Abstract
Vascular emergencies of the retroperitoneum can arise from traumatic or nontraumatic arterial or venous injuries. Advances in interventional radiology technique have permitted less invasive alternative treatments to surgery for the management of aortic and inferior vena cava acute injuries. In this chapter, the MDCT findings of large-vessel vascular emergencies within the retroperitoneum are discussed, with correlative interventional findings and treatment.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
- Vascular trauma
- Vascular emergencies
- Retroperitoneal bleeding
- Intramural hematoma
- Pseudoaneurysm
- Aortic aneurysm
- Artero-venous fistula
- Aorto-caval fistula
- Aorto-enteric fistula
- Inferior vena cava thrombosis
9.1 Introduction
Vascular emergencies of the retroperitoneum can arise from either traumatic or nontraumatic arterial or venous injuries. In patients with high-energy blunt polytrauma, vascular injuries are the second most common cause of death [1,2,3], so their identification is crucial for patient survival [4]. Vascular emergencies can occur spontaneously, particularly ruptured abdominal aortic aneurysm (AAA). The ruptured abdominal aortic aneurysm is one of the most serious emergencies, with a high morbidity and mortality rate.
The management of retroperitoneal vascular emergencies is challenging and requires a multidisciplinary approach. Advances in interventional radiology techniques allow a less invasive treatment as an alternative to surgery for the management of aortic, inferior vena cava, other retroperitoneal central vascular acute injuries, and other related conditions.
In this chapter, the MDCT findings of large-vessel emergencies of the retroperitoneum are discussed, with correlative interventional radiology findings and treatment.
9.2 Multidetector CT (MDCT) Diagnosis
MDCT with intravenous contrast is the primary imaging modality for the assessment of suspected vascular injuries and other related acute conditions, to assess whether medical, interventional, or surgical management is necessary [4].
9.2.1 MDCT Protocol
The MDCT protocol includes two acquisitions. The arterial phase is acquired after the injection of 100–120 mL of iodinated intravenous (iv) contrast medium (cm) injected at 4–5 mL/s. In our practice, this is followed by 40 mL of saline chaser at the same flow rate. Automated bolus tracking is used, with a region of interest placed in the aortic arch at an attenuation threshold of 100 HU. To evaluate the aorto-iliac axis and the central venous vessels in their entire length, the venous phase is acquired at a 60–70 s delay from the end of the injection.
The examination is supervised by a consultant radiologist, who reviews the images before the patient leaves the CT scanner.
Three-dimensional multiplanar reformation (MPR) and maximum intensity projection (MIP) images are also obtained to display the anatomy, including anatomic variants.
9.3 Traumatic Vascular Emergencies
9.3.1 Trauma Mechanisms
Arterial injuries are caused by either blunt or penetrating trauma.
Blunt traumatic aortic injuries arise from sudden stretching mechanism of the vessels on a fixed axis [4]. Blunt traumatic abdominal aortic injury is an uncommon occurrence and is typically associated with other abdominal injuries [5, 6]. The severity of injury varies from a simple subtle intimal flap to a complete transection [7] and may arise from direct trauma to the abdominal aorta (e.g., a seat belt injury) or from indirect forces transmitted to the aorta through contiguous organs [6, 7]. Also, visceral branches may be involved, including the renal, mesenteric, and iliac arteries [8], the latter of which are associated with pelvic fractures [6] (Fig. 9.1 ).
Active arterial bleeding in the obturator region related to pelvic trauma treated with endovascular embolization, in an 81-year-old man. (a) Axial nonenhanced CT image showing acetabular fracture (arrows); (b) axial IV contrast-enhanced CT image in the arterial phase showing a hematoma adjacent to the acetabular fracture, with active bleeding (arrows); (c) axial IV contrast-enhanced CT image in the venous phase showing an increase of the hemorrhage due to obturator artery injury (arrows); (d) selective angiogram of the right internal iliac artery showing active extravasation of contrast from the distal branches of right obturator artery (arrow); (e) super-selective embolization of the bleeding vessel with multiple steel coils (3-mm diamond-shaped), followed by gelfoam suspension embolization (arrow); (f) postprocedural selective angiogram of the right internal iliac artery, showing complete exclusion of bleeding (arrow). Fig. 1 is reprinted with permission from C arrafiello G, Mangini M, Ierardi AM, Recaldini C, Cotta E, Piacentino F, Fugazzola C. Vascular Emergencies of the Retroperitoneum. Book chapter in: M. Scaglione et al., Emergency Radiology of the Abdomen, Medical Radiology. Diagnostic Imaging Editor: Springer-Verlag Berlin Heidelberg 2012; pp. 189–205
Penetrating trauma involving the great vessels of the retroperitoneum is caused by a stab wound to the back, flank, or more commonly due to a gunshot wound [9]. The mechanism of injury, the velocity of the object, and the trajectory can all be used to predict the severity of injury [9]. MDCT is useful for assessing the course of the penetrating injury. It also offers valuable information on the location and extent of the injured vessel, as well as on associated parenchymal and other injuries [10]. Large arterial vascular injuries due to penetrating trauma are associated with pseudoaneurysms and/or active contrast extravasation [6, 11] (Fig. 9.1 ). According to Azizzadeh et al. [12], traumatic aortic injuries are classified into four degrees of severity: grade I (intimal tear), grade II (intramural hematoma), grade III (pseudoaneurysm), and grade IV (rupture).
9.3.2 Management
The management of retroperitoneal vascular trauma, especially in the emergency setting, is challenging and requires a multidisciplinary approach [13].
Within the past decade, endovascular techniques performed by interventional radiologists have become an alternative to surgery at many level-1 trauma centers [14].
Regardless of the etiology of the retroperitoneal bleeding (RB), all patients should initially be managed in an intensive care unit, with careful monitoring, fluid resuscitation, blood transfusion, and normalization of coagulation factors [15]. There are no specific guidelines to suggest when an endovascular versus surgical intervention is recommended, to our knowledge. However, if the patient is hemodynamically stable without evidence of obvious hemorrhage, nonsurgical management or careful observation should be considered [7].
Currently there is a growing trend in the use of interventional radiology (IR) techniques as an alternative to open surgery in the management of retroperitoneal hemorrhage in trauma patients. The advancement in endovascular techniques over the last few decades has enabled hemostasis to be achieved safely and rapidly, by using several methods including embolization, balloon occlusion, and stent-grafting [15, 16].
Intra-arterial transcatheter embolization (TAE) is being used with increasing frequency in patients whose angiograms show active bleeding sites [17]. Coils are probably the safest agents (Fig. 9.1), but Isokangas and Perala [17] have commented that proximal coiling of the bleeding artery is not sufficient with retroperitoneal hemorrhage, where there is a rich network of collateral arteries, and new arterial pathways may develop after obliteration of the lumbar arteries. It is critical to place embolic agents both proximally and distally relative to the bleeding site, to prevent re-occurrence of hemorrhage. The indications for embolization are based on the hemodynamic stability of the patient and the extent of hemorrhage [7].
These advancements have become a useful adjunct in the treatment of persistent or recurrent bleeding and have also been used as a primary therapeutic approach in selected patients [18].
9.3.3 Arterial Injuries
9.3.3.1 Intimal Tear
Intimal disruption is one of the most common types of blunt aortic injuries detected in patients with blunt trauma [6]. It is due to an injury of the intima, while the media and adventitia remain intact, so a focal detachment of the internal aortic layer occurs [4]. The intimal flap, dissected by the blood flow, may lead to thrombosis [6] due to the exposure of thrombotic subintimal factors, or can progress into a complete vessel dissection [4].
On MDCT, an intimal tear is identified as a linear endoluminal defect connected with the inner layer of the aortic wall [4, 7]. If the intimal flap measures less than 10 mm, conservative management is usually performed, and short-term follow-up imaging is indicated [4, 5]. If the flap evolves into a dissection, endovascular intervention is recommended.
9.3.3.2 IR Management
Appropriate management of traumatic aortic dissection isolated to the abdomen is not well defined to our knowledge [15]. The failure of a nonoperative approach to this condition suggests that disease progression may be inevitable [15]. Some authors suggest that all traumatic abdominal aortic injuries should be treated either by endovascular stenting, if feasible, or by conventional prosthetic graft replacement in selected patients [19]. In comparison with spontaneous abdominal aortic dissection, which will be described later in the chapter, the surgical mortality is higher in trauma because of associated retroperitoneal venous injury and sepsis [20, 21]. Emergency surgery is recommended when medically uncontrolled hemodynamic shock, lower limb acute ischemia, proven ischemic medullar paraplegia, or tortuous iliac arteries are present [19].
Excluding these conditions, IR techniques provide a less invasive alternative [16]. Several studies [22,23,24,25,26] provide encouraging results with regard to IR treatment of acute traumatic dissection of the retroperitoneal aorta, especially in the inferior inframesenteric segment. Nevertheless, the potential complexity of the entry-re-entry scenario, and the risk of aortic wall injury, may require immediate laparotomy [27].
Deployment of an endoluminal stent-graft in a dissected abdominal aorta is challenging and has a high morbidity. Most of the problems occur from the anatomical variability, where the dissection flap can propagate distally [28]. As a result, the true and false lumens may appear in complex configurations, and the branch vessel origins may be distributed in unpredictable patterns, occasionally in association with life-threatening ischemia of the viscera and extremities. It is imperative to locate the primary entry tear, as aortic rupture is most frequent cause of death in patients with abdominal aortic dissection [29, 30].
In 1995, Peterson et al. [8] and Nishimura et al. [24] successfully treated an aortic dissection by percutaneous balloon fenestration followed by stent placement. In both patients, lower limb ischemia was relieved, and a 2-year follow-up did not reveal any abnormality. Later, Berthet et al. [19] published their results of small series of patients with traumatic abdominal aortic dissection who were treated using endovascular techniques by percutaneous stent placement. No deaths were related to the aortic dissection or its treatment, and no patient underwent endovascular fenestration. The authors proposed that isolated fenestration only treats the ischemic consequences of the dissection without treating the cause; however, this is useful when the dissection extends above the renal arteries.
The placement of an aortic stent within the dissection is an alternative to fenestration, and experimental studies [31, 32] have demonstrated that stenting is more efficient if stents are inserted at the site of the intimal tear and cover the entire dissected lumen.
IR treatment could be the first therapeutic option for the following reasons:
-
endovascular approach may be performed immediately after closure of a laparotomy for septic injuries [19]
-
a less invasive technique avoids aortic cross-clamping and retroperitoneal dissection [19]
-
the limited length of dissection is a characteristic feature of aortic injuries, making it easier to cover the whole dissection by stenting [23]
-
the use of short stent-grafts and deployment far from the T8 to L2 vertebrae further minimize the risk of paraplegia, as compared with the risk of surgical aortic replacement and graft interposition [29, 30]
Endoluminal stent placement is a faster method than conventional surgery because it avoids the need for circulatory arrest and cross-clamping of the aorta, and the associated ischemia and reperfusion injury [28].
9.3.3.3 Intramural Hematoma
Intramural hematoma of the abdominal aorta may be due to blunt trauma, and arises from a minimal intimal tear causing bleeding in the media tunic of the vessel wall [4, 34]. The sensitivity of MDCT for the depiction of intramural hematoma is high, as it appears as hyperdense mural vessel thickening (Fig. 9.2). On such scans a manual adjustment of the window settings is recommended. Intramural hematoma is needed to treat because it can progress to frank aortic dissection [4, 33, 34].
