Abstract
ECMO support should be considered whenever a critically ill patient has severe respiratory or cardiorespiratory failure and conventional circulatory or respiratory therapy has failed or is anticipated to be inadequate to support vital organ function. Intuitively, like most acute life-supportive interventions, the earlier ECMO support is initiated when required, the more likely a desired clinical outcome can be achieved. Patients considered appropriate for ECMO support should have reversible clinical conditions that are expected to have realistic chances of recovery or have available options for more durable, long-term support when the primary indication is for circulatory failure. The variety of different clinical scenarios in which ECMO support has been utilized has markedly expanded over the last two decades, particularly as management of critically ill patients has improved and ECMO technology continues to evolve and is applied by a wider clinical audience. It is now important for all intensivists to have a basic understanding of ECMO principles and practices and particularly the indications for referral for consideration of ECMO.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
- ECMO
- Respiratory failure
- Acute respiratory distress syndrome
- Extracorporeal membrane oxygenation
- Venovenous
- Venoarterial
Indications for ECMO
ECMO support should be considered whenever a critically ill patient has severe respiratory or cardiorespiratory failure and conventional circulatory or respiratory therapy has failed or is anticipated to be inadequate to support vital organ function. Intuitively, like most acute life-supportive interventions, the earlier ECMO support is initiated when required, the more likely a desired clinical outcome can be achieved. Patients considered appropriate for ECMO support should have reversible clinical conditions that are expected to have realistic chances of recovery or have available options for more durable, long-term support when the primary indication is for circulatory failure.
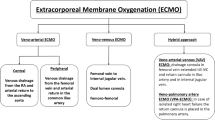
The variety of different clinical scenarios in which ECMO support has been utilized has markedly expanded over the last two decades, particularly as management of critically ill patients has improved and ECMO technology continues to evolve and is applied by a wider clinical audience. Acute refractory hypoxemic respiratory failure and cardiogenic shock remain the most common diagnoses for which ECMO support is instituted, but as shown by the illustration below (Fig. 67.1), the role of ECMO continues to expand.
Patients who decompensate due to progressive deterioration of chronic diseases which have failed treatment, those who have suffered acute devastating neurocognitive injury, and patients who have terminal illnesses with poor life expectancy are generally not considered appropriate for ECMO support. Also, the initiation of ECMO would be inappropriate for individual patients whose known goals of care would be clearly incompatible with ECMO support. As such, there currently remains no clearly defined standard in which ECMO support should or should not be utilized in critically ill patients. The Extracorporeal Life Support Organization (ELSO), a nonprofit international consortium for ECMO education and implementation, has presented its organizational guidelines for general indications and contraindications for ECMO support as outlined in Table 67.1 and available on their website: www.elso.org.
ECMO Circuit and Configurations
All current ECMO circuits consist of the following basic components: (1) large-diameter cannula(s) for drainage of deoxygenated venous blood from the patient, (2) cannula(s) for returning oxygenated blood back to the patients’ circulatory system, (3) an extracorporeal regulated pump to “circulate” blood between the patient and (4) gas-exchange device (aka, oxygenator), and a (5) heat exchanger to help regulate body temperature; these components are connected with circuit blood tubing. Additional devices can increase the complexity of the basic ECMO circuit and include various monitoring and measuring devices and different connectors to allow access for additional therapeutic modalities such as hemofiltration and renal replacement therapy.
Establishing good vascular access for cannula insertion and positioning is critical to achieving adequate ECMO support. The selection of vascular access is determined by various factors which should take into account the patient’s age and size, the type and amount of support required, and the individual clinical scenario. The sites of vascular access for ECMO support determine the ECMO configuration: whether it is for respiratory support only via venovenous ECMO (VV-ECMO) or for both cardiorespiratory support via venoarterial ECMO (VA-ECMO). In VV-ECMO, vascular access for cannulation of the drainage cannula for deoxygenated blood and of the return cannula for oxygenated blood to the patient are both in the venous system (Fig. 67.2). In VA-ECMO, vascular access for the drainage cannula is in the patient’s venous system, but the return cannula is inserted in the arterial system, typically the femoral artery.
