences of the United States of America, 94(13), 6646–6651.
Füzesi, T., Wittmann, G., Liposits, Z., Lechan, R. M., & Fekete, C. (2007). Contribution of noradrenergic and adrenergic cell groups of the brainstem and agouti-related protein-synthesizing neurons of the arcuate nucleus to neuropeptide-y innervation of corticotropin-releasing hormone neurons in hypothalamic paraventricular nucleus of the rat. Endocrinology, 148(11), 5442–5450.
Galanopoulou, A. S., Kent, G., Rabbani, S. N., Seidah, N. G., & Patel, Y. C. (1993). Heterologous processing of prosomatostatin in constitutive and regulated secretory pathways. Putative role of the endoproteases furin, PC1, and PC2. The Journal of Biological Chemistry, 268(8), 6041–6049.
Gan, X., Wang, J., Wang, C., Sommer, E., Kozasa, T., Srinivasula, S., Alessi, D., Offermanns, S., Simon, M. I., & Wu, D. (2012). PRR5L degradation promotes mTORC2-mediated PKC-δ phosphorylation and cell migration downstream of Gα12. Nature Cell Biology, 14(7), 686–696.
Gao, Q., Wolfgang, M. J., Neschen, S., Morino, K., Horvath, T. L., Shulman, G. I., & Fu, X.-Y. (2004). Disruption of neural signal transducer and activator of transcription 3 causes obesity, diabetes, infertility, and thermal dysregulation. Proceedings of the National Academy of Sciences of the United States of America, 101(13), 4661–4666.
Gao, S., Kinzig, K. P., Aja, S., Scott, K. A., Keung, W., Kelly, S., Strynadka, K., Chohnan, S., Smith, W. W., Tamashiro, K. L. K., Ladenheim, E. E., Ronnett, G. V., Tu, Y., Birnbaum, M. J., Lopaschuk, G. D., & Moran, T. H. (2007). Leptin activates hypothalamic acetyl-CoA carboxylase to inhibit food intake. Proceedings of the National Academy of Sciences of the United States of America, 104(44), 17358–17363.
Garfield, A. S., Shah, B. P., Burgess, C. R., Li, M. M., Li, C., Steger, J. S., Madara, J. C., Campbell, J. N., Kroeger, D., Scammell, T. E., Tannous, B. A., Myers, M. G., Jr., Andermann, M. L., Krashes, M. J., & Lowell, B. B. (2016). Dynamic GABAergic afferent modulation of AgRP neurons. Nature Neuroscience, 19(12), 1628–1635.
Ghosh, H. S., McBurney, M., & Robbins, P. D. (2010). SIRT1 negatively regulates the mammalian target of rapamycin. PLoS One, 5(2), e9199.
Gill, J. F., Delezie, J., Santos, G., & Handschin, C. (2016). PGC-1α expression in murine AgRP neurons regulates food intake and energy balance. Molecular Metabolism, 5(7), 580–588.
Gillum, M. P., Kotas, M. E., Erion, D. M., Kursawe, R., Chatterjee, P., Nead, K. T., Muise, E. S., Hsiao, J. J., Frederick, D. W., Yonemitsu, S., Banks, A. S., Qiang, L., Bhanot, S., Olefsky, J. M., Sears, D. D., Caprio, S., & Shulman, G. I. (2011). SirT1 regulates adipose tissue inflammation. Diabetes, 60(12), 3235–3245.
Good, D. J., Li, M., & Deater-Deckard, K. (2015). A genetic basis for motivated exercise. Exercise and Sport Sciences Reviews, 43(4), 231–237.
Gwinn, D. M., Shackelford, D. B., Egan, D. F., Mihaylova, M. M., Mery, A., Vasquez, D. S., Turk, B. E., & Shaw, R. J. (2008). AMPK phosphorylation of raptor mediates a metabolic checkpoint. Molecular Cell, 30(2), 214–226.
Hagiwara, A., Cornu, M., Cybulski, N., Polak, P., Betz, C., Trapani, F., Terracciano, L., Heim, M. H., Rüegg, M. A., & Hall, M. N. (2012). Hepatic mTORC2 activates glycolysis and lipogenesis through Akt, glucokinase, and SREBP1c. Cell Metabolism, 15(5), 725–738.
Hahn, T. M., Breininger, J. F., Baskin, D. G., & Schwartz, M. W. (1998). Coexpression of Agrp and NPY in fasting-activated hypothalamic neurons. Nature Neuroscience, 1(4), 271–272.
Haigis, M. C., & Sinclair, D. A. (2010). Mammalian sirtuins: Biological insights and disease relevance. Annual Review of Pathology, 5, 253–295.
Hao, H.-X., Cardon, C. M., Swiatek, W., Cooksey, R. C., Smith, T. L., Wilde, J., Boudina, S., Abel, E. D., McClain, D. A., & Rutter, J. (2007). PAS kinase is required for normal cellular energy balance. Proceedings of the National Academy of Sciences of the United States of America, 104(39), 15466–15471.
Hardie, D. G., & Pan, D. A. (2002). Regulation of fatty acid synthesis and oxidation by the AMP-activated protein kinase. Biochemical Society Transactions, 30(Pt 6), 1064–1070.
Harlan, S. M., Guo, D.-F., Morgan, D. A., Fernandes-Santos, C., & Rahmouni, K. (2013). Hypothalamic mTORC1 signaling controls sympathetic nerve activity and arterial pressure and mediates leptin effects. Cell Metabolism, 17(4), 599–606.
Hasegawa, K., Kawahara, T., Fujiwara, K., Shimpuku, M., Sasaki, T., Kitamura, T., & Yoshikawa, K. (2012). Necdin controls Foxo1 acetylation in hypothalamic arcuate neurons to modulate the thyroid axis. The Journal of Neuroscience, 32(16), 5562–5572.
Hawley, S. A., Boudeau, J., Reid, J. L., Mustard, K. J., Udd, L., Mäkelä, T. P., Alessi, D. R., & Hardie, D. G. (2003). Complexes between the LKB1 tumor suppressor, STRAD alpha/beta and MO25 alpha/beta are upstream kinases in the AMP-activated protein kinase cascade. Journal of Biology, 2(4), 28.
Hawley, S. A., Pan, D. A., Mustard, K. J., Ross, L., Bain, J., Edelman, A. M., Frenguelli, B. G., & Hardie, D. G. (2005). Calmodulin-dependent protein kinase kinase-beta is an alternative upstream kinase for AMP-activated protein kinase. Cell Metabolism, 2(1), 9–19.
Hawley, S. A., Ross, F. A., Gowans, G. J., Tibarewal, P., Leslie, N. R., & Hardie, D. G. (2014). Phosphorylation by Akt within the ST loop of AMPK-α1 down-regulates its activation in tumour cells. The Biochemical Journal, 459(2), 275–287.
Heathcote, H. R., Mancini, S. J., Strembitska, A., Jamal, K., Reihill, J. A., Palmer, T. M., Gould, G. W., & Salt, I. P. (2016). Protein kinase C phosphorylates AMP-activated protein kinase α1 Ser487. The Biochemical Journal, 473(24), 4681–4697.
Heinrichs, S. C., Menzaghi, F., Pich, E. M., Hauger, R. L., & Koob, G. F. (1993). Corticotropin-releasing factor in the paraventricular nucleus modulates feeding induced by neuropeptide Y. Brain Research, 611(1), 18–24.
Henckens, M. J. A. G., Deussing, J. M., & Chen, A. (2016). Region-specific roles of the corticotropin-releasing factor-urocortin system in stress. Nature Reviews. Neuroscience, 17(10), 636–651.
Heuer, H., Maier, M. K., Iden, S., Mittag, J., Friesema, E. C. H., Visser, T. J., & Bauer, K. (2005). The monocarboxylate transporter 8 linked to human psychomotor retardation is highly expressed in thyroid hormone-sensitive neuron populations. Endocrinology, 146(4), 1701–1706.
Hill, J. W., Williams, K. W., Ye, C., Luo, J., Balthasar, N., Coppari, R., Cowley, M. A., Cantley, L. C., Lowell, B. B., & Elmquist, J. K. (2008). Acute effects of leptin require PI3K signaling in hypothalamic proopiomelanocortin neurons in mice. The Journal of Clinical Investigation, 118(5), 1796–1805.
Hill, J. W., Xu, Y., Preitner, F., Fukuda, M., Cho, Y.-R., Luo, J., Balthasar, N., Coppari, R., Cantley, L. C., Kahn, B. B., Zhao, J. J., & Elmquist, J. K. (2009). Phosphatidyl inositol 3-kinase signaling in hypothalamic proopiomelanocortin neurons contributes to the regulation of glucose homeostasis. Endocrinology, 150(11), 4874–4882.
Hisahara, S., Chiba, S., Matsumoto, H., Tanno, M., Yagi, H., Shimohama, S., Sato, M., & Horio, Y. (2008). Histone deacetylase SIRT1 modulates neuronal differentiation by its nuclear translocation. Proceedings of the National Academy of Sciences of the United States of America, 105(40), 15599–15604.
Hou, X., Xu, S., Maitland-Toolan, K. A., Sato, K., Jiang, B., Ido, Y., Lan, F., Walsh, K., Wierzbicki, M., Verbeuren, T. J., Cohen, R. A., & Zang, M. (2008). SIRT1 regulates hepatocyte lipid metabolism through activating AMP-activated protein kinase. The Journal of Biological Chemistry, 283(29), 20015–20026.
Houtkooper, R. H., Pirinen, E., & Auwerx, J. (2012). Sirtuins as regulators of metabolism and healthspan. Nature Reviews. Molecular Cell Biology, 13(4), 225–238.
Hu, Z., Cha, S. H., Chohnan, S., & Lane, M. D. (2003). Hypothalamic malonyl-CoA as a mediator of feeding behavior. Proceedings of the National Academy of Sciences of the United States of America, 100(22), 12624–12629.
Huang, H., & Tindall, D. J. (2007). Dynamic FoxO transcription factors. Journal of Cell Science, 120(Pt 15), 2479–2487.
Hurtado-Carneiro, V., Roncero, I., Blazquez, E., Alvarez, E., & Sanz, C. (2013). PAS kinase as a nutrient sensor in neuroblastoma and hypothalamic cells required for the normal expression and activity of other cellular nutrient and energy sensors. Molecular Neurobiology, 48(3), 904–920.
