Abstract
Stromal cells and the immune functions that they regulate underpin multiple aspects of host defence, but the study of stromal cells as targets of infection and as regulators of anti-infective immunity is in its infancy and still limited to a few well-worked examples. In this review, the role of stromal cells at each sequential stage of infection is discussed, with examples drawn from across the spectrum of infectious agents, from prions to the parasitic helminths. Gaps in knowledge are identified, the challenges in studying stromal cell biology in the context of infection are highlighted, and the potential for stromal cell-targeted therapeutics is briefly discussed.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
2.1 Introduction
The pathogenesis of infectious disease is complex and involves a myriad of processes, some but not all related to core immune mechanisms, most of which in one form or another are underpinned by features of stromal cell biology. Stromal cells provide the tissue architecture at the primary interface with infectious agents (e.g. the skin or mucosa), act as a potential cellular target for infection, provide the framework for the compartmentalized functions of lymphoid tissue and immune response induction and generate and maintain the vascular environment that allows for effector cell trafficking to the sites of infection. At the end of infection, stromal cells play a role in resolution of immune-mediated pathology and the return to homoeostasis. Details of many of these functions of stromal cells during development, under homoeostatic conditions and during cancer are described in detail elsewhere in this volume. Here, the focus will be on providing key exemplars of how stromal cells interact, directly or indirectly, with pathogens and help to orchestrate subsequent inflammatory and immune responses that ultimately lead either to pathogen elimination or the establishment of a chronic persistent infection. The broader role of stromal cells in the resolution of infection-associated pathology is also discussed, although to date there are few studies addressing this important aspect of infection biology. For the sake of brevity, discussion regarding stromal cell interactions with pathogens associated with the development of cancer (e.g. Epstein-Barr Virus) has been omitted (Fig. 2.1).
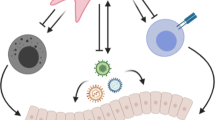
The temporal role of stromal cells in regulating immunity to infection. Schematic shows a stylized time course depicting the events from initial pathogen contact through to disease resolution that may be influenced by stromal cell interactions and functions. For examples of stromal cell interactions with specific pathogens at each stage (numbered stars), see main text
2.2 Initiation of Infection: Stromal Cells as Targets for Adhesion and Infection
For successful infection to begin, pathogens require means to adhere to and/or penetrate external barriers. For mucosal pathogens, epithelial cells represent a major site of pathogen attachment at mucosal surfaces, and an intact epithelium provides a barrier to direct interaction between these infectious agents and underlying mesenchymal stromal cells. The role of crosstalk between epithelial cells and stromal cells, serving as an integral part of the signaling required for epithelial barrier function and maintenance, is well documented, notably in the female reproductive tract and mammary gland [1, 2]. This establishes a paradigm likely to be operating at most epithelial sites and suggests that any disruption to this functional unit may occur through either epithelial or stromal cell changes.
Some mucosal pathogens such as Entamoeba histolytica [3] are professional tissue invaders and use a variety of molecular and cellular strategies to penetrate deep into the mucosa and submucosa; yet little attention has been paid to the consequences of such local tissue trauma for stromal cell function. Gastrointestinal worms have a variety of ways to interact with and manipulate the mucosal epithelium and otherwise disrupt local immunity [4, 5]; however, the direct action of helminth-derived molecules on intestinal stromal cells has not been reported in any detail.
Many of the major pathogens of man are transmitted via breaks in skin barrier that occur during blood feeding by their arthropod vector. For these pathogens , there may be more direct and immediate access to tissue stromal cells, and the tropism of intracellular pathogens to stromal cells after epithelial barrier breach provides an opportunity for their establishment and long-term survival. In addition to facilitating access to stromal cells, the tissue trauma caused by the bite of haematophagous insects may also directly trigger stromal cell release of alarmins (see below) [6]. In the case of the intracellular parasites, the reduced microbicidal capacity of stromal cells compared to myeloid cells may provide a driver for this behaviour. For example, the parasitic protozoan Trypanosoma cruzi invades the skin and oral mucosa in a process of contaminative transmission, with infectious metacyclic parasites being deposited in the faeces of feeding triatomine bugs. T. cruzi has a broad host cell range, making use of a plethora of attachment molecules and active processes to invade stromal cells in a process of triggered phagocytosis [7]. Other vector-borne parasites such as Leishmania, whilst historically regarded as having a more limited host cell range, have also been noted as intracellular parasites of stromal cells at chronic stages of infection [8, 9]. It is not clear whether stromal cell infection also occurs early after infection and has hitherto gone unrecognized.
In experimental models of infection, stromal cell tropism is also noted and may illustrate how tropism may be both cell- and tissue-specific in nature. For example, after intraperitoneal murine cytomegalovirus (MCMV) infection, ERTR7+ marginal zone reticular cells represent the major target for infection within the spleen, with subsequent viral spread to red pulp fibroblasts. In contrast, in the lymph node primary infection occurs within CD169+ subcapsular sinus macrophages [10].
These various studies also raise an important question regarding the temporal regulation of adhesion and/or pathogen selective receptors on stromal cells (e.g. by mediators involved in inflammation) and whether this may also contribute to the patterns of cellular tropism that are observed. For example, in the case of corneal infection with HSV , the major viral receptor nectin-1 is initially absent from stromal cells, but expression is induced early during inflammation allowing increased viral host cell range [11, 12]. Further studies in a variety of infection models to address the role of direct stromal cell infection at different stages of the infection process would clearly help in delineating the importance of such interactions to disease progression and pathogen life cycle maintenance.
