Abstract
Cherry is a member of the Rosaceae family, subfamily Prunoideae, subgenus Cerasus. It is the common name of several Prunus species such as P. avium, P. cerasus, P. mahaleb, P. serotina, P. serrulata, P. incisa and many interspecific hybrids (P. canescens x P. incisa, P. avium x P. cerasus, P. incisa x serrula, etc.).
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
9.1 Introduction
Cherry is a member of the Rosaceae family, subfamily Prunoideae, subgenus Cerasus. It is the common name of several Prunus species such as P. avium, P. cerasus, P. mahaleb, P. serotina, P. serrulata, P. incisa and many interspecific hybrids (P. canescens x P. incisa, P. avium x P. cerasus, P. incisa x serrula, etc.). Cherry is one of the economically important species used for direct fruit production or as rootstock for many cherry varieties (Brown et al. 1996). The estimated world annual production of cherries is over 3 million tons, and has increased steadily since 1990. Turkey is the leading producer of cherries, followed by the USA and Russia (Jayasankar and Kappel 2011).
Cherry can be propagated by seeds only for breeding , as this leads to genetic segregation due to its heterozygous nature. Therefore, for better homogeneity, this species can only be multiplied by vegetative means. Although vegetative propagation by conventional methods does not guarantee the phytosanitary status of the results, in vitro tissue culture could be considered as an alternative method. Among tissue culture techniques, somatic embryogenesis is preferred, especially for woody plants that have a long life cycle and are difficult to propagate by conventional methods (Isah 2016). Compared to organogenesis , somatic embryogenesis may offer many advantages for breeding programs thanks to the single-cell origin of the regenerated embryos, as well as for large-scale production of embryos in bioreactors (Giri et al. 2004). Moreover, somatic embryos have a bipolar structure (stem and root) that allows them to regenerate directly as rooted plantlets (Von Arnold et al. 2002).
However, the application of somatic embryogenesis in a wide range of woody plants is limited by many difficulties. First of all, the ability of cells to acquire embryogenic capacity depends on whether the genotype is competent or recalcitrant (Verdeil et al. 2007; Isah 2016). Complete recalcitrance to somatic embryogenesis is encountered in various groups, families and genera such as Prunus (Druart 1999). Even competent genotypes may show some limitations during the different steps of the embryogenic process. Among these limitations, the poor conversion rate of induced embryos to plantlets constitutes the major problem. These embryos tend to show a variety of morphological abnormalities, including variation in size, in shape and in number of cotyledons in later stages. These abnormalities hamper their further development in the used culture media , as there generation of somatic embryos depends on induction conditions, among which hormonal balance in culture media has a primary role (Feher et al. 2003). Moreover, other medium components such as carbohydrates are known to influence somatic embryo induction and expression. In this regard, it is well documented that specific carbohydrates may have differential effects on morphogenesis in Prunus genera, such as Prunus persica (Raj Bhansali et al. 1990), Prunus avium (Reidiboym-Talleux et al. 1999) and Prunus incisa x serrula (Druart 1999). Also, modification of MS salt solution has been reported to influence embryogenic induction and embryo quality (Lee et al. 2001; Samson et al. 2006).
This chapter focuses on the study of differential reactions to somatic embryogenesis in recalcitrant genotype CAB 6P and competent genotype No 131 at the morphological and molecular levels. CAB 6P (Prunus cerasus) is a dwarfing cherry rootstock. Genotype 131 (Prunus incisa) is a parental genotype of cherry dwarfing rootstock ‘Inmil’ (P. incisa x serrula) and is known for its embryogenic capacity. It has therefore been used as a positive control.
