Abstract
Ethanol, as an alternative energy resource, has become a subject of great interest due to the current surge in price of crude oil. Environmental concerns have promoted new applications and markets for ethanol. Kinetic modeling of ethanol production is very important from design and scale-up aspects of fermentors. In the present work, a kinetic model has been developed for the batch fermentation of crude whey for ethanol production by Kluyveromyces marxianus. Parameters of the kinetic model have been determined based on experimental data given by Zafar and Owais (Biochem Eng J 27, 295–298, 2006). Results have been compared by carrying out computer simulation. The kinetic model proposed in this study provides good predictions for growth of biomass, substrate consumption and ethanol production for all types of substrate-microbe systems.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Ethanol has tremendous applications in chemical, pharmaceutical and food industries in the form of raw material, solvent and fuel. The annual production of industrial ethanol is about four million tons, 80% of which is produced by fermentation. Biological fuel production might serve as a sustainable, carbon-neutral energy source compatible with current engine technology. In an effort to offset increases in consumption and to limit the fossil fuel-related negative impacts on the environment, the US Department of Energy has established the goal of supplanting 30% of gasoline consumption with cellulosic ethanol by 2030 (Bonkers 2006). With the increasing shortage of petroleum, urban air pollution and accumulation of carbon dioxide in the atmosphere, ethanol is expected to play a more significant role in the future. Government of India through a notification dated September 2002 made 5% ethanol-blending mandatory in petrol, in nine states and three Union Territories (MPNGR 2002). In the next phase, supply of ethanol-blended petrol would be extended to the whole country and efforts would be made to increase the percentage of ethanol mixture in petrol to 10% (Suresh and Chandrasekhar 2009; MPNGR 2002). The increased realization of the finite nature of the world’s oil supplies and vagaries in oil prices have rekindled interest in production of potable and industrial alcohol by fermentation of carbohydrate containing raw materials. Brazilian effort to reduce petroleum imports by adding ethanol to motor fuels is an interesting attempt in this direction.
1.1 Production of Ethanol by Using Fermentation
Ethanol Fermentation is a biological process in which sugars like glucose, fructose and sucrose are converted into cellular energy along with production of ethanol and carbon di oxide. Because yeast perform anaerobic conversion in the absence of oxygen. In ethanol fermentation, one glucose molecule breaks into two pyruvates. The energy released from this exothermic reaction is utilized in binding inorganic phosphate Adenosine di phosphate (ADP) and convert Nicotinamide Adenine Dinucleotide (NAD+) to Nicotinamide Adenine Dinucleotide Hydrogen (NADH). The two pyruvates are then broken down into two acetaldehydes and give off two CO2 as a waste product. The two acetaldehydes are then converted to two ethanol by using the H-ions from NADH; converting NADH back into NAD+.
Many of the researchers were developed and optimized the non-linear mathematical models of fermentation of ethanol using different microbios (Starzak et al., 1994; Veeramallu and Agrawal, 1990; Chouakri et al. 1994; Cazzador and Lubenova 1995; Farza et al. 1997). Further, Baltes et al. (1994) demonstrated sensitivity analysis of different parameters over production. Tao et al. (2005) evaluated ethanol production by an acid-tolerant Zymomonas mobilis under non-sterilized condition. They found theoretical yield of ethanol from glucose was 0.488 g/g. In general, most of non-linear kinetic model established from batch experimental observations to evaluate concentration profiles for fermentation process may not perfectly fit. However, Wang and Sheu (2000) applied multi-objective optimization to estimate the kinetic model parameters of batch and fed-batch fermentation processes for ethanol production using Saccharomyces diastaticus in the 5 L fermentor. They found that estimated model was fitted through hybrid differential evolution of parameters.
