Abstract
Some Scydmaeninae are strict specialists that feed exclusively on heavily sclerotized oribatid or uropodine mites. The chapter reviews the available literature on the feeding habits of Euconnus , Stenichnus , Scydmaenus , and Cephennium beetles and presents previously unpublished observations on Neuraphes and Microscydmus species. Species with unspecialized mouthparts attack the mite’s gnathosoma, removing movable parts to gain access to soft tissues. They also often remove genital or anal plates to feed through the resulting openings. In Euconnus that are specialized to feed on ptyctimous (i.e., capable of encapsulating) oribatids, a sticky droplet of digestive juice exuded onto the predator’s mouthparts is used to capture mites. The prey is then lifted and covered with noxious digestive juice, which weakens or kills the encapsulated mite . Once the muscles responsible for maintaining the encapsulation are relaxed, the prey’s prodorsum opens, and Euconnus beetles use their mandibles to crush the mite’s ventral plates and gain access to the flesh. In Scydmaenus that are specialized to feed on non-ptyctimous Oribatida and Uropodina, the mandibles play a major role both in capturing prey and in breaching the mite’s defenses. The prey’s legs are often cut off if they are long or spiny, which facilitates the subsequent attack on the gnathosoma. Cephenniini are the “hole scrapers”: they have paired labial suckers on the prementum, which are used to immobilize their prey. Once the mite adheres to the suckers, the predator’s mandibles slowly grind a small hole in the prey’s cuticle. Digestive juices are then injected; through the same puncture, liquefied tissues are ingested. The entire feeding process can take many hours. Some species show preferences toward particular mite taxa and may play a significant role in the oribatid or uropodine mite population dynamics.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
1 Introduction
Over a century ago, Reitter (1909) noticed that Scydmaeninae (Scydmaenidae at that time) seemed to feed on mites. This view was supported by Schuster (1966a, b), who observed the feeding of adult Cephennium majus Reitter and larvae of C. majus and C. thoracicum Müller and Kunze; Schuster carried out the first published prey preference experiments with these minute (1–1.5 mm) beetles. Scydmaeninaes were given a broad spectrum of potential prey, including Oribatida, Uropodina, and Gamasida mites, as well as Collembola, Protura, and oligochaete Enchytraeidae. They were found to feed mostly on the armored Oribatida, and only rarely on Uropodina and Gamasida.
A classic work was published by Schmid (1988), who made systematic observations of the feeding preferences and techniques of adults of many species belonging to the genera Cephennium Müller and Kunze (Cephenniini), Neuraphes Thomson, Scydmoraphes Reitter, Stenichnus Thomson, Microscydmus Saulcy and Croissandeau, Euconnus Thomson (Glandulariini), and Scydmaenus Latreille (Scydmaenini), as well as larvae of Cephennium , Stenichnus , and Scydmoraphes . A broad spectrum of Oribatida and Uropodina species was tested (approximately 200 species). Various structures of the mouthparts and legs of Scydmaeninae were interpreted as adaptations for feeding on these heavily armored Acari.
Later, Molleman and Walter (2001) demonstrated that some Australian Glandulariini and Scydmaenini showed strong preferences toward armored mites. However, they also scavenged on dead ants, beetles, springtails, and unarmored nymphs of galumnid mites (Oribatida). Jałoszyński and Olszanowski (2013, 2015, 2016) carried out prey choice experiments with a broad spectrum of possible prey mites offered to four species of Scydmaeninae under laboratory conditions. They obtained results concerning their prey preferences and interesting details of their feeding techniques.
The data obtained so far demonstrate that Scydmaeninae not only use different methods to breach their prey’s defenses but also show relatively narrow preferences toward certain taxa or particular body forms of oribatids or (less frequently) uropodines. Although our knowledge is still fragmentary, two distinct feeding techniques can be defined, depending on the morphological specialization of the predator’s mouthparts. Some behavioral variants were also discovered, which evolved to cope with the different and often sophisticated defense systems of armored mites.
It should be noted that not all Scydmaeninae are specialist predators feeding on heavily sclerotized mites. Leleup (1968) noticed that South African Mastigini carry small larvae and springtails in their mandibles. Furthermore, O’Keefe and Monteith (2000) mentioned observations of the only Australian Clidicini species carrying large neanurine springtails in their mandibles. Jałoszyński (2012a, b) demonstrated that two European species of Scydmaenus preferred either springtails or soft-bodied Acari and, under laboratory conditions, showed no interest in Oribatida; scavenging dead arthropods and cannibalism were also reported by the same author.
Because many other arthropods feed on soft-bodied prey and very few are strictly specialized to utilize heavily protected armored mites as the only source of food, the prey preferences and feeding techniques of acarophagous Scydmaeninae have attracted much attention. Oribatida were once believed to have evolved their defense systems in response to predation by prostigmatan and mesostigmatan mites; because this pressure is now low, they currently live in an “enemy-free space” (e.g., Jeffries and Lawton 1984; Peschel et al. 2006). Oribatids are indeed well protected against most invertebrate predators. Their defense systems include thick cuticles (which are often reinforced by mineralization, carinae, or reticulation). Furthermore, depending on taxon, they also have long setae on the idiosoma or spiny legs that make it difficult for a predator to attack the mite ’s body. Some produce repellents or toxins in the so-called oil glands, whereas others accumulate soil particles on their body surface that form an additional protective crust. Many oribatids have pteromorphs, which are lateral laminar projections over their coxae that protect the legs from being cut off by predators. The so-called ptyctimous mites can “encapsulate”—that is, they adopt a compact defensive posture with all appendages and vulnerable ventral membranous structures hidden under their closed prodorsum, which is shield-like and can move to open/close the encapsulation (Pachl et al. 2012; Schmelzle et al. 2008, 2009, 2010). A combination of several defensive mechanisms or structures in one species is not uncommon. Predators that have adopted to feed on this kind of prey are expected to use unusual techniques or to have unique tools to breach defenses of their prey. Such adaptations are summarized in this chapter on the basis of the available literature—mainly studies published by the author (Jałoszyński 2016; Jałoszyński and Olszanowski 2013, 2015, 2016) but also previously unpublished observations concerning the genera Microscydmus and Neuraphes .