Intramural hematoma following blunt trauma, in a 57-year-old man. Nonenhanced CT images in axial (a) and in coronal planes (b) show hyperdense mural thickening due to the recent bleeding, extending from the aortic arch to the abdominal aorta; (c) axial IV contrast-enhanced CT image shows that the hematoma does not enhance after contrast administration, and no intimal flap is seen. Figure 2 is reprinted with permission from C arrafiello G, Mangini M, Ierardi AM, Recaldini C, Cotta E, Piacentino F, Fugazzola C. Vascular Emergencies of the Retroperitoneum. Book chapter in: M. Scaglione et al., Emergency Radiology of the Abdomen, Medical Radiology. Diagnostic Imaging Editor: Springer-Verlag Berlin Heidelberg 2012; pp. 189–205
9.3.3.4 IR Management
Intramural hematoma (IMH) of the aorta is a variant of dissection with no entry or false lumen flow and typically occurs in hypertensive patients with severe atherosclerotic disease [35]. Traumatic IMH of the aorta is a relatively rare, but potentially life-threatening disease. Blunt trauma patients with upper or lower back pain should be considered as possibly having IMH of the descending aorta (IMH—type B) in the emergency department until it is diagnosed or excluded with imaging [36]. Fewer than 10% will resolve spontaneously [37], whereas 16–47% will progress to frank dissection [29]. Mortality may be reduced by early diagnosis and adequate treatment [7].
Indications for endovascular treatment of traumatic IMH depend on symptomatic presentation, diameter increase, pseudoaneurysm formation, or progression into acute aortic dissection during follow-up imaging. MDCT can depict the entrance tear, and endovascular aortic repair aims to close the entrance tear, with complete stent-graft coverage of the IMH having the best therapeutic outcome [33, 36].
Most studies describing IMH have looked at small patient populations due to its relative rarity [38, 39]. Thus, the optimal therapy for this condition is still largely unknown to our knowledge [38, 40]. Trauma patients with IMH present with subacute or acute pain, which can progress to aortic dissection and can eventually rupture. Dilatation of 60 mm and above should undergo surgical treatment [41]. Uncomplicated IMH is treated conservatively with blood pressure regulation and serial MDCT scans [42, 43]. Uncomplicated stable patients with retroperitoneal IMH can be treated using endovascular techniques [44, 45]. In a study [46] which compared early medical therapy with medical plus IR treatment in IMH complicated by intimal tear, at a mean follow-up of 17.6 months, 10 of 11 medically treated patients demonstrated regression, and 5 of these patients (45%) had complete resolution of their IMH. All those treated with endovascular management had resolution. Song et al. compared medical therapy with endovascular therapy in 56 patients with IMH with a thickness more than 10 mm, or with sustained chest or back pain despite maximal medical therapy. The technical success was 100%, with no progression or mortality [47].
9.3.3.5 Pseudoaneurysms
A pseudoaneurysm is false aneurysm due to the disruption of the arterial wall layer, in which the blood flow is only contained by the connective tissue surrounding the vessel [34].
On MDCT, a pseudoaneurysm is an outpouching of the arterial wall, which is well defined, with endoluminal enhancement synchronous with those of the other arterial vessels. They may occur at the aorta or may involve other splanchnic arterial vessels due to blunt or penetrating trauma. They can also occur as a sequela of prior injury [48]. The majority of blunt trauma pseudoaneurysms occur in the thoracic aorta. Post-traumatic aortic pseudoaneurysm in the abdomen is very uncommon. Post-traumatic abdominal aortic pseudoaneurysm occurs due to penetrating injury of the upper abdomen, such as from a gunshot or knife wound [11]. Multiphasic CT is helpful for correct identification, and to distinguish them from active bleeding, as a pseudoaneurysm does not change its shape and decreases in attenuation value on delayed scans, and is isodense to the arterial structures [4, 49]. Pseudoaneurysms need to be treated because several complications can occur, including rupture, fistula, mass effect upon the surrounding structures, infection, or thromboembolic events. The most severe and most common complication is rupture due to increase of the endoluminal pressure [31, 51].
9.3.3.6 IR Management
Traumatic aortic pseudoaneurysm is a potentially lethal condition [52,53,54]. Advances in endovascular techniques have provided options to treat traumatic pseudoaneurysms of the abdominal aorta and have led to a marked decrease in the morbidity and mortality rates [52]. A complete work-up to determine the location of the pseudoaneurysm and to evaluate surrounding structures and relevant vascular anatomy is essential for treatment, which should be tailored to the site, rupture risk, and clinical setting of the pseudoaneurysm, as well as to patient comorbidities [55]. IR management serves to exclude a pseudoaneurysm from the circulation and has the advantage of accessibility to most locations of the arterial system, without the potential morbidity of open surgical repair. Baltacioglu et al. [56] reported 100% technical success in the treatment of 17 retroperitoneal pseudoaneurysms with covered stents.
Selecting the optimal method depends on the size of the pseudoaneurysm neck and the expendability of the donor artery [57]. There are several visceral arteries which have a well-established collateral supply, including the gastroduodenal, hepatic, and splenic arteries, and other upper gastrointestinal arteries [55]. When embolizing arteries with numerous collateral vessels, one must embolize both proximal and distal to the pseudoaneurysm, to completely exclude it from the circulation, by preventing backflow from the collateral circulation [57]. A pseudoaneurysm arising from an inexpendable donor artery must be excluded from the circulation, while preserving the donor artery [55]. The width of the pseudoaneurysm neck relative to the diameter of the donor artery is the determining factor in the method used. A vital donor artery may be embolized in certain emergent situations (e.g., rupture with active bleeding) [55]. If the neck is narrow, the pseudoaneurysm may be embolized with catheter-directed delivery of coils (the preferred embolization material) into the sac itself [55].
In IR, coils fall into two main categories: nondetachable and detachable [55]. The nondetachable coils reassume their shape immediately after deployment from the catheter. These coils are available in a wide array of diameters and lengths. They are made of either stainless steel or, more recently, of platinum, which allows them to be softer and have a more complex (helical) shape. Consequently, such coils conform to the shape of and fill the pseudoaneurysm sac, so fewer coils are needed for the embolization [55]. However, because platinum coils are softer, initial placement of stainless steel coils may be required to act as a scaffolding. Detachable coils are held to the pusher guide wire by either a mechanical or an electrochemically dissolvable connection, which is released to deploy the coil [55]. This facilitates more accurate deployment and the possibility of readjusting the position of the coil before its final deployment [55]. A disadvantage of using coils as an embolization material is the potential for recanalization of the embolized sac, if the coils are not tightly packed [55]. However, this drawback has been largely overcome with the use of soft helical coils, which may be tightly packed in the pseudoaneurysm sac [58]. Agents such as thrombin or N-butyl 2-cyanoacrylate (glue) may also be used, either alone or in addition to coils [57, 59]. Moreover, if the neck is wide, the pseudoaneurysm can still be embolized with catheter-directed delivery of embolization materials, but remodeling is required to prevent outflow of these materials into and distal embolization of the donor artery, and to ensure adequate embolization of the pseudoaneurysm sac [55]. This remodeling may be performed with the use of a stent cage, or by trapping the coils by means of temporary balloon occlusion of the donor artery between coil deployments [60]. If distal arterial embolization is a concern, detachable balloons may be used as the embolic agent [61]. Another option, if the pseudoaneurysm neck is wide, is stent-graft placement across the neck to exclude the pseudoaneurysm [60], although this procedure requires a higher profile and a stiffer delivery system than does catheter-directed coil embolization. As a result, the arterial anatomy and the caliber of the arteries leading to and at the pseudoaneurysm site should be favorable (i.e., reduced arterial tortuosity and large-diameter arteries). An additional reason for placing stent-grafts only in larger arteries is that in small arteries they pose a higher risk of thrombosis [55]. Visceral pseudoaneurysms, which are usually smaller and located off small and tortuous donor arteries, pose a particular challenge for stent-graft placement.
Endovascular techniques have a lower complication rate for the treatment of visceral pseudoaneurysms than surgical management [59, 61]. The main complication of IR techniques is intraprocedural rupture of the pseudoaneurysm [62]. Rare cases of recanalization of the embolized vessel and reconstitution of arterial flow to the pseudoaneurysm have also been reported [62].
9.3.3.7 Artero-venous Fistula
Penetrating trauma may cause abnormal communications between arteries and veins. In the retroperitoneum, aorto-caval fistulas may be detected following trauma or interventional procedures [63].
At MDCT, these may be seen only if an arterial phase is performed; they appear as early venous opacification in the arterial phase. Furthermore, obliteration of the fat planes between the aorta and the vena cava may be detected and, less commonly, visualization of the abnormal communication may be identified [64]. Small fistulas may be asymptomatic but may enlarge over time, increasing the venous return and causing severe hemodynamic alterations with congestive heart failure [64,65,66].
9.3.3.8 IR Management
Penetrating injury remains the most common cause of abdominal and pelvic arterio-venous fistulae [67]. Although ACF due to penetrating wounds frequently have a severe acute presentation, iatrogenic fistulae produce less acute symptoms than traumatic and spontaneous aortic or iliac fistulae [7].
Surgical treatment includes repair of the fistula, usually with direct suture and reconstruction of the aortoiliac aneurysm with a dacron graft [68]. Venous bleeding from the sac of the aneurysm can be excessive, and careful and expeditious control of the bleeding using digital compression, sponge sticks, or balloon catheters is imperative. Blood salvage and rapid return of the autologous blood in these patients can be lifesaving [7]. As in other complications of AAA, open surgical repair is associated with high morbidity and mortality, which will be affected by the acute presentation and the preoperative recognition of the ACF. There are considerable difficulties with the open repair of central abdominal fistulae, which are related to the arterialization of venous structures and perivascular inflammation, resulting in an increased risk of substantial blood loss and pulmonary embolization [69].
The significance of this condition in the modern era, where endovascular aortic reconstruction (EVAR) is often performed, is when an ACF is an unexpected finding during open repair of ruptured abdominal aortic aneurysms (rAAA), this is a situation where exsanguinating hemorrhage may occur [69].
Endovascular stent-grafting offers an attractive therapeutic alternative to the open repair of ACF because it does not involve a laparotomy, there is less blood loss, it does not require general anesthesia, and therefore postoperative complication rates and costs are reduced [69]. Boudghene et al. [70] reported successful treatment of ACF with percutaneous stent-grafts in an experimental study in which the ACF was created percutaneously in eight sheep. According to the literature review reported by Antoniou et al. [71] the technical success rate is 96%; however, the mean follow-up was only 9 months, with only one study reporting a follow-up of 24 months. The most common procedure-related complication was a type II endoleak, which was found in 22% of the patients examined, but no standardized imaging modality was used at follow-up. This event was either self-limiting or required minimal percutaneous intervention to correct [71]. Lau et al. [72] summarized that endovascular repair reduces blood loss and may reduce the significant morbidity and mortality rates often associated with open surgery. In cases of rAAA with associated ACF, the technique may offer its greatest benefits. From the reported literature, it seems that endovascular repair has a higher success rate with regard to morbidity and mortality, but there is no consistency in patient selection or analysis as to whether these patients were operated on in an emergency setting or electively. In 2009, a new hybrid technique was performed to treat a patient presenting with hemodynamic instability in the context of an acute ACF complicating a large AAA which was unsuitable for standard endovascular repair. Siepe et al. treated this fistula by placing a large covered aortic stent into the inferior vena cava (IVC) [73]. This was successful in reducing central pressures, stabilizing arterial blood pressure, and allowing inotropic support to be weaned. With the patient thus stabilized, standard open surgery on the AAA was performed, without the risk of massive blood loss from the large defect that was noted in the IVC covered by the stent-graft, once the aneurysm sac was opened [73].
9.3.3.9 Bleeding
Active retroperitoneal bleeding (RB) may be seen after blunt or penetrating trauma; it represents an urgent condition that needs to be promptly treated. In blunt trauma, severe injuries to the abdominal aorta are uncommon due to the protected position of the abdominal aorta; the anatomic location of abdominal aortic injury is usually infrarenal. The most frequent sites of injury are at the level of the inferior mesenteric artery (33%), near the renal arteries (24%), and between the inferior mesenteric artery and the bifurcation (19%) [74]. In the arterial phase on CT, a focal disruption with active bleeding of the arterial wall can be detected, with hematoma in the retroperitoneal space. The extraluminal contrast medium extravasation increases, and changes its morphology in the portal venous and late acquisitions. Only occasionally, a transection or disruption of the aorta with exsanguinating hemorrhage may be seen, as these injuries quickly lead to death [6, 11].
