Abstract
Accumulating evidence has suggested that the corticothalamic system not only underlies the onset of absence seizures, but also provides functional roles in controlling absence seizures. However, few studies are involved in the roles of self-connection of thalamic reticular nucleus (TRN) in modulating absence seizures. To this end, we employ a biophysically based corticothalamic network mean-field model to explore these potential control mechanisms. We find that the inhibitory projection from the TRN to specific relay nuclei of thalamus (SRN) can shape the self-connection of TRN controlling absence seizures. Under certain condition, the self-connection of TRN can bidirectionally control absence seizures, which increasing or decreasing the coupling strength of the self-connection of TRN could successfully suppress absence seizures. These findings might provide a new perspective to understand the treatment of absence epilepsy.
Access provided by CONRICYT-eBooks. Download conference paper PDF
Similar content being viewed by others
Keywords
1 Introduction
Absence seizures, recognized as the classical characteristics of absence epilepsy, abruptly start and terminate, during which bilaterally synchronous 2–4 Hz spike and wave discharges (SWDs) can be easily observed on the electroencephalogram (EEG), especially in childhood absence epilepsy patients [1, 2]. In the past two decades, a growing body of evidence has suggested that the genesis of absence seizures is closely associated with abnormal excessive neural oscillations within corticothalamic network [3,4,5]. Based on these findings, it is reasonable to speculate that appropriately tuning interactions between cerebral cortex and thalamus to abate these abnormal neural oscillations might provide a manner to suppress absence seizures.
Both the inside and outside regulations of corticothalamic network have been suggested to successfully terminate absence seizures [6,7,8,9,10,11,12,13]. For example, in terms of the inside modulation, previous animal experimental findings suggested that both thalamic stimulation and closed-loop optogenetic control of thalamus could interrupt epileptic seizures [9, 10]. Recently, a computational model study also demonstrated that the thalamic feed-forward inhibition contributes to suppressing absence seizures [13]. Unlike these inside modulations, several external nuclei of corticothalamic network, such as basal ganglia and cerebellar nuclei, also have been suggested to play crucial roles in controlling absence seizures [6,7,8, 11, 12, 14]. Particularly, computational simulations suggested that the basal ganglia not only could bidirectionally control absence seizures through the direct nigro-thalamic pathway, but also could suppress absence seizures via the pallido-cortical pathway [11, 12]. Taken together, both animal experiments and computational simulations suggest that appropriately tuning abnormal interactions within the corticothalamic network could effectively prevent absence seizures.
Anatomically, the thalamic reticular nucleus (TRN) lies like a shell between cerebral cortex and specific relay nuclei of thalamus (SRN), and entirely composes GABAergic neurons. Note that the TRN-SRN pathway has been demonstrated to contribute to modulating absence seizures by the feed-forward or feedback inhibition for SRN neurons [13, 15]. Actually, besides the two types of inhibition, the self-inhibition of TRN neurons might also participate in suppressing absence seizures. However, few studies are involved in the potential functional roles of self-connection of TRN in controlling absence seizures [15,16,17,18]. Although past animal experimental studies have suggested that loss of recurrent inhibition in TRN in \(\beta _{3}\) knockout mice might evoke absence epilepsy [18], how this recurrent inhibition participates in suppressing absence seizures is still poorly understood. Therefore, in this study, we employ a biophysically based corticothalamic network mean-field model to investigate the potential control roles played by the self-connection of TRN. We find that the self-connection of TRN could bidirectionally control absence seizures under certain condition, which might provide some new insights into the treatment of absence epilepsy.
2 Methods and Analysis
2.1 Computational Model
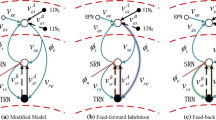
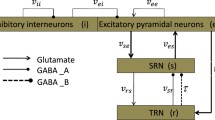
Similar to previous studies [11,12,13], we use the main epileptogenic factor that the slow kinetics of GABA\(_{\texttt {B}}\) receptors in SRN to induce absence seizures. Here, we modify the corticothalamic network by inducing the self-connection of TRN, as shown in Fig. 1. Notably, four neural populations contained in the corticothalamic network are excitatory pyramidal neurons (E, e), inhibitory interneurons (I, i), TRN (r) and SRN (s). The connections between neural populations are primarily mediated by glutamate (red arrow lines) and GABA (blue lines with round dots), which the solid and dashed blue lines represent the GABA\(_{\texttt {A}}\) and GABA\(_{\texttt {B}}\) mediated inhibitory connections, respectively. The external inputs to the SRN (\(\phi _{n}\)) show the non-specific thalamic inputs.
