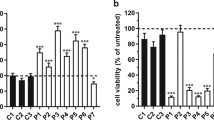
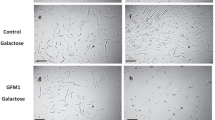
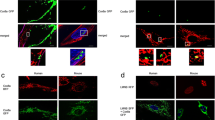
Abstract
Despite the fact that majority of studies done using different compounds with antioxidant properties showing pivotal effect on oxidative phosphorylation or glycolytic ATP production, it is still difficult to discuss efficient therapeutic solutions for patients affected by mitochondrial diseases or mitochondrial dysfunction-associated disorders. Since most of the mitochondrial disorders are manifested in tissues or organs that demand high-energy, many experimental studies have described that the pivotal effect of the tested compounds comes from the use of the skin fibroblasts from patients. In this chapter, we have gathered information about these studies and describe the effect of such treatment on mitochondrial function and the attenuation of oxidative stress in patients’ fibroblasts.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Mitochondria perform central functions in cells, such as buffering the cytosolic calcium concentration, regulating apoptosis through the mitochondrial permeability transition pore (mPTP) (Bonora et al. 2015; Ferrari et al. 2017; Morciano et al. 2015; Bonora et al. 2017), generating reactive oxygen species (ROS) (Morató et al. 2013) and many others. However, their most important role is the production of ATP (Patergnani et al. 2014). Human fibroblasts (e.g., from a skin biopsy) are a valuable and reliable source of biological material for the study of a wide range of diseases (with one limitation—mitochondrial defects must be expressed in fibroblasts), especially those caused by DNA mutations and in instances where it is not always possible to obtain fresh samples for research, such as neurodegenerative disorders (Hirashima et al. 1996). Moreover, the collection of skin biopsies is a much less invasive procedure compared to muscle or liver biopsies. Patients’ skin fibroblasts can be used directly or can be used to generate transmitochondrial cybrids or create induced pluripotent stem cells (iPSCs) (Saada 2014). Based on the available literature, fibroblasts obtained from patients suffering from mitochondrial disorders seem to be a good model not only to confirm diagnosis or find the cause of the metabolic defect but also to prove the effectiveness of potential therapies. Different classes of compounds and experimental approaches have been used to improve the mitochondrial function or to decrease mitochondrial dysfunction-related oxidative stress in patients’ fibroblasts; we describe some of them in this chapter.
2 Therapeutic Approaches
Supplementation of patients’ fibroblasts with compounds that show antioxidant properties should result in the attenuation of mitochondrial respiratory chain dysfunction caused by the intracellular oxidative stress. Very often, oxidative stress is accompanied by mitochondrial dysfunction and the oxidative phosphorylation (OXPHOS) pathology, which usually manifests as an increased level of ROS, as well as the presence of oxidatively damaged proteins, lipid peroxides and DNA (Giorgi C et al. 2010a). Several compounds have been tested in patients’ fibroblasts to investigate their positive or negative impact on mitochondrial metabolism and the ROS level. Among them are vitamins, cofactors and classical antioxidants that can enhance the cellular antioxidant capacity to remove ROS as well as improve mitochondrial function. In this group of compounds , we can find vitamin A, B vitamins, including thiamine (B1), riboflavin (B2) and nicotinamide (B3), riboflavin, folic acid and many others. Thanks to the targeting module, a special class of artificial antioxidants can be specifically targeted to the mitochondria. However, it is necessary to mention that mitochondrially-targeted antioxidants, such as ubiquinone (MitoQ) and tocopherol (MitoE), are not useful in all cases of mitochondrial dysfunction. This limitation comes from the fact that these compounds accumulate in the mitochondria due to the high mitochondrial membrane potential. However, in cases of many mitochondrial disorders, decreased mitochondrial membrane potential has been observed. This makes it impossible for the mitochondria to accumulate mitochondria-targeted antioxidants, as is observed in “healthy” fibroblasts (Smith and Murphy 2010). In cases of energy production perturbations , another approach to ameliorate mitochondrial metabolism can be the induction of mitochondrial biogenesis. In animal models of X-linked adrenoleukodystrophy , pioglitazone , a PPAR agonist, has been found to increase mitochondrial mass, decrease DNA oxidative damage, decrease the level of carbonylated proteins and improve bioenergetics parameters (Morató et al. 2013). Similarly, another PPAR agonist, bezafibrate, could improve mitochondrial parameters in the fibroblasts of patients with a complex I deficiency (caused by mutations in the NDUFS2 gene), however, there was no effect on the fibroblasts of patients with a complex I deficiency, due to mutated complex I assembly factor C20ORF7 (Golubitzky et al. 2011). In the same studies, Golubitzky et al. (2011) has shown that 5-aminoimidazole-4-carboxamide ribonucleotide (AICAR) , which is an activator of adenosine monophosphate kinase (AMPK) , significantly decreased oxidative stress and increased mitochondrial biogenesis and ATP production in fibroblasts from patients with mutations in the genes encoding NDUFS2 complex I subunit, NDUFA12L and NDUFAF4 complex I assembly factors. Interestingly, such treatment was ineffective in patients’ fibroblasts that harbored a mutation in the gene encoding the NDUFS4 subunit of complex I (Golubitzky et al. 2011). Another strategy to abrogate mitochondrial defects is pharmacologically induced metabolic reprograming. The involvement of AMPK, Sirt1 and Sirt3 activation in the metabolic adaptation of human cells harboring mitochondrial DNA mutations induced by resveratrol supplementation has been reviewed by Wu et al. (2014). Moreover, resveratrol can ameliorate the aging process in human primary keratinocytes by preventing dysfunctions in proliferation and decreasing senescence (dependent on AMPK, SIRT1 and FOXO3) (Ido et al. 2015). In addition, this polyphenolic flavonoid improved the phenotypical condition given by Graves’ Orbitopathy disease in primary cultured orbital fibroblasts of affected patients, highlighting its potential use in a wide range of disorders (Kim et al. 2015). The positive effect of resveratrol on the elevated oxidative stress of patients’ fibroblast is mostly mediated by modulation of antioxidant enzyme levels , including the superoxide dismutases, thioredoxin, glutathione peroxidase-1, heme oxygenase-1 and catalase. It has also been shown that resveratrol treatment was responsible for the increased oxygen consumption and decreased lactate production in moderately OXPHOS-deficient fibroblasts. Moreover, resveratrol has a positive effect on the mitochondrial respiratory capacities in parkin-mutated fibroblasts, which is possibly due to the up-regulation of key regulatory enzymes involved in cellular and mitochondrial metabolism (Ferretta et al. 2014). On the other hand, resveratrol was shown to have harmful effects on patients’ fibroblasts (De Paepe et al. 2014; Golubitzky et al. 2011; Lopes Costa et al. 2014). So, the positive or negative effect of resveratrol treatment depends on the type of OXPHOS defect. In general, as mentioned above, the compounds act on multiple sites and modulate mitochondrial metabolism, as well as influence the status of intracellular oxidative stress. A recent review by Koopman et al. provides an overview of the small molecules that are currently being developed for treatment of mitochondrial disease (Koopman et al. 2016). Another experimental approach that can be used to improve cellular metabolism in patients’ fibroblasts relies on the influence of proteins involved in calcium homeostasis. This issue will be discussed below.
