Abstract
Nanotechnology is promising science, which plays an important role in revolutionizing the medical and pharmaceutical fields in near future. The widespread applications of nanotechnology have already offered advantages to biomedical problem including wound healing. Generally, wound healing is a complex process; it involves coordinated interactions of diverse immunological and biological processes. However, the long-term wounds remain a challenging clinical problem due to huge chances of secondary infection by different microbes. In this context, various nanoparticles and nanobased formulations are attracting interest for their clinical applications because of their potential biological properties such as antibacterial activity, anti-inflammatory effects and wound healing efficacy. In this chapter, we have focused on role of nanotechnology, particularly the applications of various nanoparticles and their nanoformulations in the management of wound infections. The nanoformulations can be effectively used as therapeutic treatment in wound infections. However, before use of such nanoformulations, in-depth studies on toxicity need to be performed.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Skin is the most important organ, which acts as protective barrier for other human body organs and also plays a vital role in maintaining health of human being. But many factors including pathogenic microorganisms break integrity of the skin and tissues leading to the formation of wound (Solanki and Nagori 2013). A wound can be defined as an injury, which occurs quickly as a result, skin is torn, cut, punctured, or blunt force trauma causes a contusion. There are two important types of wound, i.e. acute or chronic, and they are classified depending on the time required for its healing. Among these, acute wounds heal rapidly in the predicted amount of time with no complications. However, chronic wounds take a longer time to heal and might have some complications (Fletcher 2008). Generally, the wounds with exposed tissue or organs to the outside environment are referred to as open wounds or penetrating wounds . Closed wounds have damage that occurs without exposing the underlying tissue and organs (non-penetrating wounds). Penetrating wounds mainly include stab wounds (trauma from sharp objects, such as knives), skin cuts, surgical wounds (intentional cuts in the skin to perform surgical procedures) and gunshot wounds (wounds resulting from firearms); all these generally result from trauma that breaks through the full thickness of skin, reaching down to the underlying tissue and organs (Asensio and Verde 2012). Moreover, non-penetrating wounds are usually the result of blunt trauma or friction with other surfaces; the wound does not break through the skin, and it includes abrasions (scraping of the outer skin layer), lacerations (a tear-like wound), contusions (swollen bruises due to accumulation of blood and dead cells under skin) and concussions (damage to the underlying organs and tissue on head with no significant external wound). In addition, there is third category of wounds called as miscellaneous wounds which include thermal wounds (injuries like burns, sunburns and frostbite occur due to extreme temperatures, either hot or cold), chemical wounds (resulted from contact or inhalation of chemical materials) (Chou et al. 2015), bites and stings (occur due to bites from humans, dogs, bats, rodents, snakes, scorpions, spiders and tick), electrical wounds (resulted from high-voltage electrical currents) (http://www.woundcarecenters.org/article/wound-basics/different-types-of-wounds). All the above-mentioned wounds may lead to the complications, if not treated properly. These are as follows:
-
Infections: It may cause mild to severe infections with various pathogenic microbes which further leads to pus drainage, foul odour, fever, dull throbbing pain, mild swelling and heat at wound site.
-
Inflammation: Wound may lead to inflammation in which they are hot, red, painful, and with swelling.
-
Scarring: After complete healing of wound, the new regenerated cells may be of different characteristics and sometimes leave a scar behind.
-
Loss of function: Many wounds can be disabling and life threatening if a major organ, blood vessel or nerve is damaged.
All the above-mentioned wounds are normally cured through the process of wound healing which is classically defined as a series of continuous, sometimes overlapping, events. These include haemostasis, inflammation, proliferation and remodelling of the scar tissue (Stadelmann et al. 1998; Tocco et al. 2012). The phases involved in healing of cutaneous wound are governed by coordinated action of different multiple cell types such as keratinocytes, fibroblasts immune, endothelial and progenitor cells (Fig. 9.1) (Eming et al. 2014; Pastar et al. 2014). The multiple cellular and molecular events involved in wound healing are tightly controlled by numerous growth factors, chemokines and cytokines. Apart from these, the active relationship between skin and microbiome also plays important role in wound healing process. Signalling networks which involve the interleukin (IL) family and growth factors are essentially required to coordinate cell–cell and cell–extracellular matrix (ECM) interactions for the complete healing of wound. However, the imbalances in these factors lead to a non-healing, chronic wound (Brem and Tomic-Canic 2007; Lindley et al. 2016; Hamdan et al. 2017).

(adapted and reproduced from Hamdan et al. (2017); an open-access article)
Phases of cutaneous wound healing showing the cells and molecules responsible for the regaining of a healthy barrier
The acute wounds heal rapidly, and there are no complications. But, chronic wounds require more time to heal and if not treated on time, it may result in severe infections by microbial pathogens. The bacterial pathogens such as S. aureus, P. aeruginosa and bacteria belonging to family Enterobacteriaceae are the most common causative agents responsible for wound infections (Mordi and Momoh 2009). In burn wound patients, bacterial infections may be followed by fungal infections caused by various members belonging to genera Candida, Aspergillus and Fusarium. Apart from this, viral infections by herpes simplex virus were also reported in some cases (Solanki and Nagori 2013).
Various topical agents such as antiseptics, antibiotics, granulation tissue suppressing agents, herbal therapeutics, enzymes and some other agents are available for wound treatment and healing as medications (Solanki and Nagori 2013). But some of these topical agents are facing the problem of inefficiency, side effects and increasing resistance in microbial pathogens (Howell-Jones et al. 2005). These problems create pressing need to develop alternative therapeutic agents and strategies for the treatment of wound infections and early healing of wound.
Considering the widespread biomedical applications of nanobiotechnology , it is believed that this technology has potential to address the problem associated with wound infection and its healing. Nanobiotechnology has arisen from the convergence of nanotechnology and biotechnology, leading to the development of structures, devices and systems in the form of nanomaterials having nanosize (1–100 nm) range (Rai and Ingle 2012). Due to novel properties of nanomaterials , it has tremendous applications in various fields including health care. Indeed, many areas of health care are already benefiting from the advantages of nanobiotechnology which is revolutionizing the medical and pharmaceutical fields (Gunasekaran et al. 2011). Considering the huge applicability of nanobiotechnology, Cortivo et al. (2010) proposed that “small is beautiful” should be the slogan of nanoscientists. But, we want to improvise this slogan as “small (nano) is beautiful and magic”.
As far as health care is concerned, nanomaterials are found to have broad-spectrum applications in the diagnosis, treatment and management of various microbial infections and diseases (Rai et al. 2014). Metal nanoparticles like silver have potential antimicrobial properties and hence being used as antimicrobial agents (Salunke et al. 2017), in water-disinfecting systems (Zhang et al. 2012), for the development of nanobased bone implant materials (Marsich et al. 2013), dental materials (Zhang et al. 2013), gold nanoparticles (Mendes et al. 2017) and liposomes which are the most promising nanomaterials used as vehicle for delivery of drugs and gene in life-threatening diseases (Lila and Ishida 2017). Apart from these applications, some of the metal nanoparticles can be promisingly used in the treatment and healing of wounds (Rigo et al. 2013), in burn infections (Elliott 2010), etc.
Among the various issues in health care, skin wounds and their treatments is one of the key areas of research. In last few years, nanotechnology has emerged as important platform to treat skin wounds. Various nanomaterials including metal and metal oxide nanoparticles such as silver, gold, copper, titanium and zinc oxide nanoparticles have shown significant therapeutic efficacy in wound healing (Ambrožová et al. 2017; Bui et al. 2017). The novel and characteristic properties of nanomaterials like nanocapsules, polymersomes, solid lipid nanoparticles and polymeric nanocomplexes make them vehicles of choice for the effective delivery of drugs and other molecules in wound treatment. In addition, the use of active pharmaceutical ingredients like chitosan and hyaluronate in the formulations enhances their therapeutic potential (Oyarzun-Ampuero et al. 2015).
The aim of this chapter is to review significant findings in the area of nanoparticles and wound treatment. Various topics have been focused, which include role of various nanoparticles such as silver, gold, copper, calcium, curcumin, etc., in wound healing. In addition, efficacy of combined use of nanoparticles with various drugs and antimicrobial molecules has also been discussed. Among all, we highlight the formulations of nanoparticles (nanoformulations) with drugs, natural extracts, proteins, growth factors, etc., and their applications as embedded in secondary vehicles (fibres, dressings, hydrogels, etc.) which has ability to enhance their wound healing applications.
2 Types of Wound Infections and Its Severity
Wounds are generally developed by penetrating objects or through any physical damage. However, the infection in wound depends upon the causative agent, wound depth and site. Based on above facts, the wound infections are classified into four classes as follows:
2.1 Bites
The oral flora of biting animals causes the wound infection. The majority of bites are from dogs, cats and insects which generally cause the bacterial infections (Talan et al. 1999). It can cause the morbidity from direct trauma followed by infections. Bacteriological analysis has reported the presence of mixture of both aerobes and anaerobes in such wounds (Talan et al. 2003).