The framework of the corticothalamic network. In the cerebral cortex, we mainly focus on excitatory pyramidal neurons (E, e) and inhibitory interneurons (I, i). The thalamus consists of thalamic reticular nucleus (TRN, r) and specific relay nuclei (SRN, s). Notably, the red arrow lines show the excitatory connections mediated by glutamate, and the blue lines with round dots depict the inhibitory connections mediated by GABA, including GABA\(_{\texttt {A}}\) (solid blue lines) and GABA\(_{\texttt {B}}\) (the dashed blue line). External inputs to the SRN (\(\phi _{n}\)) represent the non-specific thalamic inputs (Color figure online)
To describe the neural dynamics of corticothalamic network, we employ the mean-field model proposed by Robinson and his colleagues [19,20,21], in which for a given neural population a, three main state variables that the mean membrane potential \(V_{a}\), mean firing rate \(Q_{a}\) and presynaptic activity \(\phi _{a}\) are considered. Accordingly, we build the corticothalamic network mean-field model, as shown in the Appendix.
It is worth noting that we set \(v_{se}\) = 2.4 mV s and \(\tau \) = 50 ms to evoke absence seizures in this model, which is consistent with previous studies [13]. But for more details about the model parameter values and the related physiological significances, we refer the reader to previous literature [11,12,13, 19,20,21,22].
2.2 Data Analysis
In the current study, we use the standard fourth-order Runge-Kutta method with fixed step length of 0.05 ms to solve the corticothalamic network mean-field model, and all model simulations are performed on the MATLAB software (Mathworks, Natick, MA). Here, two primary data analysis techniques adapted from previous studies have been applied to analyze cortical neural oscillations [11,12,13]. One is the bifurcation analysis that based on the stable local minimum and maximum values of cortical neural oscillations \(\phi _{e}\) in a single oscillatory period. According to the number of extreme values and amplitude of \(\phi _{e}\), we can easily determine the dynamical states of cortical oscillations. The other one is the frequency analysis. We use the Fast Fourier transform to calculate the power spectral density of \(\phi _{e}\), based on which we can identify the dominant frequency that corresponding to the maximum of power spectral density. Combining the dynamical states and dominant frequency of cortical neural oscillations \(\phi _{e}\), we can outline the boundary of SWDs in the typical range 2–4 Hz.
Dynamical states of cortical oscillations. (A) Four different dynamical states of cortical oscillations distributed in the two-dimensional parameter space (\(-v_{sr}^{A,B}\), \(-v_{rr}\)) are saturation state (I), SWD oscillation state (II), simple oscillation state (III) including slow and fast simple oscillations, and low firing state (IV). (B) The distribution of dominant frequency of cortical oscillations in the parameter space (\(-v_{sr}^{A,B}\), \(-v_{rr}\)). Note that the regions of the typical 2–4 Hz SWDs are surrounded by white dashed lines in (A) and (B). (C-G) The time series of cortical oscillations (\(\phi _{e}\)) and the corresponding parameter values are shown in (A) with different white symbols that “\(\circ \)” (I), “\(\diamond \)” (II), “\(\triangle \)” and “\(\nabla \)” (III), and “\(\Box \)” (IV).