HIV infection provides an interesting and relevant example of how stromal cells can both be target for infection and regulator of infection in other cells. HIV infects follicular dendritic cells (FDC), which provide a reservoir for viral infection of CD4+ T cells and macrophages [13,14,15], but in addition, these infected FDCs provide TNF-dependent augmentation of HIV transcription and viral replication in CD4+ T cells [16].
Finally, to fully define pathogen cellular tropism , it may be necessary to consider the potential for lineage transformation. At least one intracellular pathogen has been shown to induce epithelial-to-mesenchymal transition (EMT). The cag pathogenicity island of Helicobacter pylori encodes a type 4 secretion system that delivers bacterial effectors into the cytosol of gastric epithelial cells and induces EMT [17]. Whether this can be induced by other intracellular pathogens remains to be determined. Another instance where lineage boundaries become blurred is the case of the fibrocyte, which shares markers of haematopoietic cells and fibroblasts [18]. Recently, the capacity of human and murine blood fibrocytes to support internalization of promastigotes of Leishmania amazonensis was reported, along with the capacity of these cells to support intracellular transformation to amastigotes [19]. Strikingly, fibrocytes produced high levels of NO and cleared parasites within a few days of infection, suggesting that transient waves of infection within fibrocytes in vivo could go unnoticed. Furthermore, a variety of Leishmania species have been shown to infect adipose-derived mesenchymal stem cells in vitro [20], though whether this alters stem cell function and differentiation capacity has yet to be determined. It seems likely that at least some intracellular pathogens will be found to have the capacity to affect the pluripotency of stem cells, but this remains an area to be explored experimentally.
2.3 Early Inflammation: Stromal Cells as Contributors to Innate Immunity
Cooperation between haematopoietic and stromal cells can play an important role in initiating inflammation. For example, TLR4 expression on stromal cells is required for optimal resistance against uropathogenic E. coli but is not sufficient for induction of inflammation in the absence of TLR4 expression on haematopoietic cells [21]. Protection in a lethal model of vesicular stomatitis virus (VSV) infection required intact TLR and retinoic acid-inducible gene I-like helicase (RLH) signaling in both radioresistant stromal cells and haematopoietic cells [22]. Likewise, effective immunity in a model of oropharyngeal candidiasis required expression of the NLRC4 inflammasome in radioresistant stromal cells, working cooperatively with NLRP3 inflammasome [23].
Recent studies in the well-established MCMV infection model have also provided insight into how stromal cells can play a direct role in innate immunity . Type I interferons are required for the early control of MCMV replication in the mouse spleen, and various studies have indicated that both plasmacytoid and conventional dendritic cells (DCs) play temporally discrete roles in producing the bulk of type I interferon detectable between 36 and 48 h postinfection. However, this wave of type I interferon is preceded (2 h postinfection) by a burst of type I interferon derived from LTβR-expressing splenic marginal zone reticular cells, acting in concert with LTαβ-producing B cells [24, 25].
Alarmins represent a constitutively available group of evolutionarily diverse molecules that normally have important intracellular roles but which can be released into the extracellular environment through tissue injury or inflammatory signaling cellular roles during regulation of innate immunity and may be triggered directly by tissue damage or indirectly through the production of other early mediators of inflammation such as IL-17. Alarmins include IL-33, various S100 molecules, HMGB1 and thymic stromal lymphopoietin (TSLP) [26, 27]. The contribution of stromal cells to the production and/or regulation of alarmins in the context of epithelial cell injury during infection is, however, poorly understood.
Some of the aforementioned papers, however, highlight a conspicuous difficulty in the field of stromal cell biology: assignment of the term “stromal” to cells that are radioresistant in radiation bone marrow chimeras. Whilst this experimental approach can identify, but not distinguish between, properties attributable to radioresistant mesenchyme-derived stromal cells and epithelial cells, the recent identification of radioresistant resident tissue macrophages of yolk sac origin [28,29,30] introduces some significant question marks over previous attribution of cell function. The ability to generate mouse strains for lineage tracing and for selective stromal cell-targeted gene ablation (e.g. [31]) is an important step towards clarifying the function of stromal cells throughout the infection process.
2.4 Induction of Acquired Immunity: Stromal Cells as APC During Infectious Disease
The notion that antigen presentation within lymphoid tissues is restricted to haematopoietic cells has been over-turned by a number of recent studies that indicate that stromal cells have all the machinery necessary for both MHCI [32, 33] and MHCII-dependent antigen presentation [34] and in addition can acquire and functionally express MHCII-peptide complexes derived from DCs [35]. Under homoeostatic conditions, this imparts an ability to effect CD8+ T cell deletion to self-antigens and regulate the extent of CD4+ T cell priming, either directly by inducing anergy [35] or indirectly by maintaining the pool of CD4+ Tregs [34]. The extent to which pathogen-derived antigens are presented in this way by stromal cells within the lymphoid tissue microenvironment is as yet unknown but clearly warrants further investigation given the potential for this route of antigen presentation to modify the quantity (and perhaps quality) of the response to infection. Whether stromal cells outside lymphoid tissue also are endowed with these properties will be important to address, as will be the question of whether stromal cell antigen presenting function “matures” through infection, in an equivalent manner to that seen in the haematopoietic lineage. In this context, it is interesting to note that inflammation, including that driven by infection, can lead to local tissue fibroblasts recapitulating the ontogeny of lymphoid tissue fibroblasts, expressing the canonical lymph node stromal marker podoplanin (gp38) [36]. Whether these cells acquire all functions associated with their lymphoid tissue resident counterparts remains to be determined, and illustrates an experimental setting where transcriptomic analysis of stromal cells might be particularly helpful.