9.3.3.10 IR Management
Traumatic RB is a life-threatening condition that requires prompt and accurate diagnosis and treatment [75]. The high morbidity (40–50%) and mortality (5–30%) rates are due to the inability to surgical control of such hemorrhage [7], which can be of arterial, venous, or osseous origin (the latter usually due to a pelvic fracture). Arterial injuries are the most common and the most severe [7].
Surgical exploration and controlling the bleeding vessels are particularly arduous for the retroperitoneum region [17]. Thus, once a bleeding artery is kept under control, collateral supply to the same territory may lead to new hemorrhage [75]. That is why most surgeons avoid exploration of the retroperitoneum in patients with RB [76]. Furthermore, surgery often carries the risk of lethal hemorrhage, by dissection of the retroperitoneal space and loss of passive tamponade of a hematoma [77].
When compared with surgical management, IR procedures are not only safe, fast, and less invasive, but also provide prompt treatment through trans-arterial embolization (TAE) of the bleeding vessel [78] (Fig. 9.3). Currently, therapeutic TAE is becoming increasingly used in the management of traumatic RB, either as definite method or as a surgical adjunct [79]. The efficacy of TAE in the management of RP due to arterial hemorrhage in trauma patients has been demonstrated by Papakostidis et al. [80], who reported a success rate, expressed in terms of hemorrhage control, and reduction in transfusion requirements, ranging from 85% to 100%. Recently, Velmahos et al. reviewed the medical records of 102 consecutive trauma patients who underwent TAE to stop RB, with angiographic and clinical bleeding control in 93 (91%). The rate of successful hemostasis by TAE was identical in blunt and penetrating trauma patients. There was no major morbidity after TAE. No factors predicted patients with a high likelihood to have a positive angiogram. Patients who had AE before or after a period of attempted hemodynamic stabilization in the intensive care unit were no different with respect to hemodynamic parameters immediately before TAE or effectiveness of TAE for bleeding control [18]. Selective catheterization and TAE of the bleeding arterial branch should be carried out as quickly as possible, to avoid dangerous delays and complications related to multiple blood transfusions, prolonged hemodynamic compromise, and extensive blood loss [18, 77]. Therefore, timing is of particular importance for TAE, but there is no consensus to our knowledge regarding the best time for embolization [18, 75]. Early TAE may be used in selected patients as a front-line therapeutic intervention that offers expeditious hemostasis and prevents delays in definitive bleeding control [16]. Active RB due to an injury of small distal vessels can be treated by embolization using particles including polyvinylalcohol (150–300 μm), embospheres (300–500 μm), or gelfoam in small segments. Microcoils have to be customized to the vessel diameter [7].
Nonenhanced axial CT image shows retroperitoneal hematoma, in a 45-year-old man (a); arterial phase demonstrates active bleeding from a lumbar arterial vessel (b, arrow); selective lumbar arteriogram confirms blush (c, arrow); postprocedural angiography shows the embolization of the lumbar artery (d, arrow)
There are a few heterogeneous case series or reports on stent-grafts in the management of RB. Watarida et al. [81] reported the successful use of a fenestrated stent-graft to manage a RB. Traumatic aortic rupture with retroperitoneal hematoma can also be treated with a combined operative and endovascular approach [82]. In conclusion, percutaneous control of RB is a valuable therapeutic option in trauma patient, especially with TAE, which is a rapid, effective, and minimally invasive technique.
9.4 Venous Injuries
Blunt injuries to the central retroperitoneal veins are very unusual and are usually associated with other abdominal injuries. Only a few cases have been reported in the literature of blunt inferior vena cava injuries, to our knowledge [83]. The mortality rate is high, ranging from 34% to 70% [84]. The CT findings depend on the location of the injury. Blunt IVC injuries can be difficult to diagnose, since the contrast material extravasation as direct evidence of vascular injury may be absent, and other extensive parenchymal injury may be associated. Retrohepatic IVC injury is usually associated with extensive liver lacerations into the porta hepatis and retrohepatic IVC region, or an irregular contour of the retrohepatic IVC [85, 86]. Injuries may vary from thrombosis to venous pseudoaneurysm formation, to active bleeding.
9.4.1 Thrombosis
Only a few cases of post-traumatic IVC thrombosis have been reported in the literature, to our knowledge [87]. There are several mechanisms that may be responsible, including endothelial injury of the caval wall with exposure of pro-thrombotic factors and secondary thrombus formation, caval stasis due to compression by a pericaval/retroperitoneal hematoma, hepatic vein thrombosis extending into the IVC, or a hypercoagulable state after major trauma [87]. The major risk of this condition is pulmonary embolism. Multiphasic MDCT examination represents the technique of choice for diagnostic purpose.
9.4.2 Traumatic Pseudoaneurysm
Similarly to arterial injuries, but much less commonly, veins may develop pseudoaneurysms after blunt or penetrating trauma due to an incomplete wall injury. Symptoms may include abdominal pain, IVC syndrome, tachycardia, upper gastrointestinal bleeding secondary to venobiliary fistulas, hemorrhagic shock secondary to rupture, and pulmonary embolus or other thromboembolic phenomenon secondary to IVC thrombosis [86]. These injuries need to be treated promptly, as they have high risk of a rupture with severe hemorrhage [88].
9.4.3 Bleeding
In IVC injury, active bleeding represents the most severe complication needing prompt treatment. At MDCT, this is evident as active extravasation of intravenous contrast media, vessel contour abnormality, and associated retroperitoneal hematoma [84].
9.5 Nontraumatic Vascular Emergencies
9.5.1 Arterial
Nontraumatic acute aortic syndromes are a spectrum of life-threatening aortic pathologies with significant implications for diagnosis, therapy, and management. There is a common etiology, leading to a breakdown of the aortic intima and media.
The subtypes of aortic syndromes are acute aortic dissection, penetrating atherosclerotic ulcer, and aortic aneurysm, with potential associated complications.
9.5.2 Management
Management of nontraumatic retroperitoneal vascular emergency is both difficult and challenging and requires a multidisciplinary approach. The control of RB can be accomplished by either surgical or endovascular approaches and, in specific circumstances (e.g., spontaneous bleeding), by conservative management. The results of surgical exploration and primary repair in hemodynamically unstable patients are well known [7]. This strategy is associated with a high mortality rate, ranging from 30% to 80%, regardless of the location [7]. The high mortality rate is linked to the opening of the retroperitoneum space, which leads to suppression of the tamponade effect, disruption of the hematoma, and destabilization of patient [16]. Embolization is becoming more common as an alternative to open surgery in the treatment of RB following iatrogenic injuries, procedures including percutaneous lumbar sympathectomy, renal biopsy, and percutaneous nephrostomy, or following iatrogenic iliofemoral vessel injuries [7]. Open surgery is indicated if the patient remains unstable despite adequate fluid and blood product resuscitation, or if IR is either not successful or is unavailable [7].
9.5.2.1 Acute Aortic Dissection
Acute aortic dissection is the most common acute aortic emergency condition. It arises from a separation of the layers of the aortic wall. Due to an intimal tear, the blood flow enters the media; this results in two lumina, a true and a false lumen. Then, pressure may increase in the false lumen, impairing the blood flow in the true lumen and its branches [6, 34, 89] (Fig. 9.4).
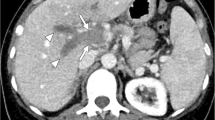
Extensive dissection of the abdominal aorta in a 64-year-old man with abdominal pain. (a) IV contrast-enhanced axial CT image in the arterial phase shows the intimal flap of the abdominal aorta at the level of the superior mesenteric artery, with intraluminal thrombosis at the origin; (b) coronal and (c) sagittal MPR reformations permit better appreciation of the extension of the dissection. Figure 4 is reprinted with permission from C arrafiello G, Mangini M, Ierardi AM, Recaldini C, Cotta E, Piacentino F, Fugazzola C. Vascular Emergencies of the Retroperitoneum. Book chapter in: M. Scaglione et al., Emergency Radiology of the Abdomen, Medical Radiology. Diagnostic Imaging Editor: Springer-Verlag Berlin Heidelberg 2012; pp. 189–205
MDCT findings of acute aortic dissection are eccentric aortic wall calcification on nonenhanced MDCT, a double aortic lumen, and direct visualization of the media-intima entrance as an intimo-medial flap defect on arterial-phase images. In the early angiographic phase, the true lumen appears smaller and more intensely opacified than the false lumen due to higher pressure and faster mixing with blood [6, 89] (Fig. 9.4). The venous phase may help in differentiating between the true lumen and partially thrombosed false lumen. Rarely, a re-entry tear may be identifiable as a small defect in the dissected intimal layer [6, 89].
9.5.2.1.1 IR Management
The optimal treatment strategy for patients with aortic dissection confined to the abdominal aorta remains controversial to our knowledge [90]. Despite remarkably improved operative techniques, surgical repair of the abdominal aorta is still associated with high morbidity and mortality [91]. Contemporary operative mortality rates of elective surgery range between 0% and 27%, but may exceed 50% in complicated dissection under emergency conditions [91, 92].
Currently, there is consensus that patients with otherwise uncomplicated abdominal aortic dissection should primarily be treated medically with tight blood pressure control, while reserving operative treatment for evolving complications [93]. Thus, the indications for operative treatment (open or endovascular repair of the abdominal aorta) of acute aortic dissection are basically the same as for IMH [34, 37], and included active rupture, lower extremity ischemia, unremitting pain, associated aortic aneurysm, and prevention of future aneurysm formation.
In 1999, endovascular stent-graft closure of the proximal entry tear was introduced as a new treatment option for patients with type B acute aortic dissection [29, 30]. Aortic remodeling is accomplished by sealing the proximal entry tear, at the same time avoiding the risks associated with open surgery [94]. This rationale was originally based on the clinical observation that patients with spontaneous thrombosis of the false lumen have a better long-term prognosis than those patients without thrombosis [90].
As dissection may extend to the iliac arteries, aorto-bi-femoral grafting is the procedure of choice [95]. Currently, endovascular treatment of isolated dissection has been associated with a high rate of technical and clinical success, with reduced morbidity and mortality rates at experienced institutions [19, 30]. A recent meta-analysis reported 30-day or in-hospital mortality for type B acute aortic dissection of 0–27% (median, 7%) for medical treatment, 13–17% (median, 16%) for open surgical procedures, and 0–18% (median, 6%) for endovascular aortic repair (EVAR) [39]. One study published in 2013 [96] compared 853 patients with medical management for type B dissection to 276 receiving EVAR in a propensity-matched analysis. Although EVAR patients presented with more complications (pulse deficit, malperfusion syndrome, shock, stroke, spinal cord ischemia, visceral ischemia, or renal failure), in-hospital mortality was not different, and 5-year cumulative probability of mortality was lower for EVAR than for medical management (15.5% vs. 29.0%, respectively). In 2005, Eggebrecht et al. [93] published a meta-analysis encompassing 609 type B dissection patients, demonstrating that endovascular stent-graft treatment of acute aortic dissection is feasible and can be performed with technical success rates of 95%. Furthermore, the acute and mid-term survival of about 90% at 2 years following stent-graft placement compares favorably with medically and surgically treated type B acute dissection patients.
Neurologic complications and paraplegia remain the most potential complications of stent-graft placement, as for surgical repair of type B dissection. Eggebrecht et al. showed that the overall risk of neurologic complications with stent-grafting ranged between 2.9% and 3.4% [93]. The 1% risk of paraplegia appears to be very low, considering that contemporary studies have suggested the risk of paraplegia after surgical repair of the descending thoracic aorta to be between 7% and 36% [91].