2.1 Patients’ Fibroblasts: Trials for Rescue of Metabolic Defects by Modulation of Calcium Homeostasis
The endoplasmic reticulum (ER) serves as the first calcium store in the cell. Calcium (Ca2+) release occurs through inositol 1,4,5-trisphosphate receptor (IP3R) channels in the cytosol and thus reaches the mitochondria and other organelles (Sbano et al. 2017). Calcium is essential for cellular bioenergetics regulation (Kaufman and Malhotra 2014), autophagy (Cárdenas and Foskett 2012; Decuypere et al. 2013), ROS production (Singh et al. 2005) and cell death (Danese et al. 2017; Giorgi et al. 2015a, 2015b, 2010; Marchi et al. 2017). Given its crucial involvement in all these physiological contexts, the modulation of calcium homeostasis with pharmacological (or genetic) approaches could be useful to amend the onset of a pathological state. Autophagy is an important response to energetic defects, as well as the lysosomal-dependent elimination of damaged organelles (Marchi et al. 2017). In addition, mitochondrial Ca2+ signaling is closely related to the fine regulation of this process (Patergnani et al. 2013; Pinton et al. 2004); thus, pharmacological (and genetic) calcium modulation could be used to regulate autophagy levels in those pathologies where cell bioenergetics properties are impaired. In a study by Granatiero et al., an important increase in the autophagic flux has been observed in fibroblasts carrying the mA13514G mutation of the MT ND5 gene encoding ND5 subunit of the mitochondrial Complex I (Granatiero et al. 2016). Due to a decrease in ER-mitochondria contact sites and defects in the mitochondrial calcium uniporter (MCU) complex, a perturbation of calcium homeostasis translated into reduced mitochondrial calcium uptake in m.A13514G cells and led to atypical MELAS and Leigh syndromes with a late onset and slow progression in patients carrying this mutation (Granatiero et al. 2016). A faster mitochondrial turnover and accelerated autophagy were associated with a milder syndrome phenotype . The authors have shown how the use of MCU activators, such as kaempferol (a plant-derived antioxidant flavonoid) (Vay et al. 2007) and SB202190 (Düzgün et al. 2017), enhanced mitochondrial Ca2+ uptake and slowed down the autophagic flux in 13514A4G fibroblasts, giving a phenotype completely comparable to that of control cell lines. These MCU activators restored a normal bioenergetics condition but their prolonged treatment with these compounds decreased cell viability. Indeed, lowering mitochondrial Ca2+ could be a compensatory and pro-survival mechanism that allows for a less severe neurodegenerative syndrome . In complex I-deficient fibroblasts from patients carrying a homozygous missense mutation (G364A) in the nuclear NDUFS7 gene, agonist-induced mitochondrial Ca2+ handling and the ensuing stimulation of mitochondrial ATP production are impaired (Visch et al. 2004). Alterations in ATP production were completely restored upon acute treatment with the CGP37157 compound (7-chloro-5-(2-chlorophenyl)-1,5-dihydro-4,1-benzothiazepin-2(3H)-one—a mitochondrial Na+-Ca2+ exchanger inhibitor), which restored the bradykinin-induced mitochondrial Ca2+ uptake. This relation can be explained simply by the fact that “repeated” agonist-induced mitochondrial Ca2+ uptake leads to an increase in mitochondrial ATP production (Jouaville et al. 1999) and CGP37157 by restoring the bradykinin-induced increase in mitochondrial calcium concentration. This was able to restore the bioenergetics state of the cell. These findings demonstrated that, although the OXPHOS machinery is composed of a defective complex I, modulation of calcium homeostasis can improve mitochondrial ATP synthesis.
Pathological conditions that could benefit from calcium modulation are countless, although, in many cases, no pharmacological treatments have been conducted. An example derives from the triad of Autism Spectrum Disorder (ASD) —Fragile X Syndrome (FXS) —Tourette Syndrome (TS) , where a perturbation in IP3-mediated Ca2+ signaling has been reported. Indeed, skin fibroblasts from patients with FXS and TS have significantly decreased Ca2+ response compared with control cell lines . This impaired signal is not due to decreased ER calcium content or a reduced expression of IP3R proteins, but to fewer sites of Ca2+ release and a general dysfunction of the IP3R channel gating (Schmunk et al. 2015, 2017).
Recently, it has been demonstrated that calcium imbalance, ER stress, unfolded protein response (UPR) and oxidative stress are consequences of skin exposure to UV radiations (Farrukh et al. 2014). Indeed, although mammalians own protective systems to overwhelm this damage starting from plasma membrane to the lipids, subcellular organelles and DNA , repeated exposures to UV lower the defenses of the human body. Additional help, such as ROS detoxification and the restoration of calcium homeostasis, may be required. It was shown that glycyrrhizic acid (GA) treatment significantly protects against Ca2+ perturbation by lowering ER stress and apoptosis in UV-B treated human skin fibroblasts (Farrukh et al. 2015).
In summary, the findings presented above have remarkable translational relevance that supports the involvement and targeting of Ca2+ signaling (and oxidative stress) in cells directly derived from patients . These studies provide important information about the use of fibroblasts from biopsy samples as a functional diagnostic tool and surrogate pharmacological trial.
3 Possible Therapeutic Approaches Carried Out in Fibroblasts Derived from Patients with Different Mitochondrial and Metabolic Abnormalities, as well as Other Disorders Characterized by Oxidative Stress
Fibroblasts derived from patients suffering from mitochondrial disorders have been used repeatedly to investigate the effect of potential pharmacological compounds designed to improve the affected cellular bioenergetics as well as to decrease oxidative stress in these cells. Below, we present examples of therapeutic trials performed with the use of patients’ fibroblasts with different abnormalities in the OXPHOS machinery.
3.1 NARP Patients’ Fibroblasts
NARP (Neuropathy, Ataxia and Retinitis Pigmentosa ) and MILS (Maternally Inherited Leigh’s Syndrome ) are mitochondrial disorders associated with mutations in the MTATP6 gene encoding subunit a of the mitochondrial ATP synthase. Typical m.T8993G mutations, which are responsible for NARP/MILS, are related to the transversion of thymine to guanine at mtDNA nucleotide 8993, which causes the conversion of a highly conserved leucine to arginine. The clinical phenotype associated with the m.T8993G mutation depends on the heteroplasmy level (White et al. 1999). The NARP phenotype is considered when the mutation load is between 70 and 90%. When it is higher, it may be responsible for fatal infantile encephalopathy MILS. Generally, mutation in the ATPase 6 gene , which encodes a subunit that is a part of the F1F0-ATPase c-ring, results in alterations to the mitochondrial ATP production process. It has been found that mitochondrial ATP synthesis can be reduced by 50–70% in cells harboring 100% m.T8993G mutation load (Vazquez-Memije et al. 1996). Detailed characterization of NARP fibroblasts made by Lebiedzinska et al. showed an increased mitochondrial membrane potential, decreased activity of the mitochondrial respiratory chain, reduced NADH/NAD ratio, alterations of mitochondrial calcium homeostasis and an increased level of mitochondrial superoxide and oxidatively damaged proteins (Lebiedzinska et al. 2013). They found that inhibition of p66Shc (an alternatively spliced isoform of the growth factor adaptor that belongs to the ShcA family) phosphorylation at Ser36 by hispidin (inhibitor of PKCβ) results in decreased mitochondrial superoxide anion production, which acts downstream of p66Shc activation and reduces the vicious cycle of ROS production in the studied NARP fibroblasts. Interestingly, in NARP fibroblasts , hispidin treatment increased the level of carbonylated proteins (Lebiedzinska et al. 2013). The other work presented by Mattiazzi et al. showed that mitochondrial dysfunction caused by m.T8993G mutation can be partially reverted by antioxidant treatment. To improve the oxygen consumption and ATP production in primary fibroblasts obtained from a patient harboring a 97% m.T8993G mutation load, 2.5 mM NAC was used (Mattiazzi et al. 2004). The studies of Wojewoda et al. showed that selenite, an inorganic form of selenium, increased the level of antioxidant enzymes, which can explain the decreased level of ROS in NARP cybrids (Wojewoda et al. 2011). Additionally, they found that selenite treated cells had a higher level of mitochondrial respiratory chain subunits, which resulted in higher intracellular ATP levels. An interesting study has been performed by Sgarbi et al. discussing whether α-ketoglutarate and aspartate treatment can have a positive impact on the viability and ATP level of NARP/MILS patients’ fibroblasts carrying 2 distinct point mutations, m.T8993G (with severe impact) and m.T8993C (with only mild impact on OXPHOS) (Sgarbi et al. 2009). Interestingly, the protective effect of α-ketoglutarate/aspartate was observed only in fibroblasts harboring the m.T8993G mutation. The treatment had absolutely no effect on the viability of cells with mildly impaired ATP synthase (m.T8993C mutation). Nevertheless, the authors believe that α-ketoglutarate/aspartate dietary supplementation can be considered a potential pharmacological therapeutic approach (Sgarbi et al. 2009).