Hurtado-Carneiro, V., Roncero, I., Egger, S. S., Wenger, R. H., Blazquez, E., Sanz, C., & Alvarez, E. (2014). PAS kinase is a nutrient and energy sensor in hypothalamic areas required for the normal function of AMPK and mTOR/S6K1. Molecular Neurobiology, 50(2), 314–326.
Ibrahim, N., Bosch, M. A., Smart, J. L., Qiu, J., Rubinstein, M., Rønnekleiv, O. K., Low, M. J., & Kelly, M. J. (2003). Hypothalamic proopiomelanocortin neurons are glucose responsive and express K(ATP) channels. Endocrinology, 144(4), 1331–1340.
Inoki, K., Zhu, T., & Guan, K.-L. (2003). TSC2 mediates cellular energy response to control cell growth and survival. Cell, 115(5), 577–590.
Iyer, A., Fairlie, D. P., & Brown, L. (2012). Lysine acetylation in obesity, diabetes and metabolic disease. Immunology and Cell Biology, 90(1), 39–46.
Jacinto, E., Loewith, R., Schmidt, A., Lin, S., Rüegg, M. A., Hall, A., & Hall, M. N. (2004). Mammalian TOR complex 2 controls the actin cytoskeleton and is rapamycin insensitive. Nature Cell Biology, 6(11), 1122–1128.
Jackson, R. S., Creemers, J. W., Ohagi, S., Raffin-Sanson, M. L., Sanders, L., Montague, C. T., Hutton, J. C., & O’Rahilly, S. (1997). Obesity and impaired prohormone processing associated with mutations in the human prohormone convertase 1 gene. Nature Genetics, 16(3), 303–306.
Jäger, S., Handschin, C., St-Pierre, J., & Spiegelman, B. M. (2007). AMP-activated protein kinase (AMPK) action in skeletal muscle via direct phosphorylation of PGC-1alpha. Proceedings of the National Academy of Sciences of the United States of America, 104(29), 12017–12022.
Jang, M., & Romsos, D. R. (1998). Neuropeptide Y and corticotropin-releasing hormone concentrations within specific hypothalamic regions of lean but not Ob/Ob mice respond to food-deprivation and refeeding. The Journal of Nutrition, 128(12), 2520–2525.
Jay, P., Rougeulle, C., Massacrier, A., Moncla, A., Mattei, M. G., Malzac, P., Roëckel, N., Taviaux, S., Lefranc, J. L., Cau, P., Berta, P., Lalande, M., & Muscatelli, F. (1997). The human necdin gene, NDN, is maternally imprinted and located in the Prader-Willi syndrome chromosomal region. Nature Genetics, 17(3), 357–361.
Jiang, H., Khan, S., Wang, Y., Charron, G., He, B., Sebastian, C., Du, J., Kim, R., Ge, E., Mostoslavsky, R., Hang, H. C., Hao, Q., & Lin, H. (2013). SIRT6 regulates TNF-α secretion through hydrolysis of long-chain fatty acyl lysine. Nature, 496(7443), 110–113.
Jing, E., Nillni, E. A., Sanchez, V. C., Stuart, R. C., & Good, D. J. (2004). Deletion of the Nhlh2 transcription factor decreases the levels of the anorexigenic peptides alpha melanocyte-stimulating hormone and thyrotropin-releasing hormone and implicates prohormone convertases I and II in obesity. Endocrinology, 145(4), 1503–1513.
Jung, J., Genau, H. M., & Behrends, C. (2015). Amino acid-dependent mTORC1 regulation by the lysosomal membrane protein SLC38A9. Molecular and Cellular Biology, 35(14), 2479–2494.
Kabra, D. G., Pfuhlmann, K., García-Cáceres, C., Schriever, S. C., Casquero García, V., Kebede, A. F., Fuente-Martin, E., Trivedi, C., Heppner, K., Uhlenhaut, N. H., Legutko, B., Kabra, U. D., Gao, Y., Yi, C.-X., Quarta, C., Clemmensen, C., Finan, B., Müller, T. D., Meyer, C. W., Paez-Pereda, M., Stemmer, K., Woods, S. C., Perez-Tilve, D., Schneider, R., Olson, E. N., Tschöp, M. H., & Pfluger, P. T. (2016). Hypothalamic leptin action is mediated by histone deacetylase 5. Nature Communications, 7, 10782.
Kanfi, Y., Peshti, V., Gozlan, Y. M., Rathaus, M., Gil, R., & Cohen, H. Y. (2008). Regulation of SIRT1 protein levels by nutrient availability. FEBS Letters, 582(16), 2417–2423.
Kang, H., Jung, J.-W., Kim, M. K., & Chung, J. H. (2009). CK2 is the regulator of SIRT1 substrate-binding affinity, deacetylase activity and cellular response to DNA-damage. PLoS One, 4(8), e6611.
Kaur, C., & Ling, E.-A. (2017). The circumventricular organs. Histology and Histopathology, 32(9), 879–892.
Kaushik, S., Rodriguez-Navarro, J. A., Arias, E., Kiffin, R., Sahu, S., Schwartz, G. J., Cuervo, A. M., & Singh, R. (2011). Autophagy in hypothalamic AgRP neurons regulates food intake and energy balance. Cell Metabolism, 14(2), 173–183.
Kaushik, S., Arias, E., Kwon, H., Lopez, N. M., Athonvarangkul, D., Sahu, S., Schwartz, G. J., Pessin, J. E., & Singh, R. (2012). Loss of autophagy in hypothalamic POMC neurons impairs lipolysis. EMBO Reports, 13(3), 258–265.
Kim, M.-S., Park, J.-Y., Namkoong, C., Jang, P.-G., Ryu, J.-W., Song, H.-S., Yun, J.-Y., Namgoong, I.-S., Ha, J., Park, I.-S., Lee, I.-K., Viollet, B., Youn, J. H., Lee, H.-K., & Lee, K.-U. (2004). Anti-obesity effects of alpha-lipoic acid mediated by suppression of hypothalamic AMP-activated protein kinase. Nature Medicine, 10(7), 727–733.
Kim, M.-S., Pak, Y. K., Jang, P.-G., Namkoong, C., Choi, Y.-S., Won, J.-C., Kim, K.-S., Kim, S.-W., Kim, H.-S., Park, J.-Y., Kim, Y.-B., & Lee, K.-U. (2006). Role of hypothalamic Foxo1 in the regulation of food intake and energy homeostasis. Nature Neuroscience, 9(7), 901–906.
Kim, E.-J., Kho, J.-H., Kang, M.-R., & Um, S.-J. (2007a). Active regulator of SIRT1 cooperates with SIRT1 and facilitates suppression of p53 activity. Molecular Cell, 28(2), 277–290.
Kim, D., Nguyen, M. D., Dobbin, M. M., Fischer, A., Sananbenesi, F., Rodgers, J. T., Delalle, I., Baur, J. A., Sui, G., Armour, S. M., Puigserver, P., Sinclair, D. A., & Tsai, L.-H. (2007b). SIRT1 deacetylase protects against neurodegeneration in models for Alzheimer’s disease and amyotrophic lateral sclerosis. The EMBO Journal, 26(13), 3169–3179.
Kim, E., Goraksha-Hicks, P., Li, L., Neufeld, T. P., & Guan, K.-L. (2008a). Regulation of TORC1 by rag GTPases in nutrient response. Nature Cell Biology, 10(8), 935–945.
Kim, J.-E., Chen, J., & Lou, Z. (2008b). DBC1 is a negative regulator of SIRT1. Nature, 451(7178), 583–586.
Kim, J., Kundu, M., Viollet, B., & Guan, K.-L. (2011). AMPK and mTOR regulate autophagy through direct phosphorylation of Ulk1. Nature Cell Biology, 13(2), 132–141.
Kinote, A., Faria, J. A., Roman, E. A., Solon, C., Razolli, D. S., Ignacio-Souza, L. M., Sollon, C. S., Nascimento, L. F., de Araújo, T. M., Barbosa, A. P. L., Lellis-Santos, C., Velloso, L. A., Bordin, S., & Anhê, G. F. (2012). Fructose-induced hypothalamic AMPK activation stimulates hepatic PEPCK and gluconeogenesis due to increased corticosterone levels. Endocrinology, 153(8), 3633–3645.
Kitamura, T., Feng, Y., Kitamura, Y. I., Chua, S. C., Jr., Xu, A. W., Barsh, G. S., Rossetti, L., & Accili, D. (2006). Forkhead protein FoxO1 mediates Agrp-dependent effects of leptin on food intake. Nature Medicine, 12(5), 534–540.
Knight, C. M., Gutierrez-Juarez, R., Lam, T. K. T., Arrieta-Cruz, I., Huang, L., Schwartz, G., Barzilai, N., & Rossetti, L. (2011). Mediobasal hypothalamic SIRT1 is essential for resveratrol’s effects on insulin action in rats. Diabetes, 60(11), 2691–2700.
Kocalis, H. E., Hagan, S. L., George, L., Turney, M. K., Siuta, M. A., Laryea, G. N., Morris, L. C., Muglia, L. J., Printz, R. L., Stanwood, G. D., & Niswender, K. D. (2014). Rictor/mTORC2 facilitates central regulation of energy and glucose homeostasis. Molecular Metabolism, 3(4), 394–407.
Kolthur-Seetharam, U., Teerds, K., de Rooij, D. G., Wendling, O., McBurney, M., Sassone-Corsi, P., & Davidson, I. (2009). The histone deacetylase SIRT1 controls male fertility in mice through regulation of hypothalamic-pituitary gonadotropin signaling. Biology of Reproduction, 80(2), 384–391.
Kong, X., Yu, J., Bi, J., Qi, H., Di, W., Wu, L., Wang, L., Zha, J., Lv, S., Zhang, F., Li, Y., Hu, F., Liu, F., Zhou, H., Liu, J., & Ding, G. (2015). Glucocorticoids transcriptionally regulate miR-27b expression promoting body fat accumulation via suppressing the browning of white adipose tissue. Diabetes, 64(2), 393–404.
Korosi, A., & Baram, T. Z. (2008). The central corticotropin releasing factor system during development and adulthood. European Journal of Pharmacology, 583(2–3), 204–214.
Kovács, K. J. (2013). CRH: The link between hormonal-, metabolic- and behavioral responses to stress. Journal of Chemical Neuroanatomy, 54, 25–33.