2.5 Maintaining the Balance: Stromal Cells and Immune Regulation
As indicated above, new evidence indicates that lymph node stromal cells can directly engage with T cells via MHCI and MHCII restricted antigen presentation, and that the primary purpose of these interactions under homeostatic conditions appears to be the induction of one or other mechanism of self-tolerance. To date, however, most attention has been focused on how stromal cells induce a regulatory environment and thus influence T cell activation indirectly rather than directly. Early studies in vitro demonstrated the capacity of stromal cell lines to drive HSPC into a programme of myelopoiesis, often generating novel subsets of dendritic like cells [37,38,39]. In a model of Leishmania donovani infection , it was shown that ex vivo isolated stromal cells were able to induce lin−c-kit+ progenitors to differentiate to a greater extent that stromal cells isolated from uninfected mice. Furthermore, the resulting CD11clo CD45RB+ IL-10-producing DCs had potent regulatory properties (defined in vitro by suppression of T cell proliferation to antigen presented by conventional CD11c DC and in vivo by the induction of antigen-specific tolerance) [9]. Subsequently, it was demonstrated that infection-associated inflammation enhanced the function of this splenic red pulp stromal haematopoietic niche, principally through aberrant expression of CCL8 [40].
A challenge for studying stromal cell biology in an infection model where stromal cells themselves can be infected, albeit at variable frequency, is to distinguish whether any changes in stromal cell function are directly attributable to intracellular parasitism (e.g. mediated via parasite-induced host cell intrinsic changes in signaling pathways) or whether they reflect the action of cytokines and or other factors operating in trans in a complex inflammatory “soup”. Importantly in the latter study [40], stromal cell lines were used to show that CCL8 expression was directly induced in stromal cell lines in vitro by infection with L. donovani and that in vivo, CCL8+ ERTR-7+ stromal cells also contained parasites. These data do not however rule out trans-acting factors as contributors to the in vivo response. Indeed, as endothelial cells and fibroblasts isolated from various tissues, with or without inflammatory stress, have been shown to induce regulatory myeloid cells capable of inhibiting T cell responses [41,42,43,44,45] and in one case to block virus-mediated activation of pDC [46], it is likely haematopoietic support is a generic tunable property of stromal cells that helps maintain immune balance.
Stromal cells have been less well studied in the context of helminth infections and immune regulation. B cells have been shown to play a role in immune regulation in some chronic infections. In the case of Schistosoma mansoni infection, regulatory B cell development has been linked to the production of BAFF, a cytokine produced by DC and stromal cells in response to helminth antigens [47]. Surprisingly, stromal cell modifications have also been implicated in the onward transmission of this parasite. S. mansoni egg excretion is essential for completion of the life cycle, and this is facilitated by entry of eggs into Peyer’s patches, which respond with extensive remodeling of their stromal elements [48].
In summary, whilst it is tempting to “hand-wave” by saying that stromal cells are almost certain to have a role in the overall generation of the changing immune environment during infection, through participation in the control of lymphocyte and dendritic cell functions or through their contribution to the cytokine environment, only carefully designed and executed studies using stromal cell specific targeting of key immune mediators will provide the answer to the question of how important stromal cell responses are relative to those of other cells in driving the ultimate phenotype – pathogen elimination or persistence of infection.
2.6 Perpetuating Chronic Infection: Breakdown of Stromal Cell Architecture
As detailed elsewhere within this volume, stromal cells play a central role in the development and maintenance of lymphoid tissue architecture. On the assumption that immune architecture is therefore integral to the efficiency of the immune system , it is perhaps not surprising that many studies of disease, including infectious disease, have noted changes in lymphoid tissue architecture and associated these with dysregulation of immunity.
Examination of the lymph nodes of patients with progressive HIV infection demonstrated marked degenerative changes to germinal centres, including the depletion of the FDC network, a process termed follicle lysis [49, 50]. Of note, a study in SIV-infected macaques demonstrated that although FDCs were also greatly reduced in number in this model infection, residual FDCs appeared to make more functionally productive interactions with B cells [51]. This study serves as a reminder that pathology-associated loss of cell number should not be equated directly with loss of function. Similar structural changes to the FDC network have also been observed in non-viral infection models, including chronic visceral leishmaniasis. Here, FDC loss was determined both by immunohistochemistry and by lack of immune complex trapping within GCs [52]. Strikingly, heavily parasitized macrophages became abundant within these GCs, resembling the tingible body macrophages described in HIV infection [49].
The demonstration that a distinct population of podoplanin+ fibroblastic reticular cells was present within the T cell zone of lymphoid tissues focused attention on how this stromal cell subset was altered in a variety of infection models. In chronic HIV infection, fibrosis of lymphoid tissue becomes apparent, and this results in loss of integrity of the FRC network. Importantly, the extent of loss of FRCs and collagen deposition are predictors of the ability of highly active antiretroviral therapy (HAART) to restore T cell count. Furthermore, HAART is most effective at restoring FRC networks when given early during disease [53, 54].
