Abstract
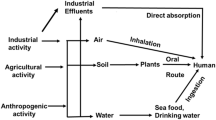
Heavy metals belong to the groups of transition elements and are defined in terms of their chemical properties, atomic weight, density, or specific gravity as compared to water. Heavy metals could be metalloids, lanthanides, and actinides. The heavy metals reach into humans and animals through contaminated air and water as well as food stuffs especially from fish, chicken, vegetables, vaccinations, dental fillings, and deodorants. Most of the heavy metals, when accumulated in excess, induce toxicity by damaging the central nervous system (CNS), energy metabolism, ion-transporters , cardiovascular systems, respiratory systems, reproductory systems , and vital organs such as lungs, liver, and brain leading to the physical, physiological, and behavioral disorders. Arsenic (As) has been shown to generate skin diseases and cancer; lead (Pb) poisoning induces infertility and neurotoxicity/neurodegeneration ; and mercury (Hg) intake causes harmful effects in lactating mothers, fetuses, and children. Cadmium (Cd) , considered to act like both an occupational and non-occupational toxicant, has been reported to be one of the carcinogens . The strategies to combat heavy metals toxicity include appropriate intake of antioxidants , phytochemicals , and minerals. The present chapter is an endeavor to illustrate an updated account of various aspects of heavy metals toxicity with a particular reference to their biomedical implications as well as the use of phytochemicals and minerals toward the treatment of their adverse effects.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Heavy metals are the inorganic elements with relatively high density, high specific gravity (five times more as compared to water), and high atomic number and weight. It is naturally present in the earth crust, and anthropogenic activities of human beings lead to their accumulation in the environment above their permissible limit (Sharma et al. 2014).
Exposure of these heavy metals is a common phenomenon due to their environmental pervasiveness. The widely known consequence of these heavy metals intoxication includes the development of neurotoxicity, genotoxicity, and carcinogenicity (Fergusson 1990). Heavy metals also affect most of the organ systems including central nervous system (CNS) , peripheral nervous systems (PNS), gastrointestinal (GI) systems, cardiovascular systems, hematopoietic systems, renal systems, and reproductive system. However, the mechanism and effect of this toxicant vary with dose, duration, mode of action, chemical, their valence, and the age of the individual.
There are some heavy metals which are required for the normal biological functioning of cells like selenium, manganese, zinc, and copper which participates in regulating various metabolic and signaling pathways. These metals possess coordination chemistry and redox properties which provides them an extra advantage by which these metals could escape out of the control mechanism such as transport, homeostasis, compartmentalization, and binding to designated cell constituents. While the biggest disadvantage lies in their ability to replace other metals normally present in the binding sites, this nature of heavy metals makes them toxic and ultimately leading to malfunctioning effect. Sometimes, these metals bind with nuclear proteins and DNA causing oxidative deterioration of these biological macromolecules (Leonard et al. 2004; Flora et al. 2008).
Among all the heavy metals, arsenic, lead, cadmium, and mercury are reported to cause serious health complications in humans (Flora et al. 2008). It has been reported by various workers that the exposure of an organism to a higher level of these metals may cause the production of free radicals such as reactive oxygen species (ROS) and reactive nitrogen species (RNS) which may result in oxidative stress (Leonard et al. 2004; ATSDR 2007). Oxidative stress is considered to be one of the major mechanisms of heavy metal toxicity (Manke et al. 2013). For example, arsenic exposure induces productions of reactive oxygen species (ROS) followed by increased oxidative stress (Shi et al. 2004), and this oxidative stress is often found to be associated with the development of tumors in lung, skin, liver, bladder, and kidney (Waalkes et al. 2004). Lead is known to induce a broad range of physiological, biochemical, and behavioral dysfunctions in laboratory animals and humans (Goyer 1996; Flora et al. 2008) including central and peripheral nervous systems, haemopoietic system, cardiovascular system (Lanphear et al. 2000), kidneys (Damek-Poprawa and Sawicka-Kapusta 2004), liver (Sharma and Street 1980), and the reproductive systems of males and females (Ronis et al. 1998). Cadmium exposure also induces ROS production and thereby mutagenesis (Filipic et al. 2006). Similarly, the intoxication of mercury affects different organ systems as well as mitochondrial function (Lund et al. 1993; Sharma et al. 2014; Gupta and Sharma 2015).
The present chapter covers the overview of the source, mechanisms, biochemical, and molecular targets as well as the phytochemicals against toxic metals such as Pb, Cd, As, and Hg. The current knowledge of toxic effects of metal-induced oxidative stress suggests that possible measures should be taken to reduce their toxic effects and to achieve physiological recoveries. This chapter also illustrates the role of essential trace metals such as Zn, Cu, and the Se in proper biological maintenance and also the toxicity induced by them when used in excess.
2 Sources of Heavy Metals in Environment
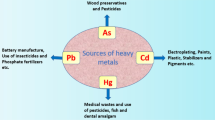
Heavy metals are naturally and ubiquitously present in earth’s crust. These elements are the most ancient toxins against humans, having been utilized for several years. Several natural and anthropogenic processes are involved in providing entry of heavy metals into the environment (VanDam et al. 1995; Pacyna 1996; Shallari et al. 1998; Bradl 2002; Waalkes et al. 2007; Strater et al. 2010). The most noteworthy natural sources are erosion, weathering of minerals, and volcanic action, while anthropogenic sources include smelting, mining, electroplating, utilization of pesticides , fertilizers and also biosolids in farming, sludge dumping, industrial release , atmospheric fixation, etc. (Modaihsh et al. 2004; Chehregani and Malayeri 2007; Fulekar et al. 2009; Wuana and Okieimen 2011). The anthropogenic sources of heavy metals are summarized in Table 14.1.
Use of cadmium is common in various industrial activities, and major application of cadmium includes pigments, alloy, and batteries (Wilson 1988). Other sources of cadmium include emissions from mining, industrial activities, smelting, and manufacturing of batteries, pigments, stabilizers, and alloys (ATSDR 2008). Foodstuffs are also contributing as a major source of cadmium exposure such as leafy vegetables, grain and seeds, potatoes and molluscs, and crustaceans (Satarug et al. 2003).
Volcanic eruptions and soil erosions are the natural phenomena, and these natural activities increase the environmental pollution of arsenic (ATSDR 2000). Arsenic is also used in several industrial manufactured products such as wood preservatives, agricultural application products, and dyestuffs. Mercury is highly utilized in electrical industry and used in making electric appliances such as batteries, switches, and thermostats. Also, it is used in dentistry, in the production of caustic soda and as solvents for various precious metals (Tchounwou et al. 2003a, b).
3 Role in Biological Functions
Mostly heavy metals are nonessential for living organisms. Some of the heavy metals serve as cofactors in several enzymes. The only known favorable biological function of cadmium is observed in diatoms (Thalassiosira weissflogii). The marine diatoms use cadmium as cofactors for their enzymes. Arsenic is used as drug for treatment of many veterinary diseases. Drugs based on Arsenic are useful and very effective against some tropical diseases such as amoebic dysentery and sleeping sickness (African). It is also used in treatment of parasitic diseases including filariasis in animals (Centeno et al. 2005). Recently, arsenic trioxide has been approved by the Food and Drug Administration (FDA) as an anticancer agent in the treatment of acute promyelocytic leukaemia. Its therapeutic action has been attributed to the induction of programmed cell death (apoptosis) in leukaemia cells (Yedjou and Tchounwou 2007). Lead and mercury have no any beneficial biological functions.
4 Biochemical Targets for Heavy Metals Toxicity
4.1 Cadmium
Cadmium is a naturally occurring metal and it is situated in between zinc (Zn) and mercury (Hg) in periodic table. Its chemical behavior is similar to Zn and it forms divalent cation complexes with other elements. Cadmium toxicity is well reported in various organs and tissues. The most prominent targets of cadmium are nervous system, cardiovascular system, respiratory system, excretory, and reproductive system.
