Abstract
In the past decades, nanoparticles have been widely used in industry and pharmaceutical fields for drug delivery, anti-pathogen, and diagnostic imaging purposes because of their unique physicochemical characteristics such as special ultrastructure, dispersity, and effective cellular uptake properties. But the nanotoxicity has been raised over the extensive applications of nanoparticles. Researchers have elucidated series of mechanisms in nanoparticles-induced toxicity, including apoptosis, necrosis, oxidative stress, and autophagy. Among upon mechanisms, autophagy was recently recognized as an important cell death style in various nanoparticles-induced toxicity, but the role of autophagy and its related cellular and molecular mechanisms during nanoparticles-triggered toxicity were still confusing. In the chapter, we briefly introduced the general process of autophagy, summarized the different roles of autophagy in various nanoparticle-treated different in vitro/in vivo models, and deeply analyzed the physicochemical and biochemical (cellular and molecular) mechanisms of autophagy during nanoparticles-induced toxicity through listing and summarizing representative examples. Physicochemical mechanisms mainly include dispersity, size, charge, and surface chemistry; cellular mechanisms primarily focus on lysosome impairment, mitochondria dysfunction, mitophagy, endoplasmic reticulum stress and endoplasmic reticulum autophagy; while molecular mechanisms were mainly including autophagy related signaling pathways, hypoxia-inducible factor, and oxidative stress. This chapter highlighted the important role of autophagy as a critical mechanism in nanoparticles-induced toxicity, and the physicochemical and biochemical mechanisms of autophagy triggered by nanoparticles might be useful for establishing a guideline for the evaluation of nanotoxicology, designing and developing new biosafety nanoparticles in the future.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
5.1 Introduction
In the past decades, nanoparticles have been widely used in industry and pharmaceutical fields for drug delivery, anti-pathogen, and diagnostic imaging purposes because of their unique physicochemical characteristics such as special ultrastructure, dispersity, and effective cellular uptake properties [1, 2]. But the nanotoxicity has been raised over the extensive applications of nanoparticles. Researchers have elucidated series of mechanisms in nanoparticles-induced toxicity, including apoptosis, necrosis, oxidative stress, and autophagy [3, 4]. Among upon mechanisms, autophagy was recently recognized as an important cell death style in various nanoparticles-induced toxicity, but the role of autophagy and its related cellular and molecular mechanisms during nanoparticles-triggered toxicity were still confusing.
In the chapter, we briefly introduced the general process of autophagy, summarized the different roles of autophagy in various nanoparticle-treated different in vitro/in vivo models, and deeply analyzed the physicochemical and biochemical (cellular and molecular) mechanisms of autophagy during nanoparticles-induced toxicity through listing and summarizing representative examples. Physicochemical mechanisms mainly include dispersity, size, charge, and surface chemistry; Cellular mechanisms primarily focus on lysosome impairment, mitochondria dysfunction, mitophagy, endoplasmic reticulum stress and endoplasmic reticulum autophagy; while molecular mechanisms were mainly including autophagy related signaling pathways, hypoxia-inducible factor, and oxidative stress. This chapter highlighted the important role of autophagy as a critical mechanism in nanoparticles-induced toxicity, the physicochemical and biochemical mechanisms of autophagy triggered by nanoparticles might be useful for establishing a guideline for the evaluation of nanotoxicology, designing and developing new biosafety nanoparticles in the future.
5.2 Classification of Autophagy
The general term of autophagy was derived from the Greek and meaning for “self-eating”. And this evolutionarily conserved process of degradation cytoplasmic components (mis-folded protein and dysfunctional organelles) within lysosomes was first described nearly 50 years ago [5, 6]. Different with endocytosis-mediated degradation and recycle of cytoplasmic components, there are three types of autophagy including microautophagy, macroautophagy, and chaperone-mediated autophagy [7]. Microautophagy is mediated by lysosomal directly engulf cytoplasmic components in nonselective manners. Macroautophagy consists of three steps: cytoplasmic constituents (mis-folded proteins or damaged organelles) are enveloped by double-membraned autophagosome; autophagosomes are fused with lysosome to form an autolysosome; and the cytoplasmic constituents are degraded and recycled in lysosome (Fig. 5.1). Macroautophagy could degrade most specific components, such as mitochondria (mitophagy), endoplasmic reticulum (ERphagy or reticulophagy), ribosomes (ribophagy), and pexophagy (peroxisomes); and is the most popular and well investigated type of autophagy. Chaperone-mediated autophagy is the process that soluble cytosolic proteins directly translocated into lysosomes for degradation in chaperone-dependent selecting manner. Chaperone-mediated autophagy dose not require the formation of additional vesicles [7]. Autophagy can be activated by series of stressful conditions such as nutrient deprivation, oxidative stress, hypoxia, and inflammatory mediates [8]. The internalized nanoparticles were regarded as foreign materials and autophagic cargoed by cells and then triggered autophagy.
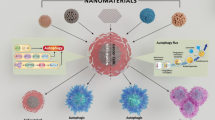
The dual role of autophagy in nanoparticles-induced toxicity. Macroautophagy consists of three steps: cytoplasmic constituents (mis-folded proteins or damaged organelles) are enveloped by double-membraned autophagosome; autophagosomes are fused with lysosome to form an autolysosome; and the cytoplasmic constituents are degraded and recycled in lysosome. Autophagy is likened to the opposite faces of Janus, as it could protect cells to survive under certain severe conditions, as well as inducing cell death when too much autophagy occurs
5.3 The Role of Autophagy in Nanoparticles-Induced Toxicity
Autophagy is likened to the opposite faces of Janus, as it could protect cells to survive under certain severe conditions, as well as inducing cell death when too much autophagy occurs [9]. Similarly, nanoparticles-mediated autophagy during nanotoxicity might be an adaptive cellular response aiding in the clearance of nanoparticles, and also might be harmful in cellular dysfunction (Fig. 5.1).
