Abstract
Improvement in the understanding of bone disease biology has led to the development of bone-targeted agents (BTAs). The most widely used BTAs are bisphosphonates, which are inhibitors of osteoclastogenesis and osteoclast activation, and the new bone-targeted therapy, which is denosumab, an inhibitor of receptor activator of nuclear factor kappa-B ligand (RANKL).
Breast cancer and prostate cancer represent the most common cancers with a high incidence of bone metastasis in their disease clinical course and in which there are several trials investigating bone health in adjuvant setting. Furthermore, it has become clear that the bone homeostasis is fundamental for the optimal management of breast cancer and prostate cancer at any stages, to prevent skeletal fractures.
The routine clinical use of BTAs in adjuvant setting is still controversial, even though evidences showed that targeting bone-cell function can provide a potential additional approach to preventing systemic relapse as a component of standard adjuvant therapy.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
The normal homeostasis of the bone is a dynamic and complex process involving a balance between osteolysis mediated by osteoclasts and osteogenesis induced by osteoblasts. The bone represents the most common site of metastasis in neoplastic disease, including breast and prostate cancer. These tumours are among the most frequent malignancies in which bone metastases can have a strong clinical impact, affecting quality of life and overall survival [1].
Alterations in the bone homeostasis and metabolism, due to the presence of cancer cells, lead to a disruption of bone integrity, which can result in skeletal morbidity, identifying the so-called skeletal-related events (SREs): bone pain, pathological fractures, need for orthopaedic surgery to prevent or repair major structural damage, spinal cord compression and hypercalcaemia [2].
In addition to the effects of cancer cells in the bone, there are relevant effects on bone health induced by cancer treatments. The cancer treatment-induced bone loss (CTIBL) represents another bone condition caused by anti-tumoural agents, which is correlated to an increased bone turnover, and risk of skeletal fractures. All of these conditions are related to a considerable morbidity and negatively impact on patients’ quality of life, affecting also healthcare resources [3].
The introduction of bone-targeted therapies showed to improve the clinical outcomes of patients with bone metastases, with the aim to prevent skeletal complications and to relieve bone pain; moreover these agents can have a role in the early stages of the disease to preserve the bone health [1, 4].
In the adjuvant setting, the use of bone-targeted therapies has the primary purpose to inhibit bone loss and to prevent adverse effects of cancer treatments on bone health. Ovarian suppression with luteinizing hormone-releasing hormone (LHRH) agonists and the use of aromatase inhibitors (AIs) in early breast cancer patients, as well as androgen deprivation therapy in high-risk prostate cancer patients, are the principal adjuvant therapies that can affect bone health. Chemotherapy can also have a direct negative impact on bone health. Interestingly, some evidences suggest that treatment with bone-targeted therapies can also prevent bone metastasis and also reduce recurrences outside the bone [5, 6].
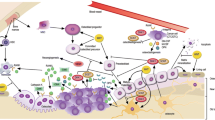
In these recent years, it has become clear that the bone microenvironment plays an important role in the bone homeostasis and metastasization process. The improvement in the understanding of bone biology has led to the identification of the crosstalk between primitive and metastatic cancer cells, cellular components of the bone marrow microenvironment and bone matrix that appears to be critical for the development and progression of bone metastases [7].
In the bone microenvironment, there are several factors with autocrine and paracrine actions that keep the balance between bone resorption and new bone formation, including transforming growth factors (TGFs), insulin-like growth factors (IGFs), platelet-derived growth factors (PDGFs), tumour necrosis factors (TNFs), interleukins (ILs), receptor activator of nuclear factor kappa-B ligand (RANK-L), RANK receptor and osteoprotegerin. These factors can also act as growth tumour factors that can cause the interactions between tumour cells and bone cells, identifying a vicious cycle in which tumour cells stimulate the bone cells to cause both bone destruction and bone formation. As a consequence, the bone microenvironment provides tumour cells with growth factors which cause tumour growth in bone.
In this scenario, the so-called pre-metastatic bone niche shows the unique characteristic to provide homing signals to cancer cells and to create a specific microenvironment for the colonization by the cancer cells [8,9,10].
Bone-targeted treatments, including bisphosphonates and denosumab, are indicated in the management of cancer patients in various settings throughout the course of the disease, including the adjuvant setting for the prevention of bone loss. They can interact with growth factors and cytokine signalling between tumour and bone cells, showing direct and indirect inhibitory effects on the vicious cycle [11].
Bisphosphonates are antiresorptive agents that inhibit specifically osteoclasts, blocking bone resorption and increasing of mineralization. They are characterized by a chemical structure of analogues of pyrophosphate, with carbon replacing the central oxygen, which promotes their binding to the mineralized bone matrix [12, 13]. There are two groups of bisphosphonates, non-nitrogen-containing and nitrogen-containing, which exhibit different effects on osteoclasts. The non-nitrogen-containing bisphosphonates are etidronate, clodronate and tiludronate, while the class of nitrogen-containing bisphosphonates, which are more potent osteoclast inhibitors, includes pamidronate, alendronate, ibandronate, risedronate and zoledronic acid.
The new bone-targeted therapy, represented by denosumab, is a fully human monoclonal antibody that specifically inhibits receptor activator of nuclear factor kappa-B ligand (RANKL) and has been demonstrated to inhibit bone destruction mediated by osteoclasts [14, 15]. RANK-L is a TNF member that is expressed by osteoblasts and is released by activated T cells. The activity of RANK-L is correlated by osteoprotegerin (OPG), another TNF family member that binds and subsequently prevents activation of its receptor, RANK. When RANK binds to RANK-L, there is an osteoclast formation, activation and survival, stimulating the bone resorption. Denosumab received the approval by the Food and Drug Administration (FDA) in November 2010 for the prevention of SREs in patients with bone metastases from solid tumours, including those from prostate cancer.
Preclinical models suggest a potential anti-tumoural activity of bone-targeted therapy, with direct and indirect effects. The direct anticancer activity consists in the inhibition of tumour cell growth, induction of tumour cell apoptosis and synergistic action with anti-tumoural treatments. The indirect anticancer effect includes the inhibition of tumour migration, invasion and metastasis, but also the inhibition of angiogenesis, the stimulation of immune surveillance and the suppression of growth factors produced by the bone [16,17,18,19].
