Abstract
Intercellular communication is a fundamental process necessary to maintain homeostasis and to mount an orchestrated response to stress. Although heat shock proteins (HSP) play a critical role by participating in the repair of damaged products as a result of the stress in the intracellular milieu, it is now evident that they play an alternative role when they escape from the cells and are placed in circulation, participating in a systemic stress response. Extracellular HSP appear as signaling molecules involved in intercellular communication during stress conditions. They have been found to modulate the function of many target cells. Moreover, extracellular HSP have been detected in several biological fluids, particularly from patients suffering from a large number of maladies. Extracellular HSP are released by many cell types and by several mechanisms, including passive dissemination after necrosis and active export by a nonclassical secretory pathway. Among several potential mechanisms for the export of HSP, their release associated with extracellular vesicles has gained increasing support. The appearance of extracellular vesicles containing HSP emerges as a new form of cellular communication during stress conditions directed at avoiding the propagation of the insult.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Cellular communication is a major physiological event that is crucial to maintain homeostasis. In this regard, unicellular organisms use chemical gradients to synchronize their metabolism and growth. Plants release volatile compounds as signals to coordinate development, attract pollinizing insects, and repel predators. Multicellular organisms send signals to their counterpart cells to regulate metabolism, growth, and stress response. Intercellular communication is particularly important in tissue homeostasis, in which a synchronized propagation of signals among cells is required to adapt to changes in nutrients and other environmental factors. For example, hepatocytes within the hepatic acinus are interconnected by various mechanisms of communication to modulate their response to changes in the delivery of nutrients, such as glucose and oxygen. Cellular communication is also critical for an efficient response of the immune system. Thus, the communication between T, B, and antigen-presenting cells is necessary to orchestrate the adaptive immune response. Similarly, macrophages, dendritic cells, and neutrophils secrete cytokines and chemokines in response to infection and injury to promote an initial response to the insult. Therefore, a coordinated intercellular communication is vital to preserve normal physiological conditions and mount a sound response to stress.
2 Types of Cellular Communication
Cells communicate by a variety of mechanisms. The most common is via soluble molecules that are placed in the extracellular environment or in circulation directed at interacting with adjacent or distant cells. A typical example of this type of communication is when hormones and cytokines are released by a certain type of cell and captured by another via specific receptors. The ligand-receptor interaction triggers a signal transduction pathway within the plasma membrane or within internal compartments directed at activating the right response to the external stimuli. In other cases, cells communicate via surface contact molecules, such as adhesion proteins, resulting in cellular synapses [1]. A great example is the immune synapse between antigen presenting cells and lymphocytes [2]. Cells in close proximity can also interact via exchanging surface molecules by the direct transfer of plasma membrane portions, which is known as trogocytosis, by membrane tethers, or by nanotubes [3]. A very important form of cellular communication is via the transfer of low-molecular-weight metabolites via gap junctions. Gap junctions are larger channels or pores formed by similar proteins within the plasma membrane of adjacent cells that allow the passage of ions (e.g., calcium), nucleotides (e.g., ATP), and other small molecules in a regulated process, creating a network of signals across the multicellular environment [4].
An alternative mechanism for cellular communication is mediated by the release of membrane vesicles into the extracellular medium. These extracellular vesicles (ECV) contain surface molecules, lipids, and cargo (e.g., proteins, nucleic acids, carbohydrates, and ions). The critical aspect of ECV is that they contain a large number of molecules within a very small volume [5,6,7]. These ECV are captured by target cells delivering the cargo, such as signaling proteins or microRNAs , which can modulate the function of the receiving cell. More importantly, the target cell senses a multiplicity of different molecules simultaneously, which is likely to result in a synergy of information. In other words, different components within ECV could concurrently activate various cellular pathways. Moreover, the concentration of a ligand within a small vesicle (e.g., 100 nm in diameter) is theoretically calculated in the millimolar range, which is much larger than the circulating concentration of any hormone. ECV could also travel long distances, delivering information to very distant cells. The fact that ECV are formed by membrane-encapsulated macromolecules assures the protection of the cargo from external environmental factors, such as circulating proteases and RNAses. The final stage for communication via ECV requires the recognition by the target cell that it could be mediated by various mechanisms. For example, ECV may contain surface molecules that are recognized by specific receptors on target cells acting as “zip codes .” In addition, ECV could be taken by cells in a non-receptor-mediated process, such as macropinocytosis, or they could fuse with the plasma membrane delivering the cargo into the cytosol.
3 Extracellular HSP as Communication Signals
Heat shock proteins (HSP) were first discovered as part of the cellular response to elevated temperatures, initiated by the discovery of the heat shock response by Ritossa [8], followed by the identification of HSP by Tissières et al. [9]. Subsequent studies showed that HSP correspond to a large family of proteins expressed after a variety of stress conditions [10, 11]. Various homologs to the stress-inducible HSP were identified afterward participating in normal basic cellular processes, including folding of newly synthesized polypeptides, translocation of polypeptides across subcellular compartments, assembly of macromolecule complexes, stabilization of receptors, and signal transduction [11, 12]. The capability of folding denatured proteins or stabilizing protein complexes gave rise to their denomination as molecular chaperones [13]. Various HSP belong to particular families that are classified according to their molecular weight, for example the Hsp70 family , which has a molecular weight of 70 kDa, is composed of four members: Hsp70 (the stress inducible form), Hsc70 (the constitutive cytosolic form), Mit70 and Grp78 (both constitutive forms located in the mitochondria and endoplasmic reticulum, respectively). Recently, a new nomenclature for HSP has been proposed [14], displayed in Table 3.1.
Although the most recognized function of HSP is as molecular chaperones in the cytosol and other subcellular compartments, they have been found outside cells. The first observations regarding the presence of HSP in the extracellular environment was made on Hsp70 by studies of Tytell et al. [15] and Hightower and Guidon [16]. These pioneer findings were followed by more recent observations documenting the presence of Hsp70 in the extracellular medium in a variety of conditions (reviewed by De Maio) [6]. Today, practically all members of the HSP family have been detected outside cells. Thus, Hsp90α (HSPC3) was identified as a secreted oxidative stress-induced factor by vascular smooth cells [17]. Hsp90β (HSPC4) was reported released by osteosarcoma cells [18]. Grp75 (HSPA9 ) or mortalin , which is a mitochondrial chaperone protein, has been shown to be released after complement treatment of cells [19]. Grp78 (HSPA5) and Grp94 (HSPC4 ), which are endoplasmic reticulum (ER) residents, have been found in the extracellular space [20,21,22]. HSP60 (HSPD1) has been detected in circulation of patients suffering from various conditions [23]. Hsp25/27 (HspB1) was observed in the extracellular environment of astrocytes [24]. Finally, a large member of the HSP family, Grp170 (HSPH4), has also been detected outside cells [25].