Kubota, N., Yano, W., Kubota, T., Yamauchi, T., Itoh, S., Kumagai, H., Kozono, H., Takamoto, I., Okamoto, S., Shiuchi, T., Suzuki, R., Satoh, H., Tsuchida, A., Moroi, M., Sugi, K., Noda, T., Ebinuma, H., Ueta, Y., Kondo, T., Araki, E., Ezaki, O., Nagai, R., Tobe, K., Terauchi, Y., Ueki, K., Minokoshi, Y., & Kadowaki, T. (2007). Adiponectin stimulates AMP-activated protein kinase in the hypothalamus and increases food intake. Cell Metabolism, 6(1), 55–68.
Kumar, M. V., Shimokawa, T., Nagy, T. R., & Lane, M. D. (2002). Differential effects of a centrally acting fatty acid synthase inhibitor in lean and obese mice. Proceedings of the National Academy of Sciences of the United States of America, 99(4), 1921–1925.
Kumar, A., Lawrence, J. C., Jr., Jung, D. Y., Ko, H. J., Keller, S. R., Kim, J. K., Magnuson, M. A., & Harris, T. E. (2010). Fat cell-specific ablation of rictor in mice impairs insulin-regulated fat cell and whole-body glucose and lipid metabolism. Diabetes, 59(6), 1397–1406.
Kurth-Kraczek, E. J., Hirshman, M. F., Goodyear, L. J., & Winder, W. W. (1999). 5’ AMP-activated protein kinase activation causes GLUT4 translocation in skeletal muscle. Diabetes, 48(8), 1667–1671.
Lafontaine-Lacasse, M., Richard, D., & Picard, F. (2010). Effects of age and gender on Sirt 1 mRNA expressions in the hypothalamus of the mouse. Neuroscience Letters, 480(1), 1–3.
Lagerlöf, O., Slocomb, J. E., Hong, I., Aponte, Y., Blackshaw, S., Hart, G. W., & Huganir, R. L. (2016). The nutrient sensor OGT in PVN neurons regulates feeding. Science, 351(6279), 1293–1296.
Lagouge, M., Argmann, C., Gerhart-Hines, Z., Meziane, H., Lerin, C., Daussin, F., Messadeq, N., Milne, J., Lambert, P., Elliott, P., Geny, B., Laakso, M., Puigserver, P., & Auwerx, J. (2006). Resveratrol improves mitochondrial function and protects against metabolic disease by activating SIRT1 and PGC-1alpha. Cell, 127(6), 1109–1122.
Lamming, D. W., & Sabatini, D. M. (2013). A central role for mTOR in lipid homeostasis. Cell Metabolism, 18(4), 465–469.
Lan, F., Cacicedo, J. M., Ruderman, N., & Ido, Y. (2008). SIRT1 modulation of the acetylation status, cytosolic localization, and activity of LKB1. Possible role in AMP-activated protein kinase activation. The Journal of Biological Chemistry, 283(41), 27628–27635.
Lane, M. D., Wolfgang, M., Cha, S.-H., & Dai, Y. (2008). Regulation of food intake and energy expenditure by hypothalamic malonyl-CoA. International Journal of Obesity, 32(Suppl 4), S49–S54.
Laplante, M., & Sabatini, D. M. (2012). mTOR signaling in growth control and disease. Cell, 149(2), 274–293.
Laryea, G., Schütz, G., & Muglia, L. J. (2013). Disrupting hypothalamic glucocorticoid receptors causes HPA axis hyperactivity and excess adiposity. Molecular Endocrinology, 27(10), 1655–1665.
Laurent, V., Kimble, A., Peng, B., Zhu, P., Pintar, J. E., Steiner, D. F., & Lindberg, I. (2002). Mortality in 7B2 null mice can be rescued by adrenalectomy: Involvement of dopamine in ACTH hypersecretion. Proceedings of the National Academy of Sciences of the United States of America, 99(5), 3087–3092.
Laurent, G., German, N. J., Saha, A. K., de Boer, V. C. J., Davies, M., Koves, T. R., Dephoure, N., Fischer, F., Boanca, G., Vaitheesvaran, B., Lovitch, S. B., Sharpe, A. H., Kurland, I. J., Steegborn, C., Gygi, S. P., Muoio, D. M., Ruderman, N. B., & Haigis, M. C. (2013). SIRT4 coordinates the balance between lipid synthesis and catabolism by repressing malonyl CoA decarboxylase. Molecular Cell, 50(5), 686–698.
Lee, K., Li, B., Xi, X., Suh, Y., & Martin, R. J. (2005). Role of neuronal energy status in the regulation of adenosine 5′-monophosphate-activated protein kinase, orexigenic neuropeptides expression, and feeding behavior. Endocrinology, 146(1), 3–10.
Lee, J. W., Park, S., Takahashi, Y., & Wang, H.-G. (2010). The association of AMPK with ULK1 regulates autophagy. PLoS One, 5(11), e15394.
Lembke, V., Goebel, M., Frommelt, L., Inhoff, T., Lommel, R., Stengel, A., Taché, Y., Grötzinger, C., Bannert, N., Wiedenmann, B., Klapp, B. F., & Kobelt, P. (2011). Sulfated cholecystokinin-8 activates phospho-mTOR immunoreactive neurons of the paraventricular nucleus in rats. Peptides, 32(1), 65–70.
Levin, B. E., Dunn-Meynell, A. A., Balkan, B., & Keesey, R. E. (1997). Selective breeding for diet-induced obesity and resistance in Sprague-Dawley rats. The American Journal of Physiology, 273(2 Pt 2), R725–R730.
Lewis, C. A., Griffiths, B., Santos, C. R., Pende, M., & Schulze, A. (2011). Regulation of the SREBP transcription factors by mTORC1. Biochemical Society Transactions, 39(2), 495–499.
Li, X., & Gao, T. (2014). mTORC2 phosphorylates protein kinase Cζ to regulate its stability and activity. EMBO Reports, 15(2), 191–198.
Libert, S., Pointer, K., Bell, E. L., Das, A., Cohen, D. E., Asara, J. M., Kapur, K., Bergmann, S., Preisig, M., Otowa, T., Kendler, K. S., Chen, X., Hettema, J. M., van den Oord, E. J., Rubio, J. P., & Guarente, L. (2011). SIRT1 activates MAO-A in the brain to mediate anxiety and exploratory drive. Cell, 147(7), 1459–1472.
Liu, K., Paterson, A. J., Chin, E., & Kudlow, J. E. (2000). Glucose stimulates protein modification by O-linked GlcNAc in pancreatic beta cells: Linkage of O-linked GlcNAc to beta cell death. Proceedings of the National Academy of Sciences of the United States of America, 97(6), 2820–2825.
Liu, Y., Dentin, R., Chen, D., Hedrick, S., Ravnskjaer, K., Schenk, S., Milne, J., Meyers, D. J., Cole, P., Yates, J., 3rd, Olefsky, J., Guarente, L., & Montminy, M. (2008). A fasting inducible switch modulates gluconeogenesis via activator/coactivator exchange. Nature, 456(7219), 269–273.
Lizcano, J. M., Göransson, O., Toth, R., Deak, M., Morrice, N. A., Boudeau, J., Hawley, S. A., Udd, L., Mäkelä, T. P., Hardie, D. G., & Alessi, D. R. (2004). LKB1 is a master kinase that activates 13 kinases of the AMPK subfamily, including MARK/PAR-1. The EMBO Journal, 23(4), 833–843.
Lloyd, D. J., Bohan, S., & Gekakis, N. (2006). Obesity, hyperphagia and increased metabolic efficiency in Pc1 mutant mice. Human Molecular Genetics, 15(11), 1884–1893.
Löffler, A. S., Alers, S., Dieterle, A. M., Keppeler, H., Franz-Wachtel, M., Kundu, M., Campbell, D. G., Wesselborg, S., Alessi, D. R., & Stork, B. (2011). Ulk1-mediated phosphorylation of AMPK constitutes a negative regulatory feedback loop. Autophagy, 7(7), 696–706.
Loftus, T. M., Jaworsky, D. E., Frehywot, G. L., Townsend, C. A., Ronnett, G. V., Lane, M. D., & Kuhajda, F. P. (2000). Reduced food intake and body weight in mice treated with fatty acid synthase inhibitors. Science, 288(5475), 2379–2381.
López, M., Lage, R., Saha, A. K., Pérez-Tilve, D., Vázquez, M. J., Varela, L., Sangiao-Alvarellos, S., Tovar, S., Raghay, K., Rodríguez-Cuenca, S., Deoliveira, R. M., Castañeda, T., Datta, R., Dong, J. Z., Culler, M., Sleeman, M. W., Alvarez, C. V., Gallego, R., Lelliott, C. J., Carling, D., Tschöp, M. H., Diéguez, C., & Vidal-Puig, A. (2008). Hypothalamic fatty acid metabolism mediates the orexigenic action of ghrelin. Cell Metabolism, 7(5), 389–399.
Lovejoy, D. A., Chang, B. S. W., Lovejoy, N. R., & del Castillo, J. (2014). Molecular evolution of GPCRs: CRH/CRH receptors. Journal of Molecular Endocrinology, 52(3), T43–T60.
Lu, X.-Y., Barsh, G. S., Akil, H., & Watson, S. J. (2003). Interaction between alpha-melanocyte-stimulating hormone and corticotropin-releasing hormone in the regulation of feeding and hypothalamo-pituitary-adrenal responses. The Journal of Neuroscience, 23(21), 7863–7872.
Lu, M., Sarruf, D. A., Li, P., Osborn, O., Sanchez-Alavez, M., Talukdar, S., Chen, A., Bandyopadhyay, G., Xu, J., Morinaga, H., Dines, K., Watkins, S., Kaiyala, K., Schwartz, M. W., & Olefsky, J. M. (2013). Neuronal Sirt1 deficiency increases insulin sensitivity in both brain and peripheral tissues. The Journal of Biological Chemistry, 288(15), 10722–10735.
MacDonald, H. R., & Wevrick, R. (1997). The necdin gene is deleted in Prader-Willi syndrome and is imprinted in human and mouse. Human Molecular Genetics, 6(11), 1873–1878.
Mao, Z., Hine, C., Tian, X., Van Meter, M., Au, M., Vaidya, A., Seluanov, A., & Gorbunova, V. (2011). SIRT6 promotes DNA repair under stress by activating PARP1. Science, 332(6036), 1443–1446.