Despite a lack of regulatory approval in most healthcare systems, the routine use of bisphosphonate as part of adjuvant therapy is considerably increasing. Current guidelines underline the importance of bone health to prevent skeletal fractures in patients with early-stage breast cancer in treatment with AIs or ovarian suppression and men with prostate cancer receiving ADT and suggest the use of bone-targeted therapy to improve clinical outcomes in these cancer populations [20,21,22,23,24].
2 The Role of Bone-Targeted Therapy on Early-Stage Breast Cancer
Breast cancer is the most frequently diagnosed cancer in women, and it is the second major cause of cancer-related death [25]. Breast cancer commonly spreads to the bone and may result in skeletal complications due to bone fragility caused by an alteration in the balance of bone homeostasis with an increase of osteoclastic activation and bone resorption. Bone-targeted therapies are routinely used in the setting of bone metastasis with the aim to prevent most of the skeletal complications.
However, the role of bone-targeted therapies in the early-stage breast cancer is less defined, even though attention to the bone status represents an important issue in this setting, because of the increased risk of fracture for the bone fragility caused by anti-tumoural treatments.
The impact of bone-targeted therapies in the setting of adjuvant breast cancer has been evaluated in several randomized clinical trials, and results depend on the type of hormonal therapy and the menopausal setting [2, 24].
Adjuvant endocrine therapy is routinely used in patients with hormone-responsive early breast cancer with the aim to prevent growth of residual tumour cells and to extend patient survival. Hormonal treatments, such as LHRH analogues with tamoxifen and AIs, may affect bone health, leading to bone metabolism changes, resulting in a rapid loss of bone mass in both premenopausal and postmenopausal women with breast cancer. These alterations in the bone structure, including osteoporosis and CTIBL, can increase the incidence of skeletal fractures [26].
Ovarian suppression in premenopausal women represents a major risk of bone loss in this population, due to the almost complete elimination of circulating oestrogens, which normally maintain bone mass with a direct action to the bone. The association with LHRH agonist, which affects the hypothalamic-pituitary-gonadal axis, causing amenorrhoea, and tamoxifen seems to be correlated to a lower impairment of bone health compared to the combination with AIs [27].
Tamoxifen, which is a selective oestrogen receptor modulator (SERM), represents the most commonly adjuvant endocrine therapy in premenopausal women with hormone receptor-positive breast cancer. This drug exhibits positive and negative effects on bone, depending on the menopausal state; in the premenopausal setting, tamoxifen can lead to a bone loss, especially in combination with LHRH agents, while in postmenopausal women, it seems to have a bone-protective effects [28,29,30,31].
Two randomized trials in postmenopausal breast cancer patients showed statistically significant increases in BMD in the groups receiving tamoxifen versus placebo. In a randomized double-blind placebo-controlled trial, including 140 postmenopausal women with negative lymph nodes breast cancer, it has been shown that tamoxifen resulted in a 0.61% increase in lumbar spine BMD compared with a 1% decrease in lumbar spine BMD for placebo-treated women (p < 0.001). Another study, evaluating postmenopausal patients with low-risk breast cancer, demonstrated an increase of about 2% in BMD in the group treated with tamoxifen compared with a 5% decrease in BMD in the group receiving placebo (p = 0.00074) [32, 33].
Treatment with AIs, such as letrozole, anastrozole or exemestane, has become a standard therapy for endocrine-responsive breast cancer in high-risk premenopausal and postmenopausal patients. These drugs prevent the conversion of androgens to oestrogen by the aromatase enzyme, reducing circulating hormonal levels. It was demonstrated that in several large trials, treatment with steroidal or nonsteroidal AIs is associated with significant bone loss that is more rapid than the one associated with menopause, with a significant increased incidence of fractures. Furthermore, the AIs treatment duration is correlated to the severity of the alteration of bone turnover [34].
The anastrozole tamoxifen alone or in combination (ATAC) study in postmenopausal women with early-stage breast cancer demonstrated the superiority of AIs over tamoxifen in terms of disease-free survival (hazard ratio [HR], 0.86; p = 0.03) and time to disease recurrence (HR, 0.83; p = 0.015). In this trial anastrozole therapy was associated with a higher incidence of fractures compared to tamoxifen alone (11% versus 7.7%) [35,36,37].
The Intergroup Exemestane Study (IES) investigated the role of exemestane in the adjuvant treatment of postmenopausal breast cancer. In this trial patients with breast cancer after 2–3 years of adjuvant tamoxifen were randomized to continue tamoxifen to 5 years or switch to exemestane until the completion of 5 years of adjuvant treatment, showing an improvement in terms of disease-free survival (DFS) and distant recurrence-free survival (RFS) in the exemestane arm. These results were confirmed also by subsequent analysis [38,39,40,41].
Moreover, results from some real-life trials suggested that the prevalence of bone fractures can be under-reported in the pivotal hormonal studies. In particular, the ABCSG-18 trial focusing on bone health, reported that the rates of bone fractures in the placebo group were higher than in previous reports from large trials of AIs [42,43,44].
Similarly, chemotherapy can have detrimental effects on the bone health by the primary ovarian dysfunction, resulting in low levels of circulating oestrogens. Moreover, chemotherapy can have both direct and indirect effects on the bone microenvironment, leading to the reduction of BMD [45].
2.1 BTAs and Prevention of Bone Loss
Many modern guidelines and recommendations suggest that patients with breast cancer in treatment with endocrine therapy should be monitored for bone loss and considered for anti-resorptive therapies [20,21,22,23,24].
The assessment of BMD, which is the most important parameter in the monitoring of bone status, is performed with routine dual-energy X-ray absorptiometry scan (DXA scan) and should be integrated with the evaluation of other risk factors, such as lack of vitamin D, and lifestyle factors, including smoking and alcohol intake, and laboratory assessment to exclude secondary causes of osteoporosis [46].
The role of bone-targeted therapies, including bisphosphonates, in the adjuvant breast cancer setting has been extensively studied in large clinical trials, with doses and schedules similar to those used in osteoporosis, showing to prevent bone loss.
An intravenous therapy with zoledronic acid every 6 months, monthly oral ibandronate and weekly oral risedronate has demonstrated to prevent bone loss in patients receiving AIs therapy for postmenopausal breast cancer [47, 50].