In the section, the dual role of autophagy in nanoparticles-induced toxicity will be reviewed. Researchers investigated the autophagic effects triggered by non-mental nanoparticles, mental nanoparticles, mental oxide nanoparticles, and polymers in various models, and found most nanoparticles could induce autophagic cell death: Yu et al., reported that silica nanoparticles induced autophagic cell death in hepatoma HepG2 cells, autophagy inhibitor could effectively impair autophagy and cell death triggered by silica nanoparticles [10]. Using different cell models, Liu et al., and Park et al., both confirmed that single-walled carbon nanotubes induced autophagic cell death in A549 human lung cells and BEAS-2B human bronchial epithelial cells [11, 12]. Besides, mental oxide nanoparticles could also trigger autopahgic cell death: Copper oxide nanoparticles induced autophagic cell death in A549 cells, and autophagy inhibitors wortmannin and 3-methyladenine could protect against copper oxide nanoparticles-induced A549 cell death [13]; Yu et al., showed that zinc oxide nanoparticle also induced autophagic cell death and mitochondrial damage via reactive oxygen species generation [14], meanwhile, Johnson et al., also found that acute exposure to zinc oxide nanoparticles could induce autophagic immune cell death [15]. And nano sized neodymium oxide particles could trigger massive vacuolization and induce autophagic cell death in lung cancer cells [16]. Apart from mental and non-mental nanoparticles, polymers could also induce autophagic cell death during their-induced toxicity: Cationic polystyrene nanoparticles induced autophagic cell death through endoplasmic reticulum stress induction in macrophage and lung epithelial cells [17]. And many research groups have confirmed that cationic poly-amidoamine dendrimers promoted liver injury, lung damage and broke neuronal function by inducting autophagic cell death in different cell and mice models [18,19,20].
Compared with massive studies on nanoparticles-induced autophagic cell death, only a few literatures showed that nanoparticles triggered cyto-protective autophagy in cells. Zhou et al., found a novel nanoparticles paramontroseite VO2 induced cyto-protective autophagy in HeLa cells, while autophagy inhibitor 3-methyladenine could obviously increase cell death rate in nanoparticels-treated HeLa cells [21]. The same research group also confirmed that silver nanoparticles induced cyto-protective autophagy during their-induced HeLa cell death, suppression of autophagy enhanced the anticancer activity of silver nanoparticles [22].
5.4 Physicochemical Mechanisms of Autophagy in Nanoparticles-Induced Toxicity
The mechanisms of autophagy in nanoparticles-induced toxicity were very complex regarding different physicochemical and biochemical properties of nanoparticles and various interactions between nanoparticles and cells during their-induced toxicity.
5.4.1 Dispersity
Huang and his colleagues explored the role of dispersity in nanoparticles-induced autophagy and found metal oxide Nanoparticles iron oxide nanoparticles could induce significant autophagic effect when in aggregated conditions; when surface modification by dopamine (DA), 3,4-dihydroxyphenylacetic acid (DOPAC), and meso-2,3-dimercaptosuccinicacid (DMSA) or protein adsorption by water dilution or bovine serum albumin (BSA) incubation improved their dispersity, the autophagic effects were also be diminished. Moreover, other kinds of nanoparticles such as mental nanoparticles (gold) and silica nanoparticles also exhibited dispersity-dependent autophagic effects. It was the first and the only study on dispersity of nanoparticles affecting autophagy, and suggested that autophagy triggered by nanoparticles could be modulated through tuning their dispersity [23].
5.4.2 Size
Compared with rare study on the role of dispersity in nanoparticles-activated autophagy, a series of literatures have widely investigated the size-dependent manner of nanoparticles during their-induced autophagy, including quantum dots, silver, gold, and silica nanoparticles.
In the year of 2006, Seleverstov O and colleagues compared the cytotoxicity and intracellular process of two different-sized quantum dots in human mesenchyal stem cells, and then reported for the first time that nanoparticles could trigger autophagy activation in a size-dependent manner [24]. Since then, more and more scientists investigated and found other nanoparticles could also induct autophagy in size-dependent manner.
Mishra and coauthors reported that silver nanoparticle could also activate autophagic-lysosomal interruption in a size-dependent manner; smaller (10 nm) silver nanoparticles exhibited highest uptake, accumulation and strongest autophagy and enhanced lysosomal activity in HepG2 cells when compared with larger (50 nm and 100 nm) silver nanoparticles [25]. Another mental gold nanoparticles could also induce autophagosome accumulation from blocking autophagy flux in a size-dependent nanoparticle uptake manner [26].
Apart from mental nanoparticles, researchers found that non-mental nanoparticles could also activate autophagy in a size-dependent manner during their-induced toxicity. Li et al., compared the difference of autophagy dysfunction triggered by nano-scale size (40 nm and 60 nm) and micro-scale size (200 nm) silica particles during their-induced cytotoxicity in human bronchial epithelial BEAS-2B cells: Nano-scale silica particles, but not micro-scale silica nanoparticles, could induce mitochondrial damage and autophagy via the PI3K/Akt/mTOR signaling pathway in a size- and dose-dependent manner in human bronchial epithelial BEAS-2B cells [27]. Using different cell model, Huang and colleagues also confirmed the size effect of silica sub-microspheres in autophagy induction; Cells treated with 0.5–0.7 μm silica particles displayed many GFP-LC3 fluorescent puncta and high expression levels of autophagy related proteins; however, when the particle size was smaller than 0.5 μm or larger than 0.7 μm, autophagic level decreased. Cells treated with 0.1 μm or 2.1 μm silica particles had negligible change in GFP-LC3 puncta or expression levels of autophagy related proteins [28].
5.4.3 Charge
The autophagy triggered by nanoparticles was found not only to be dependent on particles’ size, but also to be affected by porosity and surface charge. The polystyrene nanoparticles with neutral, anionic, and cationic surface charges could all activate autophagy and triggered the regulator of autophagy and lysosome biogenesis-transcription factor EB activation. But autophagic cargo clearance was tightly related to the charges of nanoparticles: neutral and anionic surface enhanced clearance of autophagic cargo, while cationic surface caused lysosomal dysfunction, reduced formation of autophagolysosomes, and finally blocked autophagic flux in HeLa cells [29]. There also showed different autophagic effects triggered by cationic or anionic PAMAM dendrimers during their-induced acute lung injury: Cationic PAMAM dendrimers G3 could obviously induce autophagosomes accumulation and enhance the expression of the microtubule-associated protein 1 light chain 3 in human lung A549 cells; while the same concentration of anionic PAMAM dendrimers G5.5 failed to trigger autophagy [18].