3.2 LHON Patients’ Fibroblasts
Leber Hereditary Optic Neuropathy (LHON ) is a primary mtDNA disorder that initially causes a painless and acute unilateral loss of central vision among young adults and later manifests as total bilateral vision loss and blindness. Most of the mtDNA mutations responsible for LHON affect the mitochondrial complex I subunits. Practically, three point mutations, m.G3406A (in the MT ND1 gene), m.G11778A (in the MTND4 gene) and m.T14484C (in the MT ND6 gene), are responsible for approximately 90% of all LHON cases. Interestingly, among them, the mutation in the gene encoding the ND4 subunit gene is the most prevalent (60–80%) cause of LHON. As a synthetic analog of CoQ10 in mitochondria, idebenone can act as an electron carrier in the respiratory chain, and it is considered a compound with antioxidant properties. Interestingly, in contrast to CoQ10 , idebenone participates in redox reactions outside the mitochondrial compartment (Haefeli et al. 2011). Idebenone in the cytoplasm is reduced by the NAD(P)H quinone oxidoreductase 1 (NQO1) and can be re-oxidized by complex III, which in turn enables bypass of the affected complex I (Haefeli et al. 2011). Additionally, in contrast to CoQ10, idebenone seems to stimulate complex II activity (Gueven et al. 2016). A positive effect of idebenone on fibroblasts derived from LHON patients was observed by Angebault et al. already several years ago (Angebault et al. 2011). They observed that lower activity of complex I in fibroblasts derived from LHON patients after incubation with 10 μM idebenone was increased by approximately 42%. However, idebenone treatment had variable effects on oxygen consumption , indicating that there were not equal benefits from the idebenone treatment (Angebault et al. 2011). Recently, Yu-Wai-Man et al. also evaluated the therapeutic potential of idebenone and other quinone analogues in LHON patients’ fibroblasts (Yu-Wai-Man et al. 2017). They found that idebenone treatment partially compensated for the deleterious effect of the m.G11778A mutation. Moreover, idebenone increased ATP production and reduced oxidative stress; however, this effect was observed in only a subgroup of studied patients’ fibroblasts. Other quinone analogues tested by this group, like CoQ1, CoQ10 and decylubiquinone , showed variable effects on oxygen consumption and ROS level (Yu-Wai-Man et al. 2017).
3.3 Leigh Syndrome Patients’ Fibroblasts
Leigh syndrome (Leigh disease ) (OMIM 256000) is an inherited, mitochondrial, neurodegenerative disorder mostly manifested in the central nervous system and is known as subacute necrotic encephalo(mio)pathy. First, symptoms of Leigh syndrome very often apparent in infancy; however, in some cases early symptoms can begin in the teenage or adult years. Leigh syndrome can be caused by mutations in more than 75 different genes (in mitochondrial and nuclear DNA) encoding proteins involved mostly in oxidative phosphorylation. For this reason, we can identify two groups of Leigh syndrome: (a) mitochondrial DNA-associated Leigh syndrome (approximately 20% of cases) caused by mutations in at least 11 mitochondrially-encoded genes, with an estimated incidence of 1 in 100,000 to 1 in 140,000 births, and (b) nuclear DNA-associated Leigh syndrome (approximately 80% of all cases), with an estimated incidence of 1 in 30,000 to 1 in 40,000 people at birth (Lake et al. 2015). The most common causes of Leigh syndrome are mutations in the complex I-encoding genes (mutations in at least 25 genes have been identified). Leigh syndrome is associated also with a defect in complex IV (approximately 15% of cases), with the most frequent mutations in genes encoding SURF1 and SCO2 proteins. The other frequent mutation causing Leigh syndrome affects the MTATP6 gene-encoding subunit a of mitochondrial ATP synthase (10% of Leigh cases). Leigh syndrome can also be caused by mutations in genes encoding subunits of pyruvate dehydrogenase complex or in genes encoding proteins involved in CoQ10 biosynthesis.
CoQ10 , a natural lipid-soluble quinone analogue (known also as ubiquinone ), is a component of the mitochondrial respiratory chain involved in electron transport. CoQ10 supplementation partially restores the activities of the mitochondrial respiratory chain enzymes in MELAS fibroblasts and MERRF cybrids (Cotán et al. 2011; De la Mata et al. 2012). Hirano et al. showed that coenzyme Q10 supplementation had a positive effect in fibroblasts with CoQ10 deficiency (Hirano et al. 2012). Interestingly, treatment of fibroblasts from patients with CoQ10 deficiency with coenzyme Q2, a shorter chain analogue of CoQ10, has no effect on mitochondrial parameters (López et al. 2010). Several studies demonstrated that treatment with coenzyme Q and its analogs can be beneficial in Leigh syndrome, as well as in Leigh-like syndrome (Haas 2007; Rahman 2015).
Recently, Kanabus et al. (2016) reported that decanoic acid supplementation of fibroblasts derived from patients with Leigh syndrome associated with nuclear-encoded defects of complex I increases mitochondrial biogenesis (via PPAR-γ receptor) in approximately 50% of studied fibroblast lines . Moreover, decanoic acid increases cellular resistance to oxidative stress by increasing catalase expression (Kanabus et al. 2016).
Treatment of fibroblasts derived from patients with decreased levels of mitochondrial complex I with Trolox, a water-soluble vitamin E derivate, causes a significant decrease in the ROS level (Koopman et al. 2008) and increases complex I level. Koopman et al. speculates that the level of active complex I in the mitochondria is under regulatory control of the cell’s oxidative balance (Koopman et al. 2008). Therefore, the antioxidant Trolox can mitigate complex I deficiency . Importantly, the authors claim that such treatment is beneficial to only patients with predominant expression of complex I, rather than an intrinsic catalytic defect in this respiratory chain complex. Moreover, Distelmaier et al. showed that Trolox supplementation also has a positive effect on mitochondrial membrane potential and calcium-stimulated ATP production in complex I-deficient human fibroblasts (Distelmaier et al. 2009).
It has been demonstrated that riboflavin (precursor of flavin mononucleotide—FMN) also had a positive effect on fibroblasts derived from patients with a complex I defect related to mutation in the NDUFS2 gene and genes encoding assembly factors (ACAD9 and AIF), as well as in MELAS patients’ fibroblasts (Garrido-Maraver et al. 2012; Gerards et al. 2011; Saada 2011). Vitamins such as vitamin K and vitamin C also have positive effects on patients’ fibroblasts. Vitamin K (menadione) and vitamin C (ascorbate) are potential electron donors for complexes II and IV, respectively. For this reason, these compounds have positive effects on fibroblasts with an affected complex I and fibroblasts with a CoQ deficiency (Saada 2011). Additionally, ascorbate was found to decrease superoxide levels and reduce manifestation of oxidative stress in QoQ2-deficient fibroblasts (López et al. 2010). In turn, resveratrol , a polyphenol of natural origin present in the skin of fruits, such as grapes, blueberries, raspberries and mulberries, can decrease oxidative stress in complex I-deficient patients’ cell lines, as well as restore oxygen consumption in these cells (Lopes Costa et al. 2014; Mathieu et al. 2016). On the other hand, resveratrol can inhibit mitochondrial ATP synthase, which makes possible therapy risky for individuals harboring defects in the ATP synthesis process (Gledhill et al. 2007).
Recent studies by Ehinger et al. using fibroblasts from patients with Leigh syndrome (with a recessive mutation in NDUFS2 gene) showed that cell membrane permeable succinate prodrugs (diacetoxymethyl succinate; bis-(1-acetoxy-ethyl) succinate and 1-acetoxyethyl acetoxymethyl succinate) access the intracellular space and release succinate, which enables transport of electrons from complex II and ATP production by bypassing the deficiency of complex I (Ehinger et al. 2016).
The positive effect of bezafibrate on mitochondrial parameters has been observed in fibroblasts of patients with complex IV deficiency caused by mutation in the SCO2 gene. Casarin et al. found that copper (100 μM CuCl2) and 200 μM bezafibrate had no effect on SCO2 fibroblasts when supplemented separately (Casarin et al. 2012). However, when used together, they caused a complete rescue of COX activity in SCO2 cells. Ten years earlier, the same group showed that 100 μM CuCl2 alone can fully restore activity of cytochrome c oxidase not only in fibroblasts but also in myotubes and mioblasts from patients with SCO2 gene mutation (Salviati et al. 2002).