Roles of self-connection of TRN in modulating absence seizures. (A) The dynamical state analysis of cortical oscillations \(\phi _{e}\) with \(-v_{sr}^{A,B}\) = 1.0 mV s. Gradually increasing the coupling strength of self-connection of TRN (\(-v_{rr}\)) could push cortical oscillations \(\phi _{e}\) from the SWD oscillation state (II) to simple oscillation state (III) and to saturation state (I). (B) The dominant frequency of cortical oscillations \(\phi _{e}\) that corresponding to those in (A) changes with the increase of \(-v_{rr}\). (C, D) Same as in (A) and (B), but with \(-v_{sr}^{A,B}\) = 1.46 mV s. The dynamical state analysis (C) and dominant frequency (D) of cortical oscillations \(\phi _{e}\) changes with the enhancement of \(-v_{rr}\). Notably, all gray shaded regions show the range of typical 2–4 Hz SWDs
3 Results
3.1 Dynamical States of Cortical Oscillations
In the modified corticothalamic network mean-field model, we first examine whether the model could reproduce typical absence seizures. To this end, we calculate the dynamical states and dominant frequency of cortical oscillations \(\phi _{e}\) in the two-dimensional parameter space (\(-v_{sr}^{A,B}\), \(-v_{rr}\)) (Fig. 2). We find that four dynamical states that the saturation state (I), SWD oscillation state (II), simple oscillation state (III) and low firing state (IV) are distributed in this parameter space (Fig. 2A). Correspondingly, the distribution of the dominant frequency of \(\phi _{e}\) for each dynamical state can be clearly observed (Fig. 2B). Furthermore, we outline the boundary of regions of the typical 2–4 Hz SWDs (as shown in Fig. 2A and B, the areas surrounded by white dashed lines), for which are often observed in real patients. These findings suggest that the model can replicate absence seizures, as well as other brain dynamical states. Moreover, the coupling strength of self-connection of TRN (\(-v_{rr}\)) can affect the transition between different dynamical states. Additionally, to show the firing mode of each dynamical state, we select five groups of parameters and calculate the corresponding cortical oscillations \(\phi _{e}\) (Fig. 2C–G). Interestingly, we find that the strength of \(-v_{rr}\) has a significant influence on the dominant frequency of cortical simple oscillation state (III), which relatively strong and weak \(-v_{rr}\) are corresponding to fast and slow simple oscillations, respectively (Fig. 2E and F). For this case, we speculate that strong \(-v_{rr}\) might weaken the effect of GABA\(_{\texttt {B}}\) in the TRN-SRN pathway.
3.2 The Self-connection of TRN Modulating Absence Seizures
To further uncover the roles of self-connection of TRN in controlling absence seizures, and according to the results obtained in Fig. 2A and B, we select two different values of \(-v_{sr}^{A,B}\) to observe the transition of cortical dynamical states. First, we set \(-v_{sr}^{A,B}\)=1.0 mV s. Gradually enhancing the coupling strength \(-v_{rr}\), we find that the cortical dynamical state switches from the SWD oscillation state (II) to the simple oscillation state (III), and to the saturation state (I) (Fig. 3A). Correspondingly, the dominant frequency of \(\phi _{e}\) smoothly change with the increase of \(-v_{rr}\), but significantly increase in the simple oscillation state (III) (Fig. 3B). These findings suggest that appropriately increase the coupling strength \(-v_{rr}\) can effectively interrupt absence seizures. Then, we change the value of \(-v_{sr}^{A,B}\) to 1.46 mV s. Similarly, we calculate the transition of cortical dynamical states and corresponding dominant frequency. In this case, we find that the cortical dynamical state switches from the low firing state (IV) to the slow simple oscillation state (III) at first, and then to the SWD oscillation state (II), and next to the fast simple oscillation state (III) (Fig. 3C). A sudden jump of the dominant frequency can be observed when the cortical dynamical state is in the fast simple oscillation state (III) (Fig. 3D). Obviously, these findings suggest that there exists a bidirectional control region for the coupling strength \(-v_{rr}\), in which appropriately enhancing or decreasing the coupling strength \(-v_{rr}\) can achieve the control of absence seizures, implying that \(-v_{rr}\) can bidirectionally modulate absence seizures under this condition.