5.4.4 Surface Chemistry
Multiwalled carbon nanotubes could alter autophagy via interacting with cell membranes and membrane-associated molecules. While surface ligands modified by combinatorial chemistry on multiwalled carbon nanotubes could tune cell autophagy to various levels in different cell lines. Furthermore, multiwalled carbon nanotubes with different surface chemistries could induce autophagy in mTOR signaling pathway-dependent or -independent manner via binding to different cell surface receptors [30]. Fe3O4 nanoparticles extensively impaird lysosomes and led to LC3-positive autophagosomes formation, while PLGA-coated on the surface of Fe3O4 nanoparticles reduced this damaging effect on lysosomes. Moreover, Fe3O4 nanoparticles also induced mitochondrial dysfunction and endoplasmic reticulum/Golgi body stresses, which triggered autophagy, while PLGA-coated Fe3O4 nanoparticles reduced the devastating effect on these organelles. In vivo experimental results suggested that Fe3O4 nanoparticles led to massive autophagosomes accumulation in the kidney and spleen of mice when compared with the PLGA-coated Fe3O4 and PLGA nanoparticles [31]. Besides, N-alkyl-PEI-lactobionic acid wrapped superparamagnetic iron oxide nanocomposites showed better cell viability in RAW 264.7 cells when compared with the unsubstituted ones, and PEI induced cell autophagy can be reduced via lactose modification [32], confirmed that surface chemistry played an important role in nanoparticles-induced autophagy.
5.5 Cellular Mechanisms of Autophagy in Nanoparticles-Induced Toxicity
5.5.1 Lysosome Impairment
Lysosome is a membrane-bound organelle that consists of hydrolytic enzymes containing vesicles, and is the most important organelle in the final step of autophagy steps. Lysosomal dysfunction is the major mechanism by which nanoparticles trigger autophagy, and mainly including lysosomal ultrastructures damage, changes of lysosome pH, and disactivities of lysosomal proteases (Fig. 5.2) [33]. Wang et al., reported that silica nanoparticles induced autophagy dysfunction via lysosomal impairment and inhibition of autophagosome degradation in hepatocytes. They found that silica nanoparticles could trigger autophagy formation in two kinds of hepatocytes even at the noncytotoxic level and suppress the autophagic flux at high concentration. Silica nanoparticles impaired the lysosomal function through damaging lysosomal ultrastructures, increasing membrane permeability, and downregulating the expression of lysosomal proteases, cathepsin B [34]. Meanwhile, Ji and co-authors found that graphene oxide quantum dots induced autophagosome accumulation but blocked autophagic flux by decreasing the amount and enzymatic activity of cathepsin B and inhibiting lysosome proteolytic capacity in GC-2 and TM4 cells [35]. Besides, Fe3O4 nanoparticles extensively impaired lysosomes and led to the accumulation of LC3-positive autophagosomes, while PLGA-coated Fe3O4 nanoparticles reduced this destructive effect on lysosomes [31].
Apart from lysosmal ultrastructures damage and disactivaties of lysosomal proteases, changes of pH in lysosomes were also involved in lysosome impairment. Ma et al., investigated the mechanisms of gold nanoparticles-triggered autophagy in cells and found that the internalized gold nanoparticles was finally accumulated in lysosomes and caused impaired lysosome degradation ability through alkalinization of lysosomal pH during their-induced autophagosome accumulation from blocking autophagy flux [26]. While using another nanoparticles, Schutz et al., reported that internalized silica nanoparticles accumulated in lysosomes resulted in suppression of autophagy-mediated protein recycle and impaired degradation of internalized epidermal growth factor, while endosomal recycling proceeds was not disturbed. The phenotype was caused by perturbed delivery of cargo via autophagosomes and late endosomes to silica nanoparticles-filled cathepsin B/L-containing lysosomes, rather than elevated lysosomal pH or altered mTOR activity [36].
5.5.2 Mitochondria Dysfunction and Mitophagy
Mitochondria are major places for cells to producing energy and operating oxidative reaction; besides, mitochondria were also involved in many cell activities such as cell proliferation, differentiation, and apoptosis [37, 38]. In the year of 2010, Johnson-Lyles et al., investigated that renal proximal tubule cells exposed to fullerenol showed cytoskeletion disruption, autophagic vacuole accumulation, loss of cellular mitochondrial membrane potential and ATP depletion; moreover, fullerenol-induced ATP depletion and loss of mitochondrial potential were partially ameliorated by co-treatment with the autophagy inhibitor 3-MA, which confirmed the critical relation between mitochondrial dysfunction and autophagy [39]. Wu et al., investigate that iron core and gold shell nanoparticles could cause an irreversible membrane-potential loss in the mitochondria of cancer cells. Iron elements, before oxidation, triggered mitochondria-mediated autophagy was the key factor responsible for the differential cytotoxicity observed between cancerous and healthy cells [40]. Similarly, Fe3O4 nanoparticles could also cause mitochondrial damage and ER and Golgi body stresses, which induce autophagy, while PLGA-coated Fe3O4 nanoparticles reduce the destructive effect on these organelles [31].
Apart from mitochondrial dysfunction in nanoparticles-induced autophagy during their toxicity, mitophagy is one of the most direct mechanisms linking mitochondria and autophagy. Mitophagy is a kind of autophagy, which is the process of removing abnormal mitochondria through autophagy in cells [41]. The detailed process were: abnormal conditions such as reactive oxygen species, nutrition lacking could induce depolarized and damaged mitochondrion. Dysfunctional mitochondria were packaged into autophagosome and then fused and degraded in lysosomes [42].