FRCs are also lost during infection with Leishmania donovani in mice [55]. As expected, FRC deficiency was also characterized by a loss of constitutive CCL21 and CCL19 production in the spleen of infected mice and by alterations in DC and T cell traffic. Although DC migration from the marginal zone into the T cell zone was impaired in infected mice, this was not the direct result of loss of FRCs. By adoptive transfer, it was shown that the residual FRCs and CCL21-expressing endothelium were sufficient to allow migration of DC isolated from naïve mice, and whereas conversely DCs from infected mice failed to migrate even in naïve hosts. Hence in this case, loss of CCR7 expression by DCs in infected mice, which was in turn regulated in a TNF and IL-10-dependent manner, appeared to play a more dominant role in affecting DC-T cell interactions than the loss of stromal architecture per se. Of interest in this regard, computational models have been developed to assess the impact of changes in FRC network density and inter-connectivity in regulating cellular encounters between T cells and DC [56].
Unlike the situation in chronic HIV and visceral leishmaniasis , experimental viral infections provide examples of situations where extreme but transient pathology occurs. In LCMV infection, splenomegaly is transient, peaking during the first week of infection but subsiding to normal range within 10 days. Loss of FRCs accompanies splenomegaly, as does inability to respond to challenge with exogenous antigens, but the FRC network recovers remarkably quickly as infection is cleared. Restoration of this architecture is dependent upon the presence of RORγ+ lymphoid tissue inducer cells and LTαβ signaling, suggesting that “repair” of lymphoid tissue architecture recapitulates processes that occur during lymphoid tissue development [57]. During MCMV, disruption of lymphoid stroma appears restricted to the FRCs, which show similar alterations in gp38 staining pattern and changes in CCL21 expression as observed in visceral leishmaniasis and LCMV infection. B zone stromal cells, however, appear not to be significantly affected during this infection, as judged by maintenance of CXCL13 [58].
In addition to stromal cell changes, other microarchitectural changes often accompany splenomegaly, notably loss or displacement of “stromal” macrophages within the marginal zone. Mice infected with L. donovani [59], Plasmodium chabaudi [60] and MCMV [58] all show loss of SIGNR1+ marginal zone and CD169+ marginal metallophils to a greater of lesser extent. Collectively, these data illustrate that there is a degree of commonality in the structural changes seen irrespective of infectious agent. However, the process of remodeling may be driven by quite independent mechanisms. For example, in LCMV infection , antiviral CD8+ T cells destroy FRCs [57]; in P. chabaudi infection, CD8+ T cells selectively kill CD169+ marginal metallophils through a perforin and Fas-dependent pathway [60]; and during L. donovani infection, SIGNR1+ marginal zone macrophages are lost in a TNF-dependent manner [59]. The precise impact that can be attributed to changes in secondary lymphoid tissue, in terms of immunocompetence, may not be possible to discern from simple single infection models such as those described above and may require adaptation of various co-infection models to become fully apparent.
The chronicity and extent of Leishmania donovani-induced splenomegaly also provide a context in which pathologic angiogenesis occurs to an exaggerated extent. Vascular remodeling in this disease requires the coordinated action of distinct myeloid cell populations, working in a compartment-specific manner. Thus, inflammatory monocytes regulated the expansion of the red pulp vasculature [61], whereas a population of “resident” macrophages, which were found bordering the denuded marginal zone , play a role in inducing neo-angiogenesis in the white pulp. Of note, this localized angiogenesis is controlled by the aberrant expression of the neurotrophin Bndf and its receptor Ntrk2 (Trkb) on macrophages and endothelial cells, respectively [62].
2.7 Perpetuating the Response: Ectopic Lymphoid Structures
Chronic inflammation is often associated with the generation of ectopic or tertiary lymphoid tissue, a topic summarized elsewhere in this volume. Not surprisingly, therefore, infections may also give rise to these structures, although their significance for disease progression is less well understood than in other settings.
Perhaps best characterized in animal models is the development of ectopic lymphoid structures associated with salivary gland inoculation of MCMV [63] and in the bronchus-associated tertiary lymphoid tissue associated with influenza virus infection [64]. A recent comparative study of M. tuberculosis infection in humans, in non-human primates and in mouse models provides perhaps the best characterized evaluation of the role of ectopic lymphoid tissue in disease progression [65]. Of note, whereas tuberculosis (TB) granulomas in patients and non-human primates with latent disease had associated ectopic lymphoid tissue, this was not the case for granulomas in patient or animals with active TB, suggesting a role for these structures in immune control. The finding of ectopic lymphoid tissue associated with the TB granuloma may be a special case, associated with either the chronicity of infection or the inherent adjuvant properties and/or immunogenicity of this pathogen. In other granulomatous diseases, e.g. experimental visceral leishmaniasis [66], granulomas do not acquire this feature.
2.8 Closure: Stromal Cells and the Resolution of Inflammation
Pathogen clearance ultimately leads to a reversal of most of the associated tissue pathology, through an active process of resolution. Recent evidence suggests that resolution is as complex a process as the generation of immunity and immunopathology, involving a plethora of distinct signals often mediated through shifts in metabolic profile of the tissue. As stromal cells are regarded as the key drivers for chronic inflammation [67], it goes without saying that resolution must bring about changes to the stromal compartment and that stromal cells may indeed drive this process, for example, through consumption of survival signals or the active production of resolution-promoting molecules [68].