1.2 Technologies for Ethanol Production
Chemical Route: Ethanol for use as an industrial feedstock or solvent (sometimes referred to as synthetic ethanol) is produced from petrochemical feed stocks, mainly by the acid-catalyzed hydration of ethylene:
The catalyst used in this process is mostly phosphoric acid, adsorbed on a porous support like silica gel or diatomaceous earth. Shell Oil Company was the first to use this catalyst for the production of ethanol on large scale in 1947. The reaction takes place in the presence of high pressure steam at 300 °C (572 °F) where ethylene to steam ratio is kept 1.0:0.6. In the U.S., Union Carbide Corporation and other such industries used this process, but now it is used only commercially by Lyondell Basell.
In an older process, ethylene was hydrated indirectly to produce sulfovinic acid (ethyl sulfate) by reacting ethylene with sulfuric acid (conc. H2SO4), which was further reacted with water to hydrolyze it to produce ethanol along with regeneration of sulfuric acid. Union Carbide in 1930 first practiced this process on industrial scale but now this process is completely obsolete.
Biochemical Route: Ethanol used in alcoholic beverages and as a fuel is produced through fermentation. Some particular species of yeast (e.g., Saccharomyces cerevisiae) metabolizes sugar to produce ethanol and carbon dioxide. The chemical equation given below clearly depicts this conversion:
Fermentation is the process of culturing yeast under favorable thermal conditions to produce alcohol. This process is carried out at about 35–40 °C (95–104 °F). Toxicity of ethanol to yeast restricts the concentration of ethanol obtainable by fermenting. Therefore for higher concentrations distillation or fortification is used. A maximum of 18% ethanol concentration can be tolerated by the most ethanol-tolerant yeast strains. For the production of ethanol from the starchy material such as cereal grains, first starch must be converted to sugars. In production of beer, this has traditionally been done by letting the grain to germinate, or malt which then produces the enzyme amylase. When the germinated grain is mashed, the amylase converts the remaining starches into sugars.
1.3 Crude Whey for Ethanol Production by Kluyveromyces marxianus
The dairy industry represents an important part of the food processing industry and contributes significant liquid process residues that can be used for the production of ethanol (Ghaly and El-Taweel, 1997). Cheese whey (CW), a by-product of the cheese manufacturing process whose major components are lactose (45–50 kg/m3), proteins (6–8 kg/m3), lipids (4–5 kg/m3), and mineral salts (8–10% of dried extract), constitutes an inexpensive and nutritionally rich raw material for the production of different compounds (Gonzalez Siso 1996; Panesar et al. 2007). Ethanol production by bioconversion of whey is an alternative of great interest for reuse of this industrial by-product (Dragone et al., 2009).
Biological Oxygen demand (BOD) reductions of higher than 75%, with the concomitant production of biogas, ethanol, single cell protein or another marketable product, have been achieved and about half the whey produced nowadays is not a pollutant but a resource. However, annual world cheese-whey production is increasing and new bio-productions are being sought through biotechnology in order to get full use of the whey produced. Siso (1996) reviewed that application of cheese whey being exploited for production of ethanol.
Sansonetti et al. (2009) investigated the feasibility of bio-ethanol production by batch fermentation of ricotta cheese whey (Scotta), a dairy industry waste characterized by lactose concentration ranging from 4.5 to 5.0% (w/w) and, with respect to traditional (raw) whey, by much lower protein content. The microorganism used to carry out the fermentation processes was the yeast Kluyveromyces marxianus. The experimental data have demonstrated the process feasibility: scotta is an excellent substrate for fermentation and exhibits better performance with respect to both raw cheese whey and deproteinized whey. Complete lactose consumption, indeed, was observed in the shortest time (13 h) and with the highest ethanol yield (97% of the theoretical value).