This metal has no known useful biological role in mammals and prolongs encounter with this metal show harmful consequences (Zadorozhnaja et al. 2000). The excretion rate of Cd from the body is inadequate which enhance the biological half-life around 15–30 years (Varga et al. 1993; Bhattacharyya et al. 2000; Henson and Anderson 2000). Due to the long biological half-life of Cd, it accumulates in various parts of an organism such as liver, kidney, as well as in the reproductive organs including testis, ovaries, and placenta (Paksy et al. 1997; Zadorozhnaja et al. 2000; Brohi et al. 2017). Moreover, the human exposed to this metal are more prone to health complications like renal disease , osteoporosis , hypertension, and leukaemia, as well as cancers of the lung, urinary bladder, kidney, pancreas, prostate, and breast (Satoh et al. 2002). Humans are mainly exposed to cadmium via inhalation or cigarette smoke and through ingestion of contaminated food. The blood and urine cadmium content is higher in cigarette smoker and lower in nonsmokers (Becker et al. 2002; Mannino et al. 2004). The foodstuffs that are rich in cadmium such as mushroom, seaweeds, shellfish, mussels and cocoa powder can build up more accumulation in human bodies. The U.S. National Toxicology Program and International Agency for Research on Cancer (IARC 1993) have concluded that there is satisfactory validation that cadmium is a human cancer-causing agent. Other target tissues of cadmium carcinogenesis in humans are liver, testicles, adrenals, and the hemopoietic system (IARC 1993; Waalkes et al. 1996). Some studies also reported that environmental and occupational cadmium exposure is also associated with progression and induction of cancers in kidney, prostate, and stomach (Waalkes et al. 1996).
Cadmium is a serious gastrointestinal and pulmonary irritant, which can be lethal if ingested or inhaled. Ingestion of high amount of cadmium induces several symptoms such as muscle cramps, abdominal pain, nausea, burning sensation, vomiting, salivation, shock, vertigo, loss of consciousness, and convulsions usually appear within 15–30 min (Baselt and Cravey 1995). In fact, several years before, Friberg (1948, 1950) had reported that damage to lungs and kidneys might be the earliest effects on workers exposed to cadmium. Later on, it was found that acute ingestion of cadmium can cause gastrointestinal tract erosion, pulmonary, renal or hepatic injury, and coma (Baselt and Cravey 1995; Baselt 2000). Chronic exposure to cadmium has a depressive effect on levels of several neurotransmitters such as acetylcholine, serotonin, and norepinephrine (Singhal et al. 1976). Experiments performed on rodents also explain that chronic inhalation of cadmium causes pulmonary adenocarcinomas (Waalkes and Berthan 1995; Waalkes et al. 1996) and proliferative prostatic lesions after systemic or direct exposure (Waalkes and Rehm 1992). Cadmium can also bind to E-cadherin (a cell–cell adhesion glycoprotein) at Ca (II)-binding region, disrupting cell-to-cell adhesion (Pearson and Prozialeck 2001). Toxicity of cadmium also led to an alteration in the activities of certain enzymes in the mammalian systems. The cadmium administration to rats significantly influences the activity of antioxidant enzymes such as Cu, Zn-superoxide dismutase (SOD), glutathione peroxidase (GPx), catalase (CAT), glutathione reductase (GR), and glutathione-S-transferase (GST), and increases the lipid peroxidation , and thereby causes oxidative stress (Ognjanovic et al. 2003).
4.2 Arsenic
Arsenic is a ubiquitous element and present in at low concentrations in all environmental conditions (NRCC 1978; ATSDR 2000). Nearly, 200 million people are affected globally by arsenic exposure, whereas about 70 million people are suffering in India. The trivalent arsenite and pentavalent arsenate are the major forms of inorganic arsenic. The report based on extensive surveys explained that several millions of peoples throughout the world are exposed to arsenic chronically. Peoples living in countries like India, Mexico, Taiwan, Bangladesh, and Uruguay, where the groundwater is extensively contaminated with arsenic, are mostly exposed. The arsenic exposure occurs through dermal contact, inhalation, and parental route to some extent (Tchounwou et al. 1999; ATSDR 2000). The dissolve arsenic compounds are absorbed with high efficiency than the lower solubility compounds such as lead arsenide, arsenic selenide, and gallium arsenide, whereas high acute dermal contact with inorganic arsenic solutions results in systemic skin toxicity (Smith et al. 1992). Arsenic contamination affects all organ systems, and its major targets include the renal, nervous, respiratory, gastrointestinal nervous, dermatologic, and cardiovascular systems (Tchounwou et al. 2003a, b). Research has also pointed to significantly higher standardized mortality rates for cancers of the bladder, kidney, skin, and liver in many areas of arsenic pollution . The severity of adverse health effects is related to the chemical form of arsenic, and is also time- and dose-dependent (Tchounwou et al. 2002; Yedjou et al. 2006). The evidence from studies strongly supports the carcinogenicity of arsenic in humans, but the mechanism of tumor progression in humans is not completely understood (Chappell et al. 1997). Several epidemiological studies have validated the strong association between arsenic toxicity and adverse effects on human health and increased risks of tumor formation.
One of the known adverse effects of arsenic is the reactive oxygen species (ROS) production which causes oxidative stress (Shi et al. 2004). The interaction between these reactive species and biomolecules results in alteration and loss of regulatory mechanism of the cell, which may lead to cell death. Several studies indicate that oxidative stress created by arsenic toxicity influence the antioxidant enzyme such as catalase (CAT), superoxide dismutase (SOD), glutathione peroxidase (GPx), as well as nonenzymatic factors, for example, sulfhydryl group containing peptides (Shi et al. 2004). The oxidative stress is also associated with physiological changes which cause late complications in diabetes mellitus (Parthiban et al. 1995; Rin et al. 1995). Some of the researchers are proposing that it may play a major role in vascular and neurological complications in diabetic patients (Baynes 1991; Kolb and Kolb-Bachofen 1992; Giugliano et al. 1996; Yamanaka et al. 2001; Singh et al. 2017). Arsenic also increases the rate of lipid peroxidation (LPO) , and high level of these lipid peroxides has been indicated in diabetic patients (Lyons 1991; Inada et al. 1995; Borcea et al. 1999). Arsenic may affect the central nervous system (CNS) and cause significant alterations in the behavioral pattern of exposed animals. Arsenic causes biomethylation in the brain which results in the development of neurotoxicity .
4.3 Lead
Lead is a potent occupational toxin which is non-biodegradable. It persists in the environment for a long time. In the human body, most of the absorbed lead is accumulated in kidney, followed by liver and other tissues such as brain and heart, but the skeleton represents the major fraction of overall lead content in the human body (Flora et al. 2006). The most susceptible target for lead poisoning is nervous system, and the poor attention spam, headache, loss of memory, dullness, and irritability are the early symptoms of lead exposure on the central nervous system (ATSDR 1999; CDC 2001). The major target of lead toxicity includes liver, central nervous system, reproductive system, hematopoietic system, and endocrine system (ATSDR 1999). It is most hazardous for pregnant women. The absorbed lead can readily transfer to developing embryo (Ong et al. 1985). It may cause early birth, reduce body weight and neuro development, and reproductive disability in offspring (Andrews et al. 1994).
Lead produces toxicity on most of the organ system and induces a broad range of physiological, biochemical, and genetical dysfunctions , because it is one of the most clinically significant heavy metals. The most sensitive target of lead exposure is the central nervous system. Pb causes neurotoxicity but significantly decreases pediatric cognitive functions. Lead toxicity interferes with enzymatic steps in the heme pathway and reduces the body’s capacity for formation of hemoglobin. In severe cases of lead poisoning, children or adults may present with severe cramping abdominal pain, which may be mistaken for an acute abdomen or appendicitis. Cardiovascular hypertension is a complex condition with many different causes and risk factors, including age, weight, diet, and exercise habits. Lead poisoning effects as examined in the literature include low sperm count , fertility , and pregnancy outcomes. Williams et al. (2010) have reported that the higher blood lead was associated with later pubertal onset in this prospective study of peri-pubertal Russian boys. Studies on the effects of lead on the endocrine system are mainly based on occupationally lead-exposed workers and experimental animal models. Although evidence is conflicting, it has been reported that accumulation of lead affects the majority of the endocrine glands. In particular, it appears to affect the hypothalamic–pituitary axis causing blunted TSH, GH, and FSH/LH responses to TRH, GHRH, and GnRH stimulation, respectively.