The role of pro-resolution inflammatory mediators in the resolution of inflammation during infectious disease, including the role of lipoxins in models of Toxoplasma gondii, Trypanosoma cruzi and Plasmodium berghei infections, has recently been reviewed [69], whereas the role of resolvins has been most clearly illustrated in the control of herpes simplex virus infection [70]. Nevertheless, in the context of infectious disease, this area of stromal cell biology still offers huge potential not only for uncovering new regulatory pathways per se but for the identification of novel approaches to hasten, when appropriate, the resolution of infection-associated inflammation in the clinic.
2.9 Host-Directed Therapy : Stromal-Targeted Therapeutics for Infectious Disease
The development of immunotherapies targeting stromal cells is also described in detail elsewhere in this volume. Studies in infectious disease have provided three main examples to date where stromal cell-targeted immunotherapy might have an impact on the disease outcome. In the case of HIV, there is a good correlation between the extent of FRC disruption, collagen deposition and the ability of HAART to restore CD4+ Tcell count in patients [54, 71]. In experimental MCMV infection, targeting the LTβR with an agonistic mAb was able to restore otherwise defective CCL21 production and to improve homing of T cells into the T zone of MCMV-infected mice [58]. Finally, in an experimental model of visceral leishmaniasis, therapeutic administration of the broad-spectrum tyrosine kinase inhibitor sunitinib had no direct therapeutic benefit but was able to restore FRC and FDC networks. When used in a sequential therapy regimen with conventional antimonial-based chemotherapy, a marked dose-sparing effect was observed that correlated with enhanced T cell effector function [62]. Given the narrow therapeutic window for many anti-parasite drugs and the common occurrence of lymphadenopathy and/or splenomegaly, these data suggest that immunotherapies targeted at restoring lymphoid tissue architecture or minimizing collateral damage due to fibrosis may have a unique place in the future development of anti-infective therapies.
2.10 Concluding Remarks
Although there has been an explosion in the study of stromal cells in recent years, an appreciation of their role in infectious disease pathogenesis is still in its infancy. Tools are now becoming available to fate map stromal cells under conditions of ongoing infection, conditionally deplete or modify their function and explore their characteristics at a global level, suggesting a rich harvest awaits those who choose to enter this field, bringing with them the diversity of pathogens that have contributed so much in the past in terms of understanding the biology of haematopoietic cells. Ultimately, the ability to study stromal cell populations in human infectious disease will become more tractable, and perhaps in the not too distant future, manipulating stromal cell function to combat infectious disease may become a clinical reality.
References
Donjacour AA, Cunha GR. Stromal regulation of epithelial function. Cancer Treat Res. 1991;53:335–64.
Zhang X, Martinez D, Koledova Z, Qiao G, Streuli CH, Lu P. FGF ligands of the postnatal mammary stroma regulate distinct aspects of epithelial morphogenesis. Development. 2014;141(17):3352–62.
Espinosa-Cantellano M, Martinez-Palomo A. Pathogenesis of intestinal amebiasis: from molecules to disease. Clin Microbiol Rev. 2000;13(2):318–31.
Artis D, Grencis RK. The intestinal epithelium: sensors to effectors in nematode infection. Mucosal Immunol. 2008;1(4):252–64.
Hiemstra IH, Klaver EJ, Vrijland K, Kringel H, Andreasen A, Bouma G, Kraal G, van Die I, den Haan JM. Excreted/secreted Trichuris suis products reduce barrier function and suppress inflammatory cytokine production of intestinal epithelial cells. Mol Immunol. 2014;60(1):1–7.
Kaye P, Scott P. Leishmaniasis: complexity at the host-pathogen interface. Nat Rev Microbiol. 2011;9(8):604–15.
Barrias ES, de Carvalho TM, De Souza W. Trypanosoma cruzi: entry into mammalian host cells and parasitophorous vacuole formation. Front Immunol. 2013;4:186.
Bogdan C, Donhauser N, Doring R, Rollinghoff M, Diefenbach A, Rittig MG. Fibroblasts as host cells in latent leishmaniosis. J Exp Med. 2000;191(12):2121–30.
Svensson M, Maroof A, Ato M, Kaye PM. Stromal cells direct local differentiation of regulatory dendritic cells. Immunity. 2004;21(6):805–16.
Hsu KM, Pratt JR, Akers WJ, Achilefu SI, Yokoyama WM. Murine cytomegalovirus displays selective infection of cells within hours after systemic administration. J Gen Virol. 2009;90(Pt 1):33–43.
Baldwin J, Park PJ, Zanotti B, Maus E, Volin MV, Shukla D, Tiwari V. Susceptibility of human iris stromal cells to herpes simplex virus 1 entry. J Virol. 2013;87(7):4091–6.
Valyi-Nagy T, Sheth V, Clement C, Tiwari V, Scanlan P, Kavouras JH, Leach L, Guzman-Hartman G, Dermody TS, Shukla D. Herpes simplex virus entry receptor nectin-1 is widely expressed in the murine eye. Curr Eye Res. 2004;29(4–5):303–9.
Keele BF, Tazi L, Gartner S, Liu Y, Burgon TB, Estes JD, Thacker TC, Crandall KA, McArthur JC, Burton GF. Characterization of the follicular dendritic cell reservoir of human immunodeficiency virus type 1. J Virol. 2008;82(11):5548–61.