9.2 Somatic Embryogenesis Protocols
9.2.1 Plant Material and Culture Conditions
Genotype CAB 6P belongs to the Prunus cerasus collection located in the Emilia Romagna region of Italy. It was introduced by the Laboratory of Plant Biotechnology and Physiology of the National Agronomic Research Institute of Tunisia (INRAT) as a dwarfing cherry rootstock. Genotype 131 (Prunus incisa) belongs to a Prunus collection established by the Wallon Agricultural Research Centre (CRA-W) in Gembloux (Belgium). Plant materials from these two genotypes CAB 6P and 131 were introduced in vitro through meristem culture and proliferated through axillary branching according to methods and culture conditions described elsewhere for fruit tree micropropagated (Druart 2003). Cultures of mother shoots were obtained in a growth chamber under a 16-hour photoperiod provided by a fluorescent light (Sylvania Grolux F36W) and at a constant temperature of 23 ± 1 °C. Roots and leaves derived from in vitro plantlets were used as explants for induction of somatic embryogenesis and incubated in Petri dishes (9 cm Ø) containing 25 ml of culture media . Before culturing, leaf explants were wounded at three equidistant sites on the adaxial blade surface across the midrib.
9.2.2 Somatic Embryogenesis in Genotype CAB 6P
9.2.2.1 Protocol 1
Explants consisting of roots and leaves were cultured in the dark for 4 weeks, at a temperature of 23 ± 1 °C, on MS (Murashige and Skoog 1962) medium containing 87.6 mM sucrose with added AIA or 2,4-D at different concentrations (1, 2 or 5 μM) combined with 0.2 μM BAP. These cultures were then transferred to light on MS medium containing 0.4 μM BAP and 0.05 μM ANA.
No embryogenic reaction was obtained. These explants were limited to forming amorphous compact calli without any morphogenic reaction.
9.2.2.2 Protocol 2
In the absence of any embryogenic reaction under the preceding conditions, we tested the following protocol inspired by the work of Druart (1999), which has proved favourable for other species such as Prunus incisa. Root and foliar explants were subjected to a pretreatment for 15 days at a low temperature (4 °C) in a solution which was poor in nutrients but highly concentrated in sugar (2.5 mM NH4NO3, 2 μM 2.4-D and 175.2 mM sucrose). These explants were then transplanted onto a medium containing 87.6 mM sucrose with the addition of 1 μM ANA in combination with BAP at different concentrations (2.2, 4.4 or 8.8 μM). The genotype never expressed any embryogenic reaction and then displayed recalcitrant behaviour which consists in the formation of the compact callus . However, some calli that had neoformed from root explants gave rise to budding nodules (Fig. 9.1).
Nodules observed on root explants of genotype CAB 6P (Prunus cerasus) pretreated at 4 °C in a solution composed of 2.5 mM NH4NO3, 2 μM 2.4-D and 175.2 mM sucrose for 15 days, and then cultured onto MS medium containing 87.6 mM sucrose with the addition of 1 μM ANA in combination with BAP at different concentrations (2.2, 4.4 or 8.8 μM)
9.2.2.3 Protocol 3
The recalcitrant behaviour of CAB6P to somatic embryogenesis was confirmed during this last trial based on the comparison of CAB 6P with the embryogenic control cherry genotype 131 of Prunus incisa, both cultured under the same conditions. In this experiment, leaves were used as explants, and cultured for 10, 20, 30 and 40 days in the darkonto MS medium with added picloram at different concentrations (0, 2, 4 and 6 µM). Picloram, which is known for its auxin-like activity, could be more effective than other auxins such as 2,4-D at inducing somatic embryogenesis (Steinmacher et al. 2007). After the induction phase, these explants were transplanted onto MS expression medium supplemented with 0.4 μM BAP and 0.05 μM ANA.
The results confirmed those of the previous protocols concerning the recalcitrance of the CAB 6P genotype to somatic embryogenesis process; this genotype was unable to express any embryogenic reaction. Conversely, genotype 131 confirmed its embryogenic ability and started to produce embryogenic callus after approximately four weeks of culture (Fig. 9.2). The highest rate of embryogenesis was registered in explants cultured for 30 days (D3) with the addition of 4 μM picloram (Table 9.1). Moreover, the results highlighted a tendency for the duration of induction to be inversely correlated with the concentration of picloram.