Some of the key metabolic enzymes are major target for lead toxicity. Lead can mimic the essential mineral ions such as iron, zinc, and calcium which play an important role of cofactors with several enzymes. Thus, replacement of these metals with lead will interfere with enzyme function. Lead is well known for its involvement in ROS production. The ROS-mediated damage of cell membrane and DNA damage are common in lead toxicity. Lead also interferes with antioxidant enzymes such as SOD, GPx, and CAT and nonenzymatic antioxidant molecules (Valko et al. 2005; Flora et al. 2008; Sharma et al. 2014).
4.4 Mercury
In nature, mercury is available in different physical and chemical forms, and all forms of mercury can produce toxic effects. The various forms of mercury are mercurous (Hg I), elemental mercury vapor (Hg), and mercuric (Hg II) and organic mercuric compounds (Rubino 2015). All forms of mercury are toxic to humans and have toxic effects in different organs such as kidney, brain, and lung (Fitzgerald and Clarkson 1991; Zalups and Koropatnik 2000). Exposure to mercury can induce several diseases such as Minamata disease , acrodynia (pink disease), and Hunter-Russell syndrome . The organic and elemental mercury show wide range of toxicity including gastrointestinal toxicity, neurotoxicity, and nephrotoxicity (Zalups 2002). The mercurous and mercuric ions create toxicity generally by interacting with the thiol group of essential molecules and protein such as GSH and metallothionein (MT) (Hultberg et al. 2001). Some of the researchers have reported that mammals exposed to mercuric chloride result in alterations in several antioxidants enzymes such as SOD, GPX, CAT, and GR. Exposure to mercury also causes the change in the rate of lipid peroxidation in comparison to non-contaminated groups (Yee and Choi 1996; Mahboob et al. 2001). Mercury also affects the numbers of the stress of protein including heat shock proteins and glucose-regulated proteins (Goering et al. 2000; Papaconstantinou et al. 2003). The action and targets of different chemical forms of mercury are given in Fig. 14.1. Some of the hypotheses supported that the injuries in the central nervous system caused by methylmercury are due to ROS production (Zhang et al. 2009). Studies demonstrated that methylmercury inhibits the cell division and migration in both in vivo and in vitro conditions (Grandjean et al. 1997; Graeme and Pollack 1998; Grandjean et al. 1999). Another researcher also reported that mercury intoxication is related to the increased risk of myocardial infarction , hypertension, coronary dysfunction, atherosclerosis , and increased risk of cardiovascular disease (Rhee and Choi 1989; Guallar et al. 2002; Yoshizawa et al. 2002).
5 Molecular Mechanism of Heavy Metals Toxicity
5.1 Cadmium
The mechanism of action of cadmium is not well understood. The main action of cadmium in mutagenesis is generation of ROS (Filipic et al. 2006). Due to rise in ROS level, various physiological perturbations develop such as increased permeability of blood–brain barrier and alteration in synaptic transmission. Cadmium is a non-redox active metal and cannot initiated by itself Fenton reactions (Casalino et al. 1997). Therefore, it induces oxidative stress via indirect process. Some of the known mechanism through which it act are (1) Cd combines with thiol groups of enzymes involved in antioxidant mechanisms, such as glutathione peroxidase (GPx), SOD, and catalase, and inhibits their activities (Wang et al. 2004); (2) Cd decreases in the intracellular GSH content; (3) Cd inhibits GPx activity by forming cadmium–selenium complexes; and (4) Cd inhibits complex III of the mitochondrial electronic transport chain and increases the production of ROS (Wang et al. 2004) which may trigger the apoptosis pathways.
5.2 Arsenic
Arsenic interacts with thiol group or sulfhydryl groups of protein and can inactivate around 200 enzymes, a principal mechanism of arsenic toxicity. As(V) can also replace the phosphate molecules which are actively involved in several biochemical pathways , and thereby affect that pathways (Goyer 2001; Hughes 2002). Arsenic exposure may impair the cellular respiration by inhibiting the various mitochondrial enzymes and uncoupling the oxidative phosphorylation . Several metabolic pathways may cause methylation of arsenic leading to the formation of methyl metabolites of arsenic that are more toxic than arsenite (Tchounwou et al. 2003a, b). Further, comet assay pointed out the role of arsenic trioxide in the induction of DNA damage in human lymphocytes (Anderson et al. 1994). While some of its compounds can also trigger the gene amplification, inhibit DNA repair system , and arrest cells in mitosis, it also induces the expression of the c-fos gene and oxidative stress protein heme oxygenase, and also acts as a promoter for various toxic agents (Barrett et al. 1989; Hartmann and Peit 1994; Saleha-Banu et al. 2001). Several mechanisms are reported for arsenic-induced carcinogenesis but the available information is not fulfilled to understand the actual mechanism of its action. Some of the reported mechanisms are hypoxia inducing both genetic and epigenetic changes (Salnikow and Zhitkovich 2008), modulation of gene expression (Huang et al. 2004), enhanced cell proliferation (Simeonova et al. 2000), and induction of oxidative stress (Shi et al. 2004). The regulation and mechanism of action of arsenic are given in Fig. 14.2. Arsenic can also interfere with the signaling pathways (p53 signaling pathway) that are involved in promotion and progression of several tumors in mammals (Porter et al. 1999; Vogt and Rossman 2001). A recent review discusses nine different possible modes of action of arsenic carcinogenesis: oxidative stress, induced chromosomal abnormalities, altered DNA methylation patterns, altered DNA repair, altered growth factors, suppression of p53, enhanced cell proliferation, promotion/progression, and gene amplification (Miller et al. 2002). Three modes (oxidative stress, chromosomal abnormality, and altered growth factors) of arsenic carcinogenesis have shown a degree of positive evidence, both in experimental systems (animal and human cells) and in human tissues. However, the other mechanisms do not have much evidence especially from in vivo studies.
5.3 Lead
The mechanisms which involve lead-induced toxicity primarily damage to the cell membrane and DNA as well as damage to the enzymatic antioxidant molecule such as catalase, glucose-6-phosphate dehydrogenase (G6PD), GPx, and SOD and nonenzymatic antioxidants such as GSH of animals and human systems. Several kinds of literature indicate that lead-induced toxicity might be involved in the multifactorial mechanism of action. This multifactorial mechanism can be oxidative stress, enzyme inhibition, DNA damage , change in gene expression, and adventitious like mimicry. In all mechanism, reactive oxygen species induced by lead is a well-known mechanism. The lead has electron-sharing affinities that can result in formation of covalent attachment with sulphydryl groups of cellular components. Lead is known to adversely influence the metabolism of glutathione and cause toxicity. Several mechanisms are proposed for lead-induced oxidative stress: (1) Direct effect of lead on cell membranes, (2) lead–hemoglobin interaction, (3) δ-aminolevulinic acid (δ-ALA) -induced generation of ROS, and (4) effect of lead on the antioxidant defense system of cells.
5.4 Mercury
The molecular mechanisms of toxicity of mercury are basically through the production of oxidative stress (Sharma et al. 2014). After absorption, it forms complexes with cysteine residues of proteins and diminishes the cellular antioxidants. Inorganic mercury has been reported to cause a defect in electron transport and oxidative phosphorylation at ubiquinone-cytochrome b5 step by producing ROS and creating oxidative stress (Marnett 2000). The oxidative stress also involved in the disruption of calcium homoeostasis. Both types of mercury, organic and inorganic, disrupt the calcium homoeostasis but their action of mechanisms is different. Organic mercury is supposed to increase the intracellular calcium by stimulating the influx of extracellular calcium and mobilizing intracellular stores, whereas inorganic mercury disrupts the homoeostasis by accelerating the influx of extracellular calcium (Kim et al. 2010). The link between mercury intoxication and carcinogenesis is not much clear. Some studies have confirmed the genotoxic potential of mercury, whereas others have not shown any connection between mercury exposure and its genotoxicity (Valko et al. 2004). Generation of oxidative stress and ROS production during mercury toxicity has been reported to be associated with DNA damage, which can lead to the initiation of carcinogenic process (Ogura et al. 1996; Valko et al. 2006). The free radical production may also induce the conformational changes in enzymes and other proteins that are actively involved in cell cycle regulation such as chromosomal segregation, mitotic spindle, and DNA repair (Valko et al. 2006).