Typically, the large peripheral vessels are cannulated for VV-ECMO or VA-ECMO in the adult patient, and most commonly the common femoral vein (CFV) or the internal jugular vein (IJ) and the common femoral artery (CFA) are chosen. In some circumstances where VA-ECMO support is required, transthoracic ECMO cannulation may be preferred. Typically, this occurs when patients are unable to be weaned from cardiopulmonary bypass after cardiac surgery or after post-sternotomy resuscitation. Under these circumstances, access to the right atrium for inserting a drainage cannula and the ascending aorta for the return cannula is readily available. Frequently the same vascular access used for cannulation during cardiopulmonary bypass can be used. However, since these cannulation techniques are not routinely performed by non-cardiothoracic surgeons or surgical intensivists, the procedure of transthoracic or central cannulation for VA-ECMO will not be further discussed. Procedural and technical details will focus on peripheral cannulation, which is most commonly utilized in general/trauma surgical critical care patients requiring ECMO support.
Procedure and Technique
Cannula Selection
Ideally the largest diameter and shortest drainage cannula that can be inserted in the vein is chosen. The drainage cannula usually has both end and side holes to allow blood flow to the ECMO pump and oxygenator even if the end hole becomes blocked. Various cannula manufacturers report tested flow rates with associated pressure gradients according to their cannula diameters. However, for most adult patients, our size selection ranges from 23 Fr to 29 Fr for the drainage cannula: usually for patients with smaller body habitus and BMI or those patients whom high ECMO flows are not required for cardiorespiratory support, we choose a size 23 Fr or 25 Fr cannula. We prefer a size 27 Fr or 29 Fr drainage cannula for male patients, and those we anticipate will need higher ECMO flows. The venous drainage cannula is commercially available in different lengths, and the length should be selected based on the distance of from the planned insertion site to the right atrium, which will vary depending on the patient’s torso length.
The arterial or return cannulas are smaller in diameter, and we keep available sizes from 17 Fr to 23 Fr. We customarily prefer to use a 17Fr or 19Fr size cannula for the artery for VA-ECMO and a 21 Fr or 23 Fr size return cannula for VV-ECMO, depending on the body size of the patient and the flow requirements anticipated to be required for respiratory support.
A dual-lumen single cannula (Avalon Elite®, Maquet, Wayne, NJ, USA) is also commercially available for VV-ECMO support. This single catheter cannula inserted in the right internal jugular vein has two lumens: one to allow venous drainage of deoxygenated blood from the SVC and IVC and a second lumen to allow simultaneous reinfusion of oxygenated return blood directed toward the tricuspid valve of the right atrium (Fig. 67.2b). For adult patients, the cannula sizes used typically range from 19 Fr to 31 Fr. Generally, we reserve insertion of this particular cannula in patients who we anticipate will require a long duration of VV-ECMO support, are able to be ambulatory, and can have this cannulation procedure performed in a nonurgent/emergent fashion with the availability of transesophageal echocardiography and fluoroscopy (e.g., pre-lung transplant candidate patients who acutely decompensate and are already on the waiting list). In most cases of VV-ECMO, we prefer to use separate single-lumen cannulas given the simpler technical considerations for percutaneous insertion at the patient’s bedside (Fig. 67.2a).
Cannula Insertion Site
The most common sites for peripheral cannulation for VV-ECMO and VA-ECMO in adults are the common femoral vein for drainage of deoxygenated blood and either the internal jugular vein or the common femoral artery for VV-ECMO and VA-ECMO, respectively. The direct route of the right internal jugular vein to the superior vena cava makes this vein preferred over the left internal jugular vein. Likewise, for VA-ECMO, we prefer to insert the drainage cannula via the right common femoral vein whenever possible and use the opposite left groin for the return arterial cannula. Other sites for peripheral ECMO cannulation can also be used but are less common. For VA-ECMO, axillary artery has been described for the arterial access, and for VV-ECMO, the use of both left and right common femoral veins have also been used, particularly whenever access to the left or right internal jugular vein is not available for insertion of the return cannula. The subclavian vein is uncommonly used for vascular access in VV-ECMO given the challenges with applying direct pressure to the vein during cannula removal once ECMO support is no longer required.
Vascular access of these vessels can be achieved by the percutaneous Seldinger technique or by the open cut-down approach. For obvious reasons, the percutaneous approach is preferred, but preparation and equipment to convert to an open incision approach should always be readily available at the patient’s bedside. Difficulties encountered during percutaneous insertion may warrant expedited conversion to direct open cannulation techniques. A list of basic equipment we keep in our vascular access tray is listed in Table 67.2.