Morphogenic responses of CAB 6P (P. Cerasus) (a, b, c) and 131 (P. incisa) (d, e, f) leaf explants cultured on MS medium containing 4 µM picloram : a swelling of the midrib vein of leaf on 10th day of culture, b callus formation at petiolar site of leaf on 15th day, c amorphous callus invades leaf on 25th, d swelling of the midrib vein of leaf, e callus formation at wounded sites of leaf on 15th day, f differentiation of proembryos (arrows) on 25th day of culture
9.2.3 Protocols of Somatic Embryogenesis in Genotype 131
9.2.3.1 Protocol 1
In this first protocol, leaf explants were cultured for 30 days on inductive medium consisting of MS base medium supplemented with 4 µM picloram but varying carbohydrate sources. We used sucrose (S), glucose (G), fructose (F) or maltose (M) at different concentrations: 58.4 (C1), 87.6 (C2) or 116.85 (C3) mM. Explants were incubated in the same conditions as described above. The expression step of somatic embryogenesis was realized by the transfer of the obtained calli into MS medium supplemented with 0.44 µM BAP and 0.005 µM NAA , and their exposure to a 16-hour photoperiod provided by a fluorescent light (Sylvania Grolux F36W) at 23 ± 1 °C.
The results showed that callogenesis was the first response of the leaves cultured in picloram -containing medium supplemented with sucrose, glucose and fructose. However, no callus appeared in the presence of maltose. Somatic embryogenesis has shown to be affected by various carbohydrate kinds and concentrations. It was induced in picloram-containing medium supplemented with all the sugars used, but at different rates. Frequencies (%) of embryogenic explants were significantly affected by carbohydrate sources and concentrations (Table 9.2). Two interactions (F*C1 and F*C2) were significantly superior to all other combinations for this parameter. This result is in agreement with findings in Citrus (Tomaz et al. 2001) and Coffea canephora (Fuentes et al. 2000), where fructose has also been found efficient for somatic embryogenesis . Control represented by the interaction S*C2 recorded 39% embryogenic leaves. Sucrose has also induced embryogenesis in other plant species such as Olea europea (Shibli et al. 2001), Glycin max (Omid et al. 2008), Tylophora indica (Dennis 2006). Sucrose is the most commonly used sugar for somatic embryogenesis and it partially acts as an essential source of energy supply in tissue-cultured cells and partly as an osmoticum (Lou and Kako 1995). These comparable effects of sucrose and fructose are not intriguing because fructose is a product of sucrose hydrolysis. The worst result was obtained with maltose at all concentrations and particularly at 58.4 mM (C1), where embryogenesis was completely inhibited. It has been found to be inhibitory to somatic embryogenesis in Coffea (Fuentes et al. 2000). Its metabolism produces glucose but more slowly than sucrose (Fuentes et al. 2000), and it may be suggested that this sugar was not easily available for cells during the induction period.
In our culture conditions, the morphology of somatic embryos seemed to be also affected by carbohydrate source and concentration (Table 9.3). Regenerated embryos showed some teratological abnormalities such as mono (Fig. 9.3a) or polycotyledonary embryos (Fig. 9.3b). In some cases, abnormal embryos showed fused or thickened cotyledons (Fig. 9.3c). It may be noted that the interaction G*C2 registered a significantly higher rate of abnormal embryos (Table 9.3). Conversely, the interaction S*C1 reduced the frequency of abnormality. Likewise, qualitative variation in somatic embryos has been observed in other Prunus species such as P. incisa x serrula (Druart 1999) and P. avium (Reidiboym-Talleux et al. 1999). Inadequate culture conditions were presumed to be the origin of these abnormalities.
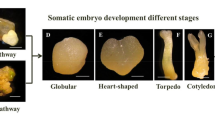
Concerning the development stage of embryos , the initiation of somatic embryos was not synchronous, and embryos could simultaneously be found at different stages ranging from the globular to the cotyledonary stage (Fig. 9.4). This morphological variability was more or less affected by the interaction carbohydrate source*concentration.