The Austrian Breast and Colorectal Cancer Study Group trial-12 (ABCSG-12) was designed to assess the clinical efficacy of goserelin-induced ovarian suppression plus tamoxifen or anastrozole with or without zoledronic acid in 1803 early breast cancer patients. A substudy was included in the study design with the aim to evaluate the long-term effects of endocrine therapy and the concomitant zoledronic acid every 6 months on BMD, which showed significant bone loss in patients who received endocrine therapy alone, and maintenance of BMD in patients who received endocrine therapy in combination with zoledronic acid [48].
The ARIBON trial analyzed the prevention of anastrozole-induced bone loss with monthly oral Ibandronate. This trial is a double-blind, randomized, placebo-controlled study evaluated the impact of bisphosphonate treatment on BMD in high-risk patients for osteoporosis during 5 years of anastrozole therapy. Results showed that oral ibandronate was able to prevent bone loss and reduce markers of bone turnover in patients with osteopenia and osteoporosis [49, 50].
In the study of anastrozole with the bisphosphonate risedronate (SABRE), breast cancer patients in treatment with anastrozole with a T score of between −1 and −2 were randomized to receive weekly risedronate or placebo. After 2 years, BMD increased by 2.2% at the lumbar spine and by 1.8% at the hip [51].
The new bone protection option, denosumab, for postmenopausal women with early breast cancer showed to be an effective intervention to prevent skeletal fractures.
In postmenopausal osteoporosis, denosumab 60 mg was approved for use by subcutaneous injection administered every 6 months based on the results of the FREEDOM study in which denosumab reduced significantly the risk of vertebral, nonvertebral and hip fractures by 68%, 20% and 40%, respectively, compared to placebo [52].
2.2 BTAs and Clinical Benefit
In addition to their effects on treatment-induced bone loss, breast cancer bone-targeted treatments in the adjuvant setting also provide the potential benefit to improve the clinical outcomes with fewer relapses of metastatic disease in bone and survival. The majority of adjuvant clinical studies with BTAs in the early stage of breast cancer are summarized in Table 3.1.
Evidences of clinical benefit with bisphosphonates therapy were initially reported with clodronate, which showed to reduce relapses and to improve overall survival (OS) and disease-free survival (DFS) in high-risk breast cancer patients in association with standard therapy [53, 54].
Other clinical trials confirmed these observations, demonstrating that zoledronic acid in combination with standard adjuvant therapy can improve the clinical outcomes. In particular, the ABCSG-12 trial, reported an improvement of DFS with a 29% of reduction for recurrences in the combination group treated with endocrine therapy and zoledronic acid [55].
In the AZURE trial, 3360 patients with stage II or III breast cancer, unselected for menopausal status or hormonal receptors status, were randomized to receive standard adjuvant systemic therapy with or without zoledronic acid every 3–4 weeks for 6 cycles, then every 3–6 months, for a total of 5 years. The authors demonstrated a 25% improvement for DFS in the predefined subgroup of patients who were postmenopausal for at least 5 years before study entry [56].
Results from the ABCSG-12 and AZURE trials suggested the initial hypothesis that adjuvant bisphosphonates may have a benefit only in women with low levels of reproductive hormones, as a result of menopause or ovarian suppression therapy.
The Zometa-Femara Adjuvant Synergy Trial (ZO-FAST Trial) enrolled 301 postmenopausal patients to receive letrozole with immediate zoledronic acid 4 mg every 6 months for 5 years or delayed zoledronate, showing a 34% relative risk reduction for recurrence and a better DFS in upfront zoledronic acid arm, compared with the delaying therapy arm. These results were confirmed at a longer follow up [57, 58].
The German Adjuvant Intergroup Node-Positive (GAIN) study investigated adjuvant ibandronate, and although no differences in DFS were reported, there was a positive trend with respect to DFS in postmenopausal patients [59].
Similar results have been shown in the National Surgical Adjuvant Breast and Bowel Project protocol B-34 (NSABP-34 Trial), in which there was a significant difference in the subgroup of patients older than 50 years of age, while the overall results did not show an outcome benefit for 3 years of oral clodronate [60].
Moreover, zoledronic acid is being investigated in the ongoing Italian multicentric HOBOE trial, which is evaluating the drug as adjuvant treatment in combination with letrozole for early breast cancer patients receiving adjuvant endocrine therapy.
Recently the meta-analysis of Early Breast Cancer Trials Collaborative Group (EBCTCG), based on individual patient data from 26 randomized trials, showed that among premenopausal women, treatment had no apparent effect on any outcome, but among 11,767 postmenopausal women, it produced highly significant reductions in recurrence (RR 0.86, 95% CI 0.78–0.94; 2p = 0.002), distant recurrence (0.82, 0.74–0.92; 2p = 0.0003), bone recurrence (0.72, 0.60–0.86; 2p = 0.0002) and breast cancer mortality (0.82, 0.73–0.93; 2p = 0.002). Even for bone recurrence, however, the heterogeneity of benefit was barely significant by menopausal status (2p = 0.06 for trend with menopausal status) or age (2p = 0.03), and it was non-significant by bisphosphonate class, treatment schedule, oestrogen receptor status, nodes, tumour grade or concomitant chemotherapy. No differences were seen in non-breast cancer mortality. Bone fractures were reduced (RR 0.85, 95% CI 0.75–0.97; 2p = 0.02) [61].
The Southwest Oncology Group (SWOG) trial confirmed the evidence that there are not differences in disease recurrence according to different dosing schedules and type of adjuvant bisphosphonates, including oral clodronate, oral ibandronate or intravenous zoledronic acid [62].
Denosumab is also investigated in the adjuvant breast cancer population to evaluate the benefit of the anti-RANKL agent in this setting, even though there are only few reported data on disease recurrence with the use of denosumab.
Results from the randomized, double-blind, placebo-controlled ABCSG-18 trial showed that in postmenopausal patients with hormone receptor-positive early-stage breast cancer who receive adjuvant aromatase inhibitor therapy, treatment with denosumab 60 mg twice per year significantly reduced the risk of clinical fractures and disease recurrence in postmenopausal women with breast cancer receiving aromatase inhibitors. Moreover, denosumab also increased the bone mineral density at the total lumbar spine, total hip and femoral neck and reduced the incidence of new and the worsening of pre-existing vertebral fractures [42].
