Abstract
The 19–24 kDa Translationally Controlled Tumor Protein (TCTP) is involved in a wide range of molecular interactions with biological and nonbiological partners of various chemical compositions such as proteins, peptides, nucleic acids, carbohydrates, or small molecules. TCTP is therefore an important and versatile binding platform. Many of these protein–protein interactions have been validated, albeit only few received an in-depth structural characterization. In this chapter, we will focus on the structural analysis of TCTP and we will review the available literature regarding its interaction network from a structural perspective.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
2.1 Introduction
This chapter will focus on the structural aspects of TCTP in the context of its wide interaction network, with the aim of being as comprehensive as possible. First we will describe the available structures of TCTP and compare them with other structurally related proteins. Then in a second part, we will discuss the properties of some amino acid regions of TCTP that are important due to their conservation and/or specific functions. Then the last two parts will describe the large interactome of TCTP involving non-proteic or proteic molecules. Two recent reviews (Kawakami et al. 2012; Amson et al. 2013) also covered part of the topics of this chapter. However, the last 4 years have witnessed astonishing progress in TCTP field, and we felt that an updated description of TCTP interactome was necessary. We believe this chapter will be useful not only for the general reader but also for TCTP experts, to overcome the difficulties associated with the multiple names of TCTP found in literature. Indeed, depending on its intra- or extracellular localization, or on the species, TCTP is also called Histamine-Releasing factor (HRF), p23, p21, Q23, fortilin, Mmi1p (yeast), or Tpt1. This confusing nomenclature undoubtedly hinders the diffusion of knowledge on this protein within the community and hence slows down the progress of its characterization.
2.2 Sequence and Structure of TCTP
2.2.1 Description of the Structure of TCTP
The high-resolution structures of TCTP from different organisms have been determined by NMR or X-ray crystallography (see Fig. 2.1). These include the malaria parasites Plasmodium falciparum (Eichhorn et al. 2013) and knowlesi (Vedadi et al. 2007), the yeast Schizosaccharomyces pombe (Thaw et al. 2001), the worm Caenorhabditis elegans (Lange et al. 2012), and wild type (Feng et al. 2007; Susini et al. 2008) or E12V mutant (Dong et al. 2009) human TCTP. The structures of TCTP are highly conserved between different organisms. TCTP is a monomeric protein, although covalent and non-covalent TCTP dimers have been observed, as discussed in Sect. 2.4.3. The TCTP fold contains three α-helices (α1, α2, α3) and eleven β-strands arranged in two small β-sheets β2–β1–β11 and β5–β6 and a larger β-sheet β7–β8–β9–β10–β4–β3 (Fig. 2.1a). The two β-sheets β2–β1–β11 and β7–β8–β9–β10–β4–β3 are twisted and their relative arrangement forms a β-tent (Lupas et al. 2015). The helices α2 and α3 are connected by a short loop that creates a kink to form a helical hairpin. This hairpin sits on one side of the large surface defined by the six-stranded β-sheet. The different TCTP structures differ by the secondary structure elements that slightly vary in length and relative positioning. For example, the β-sheet β2–β1–β11 is severely distorted in C. elegans TCTP compared to the other structures. One conserved feature of TCTP structures is the long ~30–33-amino acid loop connecting strands β5 and β6 (between residues T39 and V66 in human TCTP sequence, see sequence alignment in Fig. 2.2). This loop is highly flexible as judged from missing electron density in crystal structures (Eichhorn et al. 2013; Vedadi et al. 2007; Susini et al. 2008; Dong et al. 2009) and from the scarcity of long-range NOE restraints in this region leading to poor structural convergence in NMR structures (Thaw et al. 2001; Lange et al. 2012; Feng et al. 2007). The 15N relaxation NMR study revealed that, in human TCTP, the loop explores a wide conformation range at the pico- to nano-second timescale (Feng et al. 2007). The N- and C-extremities of the loop are more or less rigid and tend to form a short β-sheet β5–β6 that projects the loop towards the bulk solution and away from the core structure. This is clearly visible in the NMR structures of human and S. pombe TCTP (Fig. 2.1b). The NMR structure ensemble of C. elegans TCTP (Fig. 2.1b) is more compact and the loop explores a more restricted conformational space, suggesting that a few long-range NOE-derived distances bring the loop in relative close proximity to the core TCTP structure. It is therefore possible that this loop may have distinct dynamic properties in the different species.
Ribbon representation of the structures of TCTP from different organisms. (a) Crystal structure of the human TCTP [PDB Code 1YZ1 (Susini et al. 2008)]. The secondary structure elements are shown using the nomenclature from Fig. 2.2 and the α-helical, β-strand, and coil regions are colored cyan, magenta, and rose, respectively. The 30–33 amino acid long loop between strands β5 and β6 is not visible in the crystal structure and is indicated as a dotted line. Of note, the length of the dotted line does not represent the effective length of the loop. (b) TCTP structures from human (Feng et al. 2007), fission yeast Schizosaccharomyces pombe (Thaw et al. 2001), the parasites Plasmodium falciparum (Eichhorn et al. 2013) and knowlesi (Vedadi et al. 2007), and the worm Caenorhabditis elegans (Lange et al. 2012). For NMR structures, the ensemble of conformations is shown to illustrate the flexibility of the long loop due to the absence or scarcity of experimentally determined long-range distance constraints in this region
Amino acid alignment of selected TCTP sequences from mammalians, parasites, plants and insects. The amino acid numbering and the secondary structures are from the human sequences (PDB 2HR9) and are shown on the top of the alignment. The picture was prepared using the ESPript webserver (Robert and Gouet 2014) with the Blosum62 color scheme. Specific sequences such as TCTP1 and TCTP2 signatures and the BH3-like domain together with the h1, h2, h3, h4 positions conserved in conventional BH3 domains are highlighted
TCTP is a highly charged acidic protein with an isoelectric point around 4.5. Accordingly, the 172 amino acid human TCTP contains up to 31 Asp/Glu and 20 Lys/Arg amino acid residues. The vast majority of these charged residues are solvent-exposed at the surface of the protein, making TCTP a highly water-soluble molecule. TCTP is amongst the most abundant proteins in many eukaryotic cells, and the high solubility of TCTP is therefore an important feature. Several charged residues form salt bridges that are partially buried at the surface of the protein. In all TCTP structures, one aspartate residue (D6 in human TCTP sequence) located at the C-terminus of strand β1 is significantly buried in a hydrophobic environment where it makes hydrogen bond with the main chain amides of I8 and S9 forming an Asx-turn motif on the loop β1–β2. D6 forms an additional H-bond with the amide group of M145 at the N-terminus of strand β9 and, hence, creates contact between the β2–β1–β11 and β7–β8–β9–β10–β4–β3 β-sheets that define the β-tent. Another aspartate (D11), located at the beginning of strand β2, is also partially buried and form H-bond with the backbone of N139 in the loop β8–β9. Both D6 and D11 are strictly conserved, thus revealing their potential roles in the stabilization of the β-tent conformation and consequently of the TCTP fold.
2.2.2 Structural Homologues of TCTP
The long helical hairpin represents a hallmark of TCTP and shares strong structural similarity with other proteins (Susini et al. 2008). These include transmembrane domains of diphtheria toxin and bacterial colicins as well as the helices H5–H6 found in Bcl-2 family proteins, such as Bax (Susini et al. 2008). However, the similarity is restricted to structural features since there is poor amino acid homology between these proteins. The helical hairpin, and in particular residue K102, plays a role in the anti-apoptotic function of TCTP (Susini et al. 2008). Bax has a strong pro-apoptotic property, and remarkably, replacing the essential helices H5–H6 of Bax by helices α2 and α3 from TCTP does not change much Bax pro-apoptotic functions suggesting that TCTP helical hairpin can structurally and functionally replace Bax helical hairpin (Susini et al. 2008).
With the determination of the structure of TCTP, it was also realized that TCTP shares strong structural similarities with the MsrB and Mss4/Dss4 families (Thaw et al. 2001; Lowther et al. 2002) (see Fig. 2.3f,g). The methionine-R-sulfoxide reductase B (MsrB) is an enzyme involved in the protection of cell against oxidation damages by reducing methionine sulfoxide back to methionine. The Mss4/Dss4 proteins bind the GDP/GTP free form of Rab GTPase proteins and act as a poorly efficient guanine nucleotide exchange factor or guanine nucleotide-free chaperon (Itzen et al. 2006). Despite their different functions, the three protein families share a similar topology. Although the length of the β-strands differs in the different families, they all have in common the two β-sheets forming the β-tent. Nevertheless, some clear variations occur. Firstly, the long flexible loop is absent in MsrB and Mss4/Dss4 proteins and is specific to TCTP. Secondly, the MsrB and Mss4/Dss4 families do not possess the long helical hairpin present in TCTP. In the case of Mss4/Dss4 proteins, an additional two-stranded β-sheet occupies roughly the position where the helical hairpin is located in TCTP structure (see Fig. 2.3d,f,g). In MsrB, a short helical hairpin is present roughly at the same spatial position as the long helical hairpin of TCTP with respect to the β-tent; however, in MsrB the two helices represent insertions at the N-terminus instead of being inserted between strands β7–β8 as in TCTP. The size and the position of the helical hairpin in MsrB allows the positioning of the substrate on the solvent accessible surface of the larger β-sheet as seen in Fig. 2.3g. The similarity of TCTP and Mss4 folds has prompted studies to explore the role of TCTP in guanine nucleotide exchange. TCTP was found to be a GDP exchange inhibitor in the elongation step of protein synthesis (Cans et al. 2003). In contrast, it has been proposed to stimulate the GTP/GDP exchange on the Rheb GTP-binding protein to control mTORC1-dependent cell growth and proliferation (Dong et al. 2009; Hsu et al. 2007), although this function has been challenged (Rehmann et al. 2008; Wang et al. 2008).
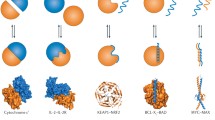
Comparison of structures showing homology to TCTP: TCTP [PDB code 1YZ1 (Susini et al. 2008)], Cereblon in complex with thalidomide [PDB Code 4CI1 (Fischer et al. 2014)], C-terminal domain of RIG-I in complex with dsRNA [PDB 3LRR (Lu et al. 2010)], DUF427 (PDB Code 3DJM, unpublished), the dimer of Mis18 [PDB Code 5HJ0 (Subramanian et al. 2016)], Mss4 in complex with Rab8 [PDB Code 2FU5, Rab8 protein is shown as a light blue surface (Itzen et al. 2006)], and MsrB in complex with the substrate Ac-Met-R-O-NHMe [PDB Code 3HCI (Ranaivoson et al. 2009)]. The structures were superimposed on their common β-tent fold. The protein ribbons are colored according to the secondary structure elements with the α-helical, β-strand, and coil regions colored cyan, magenta, and rose, respectively. For the dimeric Mis18, the two monomers are colored with different schemes. When available, zinc ions are represented as green spheres, and ligands are shown in red
The Protein DataBank was recently interrogated for structural similarities (Amson et al. 2013; Lupas et al. 2015) and several new TCTP structural homologues were identified, although the proteins shared very little sequence homology: Cereblon, Mis18, RIG-I, and DUF427 (Fig. 2.3). Because structural similarities could reveal hints about yet unknown TCTP function, we will describe those proteins from a structural but also interactome perspective.