Patients undergoing stent-graft placement for acute dissections were found to be at higher risk of death and major complications than those with chronic aortic dissection, regardless of their age [93]. However, it should be recognized that in patients with acute aortic dissection implantation of stent-grafts is often prompted by complications of the dissection, making this patients more prone for higher complications and lower survival when compared with stable patients with chronic disease undergoing elective stent-graft placement [93].
Recent data suggest a strong influence of the interventional radiologists’ experience on the results of stent-graft placement [93]. Institutes that reported an overall endovascular experience of more than 20 patients treated with stent-grafts had higher success rates and fewer complications than less experienced centers [93].
Eggebrecht’s meta-analysis highlights some technical limitations of endovascular stent-graft placement in abdominal aortic dissection [93]. Stent-grafting fails to abolish the false lumen in about a quarter of patients, suggesting that it perhaps may not be a definitive treatment for type B dissection. Even in the presence of a false lumen’s thrombosis, the aorta may enlarge during follow-up. Thus, there is a continued risk of aortic rupture (about 2% during follow-up) after stent-graft placement, and the need for adjunctive stent-graft placement or conversion to open surgery in about 12% of patients over time [93]. Nevertheless, the incidence of aortic rupture and the need of repeat endovascular or surgical interventions may also be related to progression of the disease itself, and may not necessarily reflect treatment failure. This is supported by the fact that 11–20% and 10–44% of the patients with abdominal aortic dissection need repeat surgery when treated medically or surgically, respectively [91].
9.5.2.2 Penetrating Atherosclerotic Ulcer
Penetrating atherosclerotic ulcer (PAU) is an ulceration in an atherosclerotic plaque penetrating the elastic lamina leading to hematoma formation within the media tunic of the aortic wall [7, 33, 89] (Fig. 9.5 ). Nonenhanced MDCT shows high-density hematoma surrounding the ulceration. At enhanced MDCT, a PAU is seen as a focal contrast filled outpouching of the aortic wall in the setting of an atheroma [34] (Fig. 9.5).
Penetrating atherosclerotic ulcer of the abdominal aorta in a 76-year-old man. IV contrast-enhanced CT scans in the arterial phase in axial (a) and coronal planes (b) show endoluminal outpouching along the aortic borders, and focal thickening of the aortic wall due to intramural hematoma. Figure 5 is reprinted with permission from C arrafiello G, Mangini M, Ierardi AM, Recaldini C, Cotta E, Piacentino F, Fugazzola C. Vascular Emergencies of the Retroperitoneum. Book chapter in: M. Scaglione et al., Emergency Radiology of the Abdomen, Medical Radiology. Diagnostic Imaging Editor: Springer-Verlag Berlin Heidelberg 2012; pp. 189–205
9.5.2.2.1 IR Management
Penetrating atherosclerotic ulcers (PAUs) are most often found in patients with severe atherosclerosis, usually elderly patients with several comorbidities [33].
The indications and the techniques of PAUs treatment are still controversial to our knowledge. There is currently no clear cut-off for a PAU diameter (depth) or neck diameter which warrants treatment (in one publication a depth of >20 mm or a neck >10 mm was associated with higher complication rates) [97]. Although some authors believe immediate treatment is not always required [98], as most PAUs have a benign clinical courses, early intervention has been recommended when PAU is complicated by aneurysm expansion, regardless of size, rupture, embolic symptoms, or uncontrolled pain [99]. Open surgical repair with graft interposition has been used traditionally [66], but patients with PAU are generally not ideal candidates for open surgery because of patient age and poor general status [99].
Aortic stent-grafts have changed the management strategy for PAU. This less invasive procedure is suitable for these high-risk patients, and can be useful in cases of rupture [100]. PAUs are ideal for endovascular repair, since there is usually a focal abnormality [100]. If IMH is present, the hematoma may be relatively limited, possibly due to concomitant atherosclerotic lesions causing medial fibrosis of the surrounding aorta and preventing expansion [99]. Stent-grafting of the PAU seals the diseased segment of the aorta, which reduces the wall stress and may decrease the chance of a PAU evolving into a dissection, IMH, or aneurysm [99]. Therefore, endovascular stent-grafting has been developed, and several reports about this technique applied to the treatment of PAU have been published [100, 101]. Open surgical repair of the descending aorta requires clamping of the aorta, a large thoracotomy incision, possible cardiopulmonary bypass, and prolonged mechanical ventilation. Because endovascular surgery generally requires only a femoral or iliac arterial cut down for exposure, short operative times, and no clamping of the aorta, this less invasive procedure has the potential to substantially reduce the morbidity and mortality of definitive surgical correction of PAUs [98]. Between 2003 and 2005, 21 patients with aortic PAUs were treated with Gore TAG endoluminal stent-grafts as part of a single-center investigational device exemption protocol reported by Brinster et al. [98]. Despite the high number of comorbidities and advanced age in this patient population, there was no operative mortality. Postoperative morbidities included one re-operation for an occluded limb of an iliac–femoral bypass graft, but no perioperative myocardial infarctions, strokes, or paraplegias occurred. The use of just one device in 20 (95%) of 21 patients indicates that the focal nature of PAUs is optimal for treatment by endoluminal grafts. Additionally, because there has been a correlation between the length of the descending thoracic aorta covered by stent-grafts and paraplegia rate, the single application of a single graft may have reduced the risk of postoperative neurologic dysfunction. Freedom from end-point treatment failure was 100% [98].
These mid-term results of low morbidity and mortality compare favorably with previously published studies which have examined the use of stent-grafts in the treatment of PAU [100, 102], and indicate the success of EVAR for aorta PAU. The combined success rate approaches 100%, with very low morbidity and mortality compared to the traditional open techniques. PAUs may be uniquely suited for treatment with endovascular grafts in this patient population, by allowing a minimally invasive means to treat a focal anatomic disease.
9.5.2.3 Aortic Aneurysm Rupture
Abdominal aortic aneurysm occurs in 2–9% of the population >65 years. About 80% occur between the renal arteries and the bifurcation [7]; iliac aortic aneurysm is seen in 2–10% of patients with abdominal aortic aneurysm [7, 33, 89]. Most abdominal aortic aneurysms are true aneurysms, with aortic dilation caused by weakening of all the three layers. A true aneurysm involves all three layers (intima, media, and adventitia) of the arterial wall. Ninety percent of all aortic aneurysms are caused by atherosclerotic damage to the aortic wall. Other etiologies include mycotic and inflammatory [7, 34, 89]. In the abdominal aorta, aneurysms are defined by a diameter >30 mm, regardless of the age of the patient [34]. Rupture risk increases with the diameter, with upward of 30–33% risk of rupture for aneurysms larger than 70 mm [34].
Findings at MDCT of impending rupture include:
-
size increase more than >10 mm/year
-
focal new wall discontinuity of the intimal calcifications
-
eccentric shape of the aortic lumen
-
penetrating ulcer with intramural hematoma
Rupture of an abdominal aorta aneurysm in an 80-year-old woman with abdominal pain and hypotension. (a) Note on the nonenhanced axial CT image the “crescent sign” due to acute hematoma in the left aneurysm wall; (b) IV contrast-enhanced CT image in coronal plane showing active extravasation from the ruptured aneurysm, and associated retroperitoneal hemorrhage; (c) IV contrast-enhanced CT image in the coronal plane showing also the left perirenal hematoma; IV contrast-enhanced CT image in the axial plane (d), coronal plane (e), and 3D-volume rendering (f), showing the complete exclusion of the aneurysm after endoprosthesis positioning. Figure 6 is reprinted with permission from C arrafiello G, Mangini M, Ierardi AM, Recaldini C, Cotta E, Piacentino F, Fugazzola C. Vascular Emergencies of the Retroperitoneum. Book chapter in: M. Scaglione et al., Emergency Radiology of the Abdomen, Medical Radiology. Diagnostic Imaging Editor: Springer-Verlag Berlin Heidelberg 2012; pp. 189–205
The rupture may present as a contained rupture or free rupture. Differentiation between these two conditions is crucial in selecting proper treatment. Most commonly the rupture involves the posterolateral aorta with hemorrhage into the retroperitoneum; if the anterior or anterolateral wall is involved, an intraperitoneal rupture may occur [103]. A retroperitoneal hematoma adjacent to the aortic aneurysm is the most common CT finding of abdominal aortic aneurysm rupture [104]. Another important finding that may be seen in contained rupture is the “draped aorta” sign, where the aorta is draped over and inseparable from adjacent vertebrae [7, 104]. The “crescent sign,” a peripheral crescent of increased attenuation within the thrombus of an aortic aneurysm, is a CT sign of acute or impending rupture, but it is not highly sensitive or specific as an isolated CT finding [7, 104] (Fig. 9.6). Signs of uncontained rupture are seen with active bleeding in the retroperitoneal or peritoneal spaces seen on arterial-phase images, which increases on the following phases.
9.5.2.3.1 IR Management
There is no universal agreement to our knowledge about the role of EVAR for the treatment of aortic aneurysm rupture [34], but it is established that open repair has been associated with significant morbidity and mortality, prolonged recovery, and some late complications [105, 106]. Because of these limitations, many patients and their physicians choose EVAR, which has become an effective alternative, and currently there are several studies which compared endovascular repair with surgical open repair [107,108,109,110,111,112,113,114]. Approximately 50% of all patients with rAAA are suitable for EVAR [34, 54, 115], which is less invasive and has lower overall treatment costs [116, 117], reduced morbidity and mortality, and has short- and long-time survival advantages over open surgery [105, 118]. The 30-day mortality after elective surgical repair in major randomized trials ranges from 2.7% to 5.8%, and is influenced by the volume of procedures performed at the institution and the expertise of the surgeon [116]. Logistics plays a central role in the management of traumatic patient with rAAA: EVAR may not be possible if CT angiography cannot be done immediately after the triage in emergency department and if an operating room is not available [119]. A large randomized trial of EVAR and open surgery for rAAA [119] underlined the importance of CT to confirm the suitability of endovascular repair of rAAA, without delaying treatment. Nevertheless, it is essential to have an early multidisciplinary approach [120], including a vascular surgeon, an interventional radiologist, emergency department physicians, anesthesiologists, operating room staff, and radiologic technologists [7]. Endovascular rAAA repair needs other validation in multicenter prospective studies, but is an evolving technique which offers the potential for improved outcomes in patients who otherwise have a high morbidity and mortality [15].
9.5.2.4 Aorto-enteric and Aorto-caval Fistulas
Aorto-enteric and aorto-duodenal fistulae are rare but frequently fatal complications which may follow open abdominal aneurism repair [121] or aneurysm infection. The perivascular inflammation may spread and involve the adjacent tissue leading to the formation of a fistula tract. Most of aorto-enteric fistulas involve the duodenum, most commonly its third and fourth portions. CT features of aorto-enteric fistulas include an abdominal aortic aneurysm, often with signs of rupture, and endoluminal and periaortic extraluminal gas, and contrast-enhanced CT may show contrast material extravasation from the aorta into the involved portion of the bowel [104, 121].
It has been reported that the overall prevalence of aorto-caval fistula is quite low, about 2–6%; such fistulas are caused by chronic perivascular inflammation, leading to erosion of the wall of the IVC. Aorto-caval fistula causes hemodynamic alteration with pulmonary congestion [122].