2 Cephenniini, the “Hole Scrapers”
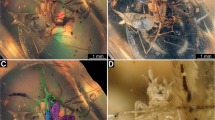
All known species of Cephenniini have highly modified mouthparts (Fig. 12.1a, b), with the labium transformed into a prey immobilizing device. The head is strongly declined, such that the mouthparts are directed downward. The labrum is typically semicircular with a membranous marginal velum and a membranous epipharynx; the mandibles are variable in shape but often short and relatively blunt; and the maxillae are generalized, as those in all Scydmaeninae. The most unusual is the labium (Fig. 12.1b), which has a highly movable prementum, with its anterior surface capable of tilting dorsally, ventrally, or/and laterally. The labial palps are exceptionally small and broadly separated, and the area between them is occupied by four or six symmetrically distributed suckers. Often, the anterior surface of the prementum is additionally divided by a median longitudinal groove, so that the lateral halves can move independently and better fit to the convex surface of oribatid mites.
Examples of Cephenniini (a–d) and their prey (e). (a) Cephennium majus, mouthparts in anteroventral view. (b) Separated labium of Cephennium majus in anterior view. (c) Cephennium majus preserved during feeding on ptyctimous oribatid mite . (d) Cephennium ruthenum preserved during feeding on non-ptyctimous oribatid mite . (e) Phthiracarus sp. (Oribatida, Phthiracaridae), prey of Cephennium majus, showing feeding damage (arrow). Abbreviations: bst basistipes, cd cardo, eph epipharynx, gal galea, lac lacinia , llh lateral lobe of hypopharynx, lp labial palp , ls labial sucker, md mandible, mn mentum, mst mediostipes, mxp maxillary palp, ntg notogaster, pmn prementum, ppf palpifer, prd prodorsum
The ultrastructure of the suckers was studied by Jałoszyński and Beutel (2012), who found that each sucker is composed of an outer oval plate connected by a circumferential ring with the inner plate bearing a median perforation; thus, the lumen between the plates is continuous with the inner space of the labium. The suckers, the labial cuticle, and the internal sclerotized scaffold of the labium, including the hypopharyngeal suspensorium, form a continuous functional unit operated by labial muscles, which can modulate the degree of concavity of the outer plates of the suckers. This sophisticated system of structures requires muscle contractions only during the attack; however, when the mite adheres to the suckers by suction forces, the muscles relax. It was suggested that the complex structural components of the suction discs have evolved by invagination of exocuticular structures and that the development of suckers was preceded by local sclerotizations of the prementum, induced by contact with soft-bodied or partly armored prey (Jałoszyński and Beutel 2012).
The labial modifications found in all extant Cephenniini suggest that they all feed on armored mites and presumably cannot feed on other types of prey. It is possible that this is an ancient adaptation: the oldest unambiguously identified fossil of Cephenniini, from Upper Cretaceous (Cenomanian) Burmese amber, is morphologically nearly identical with extant representatives of this tribe (Jałoszyński and Peris 2016). However, mouthparts of this fossil are not exposed. Thus, further study is needed to shed more light on the evolution of this intriguing, narrow feeding adaptation that most likely enabled Cephenniini to avoid competition with other small invertebrate predators of the forest floor.
Prey preferences, feeding techniques, and the functional morphology of mouthparts were studied using several species of European Cephennium as model organisms (Jałoszyński and Beutel 2012; Jałoszyński and Olszanowski 2016). Jałoszyński and Olszanowski (2016) described three phases of the feeding process on oribatid prey: (1) attack and stabilization of the attachment site (about 4−5 min), (2) penetration of the mite ’s cuticle (about 40 min), and (3) feeding (7−8 h). The mite is attacked from behind or from above (Fig. 12.3a) and lifted using the beetle’s prementum (Fig. 12.3b, c). The adhesion between the beetle’s mouthparts and the mite is so strong that it is possible to kill and preserve them, then subsequently take scanning electron microscopy images, without disrupting the connection (Fig. 12.1c, d). Within the first 1−5 min, the captured mite is rotated using the protarsi, apparently to find the best attachment site. The manipulations take place without detaching the prey; the mouthparts of the beetles appear to slide over the surface of the mite’s cuticle.
When an apparently suitable site has been chosen, both mandibles start to rhythmically spread and close, and the second phase begins. The beetles broadly open one mandible, while the other mandible makes short scraping movements within the area delimited by the labrum and the prementum. For most of the time, the prey remains lifted; however, when the grip is in the posterodorsal region of the idiosoma, the mite often manages to reach for the ground with some legs, pulls itself closer to the arena, and starts crawling forward. Beetles counteract by lifting the mite higher. The scraping movements of one mandible can be observed for 10−15 min. Then, the position of the mite is changed, with the working mandible being replaced by the previously resting one. After about 20 min, the mite’s leg movements weaken to suddenly become very rapid. This increased activity of the mite marks the moment of completing the perforation of the cuticle; however, the prey remains alive and erratically moves its legs for about half an hour. Cephennium keeps on working with one mandible, presumably broadening the hole or trying to insert a tip of the mandible deeper.