Pre-ECMO Cannulation Preparation
Once a patient has been decided to be placed on ECMO support and the decision for either VV-ECMO or VA-ECMO support has been made, it is important to optimize conditions for cannulation. Frequently, decisions will have to be made about whether first obtaining additional vascular access for administration of necessary medications and fluid therapy and hemodynamic and laboratory monitoring is necessary. Whenever feasible, it is often easier to have inserted the additional central venous catheters and invasive arterial monitoring lines needed before rather than after the ECMO cannulation procedure. This opportunity often depends on the urgency of requiring ECMO support, however. It is also important to ensure that the ECMO circuit is primed and ready to use and the ECMO personnel who will manage the ECMO device and circuit are also present and available. The provider team that will provide analgesia and sedation during the cannulation procedure should also have vasoactive medications and fluids readily available to administer as it is not uncommon that patients manifest labile hemodynamics during ECMO initiation.
The patient should be positioned supine and flat, and comfortable access to the planned vascular access sites should be prepared. Vascular access and ECMO cannulation should be performed as sterilely as possible, and therefore the skin sites should be widely prepped with chlorohexidine or other antibacterial, antiseptic topical agent. One should consider antiseptic skin prep for alternative vascular access sites should one be unsuccessful in percutaneously accessing the intended vessel for cannulation. Like any other central venous line access procedure, the patient should be fully covered with a sterile surgical drape to reduce the incidence of cannula site infection. Full personal protective equipment should be worn by those individuals who will be actually performing the cannulation procedure.
Vascular Access and ECMO Cannulation
Percutaneous access of the internal jugular vein or common femoral vein is performed similar to obtaining access for central venous line placement using the Seldinger technique. We administer of an intravenous bolus of unfractionated heparin before we start the cannulation procedure, usually 3000–5000 units, if not contraindicated by bleeding concerns. Although not required, safe practice for central venous line placement would recommend that vessel puncture should be carried out under ultrasound guidance whenever circumstances allow. Ultrasound imaging provides visual assessment of the relationship of the vein to the adjacent artery and idea of the relative depth the catheter needle trocar needs to traverse from the skin to puncture the vessel. Importantly, ultrasound allows an assessment of the vessel diameter relative to the cannula chosen. Ultrasound visualization of the needle in the desired vessel and subsequently also the guidewire is obtained. We specifically try to avoid the “through-and-through” technique with vessel puncture; purposefully “backwalling” the artery, in particular, and then slowly withdrawing the needle into the lumen has been associated with troublesome hematoma formation and subsequent difficulty passing the guidewire smoothly. We use the 6 Fr micropuncture set (Micropuncture introducer kit, Cook Medical) to obtain initial percutaneous access of the vessel initially. The 6 Fr needle trocar can accommodate a 0.035 in. guidewire which is then inserted in the vessel to be cannulated. For venous cannulation, we use a 150 cm guidewire; for cannulation of the common femoral artery, a 100 cm length guidewire is usually sufficient. It is particularly important to ensure that guidewire inserts easily through the needle catheter and passes proximally easily within the vessel.
Once sufficient guidewire length has been passed into the vessel; we use hydrophilic dilators to sequentially enlarge the vessel over the guidewire (Sorin Vascular Dilator Kit, LivaNova, USA). In our experience, it is important to dilate the vessel in a stepwise fashion and avoid skipping dilator sizes; the vessel should be dilated to the size of the intended diameter of the cannula to inserted. Care should be taken to avoid kinking the guidewire anywhere along its length when passing the dilators over the guidewire, and it is crucial to check that the guidewires move back and forth easily as the dilator is progressively inserted over the wire. Important areas where the guidewire frequently kinks is at the level of the skin, at the enveloping vascular sheath, and with the internal jugular vein, at the level of the sternocleidomastoid muscle and at the level of the sternal notch where the vessel passes through the thoracic inlet. We find it occasionally useful to enlarge the skin incision where the guidewire passes across the skin to facilitate smooth insertion of the dilators. This is especially true in obese individuals with groin cannulation. If the guidewire becomes kinked, it can be difficult to pass subsequent dilators and the cannula itself without excessive force and unsafe to do so; when this does occur, it is often easier to proceed by first exchanging the kinked guidewire for new guidewire.
The selected cannula should be able to pass along the guidewire without undue pressure. It is customary to insert the venous drainage cannula first, followed by insertion of the return/arterial cannula. If the same groin side is being cannulated for VA-ECMO, it is often easier to first obtain vascular access of both the vein and artery before proceeding with dilation and insertion of the venous cannula.
The length of venous cannula insertion can be estimated by the patient’s surface anatomy. For venous cannulation from the groin, the length to be inserted can be estimated by summing the measured distance from the groin skin puncture site to the umbilicus and then to a point just superior to the level of xiphoid. For cannula insertion of the internal jugular vein, the distance to be inserted can be estimated by the distance to the level of the sternomanubrial junction. With cannulation of the femoral artery and the cannula has side holes, it is critical that the most proximal hole is well within the vessel. We always prefer to insert the cannula deeper and have to pull the cannula back rather than have to push the cannula that has been inserted too short, particularly once the introducer and guidewire have been removed.