9.5.2.4.1 IR Management
Aorto-enteric fistulae (AEF) are rare but often fatal late complications of open repair with graft implantation or aneurysm infection [123]. The optimum management of AEF is currently not well defined to our knowledge, with a wide range of possible treatments. Several studies showed that replacement of the aneurysm with a prosthetic graft is preferable compared to other surgical treatment [124,125,126]. However, the mortality rates of in situ grafting remain 30–40% in the past decades. Axillobifemoral bypass is reserved for patients with extensive local sepsis, but this approach is associated with high mortality rates [127]. The advent of endovascular techniques has revolutionized the management of AEFs, especially for patients unsuitable for open surgery [128, 129]. Percutaneous aortic endografts are now widely used, and new endovascular approaches and other materials have been investigated [127]. A very recent systematic review by Kakkos et al. [130] showed that in a pooled analysis of 823 patients, EVAR was associated with a significantly reduced in-hospital mortality (7.1%) compared with open surgery (33.9%). Nevertheless, this difference mostly disappeared during the first 18–24 months after the procedure (2-year patient survival: 51% and 40%, respectively), concomitant with a high sepsis rate (2-year rates 42% vs. 19% for open surgery). A drawback of IR treatment is the increased risk of infection. Therefore, prophylactic broad-spectrum antibiotics should be instituted as soon as the diagnosis is suspected [127]. Moreover, patients who have undergone AAA repair may need periodic imaging to monitor treatment efficacy [128]. Endovascular closure of AEFs has proven to be less durable than other open surgery techniques, with a higher recurrence rate (100% at 2 years), which was also observed for in situ repair with homograft and impregnated prosthetic grafts (32% and 29%, respectively, at 2 years), both associated also with a poor overall survival rate (40% and 23%, respectively, at 2 years) [130]. As long-term use of multiple antibiotics cannot guarantee an uneventful outcome in patients undergoing endovascular repair [131], the correct management appears to consist of an urgent individualized interdisciplinary approach, potentially combining EVAR for bridging and open surgery (which has better results when performed in patients not actively bleeding) as the definitive treatment [34, 132]. The exact timing and also type of such a definite repair may be difficult to choose, but based on the results of the Kakkos’ review [130], in situ repair with vein grafts or prosthetic grafts covered with omentum may be the best option, and can be performed within the first few weeks.
Primary aorto-caval fistulas (ACF) are a rare complication of AAA and involve fewer than 1% of all AAAs [69]. Penetrating traumatic injury is the most common cause of IVC fistulas. Their IR management has already been discussed.
9.5.2.5 Acute Thrombosis (Acute Leriche Syndrome)
Acute thrombosis of the aorta is a rare and severe disease, which is mostly seen in patients with severe atherosclerosis of the distal abdominal aorta and the iliac arteries. At MDCT, it is seen as an opacification defect in the arterial phase due to extensive endoluminal thrombosis (Fig. 9.7 ). It is important to evaluate the location of aortic stenosis, the extension of the occlusion, the involvement of visceral arteries, and the extent of collateralization [7].
Complete aorto-bi-iliac thrombosis in a 63-year-old man. (a) IV contrast-enhanced CT image in the coronal plane in the arterial phase shows complete aorto-bi-iliac thrombosis; (b) pretreatment intraoperative digital subtraction angiography (DSA) showing the complete occlusion of the distal abdominal aorta and of the iliac branches; (c) post-treatment DSA shows the recanalization of the treated vessels after stent-graft deployment; IV contrast-enhanced CT images in the arterial phase in the sagittal (d) and coronal (e) planes and with a 3D-volume rendering (f), showing the successful procedure. Figure 7 is reprinted with permission from C arrafiello G, Mangini M, Ierardi AM, Recaldini C, Cotta E, Piacentino F, Fugazzola C. Vascular Emergencies of the Retroperitoneum. Book chapter in: M. Scaglione et al., Emergency Radiology of the Abdomen, Medical Radiology. Diagnostic Imaging Editor: Springer-Verlag Berlin Heidelberg 2012; pp. 189–205
9.5.2.5.1 IR Management
Acute thrombosis of the aorta is rare [133], and although it may cause major thromboembolic complications, there is no standardized treatment to our knowledge [134].
Thrombolysis and anticoagulation have been used with variable success, but they carry the risk of distal embolism caused by partial lysis and dislodgment of the thrombus [135]. Simple thrombectomy according to Fogarty has declined in importance because of the high recurrence rate [136]. Surgical removal is now recommended as the treatment of choice by some authors [135, 137]. However, the poor general condition of patients, especially those with associated cancer, may be less suited for standard surgery, and IR treatment will prove to be a preferred mode of management. Recently, several manuscripts have described the efficiency of stent-graft exclusion of the thrombus [138,139,140,141,142].
The advantages of IR techniques are not only to exclude the thrombus but also to cover the underlying atherosclerotic aortic wall to prevent recurrence [135]. Another benefit of IR is the possibility of combining the procedure with a peripheral embolectomy through the same surgical access. Shames et al. [138] performed endovascular stent-graft thrombus exclusion in eight patients at different locations with promising results. They observed no apparent embolic events during the stent implantation, and there was no evidence of recurrent emboli within a 12-month follow-up period, demonstrating the safety and efficacy and the safety of the procedure.
The disadvantages are the inability to perform a pathological examination (necessary to differentiate bland thrombus for aortic tumor), and the possible distal embolism and migration of the device caused by lodged thrombus between the stent-graft and the aortic wall [143]. Filter systems (e.g., temporary caval filters) are not appropriate for the aorta. Therefore, it is recommended that visceral and peripheral angiography should be routinely used at the end of the procedure, to identify potential embolic events caused by the intervention, so they can be treated simultaneously [140].
Because stent-graft placement for the acute aortic thrombosis has been rarely reported to our knowledge, there are no guidelines for the postoperative management regarding anticoagulation. Anticoagulation therapy should therefore be guided on an individual patient basis [143].
Concerning thrombosis of the superior mesenteric artery (SMA), the main nonsurgical treatments are endovascular stent placement, catheter-directed vasodilation, or thrombolytic therapy [144]. Limited studies, however, are available in the literature to our knowledge, and these procedures continue to be controversial [144]. Undoubtedly, as the average life expectancy increases and subsequently the number of elderly in our hospitals continues to grow, the need for endovascular thrombolytic therapy, angioplasty, and stent placement in either acute or chronic mesenteric ischemia will increase, especially when surgical therapy in some elderly is neither indicated nor safe [7, 144].
9.5.2.6 Spontaneous Bleeding
Spontaneous RB is a relatively uncommon but potentially lethal entity with a nonspecific presentation that can frequently lead to misdiagnosis and delayed treatment [145, 146]. If the patient is hemodynamically stable, the mainstay of management is a conservative approach, with withdrawal of anticoagulation therapy, correction of coagulopathy, volume resuscitation, and supportive measures [7, 146]. In some patients, IR procedures are necessary (Fig. 9.8). Surgery is rarely indicated and is reserved for patients with failed angiographic procedures, concurrent surgical conditions, or with significant compressive symptoms on nervous system structures from hematoma formation [146, 147].
Spontaneous active bleeding in a retroperitoneal hematoma in a 56-year-old man. IV contrast-enhanced CT images in the axial (a) and coronal (b) planes in the arterial phase show a right retroperitoneal hematoma, with active arterial bleeding; (c) selective angiogram of the feeding lumbar artery confirms a vascular “blush”; (d) angiogram performed at the end of the embolization with microcoils and sponge revealing complete embolization
There is a growing trend in the use of IR techniques as an alternative to open surgery in the management of RB, whatever the etiology [148]. The main options are selective intra-arterial embolization or stent-grafts to stop the bleeding. Intra-arterial embolization is being used with increasing frequency in patients where the angiogram shows active bleeding sites [17]. In a series reported by Isokangas et al., four patients were operated on prior to embolization, but surgery failed to control the bleeding. Embolization using a combination of agents, including coils, gelatin, and/or polyvinyl alcohol, has been used. Coils are probably the safest, but Isokangas et al. commented that proximal coiling of the bleeding artery may not be sufficient in the retroperitoneum, where there is a rich network of collateral arteries, and new arterial pathways may develop after obliteration of the lumbar arteries [6, 149], so the embolic agents should be placed both proximal and distal to the bleeding site to prevent re-bleeding.
Panetta et al. [150] stated that hemodynamic instability despite 4 or more units of blood transfusion within 24 h, or 6 or more units of blood transfusion within 48 h, is an indication for urgent investigation and IR treatment. Embolization should be performed whenever arterial extravasation is seen. Sharafuddin et al. [151] showed that selective arterial embolization was successful in a series of five patients, although re-bleeding occurred in one patient. Subsequent small case series have shown similar results [152].
9.6 Venous
9.6.1 Inferior Vena Cava Thrombosis
IVC thrombosis is a rare but severe disease which is associated with a high rate of mortality [153] (Fig. 9.9). IVC thrombosis may arise from an isolated thrombus, or may be due to propagation from the iliac veins. Isolated IVC thrombosis is commonly associated with outflow obstruction of the IVC, such as in Budd-Chiari syndrome, IVC anomalies from tumoral invasion, or from external compression by a mass or hematoma. IVC thrombosis can initially be asymptomatic and may be revealed after sudden pulmonary embolism [7, 153].
Acute left iliac vein and IVC thrombosis in a 67-year-old woman. IV contrast-enhanced CT image in the coronal plane (a) and in a coronal MIP (b), showing thrombosis of the left iliac vein and IVC; (c) transjugular vein access DSA confirms the thrombosis; (d) postprocedural DSA after caval filter placement. Figure 9 is reprinted with permission from C arrafiello G, Mangini M, Ierardi AM, Recaldini C, Cotta E, Piacentino F, Fugazzola C. Vascular Emergencies of the Retroperitoneum. Book chapter in: M. Scaglione et al., Emergency Radiology of the Abdomen, Medical Radiology. Diagnostic Imaging Editor: Springer-Verlag Berlin Heidelberg 2012; pp. 189–205
9.6.1.1 IR Management
IVC thrombosis is a rare but severe disease which is associated with a high rate of mortality. The management of IVCT has no universal agreement and continues to be the target of continued research [153]. Although anticoagulation therapy remains fundamental in treating IVCT, its inherent limitations have led to the use of minimally invasive, endovascular treatment options, including transcatheter thrombolysis, mechanical thrombectomy, or a combination of these techniques.
The primary goals of treatment for IVCT include prevention of pulmonary embolism, restoration of unobstructed venous return, prevention of recurrent deep venous thrombosis (DVT), and preservation of venous valve function [154].
To achieve these goals, the guidelines from the Society for Vascular Surgery and the American Venous Forum advocate early thrombus removal strategies for acute DVT [155], but these recommendations are mostly applicable for the treatment of primary IVCT.
The strategy of early deep venous thrombus removal is suggested if the selected patients meet the following criteria [155]:
-
first episode of acute iliofemoral DVT
-
fewer than 14 days of symptoms
-
low risk of bleeding
-
good functional capacity and acceptable life expectancy
The evidence for treatment of primary IVCT with IR procedures is predominantly extrapolated from lower extremity DVT treatment data [153]. Unless contraindicated by a significantly increased risk of RB, urgent endovascular treatment is recommended for those patients with severe acute DVT associated with limb-threatening compromise, or worsening IVCT despite anticoagulation therapy [156].
Alkhouli et al. [157] reported a total of 2674 patients with IVCT; among them 718 patients were treated with catheter-directed therapy, and the others were managed with anticoagulation alone. This study, along with the reports from the Catheter-Directed Venous Thrombolysis (CaVenT) trial [158], suggests the main benefit from IR treatment for IVCT patients is the reduced risk of developing post-thrombotic syndrome.
Limitations of the catheter-directed therapy include lengthy thrombolytic infusions in an intensive monitored setting with the inherent risks related to hemorrhage [153]. Among the 473 patients included in the national multicenter registry [156], bleeding complications were reported in 54 (11%), neurologic complications in 2 (0.4%), and death in 2 (0.4%) for the treatment of DVT.
Moreover, a current limitation is patient access to care, as not all healthcare facilities may have the availability to the endovascular interventionalists skilled in these advanced techniques to provide optimum management for IVCT [7, 153].