Data recorded after 30 days of the expression phase revealed that the interactions S*C1, S*C2, F*C1 and F*C2 were less favourable to the development of embryos into the ultimate cotyledonary stage than S*C3 and F*C3 (Table 9.3). We found that increasing the fructose or sucrose concentration in the culture medium helped embryos to reach the cotyledonary stage. Similar results have been reported for Prunus avium (Reidiboym-Talleux et al. 1999) and Daucus carota (Lee et al. 2001). This could be attributed to the increase in medium osmolarity which is required for production of cotyledonary-stage embryos (Stasolla et al. 2002).
9.2.3.2 Protocol 2
In this protocol, we carried out modifications in the macro-elements concentration of MS medium . Full strength (MS), double strength (MS × 2) and diluted strength (MS/2, MS/4) of MS macro-elements were tried. The results showed significant variability in the embryogenic response of leaf explants. All tested media improved the percentage of embryogenic leaves of genotype 131, except MS × 2 (Table 9.4). MS/2 medium allowed us to obtain a higher frequency of embryogenes is than the control. Double strength MS salts (MS × 2) inhibited the embryogenic process and gave the lowest level. Similar results have been found with different Coffea species (Samson et al. 2006) and carrot (Lee et al. 2001), in which embryogenic response increases with the dilution of the MS salt solution. This could be attributed to the reduction of total nitrogen content (Lee et al. 2001) or nitrate content (Das et al. 2001). The reduction in nitrate concentration may result in the accumulation of reserves and then trigger induction of embryogenesis (Tapan et al. 2001).
Concerning the effects of MS macro-elements concentration on quality of somatic embryos , we found that MS/2 and MS/4 media lead to an increase in typical embryos (Table 9.5). This beneficial effect on embryo quality could be correlated to stress caused by manipulations in MS media. It has been reported that stress is an important factor for the increased production of high-quality somatic embryos (Lee et al. 2001).
In addition, differences in the developmental stages of embryos were detected among different MS culture media (Table 9.5). MS/2 produced more embryos at advanced stages than at early stages. About Lee et al. (2001), MS dilution increased the percentage of cotyledonary embryos. Lowering total nitrogen by diluting the MS medium favoured the development of induced somatic embryos . The highest frequency of cotyledonary embryos was obtained with MS/2 medium . No cotyledonary embryos were formed with MS × 2 medium. High concentration of nitrogen seems to hamper the subsequent development of somatic embryos .
9.3 Molecular Study
In this section, we aim to discover the relationship between morphological reactions and molecular events that occur during the embryogenesis process in competent (131) and recalcitrant (CAB 6P ) genotypes. Molecular investigations were based on the expression of genes known for their involvement in cellular response to auxin (PiABP19) (Tromas et al. 2010), cell cycle regulation (Picdc2) (Malumbres 2014) and somatic embryogenesis process (PiSERK3) (Salvo et al. 2014).
For molecular analyses, samples consisted of leaf explants of these two genotypes cultured for 30 days on MS medium supplemented with 4 µM picloram. The samples were taken every 5 days of culture and kept at −80 °C for subsequent total RNA (ribonucleic acid) extraction according to the protocol described by Ben Mahmoud et al. (2013). These samples consisted of leaves (on the 5th and 10th days), derived calli(on the 15th and 20th days), embryogenic calli in the case of genotype 131 or non-embryogenic calli in the case of genotype CAB 6P (on the 25th and 30th days) (Fig. 9.2). The specific primers were based on expressed sequence tags (EST) available from Prunus persica, as well as ABP, cdc2 and SERK genes functionally characterized in other plant species (Ben Mahmoud et al. 2013). The 18S rRNA gene was used as a control for RNA loading and normalization. PCR (polymerase chain reaction) amplifications were carried out with 30 cycles of the following conditions: denaturation at 94 °C for 30 s, annealing for 45 s (at 55 °C for SERK and cdc2 , 58 °C for ABP19 and 55 °C for 18S rRNA), elongation at 72 °C for 1 min and final extension at 72 °C for 7 min. All reactions were performed with iCycler (Bio-Rad). The amplified cDNA (complementary deoxyribonucleic acid) fragments were of the expected sizes based on sequence information data from homologous sequences. Each PCR amplification was repeated three times. The amplified PCR products were electrophoresed on a 1% (w/v) agarose gel and visualized by staining with ethidium bromide. ABP19, cdc2 and SERK partial cDNAs were cloned, sequenced and submitted to GenBank as newly identified partial transcripts named PiABP19, Picdc2 and PiSERK3 (Ben Mahmoud et al. 2013).