2.2 Surgery
Infection at surgical site is considered to be one of the worst complications that a patient can experience after an intervention (Alfonso-Sanchez et al. 2017). Hence, avoiding the infection at surgical site has been high priority in order to reduce pathogen entry. If proper care has not been taken, the patient undergone surgery generally gets infection from the microbe present in the hospital environments (Alfonso-Sanchez et al. 2017). Hospitals are the hot spots for the antimicrobial-resistant bacteria and thus play a role in their emergence and spread (Hocquet et al. 2016).
2.3 Trauma
It results from any kind of physical damage forming the wound which is susceptible to various infections. The infectious organisms may cause deep or superficial infection depending on the depth of the wound. The wounds contaminated by dirt are at high risk of getting infected. Generally, Clostridium tetani is main bacterium to cause infection in such cases (Gulamhussein et al. 2016).
2.4 Burns
It is most common and devastating form of trauma. Burn patient needs very fast and specialized care in order to reduce morbidity and mortality. It may include the mild or severe forms of wound affecting various layers of skin. The risk of infection increases with the increasing damage to tissues. The substantial thermal injury induces the state of immunosuppression which invites the development of infections, majorly comprising of bacteria (Church et al. 2006). However, fungal and viral infections have also been observed in significant number of instances.
As far as the severity of wound is concerned, the wound developed in burn cases is more severe as compared to other types of wound. This is because in case of burn wound, there is extensive damage to the skin component. Moreover, due to tissue damage, the inner tissue gets exposed to the environment containing microbes. The damaged skin is easy portal of entry and is better platform for the growth of microbes. Additionally, a negligible immune response appears to work at the wound due to lack of organized tissue structure and cells in the wound area. The process of tissue repair and remodelling is quite lengthy and time-consuming process as compared to doubling time of most pathogens. Hence, microbes, especially the bacteria, occupy the major portion of the wound and thus hinder the wound healing process.
3 Treatment Strategies Available and Their Limitations, MDR at High Rate
The development of microbial resistance to antimicrobials is currently available in therapeutics, and it has become a growing concern in the medical and scientific community. Nowadays, many bacteria have been reported to possess resistance against various antibiotics, making them ineffective in preventing their growth. One of the advantages of the use of nanomaterials in the control and treatment of infections caused by resistant microorganisms lies in their difficulty in developing resistance to nanomaterials. This is due to the property of nanomaterials to act on different cellular mechanisms of the bacteria, making it more difficult for the appearance of resistance due to the complexity of its action (Santos et al. 2014).
The resistance of microorganisms to the most antibiotics like MRSA (methicillin-resistant S. aureus) makes the necessity of developing new, interesting nanoformulations effective and necessary weapon to control resistance against conventional therapies (Cao et al. 2017). Another important factor that has impact in wound healing is the biofilm formation, and despite several methods such as physical removal of biofilm and systemic and topical application of antimicrobials, new methods, particularly engaged with the controlled release of antimicrobial agents, should be developed to prevent biofilm-induced delayed wound healing. In this sense, the use of nanomaterials as carrier for drugs in a new system of drug delivery is also a promising alternative (Oryan et al. 2017).
Wounds are the sites of colonization of pathogenic microbes and sometimes multidrug-resistant microbes. S. aureus is an important pathogen responsible for colonization of burn wounds (Chhiber et al. 2015). Usually, fusidic acid is used in the treatment of difficult-to-treat skin infections caused by S. aureus. The authors evaluated the efficacy of topical fusidic acid (2% w/w)-loaded biocompatible microemulsion-based system (FA-ME) in removal of MSSA bacterial infections. The nanosized FA-ME system developed by them showed better wound healing with improved therapeutic potential.
Zhu et al. (2017) stated that it is urgent need to tackle the problem of wound infections caused by multidrug-resistant Gram-negative “superbugs”, such as P. aeruginosa. The authors reported the inclusion of colistin with a self-healable hydrogel. Within 24 h, the colistin is released from the hydrogel and remains active which was proved by antibacterial activity. The colistin-loaded hydrogel can be used as a wound healing formulation to treat burn wounds against infection caused by superbug like P. aeruginosa.
4 Nanotherapy for Wound Infections
4.1 Role of Nanoparticles in Wound Healing
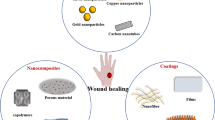
Wound healing is a complex process which requires various balanced microelements, antioxidants, matrix metalloproteins such as chemo-attractant protein 1, macrophage inflammatory protein1 and growth factors, etc. Deficiency of copper, zinc and calcium may inhibit the wound healing cascade. The best solution for wound infection is the use of antimicrobial agents, which can prevent the secondary infection of wound. Nanoparticles are antimicrobial agents, which can tackle the process of wound healing (Mohammad et al. 2008; Rakhmetova et al. 2010; Kawai et al. 2011; Ghosh et al. 2015; Chhabra et al. 2016). Nanoparticles of silver, gold, copper, zinc oxide, titanium dioxide, calcium, chitosan and curcumin can be used in wound healing. Figure 9.2 shows schematic representation of various nanoparticles involved in the recovery of wound infection and wound healing.
4.1.1 Silver Nanoparticles (AgNPs)
Over the centuries, silver and its compounds have widely been applied for treatment of burn injuries because of its strong broad-spectrum antimicrobial activity (Klasen 2000; Marx and Barillo 2014). Silver provides sanitary environment by preventing initial growth of bacteria in wound treatment. Based on type of wound and period of wound healing, various wound dressings are available. Antimicrobial wound dressings were helpful for patients to reduce the risk of infection. Infection occurs when bacteria, fungi or any other living microbe enters into wound tissues. Knowing the antimicrobial nature of silver, its ions were added to dressing surface to reduce the chances of infection. The frequent use of antibiotics, general antiseptics like chloride compounds, iodine compounds, quaternary ammonium compounds and peroxidase can exhibit antibiotic resistance, exert side effects, cause damage to surrounding tissues and systemic toxicity (Shirai et al. 2000; Fromm-Dornieden et al. 2015). Such disadvantages encouraged scientific community to look to other effective antimicrobial agent.
The introduction of nanoparticles has allowed scientific community to upgrade the antimicrobial properties of silver. AgNPs are currently in spotlight for its antibacterial and anti-inflammatory effect, which is useful in management of wound infection. Due to increase in surface area of nanoparticles, the interaction with infected surface of wound is enhanced. In the context, silver nanoparticles are also well known for its antimicrobial, anti-inflammatory, antiseptic and wound healing properties (Ahamed et al. 2010). AgNPs even at lower concentration exert same wound healing effect as compared to silver and its compounds. Because of high thermal stability, low toxicity and volatility, AgNPs exhibit high bactericidal activity against a large number of bacterial infections (Shrivastava 2007). Investigators have found antibacterial mechanism of AgNPs is due to release of silver ions from the surface of nanoparticles (Xiu et al. 2011, 2012). The major problem associated with wound is bacterial contamination. Presence of bacteria on wound surface delays healing process. E. coli, P. aeruginosa and S. aureus are mainly responsible for causing contamination on wound surface (Fong and Wood 2006; Dai et al. 2010).
Various studies have been focused on effect of AgNPs on wound healing and tissue regenerations. Recently, Pannerselvam et al. (2017) studied burn wound healing activity of AgNPs saturated cotton fabrics on Wistar albino rat (male). In this work, phytosynthesized AgNPs-incorporated cotton fabric dressing was tested to assess its wound healing ability on rat’s burn wound for 18 days. It was observed that wound healing efficacy of AgNPs-incorporated dressing (at 100 µg of AgNPs concentration) acquired high healing potential as compared to control burn heal cream dressing. These kinds of dressing are very much useful in management of wound infection and wound healing. Similar findings were reported by Hebeish et al. (2014), who studied that AgNPs-treated cotton fabric dressing containing 250 ppm AgNPs concentration exerts maximum wound healing activity as compared to dressing containing 60 and 125 ppm AgNPs concentration. Silver ions or its nanoparticles in wound dressing can react with and destroy bacteria causing wound infection.