Zhang et al., developed polyoxometalates nanoparticle-peptide conjugates targeting mitochondria to explore the interactions between nanoparticles and cells. Autophagy of Mitochondria induced by polyoxometalates nanoparticles-peptide conjugates was the cell response for the damaged organelles recycle via mitochondrial membrane potential-related molecular mediation [43]. Besides, Nano-alumina (Al2O3) could induce autophagy and mitochondria damage in primary cortical neuronal cells while the damaged mitochondria were removed by mitophagy [44].
5.5.3 Endoplasmic Reticulum Stress and Endoplasmic Reticulum Autophagy
Endoplasmic reticulum plays several common functions, including protein molecules folding and transporting synthesized proteins from vesicles to Golgi apparatus [45]. Abnormal conditions in redox and calcium regulation, glucose deficiency or viral infection can trigger endoplasmic reticulum stress response [46]. An accumulation of unfolded or misfolded proteins in the endoplasmic reticulum leads to stress conditions. Recent studies have revealed that endoplasmic reticulum stress can either stimulate or inhibit autophagy [47].
Titanium dioxide nanoparticles could induce endoplasmic reticulum stress inhuman bronchial epithelial cells and disrupted the mitochondria-associated endoplasmic reticulum membranes and calcium ion balance, thereby increasing autophagy. Tauroursodeoxycholic acid, inhibitor of endoplasmic reticulum stress, could significantly mitigated titanium dioxide nanoparticles-induced cellular toxic response [48]. In vivo experimental results also showed that endoplasmic reticulum and mitochondria were disrupted and dysfunctional in the TiO2 nanoparticles-exposed lung leading to abnormal autophagy [49]. Another mental oxide nanoparticles magnetic iron oxide nanoparticles could induce autophagy preceding apoptosis through mitochondrial damage and endoplasmic reticulum stress in RAW264.7 cells: Blocking of autophagosome formation may accelerate apoptotic cell death and endoplasmic reticulum stress [50].
Besides mental oxide nanoparticles, cationic polystyrene nanospheres could also induce autophagic cell death through the induction of endoplasmic reticulum stress. Cationic polystyrene nanospheres were highly toxic with enhanced uptake in RAW 264.7 macrophage and BEAS-2B lung epithelial cells. The nanoparticles could induce autophagic cell death, and the increased autophagic flux triggered by reactive oxygen species generation and endoplasmic reticulum stress was caused by misfolded protein accumulation. The inhibition of endoplasmic reticulum stress could impair cytotoxicity and autophagy in cationic polystyrene-treated cells [17].
The role of endoplasmic reticulum stress in nanoparticle-induced autophagy has been extensively studied, however, real time information about the endoplasmic reticulum involved autophagic process (endoplasmic reticulum autophagy) induced by nanoparticles remains confusing. Wei et al., reported that silica nanoparticles could be captured, accumulated in endoplasmic reticulum, and triggered autophagy in HCT-116 human colon cancer cells. The co-location of cells between endoplasmic reticulum, lysosomes and autophagic vacuoles confirmed that silica nanoparticles-induced endoplasmic reticulum autophagy. These findings enable us to know more about endoplasmic reticulum autophagy [51].
5.6 Molecular Mechanisms of Autophagy in Nanoparticles-Induced Toxicity
5.6.1 PI3K/Akt/mTOR Signaling Pathway
The phosphatidylinositol 3-kinase (PI3K)/Akt/mammalian target of rapamycin (mTOR) pathway plays critical role in multiple cellular functions, and is a major regulator of autophagy [52]. Upstream of mTOR PI3K/Akt signaling pathway could regulate mTOR activity [53]. Scientists have found that autophagy is negatively adjusted by the activation of mTOR as mTORC1 could regulate autophagy under unusual stressful conditions [54]. When dephosphorylated, mTOR will be inhibited and then trigger autophagy [55]. Besides, the ribosomal protein S6, one substrate of mTOR, was also involved in autophagy processes (Fig. 5.3).
Liu et al., reported that Akt-TSC2-mTOR signaling pathway played the key role in single-walled carbon nanotube-induced autophagy in A549 cells. Single-walled carbon nanotube impaired phosphorylated Akt, mTOR and its substrate ribosomal protein S6. Using siRNA to knockdown TSC2 could obviously enhance cell viability treated by carbon nanotube [11]. Using another non-mental nanoparticles in different cell model, Duan et al., confirmed that phosphorylated PI3K, Akt and mTOR were obviously suppressed in endothelial cells treated by silica nanoparticles in a dose-dependent manner [56]. For mental nanoparticles-induced autophagy, Roy et al., investigated that zinc oxide nanoparticles induced apoptosis by enhancement of autophagy via PI3K/Akt/mTOR inhibition. The phosphorylated levels of PI3K, Akt, and mTOR were significantly decreased during zinc oxide nanoparticles exposing on macrophage [57]. Apart from mental and non-mental nanoparticles, polymers could also induce autophagy via PI3K/Akt/mTOR signaling pathway: Chiu et al., found that Akt/mTOR signaling pathway was involved in cationic polystyrene nanospheres-induced autophagic cell death in macrophage and lung epithelial cells [17]. Meanwhile, Li et al., and Wang et al., both confirmed that cationic poly-amidoamine dendrimers could inhibit phosphorylation of Akt/mTOR during their-induced autophagy in hepatocyte and neuronal cells [19, 20].
5.6.2 MAPK/ERK Signaling Pathway
The mitogen-activated protein kinases (MAPKs) involving extracellular signaling-regulated kinase (ERK), was tightly related to reactive oxygen species induction [3]. Previous studies have suggested that ERK activation could contribute to autophagic effects and promote cell survival [58, 59]. Researchers also found that MAPK/ERK signaling pathway was involved in nanoparticles-induced autophagy during their-induced toxicity (Fig. 5.3).