Extracellular HSP are secreted by a variety of cell types and captured by others. The function of extracellular HSP has not been associated to their chaperone activity, which is not surprising since it requires cochaperones and nucleotides for the function. On the contrary, extracellular HSP act as signaling molecules involved in the communication between cells, inducing an array of activities. For example, Hsp70 (HSPA1) secreted by parenchymal cells has been shown to induce a robust activation of macrophages [26,27,28], dendritic cells [29], and natural killer cells [30, 31]. Extracellular Hsp70 has also been shown to modulate the response of monocytes to endotoxin [32, 33], activate chemotaxis [34], and phagocytosis [35,36,37]. They also could modulate antigen presentation [36]. Mycobacterium tuberculosis-derived DnaK has been shown to polarize macrophages to M2-like phenotype [38] and induce anti-inflammatory response [39]. Recently, Hsp70, Hsp90 (HSPC), and Hsp40 (DNAJB1) have been proposed to promote protein homeostasis in distant cells [40]. Moreover, extracellular Hsp70 has been associated with both immunostimulatory and immunosuppressive activities [41]. Extracellular Hsp90 has been shown to transport antigens from the outside to the cytosol, resulting in cross-presentation [42]. Small HSP are also secreted by cells and modulate the immune system [43]. Hsp90α was detected outside cells participating in wound reepithelialization and healing [37, 44, 45]. Extracellular Hsp70 has been shown to affect cardiomyocyte contractile dysfunction [46], and increase tumor growth and resistance to apoptosis [46]. Exogenous Hsp70 appeared to disrupt gap junction communication between human microvascular endothelial cells [47].
Extracellular HSP may be recognized by a variety of cell surface targets [48]. The list of potential receptors for extracellular HSP is large, including CD91 [49, 50], CD40 [13, 51], Scavenger receptor A [52], Lox 1 [53], mannose receptor [54], and even the β-subunit of ATP synthase [55]. Recently, Hsp70 was shown to bind to Siglec-5 and Siglec-14, which are Ig-superfamily lectins on mammalian leukocytes that recognize sialic acid-bearing glycans [56]. Some lipids have also been proposed as targets for HSP, such as sphingolipids [57, 58], phosphatidylserine [59, 60] and phospholipid bis(monoacylglycero)phosphate [61]. In general, it appears that HSP have a large appetite for molecules, raising the possibility that a single receptor model may not be correct.
4 Extracellular HSP in Pathological Conditions
Extracellular HSP has been associated with several clinical conditions , following their detection in various biological fluids (Table 3.2). In addition, antibodies against HSP have been found in the serum of a variety of patients [23, 62]. For example, circulating levels of Hsp70 and Hsp60 or their antibodies have been proposed as a risk factor for coronary heart disease [63,64,65]. Similarly, Hsp60 has been detected in circulation of individuals suffering from cardiovascular diseases [66, 67]. Extracellular Hsp25 has been shown to reduce cardiotoxicity induced by doxorubicin [68]. Hsp70 has been reported to be present in the serum of patients with chronic hepatitis, liver cirrhosis, and hepatocellular carcinoma [69]. Hsp27 has been detected in the serum of patients with chronic pancreatitis and pancreatic carcinoma [70, 71]. Hsp60 has also been observed in the saliva and serum of patients with type 2 diabetes mellitus [72] and Hsp70 in patients presenting diabetic ketoacidosis [73].
Extracellular HSP have been associated with infection and other pathologies . Thus, extracellular Hsp70 has been identified following acute infection in humans [86]. Hsp70 and Hsp60 were found in wound fluids at the site of soft tissue injury [83]. Moreover, the presence of Hsp70 in circulation has also been linked with improved survival of critically ill patients [80,81,82]. In other studies, Hsp70 was found to be released from human fetal membranes after exposure to E. coli [87]. Hsp70 has also been detected in normal and pathological pregnancies , including preeclampsia [88, 89]. Moreover, Hsp70 has been observed in amniotic fluid [90]. Circulating Hsp70 has been detected after extenuating exercise [91,92,93]. Finally, extracellular Hsp70 was present in the blood of experimental rodent models of diabetes [94], sepsis [95], and ischemia–reperfusion injury [85].
The central nervous system has also been a target for extracellular HSP activity. For example, extracellular Hsp70 has been observed after brain and spinal cord ischemia [84]. Several small HSP, including Hsp20 and Hsp22, have been detected in closed proximity of amyloid β deposits in the brains of Alzheimer disease patients [96]. Moreover, they were found to block amyloid β aggregation in vitro [96, 97]. Similar observations regarding inhibition of amyloid β aggregation have been made for Hsp70 and Hsp90 [98] and Hsp40 [99]. Moreover, HspB1 (Hsp25/27) was reported released by astrocytes in response to amyloid β exposure [24]. Extracellular Hsp70 has been shown to protect Schwann cells [100] and neurons [101]. Both αA-crystallin (HSPB4) and αB-crystallin (HSPB5) have been reported to protect astrocytes from various toxic agents [102].
5 Mechanisms of HSP Export
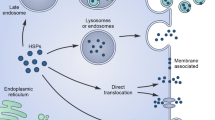
Extracellular HSP are released from at least two different sources. HSP are disseminated by a passive process secondary to cell lysis after necrosis [29, 85] or exported by an active mechanism independent of cell death, which could not be blocked by typical inhibitors of the ER-Golgi pathway, such as brefeldin A [16, 79]. The only exceptions are ER-resident HSP, Grp78 and Grp94, which are already in place within the classical secretory pathway. In contrast, the majority of HSP lack the consensus signal required for secretion via the ER-Golgi pathway. Therefore, they are likely exported by an alternative mechanism that has been named the nonclassical or unconventional secretory pathway. Many proteins besides HSP use this pathway for export, including interleukin-1β, high-mobility group box 1, galectin 1 and 3, and fibroblast growth factor 1 [103].