In conclusion, although there is a scarcity of data in the literature regarding the management of IVCT, and although there is no consensus regarding the most successful approach, accumulated evidence advocates endovascular strategies as a safe option for the treatment of IVCT [153].
Conclusion
Vascular emergencies of the retroperitoneum can be traumatic or nontraumatic, arterial, or venous in origin. Their management is challenging and requires multidisciplinary approach. MDCT with intravenous contrast is the primary imaging modality for the diagnosis of retroperitoneal vascular injuries, and to assess whether medical, interventional, or surgical management—or a combination of these—is necessary. Currently, there is a growing trend in the use of interventional radiology techniques as an alternative to open surgery. Advances in interventional radiology technique permit less invasive treatments for the management of retroperitoneal vascular emergencies, using several methods including embolization, balloon occlusion, and stent-grafting. Open surgery is still indicated if the patient remains hemodynamically unstable, or if IR techniques are either not successful or unavailable.
References
Pfeifer R, Tarkin IS, Rocos B, Pape HC. Patterns of mortality and causes of death in polytrauma patients—has anything changed? Injury. 2009;40:907–11.
Muckart DJ, Pillay B, Hardcastle TC, Skinner DL. Vascular injuries following blunt polytrauma. Eur J Trauma Emerg Surg. 2014;40:315–22.
Sobrino J, Shafi S. Timing and causes of death after injuries. Proc (Bayl Univ Med Cent). 2013;26:120–3.
Iacobellis F, Ierardi AM, Mazzei MA, Magenta Biasina A, Carrafiello G, Nicola R, Scaglione M. Dual-phase CT for the assessment of acute vascular injuries in high-energy blunt trauma: the imaging findings and management implications. Br J Radiol. 2016;89(1061):20150952.
Charlton-Ouw KM, DuBose JJ, Leake SS, et al. Observation may be safe in selected cases of blunt traumatic abdominal aortic injury. Ann Vasc Surg. 2016;30:34–9.
Genovese EA, Fonio P, Floridi C, et al. Abdominal vascular emergencies: US and CT assessment. Crit Ultrasound J. 2013;5(Suppl 1):S10.
Carrafiello G, Mangini M, Ierardi AM, et al. Vascular emergencies of the retroperitoneum. In: Scaglione M, et al., editors. Emergency radiology of the abdomen, medical radiology. Diagnostic imaging. Berlin Heidelberg: Springer-Verlag; 2012. p. 189–205.
Scaglione M, Romano L, Bocchini G, et al. Multidetector computed tomography of pancreatic, small bowel, and mesenteric traumas. Semin Roentgenol. 2012;47(4):362–70.
Romano F, Iacobellis F, Guida F, et al. Traumatic injuries: mechanisms of lesions. In: Miele V, Trinci M, editors. Diagnostic imaging in polytrauma patients. Berlin Heidelberg: Springer-Verlag; 2018. p. 35–55.
Bernstein MP, Mirvis SE. Penetrating trauma to the abdominal aorta: CT demonstration of active bleeding. Emerg Radiol. 2001;8:43–7.
Borioni R, Garofalo M, Seddio F, Colagrande L, Marino B, Albano P. Posttraumatic infrarenal abdominal aortic pseudoaneurysm. Tex Heart Inst J. 1999;26(4):312–4.
Azizzadeh A, Keyhani K, Miller CC 3rd, Coogan SM, Safi HJ, Estrera AL. Blunt traumatic aortic injury: initial experience with endovascular repair. J Vasc Surg. 2009;49(6):1403–8.
Ertel W, Keel M, Eid K, Platz A, Trentz O. Control of severe hemorrhage using C-clamp and pelvic packing in multiply injured patients with pelvic ring disruption. J Orthop Trauma. 2001;15(7):468–74.
Boufi M, Bordon S, Dona B, et al. Unstable patients with retroperitoneal vascular trauma: an endovascular approach. Ann Vasc Surg. 2011;25(3):352–8.
Nicholson AA. Vascular radiology in trauma. Cardiovasc Intervent Radiol. 2004;27(2):105–20.
Velmahos GC, Toutouzas KG, Vassiliu P, et al. A prospective study on the safety and efficacy of angiographic embolization for pelvic and visceral injuries. J Trauma. 2002;53(2):303–8. discussion 308
Isokangas JM, Perala JM. Endovascular embolization of spontaneous retroperitoneal hemorrhage secondary to anticoagulant treatment. Cardiovasc Intervent Radiol. 2004;27(6):607–11.
Velmahos GC, Chahwan S, Falabella A, Hanks SE, Demetriades D. Angiographic embolization for intraperitoneal and retroperitoneal injuries. World J Surg. 2000;24(5):539–45.
Berthet JP, Marty-Ane CH, Veerapen R, Picard E, Mary H, Alric P. Dissection of the abdominal aorta in blunt trauma: endovascular or conventional surgical management? J Vasc Surg. 2003;38(5):997–1003. discussion 1004
Sniderman KW, Sos TA, Gay WA Jr, Subramanian VA. Aortic dissection beginning in the abdomen. AJR. 1978;130(6):1115–8.
Arendrup H, Frimodt-Moller PC, Christensen JE. Acute dissection confined to the abdominal aorta. Scand J Thorac Cardiovasc Surg. 1983;17(2):121–3.
Picard E, Marty-Ane CH, Vernhet H, et al. Endovascular management of traumatic infrarenal abdominal aortic dissection. Ann Vasc Surg. 1998;12(6):515–21.
Vernhet H, Marty-Ane CH, Lesnik A, et al. Dissection of the abdominal aorta in blunt trauma: management by percutaneous stent placement. Cardiovasc Intervent Radiol. 1997;20(6):473–6.
Nishimura K, Kanaoka Y, Ikebuchi M, et al. Percutaneous balloon fenestration in a case of traumatic abdominal aortic dissection with lower extremity ischemia. J Vasc Surg. 2000;32(3):616–8.
Michaels AJ, Gerndt SJ, Taheri PA, et al. Blunt force injury of the abdominal aorta. J Trauma. 1996;41(1):105–9.
Maleux G, Soula P, Otal P, Colombier D, Cérène A, Joffre F, Rousseau H. Traumatic aortobiiliac dissection treated by kissing-stent placement. J Trauma. 1997;43(4):706–8.
Hahmann M, Richter GM, Schuhmacher H, Allenberg JR, Kauffmann GW. Post-traumatic dissection of the abdominal aorta. Radiologe. 2001;41(7):590–4.
Bortone AS, Schena S, Mannatrizio G, et al. Endovascular stent-graft treatment for diseases of the descending thoracic aorta. Eur J Cardiothorac Surg. 2001;20(3):514–9.
Nienaber CA, Sievers HH. Intramural hematoma in acute aortic syndrome: more than one variant of dissection? Circulation. 2002;106(3):284–5.
Dake MD, Kato N, Mitchell RS, et al. Endovascular stent-graft placement for the treatment of acute aortic dissection. N Engl J Med. 1999;340(20):1546–52.
Marty-Ane CH, SerreCousine O, Laborde JC, Costes V, Mary H, Senac JP. Use of a balloon-expandable intravascular graft in the management of type B aortic dissection in an animal model. J Vasc Interv Radiol. 1995;6(1):97–103.
Trent MS, Parsonnet V, Shoenfeld R, et al. A balloon-expandable intravascular stent for obliterating experimental aortic dissection. J Vasc Surg. 1990;11(5):707–17.
Voitle E, Hofmann W, Cejna M. Aortic emergencies—diagnosis and treatment: a pictorial review. Insights Imaging. 2015;6(1):17–32.
Erbel R, Aboyans V, Boileau C, et al. 2014 ESC guidelines on the diagnosis and treatment of aortic diseases: document covering acute and chronic aortic diseases of the thoracic and abdominal aorta of the adult. The task force for the diagnosis and treatment of aortic diseases of the European. Eur Heart J. 2014;35(41):2873–926.
Nienaber CA, von Kodolitsch Y, Petersen B, et al. Intramural hemorrhage of the thoracic aorta. Diagnostic and therapeutic implications. Circulation. 1995;92(6):1465–72.
Eggebrecht H, Plicht B, Kahlert P, Erbel R. Intramural hematoma and penetrating ulcers: indications to endovascular treatment. Eur J Vasc Endovasc Surg. 2009;38(6):659–65.
Daily PO, Trueblood HW, Stinson EB, Wuerflein RD, Shumway NE. Management of acute aortic dissections. Ann Thorac Surg. 1970;10(3):237–47.
von Kodolitsch Y, Csosz SK, Koschyk DH, et al. Intramural hematoma of the aorta: predictors of progression to dissection and rupture. Circulation. 2003;107(8):1158–63.
Mussa FF, Horton JD, Moridzadeh R, Nicholson J, Trimarchi S, Eagle KA. Acute aortic dissection and intramural hematoma: a systematic review. JAMA. 2016;316(7):754–63.
Braverman AC, Harris KM. Management of aortic intramural hematoma. Curr Opin Cardiol. 1995;10(5):501–4.
Motoyoshi N, Moizumi Y, Komatsu T, Tabayashi K. Intramural hematoma and dissection involving ascending aorta: the clinical features and prognosis. Eur J Cardiothorac Surg. 2003;24(2):237–42. discussion 242
Aladham F, Sundaram B, Williams DM, Quint LE. Traumatic aortic injury: computerized tomographic findings at presentation and after conservative therapy. J Comput Assist Tomogr. 2010;34(3):388–94.
Holmes JH, Bloch RD, Hall RA, Carter YM, Karmy-Jones RC. Natural history of traumatic rupture of the thoracic aorta managed nonoperatively: a longitudinal analysis. Ann Thorac Surg. 2002;73(4):1149–54.
Hirose H, Gill IS, Malangoni MA. Nonoperative management of traumatic aortic injury. J Trauma. 2006;60(3):597–601.
Kepros J, Angood P, Jaffe CC, Rabinovici R. Aortic intimal injuries from blunt trauma: resolution profile in nonoperative management. J Trauma. 2002;52(3):475–8.
Zhang G, Feng Q, Zheng D, Ma L, Li R, Jiang J, Ni Y. Early aggressive medical treatment associated with selective prophylactic aortic stent-grafting for aortic intramural hematoma. Thorac Cardiovasc Surg. 2011;59(6):342–8.
Song JK, Kim HS, Song JM, et al. Outcomes of medically treated patients with aortic intramural hematoma. Am J Med. 2002;113(3):181–7.
Abed H, Ball WR, Stone T, Houghton A. Very late rupture of a post-traumatic abdominal aortic pseudoaneurysm. BMJ Case Rep. 2017;27:2017.
Hamilton JD, Kumaravel M, Censullo ML, Cohen AM, Kievlan DS, West OC, Multidetector CT. evaluation of active extravasation in blunt abdominal and pelvic trauma patients. Radiographics. 2008;28(6):1603–16.
Peterson AH, Williams DM, Rodriguez JL, Francis IR. Percutaneous treatment of a traumatic aortic dissection by balloon fenestration and stent placement. AJR. 1995;164(5):1274–6.
Garg L, Jain N, Agrawal S, Chauhan U, Goel V, Puri SK. Juxtarenal aortic pseudoaneurysm—right renal vein fistula with circumaortic renal collar-delayed manifestation of a gunshot injury—an uncommon entity diagnosed with CT angiography. Pol J Radiol. 2016;81:114–9.
Tucker S Jr, Rowe VL, Rao R, Hood DB, Harrell D, Weaver FA. Treatment options for traumatic pseudoaneurysms of the paravisceral abdominal aorta. Ann Vasc Surg. 2005;19(5):613–8.
Mattox KL. Red River anthology. J Trauma. 1997;42(3):353–68.
Mastracci TM, Greenberg RK. Complex aortic disease: changes in perception, evaluation and management. J Vasc Surg. 2008;48(6 Suppl):17S–23S. discussion 23S.