Kluyveromyces marxianus KD-15, called flex yeast, is a strain that is in sensitive to catabolite repression and has the capacity to produce ethanol efficiently from a mixture of beet molasses and whey powder. Oda et al. (2010) conducted in 50 ml of a medium containing 200 mg/ml of sugar as sugar beet thick juice diluted with an arbitrary amount of crude whey, strain KD-15 produced over 99 mg/ml ethanol in all the media tested, and ethanol formation decreased in proportion to the volume of whey by K. marxianus NBRC 1963, the parental strain of KD-15, and Saccharomyces cerevisiae NBRC 0224, the reference strain for conventional ethanol production. Fermentation of thick juice diluted with whey alone by strain KD-15 at 30 °C initially proceeded slower than that at 33–37 °C but finally bore the highest level of ethanol. The maximum ethanol concentration obtained in 1.5L of a medium using a 2-L fermentor was elevated by aeration of 15–50 ml/min and reduced by that in excess of 100 ml/min. Under optimized conditions in 72 h, strain KD-15 converted all of the sugars derived from thick juice and whey to ethanol at 102 mg/ml, corresponding to 92.9% of the theoretical yield.
Cheese whey powder (CWP) is an attractive raw material for ethanol production since it is a dried and concentrated form of CW and contains lactose in addition to nitrogen, phosphate and other essential nutrients. Dragone et al. (2011) investigated that proteinized CWP was utilized as fermentation medium for ethanol production by Kluyveromyces fragilis. The individual and combined effects of initial lactose concentration (50–150 kg/m3), temperature (25–35 °C) and inoculum concentration (1–3 kg/m3) were investigated through a 23 full factorial central composite design, and the optimal conditions for maximizing the ethanol production were determined. According to the statistical analysis, in the studied range of values, only the initial lactose concentration had a significant effect on ethanol production, resulting in higher product formation as the initial substrate concentration was increased. Assays with initial lactose concentration varying from 150 to 250 kg/m3 were thus performed and revealed that the use of 200 kg/m3 initial lactose concentration, inoculums concentration of 1 kg/m3 and temperature of 35 °C were the best conditions for maximizing the ethanol production from CWP solution. Under these conditions, 80.95 kg/m3 of ethanol was obtained after 44 h of fermentation.
Kuznetsova et al. (2015) investigated liquid extract (lupin whey) and its perspectives for biofuel production. The optimized multienzyme complex was composed of 1.1 ± 0.2 units/g of cellulase, 5.2 ± 0.4 units/g of xylanase and 2.5 ± 0.2 units/g of α-amylase. The enzymatic treatment resulted in 19% increase of the total sugar content of lupin whey versus to the control whey obtained without enzyme addition. The lupin whey was condensed by evaporation to 48–50% dry matter content. Condensed whey was used as nutrient medium for cultivation of yeasts Saccharomyces cerevisiae. After fermentation the yield of bioethanol reached 1.6 g/l. The proposed technology of complex processing of vegetable raw materials allows to obtain lupin protein concentrates with a crude protein content up to 63.2 ± 1.3% on dry matter basis and lupin whey with a total sugar content of up to 29% on dry matter basis. The lupin whey could be used as an organic substrate for biofuel production.
It is known that the fermentation process performance is affected by operational conditions such as temperature, stirring rate, initial inoculum and substrate concentrations, dissolved oxygen, among others. A suitable control of these variables is of great importance for a good process performance and obtainment of high-quality products. So optimize the conditions for ethanol production from CWP through different optimized numerical methods. Some of the factors are selected as process variables such initial lactose concentration, temperature and inoculum concentration; ethanol concentration, substrate consumption and fermentative parameters (ethanol yield factor, Y P/S; ethanol volumetric productivity, Q P; ethanol yield per cell, Y P/x; and bioconversion efficiency, h).
In the present work, a kinetic model has been developed for the batch fermentation of crude whey for ethanol production by K. marxianus respectively. Parameters of the kinetic model have been determined based on experimental data given by Zafar and Owais (2006). Results have been compared by carrying out computer simulation.
2 Mathematical Model
In the present study, it was observed that the kinetic model used for gluconic acid fermentation by Aspergillus niger (Liu et al. 2003) may be used after suitable modifications. The logistic equation given by Eq. (1) can be used to model the cell concentration, \(X\). The logistic equation is a substrate independent model and there is inhibition of biomass on the growth model (Gong and Lun 1996).