Smith BA, Gartner S, Liu Y, Perelson AS, Stilianakis NI, Keele BF, Kerkering TM, Ferreira-Gonzalez A, Szakal AK, Tew JG, Burton GF. Persistence of infectious HIV on follicular dendritic cells. J Immunol. 2001;166(1):690–6.
Spiegel H, Herbst H, Niedobitek G, Foss HD, Stein H. Follicular dendritic cells are a major reservoir for human immunodeficiency virus type 1 in lymphoid tissues facilitating infection of CD4+ T-helper cells. Am J Pathol. 1992;140(1):15–22.
Thacker TC, Zhou X, Estes JD, Jiang Y, Keele BF, Elton TS, Burton GF. Follicular dendritic cells and human immunodeficiency virus type 1 transcription in CD4+ T cells. J Virol. 2009;83(1):150–8.
Baud J, Varon C, Chabas S, Chambonnier L, Darfeuille F, Staedel C. Helicobacter pylori initiates a mesenchymal transition through ZEB1 in gastric epithelial cells. PLoS One. 2013;8(4):e60315.
Reilkoff RA, Bucala R, Herzog EL. Fibrocytes: emerging effector cells in chronic inflammation. Nat Rev Immunol. 2011;11(6):427–35.
Macedo-Silva RM, Santos Cde L, Diniz VA, Carvalho JJ, Guerra C, Corte-Real S. Peripheral blood fibrocytes: new information to explain the dynamics of Leishmania infection. Mem Inst Oswaldo Cruz. 2014;109(1):61–9.
Allahverdiyev AM, Bagirova M, Elcicek S, Koc RC, Baydar SY, Findikli N, Oztel ON. Adipose tissue-derived mesenchymal stem cells as a new host cell in latent leishmaniasis. Am J Trop Med Hyg. 2011;85(3):535–9.
Schilling JD, Martin SM, Hung CS, Lorenz RG, Hultgren SJ. Toll-like receptor 4 on stromal and hematopoietic cells mediates innate resistance to uropathogenic Escherichia coli. Proc Natl Acad Sci U S A. 2003;100(7):4203–8.
Spanier J, Lienenklaus S, Paijo J, Kessler A, Borst K, Heindorf S, Baker DP, Kroger A, Weiss S, Detje CN, Staeheli P, Kalinke U. Concomitant TLR/RLH signaling of radioresistant and radiosensitive cells is essential for protection against vesicular stomatitis virus infection. J Immunol. 2014;193(6):3045–54.
Tomalka J, Ganesan S, Azodi E, Patel K, Majmudar P, Hall BA, Fitzgerald KA, Hise AG. A novel role for the NLRC4 inflammasome in mucosal defenses against the fungal pathogen Candida albicans. PLoS Pathog. 2011;7(12):e1002379.
Schneider K, Loewendorf A, De Trez C, Fulton J, Rhode A, Shumway H, Ha S, Patterson G, Pfeffer K, Nedospasov SA, Ware CF, Benedict CA. Lymphotoxin-mediated crosstalk between B cells and splenic stroma promotes the initial type I interferon response to cytomegalovirus. Cell Host Microbe. 2008;3(2):67–76.
Verma S, Wang Q, Chodaczek G, Benedict CA. Lymphoid-tissue stromal cells coordinate innate defense to cytomegalovirus. J Virol. 2013;87(11):6201–10.
Andersson U, Tracey KJ. HMGB1 is a therapeutic target for sterile inflammation and infection. Annu Rev Immunol. 2011;29:139–62.
Chan JK, Roth J, Oppenheim JJ, Tracey KJ, Vogl T, Feldmann M, Horwood N, Nanchahal J. Alarmins: awaiting a clinical response. J Clin Invest. 2012;122(8):2711–9.
Gomez Perdiguero E, Klapproth K, Schulz C, Busch K, Azzoni E, Crozet L, Garner H, Trouillet C, de Bruijn MF, Geissmann F, Rodewald HR. Tissue-resident macrophages originate from yolk-sac-derived erythro-myeloid progenitors. Nature. 2015;518(7540):547–51.
Schulz C, Gomez Perdiguero E, Chorro L, Szabo-Rogers H, Cagnard N, Kierdorf K, Prinz M, Wu B, Jacobsen SE, Pollard JW, Frampton J, Liu KJ, Geissmann F. A lineage of myeloid cells independent of Myb and hematopoietic stem cells. Science. 2012;336(6077):86–90.
Yona S, Kim KW, Wolf Y, Mildner A, Varol D, Breker M, Strauss-Ayali D, Viukov S, Guilliams M, Misharin A, Hume DA, Perlman H, Malissen B, Zelzer E, Jung S. Fate mapping reveals origins and dynamics of monocytes and tissue macrophages under homeostasis. Immunity. 2013;38(1):79–91.
Onder L, Narang P, Scandella E, Chai Q, Iolyeva M, Hoorweg K, Halin C, Richie E, Kaye P, Westermann J, Cupedo T, Coles M, Ludewig B. IL-7-producing stromal cells are critical for lymph node remodeling. Blood. 2012;120(24):4675–83.
Cohen JN, Guidi CJ, Tewalt EF, Qiao H, Rouhani SJ, Ruddell A, Farr AG, Tung KS, Engelhard VH. Lymph node-resident lymphatic endothelial cells mediate peripheral tolerance via Aire-independent direct antigen presentation. J Exp Med. 2010;207(4):681–8.