Cereblon (CRBN) is a multidomain protein that interacts with the damaged DNA-binding protein 1 (DDB1) and forms one component of the CUL4–RBX1–DDB1–CRBN E3 ubiquitin ligase complex to regulate the selective proteolysis of key proteins in DNA repair, replication, and transcription (Iovine et al. 2011). The N-terminal extremity of Cereblon contains the LON protease domain and the DDB1-binding region whereas the C-terminal extremity contains the CULT domain [Cereblon domain of Unknown activity, binding cellular Ligands and Thalidomide (Lupas et al. 2015)] that shows structural homology to TCTP. Cereblon has been identified as the primary teratogenic target of the well-known thalidomide drug (Ito et al. 2010), and the thalidomide-binding region is located in the CULT domain (Fischer et al. 2014) (see Fig. 2.3a). The binding of the immunomodulatory (IMiD) agents such as thalidomide to Cereblon inhibits ubiquitination of the CUL4–RBX1–DDB1–CRBN E3 ubiquitin ligase substrates and redirects the enzyme towards new protein targets such as the ikaros family of transcription factors IKZF1 and IKZF3 (Fischer et al. 2014) or casein kinase 1α (CK1α) (Kronke et al. 2015). This IMiD-induced reprogramming of CUL4–RBX1–DDB1–CRBN E3 ubiquitin ligase relies on novel interactions at the surface of the IMiD–CULT domain complex (Petzold et al. 2016) that allows new substrate recognition such as CK1α. Cereblon is involved in several protein–protein interactions including BKCa, ClC-2, AMPK, PSMB4, ikaros and aiolos (IKZF3), and MEIS2 as well as with Ago2 (Xu et al. 2016).
The protein Mis18 is a component of the kinetochore, an essential actor in centromere localization. In S. pombe, Mis18 acts as an obligatory homodimeric form mediated by the N-terminal Yippee-like domain that adopts a β-tent conformation (Subramanian et al. 2016) (see Fig. 2.3e). The interface of the dimer is stabilized by strong interactions between the three-stranded β-sheets of the two protomers. An additional α-helix at the C-terminus is involved in tetramerization (not visible in fig 2.3e). In human Mis18, oligomerization is conserved but involves an heterodimer formed between two Mis18 isoforms, Mis18α and Mis18β that share 29% identity (Subramanian et al. 2016). Within the Mis18 complex, the Mis18α and Mis18β have evolved to different functions. Mis18α interacts with the Mis18-Binding Protein 1 (Mis18BP1) through its Yippee-like domain whereas Mis18β interacts with the C terminus of CENP-C also through its Yippee-like domain (Stellfox et al. 2016). The conserved substrate-binding pocket in Mis18 is required for its function although the partners are not known exactly (Subramanian et al. 2016).
The retinoic acid-inducible gene I (RIG-I) and its homologs MDA5 and LGP2 of the RIG-I like receptors (RLR) family recognize replicating viral RNA for the innate antiviral immune response. They possess a helicase domain followed by a C-terminal conserved Yippee-like domain responsible for the binding specificity to double stranded and 5′-triphosphated single stranded RNA. RNA binding induces a major conformational change that releases RLR autoinhibition and results in the activation of type I interferon for the control of viral infection (Leung and Amarasinghe 2012, 2016). The C-terminal region that shows homology to TCTP contributes to RNA recognition through a positively charged groove formed by the large β-sheet and involves interactions essentially with strands β7–β8–β9–β10–β4–β3 (Lu et al. 2010; Cui et al. 2008) (see Fig. 2.3b).
The DUF427 protein also belongs to the same structural family as judged from its 3D structure (see Fig. 2.3c). The function of this protein is currently unknown. As proposed before (Lupas et al. 2015), the glutathione-dependent formaldehyde-activating enzyme (GFA) that catalyzes the formation of S-hydroxymethylglutathione from formaldehyde and glutathione also shares some structural similarities with the abovementioned proteins. We did not include this enzyme in the analysis because the similarities are restricted to a structural subdomain.
One common feature of a subset of proteins from the β-tent family is to bind a zinc (Zn2+) ion at the apex of the β-tent. This zinc ion is present in Mis18, Cereblon, RIG-I, and MSS4 and is coordinated by two conserved CXXC motifs. It has been demonstrated that zinc binding is essential for RIG-I in vivo (Cui et al. 2008), and the zinc-binding site may contribute to stabilize the two β-sheets forming the β-tent. In contrast, the cysteines required for zinc binding are largely absent in TCTP and DUF427 and most often are lacking in MsrB. Accordingly, zinc binding has not been reported for these proteins. Consequently, zinc likely does not play any substantial role for these proteins. Alternative processes therefore stabilize the β-tent fold in proteins lacking the zinc-binding site. We proposed that the conserved aspartates D6/D11 could contribute to the TCTP fold (vide supra).
Proteins from the β-tent fold family share no detectable sequence homology and have very distinct biological activities and functions. They bind a wide spectrum of compounds ranging from small molecules to nucleic acids and proteins. As noted in a recent survey (Lupas et al. 2015), Cereblon, RIG-I, and MsrB bind partners (small molecules or RNA) through the solvent-exposed large C-terminal β-sheet. This is clearly illustrated in Fig. 2.3 that shows the similar positioning of the ligands (shown in red) sitting on the same face of the large β-sheet. It is likely that the β-tent conformation has converged to expose this binding surface. In TCTP, the binding position is occupied by the helix hairpin, which reinforces the versatility of the C-terminal β-sheet to bind various molecular types, including internal peptide resulting from gene evolution. The presence of the helical hairpin in TCTP clearly hampers binding of TCTP ligands on the C-terminal β-sheet but, at the same time, provides a novel surface formed by the helix hairpin. As shown in Fig. 2.3, other proteins adopting the β-tent fold expose other interfaces for interaction. In Mis18, homo- or heterodimerization occur through the smaller β-sheet. Another example of the versatility of the β-tent to expose binding surface is provided by the structure of the Mss4/Rab8 complex. In this structure, the stretch encompassing helix α1 and the following strand β7 from Mss4 is largely involved in the interaction with Rab8. Taken together, these proteins most likely result from a convergent process to adopt the β-tent fold that can accommodate various binding modes and binding partners. Not surprisingly, structural elements that represent extensions when compared to the minimal fold also occupy known binding interface. This is for example illustrated by the helical hairpin of TCTP and the long insertion between strands β3 and β4 in Mss4 that both interact with the C-terminal β-sheet. Taken together, TCTP belongs to a large structural family that exposes different binding surfaces and has the ability to interact with molecules of various types. Due to this diversity, it is difficult to predict the interactome and the function for members from this family on the sole basis of the fold.
2.2.3 Functional Elements Within TCTP Sequence
2.2.3.1 Conserved Signatures
TCTP amino acid sequences are highly conserved across eukaryotic cells, including in animal and plant kingdom, as well as in yeast (Hinojosa-Moya et al. 2008) (Fig. 2.2). TCTP homologues have also been detected in spider venom, C. elegans or various parasites. Two primary regions of high sequence homology were identified and termed TCTP1 and TCTP2 signatures (Thaw et al. 2001). TCTP1 is an eight amino acid sequence (consensus sequence IG[A-G]N[A-P]SAE) located between residues 48 and 55 in the flexible loop (we use here by default human TCTP numbering, see Fig. 2.2) and is largely hydrophilic. Because this region is not involved in the stabilization of the overall fold of the protein, its conservation is most likely related to functional constraints, such as protein–protein interaction or posttranslational modifications. The residue S53 is a predicted phosphorylation site for PI3K kinase and its phosphorylation was observed in human cell line during mitosis (Dephoure et al. 2008), but not confirmed in two other studies (Maeng et al. 2015; Zhang et al. 2012). The conservation of S53 in TCTP1 signature might therefore be related to TCTP regulation. The TCTP2 signature is located between residues 133 and 151 and largely conserved residues are: F134-F135 in strand β8, G137-E138-M140-D143 in the following β7–β8 loop, and Y151 at the C-terminal extremity of strand β9. Many of these residues are solvent-exposed and do not contribute significantly to the 3D TCTP fold, suggesting that their conservation reveals nonstructural evolutionary pressure. Beyond TCTP1 and TCTP2, other residues are extremely well conserved in TCTP, including D6, D11, E12, D16, L78, K93, F114, V156, and K171. D6 and D11 have already been discussed. E12 has been shown to be essential for protein–protein interaction (Dong et al. 2009; Hsu et al. 2007; Hong and Choi 2016) and for TCTP guanine nucleotide exchange (GEF) activity (Dong et al. 2009; Hsu et al. 2007). The conserved solvent exposed residue K93 in helix α2 is involved in protein–protein interaction (Wu et al. 2015).
2.2.3.2 Functional Motifs in TCTP
2.2.3.2.1 TCTP Contains a Noncanonical Cell-Penetrating Peptide
TCTP is able to spontaneously penetrate cells of various types but also multiple organs (Kim et al. 2011a). This property is associated to a protein transduction domain (PTD) corresponding to the first ten residues of human TCTP (MIIYRDLISH) (Kim et al. 2011a). The internalization seems to involve lipid raft-mediated endocytosis and macropinocytosis (Kim et al. 2011a, 2015). The mechanism is not yet understood and seemingly differs from other known protein transduction domain, in the sense that it does not involve recognition by the cell surface heparin sulfate (Kim et al. 2011a). TCTP–PTD has been advantageously used as a cargo for the internalization of fused peptides or proteins with potential in drug delivery (Bae and Lee 2013; Kim et al. 2011b; Lee et al. 2011). Nevertheless, the biological meaning of the cell-penetrating property of TCTP is not yet clarified. A recent study pointed out that extracellular TCTP is sufficient to reprogram intracellular signaling pathways to promote migration and invasiveness in colorectal cancer cells (Xiao et al. 2016), which strongly supports the idea that TCTP cell penetration may play (patho)physiological roles. In these processes, TCTP import may mirror the exosome-mediated TCTP export process for cell-to-cell communication (Amzallag et al. 2004). From a structural perspective, the PTD domain encompasses the first strand β1 and the following loop β1–β2 that form the central part of the smaller β-sheet. In TCTP structure, most of the side chains of the PTD are accessible for interaction at the surface. However, the 3D conformation of the peptide seems not to be required for cell penetration since the 10-mer TCTP–PTD peptide can efficiently transport various molecules, although it probably lacks stable 3D conformation. Hence, it is not clear whether TCTP remains folded or undergoes severe unfolding during cell internalization.
2.2.3.2.2 TCTP Contains a Noncanonical BH3-like Domain
The anti-apoptotic Bcl-xL protein is a partner of TCTP, and the N-terminal fifty residues of TCTP were identified to contribute to TCTP/Bcl-xL interaction (Yang et al. 2005). An in-depth sequence comparison with classical BH3 domains, that are known as Bcl-xL ligands, revealed that TCTP contains a BH3-like domain between residues 16 and 27 (Thebault et al. 2016). BH3 domains usually fold as an α-helix in protein–protein complexes and are characterized by highly conserved residues at positions h1, h2, h3, and h4 that line on one face of the α-helix and that contribute to stabilize the helix in the BH3-binding groove of the partners, such as Bcl-xL. Compared to classical BH3 domains, the TCTP BH3-like domain contains the conserved residues I20, I23, and L27 at h2, h3, and h4 positions, respectively, but lacks the hydrophobic residue commonly found at h1 position in canonical BH3 domains (see Fig. 2.2). In TCTP, the h1 position is occupied by the strictly conserved D16. Accordingly, the structure of Bcl-xL in complex with a peptide derived from TCTP11–31 sequence showed that residues 16–27 of TCTP folds into a α-helix that occupies the classical BH3-binding groove of Bcl-xL (Thebault et al. 2016). Surprisingly, instead of decreasing the anti-apoptotic activity of Bcl-xL, as would be expected from competition of TCTP BH3-like domains with canonical BH3 domains at the same binding groove on Bcl-xL, TCTP appears to potentiate the anti-apoptotic activity of Bcl-xL through a yet unknown mechanism (Thebault et al. 2016). Whether TCTP BH3-like domain acts also on other BH3-binding proteins, such as Mcl-1, remains to be investigated.