6 Phytochemicals in Alleviation of Heavy Metal Toxicity
The plants are rich in antioxidant potentials such as polyphenols and flavonoids . However, plants may also have the ameliorative effect against the heavy metal toxicity. Several researchers also reported the heavy metal scavenging activity of phytochemicals . The oral administrations of soya bean supplementation and Arthrospira maxima reduce the cadmium-induced genotoxic and cardiovascular implications (Brochin et al. 2008; Argüelles et al. 2013). Many phytochemicals such as phycocyanobilin , carotenes , vitamin C, and vitamin E obtained from cyanobacterial species such as Chlorella and Spirulina have shown their protective effects against lead- and cadmium-induced toxicity (Amin et al. 2006; Shim et al. 2008; Shim and Om 2008; Yun et al. 2011; Gupta et al. 2015). Several research works provide strong evidence for antiapoptotic activity of garlic extract and its inhibitory effect on mitochondrial injury caused by cadmium and lead. Garlic is rich in phytochemical, allicin (Shahsavani et al. 2011; Sadeghi et al. 2013).
Leaf extracts of Annona muricata and Hippophae rhamnoides have been beneficial against arsenic-induced toxicity. Both are rich in vitamins, carotenoids , and organic acids which give positive effects in antidote against arsenic toxicity (Jomova et al. 2011; Gupta and Flora 2005, 2006; George et al. 2015). Some of the researchers have also shown that phytochemicals not only ameliorate the toxicity of heavy metals but also reduce the body burden of accumulated heavy metals. Tomato extracts have potential to reduce bioaccumulation of heavy metals, in particular against lead- and cadmium-induced intoxication (Nwokocha et al. 2012). An overview of some phytochemicals active against heavy metal toxicity is presented in Table 14.2.
7 Role of Essential Mineral Ions in Mitigation of Heavy Metals Toxicity
7.1 Zinc
Zinc , a ubiquitous trace element essential as a catalytic, structural, and regulatory ion, is indispensable for growth and development of the microorganisms, plants, and animals (Mocchegiani and Muzzioli 2000). Average human intake of zinc ranges between 2.5 and 10 mg/day (Letavayova et al. 2006). It is well known for its role as a cofactor for superoxide dismutase (SOD), and it protects biological structures from damage caused by free radicals by maintaining adequate levels of SOD and metallothionein , as well as preventing interaction between chemical groups with iron. It is a part of the zinc-dependent thymic hormone that is essential for thymic functions such as T-cell maturation and differentiation (Mocchegiani and Muzzioli 2000). Its antioxidant function is attributed to its function of blocking the negatively charged sites, thereby preventing lipid peroxidation. Its deficiency has mostly been associated with an increase in the levels of lipid peroxidation of mitochondrial and microsomal membranes along with osmotic fragility of the erythrocyte membrane. Zinc-binding proteins such as metallothioneins (MTs) are present in virtually all living organisms. These proteins play a significant role in zinc uptake, distribution, storage, and release, and are protective in situations of stress (exposure to oxyradicals), exposure to toxic metals, and low Zn nutrition (Vasak and Hasler 2000; Coyle et al. 2002). Zn as a part of MTs improves excretion of metals such as Pb, As, etc. from the body. In a study, Jamieson et al. (2006) found that marginal Zn deficiency enhances accumulation of Pb in bone, while its supplementation reduces its absorption and, as such, its accumulation in rats. Co-administration of zinc and lead, which compete for similar binding sites on enzymes, results in the reverse inhibition of aminolevulinic acid dehydrogenase in male Wistar rats, suggesting that administration of zinc suppresses the toxic effects of lead (Flora et al. 1989, 1999). In a similar study, supplementation of Zn was found to be associated with a reduction in the effects of HgCl2 (Franciscato et al. 2011). Possessing significant potential to displace Zn from Zn-metalloproteinases , it eliminates the effect of HgCl2 on neural development (Guzzi and Laporta 2008). Zn along with Se has been associated with a reduction in MeHg-induced toxicity. All this indicates that it plays a protective role against the damage of different metals through reduction in absorption, competing for binding to enzymes and through induction of molecules such as MTs. Besides having positive effects, supplementation of Zn has also been found associated with displacement of essential metals to substitute normal physiological activities (Briner 2014). On one side, where the supplementation of Zn seems to protect against oxidative damage of iron in the instance of iron supplementation, long-term or higher dosage treatment of Zn has been associated with the depletion of copper (Suzuki 1997; Maret and Sandstead 2006). As such, a balanced approach to the supplementation of these metal ions is necessary to prevent unwanted complications. The action of Zn in amelioration of heavy metal-induced toxicity is illustrated in Fig. 14.3.
7.2 Copper
Copper , an essential trace metal, acts as a cofactor for a variety of proteins and enzymes required for maturation of cytoplasmic cuproproteins and assembly of enzymes in different cell organelles (ceruloplasmin and tyrosinase in the case of Golgi apparatus and cytochrome c oxidase concerning mitochondria). Copper uptake occurs in a tightly regulated process through specific high-affinity plasma membrane copper transporters or low-affinity permeases (DeFeo et al. 2007; Kim et al. 2008). Binding to chaperone proteins results in the transfer of copper to its final destination or any intermediate location from which its transport to other cell compartments or efflux out of cells can occur in cases in which concentration exceeds the optimum level. It acts as a cofactor for a broad range of metal-binding enzymes , and it fluctuates between the oxidized Cu (II) and reduced Cu (I) forms. In humans, its average intake varies between 260 and 700 g/day. Although adequate intake of copper protects lead, higher consumption has been associated with increased lead absorption (Flora et al. 1982). Its presence in excess amounts led to its involvement in the generation of highly reactive oxidative species (such as hydroxyl radicals), well known for their devastating effects on cells, particularly DNA damage and oxidation of proteins and lipids (Halliwell and Gutteridge 1990). Cu (I) and Cu (II) that hold high affinity for protein sites having cysteine, methionine, and histidine side chains act as potential ligands that led to the displacement of essential metal ions from their active sites, thereby resulting in the misfolding of proteins. As such, its uptake, followed by distribution and utilization, and finally, excretion from the body, needs to be tightly regulated (O’Halloran and Culotta 2000).
7.3 Selenium
Selenium (Se) is an essential trace element found in humans, animals, and some bacteria. In humans, its sources include meat, cereal grains, and fish. Average intake required for normal body functioning varies according to the age group: from 17 g/day (children) to 45 g/day (Adults). As selenoproteins , it contributes significantly to the maintenance of essential biological functions. It exists in two forms: organic, as selenomethionine (SeMet), selenocysteine (SeCys), and methylselenocysteine (MeSeCys); and inorganic, as selenite and selenate (Letavayova et al. 2006). It has been found to play an important role in at least 25 human selenoproteins by being part of the primary amino acid sequence as selenocysteine (SeCys) (Kryukov et al. 2003; Foster et al. 2006). Among the series of selenoproteins, thioredoxin reductase and glutathione peroxidase representing selenoenzyme play critical roles in the maintenance of cellular redox homoeostasis (Rayman 2000). By acting as an antimutagenic agent, it prevents malignant transformation of normal cells. As a part of glutathione peroxidases (GSH-Pxs) and thioredoxin reductase, it is primarily associated with protecting DNA and other cellular components from oxidative damage (Trueba et al. 2004). It increases the antioxidant capacity of cells by enhancing the activity of superoxide dismutase associated with the scavenging of superoxide radicals, increasing glutathione reductase activity and, as such, glutathione content as part of its protection against heavy metals. Having the ability to enhance the levels of glutathione and metallothioneins (MTs), its supplementation has been found to be associated with reversing the effect of different metals (Abdulla and Chmielnicka 1990; Sharma et al. 2014). Its interaction with heavy metals such as mercury counteracts their adverse consequences via the formation of insoluble complexes that prevent them from exerting toxic effects on the body (Whagner 1992; Suzuki 1997). Se administration was found to have a positive effect in reducing the Pb and As toxicities through increased production of selenoproteins, competition at key enzymes, and through the formation of inert Se–metal complexes (Kalia and Flora 2005). In addition to its antioxidant property, it plays an important role in thyroid hormone metabolism and redox reactions (Cano et al. 2007; Combs et al. 2009). Bronzetti et al. (2001) have reported that within certain limits, Se may have anticarcinogenic effects. However, at concentrations higher than those necessary for nutrition, it can have adverse effects by acting as a genotoxin and a carcinogen. Besides being toxic in itself at higher concentrations, it has been found to enhance the toxicity imposed by Pb (Kalia and Flora 2005). With greater chances to cause selenosis , higher intake to combat toxicity associated with metals such as mercury does not make it an excellent choice for therapy.