The major argument against the active export of HSP is that these proteins are localized in the cytosol. In order to reach the extracellular environment, they need to cross the plasma membrane. Thermodynamically, the passing of a protein across a lipid membrane results in a less favorable change of free energy [104]. Therefore, it should not be a spontaneous process. In spite of the prior assumption, there is substantial evidence that Hsp70s can spontaneously get inserted into lipid bilayers. Indeed, our pioneering work showed that Hsc70 got inserted into artificial lipid bilayers opening ion conductance pathways [105]. This observation has been confirmed by others [106] and extended to Hsp70 [28]. Additional studies showed that both Hsp70 and Hsc70 interact with liposomes resulting in their aggregation in a process that was concentration dependent and requiring the presence of nucleotides [4]. Moreover, Hsp70 have been demonstrated to get spontaneously and selectively inserted into phosphatidylserine (PS) liposomes, forming high molecular mass oligomers [107]. The interaction of Hsp70 with PS liposomes has been confirmed by others [108]. Similarly bacterial Hsp70 (DnaK) also gets inserted into liposomes, but the translocation was not lipid specific and only forms dimeric forms within the membrane [109]. These observations suggest that HSP, at least Hsp70, could move spontaneously from the cytosol into the plasma membrane. Indeed, Hsp70 has been extensively reported to be present in the plasma membrane of transformed cells [110, 111]. The presence of Hsp70 on the plasma membrane was resistant to acid washes indicating that it was actually inserted into the lipid bilayer [28, 57]. Therefore, the question that emerges is whether Hsp70 could also spontaneously come out from the lipid membrane outside the cells. Although this option has not been demonstrated experimentally, it may be an interesting possibility to explain the extracellular release of this protein. Other mechanisms that have been proposed for the active secretion of HSP include the lysosome–endosome pathway, in which the protein translocates into the lysosome lumen via ATP-binding cassette (ABC) transport-like system and is further released outside cells via the endocytic process [112]. This pathway has also been proposed for the secretion of IL-1β, which also moves from the cytosol to outside the cell without passing through the ER-Golgi pathway [113]. Other studies have suggested the release of Hsp70 via secretory-like granules [114].
A well-accepted mechanism for the export of HSP is their association with ECV [6]. These vesicles are derived from the plasma membrane by various processes. Protuberances or blebs can be formed in the outer side of the plasma membrane by a process of ectocytosis, resulting in the release of large vesicles known as microvesicles (>1 μm) particles or smaller vesicles named ectosomes (about 100 nm). Alternatively, ECV could be produced by endocytosis of the plasma membrane-forming endosomes. The membrane of these endosomes invaginates toward the lumen resulting in the formation of new vesicles included within a large vesicle that has been named multiple vesicular bodies X. The vesicles inside the multiple vesicular bodies have the same topology of the plasma membrane. When these multiple vesicular bodies fuse with the plasma membrane the vesicle content within the lumen are released outside the cell. ECV derived from this process are known as exosomes [5, 6]. There is extensive evidence that HSP are present within different ECV that is summarized in Table 3.3. The detection of HSP within ECV has primarily been made by mass spectroscopic analysis and, in some cases, confirmed by Western blotting. Since HSP are mainly present in the cytosol, their localization within ECV was assumed to be in the lumen as a result of trapping these proteins during the formation of the vesicles. However, it has been proposed that the composition of ECV is not random but rather a very selective process [6, 115]. Other observations have shown that HSP are located within the membranes of ECV, as in the case of Hsp70 [28, 31, 116], Hsp90 [117], and Hsp60 [118, 119]. The presence of HSP on the membrane (surface) of ECV is important because it may explain a specific interaction with target cells, most likely by a process mediated by surface receptors. On the contrary, the potential biological role of HSP within the lumen of ECV is less evident, which should require the fusion of the vesicles with the plasma membrane or by the burst of ECV liberating the cargo within the extracellular milieu. The presence of HSP on the membrane of ECV has led us to postulate that insertion into the lipid bilayer is the first step in the secretion of this protein [6]. Additional observations have shown that the export of Hsp70 and Hsc70 within ECV was blocked by the reduction of membrane cholesterol levels [79, 120]. Indeed, Hsp70 within ECV was resistant to nonionic detergents, such as Triton X-100, suggesting that the protein is within lipid rafts in the vesicles [28]. In this regard, several studies have shown that Hsp70 is present within lipid rafts of cells [28, 79, 121, 122].
Although the evidence for the active secretion of HSP from living cells is well established, it cannot be ignored that, under other circumstances, HSP are released into the extracellular medium after cell necrosis. Indeed, the concentration of HSP70 released after necrosis could be potentially very high [29]. In this regard, expression of HSP70 has also been observed after ischemia–reperfusion (I/R) injury, which resulted in a necrotic focus [85].
6 The Stress Observation System
There is clear evidence that cells secrete ECV during normal physiological conditions as well as after stress. We have argued that the composition of ECV reflects the physiological stage of the cell [6]. Thus, constitutive proteins are present in ECV derived from cells under normal conditions, such as CD9 and CD63, which belong to the family of tetraspanin proteins [132]. During stress conditions , ECV should reflect the insult type, such as the presence of stress-inducible HSP. Then, ECV are recognized by other cell types, in particular cells of the immune system, as part of an assessment of the stress conditions. If there is not stress, it is unlikely that there is a response. However, ECV during stress conditions are likely to activate the immune system to prepare a preemptive response directed at avoiding the propagation of the insult (Fig. 3.1). The process of sensing stress via ECV has been termed “Stress Observation System” [6]. Thus, ECV derived from macrophages infected with intracellular pathogens were observed to activate uninfected macrophages by a Toll-like receptor and myeloid differentiation factor 88 dependent mechanism [133]. They also induced polymorphonuclear leukocyte recruitment in lungs after intranasal delivery [133]. Moreover, ECV containing Hsp70 isolated from mycobacteria-infected cells induced an inflammatory response in macrophages [134]. ECV containing Hsp70 on their surface displayed a robust and specific activation of macrophages, which was higher than the same concentration of recombinant Hsp70 in solution [28]. Finally, Hsp70-positive ECV were also found to stimulate the cytotoxic capacity of NK cells [31].
During normal physiological conditions, cells release ECV containing markers for cellular homeostasis that when captured by immune cells do not trigger any response. However, the composition of these ECV changes after stress, resulting in a signal for the immune system to mount an appropriate response directed at mitigating the insult.
7 Conclusions
HSP appear to display a different role in the extracellular environment than the well-characterized function as molecular chaperones. Extracellular HSP emerge as new signaling molecules involved in intracellular communication. The presence of extracellular HSP has been detected in biological fluids from individuals suffering from a large number of illnesses. Therefore, they are likely to become biomarkers of various disease conditions. Extracellular HSP are released by many cell types and by at least two main mechanisms, including the passive dissemination after necrosis or an active export process independent on cell death, named the nonclassical secretory pathway. Extracellular HSP come in various flavors. Thus, they can be found in a soluble form within biological fluids, trapped in the lumen of ECV or exposed to the surface of these vesicles in a membrane-bound fashion. Finally, HSP associated with ECV appear to be part of a mechanism directed at both alerting the immune system to the presence of an insult and avoiding the propagation of stress.