Semi-quantitative RT-PCR was used to monitor the relative expression of PiABP19, Picdc2 and PiSERK3 in freshly excised leaf explants (day 0) and throughout the culture period of 30 days in competent (131) and recalcitrant (CAB 6P ) cherry genotypes.
In the case of gene PiABP19, similar expression patterns were registered in the two genotypes (Fig. 9.5). The highest transcript levels were recorded in samples before culture induction (day 0). This gene has been postulated to mediate cell expansion (Chen et al. 2001; Tromas et al. 2009). During the culture period, PiABP19 transcripts recorded a significant decrease that could be attributed to the cell divisions, which leads to subsequent callogenesis observed from the 5th day of culture.
Expression profile of PiABP19 during induction period of somatic embryogenesis in embryogenic genotype 131 (P. incisa) and recalcitrant genotype CAB 6P (P. cerasus) from leaf explants cultured in picloram -containing medium for 30 days. RT-PCR amplification data were normalized to the housekeeping gene, 18S rRNA. Data are reported as mean values ± SDM, n = 3. Different letters denote significant differences (P < 0.05) according to the Newman-Keuls test
In contrast, Picdc2 (Fig. 9.6) and PiSERK3 (Fig. 9.7) showed differential and distinctive expression profiles between embryogenic and recalcitrant genotypes.
Expression profile of Picdc2 during induction period of somatic embryogenesis in embryogenic genotype 131 (P. incisa) and recalcitrant genotype CAB 6P (P. cerasus) from leaf explants cultured in picloram -containing medium for 30 days. RT-PCR amplification data were normalized to the housekeeping gene, 18S rRNA. Data are reported as mean values ± SDM, n = 3. Different letters denote significant differences (P < 0.05)
Expression profile of PiSERK3 during induction period of somatic embryogenesis in embryogenic genotype 131 (P. incisa) and recalcitrant genotype CAB 6P (P. cerasus) from leaf explants cultured in picloram -containing medium for 30 days. RT-PCR amplification data were normalized to the housekeeping gene, 18S rRNA. Data are reported as mean values ± SDM, n = 3. Different letters denote significant differences (P < 0.05)
Regarding Picdc2, transcript levels recorded in genotype 131 were stable at the beginning of the culture up to the 10th day and then increased gradually to reach a peak at day 25 (Fig. 9.6) when embryogenic structures could be observed (Fig. 9.2). They then decreased nearly twofold at the end of the culture period (Fig. 9.6). During the first ten days, declining transcript levels of this gene seem to correspond to the cell dedifferentiation process confirmed by anatomical observations (Ben Mahmoud 2012). After this, transcripts showed a considerable increase, reaching a peak at day 25, that could be associated with activation of cell proliferation and callogenesis observed from the 15th day and formation of embryogenic structures detected since day 25 of culture (Fig. 9.2). In this context, a previous study has confirmed that CDK activity is a major factor underlying cell divisions (Joubès et al. 2001; Lin et al. 2014; Malumbres 2014), callus induction and in vitro organogenesis (Cheng et al. 2015). However, in the case of CAP 6P, transcript levels of Picdc2 that were higher than in cv. No 131 until day 5 significantly decreased from the 10th day and remained low during the whole culture period (Fig. 9.6). According to these observations, accumulation of Picdc2 transcripts on the 5th day could stimulate mitotic activity leading to callus formation in leaves from the 15th day (Fig. 9.2); but the decline of these transcripts could hamper cell dedifferentiation, as proved by histological study (Ben Mahmoud 2012), and consequently the embryogenic process.