The product formation rate given by Eq. (2) depends on both the instantaneous biomass concentration, X and growth rate, \({{{\text{d}}X} \mathord{\left/ {\vphantom {{{\text{d}}X} {{\text{d}}t}}} \right. \kern-0pt} {{\text{d}}t}}\) in a linear manner and that the amount of carbon substrate used for product formation is negligible. The product formation kinetics is based on the Luedeking–Piret equation (Luedeking and Piret 1959).
When, \(\alpha \ne 0\), \(\beta = 0\), the product formation is associate-growth.
The substrate is used for the production of cells and metabolic products as well as for the maintenance of cells (Znad et al. 2004). Therefore, the amount of carbon substrate used for product formation has also been included in the substrate model for the present study.
In addition, the kinetic equations (4)–(6) previously given by Bona and Moser (1997) for L-glutamic acid production were also used for kinetic modeling for ethanol production. These equations were developed in analogy to the equations given in the literature (Bajpai and Reub 1981; Moser and Schneider 1989).
2.1 Estimation of Kinetic Parameters
In order to estimate kinetic parameters given by eqs. Given in previous section, it is required to search those values of parameters which predict values of X, S and P close to the experimental values, X exp, S exp and P exp within acceptable accuracy at all times during fermentation process. The following objective function given by Nandasana and Kumar (2008) has been used for the simulation of the kinetic data.
\(W_{X}\), \(W_{S}\) and \(W_{P}\) are weighting factors, which were assumed as the reciprocal of the maximum concentration for respective components, viz. X, S and P. Model differential equations were solved by minimizing the objective function using regression procedure or numerical technique with Microsoft EXCEL 2007 to obtain values of X, S and P.
3 Results and Discussion
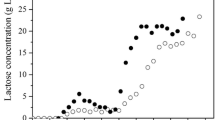
The variation of experimental and simulation values of X, S and P with respect to time is shown by data points in Figs. 1, 2 and 3, respectively. The initial value of X and S from Zafar and Owais (2006) for the experimental value were 2.82 and 35, respectively. Ethanol fermentation by K. marxianus showed a classical growth trend. After a lag phase, the cells entered the exponential growth phase. The strain started to form ethanol when the cells entered the exponential phase, and therefore, cell growth and ethanol took place simultaneously. The values of parameters of Zafar and Owais (2006) as determined by them are given in Table 1. Simulation lines of X, S and P with respect to time as per Bona and Moser (1997) and present model are shown by dotted and solid lines in Figs. 1, 2 and 3, respectively.
Simulation of the experimental data collected from Zafar and Owais (2006) in an aerobic batch culture of Kluyveromyces marxianus for cell mass concentration
Simulation of the experimental data collected from Zafar and Owais (2006) in an aerobic batch culture of Kluyveromyces marxianus for substrate concentration
Simulation of the experimental data collected from Zafar and Owais (2006) in an aerobic batch culture of Kluyveromyces marxianus for Ethanol concentration
The experimental values were fitted to the present model taking X m = 11.864 g/l. The simulated values of the parameters are given in Table 1. A comparison of calculated value of X by Bona and Moser (1997) and present model along with the experimental data is given in Fig. 1. Both models seem to well-represent the experimental data. Similarly, Bona and Moser (1997) and present model well represent the S versus t experimental data given by Fig. 2. The present model seems to better represent the P versus t experimental data given by Zafar and Owais (2006) as compared to Bona and Moser (1997) model (Fig. 3). Since, \(\alpha \ne 0, \beta = 0\), the product formation is associate-growth Values of objective function (Q) by the Bona and Moser (1997) and present model were found to be 0.217and 0.10, respectively. Therefore, it may be concluded that overall present model better represents all experimental data.