Marsin, A. S., Bertrand, L., Rider, M. H., Deprez, J., Beauloye, C., Vincent, M. F., Van den Berghe, G., Carling, D., & Hue, L. (2000). Phosphorylation and activation of heart PFK-2 by AMPK has a role in the stimulation of glycolysis during ischaemia. Current Biology, 10(20), 1247–1255.
Marsin, A.-S., Bouzin, C., Bertrand, L., & Hue, L. (2002). The stimulation of glycolysis by hypoxia in activated monocytes is mediated by AMP-activated protein kinase and inducible 6-phosphofructo-2-kinase. The Journal of Biological Chemistry, 277(34), 30778–30783.
Martin, T. L., Alquier, T., Asakura, K., Furukawa, N., Preitner, F., & Kahn, B. B. (2006). Diet-induced obesity alters AMP kinase activity in hypothalamus and skeletal muscle. The Journal of Biological Chemistry, 281(28), 18933–18941.
Martínez de Morentin, P. B., González-García, I., Martins, L., Lage, R., Fernández-Mallo, D., Martínez-Sánchez, N., Ruíz-Pino, F., Liu, J., Morgan, D. A., Pinilla, L., Gallego, R., Saha, A. K., Kalsbeek, A., Fliers, E., Bisschop, P. H., Diéguez, C., Nogueiras, R., Rahmouni, K., Tena-Sempere, M., & López, M. (2014). Estradiol regulates brown adipose tissue thermogenesis via hypothalamic AMPK. Cell Metabolism, 20(1), 41–53.
Martínez-Sánchez, N., Seoane-Collazo, P., Contreras, C., Varela, L., Villarroya, J., Rial-Pensado, E., Buqué, X., Aurrekoetxea, I., Delgado, T. C., Vázquez-Martínez, R., González-García, I., Roa, J., Whittle, A. J., Gomez-Santos, B., Velagapudi, V., YCL, T., Morgan, D. A., Voshol, P. J., Martínez de Morentin, P. B., López-González, T., Liñares-Pose, L., Gonzalez, F., Chatterjee, K., Sobrino, T., Medina-Gómez, G., Davis, R. J., Casals, N., Orešič, M., Coll, A. P., Vidal-Puig, A., Mittag, J., Tena-Sempere, M., Malagón, M. M., Diéguez, C., Martínez-Chantar, M. L., Aspichueta, P., Rahmouni, K., Nogueiras, R., Sabio, G., Villarroya, F., & López, M. (2017). Hypothalamic AMPK-ER stress-JNK1 Axis mediates the central actions of thyroid hormones on energy balance. Cell Metabolism, 26(1), 212–229.e12.
Martins, L., Fernández-Mallo, D., Novelle, M. G., Vázquez, M. J., Tena-Sempere, M., Nogueiras, R., López, M., & Diéguez, C. (2012). Hypothalamic mTOR signaling mediates the orexigenic action of ghrelin. PLoS One, 7(10), e46923.
Martins, L., Seoane-Collazo, P., Contreras, C., González-García, I., Martínez-Sánchez, N., González, F., Zalvide, J., Gallego, R., Diéguez, C., Nogueiras, R., Tena-Sempere, M., & López, M. (2016). A functional link between AMPK and orexin mediates the effect of BMP8B on energy balance. Cell Reports, 16(8), 2231–2242.
Mastorakos, G., & Zapanti, E. (2004). The hypothalamic-pituitary-adrenal axis in the neuroendocrine regulation of food intake and obesity: The role of corticotropin releasing hormone. Nutritional Neuroscience, 7(5–6), 271–280.
Meikle, L., Talos, D. M., Onda, H., Pollizzi, K., Rotenberg, A., Sahin, M., Jensen, F. E., & Kwiatkowski, D. J. (2007). A mouse model of tuberous sclerosis: Neuronal loss of Tsc1 causes dysplastic and ectopic neurons, reduced myelination, seizure activity, and limited survival. The Journal of Neuroscience, 27(21), 5546–5558.
Menzies, K. J., Zhang, H., Katsyuba, E., & Auwerx, J. (2016). Protein acetylation in metabolism - metabolites and cofactors. Nature Reviews. Endocrinology, 12(1), 43–60.
Merrill, G. F., Kurth, E. J., Hardie, D. G., & Winder, W. W. (1997). AICA riboside increases AMP-activated protein kinase, fatty acid oxidation, and glucose uptake in rat muscle. The American Journal of Physiology, 273(6 Pt 1), E1107–E1112.
Minokoshi, Y., Alquier, T., Furukawa, N., Kim, Y.-B., Lee, A., Xue, B., Mu, J., Foufelle, F., Ferré, P., Birnbaum, M. J., Stuck, B. J., & Kahn, B. B. (2004). AMP-kinase regulates food intake by responding to hormonal and nutrient signals in the hypothalamus. Nature, 428(6982), 569–574.
Moloughney, J. G., Kim, P. K., Vega-Cotto, N. M., Wu, C.-C., Zhang, S., Adlam, M., Lynch, T., Chou, P.-C., Rabinowitz, J. D., Werlen, G., & Jacinto, E. (2016). mTORC2 responds to glutamine catabolite levels to modulate the Hexosamine biosynthesis enzyme GFAT1. Molecular Cell, 63(5), 811–826.
Momcilovic, M., Hong, S.-P., & Carlson, M. (2006). Mammalian TAK1 activates Snf1 protein kinase in yeast and phosphorylates AMP-activated protein kinase in vitro. The Journal of Biological Chemistry, 281(35), 25336–25343.
Mori, H., Inoki, K., Münzberg, H., Opland, D., Faouzi, M., Villanueva, E. C., Ikenoue, T., Kwiatkowski, D., MacDougald, O. A., Myers, M. G., Jr., & Guan, K.-L. (2009). Critical role for hypothalamic mTOR activity in energy balance. Cell Metabolism, 9(4), 362–374.
Morton, G. J., Cummings, D. E., Baskin, D. G., Barsh, G. S., & Schwartz, M. W. (2006). Central nervous system control of food intake and body weight. Nature, 443(7109), 289–295.
Moynihan, K. A., Grimm, A. A., Plueger, M. M., Bernal-Mizrachi, E., Ford, E., Cras-Méneur, C., Permutt, M. A., & Imai, S.-I. (2005). Increased dosage of mammalian Sir2 in pancreatic beta cells enhances glucose-stimulated insulin secretion in mice. Cell Metabolism, 2(2), 105–117.
Muta, K., Morgan, D. A., & Rahmouni, K. (2015). The role of hypothalamic mTORC1 signaling in insulin regulation of food intake, body weight, and sympathetic nerve activity in male mice. Endocrinology, 156(4), 1398–1407.
Naggert, J. K., Fricker, L. D., Varlamov, O., Nishina, P. M., Rouille, Y., Steiner, D. F., Carroll, R. J., Paigen, B. J., & Leiter, E. H. (1995). Hyperproinsulinaemia in obese fat/fat mice associated with a carboxypeptidase E mutation which reduces enzyme activity. Nature Genetics, 10(2), 135–142.
Nakahata, Y., Kaluzova, M., Grimaldi, B., Sahar, S., Hirayama, J., Chen, D., Guarente, L. P., & Sassone-Corsi, P. (2008). The NAD+−dependent deacetylase SIRT1 modulates CLOCK-mediated chromatin remodeling and circadian control. Cell, 134(2), 329–340.
Nakahata, Y., Sahar, S., Astarita, G., Kaluzova, M., & Sassone-Corsi, P. (2009). Circadian control of the NAD+ salvage pathway by CLOCK-SIRT1. Science, 324(5927), 654–657.
Nasrin, N., Kaushik, V. K., Fortier, E., Wall, D., Pearson, K. J., de Cabo, R., & Bordone, L. (2009). JNK1 phosphorylates SIRT1 and promotes its enzymatic activity. PLoS One, 4(12), e8414.
Nie, Y., Erion, D. M., Yuan, Z., Dietrich, M., Shulman, G. I., Horvath, T. L., & Gao, Q. (2009). STAT3 inhibition of gluconeogenesis is downregulated by SirT1. Nature Cell Biology, 11(4), 492–500.
Nillni, E. A. (2010). Regulation of the hypothalamic thyrotropin releasing hormone (TRH) neuron by neuronal and peripheral inputs. Frontiers in Neuroendocrinology, 31(2), 134–156.
Nillni - Endocrinology EA. (2007). Regulation of prohormone convertases in hypothalamic neurons: implications for prothyrotropin-releasing hormone and proopiomelanocortin. press.endocrine.org 2007. Available at: http://press.endocrine.org/doi/abs/10.1210/en.2007-0173.
Nillni, E. A., Friedman, T. C., Todd, R. B., Birch, N. P., Loh, Y. P., & Jackson, I. M. (1995). Pro-thyrotropin-releasing hormone processing by recombinant PC1. Journal of Neurochemistry, 65(6), 2462–2472.
Nillni, E. A., Xie, W., Mulcahy, L., Sanchez, V. C., & Wetsel, W. C. (2002). Deficiencies in pro-thyrotropin-releasing hormone processing and abnormalities in thermoregulation in Cpefat/fat mice. The Journal of Biological Chemistry, 277(50), 48587–48595.
Oberdoerffer, P., Michan, S., McVay, M., Mostoslavsky, R., Vann, J., Park, S.-K., Hartlerode, A., Stegmuller, J., Hafner, A., Loerch, P., Wright, S. M., Mills, K. D., Bonni, A., Yankner, B. A., Scully, R., Prolla, T. A., Alt, F. W., & Sinclair, D. A. (2008). SIRT1 redistribution on chromatin promotes genomic stability but alters gene expression during aging. Cell, 135(5), 907–918.
Obici, S., Feng, Z., Morgan, K., Stein, D., Karkanias, G., & Rossetti, L. (2002). Central administration of oleic acid inhibits glucose production and food intake. Diabetes, 51(2), 271–275.
Oh, T. S., Cho, H., Cho, J. H., Yu, S.-W., & Kim, E.-K. (2016). Hypothalamic AMPK-induced autophagy increases food intake by regulating NPY and POMC expression. Autophagy, 12(11), 2009–2025.
Orozco-Solis, R., & Sassone-Corsi, P. (2014). Epigenetic control and the circadian clock: Linking metabolism to neuronal responses. Neuroscience, 264, 76–87.
Orozco-Solis, R., Ramadori, G., Coppari, R., & Sassone-Corsi, P. (2015). SIRT1 relays nutritional inputs to the circadian clock through the Sf1 neurons of the ventromedial hypothalamus. Endocrinology, 156(6), 2174–2184.