References
Ahmed KA, Xiang J (2011) Mechanisms of cellular communication through intercellular protein transfer. J Cell Mol Med 15:1458–1473
Grakoui A, Bromley SK, Sumen C, Davis MM, Shaw AS, Allen PM, Dustin ML (1999) The immunological synapse: a molecular machine controlling T cell activation. Science 285:221–227
Davis DM (2007) Intercellular transfer of cell-surface proteins is common and can affect many stages of an immune response. Nat Rev Immunol 7:238–243
Arispe N, Doh M, De Maio A (2002) Lipid interaction differentiates the constitutive and stress-induced heat shock proteins Hsc70 and Hsp70. Cell Stress Chaperones 7:330–338
Thery C, Ostrowski M, Segura E (2009) Membrane vesicles as conveyors of immune responses. Nat Rev Immunol 9:581–593
De Maio A (2011) Extracellular heat shock proteins, cellular export vesicles, and the stress observation system: a form of communication during injury, infection, and cell damage. It is never known how far a controversial finding will go! Dedicated to Ferruccio Ritossa. Cell Stress Chaperones 16:235–249
Mulcahy LA, Pink RC, Carter DR (2014) Routes and mechanisms of extracellular vesicle uptake. J Extracell Vesicles 3. https://doi.org/10.3402/jev.v3.24641
Ritossa FM (1962) A new puffing pattern induced by temperature shock and DNP in drosophila. Cell Mol Life Sci 18:571–573
Tissieres A, Mitchell HK, Tracy UM (1974) Protein synthesis in salivary glands of Drosophila Melanogaster: relation to chromosome puffs. J Mol Biol 84:389–398
De Maio A (1999) Heat shock proteins: facts, thoughts, and dreams. Shock 11:1–12
Hartl FU, Hayer-Hartl M (2009) Converging concepts of protein folding in vitro and in vivo. Nat Struct Mol Biol 16:574–581
Lindquist S, Craig EA (1988) The heat-shock proteins. Annu Rev Genet 22:631–677
Becker T, Hartl FU, Wieland F (2002) CD40, an extracellular receptor for binding and uptake of Hsp70-peptide complexes. J Cell Biol 158:1277–1285
Kampinga HH, Hageman J, Vos MJ, Kubota H, Tanguay RM, Bruford EA, Cheetham ME, Chen B, Hightower LE (2009) Guidelines for the nomenclature of the human heat shock proteins. Cell Stress Chaperones 14:105–111
Tytell M, Greenberg SG, Lasek RJ (1986) Heat shock-like protein is transferred from glia to axon. Brain Res 363:161–164
Hightower LE, Guidon PT Jr (1989) Selective release from cultured mammalian cells of heat-shock (stress) proteins that resemble glia-axon transfer proteins. J Cell Physiol 138:257–266
Liao DF, Jin ZG, Baas AS, Daum G, Gygi SP, Aebersold R, Berk BC (2000) Purification and identification of secreted oxidative stress-induced factors from vascular smooth muscle cells. J Biol Chem 275:189–196
Suzuki S, Kulkarni AB (2010) Extracellular heat shock protein HSP90beta secreted by MG63 osteosarcoma cells inhibits activation of latent TGF-beta1. Biochem Biophys Res Commun 398:525–531
Pilzer D, Fishelson Z (2005) Mortalin/GRP75 promotes release of membrane vesicles from immune attacked cells and protection from complement-mediated lysis. Int Immunol 17:1239–1248
Delpino A, Castelli M (2002) The 78 kDa glucose-regulated protein (GRP78/BIP) is expressed on the cell membrane, is released into cell culture medium and is also present in human peripheral circulation. Biosci Rep 22:407–420
Kern J, Untergasser G, Zenzmaier C, Sarg B, Gastl G, Gunsilius E, Steurer M (2009) GRP-78 secreted by tumor cells blocks the antiangiogenic activity of bortezomib. Blood 114:3960–3967
Evdokimovskaya Y, Skarga Y, Vrublevskaya V, Morenkov O (2012) Release of the glucose-regulated protein 94 by baby hamster kidney cells. Cell Biochem Funct 30:558–562
Henderson B, Pockley AG (2012) Proteotoxic stress and circulating cell stress proteins in the cardiovascular diseases. Cell Stress Chaperones 17:303–311
Nafar F, Williams JB, Mearow KM (2016) Astrocytes release HspB1 in response to amyloid-beta exposure in vitro. J Alzheimers Dis 49:251–263
Zuo D, Yu X, Guo C, Yi H, Chen X, Conrad DH, Guo TL, Chen Z, Fisher PB, Subjeck JR, Wang XY (2012) Molecular chaperoning by glucose-regulated protein 170 in the extracellular milieu promotes macrophage-mediated pathogen sensing and innate immunity. FASEB J 26:1493–1505
Asea A, Kraeft SK, Kurt-Jones EA, Stevenson MA, Chen LB, Finberg RW, Koo GC, Calderwood SK (2000) HSP70 stimulates cytokine production through a CD14-dependant pathway, demonstrating its dual role as a chaperone and cytokine. Nat Med 6:435–442
Vabulas RM, Ahmad-Nejad P, Ghose S, Kirschning CJ, Issels RD, Wagner H (2002) HSP70 as endogenous stimulus of the toll/interleukin-1 receptor signal pathway. J Biol Chem 277:15107–15112
Vega VL, Rodriguez-Silva M, Frey T, Gehrmann M, Diaz JC, Steinem C, Multhoff G, Arispe N, De Maio A (2008) Hsp70 translocates into the plasma membrane after stress and is released into the extracellular environment in a membrane-associated form that activates macrophages. J Immunol 180:4299–4307
Basu S, Binder RJ, Suto R, Anderson KM, Srivastava PK (2000) Necrotic but not apoptotic cell death releases heat shock proteins, which deliver a partial maturation signal to dendritic cells and activate the NF-kappa B pathway. Int Immunol 12:1539–1546
Gastpar R, Gross C, Rossbacher L, Ellwart J, Riegger J, Multhoff G (2004) The cell surface-localized heat shock protein 70 epitope TKD induces migration and cytolytic activity selectively in human NK cells. J Immunol 172:972–980