Moreover, denosumab is being investigated in the ongoing D-CARE trial that is evaluating the drug as adjuvant treatment for high-risk early breast cancer patients receiving neoadjuvant or adjuvant therapy (NCT01077154). Results have not yet been published at the time of this writing.
Currently, there are no trials which directly compared a bisphosphonate with denosumab for the prevention of bone fracture prevention. For this reason the choice of the bone-targeted therapy should be made considering every clinical situation and reimbursement criteria for the drugs.
3 Bone-Targeted Therapy on High-Risk Prostate Cancer
Prostate cancer is the most common cancer in men in industrialized countries and the second cause of cancer-related death in this population [25]. Since the seminal work of Huggins in 1940, it is known that the pathogenesis of prostate cancer (PC) is primarily driven by androgens and biochemical castration obtained with androgen deprivation therapy is the cornerstone of treatment for patients with prostate cancer [63].
Androgen deprivation therapy (ADT), which consists in bilateral orchiectomy or a luteinizing hormone-releasing hormone (LHRH) agonist or antagonist, with or without an antiandrogen, represents the standard therapy for metastatic prostate cancer that can be used also in high-risk prostate cancer patients [64,65,66].
High-risk prostate cancer can occur in approximately 15% of all new diagnoses [67]. The definition of high risk can vary widely, and the most significant predictive factors of disease relapse in prostate cancer include clinical tumour stage, PSA level, Gleason score and nodal status [68, 69].
ADT achieves a benefit in terms of disease-free and overall survival in various clinical settings, including adjuvant treatment in locally advanced prostate cancer patients receiving radiation therapy [65].
However, since androgens are important for the preservation of bone mass, exerting anti-apoptotic effects on osteoblasts and pro-apoptotic effects on osteoclasts, the ADT leads testosterone to castration levels (≤50 ng/dL) and determines a significant reduction of BMD with a consequent increase in bone fractures risk [70].
More than 70% of men with prostate cancer are older than 65, and already at risk for osteoporosis or fragility fracture. A correlation between bone loss and increased susceptibility to metastasis was reported in prostate cancer patients, underlining the importance to preserve bone health in high-risk prostate cancer [71]. Non-metastatic prostate cancer patients in treatment with continuous or intermittent ADT can show a significant bone loss within the first 6–12 months after starting hormonal therapy [72].
Moreover, bone fragility fractures may be associated with decreased survival and quality of life in this cancer population, and with an increased mortality [73].
In a large study included more than 50,000 patients from the Surveillance, Epidemiology and End Results database, a higher number of patients receiving ADT had osteoporosis compared to the group not receiving hormonal therapy. The risk of bone fracture at 5 years in patients receiving ADT was almost double that in patients without hormone deprivation [74].
In this context, the use of bone-targeted treatments is crucial, even though no approved therapy is indicated for the reduction of the risk of fracture in prostate cancer patients. The benefit of bone-targeted therapies in prostate cancer depends on the hormone-sensitive or castration-resistant disease status.
3.1 Hormone-Sensitive Prostate Cancer
In a randomized placebo-controlled study, intravenous pamidronate 60 mg administered every 3 months showed to reduce bone loss over 48 weeks of treatment in men receiving leuprolide [75]. However, despite the benefit in preventing osteoporosis/CTIBL in men receiving ADT for PC, intravenous pamidronate therapy was not correlated to an improvement of BMD values [76].
Interestingly, treatment with zoledronic acid every 3–12 months is able to prevent bone loss associated with therapy and also to increase BMD compared with baseline values [77].
Two placebo-controlled studies, the PR04 and PR05 trials, evaluated oral clodronate in patients with non-metastatic and metastatic PC, respectively. Treatment with clodronate was associated with an OS benefit among men with metastatic disease compared with placebo (HR for death = 0.77, 95% CI = 0.60–0.98, p = 0.032). However, among men without metastatic disease, there was no evidence of an OS benefit with clodronate compared with placebo (HR for death = 1.12; 95% CI = 0.89–1.42, p = 0.94) [66]. These trials have reported 10-year survival rates in patients with prostate cancer with (n = 311 patients) or without metastatic disease (n = 508 patients) [78].
The multi-arm and multicentre trial conducted by the Medical Research Council called the Systemic Therapy in Advanced or Metastatic Prostate Cancer: Evaluation of Drug Efficacy (STAMPEDE) is a large trial with a multistage design. This trial evaluated several drugs in combination with hormonal therapy in patients with high-risk localized or metastatic prostate cancer with the aim to investigate whether the addition of treatments at the time of long-term hormone therapy initiation improves overall survival.
The different arms comparing several treatments include docetaxel, zoledronic acid, celecoxib, abiraterone, enzalutamide and radiotherapy (only among the patients with metastatic disease) in combination with ADT versus only ADT. Recently the results of the comparison between the addition of zoledronic acid, docetaxel, or their combination to the standard of care versus the standard of care alone have been published, showing that zoledronic acid was not correlated to survival improvement, failure-free survival and skeletal-related events, while docetaxel chemotherapy, given at starting of hormone therapy, determined a benefit in overall survival, as well as improvements in prostate-cancer-specific survival, failure-free survival and skeletal-related events. The combination of zoledronic acid and docetaxel was associated with similar improvements, with a smaller benefit. Authors concluded that zoledronic acid should not become part of the standard of care [79].
Similarly, an early treatment with zoledronic acid in metastatic setting hormone-sensitive prostate cancer showed a non-decreased risk for SREs compared with the same treatment initiated after progression to castration-resistant disease [80].
Moreover, the randomized open-label multinational Zometa European Study (ZEUS) showed that treatment with zoledronic acid every 3 months was ineffective for the prevention of bone metastases in high-risk non-metastatic patients at 4 years [81].
However, in prostate cancer the role of bisphosphonates in the prevention of bone metastasis remains undefined.
The efficacy of denosumab in patients in treatment with ADT was reported in the Denosumab HALT Prostate Cancer Study Group. In this trial, patients with non-metastatic PC receiving ADT were randomized to 60 mg of denosumab or placebo every 6 months. At 24 months, the treatment arm showed a statistically significant improvement in BMD at the total hip, the femoral neck, radium and the whole body [82].