The several studies of AgNPs against a large number of bacterial species have been made (Abbasifar et al. 2017; Elemike et al. 2017; Patra and Baek 2017). The bactericidal mechanism of AgNPs is not clear, but reports suggest that silver ions interact mainly with three components of bacterial cell: (a) bacterial cell wall and plasma membrane, (b) bacterial DNA and proteins, (c) enzymes involved in cellular processes (Rai et al. 2009; Yang et al. 2009; Gunasekaran et al. 2011). A significant consideration in the application of AgNPs for wound healing has been investigated by various researchers (Tian et al. 2007; Pannerselvam et al. 2017). AgNPs-incorporated cotton fabrics and dressings have shown significant recovery at the site of burn wound infection (Rigo et al. 2013). In this in vitro study, through cellular staining technique it was observed that AgNPs induced mitochondrial activity without causing cell lysis in skin. Reduced mitochondrial activity was suggested to decrease bacterial infection in wound. Likewise, AgNPs-coated cotton wool was reported to exhibit excellent wound healing activity along with faster blood clotting rate. Additionally, AgNPs-coated cotton showed excellent UV protection properties (Kim et al. 2017). In addition to antibacterial activity, AgNPs can expedite the wound healing by anti-inflammatory activity with effect on cytokines modulations (Tian et al. 2007).
AgNPs and anticoagulating agent enoxaparin were reported to exert wound healing activity, but this activity gets enhanced when AgNPs form complex with enoxaparin (Marcato et al. 2015). Due to broad-spectrum antibacterial activity, AgNPs are effective against variety of bacterial species. The development and modification of silver at nanolevel puts a noticeable improvement in efficacy of wound treatment. This switching significantly reduces time required for wound healing and also reduces the risk of unwanted complications. AgNPs provide the medical community a new generation of highly powerful wound treatment system.
4.1.2 Gold Nanoparticles (AuNPs)
The anti-inflammatory and antiangiogenic activity of negatively charged AuNPs facilitates its use in the therapy of cutaneous wounds, diabetic wounds and rheumatoid arthritis (Pivodova et al. 2015). Kim et al. (2015) coated phytochemically synthesized AuNPs on hydrocolloid membrane and investigated wound healing activity of nanoparticles-coated membrane. In vivo activity of membrane was tested on Spargue-Dawley rats for 15 days. Histological and immunohistological studies demonstrated that synthesized nanoparticles-coated hydrocolloid membrane induces wound healing process including tissue regeneration, formation of connective tissue and the process of angiogenesis. The study also showed that nanoparticles were non-toxic to liver and kidney of rats. Application of membrane along with other antioxidant agent can be used in the cutaneous wound healing.
AuNPs have many unique properties like mechanical stability, resistance to enzymatic degradation, antimicrobial activity. AuNPs can be incorporated into scaffolds which can help in wound healing process. AuNPs and chitosan composite was fabricated, and wound healing activity of composite was evaluated on full thickness wound model of rats. The wound healing activity of composite, chitosan alone and commercially available product of chitosan was demonstrated. In vivo wound healing assay demonstrated the promising wound healing activity of nanocomposite. Combination of AuNPs with collagen scaffolds can be used in skin tissue engineering (Aktruk et al. 2016).
The cryopreserved human fibroblast cell along with AuNPs was used as therapy for wound infection. The cyropreserved fibroblast or low-temperature storage/preserved cells facilitate the availability of cell at any time, without any morphological or functional deformities. Fibroblast cell enhances the secretion of biologically active substance such as growth factors, cytokines and components of extracellular matrix. All the components which are mentioned above are required at the time of wound healing process. In vivo study was performed in the mice, and from planimetric and clinical studies, it was revealed that cryopreserved fibroblast cell with AuNPs enhanced the healing of burn wounds (Volkova et al. 2016).
Lu et al. (2012) reported the application of AuNPs in combination with antioxidants like epigallocatechin gallate (EGCG) and α lipoic acid (ALA). In vivo study found that activity of nanoparticles along with antioxidants enhances the wound healing in cutaneous wounds of mouse. Anti-inflammatory and antioxidant properties of nanoparticles increases with activity of antioxidant molecules. Lau et al. (2017) studied the effect of AuNPs in photobiomodulation therapy on wound healing process. In vivo influence of AuNPs was evaluated on Spargue-Dawley rats. The cutaneous wound of Spargue-Dawley rats was divided into three groups: one without treatment, one with gold nanoparticles and one group with combination of AuNPs in photomodulation therapy. Histological study revealed that AuNPs in photomodulation therapy were more effective in stimulating angiogenesis. Akturk and Keskin (2016) reported the novel approach in the tissue engineering of skin; AuNPs were incorporated into collagen nanofibres at different concentrations. AuNPs have demonstrated antimicrobial activity and mechanical stability. It can act as additive in skin tissue engineering.
4.1.3 Copper Nanoparticles (CuNPs)
The effect of suspension of CuNPs was evaluated on the full thickness wound infections of rats. According to planimetric measurement data, it was found that healing of wounds or regeneration ability of wound was maximum on fifth day of application of CuNPs. It was observed on twenty-first day that regeneration of skin was observed in rats. CuNPs prevent the secondary infections of the wounds and the time required for healing of wound was decreased, after using suspension of CuNPs. The suspension of CuNPs can be recommended safe for the treatment of epidermal wound infections (Babushkina et al. 2013).
Ghosh et al. (2015) reported the biological synthesis of CuNPs from Dioscorea bulbifera, antidiabetic and antioxidant activity of CuNPs was also evaluated in the study. From the study, it was found that α-amylase and α-glucosidase activity was strongly inhibited by CuNPs. Inhibition of enzyme clearly indicates the wound healing capacity of CuNPs. Rakhmetova et al. (2010) synthesized CuNPs and prepared methylcellulose-based ointment containing CuNPs. In vivo wound healing study was performed on the full thickness wound of mice. Wound healing effect of CuNPs containing ointment and ointment base (without nanoparticles) was investigated. In vivo study revealed the wound healing potential of CuNPs-based ointment.
4.1.4 Zinc Oxide Nanoparticles (ZnONPs)
Psunica-Panea et al. (2016) prepared the composite film of collagen, dextran and zinc oxide nanoparticles (ZnONPs). It was embedded for wound healing purpose. Scaffold was designed, and zinc oxide nanoparticles were decorated on it. ZnONPs can be used as promising candidate, which could be used for the regeneration of skin in wound healing process. Chhabra et al. (2016) developed novel dressing material for wound healing purpose. ZnONPs-incorporated nanofibrous scaffolds of gelatin were prepared, and its in vivo wound healing activity was evaluated on mice. In vivo study demonstrated ZnONPs-doped scaffolds of gelatin not only enhance wound healing process by promoting cell growth at the site of wound infection but also reduce the bacterial infection. ZnO-doped nanofibrous material can be used in tissue regeneration.
Sudheesh-Kumar et al. (2012) prepared nanocomposite bandages by incorporating ZnONPs into chitosan hydrogel. In vitro antimicrobial activity of composite was tested against E. coli and S. aureus, which are responsible for the microbial wound infection. In vivo wound healing activity of nanocomposite bandage was performed on Spargue-Dawley rats. Study revealed that nanocomposite bandage can enhance the wound healing and help in collagen deposition. Karahaliloglu et al. (2016) evaluated antimicrobial activity of ZnONPs-incorporated chitosan/silk sericin scaffolds. It was demonstrated that chitosan-incorporated scaffolds can be used in the wound dressing purpose.
4.1.5 Titanium Dioxide Nanoparticles (TiO2 NPs)
Sankar et al. (2014) reported the biological synthesis of TiO2NPs, and its in vivo wound healing activity was demonstrated in Winstar Albino rats. Wound healing study in albino mice includes the study of various parameters like wound closure, protein profiling and histopathology studies. In vivo study demonstrated that wound treated with TiO2NPs did not show pus formation, bleeding, microbial infection and inflammation.
Archana et al. (2013) prepared chitosan nanodressing containing TiO2NPs and pectin. In vitro study showed that nanocomposite possesses antimicrobial activity. In vivo wound healing activity of chitosan alone and TiO2 loaded chitosan-pectin nanodressing was performed on excision type of wound in Albion rats. Wound healing experiment demonstrated significant wound closure rate as compared to chitosan alone. Nanodressing acts as suitable candidate for wound healing.
Woo et al. (2015) prepared bilayer composite membrane containing TiO2NPs incorporated electrospun chitosan membrane and extracellular matrix (ECM) sheets as a wound dressing. In vitro antimicrobial activity of bilayer was evaluated against E. coli and S. aureus. The wound healing effect of bilayer composite was evaluated on full thickness wound of Spargue-Dawley rats. Histological and immunological studies demonstrated that ECM present in bilayer helps in the release of growth factor which is required at the time of wound healing. Biocomposite film can be used in wound dressing.
4.1.6 Calcium-Based Nanoparticles
The pH-sensitive calcium-based nanoparticles can be used in wound healing therapy. In vivo experiment was performed on the effect of calcium nanoparticles on mice. Calcium nanoparticles were intravenously administrated in the wound bed, and injected nanoparticles can be tracked by using FLAG detection system. It was found that pH-sensitive nanoparticles can increase the fibroblast proliferation and accelerate the wound healing process (Kawai et al. 2011).