Gastpar R, Gehrmann M, Bausero MA, Asea A, Gross C, Schroeder JA, Multhoff G (2005) Heat shock protein 70 surface-positive tumor exosomes stimulate migratory and cytolytic activity of natural killer cells. Cancer Res 65:5238–5247
Aneja R, Odoms K, Dunsmore K, Shanley TP, Wong HR (2006) Extracellular heat shock protein-70 induces endotoxin tolerance in THP-1 cells. J Immunol 177:7184–7192
Abboud PA, Lahni PM, Page K, Giuliano JS Jr, Harmon K, Dunsmore KE, Wong HR, Wheeler DS (2008) The role of endogenously produced extracellular hsp72 in mononuclear cell reprogramming. Shock 30:285–292
Ortega E, Hinchado MD, Martin-Cordero L, Asea A (2009) The effect of stress-inducible extracellular Hsp72 on human neutrophil chemotaxis: a role during acute intense exercise. Stress 12:240–249
Ortega E, Giraldo E, Hinchado MD, Martinez M, Ibanez S, Cidoncha A, Collazos ME, Garcia JJ (2006) Role of Hsp72 and norepinephrine in the moderate exercise-induced stimulation of neutrophils’ microbicide capacity. Eur J Appl Physiol 98:250–255
Wang R, Kovalchin JT, Muhlenkamp P, Chandawarkar RY (2006) Exogenous heat shock protein 70 binds macrophage lipid raft microdomain and stimulates phagocytosis, processing, and MHC-II presentation of antigens. Blood 107:1636–1642
Kovalchin JT, Wang R, Wagh MS, Azoulay J, Sanders M, Chandawarkar RY (2006) In vivo delivery of heat shock protein 70 accelerates wound healing by up-regulating macrophage-mediated phagocytosis. Wound Repair Regen 14:129–137
Lopes RL, Borges TJ, Araujo JF, Pinho NG, Bergamin LS, Battastini AM, Muraro SP, Souza AP, Zanin RF, Bonorino C (2014) Extracellular mycobacterial DnaK polarizes macrophages to the M2-like phenotype. PLoS One 9:e113441
Borges TJ, Lopes RL, Pinho NG, Machado FD, Souza AP, Bonorino C (2013) Extracellular Hsp70 inhibits pro-inflammatory cytokine production by IL-10 driven down-regulation of C/EBPbeta and C/EBPdelta. Int J Hyperth 29:455–463
Takeuchi T, Suzuki M, Fujikake N, Popiel HA, Kikuchi H, Futaki S, Wada K, Nagai Y (2015) Intercellular chaperone transmission via exosomes contributes to maintenance of protein homeostasis at the organismal level. Proc Natl Acad Sci U S A 112:E2497–E2506
Pockley AG, Muthana M, Calderwood SK (2008) The dual immunoregulatory roles of stress proteins. Trends Biochem Sci 33:71–79
Oura J, Tamura Y, Kamiguchi K, Kutomi G, Sahara H, Torigoe T, Himi T, Sato N (2011) Extracellular heat shock protein 90 plays a role in translocating chaperoned antigen from endosome to proteasome for generating antigenic peptide to be cross-presented by dendritic cells. Int Immunol 23:223–237
van Noort JM, Bsibsi M, Nacken P, Gerritsen WH, Amor S (2012) The link between small heat shock proteins and the immune system. Int J Biochem Cell Biol 44:1670–1679
Li W, Li Y, Guan S, Fan J, Cheng CF, Bright AM, Chinn C, Chen M, Woodley DT (2007) Extracellular heat shock protein-90alpha: linking hypoxia to skin cell motility and wound healing. EMBO J 26:1221–1233
Bhatia A, O'Brien K, Chen M, Woodley DT, Li W (2016) Keratinocyte-secreted heat shock protein-90alpha: leading wound reepithelialization and closure. Adv Wound Care (New Rochelle) 5:176–184
Mathur S, Walley KR, Wang Y, Indrambarya T, Boyd JH (2011) Extracellular heat shock protein 70 induces cardiomyocyte inflammation and contractile dysfunction via TLR2. Circ J 75:2445–2452
Thuringer D, Berthenet K, Cronier L, Jego G, Solary E, Garrido C (2015) Oncogenic extracellular HSP70 disrupts the gap-junctional coupling between capillary cells. Oncotarget 6:10267–10283
Calderwood SK, Gong J, Murshid A (2016) Extracellular HSPs: the complicated roles of extracellular HSPs in immunity. Front Immunol 7:159
Binder RJ, Han DK, Srivastava PK (2000) CD91: a receptor for heat shock protein gp96. Nat Immunol 1:151–155
Basu S, Binder RJ, Ramalingam T, Srivastava PK (2001) CD91 is a common receptor for heat shock proteins gp96, hsp90, hsp70, and calreticulin. Immunity 14:303–313
Wang Y, Kelly CG, Karttunen JT, Whittall T, Lehner PJ, Duncan L, MacAry P, Younson JS, Singh M, Oehlmann W, Cheng G, Bergmeier L, Lehner T (2001) CD40 is a cellular receptor mediating mycobacterial heat shock protein 70 stimulation of CC-chemokines. Immunity 15:971–983
Facciponte JG, Wang XY, Subjeck JR (2007) Hsp110 and Grp170, members of the Hsp70 superfamily, bind to scavenger receptor-a and scavenger receptor expressed by endothelial cells-I. Eur J Immunol 37:2268–2279
Theriault JR, Adachi H, Calderwood SK (2006) Role of scavenger receptors in the binding and internalization of heat shock protein 70. J Immunol 177:8604–8611
Yang S, Vigerust DJ, Shepherd VL (2013) Interaction of members of the heat shock protein-70 family with the macrophage mannose receptor. J Leukoc Biol 93:529–536
Alard JE, Hillion S, Guillevin L, Saraux A, Pers JO, Youinou P, Jamin C (2011) Autoantibodies to endothelial cell surface ATP synthase, the endogenous receptor for hsp60, might play a pathogenic role in vasculatides. PLoS One 6:e14654
Fong JJ, Sreedhara K, Deng L, Varki NM, Angata T, Liu Q, Nizet V, Varki A (2015) Immunomodulatory activity of extracellular Hsp70 mediated via paired receptors Siglec-5 and Siglec-14. EMBO J 34:2775–2788
Gehrmann M, Liebisch G, Schmitz G, Anderson R, Steinem C, De Maio A, Pockley G, Multhoff G (2008) Tumor-specific Hsp70 plasma membrane localization is enabled by the glycosphingolipid Gb3. PLoS One 3:e1925