4 Conclusions
In the present study, we found that the self-connection of TRN plays crucial roles in modulating absence seizures. Particularly, the coupling strength of TRN-SRN pathway that mediated by GABA\(_{\texttt {A}}\) and GABA\(_{\texttt {B}}\) can shape the self-connection of TRN suppressing absence seizures. Moreover, the coupling strength \(-v_{rr}\) has a significant effect on the dominant frequency of cortical oscillations in the simple oscillation state. Importantly, under certain condition, enhancing or decreasing the coupling strength \(-v_{rr}\) can terminate absence seizures, implying that the self-connection of TRN could bidirectionally control absence seizures. It should be noted that the corticothalamic network mean-field model makes specific predictions for the functional roles of recurrent inhibition of TRN in suppressing absence seizures that are testable in animal models of absence epilepsy. Therefore, these findings provide a new perspective to design animal experiments, as well as some novel insights into the treatment of absence epilepsy.
References
Crunelli, V., Leresche, N.: Childhood absence epilepsy genes, channels, neurons and networks. Nat. Rev. Neurosci. 3(5), 371–382 (2002)
Moshé, S.L., Perucca, E., Ryvlin, P., Tomson, T.: Epilepsy: new advances. Lancet 385(9971), 884–898 (2015)
Danober, L., Deransart, C., Depaulis, A., Vergnes, M., Marescaux, C.: Pathophysiological mechanisms of genetic absence epilepsy in the rat. Prog. Neurobiol. 55(1), 27–57 (1998)
Blumenfeld, H.: Cellular and network mechanisms of spike-wave seizures. Epilepsia 46, 21–33 (2005)
Avoli, M.: A brief history on the oscillating roles of thalamus and cortex in absence seizures. Epilepsia 53(5), 779–789 (2012)
Deransart, C., Vercueil, L., Marescaux, C., Depaulis, A.: The role of basal ganglia in the control of generalized absence seizures. Epilepsy Res. 32(1–2), 213–223 (1998)
Paz, J.T., Chavez, M., Saillet, S., Deniau, J.M., Charpier, S.: Activity of ventral medial thalamic neurons during absence seizures and modulation of cortical paroxysms by the nigrothalamic pathway. J. Neurosci. 27(4), 929–941 (2007)
Luo, C., Li, Q., Xia, Y., Lei, X., Xue, K., Yao, Z., Lai, Y., Martínez-Montes, E., Liao, W., Zhou, D., Valdes-Sosa, P.A., Gong, Q., Yao, D.: Resting state basal ganglia network in idiopathic generalized epilepsy. Hum. Brain Mapp. 33(6), 1279–1294 (2012)
Lüttjohann, A., van Luijtelaar, G.: Thalamic stimulation in absence epilepsy. Epilepsy Res. 106(1–2), 136–145 (2013)
Paz, J.T., Davidson, T.J., Frechette, E.S., Delord, B., Parada, I., Peng, K., Deisseroth, K., Huguenard, J.R.: Closed-loop optogenetic control of thalamus as a tool for interrupting seizures after cortical injury. Nat. Neurosci. 16(1), 64–70 (2013)
Chen, M., Guo, D., Wang, T., Jing, W., Xia, Y., Xu, P., Luo, C., Valdes-Sosa, P.A., Yao, D.: Bidirectional control of absence seizures by the basal ganglia: a computational evidence. PLoS Comput. Biol. 10(3), 1–17 (2014)
Chen, M., Guo, D., Li, M., Ma, T., Wu, S., Ma, J., Cui, Y., Xia, Y., Xu, P., Yao, D.: Critical roles of the direct gabaergic pallido-cortical pathway in controlling absence seizures. PLoS Comput. Biol. 11(10), 1–23 (2015)
Chen, M., Guo, D., Xia, Y., Yao, D.: Control of absence seizures by the thalamic feed-forward inhibition. Front. Comput. Neurosci. 11, 31 (2017)
Kros, L., Eelkman Rooda, O.H.J., Spanke, J.K., Alva, P., van Dongen, M.N., Karapatis, A., Tolner, E.A., Strydis, C., Davey, N., Winkelman, B.H.J., Negrello, M., Serdijn, W.A., Steuber, V., van den Maagdenberg, A.M.J.M., De Zeeuw, C.I., Hoebeek, F.E.: Cerebellar output controls generalized spike-and-wave discharge occurrence. Ann. Neurol. 77(6), 1027–1049 (2015)