8 Conclusions
Heavy metals have been exhaustively studied by different workers, and they have presented different mechanisms of their toxic effects on cardiovascular system, kidney, neurons, and brain of animals and humans. In humans, the treatment of heavy metal poisoning involved application of chelating agents, though the side effects of chelating agents are the issues associated with it. On the other hand, some transition elements such as vanadium, manganese, iron, cobalt, copper, zinc, selenium, strontium, and molybdenum in small quantities are required for good human health. It has been observed that the deficiency of these essential metals may increase susceptibility to heavy metal poisoning. Selenium inhibits accumulation of mercury and increases excretion of arsenic and mercury. High concentration of folic acid in blood of pregnant woman helps in reducing the blood levels of mercury and cadmium. The uses of antioxidants such as vitamin C, garlic, alpha-lipoic acid, and glutathione help to reduce the adverse effects induced by Pb, Cd, and Cu. The roles of various plant-based principles in alleviating the heavy metals toxicity in humans are significant. However, still extensive research is needed to understand the targets of actions of heavy metals and to investigate the appropriate, cost-effective and safe therapeutics to overcome their toxic effects.
References
Abdulla M, Chmielnicka J (1990) New aspects on the distribution and metabolism of essential trace elements after dietary exposure to toxic metals. Biol Trace Elem Res 23:25–53
Agency for Toxic Substances and Disease Registry (ATSDR) (1999) Public Health Service. U.S. Department of Health and Human Services; Toxicological Profile for Lead, Atlanta
Agency for Toxic Substances and Disease Registry (ATSDR) (2000) Toxicological profile for arsenic TP-92/09. Center for Disease Control, Atlanta, Georgia
Agency for Toxic Substances and Disease Registry (ATSDR) (2008) Draft toxicological profile for cadmium, Atlanta, GA
Amin A, Hamza A, Daoud S (2006) Spirulina protects against cadmium-induced hepatotoxicity in rats. Am J Pharmacol Toxicol 1:21–25
Anderson D, Yu TW, Phillips BJ, Schemezer P (1994) The effect of various antioxidants and other modifying agents on oxygen-radical-generated DNA damage in human lymphocytes in the comet assay. Mutat Res 307:261–271
Andrews KW, Savitz DA, Hertz-Picciotto I (1994) Prenatal lead exposure in relation to gestational age and birth weight: a review of epidemiologic studies. Am J Ind Med 26:13–32
Argüelles VJ, González IA, Madrigal BE, Germán CC (2013) Amelioration of cadmium-produced teratogenicity and genotoxicity in mice given Arthrospira maxima (Spirulina) treatment. Evidence-Based Complementary and Alternative Medicine 2013: Article ID 604535. http://dx.doi.org/10.1155/2013/604535
Barrett JC, Lamb PW, Wang TC, Lee TC (1989) Mechanisms of arsenic-induced cell transformation. Biol Trace Elem Res 21:421–429
Baselt RC (2000) Disposition of toxic drugs and chemicals in man, 5th edn. Chemical Toxicology Institute, Foster City, CA
Baselt RC, Cravey RH (1995) Disposition of toxic drugs and chemicals in man, 4th edn. Year Book Medical Publishers, Chicago, IL, pp 105–107
Baynes JW (1991) Role of oxidative stress in development of complications in diabetes. Diabetes 40:405–412
Becker K, Kaus S, Krause C, Lepom P, Schulz C, Seiwert M et al (2002) German Environmental Survey 1998 (GerES III): environmental pollutants in blood of the German population. Int J Hyg Environ Health 205:297–308
Bhattacharyya MH, Wilson AK, Rajan SS, Jonah M (2000) Biochemical pathways in cadmium toxicity. In: Zalup RK, Koropatnick J (eds) Molecular biology and toxicology of metals. Taylor and Francis, London, pp 1–74
Borcea V, Nourooz-Zadeh J, Wolff SP (1999) α-Lipoic acid decreases oxidative stress even in diabetic patients with poor glycemic control and albuminuria. Free Radical Biol Med 26:1495–1500
Bradl H (2002) Heavy metals in the environment: origin, interaction and remediation, vol 6. Academic Press, London
Briner W (2014) The alchemists approach to metal poisoning: transforming the metal burden. Toxics 2014(2):64–376
Brochin R, Leone S, Phillips D (2008) The cellular effect of lead poisoning and its clinical picture. Georgetown Under Graduate J Health Sci 5:1–8
Brohi RD, Wang L, Talpur HS, Wu D, Khan FA, Bhattarai D, Rehman ZU, Farmanullah F, Huo LJ (2017) Toxicity of nanoparticles on the reproductive system in animal models: A review. Front Pharmacol 8:606. https://doi.org/10.3389/fphar.2017.00606
Bronzetti G, Cini M, Andreoli E, Caltavuturo L, Panunzio M, Croce CD (2001) Protective effects of vitamins and selenium compounds in yeast. Mutat Res 496:105–115
Cano P, Poliandri AHB, Jimenez V, Cardinali DP, Esquifino AI (2007) Cadmium induced changes in Per 1 and Per 2 gene expressions in rat hypothalamus and anterior pituitary: effect of melatonin. Toxicol Lett 172:131–136
Casalino E, Sblano C, Landriscina C (1997) Enzyme activity alteration by cadmium administration to rats: the possibility of iron involvement in lipid peroxidation. Arch Biochem Biophys 346:171–179
Centeno JA, Tchounwou PB, Patlolla AK, Mullick FG, Murakat L, Meza E, Gibb H, Longfellow D, Yedjou CG (2005) Environmental pathology and health effects of arsenic poisoning: a critical review. In: Naidu R, Smith E, Smith J, Bhattacharya P (eds) Managing arsenic in the environment: from soil to human health. CSIRO Publishing Corp., Adelaide, Australia
Centers for Disease Control and Prevention (CDC) (2001) Managing elevated blood lead levels among young children: recommendations from the Advisory Committee on Childhood Lead Poisoning Prevention, Atlanta
Chappell W, Beck B, Brown K, North D, Thornton I, Chaney R, Cothern R, Cothern CR, North DW, Irgolic K, Thornton I, Tsongas T (1997) Inorganic arsenic: a need and an opportunity to improve risk assessment. Environ Health Perspect 105:1060–1067
Chehregani A, Malayeri BE (2007) Removal of heavy metals by native accumulator plants. Int J Agric Biol 9:462–465
Combs GF, Midthune DN, Patterson KY, CanWeld WK, Hill AD, Levander OA, Taylor PR, Moler JE, Patterson BH (2009) Effects of selenomethionine supplementation on selenium status and thyroid hormone concentrations in healthy adults. Am J Clin Nutr 89:1808–1814
Coyle P, Philcox JC, Carey LC, Rofe AM (2002) Metallothionein: the multipurpose protein. Cell Mol Life Sci 59:627–647
Damek-Poprawa M, Sawicka-Kapusta K (2004) Histopathological changes in the liver, kidneys, and testes of bank voles environmentally exposed to heavy metal emissions from the steelworks and zinc smelter in Poland. Environ Res 96:72–78
DeFeo CJ, Aller SG, Unger VM (2007) A structural perspective on copper uptake in eukaryotes. Biometals 20:705–716
Fergusson JE (1990) The heavy elements: chemistry, environmental impact and health effects. Pergamon Press, Oxford
Filipic M, Fatur T, Vudrag M (2006) Molecular mechanisms of cadmium induced mutagenicity. Hum Exp Toxicol 25(2):67–77