3.2 Castration-Resistant Prostate Cancer (CRPC)
The majority of patients will become resistant to the initial hormonal approach with ADT, despite castrate levels of serum androgens, developing CRPC. A considerable number of patients with CRPC continue to respond to second generation hormonal treatments, suggesting the persistence of the activity of androgen receptor (AR) in the pathogenesis of prostate cancer, also during the progression of the disease.
The AR represents the principal driver of tumour growth and the most important therapeutic target in the prostate cancer [83].
There is a particular clinical condition characterized by a progressive CRPC with no evidence of bone metastases, in which higher baseline value of PSA and shorter PSA doubling time are correlated with time to the first bone metastasis and death.
The optimal management of M0 CRPC is challenging and may represent the most interesting clinical setting in which BATs can have an important impact for the prevention of bone metastases.
Among bone-targeted therapies, denosumab reported a benefit in delaying bone metastases in non-metastatic CRPC patients. Indeed, a randomized controlled trial was designed to evaluate the effects of zoledronic acid on the time to the first bone metastasis in non-metastatic CRPC patients. It was terminated before completion of accrual after interim analyses showing that the observed event occurred less frequently than expected [84].
A randomized, double-blind, placebo-controlled, phase 3 study evaluated the efficacy of denosumab in non-metastatic CRPC. Denosumab significantly increased bone metastasis-free survival by a median of 4.2 months over placebo (HR 0.85; p = 0.028) and delayed time to symptomatic first bone metastases, but had no impact on OS [85].
These results pointed out the importance of targeting the bone microenvironment to prevent bone metastasis in prostate cancer.
4 Safety Considerations
Overall, bone-targeted therapies are well tolerated, with a low incidence of adverse effects.
Adverse effects of bisphosphonates include flu-like symptoms such as fatigue, myalgia and fever, particularly with the first infusions (44%). Other adverse effects are hypocalcaemia (6%) and osteonecrosis of the jaw (ONJ) (1–2%) [86].
Zoledronic acid has been associated with renal impairment, and dose adjustments are necessary for patients with reduced renal function. In contrast to zoledronic acid, denosumab is not correlated to renal impairment, but denosumab is associated with a higher risk of hypocalcaemia [87].
During treatment with bone-targeted therapies, patients should receive an oral intake of calcium and vitamin D, and a condition of pre-existing hypocalcaemia must also be corrected before initiating therapy.
Bisphosphonates and denosumab treatments have been associated with the development of osteonecrosis of the jaw (ONJ).
The incidence of ONJ is about 1.3% when monthly intravenous somministration for bisphosphonates is used in the setting of advanced cancer, while it is less frequent with 6 monthly somministration of intravenous bisphosphonates or with oral bisphosphonates given in adjuvant setting to preserve bone health.
Nevertheless, before bisphosphonates and denosumab are initiated, it is recommended that patients undergo a dental examination, maintaining good oral hygiene and avoiding invasive dental surgical procedures while on treatment [88, 89].
Conclusion
Bone-targeted therapies, including bisphosphonates and denosumab, are important in the management of cancer patients even in adjuvant setting for the preservation of bone health. Over the past 30 years, a prolongation in survival was reported to be correlated to an improvement of diagnostic and therapeutic interventions, and the long-term effects of treatment on the skeleton have become a relevant concern and a rationale for the use of BTAs. In addition, these therapies showed to improve clinical outcomes in patients with postmenopausal breast cancer and men with prostate cancer. The survival benefit associated with the use of adjuvant BTAs provides an important additional strategy in the treatment of early stages in breast cancer and prostate cancer.
Based on these evidences, adjuvant bisphosphonates or denosumab should be part of the standard of care, in particular for early postmenopausal breast cancer and non-metastatic CRPC.
However, the absence of adequate biomarkers and a direct comparison in clinical trials can create difficulties in the selection of patients who may benefit from a specific bone-targeted therapy.
In this context, translational and clinical research is clearly needed.
References
Ibrahim T, Farolfi A, Mercatali L, Ricci M, Amadori D. Metastatic bone disease in the era of bone-targeted therapy: clinical impact. Tumori. 2013;99(1):1–9.
Coleman RE. Risks and benefits of bisphosphonates. Br J Cancer. 2008;98(11):1736–40.
D'Oronzo S, Stucci S, Tucci M, Silvestris F. Cancer treatment-induced bone loss (CTIBL): pathogenesis and clinical implications. Cancer Treat Rev. 2015;41(9):798–808.
Brown JE, Cook RJ, Major P, Lipton A, Saad F, Smith M, Lee K-A, Zheng M, Hei Y-J, Coleman RE. Bone turnover markers as predictors of skeletal complications in prostate cancer, lung cancer, and other solid tumors. J Natl Cancer Inst. 2005;97:59–69.
Gnant M, Mlineritsch B, Stoeger H, et al. Zoledronic acid combined with adjuvant endocrine therapy of tamoxifen versus anastrozol plus ovarian function suppression in premenopausal early breast cancer: final analysis of the Austrian Breast and Colorectal Cancer Study Group trial 12. Ann Oncol. 2015;26:313–20.
Gnant M. Role of bisphosphonates in postmenopausal women with breast cancer. Cancer Treat Rev. 2014;40:476–84.
Yoneda T, Hiraga T. Crosstalk between cancer cells and bone microenvironment in bone metastasis. Biochem Biophys Res Commun. 2005;328(3):679–87.
Ibrahim T, Flamini E, Mercatali L, Sacanna E, Serra P, Amadori D. Pathogenesis of osteoblastic bone metastases from prostate cancer. Cancer. 2010;116(6):1406–18. Erratum in: Cancer. 2010 May 15;116(10):2503
Lories RJ, Luyten FP. Osteoprotegerin and osteoprotegerin-ligand balance: a new paradigm in bone metabolism providing new therapeutic targets. Clin Rheumatol. 2001;20:3–9.
Hofbauer LC, Neubader A, Heufelder AE. Receptor activator of nuclear factor-kb ligand and osteoprotegerin. Cancer. 2001;92:460–70.
Shapiro CL. Bisphosphonates in breast cancer patients with skeletal metastases. Hematol Oncol Clin North Am. 1994;8:153–63.
Rogers MJ, Gordon S, Benford HL, et al. Cellular and molecular mechanisms of action of bisphosphonates. Cancer. 2000;88:2961–78.