Park et al., explored the underlying mechanism of iron oxide nanoparticles-induced autophagy in RAW 264.7 macrophage, and found that the autophagy related protein increased in a dose-dependent manner together with phosphorylated ERK [60]. Activated phosphorylated ERK was also involved in copper oxide nanoparticle-induced cytotoxicity in HaCaT human keratinocytes and mouse embryonic fibroblasts [61]. Besides, ERK activation also played important roles in the radio-sensitivity enhancement of silver nanoparticles; suppression of ERK could reduce autophagy levels triggered by silver nanoparticles [62]. Rinna et al., also explored effects of silver nanoparticles on mitogen-activated protein kinases activation, and confirmed the role of reactive oxygen species and implication in DNA damage during silver nanoparticles-induced toxicity [63].
5.6.3 Toll-Like Receptor Signaling Pathways
Chen et al., reported that grapheme oxide nanosheets could simultaneously induce autophagy, provoke the toll-like receptor signaling cascades, and trigger ensuing cytokine responses in RAW 264.7 macrophage cells. Grapheme oxide nanoparticles-induced autophagy was regulated by toll-like receptor 4 and toll-like receptor 9, suggested that autophagy was partly regulated by the toll-like receptors pathway in grapheme oxide nanoparticles-treated immune cells [64]. Using CT26 colon cancer cell model and mice model, they also confirmed that grapheme oxide nanoparticles could induce the toll-like receptors response and autophagy in cancer cells and showed antitumor effects. The grapheme oxide nanoparticles-triggered autophagy was regulated through the myeloid differentiation primary response gene 88- and tumor necrosis factor receptor associated factor 6-associated toll-like receptor-4/9 signaling pathways [65].
5.6.4 Hypoxia-Inducible Factor-1α
Hypoxia-inducible factor-1 is a heterodimer composed of α and β subunits and is the transcription factor, which mediates adaptive responses to hypoxia [66]. Hypoxia-inducible factor-1 is mainly regulated by oxygen-dependent changes and could regulate autophagy and other hypoxia-responses [67]. Lin et al., explored the role of hypoxia-inducible factor-1α in zinc oxide nanoparticle-induced nephrotoxicity in vitro and in vivo, and found that zinc oxide nanoparticles could enhance reactive oxygen species generation, apoptosis, autophagy, and hypoxia-inducible factor-1α signaling pathway in HEK-293 human embryonic kidney cells and mouse kidney tissues. Hypoxia-inducible factor -1α knockdown resulted in significantly decreased levels of autophagy and increased cytotoxicity in HEK-293 cells [68]. Silver nanoparticles induced reactive oxygen species generation in lung cancer cells could also trigger high susceptibility to oxidative stress, whereas pre-exposure to hypoxia blocked silver nanoparticles-induced oxidative stress. Hypoxia-inducible factor-1α inhibited silver nanoparticles-induced mitochondria-mediated apoptosis by regulating autophagic flux through the regulation of ATG5, LC3-II, and p62 [69].
5.6.5 Oxidative Stress
Reactive oxygen species are highly reactive molecules containing an oxygen atom, and are mainly produced by the mitochondrial and cytoplasmic oxidation processes under physiological conditions [70]. Reactive oxygen species could induce membrane damage that could influence inner proteins, lipid denaturation, and DNA structures [71]. An increasing amount of evidence indicated that nanoparticles could induce autophagy because of their large surface and positive charges, which is capable of inducing reactive oxygen species, during their-induced toxicity (Fig. 5.4) [72]. Cadmium-based quantum dot increased intracellular reactive oxygen species levels, affected mitochondrial function and induced autophagy, led to subsequent apoptosis in mouse renal adenocarcinoma cells. Antioxidant agent N-Acetylcysteine, reduced intracellular reactive oxygen species levels and impaired quantum dots-induced autophagy but enhanced cell death. Autophagic inhibitor 3-MA also reduced cell viability in quantum dots-treated cells, suggested that oxidative stress-induced autophagy played a survival mechanism against the cytotoxicity of quantum dots [73]. On the contrary, Fan and coworkers found that another kind of cadmium-based quantum dots CdTe/CdS 655 could induce autophagic cell death in vitro and in vivo. Suppress autophagy could attenuate the toxicity induced by cadmium-based quantum dots CdTe/CdS 655 [74].
Apart from quantum dots, mental oxide nanoparticles could also trigger autophagy via reactive oxygen species induction during their-induced toxicity. Yu et al., investigated the toxicity of zinc oxide nanoparticles and explored the underlying molecular mechanisms in normal skin cell model, and found that zinc oxide nanoparticles led to cell death through autophagic vacuole accumulation and mitochondria damage in normal skin cells via reactive oxygen species induction [14]. Apart from skin cells, acute exposure of immune cells to ZnO nanoparticles resulted in autophagic death and increased levels of LC3. Accordingly, ZnO nanoparticles-mediated upregulation of LC3 and induction of immune cell death were inhibited by blocking autophagy and reactive oxygen species production. Release of Zn2+ from ZnO nanoparticles triggered excessive intracellular reactive oxygen species production, and resulted in autophagic death of immune cells [15].
Besides, reactive oxygen species were also involved in non-mental nanoparticles and polymers-induced autophagy during their-triggered nanotoxicity. Silica nanoparticles could induce autophagy in a dose-dependent manner in HepG2 cells. The elevated reactive oxygen species level was in line with the increasing of autophagy activation, while both the autophagic inhibitor (3-MA) and reactive oxygen species inhibitor (N-Acetylcysteine) effectively suppressed the autophagy and cell death induced by silica nanoparticles [10]. The role of reactive oxygen species during silica nanoparticles-induced autophagy was also been explored in two other cell models: SiO2 nanoparticles induced reactive oxygen species -mediated autophagy in MRC-5 human lung fibroblast cells as a possible mechanism of cell survival [75]. And amorphous silica nanoparticles trigger vascular endothelial cell injury through apoptosis and autophagy via reactive oxygen species-mediated MAPK/Bcl-2 and PI3K/Akt/mTOR signaling [76].