Concerning gene PiSERK3, transcript levels were detected in these two genotypes (131 and CAB 6P ) and were relatively similar on day 0. During the induction period, the expression profile of gene PiSERK3 in genotype 131 showed a biphasic pattern marked by two peaks (Fig. 9.7). A first peak was detected on the 15th day when calli were formed in different wounded sites of the leaves. A second peak was recorded on day 25 in explants developing embryogenic structures (Fig. 9.2). We suggest that this PiSERK3 biphasic pattern coincided with the cellular dedifferentiation and differentiation steps occurring through the embryogenic process. The first peak recorded on the 15th day of culture appears to coincide with the acquisition of embryogenic competence as confirmed in other plant species (Nolan et al. 2003; De Oliveira Santos et al. 2005; Shimada et al. 2005). The second peak registered on the 25th day could be associated with the differentiation of embryogenic structures and proembryo formation (Ben Mahmoud 2012). The decline of the SERK transcript level at the end of the culture period is also observed in carrot, where DcSERK expression is characteristic of embryogenic development up to the globular stage and stops thereafter (Schmidt et al. 1997). In contrast, PiSERK3 transcript levels in CAB 6P significantly declined from the beginning of the culture and remained low throughout the whole culture period (Fig. 9.7). This reduction of PiSERK3 transcript levels was accompanied by the formation of amorphous and compact calli without any embryogenic reaction (Fig. 9.2).
9.4 Research Prospects
Despite the progress achieved during the last few years in understanding the mechanisms involved in somatic embryogenesis , there are still many aspects that are not well understood and need to be elucidated.
In the case of cherry , the recalcitrant behaviour of genotype CAB 6P to somatic embryogenesis has handicapped the exploitation of this biotechnological tool especially for its propagation and genetic improvement. Deeper investigations at the molecular and cytological levels may provide more information about the relationship between the molecular and the cellular process in this recalcitrant cherry genotype. Genes associated with somatic embryogenesis could serve as biomarkers to determine embryogenic competence . In addition, data provided by proteomics and transcriptomics may lead to abetter understanding of the molecular mechanisms underlying somatic embryogenesis.
Regarding the competent genotype, the results showed that the major problem consisted of the production of a relatively high number of abnormal embryos that failed to convert into whole plantlets . Future research should therefore focus especially on culture media composition in order to improve both the quantity and quality of somatic embryos.