Once the cannula is inserted to its desired length, we remove the tapered cannula introducer and guidewire, verify that there is appropriate blood backflow into the cannula, and then occlude the proximal end of the cannula with a large atraumatic straight clamp. After both the drainage and return cannulas have been inserted, we connect and secure the proximal end of the drainage cannula to the ECMO circuit tubing, followed by next connecting the return cannula to the ECMO circuit. This is all done sterilely and taking necessary precautions to avoid introducing air bubbles inside the circuit.
Next, we confirm that there are no air bubbles detected along the circuit and cannulas, the clamp on the drainage cannula is removed first, followed by removing the clamp on the return cannula, and the ECMO flow is initiated. For VA-ECMO, we like to ensure that the ACT measurement before starting the ECMO flow is ≥220; if there is concern that the ACT level may be too low, we will empirically administer another heparin bolus (usually 2000 units) just before ECMO initiation. We are less particular with the anticoagulation with VV-ECMO flow initiation given the decreased risk of arterial system embolization. The ECMO cannulas then are sutured securely to the skin.
Cannulation with the dual-lumen, single cannula for VV-ECMO is technically more challenging. This cannula is inserted in the right internal jugular vein and is typically placed percutaneously. To achieve the necessary ECMO flow rates, the cannula needs to be larger in diameter than the single-lumen cannula sizes that are typically inserted in the RIJ vein for VV-ECMO. We typically select a 27 Fr cannula with the general size used ranging from 23 Fr to 31 Fr in adult patients. Vascular access is obtained by Seldinger technique under ultrasound guidance as is typically performed for central venous catheter insertion. Ultrasound evaluation is useful in gauging whether inserting this larger diameter cannula in the patient’s jugular vein is feasible. Once the guidewire is inserted in the jugular vein, we visualize the trajectory of the guidewire down to the infradiaphragmatic IVC. We rely on fluoroscopy to ensure that the guidewire does not unintentionally loop in the heart or traverse the tricuspid valve. If fluoroscopy is not readily available, plain X-ray films can be obtained serially as required, but it is critical that the guidewire course be direct from the R internal jugular vein across the right atrium and into the inferior vena cava without redundancy or loops. In our opinion, relying solely on echocardiography to determine guidewire location can be misleading; we have experienced circumstances where the guidewire appears by transesophageal echo to pass directly from the SVC across the right atrium and into the IVC, but when fluoroscopy is used, there is actually a loop in the guidewire.
Once guidewire access is obtained and confirmed by fluoroscopy, serial dilation of the insertion tract is performed. It useful to use a hemostat to spread and stretch the skin and subcutaneous tissue at the insertion site alongside the guidewire with each serial dilation. After the last dilator has been used, the cannula is then inserted over the guidewire with its introducer and followed directly with fluoroscopy as the cannula is guided into correct position. Again, fluoroscopy is used to ensure that the cannula and its introducer do not kink the guidewire as they pass over the wire and inadvertently cause right atrial or ventricular wall injury. The tip of the cannula should reside just caudal to the IVC-RA junction within the IVC, and the outflow opening should be rotated toward the tricuspid valve with use of echo. When the dual-lumen cannula is oriented correctly, the outflow arm of the return cannula should be directed medially and lie toward the right ear of the patient.
Cannulation Complications
Cannula placement complications are primarily related to the proficiency with the Seldinger technique and the imaging method utilized to guide percutaneous access to the vessel. In addition, some patient circumstances, such as the placement of an arterial cannula during active CPR, can lead to higher complication rates.
As part of our bedside cannulation strategy, venous access to the internal jugular and common femoral veins is consistently preformed under direct ultrasound guidance. A J-wire is then advanced and stopped when premature ventricular contractions are triggered. We then preform sequential dilations to the diameter of the selected cannula. The most frequent technical complication that we observe during this process is bending the J-wire. In our experience this occurs due to differences between the puncture access angle and the angle in which the dilators are being progressed. As a result, a false path is created. Under these circumstances the wire will need to be exchanged which may require accessing the vein at a different level or choosing a different vessel. This can be particularly challenging if the wire is compromised when trying to access the right internal jugular vein. Under these circumstances, bifemoral VV-ECMO may be the only exit strategy. We normally avoid the use of stiffer guidewires, i.e., Amplatz; in our experience, they are associated with increased vascular injury. Pneumothoraxes, right ventricle rupture, pericardial tamponade, and perforation of the IVC are some of the other complications associated with VV-ECMO cannulation.