Roelofs AJ, Thompson K, Gordon S, Rogers MJ. Molecular mechanisms of action of bisphosphonates: current status. Clin Cancer Res. 2006;12:6222s–30s.
Bekker PJ, Holloway DL, Rasmussen AS, et al. A single-dose placebo-controlled study of AMG 162, a fully human monoclonal antibody to RANKL, in postmenopausal women. J Bone Miner Res. 2004;19:1059–66.
Body JJ, Facon T, Coleman RE, et al. A study of the biological receptor activator of nuclear factor-kappa B ligand inhibitor, denosumab, in patients with multiple myeloma or bone metastases from breast cancer. Clin Cancer Res. 2006;12:1221–8.
Fromigue O, Lagneaux L, Body JJ. Bisphosphonates induce breast cancer cell death in vitro. J Bone Miner Res. 2000;15:2211–21.
Senaratne SG, Pirianov G, Mansi JL, Arnett TR, Colston KW. Bisphosphonates induce apoptosis in human breast cancer cell lines. Br J Cancer. 2000;82:1459–68.
Verdijk R, Franke HR, Wolbers F, Vermes I. Differential effects of bisphosphonates on breast cancer cell lines. Cancer Lett. 2007;246:308–12.
Mercatali L, Spadazzi C, Miserocchi G, Liverani C, De Vita A, Bongiovanni A, Recine F, Amadori D, Ibrahim T. The effect of everolimus in an in vitro model of triple negative breast cancer and osteoclasts. Int J Mol Sci. 2016;17(11):E1827.
Associazione Italiana di Oncologia Medica (AIOM) guidelines 2016.
Body JJ, Bergmann P, Boonen S, et al. Management of cancer treatment-induced bone loss in early breast and prostate cancer—a consensus paper of the Belgian Bone Club. Osteoporos Int. 2007;18:1439–50.
Reid DM, Doughty J, Eastell R, et al. Guidance for the management of breast cancer treatment-induced bone loss: a consensus position statement from a UK Expert Group. Cancer Treat Rev. 2008;34(Suppl 1):S3–S18.
Lee CE, Leslie WD, Czaykowski P, et al. A comprehensive bone-health management approach for men with prostate cancer receiving androgen deprivation therapy. Curr Oncol. 2011;18:e163–72.
Coleman R, Body JJ, Aapro M, Hadji P, J. Herrstedt on behalf of the ESMO Guidelines Working Group Bone health in Cancer Patients. ESMO clinical practice guidelines. Ann Oncol. 2014;25(Supplement 3):iii124–37.
Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66(1):7–30.
Brufsky A. Cancer treatment-induced bone loss: pathophysiology and clinical perspectives. Oncologist. 2008;13:187–95.
Gnant M, et al. Adjuvant endocrine therapy plus zoledronic acid in premenopausal women with early-stage breast cancer: 5-year follow-up of the ABCSG-12 bone-mineral density substudy. Lancet Oncol. 2008;9:840–9.
Hirbe A, Morgan EA, Uluçkan Ö, Weilbaecher K. Skeletal complications of breast cancer therapies. Clin Cancer Res. 2006;12(20 Pt 2):6309s–14s.
Cuzick J, Forbes J, Edwards R, et al. First results from the International Breast Cancer Intervention Study (IBIS-I): a randomised prevention trial. Lancet. 2002;360:817–24.
Cosman F. Selective estrogen-receptor modulators. Clin Geriatr Med. 2003;19:371–9.
Powles TJ, Hickish T, Kanis JA, Tidy A, Ashley S. Effect of tamoxifen on bone mineral density measured by dual-energy x-ray absorptiometry in healthy premenopausal and postmenopausal women. J Clin Oncol. 1996;14:78–84.
Love RR, Mazess RB, Barden HS, et al. Effects of tamoxifen on bone mineral density in postmenopausal women with breast cancer. N Engl J Med. 1992;326:852–6.
Kristensen B, Ejlertsen B, Dalgaard P, et al. Tamoxifen and bone metabolism in postmenopausal low-risk breast cancer patients: a randomized study. J Clin Oncol. 1994;12:992–7.
Eastell R, Adams JE, Coleman RE, et al. Effect of anastrozole on bone mineral density: 5-year results from the anastrozole, tamoxifen, alone or in combination trial 18233230. J Clin Oncol. 2008;26:1051–7.
Baum M, Buzdar A, Cuzick J, et al. Anastrozole alone or in combination with tamoxifen versus tamoxifen alone for adjuvant treatment of postmenopausal women with early-stage breast cancer: results of the ATAC (Arimidex, Tamoxifen Alone or in Combination) trial efficacy and safety update analyses. Cancer. 2003;98:1802–10.
Eastell R, Hannon RA, Cuzick J, et al. Effect of an aromatase inhibitor on BMD and bone turnover markers: 2-year results of the Anastrozole, Tamoxifen, Alone or in Combination (ATAC) trial (18233230). J Bone Miner Res. 2006;21:1215–23.
Eastell R, Adams J. Results of the ‘Arimidex’ (anastrozole, A), Tamoxifen (T), Alone or in Combination (C) (ATAC) trial: Effects on bone mineral density (BMD) and bone turnover (ATAC Trialists’ Group). In: Presented at the 27th Congress of the European Society for Medical Oncology, Nice, 711, 18–22 Oct 2002.
Coleman RE, Body J-J, Gralow JR, Lipton A. Bone loss in patients with breast cancer receiving aromatase inhibitors and associated treatment strategies. Cancer Treat Rev. 2008;34(Suppl 1):S31–42.
Coombes RC, Hall E, Gibson L, et al. A randomized trial of exemestane after two to three years of tamoxifen therapy in postmenopausal women with primary breast cancer. N Engl J Med. 2004;350:1081–92.
Coombes RC, Hall E, Snowdon CF, Bliss JM. The Intergroup Exemestane Study: a randomized trial in postmenopausal patients with early breast cancer who remain disease-free after two to three years of tamoxifen-updated survival analysis. Breast Cancer Res Treat. 2004;88(suppl 1):S7.
Coombes RC, Kilburn LS, Snowdon CF, et al. Survival and safety of exemestane versus tamoxifen after 2–3 years’ tamoxifen treatment (Intergroup Exemestane Study): a randomised controlled trial. Lancet. 2007;369:559–70.