Fitzgerald WF, Clarkson TW (1991) Mercury and monomethylmercury: present and future concerns. Environ Health Perspect 96:159–166
Flora SJS, Behari JR, Tandon SK (1982) Protective role of trace metals in lead intoxication. Toxicol Lett 13:51–56
Flora SJS, Kumar D, Gupta D (1999) Interaction of zinc, methionine or their combination with lead at gastrointestinal or post-absorptive levels in rats. Pharmacol Toxicol 68:3–7
Flora SJS, Singh S, Tandon SK (1989) Thiamine and zinc in prevention of lead intoxication. J Int Med Res 17:68–75
Flora SJS, Flora GJS, Saxena G (2006) Environmental occurrence, health effects and management of lead poisoning. In: Cascas SB, Sordo J (eds) Lead: chemistry, analytical aspects, environmental impacts and health effects. Elsevier Publication, Netherlands, pp 158–228
Flora SJS, Mittal M, Mehta A (2008) Heavy metal induced oxidative stress & its possible reversal by chelation therapy. Indian J Med Res 128(4):501–523
Foster CB, Aswath K, Chanock SJ, McKay HF, Peters U (2006) Polymorphism analysis of six selenoprotein genes: support for a selective sweep at the glutathione peroxidase 1 locus (3p21) in Asian populations. BMC Genet 7:56
Franciscato C, Silva LM, Duarte FA, Oliveira CS, Ineu RP, Flores EMM, Dressler VL, Piexoto NC, Pereira ME (2011) Delayed biochemical changes induced by mercury intoxication are prevented by zinc exposure. Ecotoxicol Environ Saf 74:480–486
Friberg L (1948) Proteinuria and kidney injury among workmen exposed to cadmium and nickel dust. J Ind Hyg Toxicol 30:32–36
Friberg L (1950) Health hazards in the manufacture of alkaline accumulators with special reference to chronic cadmium poisoning. Acta Medica Scandinavica 138(suppl 240):1–124
Fulekar M, Singh A, Bhaduri AM (2009) Genetic engineering strategies for enhancing phytoremediation of heavy metals. Afr J Biotech 8:529–535
George V, Kumar D, Suresh P, Kumar R (2015) In vitro protective potentials of Annona muricata leaf extracts against sodium arsenite-induced toxicity. Curr Drug Discov Technol 12:59–63
Giugliano D, Ceriello A, Paolisso G (1996) Oxidative stress and diabetic vascular complications. Diabetes Care 19:257–267
Goering PL, Fisher BR, Noren BT, Papaconstantinou A, Rojko JL, Marler RJ (2000) Mercury induces regional and cell-specific stress protein expression in rat kidney. Toxicol Sci 53:447–457
Goyer RA (1996) Toxic effects of metals. In: Klaassen CD (ed) Casarett & Doull’s toxicology: The basic science of poisons. McGraw-Hill, New York, pp 691–737
Goyer RA (2001) Toxic effects of metals. In: Klaassen CD (ed) Cassarett and Doull’s toxicology: the basic science of poisons. McGraw-Hill Publisher, New York, pp 811–867
Graeme KA, Pollack CV (1998) Heavy metal toxicity, part I: arsenic and mercury. J Emerg Med 16:45–56
Grandjean P, Budtz-Jørgensen E, White RF (1999) Methyl mercury exposure biomarkers as indicators of neurotoxicity in children aged 7 years. Am J Epidemiol 150:301–305
Grandjean P, Weihe P, White RF (1997) Cognitive deficit in 7-year-old children with prenatal exposure to methylmercury. Neurotoxicol Teratol 19:417–428
Guallar E, Sanz-Gallardo MI, Veer PVT (2002) Mercury, fish oils, and the risk of myocardial infarction. N Engl J Med 347:1747–1754
Gupta R, Flora S (2005) Therapeutic value of Hippophae rhamnoides L. against sub chronic arsenic toxicity in mice. J Med Food 8:353–361
Gupta R, Flora S (2006) Protective effects of fruit extracts of Hippophae rhamnoides L. against arsenic toxicity in Swiss albino mice. Hum Exp Toxicol 25:285–295
Gupta VK, Sharma B (2015) Environmental hazards due to xenobiotics contamination: growing risk to human health and possible remedies. In: Verma A (ed) Green social work: environmental protection, RPTU-Allahabad
Gupta VK, Singh S, Agrawal A, Siddiqi NJ, and Sharma B (2015) Phytochemicals mediated remediation of neurotoxicity induced by heavy metals. Biochemistry Research International 2015: Article ID 534769. http://dx.doi.org/10.1155/2015/534769
Guzzi G, LaPorta CAM (2008) Molecular mechanisms triggered by mercury. Toxicology 244:1–12
Halliwell B, Gutteridge JM (1990) Role of free radicals and catalytic metal ions in human disease: an overview. Methods Enzymol 186:1–85
Hartmann A, Peit G (1994) Comparative investigations of the genotoxic effects of metals in the single cell gel assay and the sister chromatid exchange test. Environ Mol Mutagen 23:299–305
Henson MC, Anderson MB (2000) The effects of cadmium on placental endocrine function. Recent Res Dev Endocrinol 1:37–47
Huang C, Costa M, Shi X (2004) Molecular mechanisms of arsenic carcinogenesis. Mol Cell Biochem 255:57–66
Hughes MF (2002) Arsenic toxicity and potential mechanisms of action. Toxicol Lett 133:1–16
Hultberg B, Anderson A, Isaksson A (2001) Interaction of metals and thiols in cell damage and glutathione distribution: potentiation of mercury toxicity by dithiothreitol. Toxicology 156:93–100
Inada C, Yamada K, Takane N, Nonaka K (1995) Poly (ADPribose) synthesis induced by nitric oxide in a mouse β-cell line. Life Sci 56:1467–1474
International Agency for Research on Cancer (IARC) (1993) Monographs cadmium. Lyon, France
Jamieson JA, Taylor CG, Weiler HA (2006) Marginal zinc deficiency exacerbates bone lead accumulation and high dietary zinc attenuates lead accumulation at the expense of bone density in growing rats. Toxicol Sci 92:286–294
Jomova K, Jenisova Z, Feszterova M (2011) Arsenic: toxicity, oxidative stress and human disease. J Appl Toxicol 31:95–107
Kalia K, Flora SJS (2005) Safe and effective therapeutic measures for chronic arsenic and lead poisoning. J Occup Health 47:1–21
Kim BE, Nevitt T, Thiele DJ (2008) Mechanisms for copper acquisition, distribution and regulation. Nat Chem Biol 4:176–185
Kim S, Dayani L, Rosenberg PA, Li J (2010) RIP1 kinase mediates arachidonic acid-induced oxidative death of oligodendrocyte precursors. Int J Physiol Pathophysiol Pharmacol 2(2):137–147
Kolb H, Kolb-Bachofen V (1992) Type I insulin dependent diabetes mellitus and nitric oxide. Diabetologia 35:796–797
Kryukov GV, Castellano S, Novoselov SV, Lobanov AV, Zehtab O, Guigo R, Gladyshev VN (2003) Characterization of mammalian selenoproteomes. Science 300:1439–1443
Lanphear BP, Dietrich K, Auinger P, Cox C (2000) Cognitive deficits associated with blood lead concentrations <10 μg/dl in US children and adolescents. Public Health Rep 115:521–529
Leonard SS, Harris GK, Shi X (2004) Metal-induced oxidative stress and signal transduction. Free Radical Biol Med 37(12):1921–1942
Letavayova L, Vlckova V, Brozmanova J (2006) Selenium: from cancer prevention to DNA damage. Toxicology 227:1–14
Lund B, Miller DM, Woods JS (1993) Studies on Hg(II)-induced H2O2 formation and oxidative stress in vivo and in vitro in rat kidney mitochondria. Biochem Pharmacol 45(10):2017–2024
Lyons TJ (1991) Oxidized low density lipoproteins: a role in the pathogenesis of atherosclerosis in diabetes? Diabet Med 8:411–419
Mahboob M, Shireen KF, Atkinson A, Khan AT (2001) Lipid peroxidation and antioxidant enzyme activity in different organs of mice exposed to low level of mercury. J Environ Sci and Health: Part B 36:687–697