4.1.7 Curcumin Nanoparticles
Krausz et al. (2014) reported curcumin-encapsulated nanoparticles as potential antimicrobial and wound healing agent. Curcumin nanoparticles were prepared by sol-gel method, and its in vitro antimicrobial activity was evaluated against methicillin-resistant S. aureus and P. aeruginosa. In vivo wound healing activity was performed on murine wound model. Curcumin nanoparticles can be used in the burn wounds and also in the therapy of other cutaneous injuries.
It was reported that some of nanoparticles may possess the ability to enhance the process of wound healing in Type 2 diabetic patient. In diabetic patients, the process of wound healing is very slow. The oxidative stress is the major problem which delayed wound healing process and resulted in the formation of reactive oxygen species (ROS). Metal oxide nanoparticles such as aluminium oxide nanoparticles, cerium oxide nanoparticles can act as ROS scavenger which enhance diabetic wound healing (Mohammad et al. 2008).
Curcumin is well-known anti-inflammatory and antioxidant agent. It plays very significant role in the proliferation phase of wound healing. Curcumin can enhance the formation of granulation tissue and also promote the synthesis of components present in the extracellular membrane. Various nanomaterials-based vehicles can be used in the controlled release of curcumin, which can help in the process of wound healing (Hamdan et al. 2017).
Curcumin-loaded chitosan nanoparticles were prepared and impregnated into collagen-alginate scaffolds. It was found that novel nanohybrid scaffolds enhance the solubility and stability of curcumin and help in reducing the process of inflammation followed by increasing the wound healing and tissue regeneration in diabetic wounds. In vivo wound healing study of nanohybrid scaffolds was conducted on Winstar rats. It was investigated that topical application of scaffolds reduces the inflammation at the site of wound. Nanoscaffold has promising properties such as biocompatibility, anti-inflammatory, cell adhesion, which are significant for the impairment of wound healing process. Hence, curcumin-impregnated scaffolds can help in the management of diabetic wounds (Karri et al. 2016).
Li et al. (2016) reported epidermal growth factor- curcumin-encapsulated hydrogel nanoparticles, and its wound healing properties were investigated. The study revealed that dual-drug-loaded hydrogel nanoparticles help in the process of skin regeneration. Epidermal growth factor (EGF) is a polypeptide which aids in the wound repair. EGF accelerates the process of wound healing by the synthesis of extracellular matrix component, cell motility and cell proliferation. Curcumin prevents the oxidative stress at the time of wound healing process. Hydrogel nanoparticles act as a topical gel which can help in wound repair.
Nguyen et al. (2013) documented curcumin-loaded gelatin and chitosan sponge, and its wound healing properties were evaluated. In vivo wound healing property was evaluated by excision wound model of rabbit. Chitosan was used in wound healing process as it acts as drug carrier which helps in the process of wound healing. Gelatin prevents the loss of fluid which can help in wound healing process. From in vivo analysis, it was found that curcumin-loaded sponge was much more effective as compared to the sponge without curcumin. The composite sponge helps in collagen formation and in closure of wound excision.
5 Nanoparticles in Combination with Antimicrobial Agents
5.1 Need of Combination Drugs
Nowadays, resistance to antimicrobial drug is a point of huge concern. Different microbes have different kinds of mechanism of resistance, which is a big burden to the researchers to exploit new drugs that will settle the need of new antimicrobials over resistant strains. But development of new drugs from the lead is quiet time-consuming and tedious process, and then also resistance to the new drugs can be developed after some time. Microorganisms can develop resistance by various means such as mutation or changes in target, over production of target site, development of alternate growth requirement, by producing drug degrading enzyme, having active drug efflux pump, multiple factors including loss of porin, production of an additional enzyme that avoids binding, cell wall thickening changes in target (Ranghar et al. 2014).
Konop et al. (2016) stated that nanoparticles are being used in a wide range of health care, food industry and domiciliary applications, materials and textiles. Nanoparticles can be applied in several ways such as in wound dressings, medical supplements, catheters and medical textiles: bedclothes, towels and clothes. Nanoparticles can also be the key component in cosmetics as antiseptic and preservative agents.
Thomas et al. (2014) performed antimicrobial activity of AgNPs, antibiotics and their combination against multidrug-resistant biofilm-forming, coagulase negative S. epidermidis strains which were isolated from the pus, catheter tips and blood samples of the patients and other organisms. S. aureus, V. cholerae, S. typhi and S. paratyphi were collected from MTCC. Synergistic effect of antibiotics with biosynthesized AgNPs against test strains was done by disc diffusion method. AgNPs were combined with standard antibiotics such as fusidic acid, gentamicin, ciprofloxacin, erythromycin, penicillin, chloramphenicol, levofloxacin, nalidixic acid and ampicillin. After evaluation, it is observed that AgNPs show highest activity against S. epidermidis strains and highest synergistic effect was observed with chloramphenicol. Shahverdi et al. (2007) also investigated synergistic effect of AgNPs with different antibiotics against S. aureus and E. coli. Their results show the hike in activities of penicillin G, amoxicillin, erythromycin, clindamycin and vancomycin in the presence of AgNPs against both bacteria.
Hassan et al. (2016) in their experiment analysed the synergistic effect of AgNPs with β-lactam cefotaxime sodium antibiotic against the test organisms, Gram-negative bacteria E. coli, P. aeruginosa and Gram-positive bacteria S. aureus and the fungal strains Candida albicans and Aspergillus niger. The combination of AgNPs with β-lactam cefotaxime sodium antibiotic increased efficiency of microbial inhibition. Similar study was conducted by Bhande et al. (2013) for the assessment of synergistic activity of ZnONPs with β-lactam antibiotics such as cefotaxime, ampicillin, ceftriaxone, cefepime against of E. coli, K. pneumoniae, S. paucimobilis and P. aeruginosa, and positive synergistic effect was observed. Baker et al. (2017) synthesized bimetallic silver–gold nanoparticles from endophyte P. veronii against bacitracin-resistant strains Bacillus subtilis, E. coli and K. pneumoniae and analysed their synergistic activity with different antibiotics bacitracin, kanamycin, streptomycin, erythromycin and chloramphenicol, and fold increase in their activity was 87.5, 18.5, 11.15, 10, 9.7 and 9.4%, respectively. Perez-Dıaz et al. (2016) also worked on antibiofilm activity of AgNPs. They conducted an experiment for the assessment of cytotoxic effect of chitosan gel formulations loaded with AgNPs on human primary fibroblasts, and silver sulfadiazine (SSD) was kept as control. Results show that chitosan gels with AgNPs have less toxicity than clinically used medication silver sulfadiazine.
Nowadays, nanoparticles-impregnated medical supplies are available in market and favourably used in medical practices. Acticoat™ Flex 3 dressing is a flexible polyethylene cloth coated with AgNPs (concentration—0.69 and 1.64 mg/cm2). Various in vitro and in vivo experiments were conducted for the analysis of toxicity of nanoparticles on cells such as treatment of Acticoat™ Flex 3 to 3D fibroblast cell culture and real partial thickness burn patient, MTT assay, transmission electron microscopy (TEM) and inductively coupled plasma mass spectrometry (ICP-MS) analysis, which shows that presence of AgNPs does not affect viability of cells, no signs of apoptosis or necrosis, does not hamper healing of wound and does not put any obstacle was observed in the proliferation of fibroblasts and keratinocytes and also suitable for prolonged use (17 days) (Rigo et al. 2013).
Velazquez-Velazquez et al. (2015) performed an experiment for evaluation of the antibiofilm activity of AgNPs impregnated in commercial dressings. They isolated bacterial strains from chronic wounds of patients suffering from diabetes type 2 and DFU. Clinical samples were identified as Gram-positive and Gram-negative P. aeruginosa, K. pneumoniae and S. epidermidis, E. faecalis, respectively, and their activity was analysed within biofilms generated under slow fluid shear conditions. So, their finding generates a view that dressings impregnated with AgNPs are able to prevent the growth and reduces microbial flora and in turn minimizes the bioburden in wound area which leads to fast healing.
Jeong et al. (2014) examined effect of silk fibroin (SF) nanofibrous matrices combined with silver sulfadiazine (SSD) on wound healing. They selected two cell lines, normal human epidermal keratinocytes (NHEK) and normal human epidermal fibroblasts (NHEF) as their model for human cells and silver sulfadiazine-modified SF were taken in different concentrations 0.1, 0.5 and 1.0 wt%. Their study depicts that number of NHEK and NHEF attached to examined nanofibres containing SSD decreased when the concentration of SSD increased and the number of attached NHEF cells was lower than attached NHEK cells. In vivo study was carried out on rat wound model against commercially available wound dressing ActicoatTM, and result shows the faster wound healing by SF matrix with 1.0wt% SSD than Acticoat.