Menzies et al. studied the effect of a thyroid hormone (3,3′,5-triiodothyronine; T3) on mitochondrial parameters and the status of oxidative stress in two patients with Leigh’s syndrome (one harboring a m.G13513A mutation in the MTND5 gene and the second with a m.T9185C mutation in the MTATP6 gene ) (Menzies et al. 2009). They observed that ROS production in T3-treated patients’ fibroblasts was decreased by 40%, accompanied by a 1.3-fold increase in complex IV activity and a 1.6-fold increase in the ATP level; moreover, the level of MnSOD was restored to control levels. The positive effect of T3 treatment was not related to the transcriptional coactivator peroxisome proliferator-activated receptor gamma coactivator 1-alpha (PGC1α) or mitochondrial transcription factor A (TFAM). This is because the level of these proteins was not changed by T3, and the mitochondrial mass was the same before and after T3 treatment of the studied Leigh’s syndrome patients ’ fibroblasts (Menzies et al. 2009).
3.4 Fibroblasts from Patients with Combined Deficiency of Complexes I, III, IV and V with a Normal Complex II Level
The combined deficiency of complexes I, III, IV and V with a normal level of complex II in patients can be caused by (a) deletions in mtDNA and point mutations in mt tRNA-encoding genes (Kemp et al. 2011); (b) mtDNA depletion related to autosomal recessive mutations in nuclear genes involved in mtDNA replication and maintenance (Spinazzola et al. 2009) and (c) mutations in nuclear-encoded components of the mitochondrial translation machinery (Smits et al. 2010). Soiferman et al. presented that ascorbate can reduce the ROS level/production in fibroblasts of patients with defects in elongation factors (EFTs) (Soiferman et al. 2014). Moreover, he found that after ascorbate administration, the activity of complex IV was increased, which was accompanied by a higher level of ATP (Soiferman et al. 2014). In the same work, he showed that another compound with antioxidant properties, N-acetyl cysteine (NAC) , which is a precursor of cysteine and glutathione, decreases the ROS level in these fibroblasts as well as in fibroblasts from patients with defects in mitochondrial t-RNA uridylation (TRMU) (Soiferman et al. 2014). Interesting studies have been performed by Wang et al. with the use of Kearns-Sayre patients’ fibroblasts (Wang et al. 1996). Kearns-Sayre syndrome , a commonly diagnosed mitochondrial cytopathy, is caused by mitochondrial DNA deletion (removal of a 4977-base pair segment of the mtDNA encoding mitochondrial respiratory chain subunits). They found that in Kearns-Sayre syndrome fibroblasts, azidothymidine and dideoxynucleosides cause a depletion of wild-type mtDNA, while increasing the number of copies of mtDNA with deletions (Wang et al. 1996).
3.5 Fibroblasts from Patients with Complex II Deficiency
Complex II deficiency is an autosomal recessive mitochondrial disorder with a highly variable phenotype that can be caused by mutations in the SDHA, SDHB, SDHC, SDHD, or SDHAF1 and SDHAF2 genes encoded in the nuclear DNA. Symptoms of mitochondrial complex II deficiency can vary from severe to life-threatening symptoms in infancy to muscle disease beginning in adulthood. It has been described that two inheriting mutations in the SDHA gene are associated with myoclonic seizures and Leigh’s syndrome. The studies performed by De Paepe et al. showed that resveratrol supplementation had no effect on complex II deficient fibroblasts (derived from patients with complex II activity close to the method detection limit; one patient with an unknown pathogenic mutation and a second one harboring a homozygous c.G622T mutation in the NFU1 gene-encoding protein involved in the formation of iron-sulfur (Fe-S) clusters) (De Paepe et al. 2014). In fibroblasts from these patients, complex II activity after resveratrol treatment was still negligible (De Paepe et al. 2014). In contrast, fibroblasts from patients harboring the homozygous c.G1663A mutation in the SDHA gene and characterized by a higher basal complex II activity (comparing to the above described two other patients with complex II deficiency) when treated with resveratrol showed a significant increase in complex II activity. This indicates that the pivotal effect of resveratrol supplementation depends on the residual complex II activity (De Paepe et al. 2014).
3.6 MERRF Patients’ Fibroblasts
Myoclonic epilepsy with ragged red fibers (MERRF) syndrome is a maternally-inherited mitochondrial encephalomyopathy. In the case of MERRF syndrome, the most common symptoms are myoclonus epilepsy, generalized seizures, ataxia and myopathy. With MERRF, four different point mutations are associated; however, the most common one (found in 80–90% of MERRF patients) is the m.A8344G mutation in the tRNALys gene of mitochondrial DNA. This mutation is associated with severe defects in mitochondrial protein synthesis, which affects the mitochondrial respiratory chain and ATP synthesis. Skin fibroblasts from patients with MERRF syndrome are characterized by significantly increased ROS production and an increased level of matrix metalloproteinases (MMPs) , which can be considered as a progressive marker of neurodegenerative diseases (Wu et al. 2010). The increased level and activity of MMPs may contribute to the cytoskeleton remodeling involved in the weakness and atrophy of muscles commonly seen in MERRF patients (Wu et al. 2010). Interestingly, no significant changes in the antioxidant defense system have been observed. Only the level and activity of SOD2 was increased in MERRF patients’ fibroblasts. Increased oxidative stress can be responsible for the oxidative damage of the voltage-dependent anion channel (VDAC) and aconitase in the MERRF fibroblasts (Wu et al. 2010). Fascinating studies with the use of MERRF patients’ fibroblasts have been performed by Chang et al. (2013). They demonstrated that, using the cell-penetrating peptide (Pep-1), they could deliver functional mitochondria isolated from “healthy” fibroblasts into the MERRF fibroblasts (peptide-mediated mitochondrial delivery). The MERRF fibroblasts receiving 3 days of treatment with peptide-mediated mitochondrial delivery restored mitochondrial respiratory chain subunits of complexes I, III and IV. This was accompanied by recovery of the mitochondrial membrane potential, ATP synthesis and a decrease in the ROS level and the recovery of the mitochondrial function has been maintained for at least 21 days (Chang et al. 2013). Interestingly, an opposite experiment where healthy cells were treated with mitochondria isolated from MERRF fibroblasts showed that previously healthy recipient cells showed a MERRF phenotype, which was characterized by increased ROS production and MMP activity (Chang et al. 2013; Clauser and Scibak 1990). More about delivering healthy mitochondria as a potential tool in the therapy of mitochondrial disorders can be found in the review by Liu et al. (2014).
3.7 Fibroblasts from Patients with Propionic Acidemia
Propionic acidemia (PA) is caused by a deficiency in propionyl-CoA carboxylase (mitochondrial enzyme) and is one of the most frequent organic acidurias in humans (incidence of 1 in 150,000 inhabitants). Propionic acidemia patients during the neonatal period develop different neurological symptoms and movement disorders. Gallego-Villar et al. found that the fibroblasts derived from PA patients have increased ROS levels (Gallego-Villar et al. 2013). Later studies of Gallego-Villar et al. showed that antioxidant treatment successfully decreases high ROS levels in PA patients’ fibroblasts, as well as the levels of mitochondrial superoxide dismutase and GPx1 (depending on used compound) (Gallego-Villar et al. 2014). They tested the effect of vitamin E, trolox, tiron, N-acetyl-cysteine (NAC), melatonin, resveratrol and MitoQ on oxidative stress manifestation in PA fibroblasts. The compounds used have different antioxidant actions. Vitamin E and Trolox neutralize lipid-derived radicals. NAC is a precursor of glutathione and can scavenge different types of ROS. Melatonin, which is a direct free radical scavenger, increases the efficiency of the antioxidant defense system. Resveratrol inhibits lipid peroxidation and is a direct ROS scavenger. MitoQ is a mitochondria-targeted antioxidant that can protect against oxidative damage within the mitochondria. Interestingly, they demonstrated that resveratrol, Trolox, Tiron and MitoQ decreased the ROS level in all studied PA-derived fibroblasts (Gallego-Villar et al. 2014). The strongest antioxidant effect was observed for Tiron (50–80%), then for MitoQ (25–30%) and finally for Trolox (15–30%). However, it is necessary to mention that the effect of individual compounds depends on the patient’s cell line. The strongest effect was observed for the fibroblasts with the highest ROS level. On the contrary , melatonin, N-acetyl cysteine (NAC) and vitamin E had absolutely no effect on the oxidative stress in these fibroblasts (Gallego-Villar et al. 2014).