Fletcher AL, Lukacs-Kornek V, Reynoso ED, Pinner SE, Bellemare-Pelletier A, Curry MS, Collier AR, Boyd RL, Turley SJ. Lymph node fibroblastic reticular cells directly present peripheral tissue antigen under steady-state and inflammatory conditions. J Exp Med. 2010;207(4):689–97.
Baptista AP, Roozendaal R, Reijmers RM, Koning JJ, Unger WW, Greuter M, Keuning ED, Molenaar R, Goverse G, Sneeboer MM, den Haan JM, Boes M, Mebius RE. Lymph node stromal cells constrain immunity via MHC class II self-antigen presentation. eLife. 2014; 3:e04433
Dubrot J, Duraes FV, Potin L, Capotosti F, Brighouse D, Suter T, LeibundGut-Landmann S, Garbi N, Reith W, Swartz MA, Hugues S. Lymph node stromal cells acquire peptide-MHCII complexes from dendritic cells and induce antigen-specific CD4(+) T cell tolerance. J Exp Med. 2014;211(6):1153–66.
Peduto L, Dulauroy S, Lochner M, Spath GF, Morales MA, Cumano A, Eberl G. Inflammation recapitulates the ontogeny of lymphoid stromal cells. J Immunol. 2009;182(9):5789–99.
Despars G, Tan J, Periasamy P, O’Neill HC. The role of stroma in hematopoiesis and dendritic cell development. Curr Stem Cell Res Ther. 2007;2(1):23–9.
O’Neill HC, Griffiths KL, Periasamy P, Hinton RA, Petvises S, Hey YY, Tan JK. Spleen stroma maintains progenitors and supports long-term hematopoiesis. Curr Stem Cell Res Ther. 2014;9(4):354–63.
Tan JK, Periasamy P, O'Neill HC. Delineation of precursors in murine spleen that develop in contact with splenic endothelium to give novel dendritic-like cells. Blood. 2010;115(18):3678–85.
Nguyen Hoang AT, Liu H, Juarez J, Aziz N, Kaye PM, Svensson M. Stromal cell-derived CXCL12 and CCL8 cooperate to support increased development of regulatory dendritic cells following Leishmania infection. J Immunol. 2010;185(4):2360–71.
Li Q, Guo Z, Xu X, Xia S, Cao X. Pulmonary stromal cells induce the generation of regulatory DC attenuating T-cell-mediated lung inflammation. Eur J Immunol. 2008;38(10):2751–61.
Tang H, Guo Z, Zhang M, Wang J, Chen G, Cao X. Endothelial stroma programs hematopoietic stem cells to differentiate into regulatory dendritic cells through IL-10. Blood. 2006;108(4):1189–97.
Xia S, Guo Z, Xu X, Yi H, Wang Q, Cao X. Hepatic microenvironment programs hematopoietic progenitor differentiation into regulatory dendritic cells, maintaining liver tolerance. Blood. 2008;112(8):3175–85.
Xu X, Yi H, Guo Z, Qian C, Xia S, Yao Y, Cao X. Splenic stroma-educated regulatory dendritic cells induce apoptosis of activated CD4 T cells via Fas ligand-enhanced IFN-gamma and nitric oxide. J Immunol. 2012;188(3):1168–77.
Zhang M, Tang H, Guo Z, An H, Zhu X, Song W, Guo J, Huang X, Chen T, Wang J, Cao X. Splenic stroma drives mature dendritic cells to differentiate into regulatory dendritic cells. Nat Immunol. 2004;5(11):1124–33.
Li L, Liu S, Zhang T, Pan W, Yang X, Cao X. Splenic stromal microenvironment negatively regulates virus-activated plasmacytoid dendritic cells through TGF-beta. J Immunol. 2008;180(5):2951–6.
Hussaarts L, van der Vlugt LE, Yazdanbakhsh M, Smits HH. Regulatory B-cell induction by helminths: implications for allergic disease. J Allergy Clin Immunol. 2011;128(4):733–9.
Turner JD, Narang P, Coles MC, Mountford AP. Blood flukes exploit Peyer’s Patch lymphoid tissue to facilitate transmission from the mammalian host. PLoS Pathog. 2012;8(12):e1003063.
Wood GS. The immunohistology of lymph nodes in HIV infection: a review. Prog AIDS Pathol. 1990;2:25–32.
Wood GS, Garcia CF, Dorfman RF, Warnke RA. The immunohistology of follicle lysis in lymph node biopsies from homosexual men. Blood. 1985;66(5):1092–7.
Rosenberg YJ, Lewis MG, Greenhouse JJ, Cafaro A, Leon EC, Brown CR, Bieg KE, Kosco-Vilbois MH. Enhanced follicular dendritic cell function in lymph nodes of simian immunodeficiency virus-infected macaques: consequences for pathogenesis. Eur J Immunol. 1997;27(12):3214–22.
Smelt SC, Engwerda CR, McCrossen M, Kaye PM. Destruction of follicular dendritic cells during chronic visceral leishmaniasis. J Immunol. 1997;158(8):3813–21.
Zeng M, Paiardini M, Engram JC, Beilman GJ, Chipman JG, Schacker TW, Silvestri G, Haase AT. Critical role of CD4 T cells in maintaining lymphoid tissue structure for immune cell homeostasis and reconstitution. Blood. 2012;120(9):1856–67.