Ortega-Molina, A., Lopez-Guadamillas, E., Mattison, J. A., Mitchell, S. J., Muñoz-Martin, M., Iglesias, G., Gutierrez, V. M., Vaughan, K. L., Szarowicz, M. D., González-García, I., López, M., Cebrián, D., Martinez, S., Pastor, J., de Cabo, R., & Serrano, M. (2015). Pharmacological inhibition of PI3K reduces adiposity and metabolic syndrome in obese mice and rhesus monkeys. Cell Metabolism, 21(4), 558–570.
Paquet, L., Massie, B., & Mains, R. E. (1996). Proneuropeptide Y processing in large dense-core vesicles: Manipulation of prohormone convertase expression in sympathetic neurons using adenoviruses. The Journal of Neuroscience, 16(3), 964–973.
Park, H.-K., & Ahima, R. S. (2014). Leptin signaling. F1000prime Reports, 6, 73.
Peek, C. B., Ramsey, K. M., Marcheva, B., & Bass, J. (2012). Nutrient sensing and the circadian clock. Trends in Endocrinology and Metabolism, 23(7), 312–318.
Perello, M., Stuart, R. C., & Nillni, E. A. (2007). Differential effects of fasting and leptin on proopiomelanocortin peptides in the arcuate nucleus and in the nucleus of the solitary tract. American Journal of Physiology. Endocrinology and Metabolism, 292(5), E1348–E1357.
Perone, M. J., Murray, C. A., Brown, O. A., Gibson, S., White, A., Linton, E. A., Perkins, A. V., Lowenstein, P. R., & Castro, M. G. (1998). Procorticotrophin-releasing hormone: Endoproteolytic processing and differential release of its derived peptides within AtT20 cells. Molecular and Cellular Endocrinology, 142(1–2), 191–202.
Pfister, J. A., Ma, C., Morrison, B. E., & D’Mello, S. R. (2008). Opposing effects of sirtuins on neuronal survival: SIRT1-mediated neuroprotection is independent of its deacetylase activity. PLoS One, 3(12), e4090.
Picard, F., Kurtev, M., Chung, N., Topark-Ngarm, A., Senawong, T., Machado De Oliveira, R., Leid, M., McBurney, M. W., & Guarente, L. (2004). Sirt1 promotes fat mobilization in white adipocytes by repressing PPAR-gamma. Nature, 429(6993), 771–776.
Plum, L., Lin, H. V., Dutia, R., Tanaka, J., Aizawa, K. S., Matsumoto, M., Kim, A. J., Cawley, N. X., Paik, J.-H., Loh, Y. P., DePinho, R. A., Wardlaw, S. L., & Accili, D. (2009). The obesity susceptibility gene Cpe links FoxO1 signaling in hypothalamic pro-opiomelanocortin neurons with regulation of food intake. Nature Medicine, 15(10), 1195–1201.
Posner, S. F., Vaslet, C. A., Jurofcik, M., Lee, A., Seidah, N. G., & Nillni, E. A. (2004). Stepwise posttranslational processing of progrowth hormone-releasing hormone (proGHRH) polypeptide by furin and PC1. Endocrine, 23(2–3), 199–213.
Price, N. L., Gomes, A. P., Ling, A. J. Y., Duarte, F. V., Martin-Montalvo, A., North, B. J., Agarwal, B., Ye, L., Ramadori, G., Teodoro, J. S., Hubbard, B. P., Varela, A. T., Davis, J. G., Varamini, B., Hafner, A., Moaddel, R., Rolo, A. P., Coppari, R., Palmeira, C. M., de Cabo, R., Baur, J. A., & Sinclair, D. A. (2012). SIRT1 is required for AMPK activation and the beneficial effects of resveratrol on mitochondrial function. Cell Metabolism, 15(5), 675–690.
Proulx, K., Cota, D., Woods, S. C., & Seeley, R. J. (2008). Fatty acid synthase inhibitors modulate energy balance via mammalian target of rapamycin complex 1 signaling in the central nervous system. Diabetes, 57(12), 3231–3238.
Prozorovski, T., Schulze-Topphoff, U., Glumm, R., Baumgart, J., Schröter, F., Ninnemann, O., Siegert, E., Bendix, I., Brüstle, O., Nitsch, R., Zipp, F., & Aktas, O. (2008). Sirt1 contributes critically to the redox-dependent fate of neural progenitors. Nature Cell Biology, 10(4), 385–394.
Pu, L. P., Ma, W., Barker, J. L., & Loh - Endocrinology YP. (1996). Differential coexpression of genes encoding prothyrotropin-releasing hormone (pro-TRH) and prohormone convertases (PC1 and PC2) in rat brain neurons: …. academic.oup.com 1996. Available at: https://academic.oup.com/endo/article-abstract/137/4/1233/3037181.
Qin, W., Yang, T., Ho, L., Zhao, Z., Wang, J., Chen, L., Zhao, W., Thiyagarajan, M., MacGrogan, D., Rodgers, J. T., Puigserver, P., Sadoshima, J., Deng, H., Pedrini, S., Gandy, S., Sauve, A. A., & Pasinetti, G. M. (2006). Neuronal SIRT1 activation as a novel mechanism underlying the prevention of Alzheimer disease amyloid neuropathology by calorie restriction. The Journal of Biological Chemistry, 281(31), 21745–21754.
Quan, W., Kim, H.-K., Moon, E.-Y., Kim, S. S., Choi, C. S., Komatsu, M., Jeong, Y. T., Lee, M.-K., Kim, K.-W., Kim, M.-S., & Lee, M.-S. (2012). Role of hypothalamic proopiomelanocortin neuron autophagy in the control of appetite and leptin response. Endocrinology, 153(4), 1817–1826.
Quiñones, M., Al-Massadi, O., Gallego, R., Fernø, J., Diéguez, C., López, M., & Nogueiras, R. (2015). Hypothalamic CaMKKβ mediates glucagon anorectic effect and its diet-induced resistance. Molecular Metabolism, 4(12), 961–970.
Raadsheer, F. C., Sluiter, A. A., Ravid, R., Tilders, F. J. H., & Swaab, D. F. (1993). Localization of corticotropin-releasing hormone (CRH) neurons in the paraventricular nucleus of the human hypothalamus; age-dependent colocalization with vasopressin. Brain Research, 615(1), 50–62.
Rafalski, V. A., Ho, P. P., Brett, J. O., Ucar, D., Dugas, J. C., Pollina, E. A., Chow, L. M. L., Ibrahim, A., Baker, S. J., Barres, B. A., Steinman, L., & Brunet, A. (2013). Expansion of oligodendrocyte progenitor cells following SIRT1 inactivation in the adult brain. Nature Cell Biology, 15(6), 614–624.
Ramadori, G., Lee, C. E., Bookout, A. L., Lee, S., Williams, K. W., Anderson, J., Elmquist, J. K., & Coppari, R. (2008). Brain SIRT1: Anatomical distribution and regulation by energy availability. The Journal of Neuroscience, 28(40), 9989–9996.
Ramadori, G., Fujikawa, T., Fukuda, M., Anderson, J., Morgan, D. A., Mostoslavsky, R., Stuart, R. C., Perello, M., Vianna, C. R., Nillni, E. A., Rahmouni, K., & Coppari, R. (2010). SIRT1 deacetylase in POMC neurons is required for homeostatic defenses against diet-induced obesity. Cell Metabolism, 12(1), 78–87.
Ramadori, G., Fujikawa, T., Anderson, J., Berglund, E. D., Frazao, R., Michán, S., Vianna, C. R., Sinclair, D. A., Elias, C. F., & Coppari, R. (2011). SIRT1 deacetylase in SF1 neurons protects against metabolic imbalance. Cell Metabolism, 14(3), 301–312.
Ramsey, K. M., Yoshino, J., Brace, C. S., Abrassart, D., Kobayashi, Y., Marcheva, B., Hong, H.-K., Chong, J. L., Buhr, E. D., Lee, C., Takahashi, J. S., Imai, S.-I., & Bass, J. (2009). Circadian clock feedback cycle through NAMPT-mediated NAD+ biosynthesis. Science, 324(5927), 651–654.
Rebsamen, M., Pochini, L., Stasyk, T., de Araújo, M. E. G., Galluccio, M., Kandasamy, R. K., Snijder, B., Fauster, A., Rudashevskaya, E. L., Bruckner, M., Scorzoni, S., Filipek, P. A., Huber, K. V. M., Bigenzahn, J. W., Heinz, L. X., Kraft, C., Bennett, K. L., Indiveri, C., Huber, L. A., & Superti-Furga, G. (2015). SLC38A9 is a component of the lysosomal amino acid sensing machinery that controls mTORC1. Nature, 519(7544), 477–481.
Ren, H., Orozco, I. J., Su, Y., Suyama, S., Gutiérrez-Juárez, R., Horvath, T. L., Wardlaw, S. L., Plum, L., Arancio, O., & Accili, D. (2012). FoxO1 target Gpr17 activates AgRP neurons to regulate food intake. Cell, 149(6), 1314–1326.
Revollo, J. R., Grimm, A. A., & Imai, S.-I. (2004). The NAD biosynthesis pathway mediated by nicotinamide phosphoribosyltransferase regulates Sir2 activity in mammalian cells. The Journal of Biological Chemistry, 279(49), 50754–50763.
Rho, J. H., & Swanson, L. W. (1987). Neuroendocrine CRF motoneurons: Intrahypothalamic axon terminals shown with a new retrograde-Lucifer-immuno method. Brain Research, 436(1), 143–147.
Richard, D., Huang, Q., & Timofeeva, E. (2000). The corticotropin-releasing hormone system in the regulation of energy balance in obesity. International Journal of Obesity, 24, S36–S39.
Rivier, J., Rivier, C., Spiess, J., & Vale, W. (1983). High-performance liquid chromatographic purification of peptide hormones: Ovine hypothalamic Amunine (Corticotropin releasing factor)1. In M. T. W. Hearn, F. E. Regnier, & C. T. Wehr (Eds.), High-performance liquid chromatography of proteins and peptides (pp. 233–241). Academic Press. Cambridge, Massachusetts
Rodgers, J. T., Lerin, C., Haas, W., Gygi, S. P., Spiegelman, B. M., & Puigserver, P. (2005). Nutrient control of glucose homeostasis through a complex of PGC-1alpha and SIRT1. Nature, 434(7029), 113–118.