3.8 Fibroblasts from Patients with Friedreich Ataxia
Friedreich ataxia (FRDA) , is the most common recessively inherited ataxia, which is caused by defective expression of frataxin (mitochondrial protein), leading to the progressive loss of neuromuscular function. The decreased level of frataxin is responsible for the accumulation of iron within the mitochondria, increased oxidative stress and decreased activity of iron-sulfur cluster-containing enzymes. Altogether, this causes mitochondrial dysfunction in FRDA patients. An interesting study performed by Jauslin et al. compared the protective effect of mitochondria-targeted and untargeted antioxidants in fibroblasts from FRDA patients (Jauslin et al. 2002). They studied the effect of these compounds on the viability of FRDA patients’ fibroblasts treated with an inhibitor of GSH biosynthesis (BSO) and thus showed an artificially-reduced glutathione level. Their experimental approach was based on the fact that BSO treatment leads to a decrease in the GSH level in control cells and FRDA fibroblasts but caused cell death only in FRDA fibroblasts (Jauslin et al. 2002). They found that MitoQ, was approximately 800-fold more effective than idebenone, the coenzyme Q10 untargeted analog, in protecting FRDA fibroblasts against GSH depletion-related cell death. Similarly, mitochondria-targeted MitoVit E was 350-fold more efficient at protecting the cells than Trolox, the water-soluble analog of vitamin E (Jauslin et al. 2003). The other classes of compounds, lipophilic iron chelators, have been investigated by Lim et al. (2008). They investigated the properties of the 2-pyridylcarboxaldehyde isonicotinoyl hydrazone (PCIH) class of chelators as agents rescuing Friedreich’s ataxia patients’ fibroblasts from H2O2-induced cytotoxicity.
3.9 Fibroblasts from Patients with Alzheimer’s Disease
Alzheimer’s Disease (AD) is a chronic neurodegenerative disease. The most common early symptom of Alzheimer’s can be difficulty remembering newly learned information (short-term memory loss), and at later stages of disease progression, symptoms can also include disorientation, problems with language and not managing self-care. Interestingly, most people with Down syndrome develop Alzheimer’s disease. The cause of Alzheimer’s disease is poorly understood, and AD is often attributed to a variety of causes. It has been found that the apolipoprotein E (APOE) gene is involved in the late-onset form of AD (symptoms become apparent in their mid-60s). APOE ε4 increases a person’s risk of developing AD. The accumulation of intracellular aggregates of tau protein in the neurofibrillary tangles and extracellular aggregates of a set of polypeptides called amyloid-β peptides in the senile plaques is the major histological hallmark of AD. Alzheimer’s disease is not a mitochondrial disease per se; however, there is direct link between AD, mitochondrial dysfunction and oxidative stress . For this reason, oxidative stress and elevated ROS production are used as a target to ameliorate cellular and mitochondrial parameters in AD patients’ fibroblasts. Among others, Moreira et al. showed that administration of lipoic acid and N-acetyl cysteine inhibits apoptosis and decreases oxidative stress in fibroblasts from patients with Alzheimer’s disease, which suggests that antioxidant therapy based on these compounds may be promising (Moreira et al. 2007).
3.10 Fibroblasts from Patients with Influenza-Associated Encephalopathy
Influenza-associated encephalopathy (IAE) is an acute brain dysfunction that usually occurs at the early stage of infectious diseases caused mainly by influenza virus, human herpes virus-6 (HHV-6) and many other viruses. IAF incidence is highest in infancy and early childhood and occurs more frequently in East Asians than in Caucasians (Kasai et al. 2000). In addition to brain dysfunction, IAE patients also show development of multiple-organ failure. Interestingly, Yao et al. showed that a high number of patients with a disabling or fatal form of IAE have a thermolabile phenotype of compound variants of carnitinepalmitoyltransferase II (CPT II) (Yao et al. 2011). Such patients are characterized by a mitochondrial energy crisis during high fever. It is related to dysfunction of the mitochondrial fatty acid β-oxidation, which is caused by heat-inactivation of carnitine palmitoyltransferase II (CPT II) in patients with the thermolabile phenotype of CPT II (Yao et al. 2011). Treatment of such fibroblasts for 24 h with bezafibrate significantly increased the CPT II activity, increased mitochondrial fatty acid β-oxidation, restored decreased ATP levels and increased the mitochondrial membrane potential in fibroblasts of IAE patients cultured at both 37 °C and 41 °C. The studies of Yamaguchi et al. indicate the possible therapeutic properties of bezafibrate in IAE patients with thermolabile variants of CPT II (Yamaguchi et al. 2012).
4 Conclusion
As presented in this chapter, several different pharmacological treatments of patients’ fibroblasts have been performed to find the most potent and appropriate way to ameliorate mitochondrial defect or mitigate oxidative stress. More details about the different strategies and treatments of mitochondrial disorders can be found in an excellent review written by Scarpelli et al. (2014). Moreover, the paper of Voets et al. elegantly shows how fibroblast analysis enables identification of patients who potentially can benefit from the antioxidant therapy (Voets et al. 2012). Similarly, a comprehensive review describing the use of individual patient fibroblasts in the search for personalized treatment has been presented by Saada (2011). Finally, we also recommend a systematic review written by Rai et al. describing pharmacological therapeutics tested using in vitro models (Rai et al. 2015), a review by Rajendran et al. summarizing the impact and involvement of antioxidants in selected human diseases (Rajendran et al. 2014), and a review by Kanabus et al. (2014) and Koopman et al. (2016).
References
Angebault C, Gueguen N, Desquiret-Dumas V, Chevrollier A, Guillet V, Verny C, Cassereau J, Ferre M, Milea D, Amati-Bonneau P, Bonneau D, Procaccio V, Reynier P, Loiseau D (2011) Idebenone increases mitochondrial complex I activity in fibroblasts from LHON patients while producing contradictory effects on respiration. BMC Res Notes 4:557
Bonora M, Wieckowski MR, Chinopoulos C, Kepp O, Kroemer G, Galluzzi L, Pinton P (2015) Molecular mechanisms of cell death: central implication of ATP synthase in mitochondrial permeability transition. Oncogene 34(12):1475–1486
Bonora M, Morganti C, Morciano G, Pedriali G, Lebiedzinska-Arciszewska M, Aquila G, Giorgi C, Rizzo P, Campo G, Ferrari R, Kroemer G, Wieckowski MR, Galluzzi L, Pinton P. (2017) Mitochondrial permeability transition involves dissociation of F1FO ATP synthase dimers and C-ring conformation. EMBO Rep 18(7):1077–1089
Cárdenas C, Foskett JK (2012) Mitochondrial Ca(2+) signals in autophagy. Cell Calcium 52(1):44–51