Archana et al. (2015) developed novel nanoparticle film exclusively for wound healing which contains chitosan, polyvinylpyrrolidone (PVP) and silver oxide nanoparticles (Ag2O), named as CPS film. In vitro antibacterial activity was assessed against E. coli and S. aureus, and it shows stronger inhibitory effect against S. aureus than E. coli. CPS films were also tested in vivo, in rats for 14 days. CPS-treated wound were healed almost completely (99%), where gauze-treated and chitosan group-treated wounds show healing rate 95 and 91%, respectively. It concludes that wounds treated with CPS healed faster than chitosan-treated groups and gauze dressing. Anisha et al. (2013) developed a chitosan, hyaluronic acid (HA) and AgNPs containing antimicrobial sponge which holds potential use in a wound dressing for diabetic foot ulcers (DFU) infected with drug-resistant bacteria. Antimicrobial assessment of this sponge was done against E. coli, S. aureus, methicillin-resistant S. aureus (MRSA), P. aeruginosa and P. pneumoniae. For the toxicity analysis of AgNPs, fibroblast cells were used which showed that the toxicity depends on the concentration of AgNPs. The sponge can be used as a potential material for wound dressing in DFU infected with antibiotic-resistant bacteria.
Ahamed et al. (2015) conducted a study regarding synthesis and antimicrobial assessment of composites containing regenerated cellulose (RC) and chitosan (Ch) in combination with silver nanoparticles (Ag). Those composites were further associated with gentamicin (G) and some were kept composites without gentamicin, for in vivo experimentation on wounds of rats. Wound healing rate in rats was examined against wound covered with soframycin, RC–Ch–Ag and RC–Ch–Ag–G. Wound covered with sterile cotton gauze and dipped in gentamicin was kept as control. Wound treated with RC–Ch–Ag composites showed 96% of contraction, RC–Ch–Ag–G composite showed completely contraction and control shows 74% of wound contraction, which showed composites having good healing properties.
Synergistic effect of nanoparticles in combination with antibiotics has potential to overcome the need of new approaches for defeating resistance. There are many postulates which explain mechanism of synergy such as:
-
1.
Antibiotics and nanoparticles can kill microbes through different mechanisms. If the microbial cell is resistant to the antibiotics, nanoparticles can kill bacteria through a completely different mechanism. Nanoparticles are having high surface-to-volume ratio, which increases drug uptake into the cells by increasing the permeability of cell membrane. Hydrophobic nature of nanoparticles also facilitates the transport of antibiotics to the cell membrane which consists of hydrophobic groups such as phospholipids and glycoprotein. There is a possibility of bonding between antibiotics and nanoparticles and thus the antibiotic—nanoparticles combination may get attached on the cell membrane and result in the lysis of cell wall of microbes, followed by the entry of combination into the cell and may result in the DNA unwinding due to spatial restrictions and cell death (Hari et al. 2014; Jamaran and Zarif 2016)
-
2.
Synergistic effect of nanoparticles with antibiotics increases fold activity against drug-resistant pathogens and provides the emerging strategy to defeat infections caused by multidrug-resistant microorganisms. (Abhishek and Hemlata 2014; baker et al. 2017)
-
3.
Encapsulation and controlled release of drugs can be possible through the synergy with nanoparticles. It can be a potential approach for the delivery of antibiotics close to the surface of microbes by promoting bacterial aggregation. Defined release of drugs thus enhances antimicrobial efficiency and potentially reduces the quantity of antimicrobial agent required for activity (Eissa et al. 2016)
-
4.
Inhibitory role of nanoparticles can be explained because they can act as an adjuvant for the treatment of infectious diseases. Furthermore, nanoparticles can generate hydroxyl radicals and dysfunctions important protective factors required for viability of microbes (Hwang et al. 2012).
In addition to antimicrobials, nanoparticles can play pivotal role in case of synergy with anticancerous drugs. Some anticancerous drugs have limitations such as poor water solubility and low oral bioavailability. Hence, nanoparticles with better encapsulation efficiency (EE), drug loading capacity (DL) and appropriate shape can improve its anticancer activity and bioavailability (Zhang et al. 2016). The synergistic activity of nanoparticles can be useful for enhancing the antimicrobial property of the antibiotic and minimizing the dosage of antibiotics against multidrug-resistant pathogens. It can also be helpful in reducing the side effects, and at the same time it is simple, eco-friendly and cost-effective also (Hwang et al. 2012; Zarina and Nanda 2014; Lakshmi and Kannan 2016).
6 Nanoparticle-Based Formulations
In the last few decades, widespread studies have been performed on the use of various methods of nanoparticle synthesis. They showed potential for various applications as said earlier. Hence, the entire available knowledge is always compelling to formulate the generated nanoparticles for formulation processes, which can lead to the product for specific needs. For instance, AgNPs synthesized by Phytophthora infestans were used in ointment and found to be effective in wound healing as compared to conventional silver sulfadiazine ointment (Thirumurugan et al. 2011). The 1% (w/w) AgNPs ointment-treated Swiss albino rats demonstrated significant wound healing. Similar pharmacological effects were found in the group of rats treated with 0.125% (w/w) AgNPs and conventional silver sulphadiazine ointment (1% w/w). The AgNPs ointment did not show any evidence of phase separation, physical instability, no objectionable odour and no effect of temperature in the storage of formulation. AgNPs-based nanoformulations were also developed for topical burn wound healing (Kaler et al. 2014). The nanoparticles of the size range 3–10 nm were synthesized by using Saccharomyces boulardii and were formulated into topical gel form (Kaler et al. 2014). It was claimed to be developed in such a way that it will keep the balance between therapeutic activity and toxicity. It was observed to possess very effective wound healing activity in vitro and in vivo on male Sprague-Dawley skin.
Sirnaomics Inc. has been investigating the nanoformulations containing SiRNA targeting for TGFβ1 and Cox-2 (cyclooxygenase-2), encapsulated into HK polymer nanoparticles. The nanoparticles formulation in methylcellulose showed to reduce the fungal growth, scar formation and accelerated wound-healing (Kalashnikova et al. 2015). Chereddy e al. (2013) developed poly (lactic-co-glycolic acid)-curcumin nanoparticles (PLGA-CC NP) formulation for effective wound healing. In this case, PLGA NPs have been used as drug carrier encapsulating CC. Both PLGA and CC showed the combined activity on topical wound healing with simultaneous utilization of therapeutic potential lactic acid in wound healing. The healing process was marked by down regulation of inflammatory responses, accelerated epithelialization and enhanced granulation in tissue formation. Skin wounds are very painful, specifically through the wound dressing changes. Ideally the dressing changes are performed after 7 days. During this procedure, there is possibility of removing the regenerating epithelia. Kuchler et al. (2010) tested the improvement of wound closure by morphine-loaded solid lipid nanoparticles (SLN). The study was performed on human-based 3D wound healing model and observed the sustained release of drug in the wound with constant opioid concentration. The formulation was claimed to be efficient as compared to the other conventional pharmaceutical formulations such as solutions or cream. Recently, nanoformulations using ZnONPs-loaded-sodium alginate-gum acacia hydrogels (SAGA-ZnONPs) were studied for its effectiveness in wound healing (Raguvaran et al. 2017). After in vitro wound healing test using skin fibroblast cells, the nanoformulation showed significant effects at different concentrations of SAGA-ZnONPs. The study claimed that use of biolpolymeric biocompatible polymers, viz. sodium alginate and gum acacia, can help to increase the efficacy of the nanoformulation and minimize their unwanted side effects. Further, there is requirement of new strategies in the development of nanoformulations having capable to heal the chronic wounds. More in vivo studies are needed to be performed which can help to develop highly efficient nanoformulations for human use.
7 Conclusions
The ultimate aim of wound healing is faster recovery with minimum scar formation. It involves many complex processes and has thus attracted many scientists across the globe to undertake intense studies. Majorly it involves the overlapping events comprising the coagulation, inflammation, proliferation and tissue remodelling. However, the infection during wound formation is also one of the major obstacles in wound healing. Particularly in case of burn patients, wound is quite difficult to heal as compared to other types of wound. Hence, there is always need of a therapy, which can be efficient in healing the wound at the earliest. Although, the available treatment methods of wound healing are effective at certain level, they also have some limitations. The development of multiple drug resistance in microbes residing in wound is of utmost importance. Hence, there is a pressing need to look for any better option. Recent advances in nanotechnology have greatly expanded our understanding of the use of nanotechnology-based products to modulate various wound healing therapeutics.