Ropelle, E. R., Fernandes, M. F. A., Flores, M. B. S., Ueno, M., Rocco, S., Marin, R., Cintra, D. E., Velloso, L. A., Franchini, K. G., Saad, M. J. A., & Carvalheira, J. B. C. (2008). Central exercise action increases the AMPK and mTOR response to leptin. PLoS One, 3(12), e3856.
Roth, S. Y., Denu, J. M., & Allis, C. D. (2001). Histone acetyltransferases. Annual Review of Biochemistry, 70, 81–120.
Rougon, G., Nédélec, J., Malapert, P., Goridis, C., & Chesselet, M. F. (1990). Post-translation modifications of neural cell surface molecules. Acta Histochemica. Supplementband, 38, 51–57.
Rouillé, Y., Duguay, S. J., Lund, K., Furuta, M., Gong, Q., Lipkind, G., Oliva, A. A., Jr., Chan, S. J., & Steiner, D. F. (1995). Proteolytic processing mechanisms in the biosynthesis of neuroendocrine peptides: The subtilisin-like proprotein convertases. Frontiers in Neuroendocrinology, 16(4), 322–361.
Ruan, H.-B., Dietrich, M. O., Liu, Z.-W., Zimmer, M. R., Li, M.-D., Singh, J. P., Zhang, K., Yin, R., Wu, J., Horvath, T. L., & Yang, X. (2014). O-GlcNAc transferase enables AgRP neurons to suppress browning of white fat. Cell, 159(2), 306–317.
Sakamoto, J., Miura, T., Shimamoto, K., & Horio, Y. (2004). Predominant expression of Sir2alpha, an NAD-dependent histone deacetylase, in the embryonic mouse heart and brain. FEBS Letters, 556(1–3), 281–286.
Sakurai, T., Amemiya, A., Ishii, M., Matsuzaki, I., Chemelli, R. M., Tanaka, H., Williams, S. C., Richardson, J. A., Kozlowski, G. P., Wilson, S., Arch, J. R., Buckingham, R. E., Haynes, A. C., Carr, S. A., Annan, R. S., McNulty, D. E., Liu, W. S., Terrett, J. A., Elshourbagy, N. A., Bergsma, D. J., & Yanagisawa, M. (1998). Orexins and orexin receptors: A family of hypothalamic neuropeptides and G protein-coupled receptors that regulate feeding behavior. Cell, 92(4), 573–585.
Sancak, Y., Thoreen, C. C., Peterson, T. R., Lindquist, R. A., Kang, S. A., Spooner, E., Carr, S. A., & Sabatini, D. M. (2007). PRAS40 is an insulin-regulated inhibitor of the mTORC1 protein kinase. Molecular Cell, 25(6), 903–915.
Sancak, Y., Peterson, T. R., Shaul, Y. D., Lindquist, R. A., Thoreen, C. C., Bar-Peled, L., & Sabatini, D. M. (2008). The rag GTPases bind raptor and mediate amino acid signaling to mTORC1. Science, 320(5882), 1496–1501.
Sancak, Y., Bar-Peled, L., Zoncu, R., Markhard, A. L., Nada, S., & Sabatini, D. M. (2010). Ragulator-rag complex targets mTORC1 to the lysosomal surface and is necessary for its activation by amino acids. Cell, 141(2), 290–303.
Sanders, M. J., Grondin, P. O., Hegarty, B. D., Snowden, M. A., & Carling, D. (2007). Investigating the mechanism for AMP activation of the AMP-activated protein kinase cascade. The Biochemical Journal, 403(1), 139–148.
Sarbassov, D. D., Ali, S. M., Kim, D.-H., Guertin, D. A., Latek, R. R., Erdjument-Bromage, H., Tempst, P., & Sabatini, D. M. (2004). Rictor, a novel binding partner of mTOR, defines a rapamycin-insensitive and raptor-independent pathway that regulates the cytoskeleton. Current Biology, 14(14), 1296–1302.
Sarbassov, D. D., Guertin, D. A., Ali, S. M., & Sabatini, D. M. (2005). Phosphorylation and regulation of Akt/PKB by the rictor-mTOR complex. Science, 307(5712), 1098–1101.
Sasaki, T., Maier, B., Koclega, K. D., Chruszcz, M., Gluba, W., Stukenberg, P. T., Minor, W., & Scrable, H. (2008). Phosphorylation regulates SIRT1 function. PLoS One, 3(12), e4020.
Sasaki, T., Kim, H.-J., Kobayashi, M., Kitamura, Y.-I., Yokota-Hashimoto, H., Shiuchi, T., Minokoshi, Y., & Kitamura, T. (2010). Induction of hypothalamic Sirt1 leads to cessation of feeding via agouti-related peptide. Endocrinology, 151(6), 2556–2566.
Sasaki, T., Kikuchi, O., Shimpuku, M., Susanti, V. Y., Yokota-Hashimoto, H., Taguchi, R., Shibusawa, N., Sato, T., Tang, L., Amano, K., Kitazumi, T., Kuroko, M., Fujita, Y., Maruyama, J., Lee, Y.-S., Kobayashi, M., Nakagawa, T., Minokoshi, Y., Harada, A., Yamada, M., & Kitamura, T. (2014). Hypothalamic SIRT1 prevents age-associated weight gain by improving leptin sensitivity in mice. Diabetologia, 57(4), 819–831.
Satoh, A., Brace, C. S., Ben-Josef, G., West, T., Wozniak, D. F., Holtzman, D. M., Herzog, E. D., & Imai, S.-I. (2010). SIRT1 promotes the central adaptive response to diet restriction through activation of the dorsomedial and lateral nuclei of the hypothalamus. The Journal of Neuroscience, 30(30), 10220–10232.
Satoh, A., Brace, C. S., Rensing, N., Cliften, P., Wozniak, D. F., Herzog, E. D., Yamada, K. A., & Imai, S.-I. (2013). Sirt1 extends life span and delays aging in mice through the regulation of Nk2 homeobox 1 in the DMH and LH. Cell Metabolism, 18(3), 416–430.
Saxton, R. A., & Sabatini, D. M. (2017). mTOR signaling in growth, metabolism, and disease. Cell, 168(6), 960–976.
Schafer, M. K., Day, R., Cullinan, W. E., Chretien, M., Seidah, N. G., & Watson, S. J. (1993). Gene expression of prohormone and proprotein convertases in the rat CNS: A comparative in situ hybridization analysis. The Journal of Neuroscience, 13(3), 1258–1279.
Schaner, P., Todd, R. B., Seidah, N. G., & Nillni, E. A. (1997). Processing of prothyrotropin-releasing hormone by the family of prohormone convertases. The Journal of Biological Chemistry, 272(32), 19958–19968.
Seidah, N. G., Gaspar, L., & Mion - DNA and cell … P. (1990). cDNA sequence of two distinct pituitary proteins homologous to Kex2 and furin gene products: tissue-specific mRNAs encoding candidates for pro-hormone …. online.liebertpub.com 1990. Available at: http://online.liebertpub.com/doi/abs/10.1089/dna.1990.9.415.
Seidah, N. G., Marcinkiewicz, M., & Benjannet - Molecular … S. (1991). Cloning and primary sequence of a mouse candidate prohormone convertase PC1 homologous to PC2, Furin, and Kex2: distinct chromosomal localization and …. academic.oup.com 1991. Available at: https://academic.oup.com/mend/article-abstract/5/1/111/2714242.
Seidah, N. G., Day, R., Hamelin, J., Gaspar, A., Collard, M. W., & Chrétien, M. (1992). Testicular expression of PC4 in the rat: Molecular diversity of a novel germ cell-specific Kex2/subtilisin-like proprotein convertase. Molecular Endocrinology, 6(10), 1559–1570.
Seidah, N. G., Chrétien, M., & Day, R. (1994). The family of subtilisin/kexin like pro-protein and pro-hormone convertases: Divergent or shared functions. Biochimie, 76(3–4), 197–209.
Seidah, N. G., Hamelin, J., Mamarbachi, M., Dong, W., Tardos, H., Mbikay, M., Chretien, M., & Day, R. (1996). cDNA structure, tissue distribution, and chromosomal localization of rat PC7, a novel mammalian proprotein convertase closest to yeast kexin-like proteinases. Proceedings of the National Academy of Sciences of the United States of America, 93(8), 3388–3393.
Seimon, R. V., Hostland, N., Silveira, S. L., Gibson, A. A., & Sainsbury, A. (2013). Effects of energy restriction on activity of the hypothalamo-pituitary-adrenal axis in obese humans and rodents: Implications for diet-induced changes in body composition. |Hormone Molecular Biology and Clinical Investigation, 15(2), 71–80.
Seto, E., & Yoshida, M. (2014). Erasers of histone acetylation: The histone deacetylase enzymes. Cold Spring Harbor Perspectives in Biology, 6(4), a018713.
Shah, Z. H., Ahmed, S. U., Ford, J. R., Allison, S. J., Knight, J. R. P., & Milner, J. (2012). A deacetylase-deficient SIRT1 variant opposes full-length SIRT1 in regulating tumor suppressor p53 and governs expression of cancer-related genes. Molecular and Cellular Biology, 32(3), 704–716.
Shimizu, H., Arima, H., Ozawa, Y., Watanabe, M., Banno, R., Sugimura, Y., Ozaki, N., Nagasaki, H., & Oiso, Y. (2010). Glucocorticoids increase NPY gene expression in the arcuate nucleus by inhibiting mTOR signaling in rat hypothalamic organotypic cultures. Peptides, 31(1), 145–149.
Sinclair, D. A., & Guarente, L. (2014). Small-molecule allosteric activators of sirtuins. Annual Review of Pharmacology and Toxicology, 54, 363–380.
Smeekens, S. P., & Steiner, D. F. (1990). Identification of a human insulinoma cDNA encoding a novel mammalian protein structurally related to the yeast dibasic processing protease Kex2. The Journal of Biological Chemistry, 265(6), 2997–3000.
Smeekens, S. P., Avruch, A. S., LaMendola, J., Chan, S. J., & Steiner, D. F. (1991). Identification of a cDNA encoding a second putative prohormone convertase related to PC2 in AtT20 cells and islets of Langerhans. Proceedings of the National Academy of Sciences of the United States of America, 88(2), 340–344.