2.2.3.2.3 TCTP Contains an ADP/Cofilin Motif
TCTP interacts with the actin cytoskeleton (Bazile et al. 2009). The comparison of the primary sequences of TCTP and of ADF/cofilin, a family of actin-binding proteins that destabilize actin filaments, unveiled a region of high sequence homology (Tsarova et al. 2010). Indeed, the stretch of residues G69 to E105 that encompasses the helices α1 and α2 and the intervening strand β7 shows significant conservation with the G-actin-binding site of cofilin (Tsarova et al. 2010). Accordingly, TCTP preferentially binds to the globular actin (G-actin) than to filamentous actin (F-actin), but TCTP binding does not alter actin dynamics (Tsarova et al. 2010).
2.2.3.3 Posttranslational Modifications of TCTP
Several posttranslational modifications are predicted on mammalian TCTP. The ELM server (Dinkel et al. 2016) (http://elm.eu.org) predicts for TCTP solvent-exposed regions the following modifications: cleavage sites for caspases 3 and 7, glycosaminoglycan or N-glycosylation attachment site (S53), CK2 phosphorylation sites (residues S9, S37), or Polo-like kinase-1 (Plk1) phosphorylation sites (S46, S64, T65 and S82). Experimentally, only a few posttranslational modifications have been observed.
Biologically important phosphorylations occur at residues S46 and S64. In vivo, the polo-like kinase Plk1 phosphorylates these two serines to detach TCTP from the mitotic spindle for proper mitosis (Yarm 2002). The Plk1-dependent phosphorylation of TCTP contributes to the subcellular localization of TCTP (Yarm 2002; Cucchi et al. 2010; Lucibello et al. 2015). S46 phosphorylation has been proposed to be a biomarker of Plk1 level and kinase activity, with potential interest in antitumor drug design strategy targeting Plk1 (Cucchi et al. 2010) and is observed in mitotic cells (Dephoure et al. 2008). In vitro, the activated Plk1 can phosphorylate TCTP at position S46 but not at position S64 (Johnson et al. 2008). A hierarchical mechanism by which S64 phosphorylation occurs only when S46 is already phosphorylated has also been proposed (Yarm 2002). The serine S46 is conserved in higher eukaryotes whereas S64 is only partially conserved (conserved in mammalian but not in chicken sequences for example, see Fig. 2.2a). Therefore, the impact of S46/S64 phosphorylations is limited to higher eukaryotes (Johnson et al. 2008). Mutations of serines 46 or 64 to glutamate residues abrogate TCTP binding to MDM2 and to the drugs sertraline and thioridazine (Amson et al. 2012). Considering that these mutations mimic phosphoserines, it might indicate that TCTP phosphorylation could also perturb TCTP interactome. Furthermore, it has been proposed that phosphorylated TCTP could be a target of dihydroartemisinin in cancer cells (Lucibello et al. 2015). More recently, the insulin-dependent phosphorylation of S9 and S15 has been reported (Maeng et al. 2015), albeit with yet unknown functional consequences. T39 and S53 phosphorylation have also been observed in mitotic human cell (Dephoure et al. 2008). Phosphorylation of TCTP at definite sites is therefore prone to play important roles in TCTP function.
Beyond phosphorylation, the N-glycosylation of TCTP has been reported (Teshima et al. 1998). The attachment site is not known but S53 is a serious candidate, as judged from ELM predictions. The BioGrid server (http://thebiogrid.org) reports several proteomics studies indicating that TCTP can be ubiquitinated or sumoylated. The Ubc9-mediated sumoylation of TCTP controls its subcellular localization, and the residue K164 was identified as a SUMO-1 substrate (Munirathinam and Ramaswamy 2012). The ubiquitination sites are not precisely known, although K19 and K112 could be potentially ubiquitinated (Kim et al. 2011c).
TCTP is known as IgE-dependent histamine-releasing factor (HRF) when it acts in the extracellular space during the human allergic response. The cytokine-like activity of TCTP seems to correlate with extensive posttranslational modifications that may include proteolytic cleavage, dimerization, or oxidation (Kim et al. 2013). According to the group of Lee (Kim et al. 2013), dimerization is the dominant process that activates TCTP for its extracellular cytokine-like function. TCTP contains two cysteine residues C28/C172, of which C172 seems more important for dimerization (Kim et al. 2009). This can be rationalized by the fact that residue C28 is located at the beginning of strand β4 and its side chain is completely buried, and in contrast the C-terminal C172 is largely solvent accessible and available for self-association (Kim et al. 2009). Dimers were also observed in the C172S mutant suggesting that intermolecular C28-mediated disulfide bridge also exists (Kim et al. 2009). Dimerization as a posttranslational modification might be required for TCTP recognition by its receptor during allergy (Kim et al. 2009). To date, there is no report of the intracellular existence of such covalent dimer, suggesting that the formation of covalent dimer would be specific of the extracellular function of TCTP. Obviously, the different redox potentials in the intra- and extracellular environments might control the formation of such dimers.
2.3 Binding Properties and Structural Aspects of TCTP in Complex with Ions, Small Molecules, Carbohydrates, Peptides, and Nucleic Acids
Since its discovery, the number of TCTP ligands has continuously increased. TCTP has the ability to interact with ions, small molecules, carbohydrates, nucleic acids, and proteins for its biological functions, and several small molecules or peptides have been designed to interfere with TCTP-based cellular processes. In this part, we will introduce the different TCTP non-proteic ligands (see Table 2.1).
2.3.1 Calcium Binding
Calcium (Ca2+) is one of the first molecules shown to interact with TCTP. The first evidence of calcium binding came in 1992 on the TCTP from Trypanosoma brucei parasite (Haghighat and Ruben 1992) and was further extended to other species such as in Wuchereria bancrofti (Gnanasekar et al. 2002), Brugia malayi (Gnanasekar et al. 2002), Schistosoma mansoni (Rao et al. 2002), rat (Kim et al. 2000), and human (Sanchez et al. 1997; Arcuri et al. 2004). Nevertheless, Ca2+-binding is not conserved across the phyla since TCTP from ixodid ticks (Mulenga and Azad 2005) and shrimp (Bangrak et al. 2004) does not bind calcium. The functional relevance of calcium binding to TCTP is not well understood. It has been proposed that TCTP may act as a calcium scavenger in the cytosol to protect cells against Ca2+-dependent apoptosis (Graidist et al. 2007). Accordingly, cells expressing TCTP mutant lacking the ability to bind calcium become more sensitive to thapsigargin-triggered apoptosis (Graidist et al. 2007). Following this idea, the anti-apoptotic role of TCTP could be due to multiple mechanisms including the direct interaction with anti-apoptoptic proteins (including Bcl-xL and Mcl-1) to control their activity but also by preventing the Ca2+-induced permeabilization of the mitochondrial membrane and the resulting release of pro-apoptotic molecules. The sequestering effect for calcium led recently to the hypothesis of a “buffer-like” role for TCTP to regulate cellular homeostasis by avoiding the unwanted excess of soluble ligands (Lucas et al. 2014). The interplay between TCTP and calcium is reinforced by the observation that calcium regulates TCTP at the transcriptional and posttranscriptional levels (Xu et al. 1999).
The binding of calcium was studied by different techniques that gave somehow inconsistent conclusions. In one study (Graidist et al. 2007), two rather high-affinity (~10 μM range) and one lower-affinity binding modes were detected. In contrast, recent NMR (Feng et al. 2007) and fluorescence (Lucas et al. 2014) studies detected low-affinity binding modes (mM range) but not the high-affinity binding modes, although both techniques are sensitive over an extreme wide range of affinity. The apparent discrepancy could be due to different purification protocols or binding conditions. From a structural perspective, the double mutant (E58A/E60A) looses the ability to bind calcium with high affinity (Graidist et al. 2007), indicating that these residues that are located in the long loop are crucial for the interaction. The same study revealed that calcium binding was accompanied by a change in the secondary structure of the protein, as judged from circular dichroism (CD) (Graidist et al. 2007). In the NMR study (Feng et al. 2007), the calcium-binding site was mapped to a region of the protein involving the C-terminal extremities of helix α3 and of strand β9 and the loop between strand β9 and helix α2. The oxygens from the side chains of residues N131, Q133, and D150 were proposed to coordinate Ca2+. The chemical shift and intensity changes upon calcium binding were rather limited in amplitude and localized to a few amino acid residues. This suggests that TCTP conformation and oligomeric state is well conserved upon Ca2+ interaction, thus corroborating another CD study (Lucas et al. 2014) in which no secondary structure nor oligomeric change was observed up to 50 mM calcium concentration. Calcium triggers monomerization of hemin-induced dimerization (Lucas et al. 2014), possibly through direct competition against hemin binding. Indeed, hemin and calcium seem to share a similar binding area on TCTP (Lucas et al. 2014). In their study, Lucas et al. also observed that the presence of calcium contributes to destabilize TCTP by reducing the urea concentration required for denaturation (Lucas et al. 2014).
2.3.2 Antihistaminic Drugs and the Related Sertraline/Thioridazine
Any compound leading to reduced TCTP levels in vivo may have potential antitumor activity. Accordingly, because TCTP is a histamine-releasing factor, the group of R. Amson and A. Telerman hypothesized that antihistaminic drugs inhibiting the histaminic pathway were interesting candidates in anticancer strategies (Tuynder et al. 2004). This approach was successful, and a few antihistaminic compounds such as hydroxyzine and promethazine (see Fig. 2.4) proved efficient to kill tumor cells and to decrease the level of TCTP either directly or indirectly (Tuynder et al. 2004). Even greater antitumor activity was reported for the structurally related thioridazine and sertraline (see Fig. 2.4), although they do not display antihistaminic properties (Amson et al. 2012; Tuynder et al. 2004). The drugs thioridazine and sertraline are used for their antipsychotic and antidepressive activities, respectively. The direct interactions of sertraline and thioridazine with TCTP have been confirmed by surface plasmon resonance (SPR) (Amson et al. 2012), and dissociation constant (K d) of 47 μM and 34 μM was estimated for sertraline and thioridazine, respectively. The interaction was later confirmed for sertraline by thermal shift assays (Zhang 2014). Surprisingly, whereas ligand binding usually stabilizes proteins, sertraline has a destabilizing effect on TCTP by reducing its melting temperature. Both thioridazine and sertraline disrupt the TCTP/MDM2 interaction in vitro which provides a rationale for the restored levels of p53 in cells treated with these pharmacological compounds (Amson et al. 2012).