When the movements of the prey’s legs definitively stop, it is usually possible to see that the tip of one mandible of the beetle is inserted into the hole and the other mandible is still broadly open. This is when the third phase starts. The beetle can now feed through the tiny hole, which is often only about 20 μm wide (Fig. 12.1e). When attached to the mite ’s cuticle, the surface of the prementum that bears the suckers is parallel to the body surface of the mite , and the labrum is strongly flipped dorsally. To feed, Cephennium beetles close their mandibles, which are bent and short enough to be contained between the labrum and labium. The attachment site is completely surrounded by the beetle’s mouthparts, which tightly seal the hole margins to enable injection of digestive juices and subsequent ingestion of liquefied tissues. The connection is sealed by the membranous marginal velum of the labrum, which laterally fits tightly into the dorsomesal notch on each closed mandible. Ventrally and laterally, the contact zone is sealed by the flexible lateral lobes of the hypopharynx, the prementum, and the maxillae; the latter fits into the concave ventral surface of the closed mandibles. The feeding can take several hours.
The only damage caused by the beetles is a tiny hole scraped through the mite ’s cuticle (Fig. 12.1e). Because the attack is directed onto the dorsolateral or posterodorsal surface of the idiosoma, Cephennium can successfully feed on both ptyctimous and non-ptyctimous mites, as the encapsulation of the former does not protect against this type of attack. Furthermore, pteromorphs are an inefficient protection against this feeding technique. However, the unique morphological and behavioral adaptation of Cephennium requires a subglobose prey with smooth and sparsely setose body to ensure the adhesion by suction forces; deeply sculptured, reticulate, or spiny mites are not attacked. In addition, those that accumulate soil particles on the body surface avoid being captured by Cephennium.
Even among “morphologically acceptable” oribatids, Cephennium species select only particular taxa, showing strong preferences toward ptyctimous Phthiracaridae and non-ptyctimous Ceratozetidae, Achipteriidae, and Liacaridae. Jałoszyński and Olszanowski (2016) observed also significant differences in prey preferences between two morphologically similar Cephennium species that differ slightly in their body size. Their choice of prey was clearly affected by this difference, as the larger C. majus was able to feed on larger prey than the smaller C. ruthenum Machulka. The feeding process clearly depends on the prey’s body size (or volume). Presumably, the structure of the mite ’s cuticle also plays an important role, as the time from attack to the killing of the mite in some instances depends on the mite taxon rather than its body length.
3 Glandulariini and Scydmaenini: Brutal Force and Leg-Cutting
Species of Glandulariini and Scydmaenini known to feed on Oribatida and/or Uropodina have unspecialized mouthparts, except for typically sharp and slender mandibular apices (Fig. 12.2a, b) that are well adapted to insert into the mite ’s natural body openings. It was demonstrated that adults of different species within the same genus, not differing in the structure of mouthparts, can feed on soft-bodied arthropods or on armored mites only. Such a pair of morphologically very similar species is Scydmaenus tarsatus Müller and Kunze and Scydmaenus rufus Müller and Kunze; the former feeds on weakly sclerotized Acaridae and springtails, whereas the latter feeds on Oribatida and Mesostigmata (Uropodina) (Jałoszyński 2012a; Jałoszyński and Olszanowski 2015). Moreover, although the adults and larvae of Sc. tarsatus have strikingly different mandibles (asymmetrical and with mesal teeth in adults vs. symmetrical, falciform, and lacking teeth in larvae), their prey choices are similar (Jałoszyński 2012a; Jałoszyński and Kilian 2012). It seems that behavioral rather than morphological adaptations play the key role in feeding for Glandulariini and Scydmaenini. Consequently, it is not possible to infer their preferred prey by studying the structure of the mouthparts; only direct behavioral observations can address the question concerning the prey choice and feeding technique.
Examples of Scydmaenini (a) and their prey (c–f) and Glandulariini (b) and their prey (g). (a) Head of Scydmaenus rufus in anterior view (after Jałoszyński and Olszanowski 2015, modified). (b) Mouthparts of Euconnus pubicollis in anterior view (after Jałoszyński and Olszanowski 2013, modified). (c–d) Oppia nitens (Oribatida, Oppiidae) before (c) and after (d) feeding of Scydmaenus rufus. (e–g) Prey of Scydmaenus rufus (e, f) and Euconnus pubicollis (g) showing feeding damage: Punctoribates punctum (Oribatida, Mycobatidae) (e), Uroobovella pyriformis (Uropodina, Urodinychidae) (f), and Phthiracarus sp. (Oribatida, Phthiracaridae) (g). Abbreviations: apt anal plate, cl clypeus, gal galea, gns gnathosoma, gpt genital plate, lac lacinia, lbr labrum, lp labial palp , md mandible , mxp maxillary palp
The compost-inhabiting European Scydmaenus rufus feeds predominantly on oribatids belonging to Scheloribatidae and Oppiidae and Urodinychidae uropodines (Jałoszyński and Olszanowski 2015). Scheloribatids and urodinychids are short-legged mites with either smooth or distinctly reticulate cuticle. Cuticular structures do not protect them against Sc. rufus because the primary target of its attack is the gnathosoma, with the secondary target being the genital or anal opening. Therefore, typical feeding damage is restricted to these body regions (Fig. 12.2e, f). The beetles attack the anterior body region of their prey, inserting one mandible into the mite’s mouth opening and breaking off all of its mouthparts by rotating the mite. The feeding takes place through the resulting opening by external digestion. The beetles inject digestive juices into the gnathosomal opening and ingest the liquefied tissues. In abandoned empty mite shells, usually some or all the legs are also removed. However, this is a secondary process that results from rotating and manipulating the dead mite during feeding; the legs, with their internal soft tissues already dissolved, are brittle and easily break off.