Sugawara S, Kawano T, Omoto T, Hosono M, Tatsuta T, Nitta K (2009) Binding of Silurus Asotus lectin to Gb3 on Raji cells causes disappearance of membrane-bound form of HSP70. Biochim Biophys Acta 1790:101–109
Arispe N, Doh M, Simakova O, Kurganov B, De Maio A (2004) Hsc70 and Hsp70 interact with phosphatidylserine on the surface of PC12 cells resulting in a decrease of viability. FASEB J 18:1636–1645
Schilling D, Gehrmann M, Steinem C, De Maio A, Pockley AG, Abend M, Molls M, Multhoff G (2009) Binding of heat shock protein 70 to extracellular phosphatidylserine promotes killing of normoxic and hypoxic tumor cells. FASEB J 23:2467–2477
Nylandsted J, Gyrd-Hansen M, Danielewicz A, Fehrenbacher N, Lademann U, Hoyer-Hansen M, Weber E, Multhoff G, Rohde M, Jaattela M (2004) Heat shock protein 70 promotes cell survival by inhibiting lysosomal membrane permeabilization. J Exp Med 200:425–435
Pockley AG, Shepherd J, Corton JM (1998) Detection of heat shock protein 70 (Hsp70) and anti-Hsp70 antibodies in the serum of normal individuals. Immunol Investig 27:367–377
Zhu J, Quyyumi AA, Wu H, Csako G, Rott D, Zalles-Ganley A, Ogunmakinwa J, Halcox J, Epstein SE (2003) Increased serum levels of heat shock protein 70 are associated with low risk of coronary artery disease. Arterioscler Thromb Vasc Biol 23:1055–1059
Zhang X, He M, Cheng L, Chen Y, Zhou L, Zeng H, Pockley AG, Hu FB, Wu T (2008) Elevated heat shock protein 60 levels are associated with higher risk of coronary heart disease in Chinese. Circulation 118:2687–2693
Zhang X, Xu Z, Zhou L, Chen Y, He M, Cheng L, Hu FB, Tanguay RM, Wu T (2010) Plasma levels of Hsp70 and anti-Hsp70 antibody predict risk of acute coronary syndrome. Cell Stress Chaperones 15:675–686
Pockley AG, Wu R, Lemne C, Kiessling R, de Faire U, Frostegard J (2000) Circulating heat shock protein 60 is associated with early cardiovascular disease. Hypertension 36:303–307
Lewthwaite J, Owen N, Coates A, Henderson B, Steptoe A (2002) Circulating human heat shock protein 60 in the plasma of British civil servants: relationship to physiological and psychosocial stress. Circulation 106:196–201
Krishnamurthy K, Kanagasabai R, Druhan LJ, Ilangovan G (2012) Heat shock protein 25-enriched plasma transfusion preconditions the heart against doxorubicin-induced dilated cardiomyopathy in mice. J Pharmacol Exp Ther 341:829–839
Gehrmann M, Cervello M, Montalto G, Cappello F, Gulino A, Knape C, Specht HM, Multhoff G (2014) Heat shock protein 70 serum levels differ significantly in patients with chronic hepatitis, liver cirrhosis, and hepatocellular carcinoma. Front Immunol 5:307
Liao WC, Wu MS, Wang HP, Tien YW, Lin JT (2009) Serum heat shock protein 27 is increased in chronic pancreatitis and pancreatic carcinoma. Pancreas 38:422–426
Melle C, Ernst G, Escher N, Hartmann D, Schimmel B, Bleul A, Thieme H, Kaufmann R, Felix K, Friess HM, Settmacher U, Hommann M, Richter KK, Daffner W, Taubig H, Manger T, Claussen U, von Eggeling F (2007) Protein profiling of microdissected pancreas carcinoma and identification of HSP27 as a potential serum marker. Clin Chem 53:629–635
Yuan J, Dunn P, Martinus RD (2011) Detection of Hsp60 in saliva and serum from type 2 diabetic and non-diabetic control subjects. Cell Stress Chaperones 16:689–693
Oglesbee MJ, Herdman AV, Passmore GG, Hoffman WH (2005) Diabetic ketoacidosis increases extracellular levels of the major inducible 70-kDa heat shock protein. Clin Biochem 38:900–904
Genth-Zotz S, Bolger AP, Kalra PR, von Haehling S, Doehner W, Coats AJ, Volk HD, Anker SD (2004) Heat shock protein 70 in patients with chronic heart failure: relation to disease severity and survival. Int J Cardiol 96:397–401
Dybdahl B, Slordahl SA, Waage A, Kierulf P, Espevik T, Sundan A (2005) Myocardial ischaemia and the inflammatory response: release of heat shock protein 70 after myocardial infarction. Heart 91:299–304
Azuma K, Shichijo S, Takedatsu H, Komatsu N, Sawamizu H, Itoh K (2003) Heat shock cognate protein 70 encodes antigenic epitopes recognised by HLA-B4601-restricted cytotoxic T lymphocytes from cancer patients. Br J Cancer 89:1079–1085
Faure O, Graff-Dubois S, Bretaudeau L, Derre L, Gross DA, Alves PM, Cornet S, Duffour MT, Chouaib S, Miconnet I, Gregoire M, Jotereau F, Lemonnier FA, Abastado JP, Kosmatopoulos K (2004) Inducible Hsp70 as target of anticancer immunotherapy: identification of HLA-A*0201-restricted epitopes. Int J Cancer 108:863–870
Wu FH, Yuan Y, Li D, Liao SJ, Yan B, Wei JJ, Zhou YH, Zhu JH, Zhang GM, Feng ZH (2012) Extracellular HSPA1A promotes the growth of hepatocarcinoma by augmenting tumor cell proliferation and apoptosis-resistance. Cancer Lett 317:157–164
Hunter-Lavin C, Davies EL, Bacelar MM, Marshall MJ, Andrew SM, Williams JH (2004) Hsp70 release from peripheral blood mononuclear cells. Biochem Biophys Res Commun 324:511–517
Pittet JF, Lee H, Morabito D, Howard MB, Welch WJ, Mackersie RC (2002) Serum levels of Hsp 72 measured early after trauma correlate with survival. J Trauma 52:611–617. discussion 617
Ziegler TR, Ogden LG, Singleton KD, Luo M, Fernandez-Estivariz C, Griffith DP, Galloway JR, Wischmeyer PE (2005) Parenteral glutamine increases serum heat shock protein 70 in critically ill patients. Intensive Care Med 31:1079–1086
Ganter MT, Ware LB, Howard M, Roux J, Gartland B, Matthay MA, Fleshner M, Pittet JF (2006) Extracellular heat shock protein 72 is a marker of the stress protein response in acute lung injury. Am J Physiol Lung Cell Mol Physiol 291:L354–L361