To date, the structural information on TCTP/ligands complexes is very limited. Two TCTP mutants (S46E and S64E) loose their ability to bind sertraline and thioridazine (Amson et al. 2012), suggesting that the residues S46 and S64 are involved in the interaction with the drugs. Accordingly, the drugs do not interfere with the interactions between MDM2 and TCTP mutants (Amson et al. 2012). As already discussed, residues S46 and S64 are located in the long inserted flexible loop. However whether these residues are directly or indirectly involved in the interaction surface with ligands remains an open question. Additional high-resolution structural information is still awaited to better characterize TCTP/ligand complexes. Interestingly, because these S->E mutants can be seen as phosphoserine mimics, it is possible that Plk1-mediated phosphorylation perturbs TCTP/ligands interaction. Drug design programs targeting TCTP should therefore take into account the potential distinct binding properties of the molecules to phosphorylated and unphosphorylated TCTP in order to inhibit the proper TCTP forms in vivo.
A recent in silico docking study (Seo and Efferth 2016) provided new insights into the molecular interaction of TCTP with 12 antihistaminic compounds. The binding of levomepromazine or buclizine (see Fig. 2.4) was confirmed in vitro by microscale thermophoresis giving dissociation constants of 57 μM and 430 μM, respectively (Seo and Efferth 2016). All tested ligands (except cetirizine) were found to dock onto TCTP at the same position, in an area of the loop encompassing the stretches T39-I48 and E60-T65 that contains both S46 and S64, thus confirming that these two serines could be binding hotspots in TCTP (Seo and Efferth 2016). However, the simulation was carried out on a single conformation of TCTP, and loop flexibility and the potential conformation change of the loop upon binding were not taken into account. Therefore, the binding site derived from this study remains to be confirmed.
2.3.3 Peptides
TCTP is now an established pharmacological target in cancer- or allergy-related diseases and hence different groups are making efforts to develop peptide-based TCTP inhibitors to interfere with the protein–protein interactions network of TCTP (Kim et al. 2011d; Kadioglu and Efferth 2016).
In their study (Kadioglu and Efferth 2016), Kadioglu and Efferth carried out an in silico screening of peptide libraries and selected octamer peptides with predicted high affinity. The peptide sequences were rather similar with a consensus sequence WGQWPYHX, where the last residue X is the only difference between the different peptides. In spite of the small sequence difference, the docking poses of the different peptides segregated into two families differing by the binding groove. One groove is defined by the bottom of the long flexible loop and strands β7, β8, and β9 on one side of the larger β-sheet whereas the other groove is located on the other side of large β-sheet and includes the C-terminus of helix α3. Although the binding with TCTP was not confirmed in vitro, one peptide WGQWPYHC induced specific cytotoxicity against tumor cells in a TCTP-dependent manner without affecting normal cells (Kadioglu and Efferth 2016).
In another study (Kim et al. 2011d), the dimeric TCTP was targeted. The covalent dimer is thought to be the active TCTP state in inflammatory processes. With the aim to inhibit TCTP in chronic allergic diseases, three peptides dTBP1/dTBP2/dTBP3 were isolated by screening a phage-displayed 7-mer peptide library. Peptides dTBP2 (WYVYPSM) and dTBP3 (WEFPGWM) were shown to interact with TCTP and with the TCTP84–108 peptide corresponding to the helix α2 in TCTP. The interaction with dTBP2 was demonstrated to be specific to the dimeric versus the monomeric TCTP, and dTBP2 inhibited the cytokine-like effect of TCTP (Kim et al. 2011d). Although they have been designed for different applications and obtained by unrelated approaches, the peptides dTBP1/dTBP2 (Kim et al. 2011d) and WGQWPYHC (Kadioglu and Efferth 2016) show striking similarities: they start by a tryptophan residue and tend to contain an aromatic residue at positions 3 or 4 and a proline residue at positions 4 or 5. However, whether all these peptides share the same binding modes remain to be investigated.
2.3.4 Heme, Artemisinine, and Analogs
P. falciparum TCTP (PfTCTP) is found to be one target of the antimalarial drug artemisinin (Krishna et al. 2004; Bhisutthibhan and Meshnick 2001) and forms complexes with artemisinin and its metabolites. Covalent but also non-covalent and reversible complexes have been reported (Eichhorn et al. 2013; Bhisutthibhan et al. 1998, 1999). The artemisinin-mediated alkylation of TCTP is facilitated by the presence of hemin in particular in a reducing environment (Bhisutthibhan et al. 1998, 1999; Zhou et al. 2016). To date, the exact residues of PfTCTP involved in alkylation are not identified but could be mapped within three peptidic fragments of PfTCTP (Eichhorn et al. 2013). A more recent study showed that multiple amino acid at the N-terminus can be modified by a reactive artemisinin analog and that F12 and C19 are key residues for the interaction (Li et al. 2016a). The reaction is thought to occur through the naturally rare endoperoxide bridge (1,2,4-trioxane structure) that becomes activated by ferrous iron, such as heme, to generate free radicals. The direct binding of heme with human TCTP has been also demonstrated (Lucas et al. 2014) and involves the dyad H76–H77. It was proposed that hemin and calcium shares a common binding pattern on TCTP (Lucas et al. 2014) and, accordingly, competes with each other. Upon complex formation with heme, TCTP forms dimers, which can be easily disrupted by calcium (Lucas et al. 2014). Therefore, ligand binding is prone to conduct to oligomers of TCTP. In P. falciparum, heme/TCTP interaction could be important for the fate of artemisinin in the parasite. Indeed, TCTP is associated with the parasite food vacuoles that are rich in hemin, as a product of degradation of hemoglobin by the intraerythrocytic parasite (Slomianny 1990; Abu Bakar et al. 2010; Klonis et al. 2011). However, it is yet not fully demonstrated if such mechanism can explain the antimalarial mode of action of artemisinin.
The heme-assisted artemisinin-alkylation of TCTP could potentially affect the various TCTP-related functions. Artemisinin can be effective in cancer (Crespo-Ortiz and Wei 2012; Krishna et al. 2008), and it has been proposed that artemisinin could adopt a similar mode of action in human cells as in parasites. Interestingly, dihydroartemisinin, a metabolite of artemisinin, binds human TCTP in vitro (K d of 38 μM) and reduces TCTP half-like in a proteasome-dependent manner by increasing its ubiquitination (Fujita et al. 2008). Furthermore, an artemisinin analog targets human TCTP in HeLa cancer cells (Zhou et al. 2016), suggesting that artemisinin might covalently interact with TCTP from different organisms. PfTCTP shares 35% sequence identity with human TCTP, and the structures of the two proteins are very similar (Eichhorn et al. 2013). Therefore, a deeper characterization of the interaction of artemisinin with TCTP and its derivatives would be helpful for a better understanding of its antimalarial activity, which is still largely unknown (O’Neill et al. 2010), but also of the role of TCTP in cancer biology.
2.3.5 Nucleic Acids
TCTP has been isolated from a search for proteins binding to the mouse oct4 promoter region using radioactively labeled DNA incubated in Xenopus oocyte extract. The direct interaction between TCTP and the steroidogenic factor-1 (Sf1) site of oct4 promoter was demonstrated in vivo from two independent studies carried out in Xenopus (Koziol et al. 2007) and in mouse pluripotent cells (Cheng et al. 2012). The first 60 amino acids of TCTP appear to be sufficient for Sf1 binding (Cheng et al. 2012). Three studies assessed the function of TCTP as a transcription factor with diverse outputs (Koziol et al. 2007; Cheng et al. 2012; Johansson and Simonsson 2010). In one study carried out in Xenopus (Koziol et al. 2007), the transcription of a subset of genes including oct4 was activated by TCTP. In their study, Johansson et al. (Johansson and Simonsson 2010) did not observe a change in oct4 transcription upon shRNA knockdown of TCTP but observed that TCTP interacts with Oct4 protein in vivo. They proposed a mechanism in which TCTP controls oct4 transcription by perturbing the self-regulatory transcriptional properties of the Oct4 transcription factor. The third study (Cheng et al. 2012) confirmed the binding of TCTP to the Sf1 site of oct4 promoter in vivo, but demonstrated that DNA binding of TCTP negatively regulated the expression of Oct4 in mouse pluripotent cells. They proposed that different epigenetic modifications in amphibian oocytes and mammalian cells could explain the conflicting results. We retain from these works that TCTP has also the ability to act as a transcription factor, although the direct interaction between TCTP and DNA has to be confirmed in vitro.
TCTP has been captured in a systematic approach targeting RNA-binding proteins in HeLa cells (Castello et al. 2012). In this study, a “zero-distance” strategy was used to select direct contacts between proteins and RNA and to avoid protein–protein crosslinks. This work therefore suggests that TCTP has also the ability to directly bind RNA in cellulo (Castello et al. 2012).
2.3.6 Bombyx mori TCTP as a Binding Platform for Saccharides
In the silkworm Bombyx mori, BmTCTP is produced in intestinal epithelial cells and is released into the hemolymph and gut lumen in response to oral microbial infection (Wang et al. 2013). A study exploring the interaction of BmTCTP with a range of pathogen-associated molecular patterns (PAMP) revealed the broad binding spectrum of BmTCTP (Wang et al. 2013). BmTCTP interacts with chitin, a polymer formed of N-acetylglucosamine, and mixtures of E. coli lipopolysaccharides (LPS) or of B. subtilis peptidoglycans (PG). The binding of TCTP with highly negatively charged bacterial wall molecules was proposed to involve the lysine residues at the surface of TCTP (Wang et al. 2013). BmTCTP also tends to bind bacteria such as Bacillus bombyseptieus or Serratia marcescens. In response to PAMP, BmTCTP induces the production of antimicrobial peptides through the ERK pathway (Wang et al. 2013). Therefore, BmTCTP contributes to the insect intestinal immunity by acting as opsonin to enhance phagocytosis. To the best of our knowledge, no mammalian TCTP has been reported to date to bind saccharides (other than nucleic acids). Considering that peptide cell-penetration often involves recognition of cell-surface carbohydrates, this study on BmTCTP could inspire future research for a better characterization of the mechanism of TCTP cell penetration.
2.4 Structural Aspects of TCTP in Complex with Proteins
2.4.1 TCTP Directly Interacts with Dozens of Proteins
Over the years, the number of proteins that interact in vivo with TCTP has progressively increased and several dozens of TCTP partners have been identified and further confirmed in vitro, by pull-down assays for example. One review by Amson et al. (2013) reported the extensive list of partners known in 2013. These partners were classified according to their functions as anti-apoptotic, GTPases, p53 axis, cytoskeleton/mitotic machinery, DNA processing and repair, and RNA/ribosome/protein biogenesis. This protein repertoire has continuously expanded to include, for example, proteins such as 14-3-3 (Le et al. 2016), Apaf-1 (Jung et al. 2014), HSPA9 (Li et al. 2016b), YBX1 (Li et al. 2016b), HSP27 (Katsogiannou et al. 2014), peroxiredoxin-1 (Chattopadhyay et al. 2016), ATG16 complex (Chen et al. 2014), nucleolin (Johansson et al. 2010a), or IgE/IgG (Kashiwakura et al. 2012).
Despite the ever-accumulating evidence of the functional importance of TCTP and of its interaction with partners, the amount of structural information regarding protein–protein interaction (PPI) is yet rather limited. Each discovery of novel TCTP-related PPI is often associated to attempts to decipher the molecular basis of the PPI through peptide fragments approaches. In such strategies, peptides derived from the native proteins are designed, and the analysis of the preservation of peptide–peptide contacts leads to the identification of the protein region(s) important for the interaction under scrutiny. The TCTP-related PPI analyzed using peptide fragments were summed up in a review in 2012 (Kawakami et al. 2012). Because several novel interactions have been identified and characterized meanwhile, we propose an updated table of interactions in Table 2.2. Partners identified by coimmunoprecipitation or two-hybrid techniques may be indirect by the implication of a third partner. It is, therefore, crucial to confirm the direct interaction in vitro between recombinant proteins. In this table, two types of interaction were selected amongst the long list of known TCTP partners. On the one hand, we listed interactions confirmed in vitro, whether the biological impact is known or not. On the other hand, we chose interactions with clear biological impacts although the involvement of a third partner is not ruled out yet. The second type was included to foster future in vitro study to confirm biologically relevant interactions.