Paz, J.T., Huguenard, J.R.: Microcircuits and their interactions in epilepsy: is the focus out of focus? Nat. Neurosci. 18(3), 351–359 (2015)
Huguenard, J.R., Prince, D.A.: Clonazepam suppresses gabab-mediated inhibition in thalamic relay neurons through effects in nucleus reticularis. J. Neurophysiol. 71(6), 2576–2581 (1994)
Kim, U., Sanchez-Vives, M.V., McCormick, D.A.: Functional dynamics of gabaergic inhibition in the thalamus. Science 278(5335), 130–134 (1997)
Huntsman, M.M., Porcello, D.M., Homanics, G.E., DeLorey, T.M., Huguenard, J.R.: Reciprocal inhibitory connections and network synchrony in the mammalian thalamus. Science 283(5401), 541–543 (1999)
Robinson, P.A., Rennie, C.J., Wright, J.J.: Propagation and stability of waves of electrical activity in the cerebral cortex. Phys. Rev. E 56, 826–840 (1997)
Robinson, P.A., Rennie, C.J., Rowe, D.L.: Dynamics of large-scale brain activity in normal arousal states and epileptic seizures. Phys. Rev. E 65, 041924 (2002)
Breakspear, M., Roberts, J.A., Terry, J.R., Rodrigues, S., Mahant, N., Robinson, P.A.: A unifying explanation of primary generalized seizures through nonlinear brain modeling and bifurcation analysis. Cereb. Cortex 16(9), 1296–1313 (2006)
Marten, F., Rodrigues, S., Benjamin, O., Richardson, M.P., Terry, J.R.: Onset of polyspike complexes in a mean-field model of human electroencephalography and its application to absence epilepsy. Philos. Trans. R. Soc. Lond. A: Math. Phys. Eng. Sci. 367(1891), 1145–1161 (2009)
Acknowledgments
This study was supported by the National Natural Science Foundation of China (Nos. 81571770, 61527815, 81401484 and 81330032).
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Appendix: The Corticothalamic Network Mean-Field Model
Appendix: The Corticothalamic Network Mean-Field Model
To obtain the corticothalamic network mean-field model, we assume that absence seizures are involved in the whole brain, so that the spatial effect is ignored in this model. Also, based on the assumption that intracortical connections are proportional to the synapses involved, we have \(V_{i}=V_{e}\) and \(Q_{i}=Q_{e}\). Note that both of these assumptions are in line with previous studies [11,12,13, 20, 21]. Accordingly, the corticothalamic network mean-field equations are given in the following:
Here, \(\phi _{a}\) (\(a={e, i, r, s}\)) shows the propagating axonal fields of the neural population a, \(v_{ab}\) describes the coupling strength from the neural population b to the neural population a. The inverses of \(\alpha \) and \(\beta \) represent the decay and rise time constants, respectively. \(\gamma _{e}\) governs the temporal damping rate of cortical excitatory pulses. \(V_{a}\) denotes the mean membrane potential, through which the mean firing rate \(Q_{a}\) can be obtained with the sigmoid function \(F[{V}_{a}(t)]\) [11, 12, 20] given by
where \(Q_{a}^{max}\) denotes the maximum firing rate, \(\theta _{a}\) shows the mean firing threshold, and \(\sigma \) is the standard deviation of the mean firing threshold.
Rights and permissions
Copyright information
© 2017 Springer International Publishing AG
About this paper
Cite this paper
Guo, D., Chen, M., Xia, Y., Yao, D. (2017). Self-connection of Thalamic Reticular Nucleus Modulating Absence Seizures. In: Liu, D., Xie, S., Li, Y., Zhao, D., El-Alfy, ES. (eds) Neural Information Processing. ICONIP 2017. Lecture Notes in Computer Science(), vol 10637. Springer, Cham. https://doi.org/10.1007/978-3-319-70093-9_65
Download citation
DOI: https://doi.org/10.1007/978-3-319-70093-9_65
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-70092-2
Online ISBN: 978-3-319-70093-9
eBook Packages: Computer ScienceComputer Science (R0)