Casarin A, Giorgi G, Pertegato V, Siviero R, Cerqua C, Doimo M, Basso G, Sacconi S, Cassina M, Rizzuto R, Brosel S, M Davidson M, Dimauro S, Schon EA, Clementi M, Trevisson E, Salviati L (2012) Copper and bezafibrate cooperate to rescue cytochrome c oxidase deficiency in cells of patients with SCO2 mutations. Orphanet J Rare Dis 7:21
Chang JC, Liu KH, Chuang CS, Su HL, Wei YH, Kuo SJ, Liu CS (2013) Treatment of human cells derived from MERRF syndrome by peptide-mediated mitochondrial delivery. Cytotherapy 15(12):1580–1596
Clauser B, Scibak JW (1990) Direct and generalized effects of food satiation in reducing rumination. Res Dev Disabil 11(1):23–36
Cotán D, Cordero MD, Garrido-Maraver J, Oropesa-Ávila M, Rodríguez-Hernández A, Gómez Izquierdo L, De la Mata M, De Miguel M, Lorite JB, Infante ER, Jackson S, Navas P, Sánchez-Alcázar JA (2011) Secondary coenzyme Q10 deficiency triggers mitochondria degradation by mitophagy in MELAS fibroblasts. FASEB J 25(8):2669–2687
Danese A, Patergnani S, Bonora M, Wieckowski MR, Previati M, Giorgi C, Pinton P (2017) Calcium regulates cell death in cancer: Roles of the mitochondria and mitochondria-associated membranes (MAMs). Biochim Biophys Acta 1858(8):615–627. pii: S0005-2728(17)30004-X. https://doi.org/10.1016/j.bbabio.2017.01.003
De la Mata M, Garrido-Maraver J, Cotán D, Cordero MD, Oropesa-Ávila M, Izquierdo LG, De Miguel M, Lorite JB, Infante ER, Ybot P, Jackson S, Sánchez-Alcázar JA (2012) Recovery of MERRF fibroblasts and cybrids pathophysiology by coenzyme Q10. Neurotherapeutics 9(2):446–463
De Paepe B, Vandemeulebroecke K, Smet J, Vanlander A, Seneca S, Lissens W, Van Hove JL, Deschepper E, Briones P, Van Coster R (2014) Effect of resveratrol on cultured skin fibroblasts from patients with oxidative phosphorylation defects. Phytother Res 28(2):312–316
Decuypere JP, Kindt D, Luyten T, Welkenhuyzen K, Missiaen L, De Smedt H, Bultynck G, Parys JB (2013) mTOR-Controlled Autophagy Requires Intracellular Ca2+. Signaling. PLoS One 8(4):e61020
Distelmaier F, Visch HJ, Smeitink JA, Mayatepek E, Koopman WJ, Willems PH (2009) The antioxidant Trolox restores mitochondrial membrane potential and Ca2+ −stimulated ATP production in human complex I deficiency. J Mol Med 87(5):515–522
Düzgün ŞA, Yerlikaya A, Zeren S, Bayhan Z, Okur E, Boyacı İ (2017) Differential effects of p38 MAP kinase inhibitors SB203580 and SB202190 on growth and migration of human MDA-MB-231 cancer cell line. Cytotechnology 69(4):711–724. https://doi.org/10.1007/s10616-017-0079-2
Ehinger JK, Piel S, Ford R, Karlsson M, Sjövall F, Frostner EÅ, Morota S, Taylor RW, Turnbull DM, Cornell C, Moss SJ, Metzsch C, Hansson MJ, Fliri H, Elmér E (2016) Cell-permeable succinate prodrugs bypass mitochondrial complex I deficiency. Nat Commun 7:12317
Farrukh MR, Nissar UA, Afnan Q, Rafiq RA, Sharma L, Amin S, Kaiser P, Sharma PR, Tasduq SA (2014) Oxidative stress mediated Ca(2+) release manifests endoplasmic reticulum stress leading to unfolded protein response in UV-B irradiated human skin cells. J Dermatol Sci 75(1):24–35
Farrukh MR, Nissar UA, Kaiser PJ, Afnan Q, Sharma PR, Bhushan S, Tasduq SA (2015) Glycyrrhizic acid (GA) inhibits reactive oxygen Species mediated photodamage by blocking ER stress and MAPK pathway in UV-B irradiated human skin fibroblasts. J Photochem Photobiol B 148:351–357
Ferrari R, Balla C, Malagù M, Guardigli G, Morciano G, Bertini M, Biscaglia S, Campo G (2017) Reperfusion damage–a story of success, failure, and hope. Circ J 81(2):131–141
Ferretta A, Gaballo A, Tanzarella P, Piccoli C, Capitanio N, Nico B, Annese T, Di Paola M, Dell'aquila C, De Mari M, Ferranini E, Bonifati V, Pacelli C, Cocco T (2014) Effect of resveratrol on mitochondrial function: implications in parkin-associated familiar Parkinson's disease. Biochim Biophys Acta 1842(7):902–915
Gallego-Villar L, Pérez-Cerdá C, Pérez B, Abia D, Ugarte M, Richard E, Desviat LR (2013) Functional characterization of novel genotypes and cellular oxidative stress studies in propionic acidemia. J Inherit Metab Dis 36(5):731–740
Gallego-Villar L, Pérez B, Ugarte M, Desviat LR, Richard E (2014) Antioxidants successfully reduce ROS production in propionic acidemia fibroblasts. Biochem Biophys Res Commun 452(3):457–461
Garrido-Maraver J, Cordero MD, Moñino ID, Pereira-Arenas S, Lechuga-Vieco AV, Cotán D, De la Mata M, Oropesa-Ávila M, De Miguel M, Bautista Lorite J, Rivas Infante E, Alvarez-Dolado M, Navas P, Jackson S, Francisci S, Sánchez-Alcázar JA (2012) Screening of effective pharmacological treatments for MELAS syndrome using yeasts, fibroblasts and cybrid models of the disease. Br J Pharmacol 167(6):1311–1328
Gerards M, van den Bosch BJ, Danhauser K, Serre V, van Weeghel M, Wanders RJ, Nicolaes GA, Sluiter W, Schoonderwoerd K, Scholte HR, Prokisch H, Rötig A, de Coo IF, Smeets HJ (2011) Riboflavin-responsive oxidative phosphorylation complex I deficiency caused by defective ACAD9: new function for an old gene. Brain 134(Pt 1):210–219
Giorgi C, Agnoletto C, Baldini C, Bononi A, Bonora M, Marchi S, Missiroli S, Patergnani S, Poletti F, Rimessi A, Zavan B, Pinton P (2010a) Redox control of protein kinase C: cell- and disease-specific aspects. Antioxid Redox Signal 13(7):1051–85
Giorgi C, Ito K, Lin HK, Santangelo C, Wieckowski MR, Lebiedzinska M, Bononi A, Bonora M, Duszynski J, Bernardi R, Rizzuto R, Tacchetti C, Pinton P, Pandolfi PP (2010b) PML regulates apoptosis at endoplasmic reticulum by modulating calcium release. Science 330(6008):1247–1251
Giorgi C, Bonora M, Sorrentino G, Missiroli S, Poletti F, Suski JM, Galindo Ramirez F, Rizzuto R, Di Virgilio F, Zito E, Pandolfi PP, Wieckowski MR, Mammano F, Del Sal G, Pinton P (2015a) p53 at the endoplasmic reticulum regulates apoptosis in a Ca2+-dependent manner. Proc Natl Acad Sci U S A 112(6):1779–1784
Giorgi C, Missiroli S, Patergnani S, Duszynski J, Wieckowski MR, Pinton P (2015b) Mitochondria-associated membranes: composition, molecular mechanisms, and physiopathological implications. Antioxid Redox Signal 22(12):995-1019
Gledhill JR, Montgomery MG, Leslie AG, Walker JE (2007) Mechanism of inhibition of bovine F1-ATPase by resveratrol and related polyphenols. Proc Natl Acad Sci U S A 104(34):13632–13637
Golubitzky A, Dan P, Weissman S, Link G, Wikstrom JD, Saada A (2011) Screening for active small molecules in mitochondrial complex I deficient patient’s fibroblasts, reveals AICAR as the most beneficial compound. PLoS One 6(10):e26883
Granatiero V, Giorgio V, Calì T, Patron M, Brini M, Bernardi P, Tiranti V, Zeviani M, Pallafacchina G, De Stefani D, Rizzuto R (2016) Reduced mitochondrial Ca(2+) transients stimulate autophagy in human fibroblasts carrying the 13514A>G mutation of the ND5 subunit of NADH dehydrogenase. Cell Death Differ 23(2):231–241
Gueven N, Nadikudi M, Daniel A, Chhetri J (2016) Targeting mitochondrial function to treat optic neuropathy. Mitochondrion S1567-7249(16):30115–30115. https://doi.org/10.1016/j.mito.2016.07.013
Haas RH (2007) The evidence basis for coenzyme Q therapy in oxidative phosphorylation disease. Mitochondrion 7(Suppl):S136–S145