Poly-amidoamine dendrimers induced reactive oxygen species and autophagy flux in PC-12 and SH-SY5Y neuronal cells and glioma cells [20]. Interestingly, autophagy might be triggered by the formation of reactive oxygen species induced by poly-amidoamine dendrimers. Suppression of reactive oxygen species could not only impair poly-amidoamine dendrimers-induced autophagic effects, but also reduce poly-amidoamine dendrimers-induced neuronal cell death [77] (Fig. 5.4).
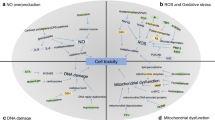
Crosstalk of physicochemical and biochemical mechanisms of autophagy in nanoparticles-induced toxicity. (1) Physicochemical mechanisms including dispersity, size, charge, and surface chemistry of nanoparticles; (2) Nanoparticles triggered reactive oxygen species induced mitochondria dysfunction and endoplasmic reticulum stress. (3) Mitochondria dysfunction and endoplasmic reticulum stress was involved in nanoparticles-induced autophagic cell death
5.7 Conclusion
As nanoparticles possess unique physical structures and specific properties, series of nanoparticles have been developed in the past few decades for wide industry or medicinal application. Because nanoparticles could highly interact with cells after they entering into bodies, they may cause nanotoxicity and induce body damage, which could seriously limit their application. Although the underlying mechanisms of nanotoxicity triggered by nanoparticles have been widely investigated, unfortunately, our understanding of the role of autophagy and its related mechanisms in nanotoxicity is still poor. Thus, it is an instant need for researchers to address the questions about the role and mechanism of autophagy in nanoparticles-induced toxicity. While evaluating the mechanisms of autophagy in nanoparticles-induced toxicity, the dispersity, size, surface charge, and surface modification of nanoparticles were expected to present critical roles in nanoparticles-triggered autophagy, and should be cautiously considered. Besides, the research initiatives highlighted in the chapter showed cellular and molecular mechanisms of autophagy during nanoparticles-induced toxicity, including lysosome impairment, mitochondria dysfunction, endoplasmic reticulum stress, PI3K/Akt/mTOR signaling pathway, MAPK/ERK signaling pathway, toll-like receptor pathway, Hypoxia-inducible factor, and oxidative stress. These mechanisms of autophagy triggered by nanoparticles might be help for evaluating nanotoxicity, and might offer a foundation for deeply design safe nanoparticles in the future.
References
Li J, Fan C, Pei H et al (2013) Smart drug delivery nanocarriers with self-assembled DNA nanostructures. Adv Mater 25:4386–4396
Linko V, Ora A, Kostiainen MA (2015) DNA nanostructures as smart drug-delivery vehicles and molecular devices. Trends Biotechnol 33:586–594
Fu PP, Xia Q, Hwang HM et al (2014) Mechanisms of nanotoxicity: generation of reactive oxygen species. J Food Drug Anal 22:64–75
Liu Y, Liang J, Wang Q et al (2016) Copper nanoclusters trigger muscle cell apoptosis and atrophy in vitro and in vivo. J Appl Toxicol 36:454–463
Deter RL, De Duve C (1967) Influence of glucagon, an inducer of cellular autophagy, on some physical properties of rat liver lysosomes. J Cell Biol 33:437–449
Glick D, Barth S, Macleod KF (2010) Autophagy: cellular and molecular mechanisms. J Pathol 221:3–12
Mizushima N (2007) Autophagy: process and function. Genes Dev 21:2861–2873
Zeng X, Zhao H, Li Y et al (2015) Targeting Hedgehog signaling pathway and autophagy overcomes drug resistance of BCR-ABL-positive chronic myeloid leukemia. Autophagy 11:355–372
Levine B, Kroemer G (2008) Autophagy in the pathogenesis of disease. Cell 132:27–42
Yu Y, Duan J, Yu Y et al (2014) Silica nanoparticles induce autophagy and autophagic cell death in HepG2 cells triggered by reactive oxygen species. J Hazard Mater 270:176–186
Liu HL, Zhang YL, Yang N et al (2011) A functionalized single-walled carbon nanotube-induced autophagic cell death in human lung cells through Akt-TSC2-mTOR signaling. Cell Death Dis 2:e159
Park EJ, Zahari NE, Lee EW et al (2014c) SWCNTs induced autophagic cell death in human bronchial epithelial cells. Toxicol In Vitro 28:442–450
Sun T, Yan Y, Zhao Y et al (2012) Copper oxide nanoparticles induce autophagic cell death in A549 cells. PLoS One 7:e43442
Yu KN, Yoon TJ, Minai-Tehrani A et al (2013) Zinc oxide nanoparticle induced autophagic cell death and mitochondrial damage via reactive oxygen species generation. Toxicol In Vitro 27:1187–1195
Johnson BM, Fraietta JA, Gracias DT et al (2015) Acute exposure to ZnO nanoparticles induces autophagic immune cell death. Nanotoxicology 9:737–748
Chen Y, Yang L, Feng C et al (2005) Nano neodymium oxide induces massive vacuolization and autophagic cell death in non-small cell lung cancer NCI-H460 cells. Biochem Biophys Res Commun 337:52–60
Chiu HW, Xia T, Lee YH et al (2015) Cationic polystyrene nanospheres induce autophagic cell death through the induction of endoplasmic reticulum stress. Nanoscale 7:736–746
Li C, Liu H, Sun Y et al (2009) PAMAM nanoparticles promote acute lung injury by inducing autophagic cell death through the Akt-TSC2-mTOR signaling pathway. J Mol Cell Biol 1:37–45
Li Y, Zeng X, Wang S et al (2015a) Inhibition of autophagy protects against PAMAM dendrimers-induced hepatotoxicity. Nanotoxicology 9:344–355