Saad NE, Saad WE, Davies MG, Waldman DL, Fultz PJ, Rubens DJ. Pseudoaneurysms and the role of minimally invasive techniques in their management. Radiographics. 2005;25(Suppl 1):S173–89.
Baltacioglu F, Cimsit NC, Cil B, Cekirge S, Ispir S. Endovascular stent-graft applications in iatrogenic vascular injuries. Cardiovasc Intervent Radiol. 2003;26(5):434–9.
Kapoor BS, Haddad HL, Saddekni S, Lockhart ME. Diagnosis and management of pseudoaneurysms: an update. Curr Probl Diagn Radiol. 2009;38(4):170–88.
Leyon JJ, Littlehales T, Rangarajan B, Hoey ET, Ganeshan A. Endovascular embolization: review of currently available embolization agents. Curr Probl Diagn Radiol. 2014;43(1):35–53.
Yamakado K, Nakatsuka A, Tanaka N, Takano K, Matsumura K, Takeda K. Transcatheter arterial embolization of ruptured pseudoaneurysms with coils and n-butyl cyanoacrylate. J Vasc Interv Radiol. 2000;11(1):66–72.
Morgan TA, Steenburg SD, Siegel EL, Mirvis SE. Acute traumatic aortic injuries: posttherapy multidetector CT findings. Radiographics. 2010;30(4):851–67.
McDermott VG, Shlansky-Goldberg R, Cope C. Endovascular management of splenic artery aneurysms and pseudoaneurysms. Cardiovasc Intervent Radiol. 1994;17(4):179–84.
Guillon R, Garcier JM, Abergel A, et al. Management of splenic artery aneurysms and false aneurysms with endovascular treatment in 12 patients. Cardiovasc Intervent Radiol. 2003;26(3):256–60.
Spencer TA, Smyth SH, Wittich G, Hunter GC. Delayed presentation of traumatic aortocaval fistula: a report of two cases and a review of the associated compensatory hemodynamic and structural changes. J Vasc Surg. 2006;43(4):836–40.
Kim H, Randolph S. Traumatic aortocaval fistula from gunshot wound, complicated by bullet embolization to the right ventricle. Radiol Case Rep. 2015;7(4):767.
Beton O, Kaplanoğlu H, Berkan Ö, Yılmaz MB. A case report of delayed diagnosed chronic aortocaval fistula: a rare complication of penetrating trauma to the abdomen. J Clin Imaging Sci. 2015;5:62.
Srisuwan T, Kanjanavanit R, Rerkasem K. Endovascular aortic stenting in patients with chronic traumatic aortocaval fistula. Ann Vasc Dis. 2013;6(4):741–4.
O'Brien GC, Murphy C, Martin Z, et al. Hybrid management of a spontaneous ilio-iliac arteriovenous fistula: a case report. J Med Case Reports. 2011;5:401.
Davis PM, Gloviczki P, Cherry KJ Jr, et al. Aorto-caval and ilio-iliac arteriovenous fistulae. Am J Surg. 1998;176(2):115–8.
Brightwell RE, Pegna V, Boyne N. Aortocaval fistula: current management strategies. ANZ J Surg. 2013;83(1–2):31–5.
Boudghene F, Sapoval M, Bonneau M, Bigot JM. Aortocaval fistulae: a percutaneous model and treatment with stent grafts in sheep. Circulation. 1996;94(1):108–12.
Antoniou GA, Koutsias S, Karathanos C, Sfyroeras GS, Vretzakis G, Giannoukas AD. Endovascular stent-graft repair of major abdominal arteriovenous fistula: a systematic review. J Endovasc Ther. 2009;16(4):514–23.
Lau LL, O’Reilly MJ, Johnston LC, Lee B. Endovascular stent-graft repair of primary aortocaval fistula with an abdominal aortoiliac aneurysm. J Vasc Surg. 2001;33(2):425–8.
Siepe M, Koeppe S, Euringer W, Schlensak C. Aorto-caval fistula from acute rupture of an abdominal aortic aneurysm treated with a hybrid approach. J Vasc Surg. 2009;49(6):1574–6.
Daly KP, Ho CP, Persson DL, Gay SB. Traumatic retroperitoneal injuries: review of multidetector CT findings. Radiographics. 2008;28(6):1571–90.
Akpinar E, Peynircioglu B, Turkbey B, Cil BE, Balkanci F. Endovascular management of life-threatening retroperitoneal bleeding. ANZ J Surg. 2008;78(8):683–7.
Marty B, Sanchez LA, Wain RA, et al. Endovascular treatment of a ruptured lumbar artery aneurysm: case report and review of the literature. Ann Vasc Surg. 1998;12(4):379–83.
Dondelinger RF, Trotteur G, Ghaye B, Szapiro D. Traumatic injuries: radiological hemostatic intervention at admission. Eur Radiol. 2002;12(5):979–93.
Scott AR, Gilani R, Tapia NM, Mattox KL, Wall MJ, Suliburk JW. Endovascular management of traumatic peripheral arterial injuries. J Surg Res. 2015;199(2):557–63.
Moore HM, List A, Holden A, Osborne TM. Therapeutic embolization for acute haemorrhage in the abdomen and pelvis. Australas Radiol. 2000;44(2):161–8.
Papakostidis C, Kanakaris N, Dimitriou R, Giannoudis PV. The role of arterial embolization in controlling pelvic fracture haemorrhage: a systematic review of the literature. Eur J Radiol. 2012;81(5):897–904.
Watarida S, Nishi T, Furukawa A, et al. Fenestrated stent-graft for traumatic juxtahepatic inferior vena cava injury. J Endovasc Ther. 2002;9(1):134–7.
Lindblad B, Brunkwall J, Lindh M, Nyman U, Malina M, Ivancev K. Traumatic aortic rupture and retroperitoneal haematoma—treatment including combined operative and endovascular approach. Eur J Vasc Endovasc Surg. 1999;17(5):451–5.
Fraga GP, Bansal V, Fortlage D, Coimbra R. A 20-year experience with portal and superior mesenteric venous injuries: has anything changed? Eur J Vasc Endovasc Surg. 2009;37:87e91.
Tsai R, Raptis C, Schuerer DJ, Mellnick VM. CT appearance of traumatic inferior vena cava injury. AJR. 2016;19:1–7.
Ierardi AM, Berselli M, Cuffari S, Castelli P, Cocozza E, Carrafiello G. Uncommon case of a post-traumatic portal vein pseudoaneurysm treated with percutaneous transhepatic stent grafting. Cardiovasc Intervent Radiol. 2016;39(10):1506–9.
Kunkala M, Jenkins DH, McEachen J, Stockland A, Zielinski MD. Nonoperative management of traumatic suprahepatic inferior vena cava pseudoaneurysms. J Vasc Surg. 2011;54(6 Suppl):80S–2S.
Chakroun A, Nakhli MS, Kahloul M, Harrathi MA, Naija W. Post traumatic inferior vena cava thrombosis: A case report and review of literature. Int J Surg Case Rep. 2017;15(36):59–63.
Cheaito A, Tillou A, Lewis C, Cryer H. Management of traumatic blunt IVC injury. Int J Surg Case Rep. 2016;28:26–30.
Valente T, Rossi G, Lassandro F, et al. MDCT evaluation of acute aortic syndrome (AAS). Br J Radiol. 2016;89(1061):20150825.
Erbel R, Alfonso F, Boileau C, et al. Task force on aortic dissection, European society of cardiology diagnosis and management of aortic dissection. Eur Heart J. 2001;22(18):1642–81.
Umana JP, Miller DC, Mitchell RS. What is the best treatment for patients with acute type B aortic dissections—medical, surgical, or endovascular stent-grafting? Ann Thorac Surg. 2002;74(5):S1840–3. discussion S1857-1863.
Brandt M, Hussel K, Walluscheck KP, et al. Stent-graft repair versus open surgery for the descending aorta: a case-control study. J Endovasc Ther. 2004;11(5):535–8.
Eggebrecht H, Nienaber CA, Neuhauser M, et al. Endovascular stent-graft placement in aortic dissection: a meta-analysis. Eur Heart J. 2006;27(4):489–98.
Nienaber CA, Erbel R, Ince H. Nihil nocere on the rocky road to endovascular stent-graft treatment. J Thorac Cardiovasc Surg. 2004;127(3):620–1.
Castelli P, Caronno R, Piffaretti G, et al. Endovascular repair for concomitant multilevel aortic disease. Eur J Cardiothorac Surg. 2005;28(3):478–82.
Fattori R, Montgomery D, Lovato L, et al. Survival after endovascular therapy in patients with type B aortic dissection: a report from the International Registry of Acute Aortic Dissection (IRAD). JACC Cardiovasc Interv. 2013;6(8):876–82.
Ganaha F, Miller DC, Sugimoto K, et al. Prognosis of aortic intramural hematoma with and without penetrating atherosclerotic ulcer: a clinical and radiological analysis. Circulation. 2002;106(3):342–8.
Brinster DR, Wheatley GH 3rd, Williams J, Ramaiah VG, Diethrich EB, Rodriguez-Lopez JA. Are penetrating aortic ulcers best treated using an endovascular approach? Ann Thorac Surg. 2006;82(5):1688–91.
Brinster DR. Endovascular repair of the descending thoracic aorta for penetrating atherosclerotic ulcer disease. J Card Surg. 2009;24(2):203–8.
Kos X, Bouchard L, Otal P, et al. Stent-graft treatment of penetrating thoracic aortic ulcers. J Endovasc Ther. 2002;9(Suppl 2):II25–31.
Piffaretti G, Tozzi M, Lomazzi C, et al. Penetrating ulcers of the thoracic aorta: results from a single-centre experience. Am J Surg. 2007;193(4):443–7.
Demers P, Miller DC, Mitchell RS, Kee ST, Chagonjian L, Dake MD. Stent-graft repair of penetrating atherosclerotic ulcers in the descending thoracic aorta: mid-term results. Ann Thorac Surg. 2004;77(1):81–6.
Schwartz SA, Taljanovic MS, Smyth S, O'Brien MJ, Rogers LF. CT findings of rupture, impending rupture, and contained rupture of abdominal aortic aneurysms. AJR. 2007;188(1):W57–62.
Rakita D, Newatia A, Hines JJ, Siegel DN, Friedman B. Spectrum of CT findings in rupture and impending rupture of abdominal aortic aneurysms. Radiographics. 2007;27(2):497–507.
Williamson WK, Nicoloff AD, Taylor LM Jr, Moneta GL, Landry GJ, Porter JM. Functional outcome after open repair of abdominal aortic aneurysm. J Vasc Surg. 2001;33(5):913–20.
Kalman PG, Rappaport DC, Merchant N, Clarke K, Johnston KW. The value of late computed tomographic scanning in identification of vascular abnormalities after abdominal aortic aneurysm repair. J Vasc Surg. 1999;29(3):442–50.
Makaroun MS, Chaikof E, Naslund T, Matsumura JS. Efficacy of a bifurcated endograft versus open repair of abdominal aortic aneurysms: a reappraisal. J Vasc Surg. 2002;35(2):203–10.
Zarins CK, White RA, Schwarten D, et al. AneuRx stent graft versus open surgical repair of abdominal aortic aneurysms: multicenter prospective clinical trial. J Vasc Surg. 1999;29(2):292–305. discussion 306-298
Zarins CK, White RA, Moll FL, et al. The AneuRx stent graft: four-year results and worldwide experience 2000. J Vasc Surg. 2001;33(2 Suppl):S135–45.