Gnant M, Pfeiler G, Dubsky PC, et al. Adjuvant denosumab in breast cancer (ABCSG-18): a multicentre, randomised, double-blind, placebo-controlled trial. Lancet. 2015;386:433–43.
Soiland H, Hagen KB, Gjerde J, Lende TH, Lien EA. Breaking away: high fracture rates may merit a new trial of adjuvant endocrine therapy in Scandinavian breast cancer patients. Acta Oncol. 2013;52:861–2.
Early Breast Cancer Trialists Collaborative Group (EBCTCG). Aromatase inhibitors versus tamoxifen in early breast cancer: patient-level meta-analysis of the randomised trials. Lancet. 2015;386:1341–52.
Lester J, Dodwell D, McCloskey E, Coleman R. The causes and treatment of bone loss associated with carcinoma of the breast. Cancer Treat Rev. 2005;31:115–42.
Wilson C, Coleman R. Adjuvant bone-targeted therapies for postmenopausal breast cancer. JAMA Oncol. 2016;2(4):423–4.
Cheung AM, Tile L, Cardew S, et al. Bone density and structure in healthy postmenopausal women treated with exemestane for the primary prevention of breast cancer: a nested substudy of the MAP.3 randomised controlled trial. Lancet Oncol. 2012;13:275–84.
Gnant MF, Mlineritsch B, Luschin-Ebengreuth G, Grampp S, Kaessmann H, Schmid M, Menzel C, Piswanger-Soelkner JC, Galid A, Mittlboeck M, Hausmaninger H, Jakesz R. Zoledronic acid prevents cancer treatment-induced bone loss in premenopausal women receiving adjuvant endocrine therapy for hormone-responsive breast cancer: a report from the Austrian Breast and Colorectal Cancer Study Group. J Clin Oncol. 2007;25:820–8.
Lester JE, Dodwell D, Purohit O, Gutcher SA, Ellis SP, Thorpe R. Prevention of anastrozole-induced bone loss with oral monthly ibandronate during aromatase inhibitor therapy for breast cancer. Clin Cancer Res. 2008;14:6336–42.
Lester JE, Dodwell D, et al. Prevention of anastrozole induced bone loss with monthly oral ibandronate: final 5 year results from the ARIBON trial. J Bone Oncol. 2012;1(2):57–62.
Van Poznak C, Hannon RA, Clack G, Campone M, Mackey JR, Apffelstaedt J, Eastell R. The SABRE (Study of Anastrozole with the Bisphosphonate RisedronatE) study: 12 month analysis. Breast Cancer Res Treat. 2007;106(suppl 1):S37.
Cummings SR, San Martin J, McClung MR, et al. Denosumab for prevention of fractures in postmenopausal women with osteoporosis. N Engl J Med. 2009;361:756–65.
Diel IJ, Jaschke A, Solomayer EF, et al. Adjuvant oral clodronate improves the overall survival of primary breast cancer patients with micrometastases to the bone marrow-a long-term follow-up. Ann Oncol. 2008;19:2007–11.
Powles TJ, Paterson A, McCloskey E, et al. Reduction in bone relapse and improved survival with oral clodronate for adjuvant treatment of operable breast cancer. Breast Cancer Res Treat. 2006;8:R13.
Gnant M, Mlineritsch B, Schippinger W, et al. Endocrine therapy plus zoledronic acid in premenopausal breast cancer. N Engl J Med. 2009;360:679–91.
Coleman RE, Marshall H, Cameron D, AZURE Investigators, et al. Breast-cancer adjuvant therapy with zoledronic acid. N Engl J Med. 2011;365(15):1396–405.
Brufsky AM, Bosserman LD, Caradonna RR, et al. Zoledronic acid effectively prevents aromatase inhibitor-associated bone loss in postmenopausal women with early breast cancer receiving adjuvant letrozole: Z-FAST study 36-month follow-up results. Clin Breast Cancer. 2009;9:77–85.
Coleman R, de Boer R, Eidtmann H, et al. Zoledronic acid (zoledronate) for postmenopausal women with early breast cancer receiving adjuvant letrozole (ZO-fast study): final 60-month results. Ann Oncol. 2013;24:398–405.
von Minckwitz G, et al. German adjuvant intergroup node-positive study: a phase III trial to compare oral ibandronate versus observation in patients with high-risk early breast cancer. J Clin Oncol. 2013;31:3531–9.
Paterson AH, Anderson SJ, Lembersky BC, Fehrenbacher L, Falkson CI, King KM, Weir LM, Brufsky AM, Dakhil S, Lad T, Baez-Diaz L, Gralow JR, Robidoux A, Perez EA, Zheng P, Geyer CE Jr, Swain SM, Costantino JP, Mamounas EP, Wolmark N. Oral clodronate for adjuvant treatment of operable breast cancer (National Surgical Adjuvant Breast and Bowel Project protocol B-34): a multicentre, placebo-controlled, randomised trial. Lancet Oncol. 2012;13(7):734–42.
Early Breast Cancer Trialists’ Collaborative Group (EBCTCG). Adjuvant bisphosphonate treatment in early breast cancer: meta-analyses of individual patient data from randomised trials. Lancet. 2015;386:1353–61.
Gralow JBW, Paterson AHG, Lew D, et al. Phase III trial of bisphosphonates as adjuvant therapy in primary breast cancer: SWOG/Alliance/ECOG-ACRIN/NCIC Clinical Trials Group/NRG oncology study S0307 [abstract 558]. J Clin Oncol. 2014;32(5(suppl)).
Huggins C, Hodges CV. Studies on prostatic cancer. I. The effect of castration, of estrogen and of androgen injection on serum phosphatases in metastatic carcinoma of the prostate. Cancer Res. 1941;1:293–7.
Heidenreich A, Pfister D, Ohlmann CH, Engelmann UH. Androgen deprivation for advanced prostate cancer. Urol A. 2008;47(3):270–83.
Denis LJ, Keuppens F, Smith PH, EORTC Genito-Urinary Tract Cancer Cooperative Group and the EORTC Data Center, et al. Maximal androgen blockade: final analysis of EORTC phase III trial 30853. Eur Urol. 1998;33:144–51.
Sharifi N, Gulley JL, Dahut WL. Androgen deprivation therapy for prostate cancer. JAMA. 2005;294(2):238–44.