Various metal nanoparticles have been shown to be efficient in inhibiting the growth of pathogenic microorganisms in wound. As far as the diabetic patients are concerned, the healing is very lengthy procedure, and hence there are many chances of secondary infections. Nanoparticles can be helpful herein to promote the early wound healing with minimal scar formation. Various nanobased formulations have been already available in market for topical application on wound. Clinical observations and experimental data have shown that these nanoformulations have a great potential in accelerating the wound healing, reducing the oxidative stress and protecting the regenerative tissues. However, more studies are recommended in this field so as to develop the nanoformulations, which can help to retain the tensile properties of the repaired skin and can closely bear a resemblance to the normal skin. It will definitely benefit to develop nanobased products in better way for clinical application in wound healing.
References
Abbasifar A, Ghani S, Irvani MA, Rafiee B, Kaji BV, Akbari A (2017) Antibacterial activity of silver nanoparticles synthesized by using extracts of Hedera helix. Zahedan. J Res Med Sci 19(1):e5920. doi:10.17795/zjrms-5920
Abhishek B, Hemlata C (2014) Antibacterial activity of silver nanoparticles conjugated with antibiotics. Bionano Frontier 7:32–35
Ahamed M, Alsalhi MS, Siddiqui MK (2010) Silver nanoparticle applications and human health. Clin Chim Acta 411(23–24):1841–1848
Ahamed M, Sankar S, Kashif P, Basha S, Sastry T (2015) Evaluation of biomaterial containing regenerated cellulose and chitosan incorporated with silver nanoparticles. Int J Biol Macromol 72:680–686
Akturk O, Keskin D (2016) Collagen/PEO/gold nanofibrous matrices for skin tissue engineering. Turk J Biol 40:380–398
Akturk O, Kismet K, Yasti AC, Kuru S, Duymus ME, Kaya F, Caydere M, Hucumenoglu S, Keskin D (2016) Collagen/gold nanoparticle nanocomposites: a potential skin wound healing biomaterial. J Biomater Appl 31(2):283–301
Alfonso-Sanchez JL, Martinez IM, Martín-Moreno JM, González RS, Botía F (2017) Analysing the risk factors influencing surgical site infections: the site of environmental factors. Can J Surg 60(3):155–161
Ambrožová N, Zálešák B, Ulrichová J, Čížková K, Galandáková A (2017) Low concentrations of silver nanoparticles have a beneficial effect on wound healing in vitro. J Nanopart Res 19:108. doi:10.1007/s11051-017-3809-7
Anisha B, Biswas R, Chennazhi K, Jayakumar R (2013) Chitosan-hyaluronic acid/nano silver composite sponges for drug resistant bacteria infected diabetic wounds. Int J Biol Macromol 62:310–320
Archana D, Singh B, Dutta J, Dutta P (2015) Chitosan- PVP-nano silver oxide wound dressing: in vitro and in vivo evaluation. Int J Biol Macromol 73:49–57
Archanaa D, Duttab J, Duttaa PK (2013) Evaluation of chitosan nano dressing for wound healing: characterization, in vitro and in vivo studies. Int J Biol Macromol 57:193–203
Asensio JA, Verde JM (2012) Penetrating wounds. In: Vincent JL, Hall JB (eds) Encyclopedia of Intensive Care Medicine. Springer-Verlag, Berlin, Heidelberg, Germany, pp 1699–1703
Babushkina IV, Gladkova EV, Mamonova IA (2013) Regeneration of conventionally aseptic experimental wounds under the action of copper Nanoparticles. World J Med Sci 8(4):318–321
Baker S, Pasha A, Sreedharamurthy S (2017) Biogenic nanoparticles bearing antibacterial activity and their synergistic effect with broad spectrum antibiotics: emerging strategy to combat drug resistant pathogens. Saudi Pharm J 25:44–51
Bhande R, Khobragade C, Mane S, Bhande S (2013) Enhanced synergism of antibiotics with zinc oxide nanoparticles against extended spectrum b-lactamase producers implicated in urinary tract infections. J Nanoparticles Res 15:1413. doi:10.1007/s11051-012-1413-4
Brem H, Tomic-Canic M (2007) Cellular and molecular basis of wound healing in diabetes. J Clin Inv 117:1219–1222
Bui VKH, Park D, Lee YC (2017) Chitosan combined with ZnO, TiO2 and Ag nanoparticles for antimicrobial wound healing applications: a mini review of the research trends. Polymers 9:21
Cao Z, Spilker T, Fan Y, Kalikin LM, Ciotti S, Li Puma JJ, Makidon PE, Wilkinson JE, Baker JR Jr, Wang SH (2017) Nanoemulsion is an effective antimicrobial for methicillin-resistant Staphylococcus aureus in infected wound. Nanomedicine 12(6):1543–1555
Chereddy KK, Coco R, Memvanga PB, Ucakar B, des Rieux A, Vandermeulen G, Préat V (2013) Combined effect of PLGA and curcumin on wound healing activity. J Controlled Release 171(2):208–215
Chhabra H, Deshpande R, Kanitkar M, Jaiswal A, Kaleb VP, Bellare JR (2016) A nano zinc oxide doped electrospun scaffold improves wound healing in a rodent model. RSC Adv 6:1428–1439
Chhibber T, Wadhwa S, Chadha P, Sharma G, Katare OP (2015) Phospholipid structured microemulsion as effective carrier system with potential in methicillin sensitive Staphylococcus aureus (MSSA) involved burn wound infection. J Drug Target 23(10):943–952
Chou CY, Chiao HY, Wang CY, Dai NT, Chen SG, Chen TM, Tzeng YS (2015) Major chemical burn injury combined with a penetrating injury of the abdomen leading to hypovolemic shock. Formosan J Surg 48(1):26–29
Church D, Elsayed S, Reid O, Winston B, Lindsay R (2006) Burn wound infections. Clin Microbiol Rev 19(2):403–434
Cortivo R, Vindigni V, Iacobellis L, Abatangelo G, Pinton P, Zavan B (2010) Nanoscale particle therapies for wounds and ulcers. Nanomedicine 5(4):641–656
Dai T, Huang YY, Sharma SK, Hashmi JT, Kurup DB, Hamblin MR (2010) Topical antimicrobials for burn wound infections. Recent Pat Anti-Infec Drug Discovery 5(2):124–151
Eissa A, Abdulkarim A, Sharples G, Cameron N (2016) Glycosylated nanoparticles as efficient antimicrobial delivery agents. Biomacromol 17:2672–2679
Elemike EE, Fayemi OE, Ekennia AC, Onwudiwe DC, Ebenso EE (2017) Silver nanoparticles mediated by Costus afer leaf extract: synthesis, antibacterial, antioxidant and electrochemical properties. Molecules 22:701. doi:10.3390/molecules22050701
Elliott C (2010) The effects of silver dressings on chronic and burns wound healing. Br J Nurs 19:S32–S36
Eming SA, Martin P, Tomic-Canic M (2014) Wound repair and regeneration: Mechanisms, signalling and translation. Sci Trans Med 6:265. doi:10.1126/scitranslmed.3009337
Fletcher J (2008) Differences between acute and chronic wounds and the role of wound bed preparation. Nurs Stan 22(24):62–68
Fong J, Wood F (2006) Nanocrystalline silver dressings in wound management: a review. Int J Nanomed 1(4):441–449
Fromm-Dornieden C, Rembe JD, Schafer N, Bohm J, Stuermer EK (2015) Cetylpyridinium chloride and miramistin as antiseptic substances in chronic wound management: prospects and limitations. J Med Microbiol 64:407–414
Ghosh S, More P, Nitnavare R, Jagtap S, Chippalkatti R, Derle A, Kitture R, Asok A, Kale S, Singh S, Shaikh ML, Ramanamurthy B, Bellare J, Chopade BA (2015) Antidiabetic and antioxidant properties of copper nanoparticle synthesized by Medicinal Plant Dioscorea bulbifera. J Nanomed Nanotechnol 7:483–496
Gulamhussein MA, Li Y, Guha A (2016) Localized tetanus in an adult patient: case report. J Orthop Case Rep 6(4):100–102
Gunasekaran T, Nigusse T, Dhanaraju MD (2011) Silver nanoparticles as real topical bullets for wound healing. J Am Coll Clini Wound Spec 3(4):82–96
Hamdan S, Pastar I, Drakulich S, Dikici E, Tomic-Canic M, Deo S, Daunert S (2017a) Nanotechnology-driven therapeutic interventions in wound healing: Potential uses and applications. ACS Cent Sci 3:163–175