Smeekens, S. P., Montag, A. G., Thomas, G., Albiges-Rizo, C., Carroll, R., Benig, M., Phillips, L. A., Martin, S., Ohagi, S., & Gardner, P. (1992). Proinsulin processing by the subtilisin-related proprotein convertases furin, PC2, and PC3. Proceedings of the National Academy of Sciences of the United States of America, 89(18), 8822–8826.
Smith, T. L., & Rutter, J. (2007). Regulation of glucose partitioning by PAS kinase and Ugp1 phosphorylation. Molecular Cell, 26(4), 491–499.
Sohn, J.-W., Oh, Y., Kim, K. W., Lee, S., Williams, K. W., & Elmquist, J. K. (2016). Leptin and insulin engage specific PI3K subunits in hypothalamic SF1 neurons. Molecular Metabolism, 5(8), 669–679.
Solomon, S. (1999). POMC-derived peptides and their biological action. Annals of the New York Academy of Sciences, 885, 22–40.
Sominsky, L., & Spencer, S. J. (2014). Eating behavior and stress: A pathway to obesity. Frontiers in Psychology, 5, 434.
Spencer, S. J., & Tilbrook, A. (2011). The glucocorticoid contribution to obesity. Stress, 14(3), 233–246.
Starheim, K. K., Gevaert, K., & Arnesen, T. (2012). Protein N-terminal acetyltransferases: When the start matters. Trends in Biochemical Sciences, 37(4), 152–161.
Steinberg, G. R., Watt, M. J., Fam, B. C., Proietto, J., Andrikopoulos, S., Allen, A. M., Febbraio, M. A., & Kemp, B. E. (2006). Ciliary neurotrophic factor suppresses hypothalamic AMP-kinase signaling in leptin-resistant obese mice. Endocrinology, 147(8), 3906–3914.
Steiner, D. F. (1998). The proprotein convertases. Current Opinion in Chemical Biology, 2(1), 31–39.
Steiner, D. F., Smeekens, S. P., Ohagi, S., & Chan, S. J. (1992). The new enzymology of precursor processing endoproteases. The Journal of Biological Chemistry, 267(33), 23435–23438.
Stevanovic, D., Trajkovic, V., Müller-Lühlhoff, S., Brandt, E., Abplanalp, W., Bumke-Vogt, C., Liehl, B., Wiedmer, P., Janjetovic, K., Starcevic, V., Pfeiffer, A. F. H., Al-Hasani, H., Tschöp, M. H., & Castañeda, T. R. (2013). Ghrelin-induced food intake and adiposity depend on central mTORC1/S6K1 signaling. Molecular and Cellular Endocrinology, 381(1–2), 280–290.
Stoppa, G. R., Cesquini, M., Roman, E. A., Prada, P. O., Torsoni, A. S., Romanatto, T., Saad, M. J., Velloso, L. A., & Torsoni, M. A. (2008). Intracerebroventricular injection of citrate inhibits hypothalamic AMPK and modulates feeding behavior and peripheral insulin signaling. The Journal of Endocrinology, 198(1), 157–168.
Sullivan, J. E., Brocklehurst, K. J., Marley, A. E., Carey, F., Carling, D., & Beri, R. K. (1994). Inhibition of lipolysis and lipogenesis in isolated rat adipocytes with AICAR, a cell-permeable activator of AMP-activated protein kinase. FEBS Letters, 353(1), 33–36.
Susanti, V. Y., Sasaki, T., Yokota-Hashimoto, H., Matsui, S., Lee, Y.-S., Kikuchi, O., Shimpuku, M., Kim, H.-J., Kobayashi, M., & Kitamura, T. (2014). Sirt1 rescues the obesity induced by insulin-resistant constitutively-nuclear FoxO1 in POMC neurons of male mice. Obesity, 22(10), 2115–2119.
Suter, M., Riek, U., Tuerk, R., Schlattner, U., Wallimann, T., & Neumann, D. (2006). Dissecting the role of 5’-AMP for allosteric stimulation, activation, and deactivation of AMP-activated protein kinase. The Journal of Biological Chemistry, 281(43), 32207–32216.
Taleux, N., De Potter, I., Deransart, C., Lacraz, G., Favier, R., Leverve, X. M., Hue, L., & Guigas, B. (2008). Lack of starvation-induced activation of AMP-activated protein kinase in the hypothalamus of the Lou/C rats resistant to obesity. International Journal of Obesity, 32(4), 639–647.
Tan, M., Peng, C., Anderson, K. A., Chhoy, P., Xie, Z., Dai, L., Park, J., Chen, Y., Huang, H., Zhang, Y., Ro, J., Wagner, G. R., Green, M. F., Madsen, A. S., Schmiesing, J., Peterson, B. S., Xu, G., Ilkayeva, O. R., Muehlbauer, M. J., Braulke, T., Mühlhausen, C., Backos, D. S., Olsen, C. A., McGuire, P. J., Pletcher, S. D., Lombard, D. B., Hirschey, M. D., & Zhao, Y. (2014). Lysine glutarylation is a protein posttranslational modification regulated by SIRT5. Cell Metabolism, 19(4), 605–617.
Tanida, M., Yamamoto, N., Shibamoto, T., & Rahmouni, K. (2013). Involvement of hypothalamic AMP-activated protein kinase in leptin-induced sympathetic nerve activation. PLoS One, 8(2), e56660.
Tanida, M., Yamamoto, N., Morgan, D. A., Kurata, Y., Shibamoto, T., & Rahmouni, K. (2015). Leptin receptor signaling in the hypothalamus regulates hepatic autonomic nerve activity via phosphatidylinositol 3-kinase and AMP-activated protein kinase. The Journal of Neuroscience, 35(2), 474–484.
Tanno, M., Sakamoto, J., Miura, T., Shimamoto, K., & Horio, Y. (2007). Nucleocytoplasmic shuttling of the NAD+−dependent histone deacetylase SIRT1. The Journal of Biological Chemistry, 282(9), 6823–6832.
Tataranni, P. A., Larson, D. E., Snitker, S., Young, J. B., Flatt, J. P., & Ravussin, E. (1996). Effects of glucocorticoids on energy metabolism and food intake in humans. The American Journal of Physiology, 271(2 Pt 1), E317–E325.
Thomas, L., Leduc, R., & Thorne - Proceedings of the … BA. (1991). Kex2-like endoproteases PC2 and PC3 accurately cleave a model prohormone in mammalian cells: evidence for a common core of neuroendocrine processing …. National Acad Sciences 1991. Available at: http://www.pnas.org/content/88/12/5297.short.
Tong, L., & Denu, J. M. (2010). Function and metabolism of sirtuin metabolite O-acetyl-ADP-ribose. Biochimica et Biophysica Acta, 1804(8), 1617–1625.
Toorie, A. M., & Nillni, E. A. (2014). Minireview: Central Sirt1 regulates energy balance via the melanocortin system and alternate pathways. Molecular Endocrinology, 28(9), 1423–1434.
Toorie, A. M., Cyr, N. E., Steger, J. S., Beckman, R., Farah, G., & Nillni, E. A. (2016). The nutrient and energy sensor Sirt1 regulates the hypothalamic-pituitary-adrenal (HPA) Axis by altering the production of the prohormone convertase 2 (PC2) essential in the maturation of Corticotropin-releasing hormone (CRH) from its prohormone in male rats. The Journal of Biological Chemistry, 291(11), 5844–5859.
Toriya, M., Maekawa, F., Maejima, Y., Onaka, T., Fujiwara, K., Nakagawa, T., Nakata, M., & Yada, T. (2010). Long-term infusion of brain-derived neurotrophic factor reduces food intake and body weight via a corticotrophin-releasing hormone pathway in the paraventricular nucleus of the hypothalamus. Journal of Neuroendocrinology, 22(9), 987–995.
Vale, W., Spiess, J., Rivier, C., & Rivier, J. (1981a). Characterization of a 41-residue ovine hypothalamic peptide that stimulates secretion of corticotropin and beta-endorphin. Science, 213(4514), 1394–1397.
Vale, W., Spiess, J., Rivier, C., & Rivier, J. (1981b). Characterization of a 41-residue ovine hypothalamic peptide that stimulates secretion of Corticotropin and β-endorphin. Science, 213(4514), 1394–1397.
Vander Haar, E., Lee, S.-I., Bandhakavi, S., Griffin, T. J., & Kim, D.-H. (2007). Insulin signalling to mTOR mediated by the Akt/PKB substrate PRAS40. Nature Cell Biology, 9(3), 316–323.
Varela, L., Martínez-Sánchez, N., Gallego, R., Vázquez, M. J., Roa, J., Gándara, M., Schoenmakers, E., Nogueiras, R., Chatterjee, K., Tena-Sempere, M., Diéguez, C., & López, M. (2012). Hypothalamic mTOR pathway mediates thyroid hormone-induced hyperphagia in hyperthyroidism. The Journal of Pathology, 227(2), 209–222.
Velásquez, D. A., Martínez, G., Romero, A., Vázquez, M. J., Boit, K. D., Dopeso-Reyes, I. G., López, M., Vidal, A., Nogueiras, R., & Diéguez, C. (2011). The central Sirtuin 1/p53 pathway is essential for the orexigenic action of ghrelin. Diabetes, 60(4), 1177–1185.
Veo, K., Reinick, C., Liang, L., Moser, E., Angleson, J. K., & Dores, R. M. (2011). Observations on the ligand selectivity of the melanocortin 2 receptor. General and Comparative Endocrinology, 172(1), 3–9.
Villanueva, E. C., Münzberg, H., Cota, D., Leshan, R. L., Kopp, K., Ishida-Takahashi, R., Jones, J. C., Fingar, D. C., Seeley, R. J., & Myers, M. G., Jr. (2009). Complex regulation of mammalian target of rapamycin complex 1 in the basomedial hypothalamus by leptin and nutritional status. Endocrinology, 150(10), 4541–4551.
Villeneuve, P., Seidah, N. G., & Beaudet, A. (2000). Immunohistochemical evidence for the implication of PCI in the processing of proneurotensin in rat brain. Neuroreport, 11(16), 3443.
Wang, R., Cherukuri, P., & Luo, J. (2005). Activation of Stat3 sequence-specific DNA binding and transcription by p300/CREB-binding protein-mediated acetylation. The Journal of Biological Chemistry, 280(12), 11528–11534.
Wang, L., Harris, T. E., Roth, R. A., & Lawrence, J. C., Jr. (2007). PRAS40 regulates mTORC1 kinase activity by functioning as a direct inhibitor of substrate binding. The Journal of Biological Chemistry, 282(27), 20036–20044.