Bastiana PJ, Boorjian SA, Bossi A, et al. High-risk prostate cancer: from definition to contemporary management. Eur Urol. 2012;61(6):1096.
D’Amico AV, Cote K, Loffredo M, et al. Determinants of prostate cancer specific survival after radiation therapy for patients with clinically localized prostate cancer. J Clin Oncol. 2002;20:4567–73.
Kattan MW, Eastham JA, Stapleton AM, Wheeler TM, Scardino PT. A preoperative nomogram for disease recurrence following radical prostatectomy for prostate cancer. J Natl Cancer Inst. 1998;90:766–71.
Manolagas SC. Birth and death of bone cells: basic regulatory mechanisms and implications for the pathogenesis and treatment of osteoporosis. Endocr Rev. 2000;21(2):115–37.
Higano CS. Bone loss and the evolving role of bisphosphonate therapy in prostate cancer. Urol Oncol. 2003;21(5):392–8.
Galvão DA, Spry NA, Taaffe DR, et al. Changes in muscle, fat and bone mass after 36 weeks of maximal androgen blockade for prostate cancer. BJU Int. 2008;102:44–7.
Center JR, Nguyen TV, Schneider D, Sambrook PN, Eisman JA. Mortality after all major types of osteoporotic fracture in men and women: an observational study. Lancet. 1999;353(9156):878–82.
Shahinian VB, Kuo YF, Freeman JL, Goodwin JS. Risk of fracture after androgen deprivation for prostate cancer. N Engl J Med. 2005;352(2):154–64.
Smith MR, McGovern FJ, Zietman AL, Fallon MA, Hayden DL, Schoenfeld DA, Kantoff PW, Finkelstein JS. Pamidronate to prevent bone loss during androgen-deprivation therapy for prostate cancer. N Engl J Med. 2001;345(13):948–55.
Lipton A, Small E, Saad F, et al. The new bisphosphonate, zometa (zoledronic acid), decreases skeletal complications in both osteolytic and osteoblastic lesions: a comparison to pamidronate. Cancer Investig. 2002;20(suppl 2):45–54.
Smith MR, Eastham J, Gleason DM, Shasha D, Tchekmedyian S, Zinner N. Randomized controlled trial of zoledronic acid to prevent bone loss in men receiving androgen deprivation therapy for nonmetastatic prostate cancer. J Urol. 2003;169(6):2008–12.
Dearnaley DP, Mason MD, Parmar MK, Sanders K, Sydes MR. Adjuvant therapy with oral sodium clodronate in locally advanced and metastatic prostate cancer: long-term overall survival results from the MRC PR04 and PR05 randomised controlled trials. Lancet Oncol. 2009;10(9):872–6.
James ND, Sydes MR, Clarke NW, Mason MD, Dearnaley DP, et al. Addition of docetaxel, zoledronic acid, or both to first-line long-term hormone therapy in prostate cancer (STAMPEDE): survival results from an adaptive, multiarm, multistage, platform randomised controlled trial. Lancet. 2016;387:1163–77.
Smith MR, Halabi S, Ryan CJ, et al. Randomized controlled trial of early zoledronic acid in men with castration-sensitive prostate cancer and bone metastases: results of CALGB 90202 (alliance). J Clin Oncol. 2014;32:1143–50.
Wirth M, Tammela T, Cicalese V, Gomez Veiga F, Delaere K, Miller K, Tubaro A, Schulze M, Debruyne F, Huland H, Patel A, Lecouvet F, Caris C, Witjes W. Prevention of bone metastases in patients with high-risk nonmetastatic prostate cancer treated with zoledronic acid: efficacy and safety results of the Zometa European Study (ZEUS). Eur Urol. 2015;67(3):482–91.
Smith MR, Egerdie B, Hernández Toriz N, Feldman R, Tammela TL, Saad F, Heracek J, Szwedowski M, Ke C, Kupic A, Leder BZ, Goessl C, Denosumab HALT Prostate Cancer Study Group. Denosumab in men receiving androgen-deprivation therapy for prostate cancer. N Engl J Med. 2009;361(8):745–55.
Chen Y, Sawyers CL, Scher HI. Targeting the androgen receptor pathway in prostate cancer. Curr Opin Pharmacol. 2008;8(4):440–8.
Smith MR, Kabbinavar F, Saad F, Hussain A, Gittelman MC, Bilhartz DL, Wynne C, Murray R, Zinner NR, Schulman C, Linnartz R, Zheng M, Goessl C, Hei YJ, Small EJ, Cook R, Higano CS. Natural history of rising serum prostate-specific antigen in men with castrate nonmetastatic prostate cancer. J Clin Oncol. 2005;23(13):2918–25.
Smith MR, Saad F, Coleman R, et al. Denosumab and bone-metastasis-free survival in men with castration-resistant prostate cancer: results of a phase 3, randomised, placebo-controlled trial. Lancet. 2012;379:39–46.
Ibrahim T, Barbanti F, Giorgio-Marrano G, et al. Osteonecrosis of the jaw in patients with bone metastases treated with bisphosphonates: a retrospective study. Oncologist. 2008;13(3):330–6.
European Medicines Agency. Xgeva (denosumab) summary of product characteristics. 2014. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_Product_Information/human/002173/WC500110381.pdf.
Saad F, Brown JE, Van Poznak C, et al. Incidence, risk factors, and outcomes of osteonecrosis of the jaw: integrated analysis from three blinded active-controlled phase III trials in cancer patients with bone metastases. Ann Oncol. 2012;23:1341–7.
Migliorati CA, Epstein JB, Abt E, Berenson JR. Osteonecrosis of the jaw and bisphosphonates in cancer: a narrative review. Nat Rev Endocrinol. 2011;7:34–42.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2019 Springer International Publishing AG, part of Springer Nature
About this chapter
Cite this chapter
Ibrahim, T., Recine, F. (2019). Bone-Targeted Therapies in Adjuvant Setting. In: Denaro, V., Di Martino, A., Piccioli, A. (eds) Management of Bone Metastases. Springer, Cham. https://doi.org/10.1007/978-3-319-73485-9_3
Download citation
DOI: https://doi.org/10.1007/978-3-319-73485-9_3
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-73484-2
Online ISBN: 978-3-319-73485-9
eBook Packages: MedicineMedicine (R0)