Once ECMO is initiated, migration of the cannula can result in lower oxygenation and flow, particularly with the use of bi-caval dual-lumen cannulas. Migration of the distal lumen into the right atrium can increase recirculation and decrease flow. Reduced drainage can be observed when the cannula advances into the suprahepatic veins.
Gaining access to the common femoral artery (CFA) for VA-ECMO initiation can result in hematomas, artery dissection, limb ischemia, and, in severe cases, amputation. To reduce arterial complications, we have a consistent approach to cannulation of the CFA and the superficial femoral artery (SFA). Using ultrasound, we identify the common, the superficial, and the profunda femoral arteries. Then we place a micropuncture into the SFA and a micropunture into the CFA. By wire exchange, a 6F wire reinforced cannula is placed in the SFA. Now, we advance a J-wire 30–40 cm into the abdominal aorta via the micropunture in the CFA. After sequential dilation, again ensuring that the wire is not bending or a false path is created, we insert our arterial cannula and connect the 6 F cannula to its side port (Fig. 67.3).
By following this technique and in cases in which urgent arterial cannulation is not necessary, CPR, our rate of arterial complications is lower than 5%.
ECMO Initiation
Once the cannulas are connected and secured to the ECMO circuit and air bubbles have been purged, we increase ECMO flows gradually to achieve the highest flow rates possible. Flow is defined by a negative pressure not > −100 mmHg in the drainage cannula to avoid endothelial injury and hemolysis. We set the oxygen delivery at 100%, and the sweep is set to match the flow rates 1:1. We carefully monitor the decrease of pCO2 to normal values over the next 24 h. Acute shifts in pCO2 after ECMO initiation have been associated with brain injury. ECMO flows are adjusted to maintain a cardiac index greater than 2 L/m2 and a mixed venous oxygen saturation >60%. When a patient is placed on VA-ECMO, we perform a transthoracic ECHO to define flows. ECMO flows are determined by evaluating right heart drainage and degree of left ventricular distention. In addition, inotropic support can be initiated to promote contractility. In cases where left ventricular distention and low contractility persist, the need for left ventricular venting is discussed.
As ECMO flows are optimized and if the patient is intubated, we initiate a lung-protective strategy using bi-level settings with a PEEP of 10, a peak airway pressure lower than 25 cmH2O, and FiO2 of 40% .
ECMO Weaning
We base our decision to wean from VV-ECMO on improving lung compliance, in the setting of an improving arterial pO2 and decreasing sweep gas for CO2 clearance. In most cases, decannulation from VV-ECMO can be accomplished at bedside with a purse string around the cannula insertion site and moderate compression of the area for 15 min.
Weaning from VA-ECMO in cases which ECMO was used as a bridge to recovery requires resolution or improvement of the pathology that triggered cannulation. For example, in cases of massive pulmonary embolism, emboli resolution is necessary before the patient can be weaned from ECMO. This is also the case in myocarditis, post-cardiectomy, ischemic myocardiopathy, or graft failure after transplant. In these cases contractility needs to be reestablished to the point that the patient’s perfusion can be achieved without or with minimal ECMO and inotropic support. Improvement in pulsatility and right brachial artery waveform and improving ejection fraction evaluated by transthoracic ECHO (TTE) or transesophageal ECHO (TEE) are good indicators to define VA-ECMO weaning. Our ECHO studies include a stepwise approach of reducing VA-ECMO flows and simultaneously evaluating contractility.
The most simple method to remember how to approach weaning from ECMO is to remember that the goal to VV-ECMO is to wean sweep and in VA-ECMO is to wean flow. Weaning sweep in VA-ECMO can result in decreased oxygenation and end-organ dysfunction.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 Springer International Publishing AG, part of Springer Nature
About this chapter
Cite this chapter
Sanchez, P.G., Cheng, A.M. (2018). Extracorporeal Membrane Oxygenation: How Do We Do It?. In: Salim, A., Brown, C., Inaba, K., Martin, M. (eds) Surgical Critical Care Therapy . Springer, Cham. https://doi.org/10.1007/978-3-319-71712-8_67
Download citation
DOI: https://doi.org/10.1007/978-3-319-71712-8_67
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-71711-1
Online ISBN: 978-3-319-71712-8
eBook Packages: MedicineMedicine (R0)