Wang, S., Tsun, Z.-Y., Wolfson, R. L., Shen, K., Wyant, G. A., Plovanich, M. E., Yuan, E. D., Jones, T. D., Chantranupong, L., Comb, W., Wang, T., Bar-Peled, L., Zoncu, R., Straub, C., Kim, C., Park, J., Sabatini, B. L., & Sabatini, D. M. (2015). Metabolism. Lysosomal amino acid transporter SLC38A9 signals arginine sufficiency to mTORC1. Science, 347(6218), 188–194.
Wardlaw, S. L. (2011). Hypothalamic proopiomelanocortin processing and the regulation of energy balance. European Journal of Pharmacology, 660(1), 213–219.
Wellen, K. E., Hatzivassiliou, G., Sachdeva, U. M., Bui, T. V., Cross, J. R., & Thompson, C. B. (2009). ATP-citrate lyase links cellular metabolism to histone acetylation. Science, 324(5930), 1076–1080.
Westphal, C. H., Muller, L., Zhou, A., Zhu, X., Bonner-Weir, S., Schambelan, M., Steiner, D. F., Lindberg, I., & Leder, P. (1999). The neuroendocrine protein 7B2 is required for peptide hormone processing in vivo and provides a novel mechanism for pituitary Cushing’s disease. Cell, 96(5), 689–700.
Williams, G., Bing, C., Cai, X. J., Harrold, J. A., King, P. J., & Liu, X. H. (2001). The hypothalamus and the control of energy homeostasis: Different circuits, different purposes. Physiology & Behavior, 74(4–5), 683–701.
Williams, K. W., Sohn, J.-W., Donato, J., Jr., Lee, C. E., Zhao, J. J., Elmquist, J. K., & Elias, C. F. (2011). The acute effects of leptin require PI3K signaling in the hypothalamic ventral premammillary nucleus. The Journal of Neuroscience, 31(37), 13147–13156.
Winder, W. W., & Hardie, D. G. (1996). Inactivation of acetyl-CoA carboxylase and activation of AMP-activated protein kinase in muscle during exercise. The American Journal of Physiology, 270(2 Pt 1), E299–E304.
Winsky-Sommerer, R., Benjannet, S., Rovère, C., Barbero, P., Seidah, N. G., Epelbaum, J., & Dournaud, P. (2000). Regional and cellular localization of the neuroendocrine prohormone convertases PC1 and PC2 in the rat central nervous system. The Journal of Comparative Neurology, 424(3), 439–460.
Witters, L. A., Nordlund, A. C., & Marshall, L. (1991). Regulation of intracellular acetyl-CoA carboxylase by ATP depletors mimics the action of the 5’-AMP-activated protein kinase. Biochemical and Biophysical Research Communications, 181(3), 1486–1492.
Wittmann, G., Lechan, R. M., Liposits, Z., & Fekete, C. (2005). Glutamatergic innervation of corticotropin-releasing hormone- and thyrotropin-releasing hormone-synthesizing neurons in the hypothalamic paraventricular nucleus of the rat. Brain Research, 1039(1–2), 53–62.
Woods, A., Dickerson, K., Heath, R., Hong, S.-P., Momcilovic, M., Johnstone, S. R., Carlson, M., & Carling, D. (2005). Ca2+/calmodulin-dependent protein kinase kinase-beta acts upstream of AMP-activated protein kinase in mammalian cells. Cell Metabolism, 2(1), 21–33.
Wu, D., Qiu, Y., Gao, X., Yuan, X.-B., & Zhai, Q. (2011). Overexpression of SIRT1 in mouse forebrain impairs lipid/glucose metabolism and motor function. PLoS One, 6(6), e21759.
Wu, N., Zheng, B., Shaywitz, A., Dagon, Y., Tower, C., Bellinger, G., Shen, C.-H., Wen, J., Asara, J., McGraw, T. E., Kahn, B. B., & Cantley, L. C. (2013a). AMPK-dependent degradation of TXNIP upon energy stress leads to enhanced glucose uptake via GLUT1. Molecular Cell, 49(6), 1167–1175.
Wu, W.-N., Wu, P.-F., Zhou, J., Guan, X.-L., Zhang, Z., Yang, Y.-J., Long, L.-H., Xie, N., Chen, J.-G., & Wang, F. (2013b). Orexin-a activates hypothalamic AMP-activated protein kinase signaling through a Ca2+−dependent mechanism involving voltage-gated L-type calcium channel. Molecular Pharmacology, 84(6), 876–887.
Xia, N., Strand, S., Schlufter, F., Siuda, D., Reifenberg, G., Kleinert, H., Förstermann, U., & Li, H. (2013). Role of SIRT1 and FOXO factors in eNOS transcriptional activation by resveratrol. Nitric Oxide, 32, 29–35.
Xiao, B., Sanders, M. J., Underwood, E., Heath, R., Mayer, F. V., Carmena, D., Jing, C., Walker, P. A., Eccleston, J. F., Haire, L. F., Saiu, P., Howell, S. A., Aasland, R., Martin, S. R., Carling, D., & Gamblin, S. J. (2011). Structure of mammalian AMPK and its regulation by ADP. Nature, 472(7342), 230–233.
Xin, X., Varlamov, O., Day, R., Dong, W., Bridgett, M. M., Leiter, E. H., & Fricker, L. D. (1997). Cloning and sequence analysis of cDNA encoding rat carboxypeptidase D. DNA and Cell Biology, 16(7), 897–909.
Xiong, S., Salazar, G., Patrushev, N., & Alexander, R. W. (2011). FoxO1 mediates an autofeedback loop regulating SIRT1 expression. The Journal of Biological Chemistry, 286(7), 5289–5299.
Xu, Y., Elmquist, J. K., & Fukuda, M. (2011). Central nervous control of energy and glucose balance: Focus on the central melanocortin system. Annals of the New York Academy of Sciences, 1243, 1–14.
Yamada, E., Pessin, J. E., Kurland, I. J., Schwartz, G. J., & Bastie, C. C. (2010). Fyn-dependent regulation of energy expenditure and body weight is mediated by tyrosine phosphorylation of LKB1. Cell Metabolism, 11(2), 113–124.
Yamada, E., Okada, S., Bastie, C. C., Vatish, M., Nakajima, Y., Shibusawa, R., Ozawa, A., Pessin, J. E., & Yamada, M. (2016). Fyn phosphorylates AMPK to inhibit AMPK activity and AMP-dependent activation of autophagy. Oncotarget, 7(46), 74612–74629.
Yang, X., & Qian, K. (2017). Protein O-GlcNAcylation: Emerging mechanisms and functions. Nature Reviews. Molecular Cell Biology, 18(7), 452–465.
Yang, G., Lim, C.-Y., Li, C., Xiao, X., Radda, G. K., Li, C., Cao, X., & Han, W. (2009). FoxO1 inhibits leptin regulation of pro-opiomelanocortin promoter activity by blocking STAT3 interaction with specificity protein 1. The Journal of Biological Chemistry, 284(6), 3719–3727.
Yang, C. S., Lam, C. K. L., Chari, M., Cheung, G. W. C., Kokorovic, A., Gao, S., Leclerc, I., Rutter, G. A., & Lam, T. K. T. (2010). Hypothalamic AMP-activated protein kinase regulates glucose production. Diabetes, 59(10), 2435–2443.
Yang, S.-B., Tien, A.-C., Boddupalli, G., Xu, A. W., Jan, Y. N., & Jan, L. Y. (2012). Rapamycin ameliorates age-dependent obesity associated with increased mTOR signaling in hypothalamic POMC neurons. Neuron, 75(3), 425–436.
Yuan, Z.-L., Guan, Y.-J., Chatterjee, D., & Chin, Y. E. (2005). Stat3 dimerization regulated by reversible acetylation of a single lysine residue. Science, 307(5707), 269–273.
Yuan, M., Pino, E., Wu, L., Kacergis, M., & Soukas, A. A. (2012). Identification of Akt-independent regulation of hepatic lipogenesis by mammalian target of rapamycin (mTOR) complex 2. The Journal of Biological Chemistry, 287(35), 29579–29588.
Zakhary, S. M., Ayubcha, D., Dileo, J. N., Jose, R., Leheste, J. R., Horowitz, J. M., & Torres, G. (2010). Distribution analysis of deacetylase SIRT1 in rodent and human nervous systems. The Anatomical Record, 293(6), 1024–1032.
Zhang, T., Berrocal, J. G., Frizzell, K. M., Gamble, M. J., DuMond, M. E., Krishnakumar, R., Yang, T., Sauve, A. A., & Kraus, W. L. (2009). Enzymes in the NAD+ salvage pathway regulate SIRT1 activity at target gene promoters. The Journal of Biological Chemistry, 284(30), 20408–20417.
Zhao, M., & Klionsky, D. J. (2011). AMPK-dependent phosphorylation of ULK1 induces autophagy. Cell Metabolism, 13(2), 119–120.
Zhu, X., Zhou, A., Dey, A., Norrbom, C., Carroll, R., Zhang, C., Laurent, V., Lindberg, I., Ugleholdt, R., Holst, J. J., & Steiner, D. F. (2002). Disruption of PC1/3 expression in mice causes dwarfism and multiple neuroendocrine peptide processing defects. Proceedings of the National Academy of Sciences of the United States of America, 99(16), 10293–10298.
Ziegler, C. G., Krug, A. W., Zouboulis, C. C., & Bornstein, S. R. (2007). Corticotropin releasing hormone and its function in the skin. Hormone and Metabolic Research, 39(2), 106–109.
Zoncu, R., Bar-Peled, L., Efeyan, A., Wang, S., Sancak, Y., & Sabatini, D. M. (2011). mTORC1 senses lysosomal amino acids through an inside-out mechanism that requires the vacuolar H(+)-ATPase. Science, 334(6056), 678–683.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 Springer International Publishing AG, part of Springer Nature
About this chapter
Cite this chapter
Cakir, I., Nillni, E.A. (2018). Nutrient Sensors Regulating Peptides. In: Nillni, E. (eds) Textbook of Energy Balance, Neuropeptide Hormones, and Neuroendocrine Function. Springer, Cham. https://doi.org/10.1007/978-3-319-89506-2_6
Download citation
DOI: https://doi.org/10.1007/978-3-319-89506-2_6
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-89505-5
Online ISBN: 978-3-319-89506-2
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)