Flohe SB, Bangen JM, Flohe S, Agrawal H, Bergmann K, Schade FU (2007) Origin of immunomodulation after soft tissue trauma: potential involvement of extracellular heat-shock proteins. Shock 27:494–502
Hecker JG, McGarvey M (2011) Heat shock proteins as biomarkers for the rapid detection of brain and spinal cord ischemia: a review and comparison to other methods of detection in thoracic aneurysm repair. Cell Stress Chaperones 16:119–131
De Maio A, Vazquez D (2013) Extracellular heat shock proteins: a new location, a new function. Shock 40:239–246
Njemini R, Lambert M, Demanet C, Mets T (2003) Elevated serum heat-shock protein 70 levels in patients with acute infection: use of an optimized enzyme-linked immunosorbent assay. Scand J Immunol 58:664–669
Osorio-Caballero M, Perdigon-Palacio C, Garcia-Lopez G, Flores-Herrera O, Olvera-Sanchez S, Morales-Mendez I, Sosa-Gonzalez I, Acevedo JF, Guzman-Grenfell AM, Molina-Hernandez A, Diaz NF, Flores-Herrera H (2015) Escherichia coli-induced temporal and differential secretion of heat-shock protein 70 and interleukin-1beta by human fetal membranes in a two-compartment culture system. Placenta 36:262–269
Molvarec A, Prohaszka Z, Nagy B, Szalay J, Fust G, Karadi I, Rigo J Jr (2006) Association of elevated serum heat-shock protein 70 concentration with transient hypertension of pregnancy, preeclampsia and superimposed preeclampsia: a case-control study. J Hum Hypertens 20:780–786
Molvarec A, Tamasi L, Losonczy G, Madach K, Prohaszka Z, Rigo J Jr (2010) Circulating heat shock protein 70 (HSPA1A) in normal and pathological pregnancies. Cell Stress Chaperones 15:237–247
Asea A, Jean-Pierre C, Kaur P, Rao P, Linhares IM, Skupski D, Witkin SS (2008) Heat shock protein-containing exosomes in mid-trimester amniotic fluids. J Reprod Immunol 79:12–17
Walsh RC, Koukoulas I, Garnham A, Moseley PL, Hargreaves M, Febbraio MA (2001) Exercise increases serum Hsp72 in humans. Cell Stress Chaperones 6:386–393
Febbraio MA, Ott P, Nielsen HB, Steensberg A, Keller C, Krustrup P, Secher NH, Pedersen BK (2002) Exercise induces hepatosplanchnic release of heat shock protein 72 in humans. J Physiol 544:957–962
Periard JD, Ruell P, Caillaud C, Thompson MW (2012) Plasma Hsp72 (HSPA1A) and Hsp27 (HSPB1) expression under heat stress: influence of exercise intensity. Cell Stress Chaperones 17:375–383
Santos TM, Sinzato YK, Gallego FQ, Iessi IL, Volpato GT, Dallaqua B, Damasceno DC (2015) Extracellular HSP70 levels in diabetic environment in rats. Cell Stress Chaperones 20:595–603
Tsai TN, Lee TY, Liu MS, Chuang IC, Lu MC, Dong HP, Lue SI, Yang RC (2015) Release of endogenous heat shock protein 72 on the survival of sepsis in rats. J Surg Res 198:165–174
Wilhelmus MM, Boelens WC, Otte-Holler I, Kamps B, de Waal RM, Verbeek MM (2006) Small heat shock proteins inhibit amyloid-beta protein aggregation and cerebrovascular amyloid-beta protein toxicity. Brain Res 1089:67–78
Wilhelmus MM, Boelens WC, Otte-Holler I, Kamps B, Kusters B, Maat-Schieman ML, de Waal RM, Verbeek MM (2006) Small heat shock protein HspB8: its distribution in Alzheimer's disease brains and its inhibition of amyloid-beta protein aggregation and cerebrovascular amyloid-beta toxicity. Acta Neuropathol 111:139–149
Evans CG, Wisen S, Gestwicki JE (2006) Heat shock proteins 70 and 90 inhibit early stages of amyloid beta-(1-42) aggregation in vitro. J Biol Chem 281:33182–33191
Carnini A, Scott LO, Ahrendt E, Proft J, Winkfein RJ, Kim SW, Colicos MA, Braun JE (2012) Cell line specific modulation of extracellular abeta42 by Hsp40. PLoS One 7:e37755
Luo X, Tao L, Lin P, Mo X, Chen H (2012) Extracellular heat shock protein 72 protects schwann cells from hydrogen peroxide-induced apoptosis. J Neurosci Res 90:1261–1269
Guzhova I, Kislyakova K, Moskaliova O, Fridlanskaya I, Tytell M, Cheetham M, Margulis B (2001) In vitro studies show that Hsp70 can be released by glia and that exogenous Hsp70 can enhance neuronal stress tolerance. Brain Res 914:66–73
Zhu Z, Li R, Stricker R, Reiser G (2015) Extracellular alpha-crystallin protects astrocytes from cell death through activation of MAPK, PI3K/Akt signaling pathway and blockade of ROS release from mitochondria. Brain Res 1620:17–28
Nickel W, Seedorf M (2008) Unconventional mechanisms of protein transport to the cell surface of eukaryotic cells. Annu Rev Cell Dev Biol 24:287–308
Wimley WC, Hristova K, Ladokhin AS, Silvestro L, Axelsen PH, White SH (1998) Folding of beta-sheet membrane proteins: a hydrophobic hexapeptide model. J Mol Biol 277:1091–1110
Arispe N, De Maio A (2000) ATP and ADP modulate a cation channel formed by Hsc70 in acidic phospholipid membranes. J Biol Chem 275:30839–30843
Macazo FC, White RJ (2014) Monitoring charge flux to quantify unusual ligand-induced ion channel activity for use in biological nanopore-based sensors. Anal Chem 86:5519–5525
Armijo G, Okerblom J, Cauvi DM, Lopez V, Schlamadinger DE, Kim J, Arispe N, De Maio A (2014) Interaction of heat shock protein 70 with membranes depends on the lipid environment. Cell Stress Chaperones 19:877–886
McCallister C, Kdeiss B, Nikolaidis N (2016) Biochemical characterization of the interaction between HspA1A and phospholipids. Cell Stress Chaperones 21:41–53
Lopez V, Cauvi DM, Arispe N, De Maio A (2016) Bacterial Hsp70 (DnaK) and mammalian Hsp70 interact differently with lipid membranes. Cell Stress Chaperones 21:609–616