Manke A, Wang L, Rojanasakul Y (2013) Mechanisms of nanoparticle-induced oxidative stress and toxicity. BioMed Research International 2013: Article ID 942916. http://dx.doi.org/10.1155/2013/942916
Mannino DM, Holguin F, Greves HM, Savage-Brown A, Stock AL, Jones RL (2004) Urinary cadmium levels predict lower lung function in current and former smokers: data from the Third National Health and Nutrition Examination Survey. Thorax 59:194–198
Maret W, Sandstead HH (2006) Zinc requirements and the risks and benefits of zinc supplementation. J Trace Elem Med Biol 20:3–18
Marnett LJ (2000) Oxyradicals and DNA damage. Carcinogenesis 21(3):361–370
Memon AR, Aktoprakligil D, Ozdemir A, Vertii A (2001) Heavy metal accumulation and detoxification mechanisms in plants. Turk J Bot 25:111–121
Miller WH, Schipper HM, Lee JS, Singer J, Waxman S (2002) Mechanisms of action of arsenic trioxide review. Can Res 62:3893–3903
Mocchegiani E, Muzzioli M (2000) Zinc, metallothioneins, immune response, survival and ageing. Biogerontology 1:133–143
Modaihsh A, Al-Swailem M, Mahjoub M (2004) Heavy metal contents of commercial inorganic fertilizer used in the Kingdom of Saudi Arabia. Agric Mar Sci 9:21–25
National Research Council Canada (NRCC) (1978) Effects of arsenic in the environment. National Research Council of Canada, pp 1–349
Nwokocha R, Nwokocha M, Aneto I (2012) Comparative analysis on the effect of Lycopersicon esculentum (tomato) in reducing cadmium, mercury and lead accumulation in liver. Food Chem Toxicol 50:2070–2073
O’Halloran TV, Culotta VC (2000) Metallochaperones: an intracellular shuttle service for metal ions. J Biol Chem 275:25057–25060
Ognjanovic B, Pavloic SZ, Maletic SD, Žikic RV, Štajn A, Radojicic RM, Saicic ZS, Petrovic VM (2003) Protective influence of vitamin E on antioxidant defense system in the blood of rats treated with cadmium. Physiol Res 52:563–570
Ogura H, Takeuchi T, Morimoto KA (1996) A comparison of the 8-hydroxyl-deoxyguanosine, chromosome aberrations and micronucleus techniques for the assessment of the genotoxicity of mercury compounds in human blood lymphocytes. Mutat Res 340:175–182
Ong CN, Phoon WO, Law HY, Tye CY, Lim HH (1985) Concentrations of lead in maternal blood, cord blood, and breast milk. Arch Dis Child 60:756–759
Pacyna JM (1996) Monitoring and assessment of metal contaminants in the air. In: Chang LW, Magos L, Suzuli T (eds) Toxicology of metals. CRC Press, Boca Raton, FL, pp 9–28
Paksy K, Rajczy K, Forgacs Z, Lazar P, Bernard A, Gati I, Kaali GS (1997) Effect of cadmium on morphology and steroidogenesis of cultured human ovarian granulosa cells. J Appl Toxicol 17:321–327
Papaconstantinou AD, Brown KM, Noren BT, McAlister T, Fisher BR, Goering PLV (2003) Mercury, cadmium, and arsenite enhance heat shock protein synthesis in chick embryos prior to embryo toxicity. Birth Defects Res. Part B, Dev Reprod Toxicol 68:456–464
Parthiban A, Vijayalingam S, Shanmugasundaram KR, Mohan R (1995) Oxidative stress and the development of diabetic complications antioxidants and lipid peroxidation in erythrocytes and cell membrane. Cell Biol Int 19:987–993
Pearson CA, Prozialeck WC (2001) E-Cadherin, beta-Catenin and cadmium carcinogenesis. Med Hypotheses 56:573–581
Porter AC, Fanger GR, Vaillancourt RR (1999) Signal transduction pathways regulated by arsenate and arsenite. Oncogene 18(54):7794–7802
Pulford I, Watson C (2003) Phytoremediation of heavy metal-contaminated land by trees-a review. Environ Int 29:529–540
Rayman MP (2000) The importance of selenium to human health. Lancet 356:233–241
Rhee HM, Choi BH (1989) Hemodynamic and electrophysiological effects of mercury in intact anesthetized rabbits and in isolated perfused hearts. Exp Mol Pathol 50:281–290
Rin K, Kawaguchi K, Yamanaka K, Tezuka M, Oku N, Okada S (1995) DNA-Strand breaks induced by dimethyl arsenic acid, a metabolite of inorganic arsenics, are strongly enhanced by superoxide anion radicals. Biol Pharm Bull 18:45–48
Rodrigues S, Henriques B, Reis A, Duarte A, Pereira E, Romkens PFAM (2012) Hg transfer from contaminated soils to plants and animals. Environ Chem Lett 10:61–67
Ronis MJJ, Bedger TM, Shema SJ (1998) Endocrine mechanism underlying the growth effects of developmental lead exposure in rat. J Toxicol Environ Health 54:101–120
Rubino FM (2015) Toxicity of glutathione-binding metals: a review of targets and mechanisms. Toxics 3:20–62
Sadeghi A, Bideskan A, Alipour F, Fazel A, Haghir H (2013) The effect of ascorbic acid and garlic administration on lead induced neural damage in rat offspring’s hippocampus. Iran J Basic Med Sci 16:157–164
Saleha-Banu B, Danadevi K, Jamil Kaiser, Ahuja YR, Visweswara Rao K, Ishap M (2001) In vivo genotoxic effect of arsenic trioxide in mice using comet assay. Toxicology 162:171–177
Salem HM, Eweida EA, Farag A (2000) Heavy metals in drinking water and their environmental impact on human health. ICEHM2000, Cairo University, Egypt, pp 542–556
Salnikow K, Zhitkovich A (2008) Genetic and epigenetic mechanisms in metal carcinogenesis and cocarcinogenesis: nickel, arsenic, and chromium. Chem Res Toxicol 21:28–44
Satarug S, Baker JR, Urbenjapol S, Haswell-Elkins M, Reilly PE, Williams DJ et al (2003) A global perspective on cadmium pollution and toxicity in non-occupationally exposed population. Toxicol Lett 137:65–83
Satoh M, Koyama H, Kaji T, Kito H, Tohyama C (2002) Perspectives on cadmium research. Tohoku J Exp Med 196:23–32
Shahsavani D, Baghshani H, Alishahi E (2011) Efficacy of allicin in decreasing lead (Pb) accumulation in selected tissues of lead-exposed common carp (Cyprinus carpio). Biol Trace Elem Res 142:572–580
Shallari S, Schwartz C, Hasko A, Morel JL (1998) Heavy metals in soils and plants of serpentine and industrial sites of Albania. Sci Total Environ 19209:133–142
Sharma B, Singh S, Siddiqi NJ (2014) Biomedical Implications of heavy metals induced imbalances in redox systems. BioMed Research International 2014: Article ID 640754. http://dx.doi.org/10.1155/2014/640754
Sharma RP, Street JC (1980) Public health aspects of toxic heavy metals in animal feeds. J Am Vet Med Assoc 177:149–153
Shi H, Shi X, Liu KJ (2004) Oxidative mechanism of arsenic toxicity and carcinogenesis. Mol Cell Biochem 255:67–78
Shim JY, Om AS (2008) Chlorella vulgaris has preventive effect on cadmium induced liver damage in rats. Mol Cell Toxicol 4:138–143
Shim JY, Shin HS, Han JG (2008) Protective effects of Chlorella vulgaris on liver toxicity in cadmium-administered rats. J Med Food 11:479–485
Simeonova P, Wang S, Toriuma W, Kommineni V, Matheson J, Unimye N, Kayama F, Harki D, Ding M, Vallyathan V, Luster M (2000) Arsenic mediates cell proliferation and gene expression in the bladder epithelium: association with activating protein-1 transactivation. Cancer Res 60:3445–3453
Singh N, Gupta VK, Kumar A, Sharma B (2017) Synergistic effects of heavy metals and pesticides in living systems. Front Chem. https://doi.org/10.3389/fchem.2017.00070