References
Ben Mahmoud K (2012) Etude de l’aptitude à l’embryogenèse somatique du porte-greffe de cerisier CAB 6P (Prunus cerasus L.) et des mécanismes histologiques et moléculaires associés. Ph.D., National Agronomic Institute of Tunisia, Tunis, Tunisia
Ben Mahmoud K, Delporte F, Muhovski Y, Elloumi N, Jemmali A, Druart Ph (2013) Expression of PiABP19, Picdc2 and PiSERK3 during induction of somatic embryogenesis in leaflets of Prunus incisa (Thunb.). Mol Biol Rep 40:1569–1577
Brown SK, Iezzoni AF, Fogle HW (1996) Cherries. In: Janick J, Moore JN (ed) Fruit breeding: tree and tropical fruits, 1st edn. Wiley, New York, USA, pp 213–255
Chen JG, Shimomura S, Sitbon F, Stanberg G, Jones AM (2001) The role of auxin-binding protein 1 in the expansion of tobacco leaf cells. Plant J 28:607–617
Cheng Y, Liu H, Cao L, Wang S, Li Y, Zhang Y, Jiang W, Zhou Y, Wang H (2015) Down regulation of multiple CDK inhibitor ICK/KRP genes promotes cell proliferation, callus induction and plant regeneration in Arabidopsis. Front Plant Sci 6:1–12
Das S, Ray S, Dey S, Dasgupta S (2001) Optimisation of sucrose, inorganic nitrogen and abscisic acid levels for Santalum album L. somatic embryo production in suspension culture. Process Biochem 37:51–56
De Oliveira Santos M, Romano E, Yotoko KSC, Tinoco MLP, Dias BBA, Araga FJL (2005) Characterization of the cacao somatic embryogenesis receptor-like kinase (SERK) gene expressed during somatic embryogenesis. Plant Sci 168:723–729
Dennis TT (2006) Effect of sugars, gibberellic acid and abscisic acid on somatic embryogenesis in Tylophora indica (Burm. f.) Merrill. Chin J Biotech 22:465–471
Druart Ph (1999) Somatic embryogenesis in Prunus species. In: Gupta PK, Newton RJ, Mohain JS (eds) Somatic embryogenesis in woody plants, 1st edn. Kluwer Academic, Dordrecht, Netherlands, pp 215–235
Druart Ph (2003) Micropropagation of apples (Malus sp.). In: Jain SM, Ishii K (eds) Micropropagation. Kluwer Academic, The Netherlands, pp 433–463
Feher A, Pasternak TP, Dudits D (2003) Transition of somatic plant cells to an embryogenic state. Plant Cell Tiss Org Cult 74:201–228
Fuentes SRL, Calheiros MBP, Manetti-Filho J, Vieira LGE (2000) The effects of silver nitrate and different carbohydrates sources on somatic embryogenesis in Coffea canephora. Plant Cell Tiss Org Cult 60:5–13
Giri C, Shyamkumar B, Anjaneyulu C (2004) Progress in tissue culture, genetic transformation and applications of biotechnology to tress: an overview. Trees 18:115–135
Isah T (2016) Induction of somatic embryogenesis in woody plants. Acta Physiol Plant 38:1–22
Jayasankar S, Kappel F (2011) Recent advances in cherry breeding. Fruit Vegetable Cereal Biotechnol 5:63–67
Joubès J, Lemaire-Chamley M, Delmas F, Walter J, Hernould M, Mouras A, Raymond P, Chevalier C (2001) A new C-type cyclin-dependent kinase from tomato expressed in dividing tissues does not interact with mitotic and G1 cyclins. Plant Phys 126:1403–1415
Lee EK, Cho DY, Soh WY (2001) Enhanced production and germination of somatic embryos by temporary starvation in tissue cultures of Daucus carota. Plant Cell Tiss Org Cult 20:408–415
Lin HY, Chen JC, Wei MJ, Lien YC, Li HH, Ko SS, Liu ZH, Fang SC (2014) Genome-wide annotation, expression profiling and protein interaction studies of the core cell-cycle genes in Phalaenopsis Aphrodite. Plant Mol Biol 84:203–226
Lou H, Kako S (1995) Role of high sugar concentrations in inducing somatic embryogenesis from cucumber cotyledons. Sci Hort 64:11–20
Malumbres M (2014) Cyclin-dependent kinases. Genome Biol 15:122–131
Murashige T, Skoog F (1962) A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol Plant 15:473–497
Nolan KE, Irwanto RR, Rose RJ (2003) Auxin up-regulates MtSERK1 expression in both Medicago truncatula root-forming and embryogenic cultures. Plant Phys 133:218–230