Table 2.2 clearly illustrates the versatility of TCTP to bind proteins of distinct cellular functions but also biochemical functions (enzymes, DNA/RNA/protein-binding proteins, scaffold proteins, …). The consequences of TCTP binding range from direct enzyme activation or inhibition, protein stabilization by promoting or preventing ubiquitination, protein stabilization in response to heat shock, facilitating or hindering the recruitment of other partners, and the control of phosphorylation of the partner. To play all these functions, TCTP evolved to interact with a large interactome and despite its relatively small size, it proposes different binding modes. Table 2.2 suggests that almost all TCTP regions are potentially involved in the direct interaction with partners. For example, when mutated, residues Y4, E12, I20, R21, E22, D25, E138, or E168 abrogate binding to a range of partners. All these residues cover a wide surface on TCTP, reinforcing the idea that TCTP does not expose a unique interface for interaction. This parallels the many binding modes observed for the proteins from the β-tent family (see Fig. 2.3).
2.4.2 Structural Information on Native Complexes
The strategy consisting in deleting large portions of protein is extremely efficient when it comes to isolate interacting domains from multidomain proteins. This approach is also useful to identify short peptide fragments from independent folding units such as protein globular domains, in particular when these fragments folds as helices at the interface of protein–protein complex. Nevertheless, short fragments might not properly fold or keep the same 3D conformation as in the native protein. In such situation, the peptide fragment approach is prone to give false negative results. Oppositely, the disruption of the 3D native fold in short fragments is prone to facilitate nonnative interaction, leading to potential false positive results. These limitations may explain some discrepancies observed in the dissection of TCTP interactions as reported in Table 2.2, such as with the p53 or MDM2 partners. For these reasons, proper interaction analysis are better carried out with native proteins, preferentially with full length proteins or at least by preserving folding units, followed by point mutations. To date, our understanding of TCTP interactions using native proteins is limited to the TCTP/eEF1Bδ complex, for which a high-resolution structure have been obtained from a mixed approach based on classical NMR-based structure determination followed by molecular docking driven by experimental NMR data (Wu et al. 2015) (see Fig. 2.5a). This structure was validated by extensive site-directed mutagenesis and highlighted the role of the helical hairpin (Site I) and of a surface patch (Site II) formed by the stretch connecting helices α1 and α2 and including strand β7 (around F83) and the loop β8–β9 (containing M140 and P142) in the interaction with the CAR domain from eEF1Bδ (Fig. 2.5a). In the complex, the negatively charged N-terminus of CAR adopts an extended conformation that wraps around the positively charged helical hairpin, including residues K90, I92, K93, M96, K97, K100, M115, T116, A118, A119, and I122. At its C-terminus, CAR adopts an α-helical conformation that docks on a surface overlapping sites I and II through hydrophobic contacts with F83, M140, P142 and electrostatic contacts with D143 and D94. Electrostatic and hydrophobic interactions both contribute to stabilize the complex.
High-resolution structures or structural models of TCTP in interaction with protein partners. (a) NMR-based structure of TCTP in interaction with the CAR domain of eEF1Bδ (Wu et al. 2015). The two proteins are shown as ribbons with TCTP and eEF1Bδ in green and cyan, respectively. Two orthogonal views are shown. Picture taken with permission from Wu et al. (2015). (b) Energy-minimized structural model of the complex between TCTP and the N-terminal domain of human MDM2 obtained from molecular docking based on peptide deletion analysis and MDM2 M62A mutant (Funston et al. 2012). Picture taken with permission from Funston et al. (2012). (c) Structure of the full-length Bcl-xL in complex with the BH3-like domain of TCTP (residues 16–27) [PDB code 4Z9V (Thebault et al. 2016)]. The proteins are shown as ribbons. Bcl-xL (cyan and green) crystallized as a swap dimer and six BH3-like peptides were observed (magenta, yellow, orange, grey, salmon, marine). Only the magenta and yellow peptides were considered meaningful, while the others were considered as crystallization artifacts (Thebault et al. 2016). (d) Potential TCTP dimer structure. The chains A and D from the 1YZ1 crystal structure of TCTP are represented as ribbons. The two monomers have different color codes for their secondary structures. The amino acid residues at the dimer interface are labeled
This work provides the most convincing study of the key role of the helical hairpin in TCTP PPI. It is most likely that this structure element is also involved in other PPIs as suggested from other studies (Amson et al. 2012; Kashiwakura et al. 2012; Rid et al. 2010; Gachet et al. 1999; Rho et al. 2011; Funston et al. 2012), with strong functional impacts such as in apoptosis (Susini et al. 2008). Another hint about the key role of the helical hairpin is provided by a structural model of the complex formed by TCTP and the N-terminal domain of MDM2 (Fig. 2.5b). The molecular interface of this complex has been well characterized on MDM2 side by competition with with a well-known MDM2 binder (nutlin-3) or by using MDM2 mutants (M62A). However, the interface on TCTP still awaits validation with high resolution structural data from native protein and/or from point mutations. This could also resolve the somehow inconsistent results obtained from independent studies on TCTP/MDM2 interaction (Amson et al. 2012; Funston et al. 2012).
The TCTP/Bcl-xL complex is the only TCTP complex for which a crystal structure has been obtained (Fig. 2.5c). The TCTP/Bcl-xL complex with full-length proteins could be purified but only a complex of Bcl-xL with a peptide derived from the TCTP BH3-like domain was crystallized. This structure illustrates how the TCTP BH3-like peptide binds to the BH3-binding groove of Bcl-xL (Thebault et al. 2016). The crystal contained a swapped dimeric Bcl-xL, which appears to be an hallmark of this protein. Six TCTP peptides were observed, although only two of them were considered as significant. This work (Thebault et al. 2016) provided sound basis to demonstrate the existence of a functional BH3-like element in TCTP, but at the same time, raises novel questions. The BH3-like domain (residues 16 to 27) folds as strand β3 in the native TCTP structure and undergoes a severe conformational rearrangement in the complex with Bcl-xL. Because residues 16 to 27 play a key role in stabilizing TCTP larger β-sheet, it is difficult to predict the impact of the global conformation change of TCTP in the complex: is strand β3 the only element being affected? does TCTP completely unfold and remain unfolded at the exception of the helical BH3 region? does TCTP remold into another stable conformation unrelated to the fold of the unbound protein? does other region than the BH3 region of TCTP interact with Bcl-xL? Because TCTP binds at the Bax-binding groove of Bcl-xL, one would expect that TCTP competes with Bax to inhibit the Bax-mediated anti-apoptotic activity of Bcl-xL. Paradoxically, TCTP appears to potentiate the anti-apoptotic activity of Bcl-xL (Thebault et al. 2016). The apparently counter-intuitive structural mechanism by which TCTP activates Bcl-xL remains largely unknown. Clearly, additional structural, thermodynamic, and kinetic studies on full-length proteins are required to assess the plasticity of TCTP and to understand its role to control apoptosis.
2.4.3 TCTP Tends to Self-associate
TCTP tends to self-associate. For example, non-covalent dimers/oligomers of rat TCTP have been detected from yeast two-hybrid system (Yoon et al. 2000). The deletion of the region 126–172 resulted in loss of self-interaction of TCTP in vivo (Yoon et al. 2000). The authors concluded that this region was involved in oligomer formation. The amino acids 126–172 encompass the last four β-strands of TCTP that largely contribute to create the large β-sheet at the core of TCTP structure. Hence, its deletion is prone to severely impact the proper folding of TCTP and its oligomerization properties. It is therefore highly possible that the peptide missing residues 126–172 is not able to oligomerize because of the global unfolding of the protein, which questions the involvement of these residues in oligomerization of the native TCTP. As noted before, heme tends to favor TCTP dimer in vitro with potential role in cellular homeostasis (Lucas et al. 2014). The details of the dimer interface have not been investigated yet. In the analysis of TCTP structures so far available, we have noticed that human wild-type and E12V mutant TCTP crystallize with four molecules in the unit cell although they were obtained in different space groups. Interestingly, in the two crystals, an intermolecular interface and a relative protein orientation were clearly conserved within a pair of molecules. This is illustrated by the contacts between chains A and D from the human wild-type structure (Fig. 2.5d). Because this self-association mode is observed in different crystal packings, it is possible that the interactions at this interface of these two molecules are strong enough to exist in solution. We propose that the complex observed between chains A and D could represent the structure of TCTP dimer in solution. The proposed interface is formed by the hydrophilic and charged residues such as E80, S82, T84, Q130, and K133 as highlighted in Fig. 2.5d.
2.5 Conclusions
In this chapter, we have presented the structural features of TCTP with a focus on its posttranslational modifications and interaction network. Despite its relative small size and globular nature, the TCTP structure is extremely versatile and is able to interact with ions, small molecules, carbohydrates, nucleic acids, and proteins. Although high-resolution structures and more precise delineation of complex interfaces are still required, it seems that most TCTP surface patches are potential binding hotspots, which might be the hallmark of proteins from the β-tent family.
To date, our knowledge of the structural property of TCTP in interaction is still very limited. Although the structure of TCTP is apparently highly stable, there are some lines of evidence that TCTP is prone to major rearrangement upon interaction. On the one hand, TCTP interacts with Bcl-xL by the BH3-like domain that is partially buried in the unbound TCTP, suggesting that TCTP undergoes a severe conformational change in the complex. This could explain the difficulty to form the TCTP/Bcl-xL complex (Thebault et al. 2016). In this regard, TCTP also interacts with the Bcl-xL-related Mcl-1, although it is not clear yet if the interaction is also mediated by the BH3-like domain. The formation of TCTP/Mcl-1 complex is also rather difficult and is greatly facilitated by the truncation of the first ten residues (Liu et al. 2005). It is likely that the removal of the N-terminal residues prevents the proper folding of TCTP. Thus, by alleviating the kinetically unfavorable unfolding barrier, this truncated TCTP form probably already exposes interacting residues, possibly in the BH3-like region, thus making the formation of the complex easier. Such a truncated form was also proved to be more active to trigger the IgE response (Kim et al. 2009). Shortened TCTP has not yet been observed in vivo but a TCTP isoform lacking the first 34 amino acids is reported by UniProt (P13693-2), which could have variable binding properties compared to the canonical TCTP isoform. On the other hand, TCTP penetrates cells by using a protein transduction domain that folds as a β-strand. It is possible that TCTP also undergoes a significant fold rearrangement during cell entry to facilitate recognition and internalization. Future researches are necessary to confirm these hypotheses. Importantly, it will be crucial to identify the molecular triggers, to assess the extent of the conformational change, and to assess the functional consequences of the rearrangement on the partners. We speculate that TCTP plasticity greatly contributes to the versatility of its effects on partners and to its multifunctional nature.