A modification of this simple mechanism is required when Sc. rufus attacks Oppiidae mites, which have long and spiny legs (Figs. 12.2c and 12.3g). The legs are a part of the mites’ defense system and mechanically interfere with a predator’s attempts to get close to the vital regions of the gnathosoma or ventral structures. Consequently, the attack typically begins with the cutting off of some legs. An experiment with 60 beetles, each attacking a single Oppia mite, took observations 10–100 min after the attack (Jałoszyński and Olszanowski 2015). After just 10 min, one or two of the mite’s legs or at least some podites were removed in 40% of mites. After 30 min, several legs were removed in 70% cases and the first successful attempts to attack the gnathosoma were noticed. After 100 min, all of the mite prey had some or all of their legs cut off; their mouthparts were also completely removed. In some cases, the secondary targets—the genital plates—were also removed at this phase (Fig. 12.2d).
Scydmaeninae feeding on armored mites. (a–c) Cephennium majus feeding on ptyctimous (a, c) and non-ptyctimous (b) Oribatida. (d, e) Euconnus pubicollis feeding on Phthiracarus sp. (f) Microscydmus sp. feeding on Oribatida. (g) Scydmaenus rufus feeding on Oppia nitens. (h) Stenichnus godarti feeding on Uropodina. (i) Neuraphes elongatulus feeding on juvenile Damaeidae
Beetles that manage to successfully attack the gnathosoma can be observed exuding a droplet of digestive juices onto their prey and then sucking it back; one mandible remains inserted into the gnathosomal (or genital) opening during the entire feeding process. Beetles select their prey based on unknown factors. However, body size is certainly one of them because attacks on too-large prey (e.g., some Liacaridae oribatids) are usually unsuccessful. It is worth noting, however, that beetles do make such attempts despite poor chances to succeed. Tactile or chemical (and less so, visual) stimuli seem to play an important role in initiating attacks.
A different technique of attack, but a similar method of feeding, is used by a common European inhabitant of the forest leaf litter, Euconnus pubicollis (Müller & Kunze), a glandulariine ant-like stone beetle (Jałoszyński and Olszanowski 2013). When presented with a broad spectrum of oribatid and uropodine mite taxa, this species strongly prefers the ptyctimous Phthiracaridae ( Phthiracarus spp.). Several other oribatid families (both ptyctimous and non-ptyctimous) represented less than 8% of its diet in laboratory prey choice experiments (based on 30 beetles observed for a month that chose their prey from more than 1400 living mites belonging to 24 families and 50 species; Jałoszyński and Olszanowski 2013). The mouthparts of E. pubicollis are similar to those of most Glandulariini (Fig. 12.2b), with unmodified labrum, maxillae, and labium, as well as elongated, curved mandibles that each have a small preapical mesal tooth and a slender apical portion. The preferred prey of this species is ptyctimous and therefore is capable of encapsulation; consequently, when attacked, the mites retract and protect all vulnerable body parts under their shield-like prodorsum. This defensive posture does not leave any externally accessible grip sites or intersegmental membranes that could be pierced. Euconnus pubicollis uses a different technique to capture its prey and breach its defenses than does Cephennium or Sc. rufus.
Euconnus pubicollis, when confronted with its preferred prey, rapidly moves its head and mouthparts toward the cuticle of the mite, exudes a droplet of sticky liquid from its mouth, and lifts the mite ; furthermore, it often additionally uses the protibial apices with spatulate, adhesive setae to manipulate its prey. However, the beetles are able to lift their prey without using their fore legs, only by means of the sticky properties of the liquid on their mouthparts or/and capillary forces. The anterior portion of the beetle’s labrum or its dorsal surface adheres to the mite cuticle by means of the liquid exuded from the mouth; the mandibles remain widely spread and only their apices touch the mite. The maxillae are protruded anteriorly, with galea and lacinia covered with the liquid and adhering to the prey cuticle. The maxillary palps are spread laterally and occasionally touch the prey’s cuticle. The labium is protruded anteriorly; its anterior part is also covered with the liquid and adheres to the mite. This arrangement of mouthparts allows the formation of a large contact area from the labrum to the galea and lacinia, bearing dense trichia covered with the liquid.
The beetles typically adopt a posture of a raised head and prothorax while standing on their middle and hind legs; alternatively, they use substrate particles to attack from above, standing head down, with hind and middle legs on the side of a soil or wood particle, and the anterior part of the body with the captured mite hanging down above the ground (Fig. 12.3d, e). The attack phase, if undisturbed by other beetles, is immediately followed by manipulating the prey and searching for access to fragile or movable parts of the integument. If there are other beetles frequently disturbing the successful predator, the latter walks for minutes or even hours with the prey held in its mouthparts, searching for shelter under soil particles or in narrow spaces between them, where it could continue to manipulate the mite to overcome its encapsulation.
Adult Euconnus beetles that have already captured a phthiracarid mite frequently rotate their prey using their protibiae, and often detach and attach their mouthparts to the prey. A voluminous droplet of liquid is regularly produced and sucked back in cycles of a few seconds, with the droplet first increasing in size then rapidly decreasing in volume. This is repeated from about 90 min to more than 20 h, during which time the manipulated mite remains motionless and encapsulated. During this prolonged activity, Euconnus covers the entire body surface of its prey with the liquid exuded from the mouthparts. The moving and rotating of the mite is occasionally accompanied by movements of the mandibles; apparently, the beetle searches for a grip on the margins of the closed prodorsum or the genital and anal plates. It seems that toxic or otherwise noxious properties of the exuded liquid (presumably digestive juice) are responsible for slowly weakening the mite; eventually, the muscles that maintain the encapsulation relax.