Hamdan S, Pastar I, Drakulich S, Dikici E, Tomic-Canic M, Deo S, Daunert S (2017b) Nanotechnology driven therapeutic interventions in wound healing: potential uses and applications. ACS Cent Sci 3:163–175
Hari N, Thomas T, Nair A (2014) Comparative study on the synergistic action of differentially synthesized silver nanoparticles with β-cephem antibiotics and chloramphenicol. J Nanosci Article ID 201482. doi:http://dx.doi.org/10.1155/2014/201482
Hassan M, Ismail M, Moharram A, Shoreit A (2016) Synergistic effect of biogenic silver nanoparticles with β. lactam cefotaxime against resistant Staphylococcus arlettae AUMC b-163 isolated from T3A pharmaceutical cleanroom, Assiut Egypt. Am J Microbiol Res 4:132–137
Hebeish A, El-Rafie MH, El-Sheikh MA, Seleem AA, El-Naggar ME (2014) Antimicrobial wound dressing and anti-inflammatory efficacy of silver nanoparticles. Int J Biol Macromol 65:509–515
Hocquet D, Muller A, Bertrand X (2016) What happens in hospitals does not stay in hospitals: antibiotic-resistant bacteria in hospital wastewater systems. J Hosp Infect 93(4):395–402
Howell-Jones RS, Wilson MJ, Hill KE, Howard AJ, Price PE, Thomas DW (2005) A review of the microbiology, antibiotic usage and resistance in chronic skin wounds. J Antimicrob Chemother 55(2):143–149
Hwang I, Hwang J, Choi H, Kim K, Lee D (2012) Synergistic effects between silver nanoparticles and antibiotics and the mechanisms involved. J Med Microbiol 61:1719–1726
Jamaran S, Zarif R (2016) Synergistic effect of silver nanoparticles with neomycin or gentamicin antibiotics on mastitis-causing Staphylococcus aureus. Open J Ecol 6:452–459
Jeong L, Kim M, Jung J, Min B, Park W (2014) Effect of silk fibroin nanofibers containing silver sulfadiazine on wound healing. Int J Nanomed 9:5277–5287
Kalashnikova I, Das S, Seal S (2015) Nanomaterials for wound healing: scope and advancement. Nanomedicine 10(16):2593–2612
Kaler A, Mittal AK, Katariya M, Harde H, Agrawal AK, Jain S, Banerjee UC (2014) An investigation of in vivo wound healing activity of biologically synthesized silver nanoparticles. J Nanoparticles Res 16:2605. doi:10.1007/s11051-014-2605-x
Karahaliloglu Z, Kilicay E, Denkbas EB (2016) Antibacterial chitosan/silk sericin 3D porous scaffolds as a wound dressing material. Nanomed Biotechnol 45(6):1–14
Karri VVS, Kuppusamy G, Talluri SV, Mannemala SS, Kollipara R, Wadhwani AD, Mulukutla S, Raju KRS, Malayandi R (2016) Curcumin loaded chitosan nanoparticles impregnated into collagen alginate scaffolds for diabetic wound healing. Int J Biol Macromol 93:1519–1529
Kawai K, Larson BJ, Ishise H, Carrel AL, Nishimoto S, Longaker M, Lorenz M (2011) Calcium-based nanoparticles accelerate skin wound healing. PLoS ONE 6(11):e27106. doi:10.1371/journal.pone.0027106
Kim JE, Lee J, Jang M, Kwak MH, Go J, Kho EK, Song SH, Sung JE, Lee J, Hwang DY (2015) Accelerated healing of cutaneous wounds using phytochemically stabilized gold nanoparticle deposited hydrocolloid membranes. Biomater Sci 3(3):509–519
Kim TS, Cha JR, Gong MS (2017) Investigation of the antimicrobial and wound healing properties of silver nanoparticle-loaded cotton prepared using silver carbamate. Text Res J. doi:10.1177/0040517516688630
Klasen HJ (2000) Historical review of the use of silver in the treatment of burns. I Early Uses Burns 26(2):117–130
Konop M, Damps T, Misicka A, Rudnicka L (2016) Certain aspects of silver and silver nanoparticles in wound care: a mini review. J Nanomater Article ID 7614753. doi:http://dx.doi.org/10.1155/2016/7614753
Krausz AE, Adler BL, Cabral V, Navati M, Doerner J, Charafeddine RA, Chandra D, Liang H, Gunther L, Clendaniel A, Harper BSS, Friedman JM, Nosanchuk JD, Friedman AJ (2014) Curcumin encapsulated nanoparticles as innovative antimicrobial and wound healing agent. Nanomed Nanotechnol Biol Med 11(1):195–206
Kuchler S, Wolf NB, Heilmann S, Weindl G, Helfmann J, Yahya MM, Stein C, Schäfer-Korting M (2010) 3D-wound healing model: influence of morphine and solid lipid nanoparticles. J Biotechnol 148(1):24–30
Lakshmi V, Kannan K (2016) In vitro evaluation of antibiotic conjugated biogenic gold nanoparticles by Neosartorya udagawae. Int J Pharma Bio Sci 7:83–89
Lau P, Bidin N, Islam S, Binti WN, Mohd Shukri W, Dip NZ, Musa N, Krishnan G (2017) Influence of gold nanoparticles on wound healing treatment in rat model: photobiomodulation therapy. Lasers Surg Med 49(4):380–386
Leu J, Chen SA, Chen HM, Wu WM, Hung CF, Yao YD, Tu CS, Liang YJ (2012) The effects of gold nanoparticles in wound healing with antioxidant epigallocatechin gallate and αlipoic acid. Nanomed Nanotechnol Biol Med 8(5):767–775
Li X, Ye X, Qi J, Fan R, Gao X, Wu Y, Zhou L, Tong A, Guo G (2016) EGF and curcumin co-encapsulated nanoparticle/hydrogel system as potent skin regeneration agent. Int J Nanomed 11:3993–4009
Lila ASA, Ishida T (2017) Liposomal delivery systems: design, optimization and current applications. Biolog Pharma Bull 40(1):1–10
Lindley LE, Stojadinovic O, Pastar I, Tomic-Canic M (2016) Biology and biomarkers for wound healing. Plast Reconstr Surg 138:18S–28S
Marcato PD, De Paula LB, Melo PS, Ferreira IR, Almeida ABA, Torsoni AS, Alves OL (2015) In vivo evaluation of complex biogenic silver nanoparticle and enoxaparin in wound healing. J Nanomater Article ID439820. doi:http://dx.doi.org/10.1155/2015/439820
Marx DE, Barillo DJ (2014) Silver in medicine: the basic science. Burns 40(1):9–18
Marsich E, Travan A, Donati I, Turco G, Kulkova J, Moritz N 2(013) Biological responses of silver-coated thermosets: an In vitro and In vivo study. Acta Biomater 9:5088–5099
Mendes R, Fernandes AR, Baptista PV (2017) Gold nanoparticle approach to the selective delivery of gene silencing in cancer: the case for combined delivery? Genes 8: 94. doi:10.3390/genes8030094
Mohammad G, Pandey HP, Tripathib K (2008) Diabetic wound healing and its angiogenesis with special reference to nanoparticles. Digest J Nanomater Biostructures 3(4):203–208
Mordi RM, Momoh MI (2009) Incidence of Proteus species in wound infections and their sensitivity pattern in the University of Benin teaching hospital. Afr J Biotech 8(5):725–730
Nguyen VC, Nguyen VB, Hsieh MF (2013) Curcumin-loaded chitosan/gelatin composite sponge for wound healing application. Int J Polymer Science. 2013: Article ID 106570. doi:http://dx.doi.org/10.1155/2013/106570
Oryan AE, Alemzadeh A, Moshiri A (2017) Burn wound healing: present concepts, treatment strategies and future directions. J Wound Care 26(1):5–19
Oyarzun-Ampuero F, Vidal A, Concha M, Morales J, Orellana S, Moreno-Villoslada I (2015) Nanoparticles for the treatment of wounds. Curr Pharm Des 21(29):4329–4341
Pannerselvam B, Jothinathan MD, Rajenderan M, Perumal P, Thangavelu KP, Kim HJ, Singh V (2017) An In vitro study on the burn wound healing activity of cotton fabrics incorporated with phytosynthesized silver nanoparticles in male Wistar albino rats. Eur J Pharm Sci 100:187–196
Pastar I, Stojadinovic O, Yin NC, Ramirez H, Nusbaum AG, Sawaya A, Patel SB, Khalid L, Isseroff RR, Tomic-Canic M (2014) Epithelialization in wound healing: A comprehensive review. Adv Wound Care 3:445–464
Patra JK, Baek KH (2017) Antibacterial activity and synergistic antibacterial potential of biosynthesized silver nanoparticles against foodborne pathogenic bacteria along with its anticandidal and antioxidant effects. Front Microbiol 8:167. doi:10.3389/fmicb.2017.00167
Perez-Díaz M, Alvarado-Gomez E, Magaña-Aquino M, Sánchez-Sánchez R, Velasquillo C, Gonzalez C, Ganem-Rondero A, Martínez-Castañon G, Zavala-Alonso N, Martinez-Gutierrez F (2016) Anti-biofilm activity of chitosan gels formulated with silver nanoparticles and their cytotoxic effect on human fibroblasts. Mater Sci Eng 60:317–323