4 Conclusion
Fermentation is a very complex process, and it is often very difficult to obtain a complete picture of what is actually going on in a particular fermentation. The model presented in this work is able to fit the experimental data with minimum value of the objective function (\(Q\)) of 0.10. The kinetic model proposed in this study provides good predictions for growth of biomass, substrate consumption and ethanol production by batch fermentation of crude whey by Kluyveromyces marxianus.
Abbreviations
- \(K_{\text{ipx}}\) :
-
Inhibition constant of growth by product (g/l)
- \(K_{\text{s}}\) :
-
Monod growth constant for the substrate (g/l)
- \(K_{\text{sI}}\) :
-
Inhibition coefficient for cell growth on glucose
- \(K_{\text{p}}\) :
-
Saturation coefficient for cell growth on ethanol
- \(K_{\text{pI}}\) :
-
Inhibition coefficient for cell growth on ethanol
- \(K{}_{\text{p/s}}\) :
-
Monod product constant for the substrate (g/l)
- \(K_{\text{r}}\) :
-
Monod growth constant for the specific biotin concentration (g/l)
- \(m_{\text{s}}\) :
-
Maintenance coefficient (g substrate/(g cells h))
- \(P\) :
-
Product (L-glutamic acid) concentration (g/l)
- \(q_{{{\text{p}},\hbox{max} }}\) :
-
Maximal specific production rate (1/h)
- \(S\) :
-
Substrate (glucose) concentration (g/l)
- \(t\) :
-
Time (h)
- \(X\) :
-
Biomass concentration (g/l)
- \(X_{\text{m}}\) :
-
Maximum cell concentration (g/l)
- \(Y_{\text{x/s}}\) :
-
Yield coefficient biomass from substrate (g/g)
- \(Y_{\text{p/s}}\) :
-
Yield coefficient product from substrate (g/g)
- \(\mu\) :
-
Speicific growth rate (1/h)
- \(\mu_{\hbox{max} }\) :
-
Maximal specific growth rate (1/h)
- \(\alpha\) :
-
Growth-associated product formation coefficient (g/g)
- \(\beta\) :
-
Non-growth associated product formation coefficient(g/g h)
References
Bajpai RK, Reub M (1981) Evaluation of feeding strategies in carbon regulated secondary metabolite production through mathematical modeling. Biotechnol Bioeng 23:717–738
Baltes M, Schneider R, Sturm C, Reuss M (1994) Optimal experimental design for parameter estimation in unstructured growth models. Biotechnol Prog 10:480–488
Bona R, Moser A (1997) Modeling of l-glutamic acid production with Corynebacterium glutamicum under biotin limitation. Bioprocess Eng 17:139–142
Bonkers HS (2006) About biofuels. Nat Biotechnol 24:755–760
Cazzador L, Lubenova V (1995) Non-linear estimation of specific growth rate for aerobic fermentation processes. Biotechnol Bioeng 47:626–632
Chouakri N, Fonteix C, Marc I, Corriou JP (1994) Parameter estimation of a Monod-type model. Part-I: theoretical identifiability and sensitivity analysis. Biotechnol Tech 8:683–688
Dragone G, Mussatto SI, Almeida e Silva JB, Teixeira JA (2011) Optimal fermentation conditions for maximizing the ethanol production by Kluyveromyces fragilis from cheese whey powder. Biomass Bioenerg 35:1977–1982
Dragone G, Mussatto SI, Oliveira JM, Teixeira JA (2009) Characterisation of volatile compounds in an alcoholic beverage produced by whey fermentation. Food Chem 112:35–929
Farza M, Hammouri H, Othman S, Busawon K (1997) Nonlinear observers for parameter estimation in bioprocesses. Chem Eng Sci 52:4251–4267
Ghaly AE, El-Taweel AA (1997) Kinetic modelling of continuous production of ethanol from cheese whey. Biomass Bioenerg 6:61–72
Gong H, Lun S (1996) The kinetics of lysine batch fermentation. Chin J Biotechnol 12:219–225