The moment when the prodorsum is at least partly lifted marks the end of the long struggle and the beginning of the last phase. The time from the attack to the lifting of the prodorsum can range from approximately 1 to more than 20 h and depends on the body length of the prey. To gain access to the flesh after opening the prodorsum of the prey, the Euconnus beetle presses the mite’s ventral (genital and anal) plates. To this aim, the mite is usually pressed against the ground; the beetle adopts a posture with its head and pronotum lowered toward the prey while standing on all six legs. The mandibles are used to grip the margin of genital or anal valves. Gradually, the entire complex of ventral plates is pressed into the notogaster and often crushed, exposing the flesh. The mouthparts and nearly the entire head of Euconnus are gradually inserted deeper and deeper into the notogaster, if the mite is large enough. During feeding, the mandibles rapidly chew away the soft internal tissues. At this stage, the beetle exudes a small amount of digestive juice from its mouth. When the prey is too small for the beetle’s head to be inserted into the opening, only the mandibles (or even only one mandible) are inserted into the mite ’s body. The mite is rotated around the inserted mandible while copious amounts of digestive juice are exuded and then ingested.
Empty phthiracarid mite shells that were abandoned after feeding by E. pubicollis have characteristic feeding damage patterns (Fig. 12.2g). The prodorsum is either broadly open or (frequently) completely removed. The ventral plates are typically deeply pressed inside the idiosoma, often crushed and fragmented. The entire feeding process shows a strong linear correlation with the prey length; it can take from several hours to more than 30 h.
Euconnus pubicollis can also feed on some non-ptyctimous oribatid mites (but not on uropodines). Species that were successfully attacked by beetles in prey choice experiments belong to Achipteriidae, Chamobatidae, Oribatellidae, Ceratozetidae, and Galumnidae (Jałoszyński and Olszanowski 2013). Their defense systems do not rely on encapsulation, but solely on the strength of their smooth and sparsely setose cuticle. Additionally, some of them (e.g., galumnids) have pteromorphs—lateral cuticular lobes that protect their legs from being cut off by predators. These structures do not protect mites from being killed by E. pubicollis, whose technique does not involve cutting the legs prior to attacking other body parts. Non-ptyctimous oribatids are captured and lifted in a similar way as the beetles handle phthiracarids (i.e., by a droplet of sticky liquid produced from the predator’s mouth). However, further manipulations are clearly different. The mite, which adheres to the mouthparts (and often to the protibiae) of E. pubicollis, is moved, rotated, and frequently pressed against the ground or soil particles when the predator detaches its tibiae to change its grip. Euconnus tries to insert the slender and pointed tip of one mandible into the gnathosoma, the genital or anal valves. When successful, the leg movements of the prey stop, apparently marking the moment of death; the beetle rotates the mite around the inserted mandible to tear off movable structures around the opening. The time from the attack to inserting one mandible into the prey is 25–190 min. During feeding, beetles usually remove all or most of the legs of the mite. However, this is secondary damage: the legs are broken off during the last 5–20 min by the mandible, which remains outside the mite during rotations. Purposeful severing of the legs with both mandibles, as a prerequisite for attacking the body openings, was not observed.
Euconnus beetles often take short breaks during feeding on both ptyctimous and non-ptyctimous oribatids. The dead mite is placed on the ground and the predator spends a short time (up to slightly over 2 min) with self-grooming. The cleaning is restricted to the antennae and distal portions of the fore legs, which are passed through the mouthparts. Additionally, the middle legs are used to clean the elytra. Then, the beetles resume their feeding.
Thus far, all observed Scydmaeninae that feed on armored mites and have an unmodified labium feed in a similar way as Scydmaenus rufus or Euconnus pubicollis (although lifting of the prey using a droplet of sticky liquid was observed only for the latter species). Many observations were made by the author of this chapter; however, most of them remain unpublished because collecting a sufficient number of specimens for conclusive prey choice experiments is a difficult task. Acarophagous Glandulariini include one of the smallest predaceous beetles; among them are the genus Microscydmus Saulcy and Croissandeau (Fig. 12.3f), which comprise species with adults as small as 0.6–0.8 mm in body length. Such small beetles attack only oribatids with the smallest adults; however, they feed in the same way as the much larger Scydmaenus or Euconnus (i.e., through the damaged gnathosoma of non-ptyctimous mites).
A notable example of acarophagous Glandulariini showing a mixed mechanism of capturing mites is the genus Stenichnus Thomson. These middle-sized (typically 1.5–2.5 mm) Holarctic beetles have long and very slender falciform mandibles, usually with finely serrated mesal margins, and one pair of membranous suckers or adhesive discs on their prementum (Jałoszyński 2013). The ultrastructure of these organs remains unknown, but they seem to be simpler than those in specialized Cephenniini. Moreover, the labial palps in Stenichnus are large and not reduced, as those in Cephenniini. Little is known about feeding habits of this genus; most observations so far have remained unpublished. It seems that Stenichnus shows preferences toward feeding on armored and relatively large Uropodina. Jałoszyński (2016) reared an adult of the European St. godarti (Latreille) ex larva and fed it with uropodines; this single beetle ate 112 individuals of Trichouropoda sp. within 92 days of its life. Adults of Stenichnus seem to use their labial suckers only during the initial phase of capturing the prey to adhere to the mite ’s cuticle and lift the uropodine mite. Then, the long mandibles take over and further manipulations lead to their insertion into the mite’s gnathosoma. This mixed mechanism can be expected to allow for feeding on various mites. Indeed, some observations of several species of Stenichnus showed that they can feed on smooth and finely reticulated Oribatida and finely or coarsely reticulated Uropodina (Jałoszyński, unpublished data).