Singhal RL, Merali Z, Hrdina PD (1976) Aspects of the biochemical toxicology of cadmium. Fed Proc 35(1):75–80
Smith AH, Hopenhayn-Rich C, Bates MN (1992) Cancer risks from arsenic in drinking water. Environ Health Perspect 97:259–267
Strater E, Westbeld A, Klemm O (2010) Pollution in coastal fog at Alto Patache, Northern Chile. Environ Sci Pollut Res 17(9):1563–1573
Suzuki KT (1997) Equimolar Hg–Se complex binds to selenoprotein P. Biochem Biophys Res Commun 231:7–11
Tchounwou PB, Ayensu WK, Ninashvilli N, Sutton D (2003a) Environmental exposures to mercury and its toxicopathologic implications for public health. Environ Toxicol 18:149–175
Tchounwou PB, Patlolla AK, Centeno JA (2003b) Carcinogenic and systemic health effects associated with arsenic exposure—a critical review. Toxicol Pathol 31(6):575–588
Tchounwou PB, Wilson B, Ishaque A (1999) Important considerations in the development of public health advisories for arsenic and arsenic-containing compounds in drinking water. Rev Environ Health 14(4):211–229
Tchounwou PB, Wilson BA, Abdelgnani AA, Ishaque AB, Patlolla AK (2002) Differential cytotoxicity and gene expression in human liver carcinoma (HepG2) cells exposed to arsenic trioxide and monosodium acid methanearsonate (MSMA). Int J Mol Sci 3(11):1117–1132
Thangavel P, Subbhuraam C (2004) Phytoextraction: role of hyperaccumulators in metal contaminated soils. Proc Nat Acad Sci India, Section B: Biol Sci 70:109–130
Trueba GP, Sanchez GM, Giuliani A (2004) Oxygen free radical and antioxidant defense mechanism in cancer. Front Biosci 9:2029–2044
US Department of Health and Human services, Agency for Toxic substance and Disease Registry (ATSDR) (2007) CERCLA priority list of substances
Valko M, Izakovic M, Mazur M, Rhodes CJ, Tesler J (2004) Role of oxygen radicals in DNA damage and cancer incidence. Mol Cell Biochem 266:79–110
Valko M, Morris H, Cronin MTD (2005) Metals, toxicity and oxidative stress. Curr Med Chem 12:1161–1208
Valko M, Rhodes CJ, Monocol J, Izakovic-Mazur M (2006) Free radicals, metals and antioxidants in oxidative stress-induced cancer. Chem Biol Interact 160:1–40
VanDam PS, VanAsbeck BS, Erkelens DW, Marx JJM, Gispen W, Bravenboer B (1995) The role of oxidative stress in neuropathy and other diabetic complications. Diab Metab Rev 11:181–192
Varga B, Zsolnai B, Paksy K, Naray M, Ungvary GY (1993) Age dependent accumulation of cadmium in the human ovary. Reprod Toxicol 7:225–228
Vasak M, Hasler DW (2000) Metallothioneins: new functional and structural insights. Curr Opin Chem Biol 4:177–183
Vogt BL, Rossman TG (2001) Effects of arsenite on p53, p21 and cyclin D expression in normal human fibroblasts—a possible mechanism for arsenite’s comutagenicity. Mutat Res 478(1–2):159–168
Waalkes MP, Liu J, Diwan BA (2007) Transplacental arsenic carcinogenesis in mice. Toxicol Appl Pharmacol 222(3):271–280
Waalkes MP, Rehm S (1992) Carcinogenicity of oral cadmium in the male Wistar (WFNCr) rat: effect of chronic dietary zinc deficiency. Fundam Appl Toxicol 19:512–520
Waalkes MP, Berthan G (eds) (1995) Handbook on metal-ligand interactions of biological fluids, vol 2. Marcel Dekker, New York, pp 471–482
Waalkes MP, Liu J, Ward JM, Diwan BA (2004) Mechanisms underlying arsenic carcinogenesis: hypersensitivity of mice exposed to inorganic arsenic during gestation. Toxicology 198:31–38
Waalkes MP, Misra RR, Chang LW (eds) (1996) Toxicology of metals. CRC Press, Boca Raton, FL, pp 231–244
Wang Y, Fang J, Leonard SS, Rao KMK (2004) Cadmium inhibits the electron transfer chain and induces reactive oxygen species. Free Radical Biol Med 11:1434–1443
Whagner PD (1992) Selenium in the treatment of heavy metal poisoning and chemical carcinogenesis. J Trace Elem Electrolytes Health Dis 6:209–221
Williams PL, Sergeyev O, Lee MM, Korrick SA, Burns JS, Humblet O, DelPrato J, Revich B, Hauser R (2010) Blood lead levels and delayed onset of puberty in a longitudinal study of Russian boys. Pediatrics 125(5):1088–1096
Wilson DN (1988) Association cadmium. Cadmium—market trends and influences. In: Cadmium 87 Proceedings of the 6th international cadmium conference, London, pp 9–16
Wuana RA, Okieimen FE (2011) Heavy metals in contaminated soils: a review of sources, chemistry, risks and best available strategies for remediation. ISRN Ecology 2011: Article ID 402647. http://dx.doi.org/10.5402/2011/402647
Yamanaka K, Takabayashi F, Mizoi M, An Y, Hasegawa A, Okada S (2001) Oral exposure of dimethylarsinic acid, a main metabolite of inorganic arsenics, in mice leads to an increase in 8-oxo-2′-deoxyguanosine level, specifically in the targetorgans for arsenic carcinogenesis. Biochem Biophys Res Commun 287:66–70
Yedjou GC, Moore P, Tchounwou PB (2006) Dose and time dependent response of human leukemia (HL-60) cells to arsenic trioxide. Int J Environ Res Public Health 3(2):136–140
Yedjou GC, Tchounwou PB (2007) In vitro cytotoxic and genotoxic effects of arsenic trioxide on human leukemia cells using the MTT and alkaline single cell gel electrophoresis (comet) assays. Mol Cell Biochem 301:123–130
Yee S, Choi BH (1996) Oxidative stress in neurotoxic effects of methyl mercury poisoning. Neurotoxicology 17:17–26
Yoshizawa K, Rimm EB, Morris JS (2002) Mercury and the risk of coronary heart disease in men. N Engl J Med 347:1755–1760
Yun H, Kim I, Kwon SH, Kang JS, Om AS (2011) Protective effect of Chlorella vulgaris against lead-induced oxidative stress in rat brains. J Health Sci 57:245–254
Zadorozhnaja TD, Little RE, Miller RK, Mendel NA, Taylor RJ, Presley BJ, Gladen BC (2000) Concentrations of arsenic, cadmium, copper, lead, mercury, and zinc in human placentas from two cities in Ukraine. J Toxicol Environ Health 61:255–263
Zalups RK, Koropatnik DJ (2000) Molecular biology and toxicology of metals. Taylor & Francis, London
Zalups RK (2002) Molecular interactions with mercury in the kidney. Pharmacol Rev 52:113–143
Zhang P, Xu Y, Sun J, Li X, Wang L, Jin L (2009) Protection of pyrroloquinoline quinone against methyl mercury induced neurotoxicity via reducing oxidative stress. Free Radical Res 43:224–233
Acknowledgements
AK, NS, and VKG are grateful to University Grants Commission-New Delhi for providing financial support in the form of research fellowships. RP acknowledges UGC-New Delhi for providing financial support. The work is supported by DST-FIST and UGC-SAP programs in the department.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 Springer International Publishing AG
About this chapter
Cite this chapter
Kumar, A., Singh, N., Pandey, R., Gupta, V.K., Sharma, B. (2018). Biochemical and Molecular Targets of Heavy Metals and Their Actions. In: Rai, M., Ingle, A., Medici, S. (eds) Biomedical Applications of Metals. Springer, Cham. https://doi.org/10.1007/978-3-319-74814-6_14
Download citation
DOI: https://doi.org/10.1007/978-3-319-74814-6_14
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-74813-9
Online ISBN: 978-3-319-74814-6
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)