Multhoff G, Hightower LE (1996) Cell surface expression of heat shock proteins and the immune response. Cell Stress Chaperones 1:167–176
Multhoff G (2007) Heat shock protein 70 (Hsp70): membrane location, export and immunological relevance. Methods 43:229–237
Mambula SS, Calderwood SK (2006) Heat shock protein 70 is secreted from tumor cells by a nonclassical pathway involving lysosomal endosomes. J Immunol 177:7849–7857
Andrei C, Dazzi C, Lotti L, Torrisi MR, Chimini G, Rubartelli A (1999) The secretory route of the leaderless protein interleukin 1beta involves exocytosis of endolysosome-related vesicles. Mol Biol Cell 10:1463–1475
Evdonin AL, Martynova MG, Bystrova OA, Guzhova IV, Margulis BA, Medvedeva ND (2006) The release of Hsp70 from A431 carcinoma cells is mediated by secretory-like granules. Eur J Cell Biol 85:443–455
Janas T, Janas MM, Sapon K, Janas T (2015) Mechanisms of RNA loading into exosomes. FEBS Lett 589:1391–1398
Chalmin F, Ladoire S, Mignot G, Vincent J, Bruchard M, Remy-Martin JP, Boireau W, Rouleau A, Simon B, Lanneau D, De Thonel A, Multhoff G, Hamman A, Martin F, Chauffert B, Solary E, Zitvogel L, Garrido C, Ryffel B, Borg C, Apetoh L, Rebe C, Ghiringhelli F (2010) Membrane-associated Hsp72 from tumor-derived exosomes mediates STAT3-dependent immunosuppressive function of mouse and human myeloid-derived suppressor cells. J Clin Invest 120:457–471
Li W, Sahu D, Tsen F (2012) Secreted heat shock protein-90 (Hsp90) in wound healing and cancer. Biochim Biophys Acta 1823:730–741
Gupta S, Knowlton AA (2007) HSP60 trafficking in adult cardiac myocytes: role of the exosomal pathway. Am J Physiol Heart Circ Physiol 292:H3052–H3056
Merendino AM, Bucchieri F, Campanella C, Marciano V, Ribbene A, David S, Zummo G, Burgio G, Corona DF, Conway de Macario E, Macario AJ, Cappello F (2010) Hsp60 is actively secreted by human tumor cells. PLoS One 5:e9247
Evdokimovskaya Y, Skarga Y, Vrublevskaya V, Morenkov O (2010) Secretion of the heat shock proteins HSP70 and HSC70 by baby hamster kidney (BHK-21) cells. Cell Biol Int 34:985–990
Broquet AH, Thomas G, Masliah J, Trugnan G, Bachelet M (2003) Expression of the molecular chaperone Hsp70 in detergent-resistant microdomains correlates with its membrane delivery and release. J Biol Chem 278:21601–21606
Chen S, Bawa D, Besshoh S, Gurd JW, Brown IR (2005) Association of heat shock proteins and neuronal membrane components with lipid rafts from the rat brain. J Neurosci Res 81:522–529
Thery C, Regnault A, Garin J, Wolfers J, Zitvogel L, Ricciardi-Castagnoli P, Raposo G, Amigorena S (1999) Molecular characterization of dendritic cell-derived exosomes. Selective accumulation of the heat shock protein hsc73. J Cell Biol 147:599–610
Chaput N, Flament C, Viaud S, Taieb J, Roux S, Spatz A, Andre F, LePecq JB, Boussac M, Garin J, Amigorena S, Thery C, Zitvogel L (2006) Dendritic cell derived-exosomes: biology and clinical implementations. J Leukoc Biol 80:471–478
Lancaster GI, Febbraio MA (2005) Exosome-dependent trafficking of HSP70: a novel secretory pathway for cellular stress proteins. J Biol Chem 280:23349–23355
Clayton A, Turkes A, Navabi H, Mason MD, Tabi Z (2005) Induction of heat shock proteins in B-cell exosomes. J Cell Sci 118:3631–3638
Mathew A, Bell A, Johnstone RM (1995) Hsp-70 is closely associated with the transferrin receptor in exosomes from maturing reticulocytes. Biochem J 308(Pt 3):823–830
Conde-Vancells J, Rodriguez-Suarez E, Embade N, Gil D, Matthiesen R, Valle M, Elortza F, Lu SC, Mato JM, Falcon-Perez JM (2008) Characterization and comprehensive proteome profiling of exosomes secreted by hepatocytes. J Proteome Res 7:5157–5166
McCready J, Sims JD, Chan D, Jay DG (2010) Secretion of extracellular hsp90alpha via exosomes increases cancer cell motility: a role for plasminogen activation. BMC Cancer 10:294
Hegmans JP, Bard MP, Hemmes A, Luider TM, Kleijmeer MJ, Prins JB, Zitvogel L, Burgers SA, Hoogsteden HC, Lambrecht BN (2004) Proteomic analysis of exosomes secreted by human mesothelioma cells. Am J Pathol 164:1807–1815
Anand PK, Anand E, Bleck CK, Anes E, Griffiths G (2010) Exosomal Hsp70 induces a pro-inflammatory response to foreign particles including mycobacteria. PLoS One 5:e10136
Maecker HT, Todd SC, Levy S (1997) The tetraspanin superfamily: molecular facilitators. FASEB J 11:428–442
Bhatnagar S, Shinagawa K, Castellino FJ, Schorey JS (2007) Exosomes released from macrophages infected with intracellular pathogens stimulate a proinflammatory response in vitro and in vivo. Blood 110:3234–3244
O'Neill HC, Quah BJ (2008) Exosomes secreted by bacterially infected macrophages are proinflammatory. Sci Signal 1:pe8
Acknowledgments
This work was supported by the National Institutes of Health, grant number GM R01 09845.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 Springer International Publishing AG, part of Springer Nature
About this chapter
Cite this chapter
De Maio, A. (2018). Extracellular Heat Shock Proteins as Stress Communication Signals. In: Binder, R., Srivastava, P. (eds) Heat Shock Proteins in the Immune System. Springer, Cham. https://doi.org/10.1007/978-3-319-69042-1_3
Download citation
DOI: https://doi.org/10.1007/978-3-319-69042-1_3
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-69040-7
Online ISBN: 978-3-319-69042-1
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)