Schmid (1988) suggested that the mouthparts of Neuraphes Thomson are used to grasp the legs of Damaeoidea (as Belboidea) oribatids, but no further details were given. Damaeoidea include mites that were not attacked by any Scydmaeninae species tested by Jałoszyński and Olszanowski (2013, 2015, 2016); they seem to be especially well protected against predators by their morphological structures. Some species are exceptionally large and have particularly thick and hard cuticles. Some Damaeidae accumulate soil particles on their idiosoma to form an additional protecting crust, and many have very long and spiny legs. Previously unpublished observations made by the author of this chapter show that several Central European species of Neuraphes indeed feed exclusively on Damaeidae—but on juveniles, not on heavily sclerotized adults (Fig. 12.3i). Juveniles are spiny but soft-bodied; their main protection are long and spiny legs, which prevent predators from getting close to the vulnerable body. Neuraphes beetles grasp the mite ’s legs to turn their prey upside down, then attack soft ventral structures. Neither living juveniles nor their remains abandoned after feeding can be identified to the genus or species level. Thus, it is especially difficult to study prey preferences of Neuraphes. Besides the general technique they use, nothing else is known about their prey choice.
4 Problems and Perspectives
Defensive adaptations of Oribatida—and to a lesser extent, those of similarly armored mesostigmatan Uropodina—are relatively well studied. They seem so efficient that acarologists proposed the hypothesis of an “enemy-free space” where extant mite taxa live after having developed impenetrable protection during co-evolution with predatory prostigmatan and mesostigmatan mites (e.g., Jeffries and Lawton 1984; Peschel et al. 2006). Indeed, oribatids are particularly difficult prey because of their thick and mineralized cuticle, which is often reinforced by a system of grooves, carinae, or reticulation; they are also protected by long spines or accumulated soil particles, as well as the presence of defensive glands in many taxa. However, it is well-known that various oribatids can be successfully attacked and eaten by some rove beetles, as Pselaphinae and Scydmaeninae, and by some ants.
Park (1947) mentioned that Batrisodes Reitter (Pselaphinae, Batrisini) feeds on oribatids, but no further details concerning the feeding technique or mite taxa were given. Two species of Japanese ants in the genus Myrmecina Curtis (Myrmicinae, Crematogastrini) showed some behavioral and morphological adaptations to use oribatids as a major or sole source of food. The worker ants crush and tear off a large portion of the mite’s cuticle to feed larvae; the latter have elongate and narrow heads that can be easily inserted into the partly damaged mite shell to feed on the flesh (Masuko 1994). Early reports concerning the featherwing beetles (Ptiliidae) being capable of feeding on Oribatida (Riha 1951) have never been confirmed and seem dubious, as ptiliids are currently recognized as a group of fungivorous or spore-feeding beetles (e.g., Betz et al. 2003; Jałoszyński 2015). Therefore, the Scydmaeninae are currently the best studied examples of arthropod predators specialized to feed on armored mites, which are one of the best protected prey among thousands of soft-bodied invertebrates that inhabit the soil, leaf litter, rotten wood, or decomposing plant remains.
There are currently more than 5300 nominal species of Scydmaeninae known. Prey preferences and feeding-related behaviors have been studied under laboratory conditions in a few of them, including only four that feed on oribatid or uropodine mites (Jałoszyński and Olszanowski 2013, 2015, 2016). However, already in such a tiny fraction of known scydmaeninae diversity, the observed spectrum of behavioral and morphological adaptations and differences in prey preferences are astounding. Cephennium species are “hole scrapers” and use sophisticated structures of their modified, specialized mouthparts to capture subglobose and smooth oribatids. When given a choice between more than 40 species representing more than 20 families of Oribatida and Uropodina, they predominantly fed on Phthiracaridae, Ceratozetidae, and Achipteriidae; the larger of two tested species also fed on Liacaridae. The choice of prey was apparently not affected by the ptyctimous versus non-ptyctimous body form of the prey, and the entire feeding process took place through a tiny hole ground in the mite ’s cuticle (Jałoszyński and Olszanowski 2016).
Euconnus pubicollis, when given a choice between mites belonging to 50 species and representing 25 families of Oribatida and Uropodina, predominantly fed on one family only—the Phthiracaridae—showing strong preferences toward the ptyctimous body form of its prey. This species captures mites using a droplet of sticky liquid exuded from its mouth, to which the prey adheres and can be further manipulated and “opened” by a slow process in which copious amounts of digestive juices weaken the mite; mandibles are only used in the final coup de grâce (Jałoszyński and Olszanowski 2013). Scydmaenus rufus, when offered more than 20 species representing 15 families of Oribatida and Uropodina, predominantly fed on the oribatid Scheloribatidae and Oppiidae, and only marginally on the uropodine Urodinychidae and other taxa. This species also has unspecialized mouthparts, which are used to attack the mite ’s gnathosoma to feed through a large opening left after tearing off the prey’s mouthparts. If the prey mites have long and spiny legs, they are partly removed before the predator can gain access to the gnathosoma (Jałoszyński and Olszanowski 2015). Unpublished observations of the author of this chapter on several other Scydmaeninae species show an even broader spectrum of adaptations and narrower prey preferences, as those of Neuraphes , which seems to feed exclusively on juvenile Damaeidae.
It seems that gaining access to armored mites as a source of food might have been an important event in the evolution of Scydmaeninae. An unnamed species that is morphologically very similar to the extant acarophagous Cephenniini is known from the Cenomanian (Jałoszyński and Peris 2016), and a Stenichnus -like glandulariine species with a specialized prementum bearing a pair of suckers was recently discovered in Turonian amber (Jałoszyński et al. 2017). Oribatids are beyond doubt a much more ancient group than scydmaeninaes; the oldest fossils of Oribatida date to the Middle and Upper Devonian (e.g., Norton et al. 1988; Subías and Arillo 2002; reviewed by Arillo et al. 2012), whereas ant-like stone beetles are known from the Upper Cretaceous (reviewed by Jałoszyński and Peris 2016). It remains unknown how scydmaeninaes adapted to feed on armored mites or what was the food of their ancestors. Oribatids—and to a lesser extent, uropodines—are very rich food sources in terrestrial ecosystems; however, they are so well protected against predators that only few can feed on these mites. Species that are able to breach the defenses of this prey, can escape the competition that shapes relationships between numerous small soil predators, such as ants, ground beetles, spiders, pseudoscorpions, mesostigmatan mites, and others. Furthermore, various species of Scydmaeninae that co-occur in the forest floor can avoid competition by specializing to feed on particular mite taxa or mite body forms. This seems to be a major achievement for a large group of predators that live in highly competitive environments.