Gonzalez Siso MI (1996) The biotechnological utilization of cheese whey: a review. Bioresour Technol 57:1–11
Kuznetsova L, Zabodalova L, Domoroshchenkova M (2015) Lupin whey as a perspective substrate for bioethanol production. Energy Procedia 72:103–110
Liu JZ, Weng LP, Zhang Q-L, Xu H, Ji L-N (2003) A mathematical model for gluconic acid fermentation by Aspergillus niger. Biochem Eng J 14:137–141
Luedeking R, Piret EL (1959) A kinetic study of the lactic acid fermentation: batch process at controlled pH. J Biochem Microbiol Technol Eng 4:231–241
Moser A, Schneider H (1989) Modeling secondary metabolite production—case Pleuromulin. In: Computer applications in fermentation technology: modeling and control of biotechnological processes. Elsevier Science Publishers Ltd., Amsterdam, pp. 93–103
Nandasana AD, Kumar S (2008) Kinetic modeling of lactic acid production from molasses using Enterococcus faecalis RKY1. Biochem Eng J 38:277–284
Oda Y, Nakamura K, Shinomiya N, Ohba K (2010) Ethanol fermentation of sugar beet thick juice diluted with crude cheese whey by the flex yeast Kluyveromyces marxianus KD-15. Biomass Bioenerg 34:1263–1266
Panesar PS, Kennedy JF, Gandhi DN, Bunko K (2007) Bioutilisation of whey for lactic acid production. Food Chem 105:1–14
Sansonetti S, Curcio S, Calabro V, Iorio G (2009) Bio-ethanol production by fermentation of ricotta cheese whey as an effective alternative non-vegetable source. Biomass Bioenergy 33:1687–1692
Siso MIG (1996) The biotechnological utilization of cheese whey: a review. Biores Technol 57:1–11
Starzak M, Krzystek L, Nowicki L, Miehalski H (1994) Macro-approach kinetics of ethanol fermentation by Saccharomyces cerevisiae: experimental studies and mathematical modelling. Chem Eng Journal 54:221–240
Suresh S, Chandrasekhar G (2009 )Production of bioethanol from cashew wastes. ACT, sponsored by Petroleum conservation research association. Oct–Dec, pp 16–17 2009
Tao F, Miao JY, Shi GY, Zhang KC (2005) Ethanol fermentation by an acid-tolerant Zymomonas mobilis under non-sterilized condition. Process Biochem 40:183–187
Ministry of Petroleum and Natural Gas Resolution (2002) The gazette of India: extraordinary [part I, sec. I]. Ministry of Petroleum and Natural Gas Resolution. New Delhi, 3 Sept, No. P-45018/28/2000-C. C, (2002)
Veeramallu U, Agrawal P (1990) A structured kinetic model for Zymomonas mobilis ATC10988. Biotechnol Bioeng 36:694–704
Wang F-S, Sheu J-W (2000) Multiobjective parameter estimation problems of fermentation processes using a high ethanol tolerance yeast. Chem Eng Sci 55:3684–3695
Zafar S, Owais M (2006) Ethanol production from crude whey by Kluyveromyces marxianus. Biochem Eng J 27:295–298
Znad H, Blazej M, Bales V, Markos J (2004) Kinetic model for gluconic acid production by Aspergillus niger. Chem Pap 58:23–28
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 Springer International Publishing AG
About this chapter
Cite this chapter
Suresh, S., Srivastava, V.C., Sakthivel, S., Arisutha, S. (2018). Kinetic Modeling of Ethanol Production for Substrate–Microbe System. In: Kumar, S., Sani, R. (eds) Biorefining of Biomass to Biofuels. Biofuel and Biorefinery Technologies, vol 4. Springer, Cham. https://doi.org/10.1007/978-3-319-67678-4_16
Download citation
DOI: https://doi.org/10.1007/978-3-319-67678-4_16
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-67677-7
Online ISBN: 978-3-319-67678-4
eBook Packages: EnergyEnergy (R0)