One intriguing feature of TCTP is the 30–33 amino acid long flexible loop. This region is very well conserved throughout the phylum, both in length and amino acid composition and contains the TCTP1 signature at its center. Compared to structured regions, flexible regions are in general less under evolution constraints of keeping structurally important amino acids and are therefore prone to vary in length and composition. The conservation of the loop in TCTP therefore suggests that other forces drive its conservation during evolution. In particular, one may wonder if the loop directly interacts with partners, if it controls the access to other binding hotspots on TCTP, or if it contributes to the proposed conformational rearrangement. For this, it is crucial to characterize complexes between native proteins, with a focus on the dynamics of this loop. The loop might be involved in other regulatory events such as the phosphorylation of serines S46, S53, or S64. Being part of TCTP1 signature, S53 is strictly conserved and its phosphorylation could regulate biological functions shared throughout the phylum. In contrast, S46 and S64 are found only in mammalian TCTP and most likely regulate mammalian-specific functions. The impact of phosphorylation on the dynamics of the loop and, beyond, on the structure of the protein will also provide insights into the role of the loop.
Although our knowledge of TCTP functions has greatly expanded over the last years, much remains to be done to characterize the biochemical and structural features of TCTP. No doubt that such gain in knowledge will contribute to decipher the multiple functions of TCTP in physiological and pathophysiological processes.
References
Abu Bakar N, Klonis N, Hanssen E, Chan C, Tilley L (2010) Digestive-vacuole genesis and endocytic processes in the early intraerythrocytic stages of Plasmodium falciparum. J Cell Sci 123(Pt 3):441–450
Amson R, Pece S, Lespagnol A, Vyas R, Mazzarol G, Tosoni D, Colaluca I, Viale G, Rodrigues-Ferreira S, Wynendaele J, Chaloin O, Hoebeke J, Marine JC, Di Fiore PP, Telerman A (2012) Reciprocal repression between P53 and TCTP. Nat Med 18(1):91–99
Amson R, Pece S, Marine JC, Di Fiore PP, Telerman A (2013) TPT1/TCTP-regulated pathways in phenotypic reprogramming. Trends Cell Biol 23(1):37–46
Amzallag N, Passer BJ, Allanic D, Segura E, Thery C, Goud B, Amson R, Telerman A (2004) TSAP6 facilitates the secretion of translationally controlled tumor protein/histamine-releasing factor via a nonclassical pathway. J Biol Chem 279(44):46104–46112
Arcuri F, Papa S, Carducci A, Romagnoli R, Liberatori S, Riparbelli MG, Sanchez JC, Tosi P, del Vecchio MT (2004) Translationally controlled tumor protein (TCTP) in the human prostate and prostate cancer cells: expression, distribution, and calcium binding activity. Prostate 60(2):130–140
Bae HD, Lee K (2013) On employing a translationally controlled tumor protein-derived protein transduction domain analog for transmucosal delivery of drugs. J Control Release 170(3):358–364
Bangrak P, Graidist P, Chotigeat W, Phongdara A (2004) Molecular cloning and expression of a mammalian homologue of a translationally controlled tumor protein (TCTP) gene from Penaeus monodon shrimp. J Biotechnol 108(3):219–226
Baylot V, Katsogiannou M, Andrieu C, Taieb D, Acunzo J, Giusiano S, Fazli L, Gleave M, Garrido C, Rocchi P (2012) Targeting TCTP as a new therapeutic strategy in castration-resistant prostate cancer. Mol Ther 20(12):2244–2256
Bazile F, Pascal A, Arnal I, Le Clainche C, Chesnel F, Kubiak JZ (2009) Complex relationship between TCTP, microtubules and actin microfilaments regulates cell shape in normal and cancer cells. Carcinogenesis 30(4):555–565
Bhisutthibhan J, Meshnick SR (2001) Immunoprecipitation of [(3)H]dihydroartemisinin translationally controlled tumor protein (TCTP) adducts from Plasmodium falciparum-infected erythrocytes by using anti-TCTP antibodies. Antimicrob Agents Chemother 45(8):2397–2399
Bhisutthibhan J, Pan XQ, Hossler PA, Walker DJ, Yowell CA, Carlton J, Dame JB, Meshnick SR (1998) The Plasmodium falciparum translationally controlled tumor protein homolog and its reaction with the antimalarial drug artemisinin. J Biol Chem 273(26):16192–16198
Bhisutthibhan J, Philbert MA, Fujioka H, Aikawa M, Meshnick SR (1999) The Plasmodium falciparum translationally controlled tumor protein: subcellular localization and calcium binding. Eur J Cell Biol 78(9):665–670
Burgess A, Labbe JC, Vigneron S, Bonneaud N, Strub JM, Van Dorsselaer A, Lorca T, Castro A (2008) Chfr interacts and colocalizes with TCTP to the mitotic spindle. Oncogene 27(42):5554–5566
Cans C, Passer BJ, Shalak V, Nancy-Portebois V, Crible V, Amzallag N, Allanic D, Tufino R, Argentini M, Moras D, Fiucci G, Goud B, Mirande M, Amson R, Telerman A (2003) Translationally controlled tumor protein acts as a guanine nucleotide dissociation inhibitor on the translation elongation factor eEF1A. Proc Natl Acad Sci USA 100(24):13892–13897
Castello A, Fischer B, Eichelbaum K, Horos R, Beckmann BM, Strein C, Davey NE, Humphreys DT, Preiss T, Steinmetz LM, Krijgsveld J, Hentze MW (2012) Insights into RNA biology from an atlas of mammalian mRNA-binding proteins. Cell 149(6):1393–1406
Chattopadhyay A, Pinkaew D, Doan HQ, Jacob RB, Verma SK, Friedman H, Peterson AC, Kuyumcu-Martinez MN, McDougal OM, Fujise K (2016) Fortilin potentiates the peroxidase activity of Peroxiredoxin-1 and protects against alcohol-induced liver damage in mice. Sci Rep 6:18701
Chen Y, Fujita T, Zhang D, Doan H, Pinkaew D, Liu Z, Wu J, Koide Y, Chiu A, Lin CC, Chang JY, Ruan KH, Fujise K (2011) Physical and functional antagonism between tumor suppressor protein p53 and fortilin, an anti-apoptotic protein. J Biol Chem 286(37):32575–32585
Chen K, Chen S, Huang C, Cheng H, Zhou R (2013) TCTP increases stability of hypoxia-inducible factor 1alpha by interaction with and degradation of the tumour suppressor VHL. Biol Cell 105(5):208–218
Chen K, Huang C, Yuan J, Cheng H, Zhou R (2014) Long-term artificial selection reveals a role of TCTP in autophagy in mammalian cells. Mol Biol Evol 31(8):2194–2211
Cheng X, Li J, Deng J, Li Z, Meng S, Wang H (2012) Translationally controlled tumor protein (TCTP) downregulates Oct4 expression in mouse pluripotent cells. BMB Rep 45(1):20–25
Choi KW, Hsu YC (2007) To cease or to proliferate: new insights into TCTP function from a Drosophila study. Cell Adhes Migr 1(3):129–130
Crespo-Ortiz MP, Wei MQ (2012) Antitumor activity of artemisinin and its derivatives: from a well-known antimalarial agent to a potential anticancer drug. J Biomed Biotechnol 2012:247597
Cucchi U, Gianellini LM, De Ponti A, Sola F, Alzani R, Patton V, Pezzoni A, Troiani S, Saccardo MB, Rizzi S, Giorgini ML, Cappella P, Beria I, Valsasina B (2010) Phosphorylation of TCTP as a marker for polo-like kinase-1 activity in vivo. Anticancer Res 30(12):4973–4985
Cui S, Eisenacher K, Kirchhofer A, Brzozka K, Lammens A, Lammens K, Fujita T, Conzelmann KK, Krug A, Hopfner KP (2008) The C-terminal regulatory domain is the RNA 5′-triphosphate sensor of RIG-I. Mol Cell 29(2):169–179
Dephoure N, Zhou C, Villen J, Beausoleil SA, Bakalarski CE, Elledge SJ, Gygi SP (2008) A quantitative atlas of mitotic phosphorylation. Proc Natl Acad Sci USA 105(31):10762–10767
Dinkel H, Van Roey K, Michael S, Kumar M, Uyar B, Altenberg B, Milchevskaya V, Schneider M, Kuhn H, Behrendt A, Dahl SL, Damerell V, Diebel S, Kalman S, Klein S, Knudsen AC, Mader C, Merrill S, Staudt A, Thiel V, Welti L, Davey NE, Diella F, Gibson TJ (2016) ELM 2016–data update and new functionality of the eukaryotic linear motif resource. Nucleic Acids Res 44(D1):D294–D300
Dong X, Yang B, Li Y, Zhong C, Ding J (2009) Molecular basis of the acceleration of the GDP-GTP exchange of human ras homolog enriched in brain by human translationally controlled tumor protein. J Biol Chem 284(35):23754–23764
Eichhorn T, Winter D, Buchele B, Dirdjaja N, Frank M, Lehmann WD, Mertens R, Krauth-Siegel RL, Simmet T, Granzin J, Efferth T (2013) Molecular interaction of artemisinin with translationally controlled tumor protein (TCTP) of Plasmodium falciparum. Biochem Pharmacol 85(1):38–45
Feng Y, Liu D, Yao H, Wang J (2007) Solution structure and mapping of a very weak calcium-binding site of human translationally controlled tumor protein by NMR. Arch Biochem Biophys 467(1):48–57
Fischer ES, Bohm K, Lydeard JR, Yang H, Stadler MB, Cavadini S, Nagel J, Serluca F, Acker V, Lingaraju GM, Tichkule RB, Schebesta M, Forrester WC, Schirle M, Hassiepen U, Ottl J, Hild M, Beckwith RE, Harper JW, Jenkins JL, Thoma NH (2014) Structure of the DDB1-CRBN E3 ubiquitin ligase in complex with thalidomide. Nature 512(7512):49–53
Fleischer TC, Weaver CM, McAfee KJ, Jennings JL, Link AJ (2006) Systematic identification and functional screens of uncharacterized proteins associated with eukaryotic ribosomal complexes. Genes Dev 20(10):1294–1307
Fujita T, Felix K, Pinkaew D, Hutadilok-Towatana N, Liu Z, Fujise K (2008) Human fortilin is a molecular target of dihydroartemisinin. FEBS Lett 582(7):1055–1060
Funston G, Goh W, Wei SJ, Tng QS, Brown C, Jiah Tong L, Verma C, Lane D, Ghadessy F (2012) Binding of translationally controlled tumour protein to the N-terminal domain of HDM2 is inhibited by nutlin-3. PLoS One 7(8):e42642
Gachet Y, Tournier S, Lee M, Lazaris-Karatzas A, Poulton T, Bommer UA (1999) The growth-related, translationally controlled protein P23 has properties of a tubulin binding protein and associates transiently with microtubules during the cell cycle. J Cell Sci 112(Pt 8):1257–1271
Gnanasekar M, Rao KV, Chen L, Narayanan RB, Geetha M, Scott AL, Ramaswamy K, Kaliraj P (2002) Molecular characterization of a calcium binding translationally controlled tumor protein homologue from the filarial parasites Brugia malayi and Wuchereria bancrofti. Mol Biochem Parasitol 121(1):107–118