Have oribatid mites evolved defense mechanisms during at least 100 Ma of co-evolution with specialized predators? To date, it has not been possible to answer this question. Apparently, some of the most efficient defense mechanisms, such as the encapsulation of ptyctimous mites, are easily overcome by scydmaeninaes, and oribatids seem helpless during attacks. Even toxic secretions of their defensive glands do not protect them against scydmaeninaes; for example, Scheloribates laevigatus (Koch), readily eaten by Scydmaenus rufus, is a well-known producer of highly toxic alkaloids—among others the infamous pumiliotoxins, which are components of skin secretions of dendrobatid poisonous frogs (Saporito et al. 2007, 2011). On the other hand, Jałoszyński and Olszanowski (2016) analyzed the morphological characters of mites not eaten by any species of Scydmaeninae tested so far. They concluded that adults of oribatid taxa with particularly thick and typically densely sculptured cuticles, such as Carabodidae, Nothridae, Damaeidae, and Hermanniellidae, avoid predation by ant-like stone beetles.
It seems that Scydmaeninae may exert some pressure on the local population dynamics of their prey. Although under laboratory conditions Euconnus pubicollis consumed on average only 1 mite per 3.7 days, Scydmaenus rufus was able to consume approximately 1.4 mites per day (Jałoszyński and Olszanowski 2013, 2015). Assuming that Sc. rufus is active only during the warm season in Central Europe and feeding rates remain constant over time, then 100 beetles might consume nearly 26,000 mites from April to September (Jałoszyński and Olszanowski 2015). Sc. rufus commonly inhabits compost, from which more than 50 beetles were collected from 10 L of the substrate taken only from the upper compost layer (Jałoszyński and Olszanowski 2015). Thus, it seems possible that a population of this species contained within a typical garden compost heap may significantly affect the population dynamics of their most preferred prey—that is, scheloribatids and oppiids. Because oribatids are known to alter the chemistry and nutrient cycling in decomposing plant matter (e.g., Wickings and Grandy 2011), these processes may also be affected by their dedicated predators.
A major open research question in studies of the specialized feeding of scydmaeninaes on armored mites is the astonishingly long feeding process. It may take over 10 h to complete feeding by Cephennium beetles and more than 30 h for Euconnus (Jałoszyński and Olszanowski 2013, 2016). During this process, the mandibles of the beetle may be buried deeply in the idiosoma (or one mandible in the gnathosoma) of the mite ( Euconnus ), or the tip of one mandible may be inserted into the tiny hole drilled by Cephennium. This is not a good position to escape from larger generalist predators of soil and leaf litter, such as ants or ground beetles, which are common in this habitat. The effort and energy investment made into the slow process of penetrating the mite ’s cuticle or breaking off its mouthparts must be awarded by feeding long enough to gain energy, not to lose it. A disturbance from numerous soil invertebrates, and especially predators that could attack scydmaeninaes, is likely to disrupt the feeding before the energy balance reaches a positive value. How the beetles protect themselves while being attached to prey that is often nearly as large as themselves, and how they manage to complete their feeding undisturbed, remain major questions in the study of Scydmaeninae biology.
References
Arillo A, Subías LS, Shtanchaeva U (2012) A new species of fossil oribatid mite (Acariformers, Oribatida, Trhypochthoniidae) from the Lower Cretaceous amber of San Just (Terruel Province, Spain). Syst Appl Acarol 17(1):106–112
Betz O, Thayer M, Newton AF (2003) Comparative morphology and evolutionary pathways of the mouthparts in spore-feeding Staphylinoidea (Coleoptera). Acta Zool 84(3):179–238
Jałoszyński P (2012a) Adults of European ant-like stone beetles (Coleoptera: Staphylinidae: Scydmaeninae) Scydmaenus tarsatus Müller & Kunze and S. hellwigii (Herbst) prey on soft-bodied arthropods. Entomol Sci 15:35–41
Jałoszyński P (2012b) Observations on cannibalism and feeding on dead arthropods in Scydmaenus tarsatus Müller & Kunze. Genus 23(1):25–31
Jałoszyński P (2013) Revision of subgenera of Stenichnus Thomson, with review of Australo-Pacific species (Coleoptera, Staphylinidae, Scydmaeninae). Zootaxa 3630(1):39–79
Jałoszyński P (2015) Ptenidium pusillum (Gyllenhal, 1808) from egg to pupa (Coleoptera: Ptiliidae). Zootaxa 3948(3):361–421
Jałoszyński P (2016) Mature larva of Stenichnus godarti (Latreille) (Coleoptera: Staphylinidae, Scydmaeninae): redescription, hypothesis of displaced epicranial sutures and alternative interpretation of homology between chaetotaxic structures. Zootaxa 4196(1):77–94
Jałoszyński P, Beutel R (2012) Functional morphology and evolution of specialized mouthparts of Cephenniini (Scydmaeninae, Staphylinidae). Arthr Str Dev 41:593–607
Jałoszyński P, Kilian A (2012) Larval morphology of Scydmaenus tarsatus and S. hellwigii, with notes on feeding behavior and a review of bibliography on preimaginal stages of ant-like stone beetles (Coleoptera: Staphylinidae, Scydmaeninae). Eur J Entomol 109:587–601