May J, White GH, Yu W, et al. Concurrent comparison of endoluminal versus open repair in the treatment of abdominal aortic aneurysms: analysis of 303 patients by life table method. J Vasc Surg. 1998;27(2):213–20. discussion 220-211
White GH, May J, McGahan T, et al. Historic control comparison of outcome for matched groups of patients undergoing endoluminal versus open repair of abdominal aortic aneurysms. J Vasc Surg. 1996;23(2):201–11. discussion 211-202
Brewster DC, Geller SC, Kaufman JA, et al. Initial experience with endovascular aneurysm repair: comparison of early results with outcome of conventional open repair. J Vasc Surg. 1998;27(6):992–1003. discussion 1004-1005
Moore WS, Kashyap VS, Vescera CL, Quinones-Baldrich WJ. Abdominal aortic aneurysm: a 6-year comparison of endovascular versus transabdominal repair. Ann Surg. 1999;230(3):298–306. discussion 306-298
Moore WS, Brewster DC, Bernhard VM. Aorto-uni-iliac endograft for complex aortoiliac aneurysms compared with tube/bifurcation endografts: results of the EVT/Guidant trials. J Vasc Surg. 2001;33(2 Suppl):S11–20.
Mastracci TM, Clase CM, Devereaux PJ, Cina CS. Open versus endovascular repair of abdominal aortic aneurysm: a survey of Canadian vascular surgeons. Can J Surg. 2008;51(2):142–8. quiz 149
Greenhalgh RM, Brown LC, Kwong GP, Powell JT, Thompson SG. EVAR trial participants. Comparison of endovascular aneurysm repair with open repair in patients with abdominal aortic aneurysm (EVAR trial 1), 30-day operative mortality results: randomised controlled trial. Lancet. 2004;364(9437):843–8.
Sadat U, Cooper DG, Cousins C, Boyle JR. Endovascular stenting of large popliteal artery aneurysm is feasible! Adv Med Sci. 2008;53(2):335–7.
Investigators IT, Powell JT, Sweeting MJ, et al. Endovascular or open repair strategy for ruptured abdominal aortic aneurysm: 30 day outcomes from IMPROVE randomised trial. BMJ. 2014;348:f7661.
Hinchliffe RJ, Bruijstens L, MacSweeney ST, Braithwaite BD. A randomised trial of endovascular and open surgery for ruptured abdominal aortic aneurysm—results of a pilot study and lessons learned for future studies. Eur J Vasc Endovasc Surg. 2006;32(5):506–13. discussion 514-505
Klocker J, Falkensammer J, Pellegrini L, Biebl M, Tauscher T, Fraedrich G. Repair of arterial injury after blunt trauma in the upper extremity—immediate and long-term outcome. Eur J Vasc Endovasc Surg. 2010;39(2):160–4.
Valente T, Rossi G, Rea G, et al. Multidetector CT findings of complications of surgical and endovascular treatment of aortic aneurysms. Radiol Clin North Am. 2014;52(5):961–89.
Wang T, Huang B, Zhao J, Yang Y, Yuan D. Aortocaval fistula resulting from rupture of abdominal aortic dissecting aneurysm treated by delayed endovascular repair: a case report. Medicine (Baltimore). 2016;95(18):e3570.
Saratzis N, Saratzis A, Melas N, Ktenidis K, Kiskinis D. Aortoduodenal fistulas after endovascular stent-graft repair of abdominal aortic aneurysms: single-center experience and review of the literature. J Endovasc Ther. 2008;15(4):441–8.
Oderich GS, Bower TC, Hofer J, et al. In situ rifampin-soaked grafts with omental coverage and antibiotic suppression are durable with low reinfection rates in patients with aortic graft enteric erosion or fistula. J Vasc Surg. 2011;53(1):99–106. 107 e101–107; discussion 106-107
Danneels MI, Verhagen HJ, Teijink JA, Cuypers P, Nevelsteen A, Vermassen FE. Endovascular repair for aorto-enteric fistula: a bridge too far or a bridge to surgery? Eur J Vasc Endovasc Surg. 2006;32(1):27–33.
Kakkos SK, Antoniadis PN, Klonaris CN, et al. Open or endovascular repair of aortoenteric fistulas? A multicentre comparative study. Eur J Vasc Endovasc Surg. 2011;41(5):625–34.
Valentine RJ, Timaran CH, Modrall GJ, Smith ST, Arko FR, Clagett GP. Secondary aortoenteric fistulas versus paraprosthetic erosions: is bleeding associated with a worse outcome? J Am Coll Surg. 2008;207(6):922–7.
Song Y, Liu Q, Shen H, Jia X, Zhang H, Qiao L. Diagnosis and management of primary aortoenteric fistulas—experience learned from eighteen patients. Surgery. 2008;143(1):43–50.
Chuter TA, Lukaszewicz GC, Reilly LM, et al. Endovascular repair of a presumed aortoenteric fistula: late failure due to recurrent infection. J Endovasc Ther. 2000;7(3):240–4.
Kakkos SK, Bicknell CD, Tsolakis IA, Bergqvist D, Hellenic Co-operative Group on Aortic Surgery. Editor’s choice—management of secondary aorto-enteric and other abdominal arterio-enteric fistulas: a review and pooled data analysis. Eur J Vasc Endovasc Surg. 2016;52(6):770–86.
Kakkos SK, Papadoulas S, Tsolakis IA. Endovascular management of arterioenteric fistulas: a systemic review and meta-analysis of the literature. J Endovasc Ther. 2011;18(1):66–77.
Bergqvist D, Bjorck M. Secondary arterioenteric fistulation—a systematic literature analysis. Eur J Vasc Endovasc Surg. 2009;37(1):31–42.
Bogie R, Willigendael EM, de Booij M, Meesters B, Teijink JA. Acute thrombosis of an abdominal aortic aneurysm: a short report. Eur J Vasc Endovasc Surg. 2008;35(5):590–2.
Prandoni P, Lensing AW, Piccioli A, et al. Recurrent venous thromboembolism and bleeding complications during anticoagulant treatment in patients with cancer and venous thrombosis. Blood. 2002;100(10):3484–8.
Nakao Y, Akagi H, Irie H, Sakaguchi S, Sakai K. Successful endovascular repair of a ruptured thoracoabdominal aortic aneurysm with severe mural thrombus. Kyobu Geka. 2014;67(12):1051–5.
Lozano P, Gomez FT, Julia J, MR E, Garcia F. Recurrent embolism caused by floating thrombus in the thoracic aorta. Ann Vasc Surg. 1998;12(6):609–11.
Dua A, Desai SS, Holcomb JB, Burgess AR, Freischlag JA. Clinical review of vascular trauma. Berlin Heidelberg: Springer; 2014. p. 191–9. ISBN 978-3-642-39100-2
Shames ML, Rubin BG, Sanchez LA, Thompson RW, Sicard GA. Treatment of embolizing arterial lesions with endoluminally placed stent grafts. Ann Vasc Surg. 2002;16(5):608–12.
Criado E, Wall P, Lucas P, Gasparis A, Proffit T, Ricotta J. Transesophageal echo-guided endovascular exclusion of thoracic aortic mobile thrombi. J Vasc Surg. 2004;39(1):238–42.
Fueglistaler P, Wolff T, Guerke L, Stierli P, Eugster T. Endovascular stent graft for symptomatic mobile thrombus of the thoracic aorta. J Vasc Surg. 2005;42(4):781–3.
Zhang WW, Abou-Zamzam AM, Hashisho M, Killeen JD, Bianchi C, Teruya TH. Staged endovascular stent grafts for concurrent mobile/ulcerated thrombi of thoracic and abdominal aorta causing recurrent spontaneous distal embolization. J Vasc Surg. 2008;47(1):193–6.
Alhan C, Karabulut H, Senay S, Cagil H, Toraman F. Endovascular treatment of occlusive abdominal aortic thrombosis. Heart Vessels. 2010;25(1):70–2.
Akagawa E, Ookawa K, Ohshima N. Endovascular stent configuration affects intraluminal flow dynamics and in vitro endothelialization. Biorheology. 2004;41(6):665–80.
Schoots IG, Levi MM, Reekers JA, Lameris JS, van Gulik TM. Thrombolytic therapy for acute superior mesenteric artery occlusion. J Vasc Interv Radiol. 2005;16(3):317–29.
Gonzalez C, Penado S, Llata L, Valero C, Riancho JA. The clinical spectrum of retroperitoneal hematoma in anticoagulated patients. Medicine (Baltimore). 2003;82(4):257–62.
Caleo O, Bocchini G, Paoletta S, et al. Spontaneous non-aortic retroperitoneal hemorrhage: etiology, imaging characterization and impact of MDCT on management. A multicentric study. Radiol Med. 2015;120(1):133–48.
Sunga KL, Bellolio MF, Gilmore RM, Cabrera D. Spontaneous retroperitoneal hematoma: etiology, characteristics, management, and outcome. J Emerg Med. 2012;43(2):e157–61.
Chan YC, Morales JP, Reidy JF, Taylor PR. Management of spontaneous and iatrogenic retroperitoneal haemorrhage: conservative management, endovascular intervention or open surgery? Int J Clin Pract. 2008;62(10):1604–13.
Kauppila LI. Prevalence of stenotic changes in arteries supplying the lumbar spine. A postmortem angiographic study on 140 subjects. Ann Rheum Dis. 1997;56(10):591–5.
Panetta T, Sclafani SJ, Goldstein AS, Phillips TF, Shaftan GW. Percutaneous transcatheter embolization for massive bleeding from pelvic fractures. J Trauma. 1985;25(11):1021–9.
Sharafuddin MJ, Andresen KJ, Sun S, Lang E, Stecker MS, Wibbenmeyer LA. Spontaneous extraperitoneal hemorrhage with hemodynamic collapse in patients undergoing anticoagulation: management with selective arterial embolization. J Vasc Interv Radiol. 2001;12(10):1231–4.
Pathi R, Voyvodic F, Thompson WR. Spontaneous extraperitoneal haemorrhage: computed tomography diagnosis and treatment by selective arterial embolization. Australas Radiol. 2004;48(2):123–8.
Shi W, Dowell JD. Etiology and treatment of acute inferior vena cava thrombosis. Thromb Res. 2017;149:9–16.
Kasirajan K, Gray B, Ouriel K. Percutaneous AngioJet thrombectomy in the management of extensive deep venous thrombosis. J Vasc Interv Radiol. 2001;12(2):179–85.
Meissner MH, Gloviczki P, Comerota AJ, et al. Society for Vascular Surgery; American Venous Forum. Early thrombus removal strategies for acute deep venous thrombosis: clinical practice guidelines of the Society for Vascular Surgery and the American Venous Forum. J Vasc Surg. 2012;55(5):1449–62.
Mewissen MW, Seabrook GR, Meissner MH, Cynamon J, Labropoulos N, Haughton SH. Catheter-directed thrombolysis for lower extremity deep venous thrombosis: report of a national multicenter registry. Radiology. 1999;211(1):39–49.
Alkhouli M, Zack CJ, Zhao H, Shafi I, Bashir R. Comparative outcomes of catheter-directed thrombolysis plus anticoagulation versus anticoagulation alone in the treatment of inferior vena caval thrombosis. Circ Cardiovasc Interv. 2015;8(2):e001882.
Enden T, Haig Y, Klow NE, et al. Long-term outcome after additional catheter-directed thrombolysis versus standard treatment for acute iliofemoral deep vein thrombosis (the CaVenT study): a randomised controlled trial. Lancet. 2012;379(9810):31–8.
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 Springer International Publishing AG, part of Springer Nature
About this chapter
Cite this chapter
Ierardi, A.M., Iacobellis, F., Carrafiello, G., Pesapane, F., Nicola, R., Scaglione, M. (2018). Vascular Emergencies of the Retroperitoneum: Recent Advances in MDCT and Interventional Radiology. In: Patlas, M., Katz, D., Scaglione, M. (eds) MDCT and MR Imaging of Acute Abdomen. Springer, Cham. https://doi.org/10.1007/978-3-319-70778-5_9
Download citation
DOI: https://doi.org/10.1007/978-3-319-70778-5_9
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-70777-8
Online ISBN: 978-3-319-70778-5
eBook Packages: MedicineMedicine (R0)