Wang S, Li Y, Fan J et al (2014) The role of autophagy in the neurotoxicity of cationic PAMAM dendrimers. Biomaterials 35:7588–7597
Zhou W, Miao Y, Zhang Y et al (2013) Induction of cyto-protective autophagy by paramontroseite VO2 nanocrystals. Nanotechnology 24:165102
Lin J, Huang Z, Wu H et al (2014) Inhibition of autophagy enhances the anticancer activity of silver nanoparticles. Autophagy 10:2006–2020
Huang D, Zhou H, Gao J (2015) Nanoparticles modulate autophagic effect in a dispersity-dependent manner. Sci Rep 5:14361
Seleverstov O, Zabirnyk O, Zscharnack M et al (2006) Quantum dots for human mesenchymal stem cells labeling. A size-dependent autophagy activation. Nano Lett 6:2826–2832
Mishra AR, Zheng J, Tang X et al (2016) Silver nanoparticle-induced autophagic-lysosomal disruption and NLRP3-inflammasome activation in HepG2 cells is size-dependent. Toxicol Sci 150:473–487
Ma X, Wu Y, Jin S et al (2011) Gold nanoparticles induce autophagosome accumulation through size-dependent nanoparticle uptake and lysosome impairment. ACS Nano 5:8629–8639
Li Q, Hu H, Jiang L et al (2016) Cytotoxicity and autophagy dysfunction induced by different sizes of silica particles in human bronchial epithelial BEAS-2B cells. Toxicol Res 5:1216–1228
Huang D, Zhou H, Gong X et al (2017) CSilica sub-microspheres induce autophagy in an endocytosis dependent manner. RSC Adv 7:12496–12502
Song W, Popp L, Yang J et al (2015) The autophagic response to polystyrene nanoparticles is mediated by transcription factor EB and depends on surface charge. J Nanobiotechnol 13:87
Wu L, Zhang Y, Zhang C et al (2014) Tuning cell autophagy by diversifying carbon nanotube surface chemistry. ACS Nano 8:2087–2099
Zhang X, Zhang H, Liang X et al (2016) Iron oxide nanoparticles induce autophagosome accumulation through multiple mechanisms: lysosome impairment, mitochondrial damage, and ER stress. Mol Pharm 13:2578–2587
Du J, Zhu W, Yang L et al (2016) Reduction of polyethylenimine-coated iron oxide nanoparticles induced autophagy and cytotoxicity by lactosylation. Regen Biomater 3:223–229
Stern ST, Adiseshaiah PP, Crist RM (2012) Autophagy and lysosomal dysfunction as emerging mechanisms of nanomaterial toxicity. Part Fibre Toxicol 9:20
Wang J, Yu Y, Lu K et al (2017) Silica nanoparticles induce autophagy dysfunction via lysosomal impairment and inhibition of autophagosome degradation in hepatocytes. Int J Nanomedicine 12:809–825
Ji X, Xu B, Yao M et al (2016) Graphene oxide quantum dots disrupt autophagic flux by inhibiting lysosome activity in GC-2 and TM4 cell lines. Toxicology 374:10–17
Schutz I, Lopez-Hernandez T, Gao Q et al (2016) Lysosomal dysfunction caused by cellular accumulation of silica nanoparticles. J Biol Chem 291:14170–14184
Miettinen TP, Bjorklund M (2017) Mitochondrial function and cell size: an allometric relationship. Trends Cell Biol 27:393–402
Altshuler-Keylin S, Kajimura S (2017) Mitochondrial homeostasis in adipose tissue remodeling. Sci Signal 10:eaai9248
Johnson-Lyles DN, Peifley K, Lockett S et al (2010) Fullerenol cytotoxicity in kidney cells is associated with cytoskeleton disruption, autophagic vacuole accumulation, and mitochondrial dysfunction. Toxicol Appl Pharmacol 248:249–258
Wu YN, Yang LX, Shi XY et al (2011) The selective growth inhibition of oral cancer by iron core-gold shell nanoparticles through mitochondria-mediated autophagy. Biomaterials 32:4565–4573
Fivenson EM, Lautrup S, Sun N et al (2017) Mitophagy in neurodegeneration and aging. Neurochem Int 109:202–209
Kim MJ, Yoon JH, Ryu JH (2016) Mitophagy: a balance regulator of NLRP3 inflammasome activation. BMB Rep 49:529–535
Zhang Z, Zhou L, Zhou Y et al (2015) Mitophagy induced by nanoparticle-peptide conjugates enabling an alternative intracellular trafficking route. Biomaterials 65:56–65
Chang L, Guo W, Ge C et al (2014) Effect of nano-alumina on mitophagy in primary cortical neuronal cells from Wistar newborn rats. Chin J Pharmacol Toxicol 5:737–742
Preston GM, Brodsky JL (2017) The evolving role of ubiquitin modification in endoplasmic reticulum-associated degradation. Biochem J 474:445–469
Lee WS, Yoo WH, Chae HJ (2015) ER stress and autophagy. Curr Mol Med 15:735–745
Rashid HO, Yadav RK, Kim HR et al (2015) ER stress: autophagy induction, inhibition and selection. Autophagy 11:1956–1977
Yu KN, Chang SH, Park SJ et al (2015a) Titanium dioxide nanoparticles induce endoplasmic reticulum stress-mediated autophagic cell death via mitochondria-associated endoplasmic reticulum membrane disruption in normal lung cells. PLoS One 10:e0131208
Yu KN, Sung JH, Lee S et al (2015b) Inhalation of titanium dioxide induces endoplasmic reticulum stress-mediated autophagy and inflammation in mice. Food Chem Toxicol 85:106–113
Park EJ, Choi DH, Kim Y et al (2014a) Magnetic iron oxide nanoparticles induce autophagy preceding apoptosis through mitochondrial damage and ER stress in RAW264.7 cells. Toxicol In Vitro 28:1402–1412
Wei F, Wang Y, Luo Z et al (2017) New findings of silica nanoparticles induced ER autophagy in human colon cancer cell. Sci Rep 7:42591
McAuliffe PF, Meric-Bernstam F, Mills GB et al (2010) Deciphering the role of PI3K/Akt/mTOR pathway in breast cancer biology and pathogenesis. Clin Breast Cancer 10(Suppl 3):S59–S65
Heras-Sandoval D, Perez-Rojas JM, Hernandez-Damian J et al (2014) The role of PI3K/AKT/mTOR pathway in the modulation of autophagy and the clearance of protein aggregates in neurodegeneration. Cell Signal 26:2694–2701