Graidist P, Yazawa M, Tonganunt M, Nakatomi A, Lin CC, Chang JY, Phongdara A, Fujise K (2007) Fortilin binds Ca2+ and blocks Ca2+-dependent apoptosis in vivo. Biochem J 408(2):181–191
Gu X, Yao L, Ma G, Cui L, Li Y, Liang W, Zhao B, Li K (2014) TCTP promotes glioma cell proliferation in vitro and in vivo via enhanced beta-catenin/TCF-4 transcription. Neuro-oncology 16(2):217–227
Haghighat NG, Ruben L (1992) Purification of novel calcium binding proteins from Trypanosoma brucei: properties of 22-, 24- and 38-kilodalton proteins. Mol Biochem Parasitol 51(1):99–110
Hinojosa-Moya J, Xoconostle-Cazares B, Piedra-Ibarra E, Mendez-Tenorio A, Lucas WJ, Ruiz-Medrano R (2008) Phylogenetic and structural analysis of translationally controlled tumor proteins. J Mol Evol 66(5):472–483
Hong ST, Choi KW (2013) TCTP directly regulates ATM activity to control genome stability and organ development in Drosophila melanogaster. Nat Commun 4:2986
Hong ST, Choi KW (2016) Antagonistic roles of Drosophila Tctp and Brahma in chromatin remodelling and stabilizing repeated sequences. Nat Commun 7:12988
Hsu YC, Chern JJ, Cai Y, Liu M, Choi KW (2007) Drosophila TCTP is essential for growth and proliferation through regulation of dRheb GTPase. Nature 445(7129):785–788
Iovine B, Iannella ML, Bevilacqua MA (2011) Damage-specific DNA binding protein 1 (DDB1): a protein with a wide range of functions. Int J Biochem Cell Biol 43(12):1664–1667
Ito T, Ando H, Suzuki T, Ogura T, Hotta K, Imamura Y, Yamaguchi Y, Handa H (2010) Identification of a primary target of thalidomide teratogenicity. Science 327(5971):1345–1350
Itzen A, Pylypenko O, Goody RS, Alexandrov K, Rak A (2006) Nucleotide exchange via local protein unfolding--structure of Rab8 in complex with MSS4. EMBO J 25(7):1445–1455
Jaglarz MK, Bazile F, Laskowska K, Polanski Z, Chesnel F, Borsuk E, Kloc M, Kubiak JZ (2012) Association of TCTP with centrosome and microtubules. Biochem Res Int 2012:541906
Jeon HJ, You SY, Park YS, Chang JW, Kim JS, Oh JS (2016) TCTP regulates spindle microtubule dynamics by stabilizing polar microtubules during mouse oocyte meiosis. Biochim Biophys Acta 1863(4):630–637
Johansson H, Simonsson S (2010) Core transcription factors, Oct4, Sox2 and Nanog, individually form complexes with nucleophosmin (Npm1) to control embryonic stem (ES) cell fate determination. Aging 2(11):815–822
Johansson H, Svensson F, Runnberg R, Simonsson T, Simonsson S (2010a) Phosphorylated nucleolin interacts with translationally controlled tumor protein during mitosis and with Oct4 during interphase in ES cells. PLoS One 5(10):e13678
Johansson H, Vizlin-Hodzic D, Simonsson T, Simonsson S (2010b) Translationally controlled tumor protein interacts with nucleophosmin during mitosis in ES cells. Cell Cycle 9(11):2160–2169
Johnson TM, Antrobus R, Johnson LN (2008) Plk1 activation by Ste20-like kinase (Slk) phosphorylation and polo-box phosphopeptide binding assayed with the substrate translationally controlled tumor protein (TCTP). Biochemistry 47(12):3688–3696
Jung J, Kim M, Kim MJ, Kim J, Moon J, Lim JS, Kim M, Lee K (2004) Translationally controlled tumor protein interacts with the third cytoplasmic domain of Na,K-ATPase alpha subunit and inhibits the pump activity in HeLa cells. J Biol Chem 279(48):49868–49875
Jung J, Kim HY, Maeng J, Kim M, Shin DH, Lee K (2014) Interaction of translationally controlled tumor protein with Apaf-1 is involved in the development of chemoresistance in HeLa cells. BMC Cancer 14:165
Kadioglu O, Efferth T (2016) Peptide aptamer identified by molecular docking targeting translationally controlled tumor protein in leukemia cells. Invest New Drugs 34(4):515–521
Kashiwakura JC, Ando T, Matsumoto K, Kimura M, Kitaura J, Matho MH, Zajonc DM, Ozeki T, Ra C, MacDonald SM, Siraganian RP, Broide DH, Kawakami Y, Kawakami T (2012) Histamine-releasing factor has a proinflammatory role in mouse models of asthma and allergy. J Clin Invest 122(1):218–228
Katsogiannou M, Andrieu C, Baylot V, Baudot A, Dusetti NJ, Gayet O, Finetti P, Garrido C, Birnbaum D, Bertucci F, Brun C, Rocchi P (2014) The functional landscape of Hsp27 reveals new cellular processes such as DNA repair and alternative splicing and proposes novel anticancer targets. Mol Cell Proteomics 13(12):3585–3601
Kawakami T, Ando T, Kawakami Y (2012) HRF-interacting molecules. Open Allergy J 5(41–46)
Kawakami T, Kashiwakura J, Kawakami Y (2014) Histamine-releasing factor and immunoglobulins in asthma and allergy. Allergy Asthma Immunol Res 6(1):6–12
Kim M, Jung Y, Lee K, Kim C (2000) Identification of the calcium binding sites in translationally controlled tumor protein. Arch Pharm Res 23(6):633–636
Kim M, Min HJ, Won HY, Park H, Lee JC, Park HW, Chung J, Hwang ES, Lee K (2009) Dimerization of translationally controlled tumor protein is essential for its cytokine-like activity. PLoS One 4(7):e6464
Kim M, Kim M, Kim HY, Kim S, Jung J, Maeng J, Chang J, Lee K (2011a) A protein transduction domain located at the NH2-terminus of human translationally controlled tumor protein for delivery of active molecules to cells. Biomaterials 32(1):222–230
Kim HY, Kim S, Youn H, Chung JK, Shin DH, Lee K (2011b) The cell penetrating ability of the proapoptotic peptide, KLAKLAKKLAKLAK fused to the N-terminal protein transduction domain of translationally controlled tumor protein, MIIYRDLISH. Biomaterials 32(22):5262–5268
Kim W, Bennett EJ, Huttlin EL, Guo A, Li J, Possemato A, Sowa ME, Rad R, Rush J, Comb MJ, Harper JW, Gygi SP (2011c) Systematic and quantitative assessment of the ubiquitin-modified proteome. Mol Cell 44(2):325–340
Kim M, Chung J, Lee C, Jung J, Kwon Y, Lee K (2011d) A peptide binding to dimerized translationally controlled tumor protein modulates allergic reactions. J Mol Med 89(6):603–610
Kim M, Maeng J, Lee K (2013) Dimerization of TCTP and its clinical implications for allergy. Biochimie 95(4):659–666
Kim HY, Kim S, Pyun HJ, Maeng J, Lee K (2015) Cellular uptake mechanism of TCTP-PTD in human lung carcinoma cells. Mol Pharm 12(1):194–203
Klonis N, Crespo-Ortiz MP, Bottova I, Abu-Bakar N, Kenny S, Rosenthal PJ, Tilley L (2011) Artemisinin activity against Plasmodium falciparum requires hemoglobin uptake and digestion. Proc Natl Acad Sci U S A 108(28):11405–11410
Koziol MJ, Garrett N, Gurdon JB (2007) Tpt1 activates transcription of oct4 and nanog in transplanted somatic nuclei. Curr Biol 17(9):801–807
Krishna S, Uhlemann AC, Haynes RK (2004) Artemisinins: mechanisms of action and potential for resistance. Drug Resist Updates 7(4–5):233–244
Krishna S, Bustamante L, Haynes R, Staines H (2008) Artemisinins: their growing importance in medicine. Trends Pharmacol Sci 29(10):520–527
Kronke J, Fink EC, Hollenbach PW, MacBeth KJ, Hurst SN, Udeshi ND, Chamberlain PP, Mani DR, Man HW, Gandhi AK, Svinkina T, Schneider RK, McConkey M, Jaras M, Griffiths E, Wetzler M, Bullinger L, Cathers BE, Carr SA, Chopra R, Ebert BL (2015) Lenalidomide induces ubiquitination and degradation of CK1alpha in del(5q) MDS. Nature 523(7559):183–188
Langdon JM, Vonakis BM, MacDonald SM (2004) Identification of the interaction between the human recombinant histamine releasing factor/translationally controlled tumor protein and elongation factor-1 delta (also known as elongation factor-1B beta). Biochim Biophys Acta 1688(3):232–236
Lange OF, Rossi P, Sgourakis NG, Song Y, Lee HW, Aramini JM, Ertekin A, Xiao R, Acton TB, Montelione GT, Baker D (2012) Determination of solution structures of proteins up to 40 kDa using CS-Rosetta with sparse NMR data from deuterated samples. Proc Natl Acad Sci U S A 109(27):10873–10878
Le TP, Vuong LT, Kim AR, Hsu YC, Choi KW (2016) 14-3-3 proteins regulate Tctp-Rheb interaction for organ growth in Drosophila. Nat Commun 7:11501
Leclercq TM, Moretti PA, Pitson SM (2011) Guanine nucleotides regulate sphingosine kinase 1 activation by eukaryotic elongation factor 1A and provide a mechanism for eEF1A-associated oncogenesis. Oncogene 30(3):372–378
Lee JH, Rho SB, Park SY, Chun T (2008) Interaction between fortilin and transforming growth factor-beta stimulated clone-22 (TSC-22) prevents apoptosis via the destabilization of TSC-22. FEBS Lett 582(8):1210–1218
Lee J, Kim S, Shin DH, Kim HJ, Lee K (2011) Neuroprotective effect of Cu,Zn-superoxide dismutase fused to a TCTP-derived protein transduction domain. Eur J Pharmacol 666(1–3):87–92
Leung DW, Amarasinghe GK (2012) Structural insights into RNA recognition and activation of RIG-I-like receptors. Curr Opin Struct Biol 22(3):297–303
Leung DW, Amarasinghe GK (2016) When your cap matters: structural insights into self vs non-self recognition of 5′ RNA by immunomodulatory host proteins. Curr Opin Struct Biol 36:133–141
Li W, Zhou Y, Tang G, Xiao Y (2016a) Characterization of the artemisinin binding site for translationally controlled tumor protein (TCTP) by bioorthogonal click chemistry. Bioconjug Chem 27(12):2828–2833
Li S, Chen M, Xiong Q, Zhang J, Cui Z, Ge F (2016b) Characterization of the translationally controlled tumor protein (TCTP) interactome reveals novel binding partners in human cancer cells. J Proteome Res 15:3741–3751
Liu H, Peng HW, Cheng YS, Yuan HS, Yang-Yen HF (2005) Stabilization and enhancement of the antiapoptotic activity of mcl-1 by TCTP. Mol Cell Biol 25(8):3117–3126
Lowther WT, Weissbach H, Etienne F, Brot N, Matthews BW (2002) The mirrored methionine sulfoxide reductases of Neisseria gonorrhoeae pilB. Nat Struct Biol 9(5):348–352
Lu C, Xu H, Ranjith-Kumar CT, Brooks MT, Hou TY, Hu F, Herr AB, Strong RK, Kao CC, Li P (2010) The structural basis of 5′ triphosphate double-stranded RNA recognition by RIG-I C-terminal domain. Structure 18(8):1032–1043
Lucas AT, Fu X, Liu J, Brannon MK, Yang J, Capelluto DG, Finkielstein CV (2014) Ligand binding reveals a role for heme in translationally-controlled tumor protein dimerization. PLoS One 9(11):e112823