Omid K, Deljou A, Kordestani GK (2008) Secondary somatic embryogenesis of carnation (Dianthus caryophyllus L.). Plant Cell Tiss Org Cult 92:273–280
Raj Bhansali R, Driver JA, Durzan DJ (1990) Rapid multiplication of adventitious somatic embryos in peach and nectarine by secondary embryogenesis. Plant Cell Rep 9:280–284
Reidiboym-Talleux L, Diemer F, Sourdioux M, Chapelain K, De March G (1999) Improvement of somatic embryogenesis in wild cherry (Prunus avium). Effect of maltose and ABA supplements. Plant Cell Tiss Org Cult 55:199–209
Salvo SAGD, Hirsch CN, Buell CR, Kaeppler SM, Kaeppler HF (2014) Whole transcriptome profiling of maize during early somatic embryogenesis reveals altered expression of stress factors and embryogenesis-related genes. PLoS One 9:1–17
Samson NP, Campa C, Le Gal L, Noirot M, Thomas G, Lokeswari TS, de Kochko A (2006) Effect of primary culture medium composition on high frequency somatic embryogenesis in different Coffea species. Plant Cell Tiss Org Cult 86:37–45
Schmidt ED, Guzzo F, Toonen MA, de Vries SC (1997) A leucine rich repeat containing receptor-like kinase marks somatic plant cells competent to form embryos. Development 124:2049–2062
Shibli RA, Shatnawi M, Abu-Ein, Al-Juboory KH (2001) Somatic embryogenesis and plant recovery from callus of ‘Nabali’ Olive (Olea europea L.). Sci Hort 88:243–256
Shimada T, Hirabayashi T, Endo T, Fuji H, Kita M, Omura M (2005) Isolation and characterization of the somatic embryogenesis receptor-like kinase gene homologue (CiSERK1) from Citrus unshiu Marc. Sci Hort 103:233–238
Stasolla C, Kong L, Yeung EC, Thorpe TA (2002) Maturation of somatic embryos in conifers: morphogenesis, physiology, biochemistry and molecular biology. In vitro Cell Dev Biol-Plant 38:93–105
Steinmacher DA, Clement CR, Guerra MP (2007) Somatic embryogenesis from immature peach palm inflorescence explants: towards development of an efficient protocol. Plant Cell Tiss Org 89:15–22
Tapan KM, Bhattacharya A, Ahuja PS (2001) Induction of synchronous secondary somatic embryogenesis in Camellia sinensis (L.) O. Kuntze. J Plant Physiol 158:945–951
Tomaz ML, Januzzi Mendes BM, Mourao Filho DAA, Demétrio CGB, Jansakul N, Rodriguez APM (2001) Somatic emrbyogenesis in citrus spp.: carbohydrate stimulation and histodifferentiation. In vitro Cell Dev Biology-Plant 37:446–450
Tromas A, Braun N, Muller P, Khodus T, Paponov IA, Palme A, Liung K, Lee JY, Benfey P, Murray JAH, Scheres B, Perrot-Rechenmann C (2009) The auxin binding protein 1 is required for differential auxin responses mediating root growth. PLoS One 4:1–11
Tromas A, Paponov I, Perrot-Rechemmann C (2010) Auxin binding protein 1: functional and evolutionary aspects. Trends Plant Sci 8:436–446
Verdeil JL, Alemanno L, Niemenak N, Tranbarger TJ (2007) Pluripotent versus totipotent plant stem cells: dependence versus autonomy? Trends Plant Sci 12:245–252
Von Arnold S, Sabala I, Bozhkov P, Dyachok J, Filonova L (2002) Developmental pathways of somatic embryogenesis. Plant Cell Tiss Org Cult 69:233–249
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 Springer International Publishing AG, part of Springer Nature
About this chapter
Cite this chapter
Ben Mahmoud, K., Muhovski, Y., Delporte, F., Jemmali, A., Druart, P. (2018). Somatic Embryogenesis in Cherry (Prunus sp.). In: Jain, S., Gupta, P. (eds) Step Wise Protocols for Somatic Embryogenesis of Important Woody Plants. Forestry Sciences, vol 85. Springer, Cham. https://doi.org/10.1007/978-3-319-79087-9_9
Download citation
DOI: https://doi.org/10.1007/978-3-319-79087-9_9
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-79086-2
Online ISBN: 978-3-319-79087-9
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)