Zeng M, Southern PJ, Reilly CS, Beilman GJ, Chipman JG, Schacker TW, Haase AT. Lymphoid tissue damage in HIV-1 infection depletes naive T cells and limits T cell reconstitution after antiretroviral therapy. PLoS Pathog. 2012;8(1):e1002437.
Ato M, Stager S, Engwerda CR, Kaye PM. Defective CCR7 expression on dendritic cells contributes to the development of visceral leishmaniasis. Nat Immunol. 2002;3(12):1185–91.
Graw F, Regoes RR. Influence of the fibroblastic reticular network on cell-cell interactions in lymphoid organs. PLoS Comput Biol. 2012;8(3):e1002436.
Scandella E, Bolinger B, Lattmann E, Miller S, Favre S, Littman DR, Finke D, Luther SA, Junt T, Ludewig B. Restoration of lymphoid organ integrity through the interaction of lymphoid tissue-inducer cells with stroma of the T cell zone. Nat Immunol. 2008;9(6):667–75.
Benedict CA, De Trez C, Schneider K, Ha S, Patterson G, Ware CF. Specific remodeling of splenic architecture by cytomegalovirus. PLoS Pathog. 2006;2(3):e16.
Engwerda CR, Ato M, Cotterell SE, Mynott TL, Tschannerl A, Gorak-Stolinska PM, Kaye PM. A role for tumor necrosis factor-alpha in remodeling the splenic marginal zone during Leishmania donovani infection. Am J Pathol. 2002;161(2):429–37.
Beattie L, Engwerda CR, Wykes M, Good MF. CD8+ T lymphocyte-mediated loss of marginal metallophilic macrophages following infection with Plasmodium chabaudi chabaudi AS. J Immunol. 2006;177(4):2518–26.
Yurdakul P, Dalton J, Beattie L, Brown N, Erguven S, Maroof A, Kaye PM. Compartment-specific remodeling of splenic micro-architecture during experimental visceral leishmaniasis. Am J Pathol. 2011;179(1):23–9.
Dalton JE, Maroof A, Owens BM, Narang P, Johnson K, Brown N, Rosenquist L, Beattie L, Coles M, Kaye PM. Inhibition of receptor tyrosine kinases restores immunocompetence and improves immune-dependent chemotherapy against experimental leishmaniasis in mice. J Clin Invest. 2010;120(4):1204–16.
Grewal JS, Pilgrim MJ, Grewal S, Kasman L, Werner P, Bruorton ME, London SD, London L. Salivary glands act as mucosal inductive sites via the formation of ectopic germinal centers after site-restricted MCMV infection. FASEB J. 2011;25(5):1680–96.
GeurtsvanKessel CH, Willart MA, Bergen IM, van Rijt LS, Muskens F, Elewaut D, Osterhaus AD, Hendriks R, Rimmelzwaan GF, Lambrecht BN. Dendritic cells are crucial for maintenance of tertiary lymphoid structures in the lung of influenza virus-infected mice. J Exp Med. 2009;206(11):2339–49.
Slight SR, Rangel-Moreno J, Gopal R, Lin Y, Fallert Junecko BA, Mehra S, Selman M, Becerril-Villanueva E, Baquera-Heredia J, Pavon L, Kaushal D, Reinhart TA, Randall TD, Khader SA. CXCR5(+) T helper cells mediate protective immunity against tuberculosis. J Clin Invest. 2013;123(2):712–26.
Moore JW, Beattie L, Dalton JE, Owens BM, Maroof A, Coles MC, Kaye PM. B cell: T cell interactions occur within hepatic granulomas during experimental visceral leishmaniasis. PLoS One. 2012;7(3):e34143.
Barone F, Nayar S, Buckley CD. The role of non-hematopoietic stromal cells in the persistence of inflammation. Front Immunol. 2012;3:416.
Serhan CN, Brain SD, Buckley CD, Gilroy DW, Haslett C, O’Neill LA, Perretti M, Rossi AG, Wallace JL. Resolution of inflammation: state of the art, definitions and terms. FASEB J. 2007;21(2):325–32.
Russell CD, Schwarze J. The role of pro-resolution lipid mediators in infectious disease. Immunology. 2014;141(2):166–73.
Rajasagi NK, Reddy PB, Suryawanshi A, Mulik S, Gjorstrup P, Rouse BT. Controlling herpes simplex virus-induced ocular inflammatory lesions with the lipid-derived mediator resolvin E1. J Immunol. 2011;186(3):1735–46.
Zeng M, Haase AT, Schacker TW. Lymphoid tissue structure and HIV-1 infection: life or death for T cells. Trends Immunol. 2012;33(6):306–14.
Acknowledgements
The author thanks his numerous colleagues who have contributed to the study of stromal cell biology in his laboratory and the Medical Research Council and the Wellcome Trust for providing long-term research support. The author also apologizes to the many investigators whose work has been omitted for the sake of brevity but who have set the scene for future studies of the role of stromal cells in infection.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 Springer International Publishing AG, part of Springer Nature
About this chapter
Cite this chapter
Kaye, P.M. (2018). Stromal Cell Responses in Infection. In: Owens, B., Lakins, M. (eds) Stromal Immunology. Advances in Experimental Medicine and Biology, vol 1060. Springer, Cham. https://doi.org/10.1007/978-3-319-78127-3_2
Download citation
DOI: https://doi.org/10.1007/978-3-319-78127-3_2
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-78125-9
Online ISBN: 978-3-319-78127-3
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)