Lucibello M, Adanti S, Antelmi E, Dezi D, Ciafre S, Carcangiu ML, Zonfrillo M, Nicotera G, Sica L, De Braud F, Pierimarchi P (2015) Phospho-TCTP as a therapeutic target of Dihydroartemisinin for aggressive breast cancer cells. Oncotarget 6(7):5275–5291
Lupas AN, Zhu H, Korycinski M (2015) The thalidomide-binding domain of cereblon defines the CULT domain family and is a new member of the beta-tent fold. PLoS Comput Biol 11(1):e1004023
Maeng J, Kim M, Lee H, Lee K (2015) Insulin induces phosphorylation of serine residues of translationally controlled tumor protein in 293T cells. Int J Mol Sci 16(4):7565–7576
Mulenga A, Azad AF (2005) The molecular and biological analysis of ixodid ticks histamine release factors. Exp Appl Acarol 37(3–4):215–229
Munirathinam G, Ramaswamy K (2012) Sumoylation of human translationally controlled tumor protein is important for its nuclear transport. Biochem Res Int 2012:831940
Niikura M, Liu HC, Dodgson JB, Cheng HH (2004) A comprehensive screen for chicken proteins that interact with proteins unique to virulent strains of Marek’s disease virus. Poult Sci 83(7):1117–1123
O’Neill PM, Barton VE, Ward SA (2010) The molecular mechanism of action of artemisinin–the debate continues. Molecules 15(3):1705–1721
Panrat T, Sinthujaroen P, Nupan B, Wanna W, Tammi MT, Phongdara A (2012) Characterization of a novel binding protein for Fortilin/TCTP–component of a defense mechanism against viral infection in Penaeus monodon. PLoS One 7(3):e33291
Petzold G, Fischer ES, Thoma NH (2016) Structural basis of lenalidomide-induced CK1alpha degradation by the CRL4(CRBN) ubiquitin ligase. Nature 532(7597):127–130
Ranaivoson FM, Neiers F, Kauffmann B, Boschi-Muller S, Branlant G, Favier F (2009) Methionine sulfoxide reductase B displays a high level of flexibility. J Mol Biol 394(1):83–93
Rao KV, Chen L, Gnanasekar M, Ramaswamy K (2002) Cloning and characterization of a calcium-binding, histamine-releasing protein from Schistosoma mansoni. J Biol Chem 277(34):31207–31213
Rehmann H, Bruning M, Berghaus C, Schwarten M, Kohler K, Stocker H, Stoll R, Zwartkruis FJ, Wittinghofer A (2008) Biochemical characterisation of TCTP questions its function as a guanine nucleotide exchange factor for Rheb. FEBS Lett 582(20):3005–3010
Rho SB, Lee JH, Park MS, Byun HJ, Kang S, Seo SS, Kim JY, Park SY (2011) Anti-apoptotic protein TCTP controls the stability of the tumor suppressor p53. FEBS Lett 585(1):29–35
Rid R, Onder K, Trost A, Bauer J, Hintner H, Ritter M, Jakab M, Costa I, Reischl W, Richter K, MacDonald S, Jendrach M, Bereiter-Hahn J, Breitenbach M (2010) H2O2-dependent translocation of TCTP into the nucleus enables its interaction with VDR in human keratinocytes: TCTP as a further module in calcitriol signalling. J Steroid Biochem Mol Biol 118(1–2):29–40
Robert X, Gouet P (2014) Deciphering key features in protein structures with the new ENDscript server. Nucleic Acids Res 42(Web Server issue):W320–W324
Sanchez JC, Schaller D, Ravier F, Golaz O, Jaccoud S, Belet M, Wilkins MR, James R, Deshusses J, Hochstrasser D (1997) Translationally controlled tumor protein: a protein identified in several nontumoral cells including erythrocytes. Electrophoresis 18(1):150–155
Seo EJ, Efferth T (2016) Interaction of antihistaminic drugs with human translationally controlled tumor protein (TCTP) as novel approach for differentiation therapy. Oncotarget 7(13):16818–16839
Slomianny C (1990) Three-dimensional reconstruction of the feeding process of the malaria parasite. Blood Cells 16(2–3):369–378
Stellfox ME, Nardi IK, Knippler CM, Foltz DR (2016) Differential binding partners of the Mis18alpha/beta YIPPEE domains regulate Mis18 complex recruitment to centromeres. Cell Rep 15(10):2127–2135
Subramanian L, Medina-Pritchard B, Barton R, Spiller F, Kulasegaran-Shylini R, Radaviciute G, Allshire RC, Arockia Jeyaprakash A (2016) Centromere localization and function of Mis18 requires Yippee-like domain-mediated oligomerization. EMBO Rep 17(4):496–507
Susini L, Besse S, Duflaut D, Lespagnol A, Beekman C, Fiucci G, Atkinson AR, Busso D, Poussin P, Marine JC, Martinou JC, Cavarelli J, Moras D, Amson R, Telerman A (2008) TCTP protects from apoptotic cell death by antagonizing bax function. Cell Death Differ 15(8):1211–1220
Telerman A, Amson R, Cans C, Nancy-Portebois V, Passer BJ (2006) US Patent 20060140970 (06/29/2006)
Teshima S, Rokutan K, Nikawa T, & Kishi K (1998) Macrophage colony-stimulating factor stimulates synthesis and secretion of a mouse homolog of a human IgE-dependent histamine-releasing factor by macrophages in vitro and in vivo. J Immunol 161(11):6356–6366
Thaw P, Baxter NJ, Hounslow AM, Price C, Waltho JP, Craven CJ (2001) Structure of TCTP reveals unexpected relationship with guanine nucleotide-free chaperones. Nat Struct Biol 8(8):701–704
Thebault S, Agez M, Chi X, Stojko J, Cura V, Telerman SB, Maillet L, Gautier F, Billas-Massobrio I, Birck C, Troffer-Charlier N, Karafin T, Honore J, Senff-Ribeiro A, Montessuit S, Johnson CM, Juin P, Cianferani S, Martinou JC, Andrews DW, Amson R, Telerman A, Cavarelli J (2016) TCTP contains a BH3-like domain, which instead of inhibiting, activates Bcl-xL. Sci Rep 6:19725
Tonganunt M, Nupan B, Saengsakda M, Suklour S, Wanna W, Senapin S, Chotigeat W, Phongdara A (2008) The role of Pm-fortilin in protecting shrimp from white spot syndrome virus (WSSV) infection. Fish Shellfish Immunol 25(5):633–637
Tsarova K, Yarmola EG, Bubb MR (2010) Identification of a cofilin-like actin-binding site on translationally controlled tumor protein (TCTP). FEBS Lett 584(23):4756–4760
Tuynder M, Fiucci G, Prieur S, Lespagnol A, Geant A, Beaucourt S, Duflaut D, Besse S, Susini L, Cavarelli J, Moras D, Amson R, Telerman A (2004) Translationally controlled tumor protein is a target of tumor reversion. Proc Natl Acad Sci U S A 101(43):15364–15369
Vedadi M, Lew J, Artz J, Amani M, Zhao Y, Dong A, Wasney GA, Gao M, Hills T, Brokx S, Qiu W, Sharma S, Diassiti A, Alam Z, Melone M, Mulichak A, Wernimont A, Bray J, Loppnau P, Plotnikova O, Newberry K, Sundararajan E, Houston S, Walker J, Tempel W, Bochkarev A, Kozieradzki I, Edwards A, Arrowsmith C, Roos D, Kain K, Hui R (2007) Genome-scale protein expression and structural biology of Plasmodium falciparum and related Apicomplexan organisms. Mol Biochem Parasitol 151(1):100–110
Wang X, Fonseca BD, Tang H, Liu R, Elia A, Clemens MJ, Bommer UA, Proud CG (2008) Re-evaluating the roles of proposed modulators of mammalian target of rapamycin complex 1 (mTORC1) signaling. J Biol Chem 283(45):30482–30492
Wang F, Hu C, Hua X, Song L, Xia Q (2013) Translationally controlled tumor protein, a dual functional protein involved in the immune response of the silkworm, Bombyx mori. PLoS One 8(7):e69284
Wu H, Gong W, Yao X, Wang J, Perrett S, Feng Y (2015) Evolutionarily conserved binding of translationally controlled tumor protein to eukaryotic elongation factor 1B. J Biol Chem 290(14):8694–8710
Xiao B, Chen D, Luo S, Hao W, Jing F, Liu T, Wang S, Geng Y, Li L, Xu W, Zhang Y, Liao X, Zuo D, Wu Y, Li M, Ma Q (2016) Extracellular translationally controlled tumor protein promotes colorectal cancer invasion and metastasis through Cdc42/JNK/MMP9 signaling. Oncotarget 7(31):50057–50073
Xu A, Bellamy AR, Taylor JA (1999) Expression of translationally controlled tumour protein is regulated by calcium at both the transcriptional and post-transcriptional level. Biochem J 342:683–689
Xu Q, Hou YX, Langlais P, Erickson P, Zhu J, Shi CX, Luo M, Zhu Y, Xu Y, Mandarino LJ, Stewart K, Chang XB (2016) Expression of the cereblon binding protein argonaute 2 plays an important role for multiple myeloma cell growth and survival. BMC Cancer 16:297
Yang Y, Yang F, Xiong Z, Yan Y, Wang X, Nishino M, Mirkovic D, Nguyen J, Wang H, Yang XF (2005) An N-terminal region of translationally controlled tumor protein is required for its antiapoptotic activity. Oncogene 24(30):4778–4788
Yarm FR (2002) Plk phosphorylation regulates the microtubule-stabilizing protein TCTP. Mol Cell Biol 22(17):6209–6221
Yoon T, Jung J, Kim M, Lee KM, Choi EC, Lee K (2000) Identification of the self-interaction of rat TCTP/IgE-dependent histamine-releasing factor using yeast two-hybrid system. Arch Biochem Biophys 384(2):379–382
Yoon T, Kim M, Lee K (2006) Inhibition of Na,K-ATPase-suppressive activity of translationally controlled tumor protein by sorting nexin 6. FEBS Lett 580(14):3558–3564
Zhang X (2014) Etude de complexes protéine-protéine impliquant la chaperone de bas poids moléculaire HSP27: Implications dans le cancer de la prostate. PhD Thesis, Aix-Marseille Université
Zhang D, Li F, Weidner D, Mnjoyan ZH, Fujise K (2002) Physical and functional interaction between myeloid cell leukemia 1 protein (MCL1) and Fortilin. The potential role of MCL1 as a fortilin chaperone. J Biol Chem 277(40):37430–37438
Zhang J, de Toledo SM, Pandey BN, Guo G, Pain D, Li H, Azzam EI (2012) Role of the translationally controlled tumor protein in DNA damage sensing and repair. Proc Natl Acad Sci U S A 109(16):E926–E933
Zhou Y, Li W, Xiao Y (2016) Profiling of multiple targets of artemisinin activated by hemin in cancer cell proteome. ACS Chem Biol 11(4):882–888
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 Springer International Publishing AG
About this chapter
Cite this chapter
Assrir, N., Malard, F., Lescop, E. (2017). Structural Insights into TCTP and Its Interactions with Ligands and Proteins. In: Telerman, A., Amson, R. (eds) TCTP/tpt1 - Remodeling Signaling from Stem Cell to Disease. Results and Problems in Cell Differentiation, vol 64. Springer, Cham. https://doi.org/10.1007/978-3-319-67591-6_2
Download citation
DOI: https://doi.org/10.1007/978-3-319-67591-6_2
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-67590-9
Online ISBN: 978-3-319-67591-6
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)