Pivodova V, Franková J, Galandáková A, Ulrichová J (2015) In vitro AuNPs’ cytotoxicity and their effect on wound healing. Nanobiomedicine 2:7. doi:10.5772/61132
Psunica-Panea G, Ficai A, MariaMarin M, tefaniaMarin S, Albu MG, Constantin DV, Dinu-Pîrvu C, Vuluga Z, Corobea CM, Ghica MV (2016) New collagen-dextran-zinc oxide composites for wound dressing. J Nanomater Article ID 5805034. doi:http://dx.doi.org/10.1155/2016/5805034
Raguvaran R, Manuja BK, Chopra M, Thakur R, Anand T, Kalia A, Manuja A (2017) Sodium alginate and gum acacia hydrogels of ZnO nanoparticles show wound healing effect on fibroblast cells. Int J Biol Macromol 96:185–191
Rai M, Ingle A (2012) Role of nanotechnology in agriculture with special reference to management of insect pests. Appl Microbiol Biotechnol 94:287–293
Rai M, Kon K, Ingle AP, Duran N, Galdiero S, Galdiero M (2014) Broad-spectrum bioactivities of silver nanoparticles: The emerging trends and future prospects. Appl Microbiol Biotechnol 98:1951–1961
Rai M, Yadav A, Gade A (2009) Silver nanoparticles as a new generation of antimicrobials. Biotechnol Adv 27(1):76–83
Rakhmetova AA, Alekseeva TP, Bogoslovskaya OA, Leipunskii IO, Ol’khovskaya IP, Zhigach AN, Glushchenko N (2010) Wound-healing properties of copper nanoparticles as a function of physicochemical parameters. Nanotechnol Russ 5(3):271–276
Ranghar S, Sirohi P, Verma P, Agarwal V (2014) Nanoparticle-based drug delivery systems: promising approaches against infections. Braz Arch Biol Technol 57:209–222
Rigo C, Ferroni L, Tocco I, Roman M, Munivrana I, Gardin C, Cairns W, Vindigni V, Azzena B, Barbante C, Zavan B (2013) Active silver nanoparticles for wound healing. Int J Mol Sci 14:4817–4840
Salunke BK, Sathiyamoorthi E, Tran TK, Kim BS (2017) Phytosynthesized silver nanoparticles for biological applications. Korean J Chem Eng 34(3):1–9
Sankar R, Dhivya R, Shivashangari SK, Ravikumar V (2014) Wound healing activity of Origanum vulgare engineered titanium dioxide nanoparticles in Wistar Albino rats. J Mater Sci Mater Med 25:1701–1708
Santos CA, Seckler MM, Ingle A, Gupta I, Galdieiro S, Galdieiro M, Gade A, Rai M (2014) Silver nanoparticles: therapeutical uses, toxicity and safety issues. J Pharm Sci 103(7):1931–1944
Shahverdi A, Fakhimi A, Shahverdi H, Minaian S (2007) Synthesis and effect of silver nanoparticles on the antibacterial activity of different antibiotics against Staphylococcus aureus and Escherichia coli. Nanomed NanotechnolBiol Med 3:168–171
Shirai J, Kanno T, Tsuchiya Y, Mitsubayashi S, Seki R (2000) Effects of chlorine, iodine, and quaternary ammonium compound disinfectants on several exotic disease viruses. J Vet Med Sci 62(1):85–92
Shrivastava S (2007) Characterization of enhanced antibacterial effects of novel silver nanoparticles. Nanotechnology 18:225103–225112
Solanki R, Nagori BP (2013) A review on microorganisms causing wound infections on skin. Asian J Pharm Technol 3(3):119–122
Stadelmann WK, Digenis AG, Tobin GR (1998) Physiology and healing dynamics of chronic cutaneous wounds. Am J Surg 176(2):26S–38S
Sudheesh-Kumar PT, Lakshmanan VK, Anilkumar TV, Ramya C, Reshmi P, Unnikrishnan AG, Nair SV, Jayakumar R (2012) Flexible and microporous chitosan hydrogel/nano ZnO composite bandages for wound dressing: In vitro and in vivo evaluation. ACS Appl Mater Interfaces 4:2618–2629
Talan DA, Abrahamian FM, Moran GJ, Citron DM, Tan JO, Goldstein EJC (2003) Clinical presentation and bacteriologic analysis of infected human bites in patients presenting to emergency departments. Clin Infect Dis 37(11):1481–1489
Talan DA, Citron DM, Abrahamian FM, Moran GJ, Goldstein EJ (1999) Bacteriologic analysis of infected dog and cat bites. New Eng J Med 340(2):85–92
Thirumurugan G, Veni VS, Ramachandran S, Rao JV, Dhanaraju MD (2011) Superior wound healing effect of topically delivered silver nanoparticle formulation using eco-friendly potato plant pathogenic fungus: synthesis and characterization. J Biomed Nanotechnol 7(5):659–666
Thomas R, Nair A, Kr S, Mathew J, Ek R (2014) Antibacterial activity and synergistic effect of biosynthesized AgNPs with antibiotics against multidrug-resistant biofilm-forming coagulase-negative staphylococci isolated from clinical samples. Appl Biochem Biotechnol 173:449–460
Tian J, Wong KKY, Ho CM, Lok CN, Yu WY, Che CM, Chiu JF, Tam PK (2007) Topical delivery of silver nanoparticles promotes wound healing. Chem Med Chem 2(1):129–136
Tocco I, Zavan B, Bassetto F, Vindigni V (2012) Nanotechnology-based therapies for skin wound regeneration. J Nanomater Article ID 714134. doi:10.1155/2012/714134
Velazquez-Velazquez J, Santos-Flores A, Araujo-Mel´endez J (2015) Anti-biofilm and cytotoxicity activity of impregnated dressings with silver nanoparticles. Mater Sci Eng 49:604–611
Volkova N, Yukhta M, Pavlovich O, Goltsev A (2016) Application of cryopreserved fibroblast culture with Au nanoparticles to treat burns. Nanoscale Res Lett 11:22. doi:10.1186/s11671-016-1242-y
Woo CH, Choia YC, Choia JS, Leeb HY, Choa YW (2015) A bilayer composite composed of TiO2-incorporated electrospun chitosan membrane and human extracellular matrix sheet as a wound dressing. J Biomater Sci Polym Ed 26(13):841–854
Xiu Z, Zhang Q, Puppala HL, Colvin VL, Alvarez PJ (2012) Negligible particle-specific antibacterial activity of silver nanoparticles. Nano Lett 12(8):4271–4275
Xiu ZM, Ma J, Alvarez PJJ (2011) Differential effect of common ligands and molecular oxygen on antimicrobial activity of silver nanoparticles versus silver ions. Environ Sci Technol 45(20):9003–9008
Yang W, Shen C, Ji Q, An H, Wang J, Liu Q, Zhang Z (2009) Food storage material silver nanoparticles interfere with DNA replication fidelity and bind with DNA. Nanotechnology 20(8):085102. doi:10.1088/0957-4484/20/8/085102
Zarina A, Nanda A (2014) Combined efficacy of antibiotics and biosynthesized silver nanoparticles from Streptomyces albaduncus. Int J PharmTech Res 6:1862–1869
Zhang H, Smith JA, Oyanedel-Craver V (2012) The effect of natural water conditions on the anti-bacterial performance and stability of silver nanoparticles capped with different polymers. Water Res 46:691–699
Zhang K, Li F, Imazato S, Cheng L, Liu H, Arola DD, Bai Y, Xu HH (2013) Dual antibacterial agents of nano-silver and 12- methacryloyloxydodecylpyridinium bromide in dental adhesive to inhibit caries. J Biomed Mater Res B Appl Biomater 101:929–938
Zhang H, Wu F, Li Y, Yang X, Huang J, Tingting L, Zhang Y, Chen J, Chen H, Gao Y, Liu G, Jia L (2016) Chitosan-based nanoparticles for improved anticancer efficacy and bioavailability of mifepristone. Beilstein J Nanotechnol 7:1861–1870
Zhu C, Zhao J, Kempe K, Wilson P, Wang J, Velkov T, Li J, Davis TP, Whittaker MR, Haddleton DM. (2017). A Hydrogel-based localized release of colistin for antimicrobial treatment of burn wound infection. Macromol Biosci 17(2):1600320. doi:10.1002/mabi.201600320
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 Springer International Publishing AG
About this chapter
Cite this chapter
Ingle, A.P. et al. (2017). Nanoformulations for Wound Infections. In: Rai, M., Alves dos Santos, C. (eds) Nanotechnology Applied To Pharmaceutical Technology. Springer, Cham. https://doi.org/10.1007/978-3-319-70299-5_9
Download citation
DOI: https://doi.org/10.1007/978-3-319-70299-5_9
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-70298-8
Online ISBN: 978-3-319-70299-5
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)