Jung CH, Ro SH, Cao J et al (2010) mTOR regulation of autophagy. FEBS Lett 584:1287–1295
Yu X, Long YC, Shen HM (2015c) Differential regulatory functions of three classes of phosphatidylinositol and phosphoinositide 3-kinases in autophagy. Autophagy 11:1711–1728
Duan J, Yu Y, Yu Y et al (2014) Silica nanoparticles induce autophagy and endothelial dysfunction via the PI3K/Akt/mTOR signaling pathway. Int J Nanomedicine 9:5131–5141
Roy R, Singh SK, Chauhan LK et al (2014) Zinc oxide nanoparticles induce apoptosis by enhancement of autophagy via PI3K/Akt/mTOR inhibition. Toxicol Lett 227:29–40
Ogier-Denis E, Pattingre S, El Benna J et al (2000) Erk1/2-dependent phosphorylation of Galpha-interacting protein stimulates its GTPase accelerating activity and autophagy in human colon cancer cells. J Biol Chem 275:39090–39095
Cagnol S, Chambard JC (2010) ERK and cell death: mechanisms of ERK-induced cell death – apoptosis, autophagy and senescence. FEBS J 277:2–21
Park EJ, Umh HN, Kim SW et al (2014b) ERK pathway is activated in bare-FeNPs-induced autophagy. Arch Toxicol 88:323–336
Luo C, Li Y, Yang L et al (2014) Activation of Erk and p53 regulates copper oxide nanoparticle-induced cytotoxicity in keratinocytes and fibroblasts. Int J Nanomedicine 9:4763–4772
Wu H, Lin J, Liu P et al (2015) Is the autophagy a friend or foe in the silver nanoparticles associated radiotherapy for glioma? Biomaterials 62:47–57
Rinna A, Magdolenova Z, Hudecova A et al (2015) Effect of silver nanoparticles on mitogen-activated protein kinases activation: role of reactive oxygen species and implication in DNA damage. Mutagenesis 30:59–66
Chen GY, Yang HJ, Lu CH et al (2012) Simultaneous induction of autophagy and toll-like receptor signaling pathways by graphene oxide. Biomaterials 33:6559–6569
Chen GY, Chen CL, Tuan HY et al (2014) Graphene oxide triggers toll-like receptors/autophagy responses in vitro and inhibits tumor growth in vivo. Adv Healthc Mater 3:1486–1495
Semenza GL, Wang GL (1992) A nuclear factor induced by hypoxia via de novo protein synthesis binds to the human erythropoietin gene enhancer at a site required for transcriptional activation. Mol Cell Biol 12:5447–5454
Bruick RK, McKnight SL (2001) A conserved family of prolyl-4-hydroxylases that modify HIF. Science 294:1337–1340
Lin YF, Chiu IJ, Cheng FY et al (2016) The role of hypoxia-inducible factor-1alpha in zinc oxide nanoparticle-induced nephrotoxicity in vitro and in vivo. Part Fibre Toxicol 13:52
Jeong JK, Gurunathan S, Kang MH et al (2016) Hypoxia-mediated autophagic flux inhibits silver nanoparticle-triggered apoptosis in human lung cancer cells. Sci Rep 6:21688
Indo HP, Hawkins CL, Nakanishi I et al (2017) Role of mitochondrial reactive oxygen species in the activation of cellular signals, molecules, and function. Handb Exp Pharmacol 240:439–456
Chen Y, Gibson SB (2008) Is mitochondrial generation of reactive oxygen species a trigger for autophagy? Autophagy 4:246–248
Anozie UC, Dalhaimer P (2017) Molecular links among non-biodegradable nanoparticles, reactive oxygen species, and autophagy. Adv Drug Deliv Rev 17:30001–30007
Luo YH, Wu SB, Wei YH et al (2013) Cadmium-based quantum dot induced autophagy formation for cell survival via oxidative stress. Chem Res Toxicol 26:662–673
Fan J, Sun Y, Wang S et al (2016) Inhibition of autophagy overcomes the nanotoxicity elicited by cadmium-based quantum dots. Biomaterials 78:102–114
Petrache Voicu SN, Dinu D, Sima C et al (2015) Silica nanoparticles induce oxidative stress and autophagy but not apoptosis in the MRC-5 cell line. Int J Mol Sci 16:29398–29416
Guo C, Yang M, Jing L et al (2016) Amorphous silica nanoparticles trigger vascular endothelial cell injury through apoptosis and autophagy via reactive oxygen species-mediated MAPK/Bcl-2 and PI3K/Akt/mTOR signaling. Int J Nanomedicine 11:5257–5276
Li Y, Zhu H, Wang S et al (2015b) Interplay of oxidative stress and autophagy in PAMAM dendrimers-induced neuronal cell death. Theranostics 5:1363–1377
Acknowledgments
This work was supported by grants from the National Key Basic Research Program of China (2015CB931800, 2013CB932502), and the National Natural Science Foundation of China (81573332, 81773620). Yubin Li would like to acknowledge his baby girl Charlotte (Qianxun) Li.
Conflict of Interests
All the Figures in “The Role of Autophagy in Nanoparticles-Induced Toxicity and Its Related Cellular and Molecular Mechanisms” are original, unpublished materials designed and prepared by Yubin Li and Dianwen Ju. The authors declared that there’s no conflict of interests.
Author information
Authors and Affiliations
Corresponding authors
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 The Author(s)
About this chapter
Cite this chapter
Li, Y., Ju, D. (2018). The Role of Autophagy in Nanoparticles-Induced Toxicity and Its Related Cellular and Molecular Mechanisms. In: Saquib, Q., Faisal, M., Al-Khedhairy, A., Alatar, A. (eds) Cellular and Molecular Toxicology of Nanoparticles. Advances in Experimental Medicine and Biology, vol 1048. Springer, Cham. https://doi.org/10.1007/978-3-319-72041-8_5
Download citation
DOI: https://doi.org/10.1007/978-3-319-72041-8_5
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-72040-1
Online ISBN: 978-3-319-72041-8
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)