Jałoszyński P, Olszanowski Z (2013) Specialized feeding of Euconnus pubicollis (Coleoptera: Staphylinidae, Scydmaeninae) on oribatid mites: prey preferences and hunting behaviour. Eur J Entomol 110:339–353
Jałoszyński P, Olszanowski Z (2015) Feeding of Scydmaenus rufus (Coleoptera: Staphylinidae, Scydmaeninae) on oribatid and uropodine mites: prey preferences and hunting behaviour. Eur J Entomol 112:151–164
Jałoszyński P, Olszanowski Z (2016) Feeding of two species of Scydmaeninae ‘hole scrapers’, Cephennium majus and C. ruthenum (Coleoptera: Staphylinidae), on oribatid mites. Eur J Entomol 113:372–386
Jałoszyński P, Peris D (2016) Cretaceous amber inclusions of Spain and Myanmar demonstrate early diversification and wide dispersal of Cephenniitae (Coleoptera: Staphylinidae: Scydmaeninae). Cret Res 57:190–198
Jałoszyński P, Perrichot V, Peris D (2017) Ninety million years of chasing mites by ant-like stone beetles. Gondwana Res 48:1–6
Jeffries MJ, Lawton JH (1984) Enemy free space and the structure of ecological communities. Biol J Linn Soc 23:269–286
Leleup N (1968) Révision des Mastigini de l’Afrique du Sud. Ann Mus Roy Afr Centr, Tervuren 166:1–107
Masuko K (1994) Specialized predation on oribatid mites by two species of the ant genus Myrmecina (Hymenoptera: Formicidae). Psyche 101:159–173
Molleman F, Walter DE (2001) Niche segregation and can-openers: Scydmaenid beetles as predators of armoured mites in Australia. In: Halliday RB, Walter DE, Proctor HC, Norton RA, Colloff MJ (eds) Acarology: Proceedings of the 10th international congress. CSIRO Publishing, Melbourne, pp 283–288
Norton RA, Bonamo PM, Grierson JD, Shear WA (1988) Oribatid mite fossils from a terrestrial deposit near Gilboa, New York. J Paleontol 62(2):259–269
O’Keefe ST, Monteith GB (2000) Clidicus abbotensis O’Keefe, a new species of Scydmaenidae (Coleoptera: Staphylinoidea) from Australia with description of the larva. Mem Queensland Mus 46:211–223
Pachl P, Domes K, Schulz G, Norton RA, Scheu S, Schaefer I, Maraun M (2012) Convergent evolution of defense mechanisms in oribatid mites (Acari, Oribatida) shows no “ghosts of predation past”. Mol Phylogenet Evol 65:412–420
Park O (1947) Observations on Batrisodes (Coleoptera: Pselaphidae), with particular reference to the American species east of the Rocky Mountains. Bull Chicago Acad Sci 8:43–132
Peschel K, Norton RA, Scheu S, Maraun M (2006) Do oribatid mites live in enemy-free space? Evidence from feeding experiments with the predatory mite Pergamasus septentrionalis. Soil Biol Biochem 38:2985–2989
Reitter E (1909) Fauna Germanica. Die Käfer des Deutschen Reiches, vol 2. Lutz KG, Stuttgart
Riha G (1951) Zur Ökologie der Oribatiden in Kalksteinböden. Zool Jb Syst 80:408–450
Saporito RA, Donnelly MA, Norton RA, Garraffo HM, Spande TF, Daly JW (2007) Oribatid mites as a major dietary source for alkaloids in poison frogs. Proc Natl Acad Sci U S A 104:8885–8890
Saporito RA, Norton RA, Andriamaharavo NR, Garraffo HM, Spande TF (2011) Alkaloids in the mite Scheloribates laevigatus: further alkaloids common to oribatid mites and poison frogs. J Chem Ecol 37:213–218
Schmelzle S, Helfen L, Norton RA, Heethoff M (2008) The ptychoid defensive mechanism in Euphthiracaroidea (Acari, Oribatida): a comparison of exoskeletal elements. Soil Org 80:233–247
Schmelzle S, Helfen L, Norton RA, Heethoff M (2009) The ptychoid defensive mechanism in Euphthiracaroidea (Acari: Oribatida): a comparison of muscular elements with functional considerations. Arthr Struct Dev 38:461–472
Schmelzle S, Helfen L, Norton RA, Heethoff M (2010) The ptychoid defensive mechanism in Phthiracarus longulus (Acari, Oribatida, Phthiracaroidea): exoskeletal and muscular elements. Soil Org 82:253–273
Schmid R (1988) Morphologische Anpassungen in einem Räuber-Beute-System: Ameisenkäfer (Scydmaenidae, Staphylinoidea) und gepanzerte Milben (Acari). Zool Jahrb, Abt Syst, Ökol Geogr Tiere 115:207–228
Schuster R (1966a) Über den Beutefang des Ameisenkäfers Cephennium austriacum Reitter. Naturwiss 53:113
Schuster R (1966b) Scydmaeniden-Larven als Milbenräuber. Naturwiss 53:439–440
Subías LS, Arillo A (2002) Oribatid fossil mites from the Upper Devonian of South Mountain, New York and the Lower Carboniferous of County Antrim, North Ireland (Acariformes, Oribatida). Estud Mus Cienc Nat Álava 17:93–106
Wickings K, Grandy AS (2011) The oribatid mite Scheloribates moestus (Acari: Oribatida) alters litter chemistry and nutrient cycling during decomposition. Soil Biol Biochem 43:351–358
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 Crown
About this chapter
Cite this chapter
Jałoszyński, P. (2018). Biology of Acarophagous Scydmaeninae. In: Betz, O., Irmler, U., Klimaszewski, J. (eds) Biology of Rove Beetles (Staphylinidae). Springer, Cham. https://doi.org/10.1007/978-3-319-70257-5_12
Download citation
DOI: https://doi.org/10.1007/978-3-319-70257-5_12
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-70255-1
Online ISBN: 978-3-319-70257-5
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)