Haefeli RH, Erb M, Gemperli AC, Robay D, Courdier Fruh I, Anklin C, Dallmann R, Gueven N (2011) NQO1-dependent redox cycling of idebenone: effects on cellular redox potential and energy levels. PLoS One 6(3):e17963
Hirano M, Garone C, Quinzii CM (2012) CoQ(10) deficiencies and MNGIE: two treatable mitochondrial disorders. Biochim Biophys Acta 1820(5):625–631
Hirashima N, Etcheberrigaray R, Bergamaschi S, Racchi M, Battaini F, Binetti G, Govoni S, Alkon DL (1996) Calcium responses in human fibroblasts: a diagnostic molecular profile for Alzheimer's disease. Neurobiol Aging 17(4):549–555
Ido Y, Duranton A, Lan F, Weikel KA, Breton L, Ruderman NB (2015) Resveratrol prevents oxidative stress-induced senescence and proliferative dysfunction by activating the AMPK-FOXO3 cascade in cultured primary human keratinocytes. PLoS One 10(2):e0115341
Jauslin ML, Wirth T, Meier T, Schoumacher F (2002) A cellular model for Friedreich Ataxia reveals small-molecule glutathione peroxidase mimetics as novel treatment strategy. Hum Mol Genet 11(24):3055–3063
Jauslin ML, Meier T, Smith RA, Murphy MP (2003) Mitochondria-targeted antioxidants protect Friedreich Ataxia fibroblasts from endogenous oxidative stress more effectively than untargeted antioxidants. FASEB J 17(13):1972–1974
Jouaville LS, Pinton P, Bastianutto C, Rutter GA, Rizzuto R (1999) Regulation of mitochondrial ATP synthesis by calcium: evidence for a long-term metabolic priming. Proc Natl Acad Sci U S A 96(24):13807–13812
Kanabus M, Heales SJ, Rahman S (2014) Development of pharmacological strategies for mitochondrial disorders. Br J Pharmacol 171(8):1798–1817
Kanabus M, Fassone E, Hughes SD, Bilooei SF, Rutherford T, Donnell MO, Heales SJ, Rahman S (2016) The pleiotropic effects of decanoic acid treatment on mitochondrial function in fibroblasts from patients with complex I deficient Leigh syndrome. J Inherit Metab Dis 39(3):415–426
Kasai T, Togashi T, Morishima T (2000) Encephalopathy associated with influenza epidemics. Lancet 355(9214):1558–1559
Kaufman RJ, Malhotra JD (2014) Calcium trafficking integrates endoplasmic reticulum function with mitochondrial bioenergetics. Biochim Biophys Acta 1843(10):2233–2239
Kemp JP, Smith PM, Pyle A, Neeve VC, Tuppen HA, Schara U, Talim B, Topaloglu H, Holinski-Feder E, Abicht A, Czermin B, Lochmüller H, McFarland R, Chinnery PF, Chrzanowska-Lightowlers ZM, Lightowlers RN, Taylor RW, Horvath R (2011) Nuclear factors involved in mitochondrial translation cause a subgroup of combined respiratory chain deficiency. Brain 134(Pt 1):183–195
Kim CY, Lee HJ, Chae MK, Byun JW, Lee EJ, Yoon JS (2015) Therapeutic effect of resveratrol on oxidative stress in graves’ orbitopathy orbital fibroblasts. Invest Ophthalmol Vis Sci 56(11):6352–6361
Koopman WJ, Verkaart S, van Emst-de Vries SE, Grefte S, Smeitink JA, Nijtmans LG, Willems PH (2008) Mitigation of NADH: ubiquinone oxidoreductase deficiency by chronic Trolox treatment. Biochim Biophys Acta 1777(7–8):853–859
Koopman WJ, Beyrath J, Fung CW, Koene S, Rodenburg RJ, Willems PH, Smeitink JA (2016) Mitochondrial disorders in children: toward development of small-molecule treatment strategies. EMBO Mol Med 8(4):311–327
Lake NJ, Bird MJ, Isohanni P, Paetau A (2015) Leigh syndrome: neuropathology and pathogenesis. J Neuropathol Exp Neurol 74(6):482–492
Lebiedzinska M, Karkucinska-Wieckowska A, Wojtala A, Suski JM, Szabadkai G, Wilczynski G, Wlodarczyk J, Diogo CV, Oliveira PJ, Tauber J, Ježek P, Pronicki M, Duszynski J, Pinton P, Wieckowski MR (2013) Disrupted ATP synthase activity and mitochondrial hyperpolarisation-dependent oxidative stress is associated with p66Shc phosphorylation in fibroblasts of NARP patients. Int J Biochem Cell Biol 45(1):141–150
Lim CK, Kalinowski DS, Richardson DR (2008) Protection against hydrogen peroxide-mediated cytotoxicity in Friedreich's ataxia fibroblasts using novel iron chelators of the 2-pyridylcarboxaldehyde isonicotinoyl hydrazone class. Mol Pharmacol 74(1):225–235
Liu CS, Chang JC, Kuo SJ, Liu KH, Lin TT, Cheng WL, Chuang SF (2014) Delivering healthy mitochondria for the therapy of mitochondrial diseases and beyond. Int J Biochem Cell Biol 53:141–146
Lopes Costa A, Le Bachelier C, Mathieu L, Rotig A, Boneh A, De Lonlay P, Tarnopolsky MA, Thorburn DR, Bastin J, Djouadi F (2014) Beneficial effects of resveratrol on respiratory chain defects in patients’ fibroblasts involve estrogen receptor and estrogen-related receptor alpha signaling. Hum Mol Genet 23(8):2106–2119
López LC, Quinzii CM, Area E, Naini A, Rahman S, Schuelke M, Salviati L, Dimauro S, Hirano M (2010) Treatment of CoQ(10) deficient fibroblasts with ubiquinone, CoQ analogs, and vitamin C: time- and compound-dependent effects. PLoS One 5(7):e11897
Marchi S, Bonora M, Patergnani S, Giorgi C, Pinton P (2017) Methods to assess mitochondrial morphology in mammalian cells mounting autophagic or mitophagic responses. Methods Enzymol 588:171–186
Mathieu L, Costa AL, Le Bachelier C, Slama A, Lebre AS, Taylor RW, Bastin J, Djouadi F (2016) Resveratrol attenuates oxidative stress in mitochondrial Complex I deficiency: Involvement of SIRT3. Free Radic Biol Med 96:190–198
Mattiazzi M, Vijayvergiya C, Gajewski CD, DeVivo DC, Lenaz G, Wiedmann M, Manfredi G (2004) The mtDNA T8993G (NARP) mutation results in an impairment of oxidative phosphorylation that can be improved by antioxidants. Hum Mol Genet 13(8):869–879
Menzies KJ, Robinson BH, Hood DA (2009) Effect of thyroid hormone on mitochondrial properties and oxidative stress in cells from patients with mtDNA defects. Am J Physiol Cell Physiol 296(2):C355–C362
Morató L, Galino J, Ruiz M, Calingasan NY, Starkov AA, Dumont M, Naudí A, Martínez JJ, Aubourg P, Portero-Otín M, Pamplona R, Galea E, Beal MF, Ferrer I, Fourcade S, Pujol A (2013) Pioglitazone halts axonal degeneration in a mouse model of X-linked adrenoleukodystrophy. Brain 136(Pt 8):2432–2443
Morciano G, Giorgi C, Bonora M, Punzetti S, Pavasini R, Wieckowski MR, Campo G, Pinton P (2015) Molecular identity of the mitochondrial permeability transition pore and its role in ischemia-reperfusion injury. J Mol Cell Cardiol 78:142–153
Moreira PI, Harris PL, Zhu X, Santos MS, Oliveira CR, Smith MA, Perry G (2007) Lipoic acid and N-acetyl cysteine decrease mitochondrial-related oxidative stress in Alzheimer disease patient fibroblasts. J Alzheimers Dis 12(2):195–206
Patergnani S, Marchi S, Rimessi A, Bonora M, Giorgi C, Mehta KD, Pinton P (2013) PRKCB/protein kinase C, beta and the mitochondrial axis as key regulators of autophagy. Autophagy 9(9):1367–1385
Patergnani S, Baldassari F, De Marchi E, Karkucinska-Wieckowska A, Wieckowski MR, Pinton P (2014) Methods to monitor and compare mitochondrial and glycolytic ATP production. Methods Enzymol 542:313–332
Pinton P, Leo S, Wieckowski MR, Di Benedetto G, Rizzuto R (2004) Long-term modulation of mitochondrial Ca2+ signals by protein kinase C isozymes. J Cell Biol 165(2):223–32
Rahman S (2015) Emerging aspects of treatment in mitochondrial disorders. J Inherit Metab Dis 38(4):641–653
Rai PK, Russell OM, Lightowlers RN, Turnbull DM (2015) Potential compounds for the treatment of mitochondrial disease. Br Med Bull 116:5–18
Rajendran P, Nandakumar N, Rengarajan T, Palaniswami R, Gnanadhas EN, Lakshminarasaiah U, Gopas J, Nishigaki I (2014) Antioxidants and human diseases. Clin Chim Acta 436:332–347
Saada A (2011) The use of individual patient’s fibroblasts in the search for personalized treatment of nuclear encoded OXPHOS diseases. Mol Genet Metab 104(1–2):39–47
Saada A (2014) Mitochondria: mitochondrial OXPHOS (dys) function ex vivo–the use of primary fibroblasts. Int J Biochem Cell Biol 48:60–65
Salviati L, Hernandez-Rosa E, Walker WF, Sacconi S, DiMauro S, Schon EA, Davidson MM (2002) Copper supplementation restores cytochrome c oxidase activity in cultured cells from patients with SCO2 mutations. Biochem J 363(Pt 2):321–327
Sbano L, Bonora M, Marchi S, Baldassari F, Medina DL, Ballabio A, Giorgi C, Pinton P (2017) TFEB-mediated increase in peripheral lysosomes regulates store-operated calcium entry. Sci Rep 7:40797
Scarpelli M, Todeschini A, Rinaldi F, Rota S, Padovani A, Filosto M (2014) Strategies for treating mitochondrial disorders: an update. Mol Genet Metab 113(4):253–260
Schmunk G, Boubion BJ, Smith IF, Parker I, Gargus JJ (2015) Shared functional defect in IP3R-mediated calcium signaling in diverse monogenic autism syndromes. Transl Psychiatry 5:e643
Schmunk G, Nguyen RL, Ferguson DL, Kumar K, Parker I, Gargus JJ (2017) High-throughput screen detects calcium signaling dysfunction in typical sporadic autism spectrum disorder. Sci Rep 7:40740
Sgarbi G, Casalena GA, Baracca A, Lenaz G, DiMauro S, Solaini G (2009) Human NARP mitochondrial mutation metabolism corrected with alpha-ketoglutarate/aspartate: a potential new therapy. Arch Neurol 66(8):951–957
Singh DK, Kumar D, Siddiqui Z, Basu SK, Kumar V, Rao KV (2005) The strength of receptor signaling is centrally controlled through a cooperative loop between Ca2+ and an oxidant signal. Cell 121(2):281–293
Smith RA, Murphy MP (2010) Animal and human studies with the mitochondria-targeted antioxidant MitoQ. Ann N Y Acad Sci 1201:96–103
Smits P, Smeitink J, van den Heuvel L (2010) Mitochondrial translation and beyond: processes implicated in combined oxidative phosphorylation deficiencies. J Biomed Biotechnol 2010:737385
Soiferman D, Ayalon O, Weissman S, Saada A (2014) The effect of small molecules on nuclear-encoded translation diseases. Biochimie 100:184–191
Spinazzola A, Invernizzi F, Carrara F, Lamantea E, Donati A, Dirocco M, Giordano I, Meznaric-Petrusa M, Baruffini E, Ferrero I, Zeviani M (2009) Clinical and molecular features of mitochondrial DNA depletion syndromes. J Inherit Metab Dis 32(2):143–158
Vay L, Hernández-Sanmiguel E, Santo-Domingo J, Lobatón CD, Moreno A, Montero M, Alvarez J (2007) Modulation of Ca(2+) release and Ca(2+) oscillations in HeLa cells and fibroblasts by mitochondrial Ca(2+) uniporter stimulation. J Physiol Lond 580(Pt 1):39–49
Vazquez-Memije ME, Shanske S, Santorelli FM, Kranz-Eble P, Davidson E, DeVivo DC, DiMauro S (1996) Comparative biochemical studies in fibroblasts from patients with different forms of Leigh syndrome. J Inherit Metab Dis 19(1):43–50
Visch HJ, Rutter GA, Koopman WJ, Koenderink JB, Verkaart S, de Groot T, Varadi A, Mitchell KJ, van den Heuvel LP, Smeitink JA, Willems PH (2004) Inhibition of mitochondrial Na+-Ca2+ exchange restores agonist-induced ATP production and Ca2+ handling in human complex I deficiency. J Biol Chem 279(39):40328–40336
Voets AM, Lindsey PJ, Vanherle SJ, Timmer ED, Esseling JJ, Koopman WJ, Willems PH, Schoonderwoerd GC, De Groote D, Poll-The BT, de Coo IF, Smeets HJ (2012) Patient-derived fibroblasts indicate oxidative stress status and may justify antioxidant therapy in OXPHOS disorders. Biochim Biophys Acta 1817(11):1971–1978
Wang H, Lemire BD, Cass CE, Weiner JH, Michalak M, Penn AM, Fliegel L (1996) Zidovudine and dideoxynucleosides deplete wild-type mitochondrial DNA levels and increase deleted mitochondrial DNA levels in cultured Kearns-Sayre syndrome fibroblasts. Biochim Biophys Acta 1316(1):51–59
White SL, Collins VR, Wolfe R, Cleary MA, Shanske S, DiMauro S, Dahl HH, Thorburn DR (1999) Genetic counseling and prenatal diagnosis for the mitochondrial DNA mutations at nucleotide 8993. Am J Hum Genet 65(2):474–482
Wojewoda M, Duszyński J, Szczepanowska J (2011) NARP mutation and mtDNA depletion trigger mitochondrial biogenesis which can be modulated by selenite supplementation. Int J Biochem Cell Biol 43(8):1178–1186
Wu SB, Ma YS, Wu YT, Chen YC, Wei YH (2010) Mitochondrial DNA mutation-elicited oxidative stress, oxidative damage, and altered gene expression in cultured cells of patients with MERRF syndrome. Mol Neurobiol 41(2–3):256–266
Wu YT, Wu SB, Wei YH (2014) Metabolic reprogramming of human cells in response to oxidative stress: implications in the pathophysiology and therapy of mitochondrial diseases. Curr Pharm Des 20(35):5510–5526
Yamaguchi S, Li H, Purevsuren J, Yamada K, Furui M, Takahashi T, Mushimoto Y, Kobayashi H, Hasegawa Y, Taketani T, Fukao T, Fukuda S (2012) Bezafibrate can be a new treatment option for mitochondrial fatty acid oxidation disorders: evaluation by in vitro probe acylcarnitine assay. Mol Genet Metab 107(1–2):87–91
Yao M, Yao D, Yamaguchi M, Chida J, Yao D, Kido H (2011) Bezafibrate upregulates carnitine palmitoyltransferase II expression and promotes mitochondrial energy crisis dissipation in fibroblasts of patients with influenza-associated encephalopathy. Mol Genet Metab 104(3):265–272
Yu-Wai-Man P, Soiferman D, Moore DG, Burté F, Saada A (2017) Evaluating the therapeutic potential of idebenone and related quinone analogues in Leber hereditary optic neuropathy. Mitochondrion S1567-7249(17):30012–30010. https://doi.org/10.1016/j.mito.2017.01.004
Acknowledgments
This work was supported by the Polish National Science Centre grant (UMO-2014/15/B/NZ1/00490) to MRW and AKW and by the Internal Projects of the Children’s Memorial Health Institute No S125/2012 for MP and AKW. PP is grateful to Camilla degli Scrovegni for providing continuous support. PP is grateful to Camilla degli Scrovegni for continuous support. PP is supported by the Italian Ministry of Education, University and Research; the Italian Ministry of Health; Telethon (GGP15219/B); the Italian Association for Cancer Research (AIRC: IG-18624); and by local funds from the University of Ferrara).
Conflict of Interest
The authors state that there are no conflicts of interest relevant for this publication.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 Springer International Publishing AG, part of Springer Nature
About this chapter
Cite this chapter
Wieckowski, M.R. et al. (2018). Recovering Mitochondrial Function in Patients’ Fibroblasts. In: Oliveira, P. (eds) Mitochondrial Biology and Experimental Therapeutics. Springer, Cham. https://doi.org/10.1007/978-3-319-73344-9_17
Download citation
DOI: https://doi.org/10.1007/978-3-319-73344-9_17
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-73343-2
Online ISBN: 978-3-319-73344-9
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)