Abstract
The host immune system is continuously exposed to dying cells and has evolved to distinguish between cell death events signaling potential threats and physiological apoptosis that should be tolerated. Tumors can use this distinction to their advantage, promoting apoptotic death of cancer cells to induce tolerance and evasion of immunosurveillance. On the other hand, stimuli that cause immunogenic death of cancer cells can induce an effective anti-tumor immune response. In this chapter we discuss different forms of cell death in the tumor microenvironment, and how these interact with host immune cells to impact tumor progression and cancer therapy. We focus on how cancer cells hijack aspects of cell death to promote tumor survival, and how anti-cancer treatments that activate immunogenic death modalities give strong and durable clinical efficacy.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
5.1 Introduction
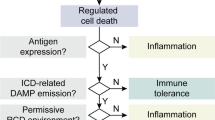
Cell death is essential to the turnover of healthy tissues in the steady state, and in an organism’s defense against pathogen infection and malignant cells. In adult humans, approximately 50–70 billion cells die each day during the normal turnover of body tissues [1]. An intricate cross-talk has evolved between cell death circuitries and the vertebrate immune system to allow distinction of potential threats from physiological cell death in healthy tissues. Physiological cell death (better known as apoptosis) is a fundamentally tolerogenic process that prevents autoreactivity to normal host tissues. Cells undergoing physiological apoptosis are cleared by phagocytic cells in a “silent” manner, thus concealing cellular components that might otherwise induce inflammation [2]. In contrast, pathological cell death (often termed necrosis) is an inherently immunogenic and inflammatory process, which alerts the immune system of an abnormality or potential threat. However, this apparent cell death dichotomy may not be as clear-cut as once thought. For example, alternative subtypes of apoptosis that are immunogenic can be initiated under certain circumstances [3, 4].
The characteristics of an immune response to cell death (e.g., immunogenic vs. tolerogenic responses) are determined by the precise molecular signaling between dying cells and local immune cells. Immune cells recognize pathogen-infected cells through pathogen-associated molecular patterns (PAMPs). PAMPs exposed by infected cells target them for immune cell-mediated killing or for phagocytosis and subsequent antigen presentation by antigen-presenting cells (APCs), which is required to induce antigen-specific immune responses [5,6,7]. Examples of PAMPs include components of viruses or bacteria, and certain sequences of the nucleic acids that form their genomes [6, 7]. Appropriate immune responses are also required in cases where there is no pathogen involved in the abnormal dying or stressing of a cell, for example in malignant pre-cancerous cells. In 1994, Polly Matzinger proposed the “danger theory,” stating that the immune system has evolved an inherent capacity to distinguish between dangerous and innocuous endogenous signals [8]. The theory is supported by evidence that malignant dying cells expose damage-associated molecular patterns (DAMPs), which function as danger signals for the host immune system [9]. PAMPs and DAMPs are recognized by germline-encoded pattern recognition receptors (PRRs) present on immune cells. Toll-like receptors (TLRs) and nucleotide oligodimerization domain (NOD)-like receptors (NLRs) are examples of two important PRR families in immune signaling [5, 10]. PRR recognition of cognate ligand can activate downstream effects such as immune cell differentiation and pro-inflammatory cytokine production, which initiate or propagate immune responses localized around abnormal tissues.
Defining cell death by biomolecular patterns has highlighted many different cell death subroutines, which have extended the original apoptosis and necrosis dichotomy [11]. This greater level of contrast has revealed new opportunities for therapeutic intervention, notably in cancer treatment. In this chapter, we discuss how different cell death modalities impact host immunosurveillance in the tumor microenvironment. We pay particular attention to cell death modalities that occur following administration of certain cancer therapies.
5.2 Tolerogenic Cell Death in the Tumor Microenvironment
Various elements work in unison to determine whether cell death is immunogenic or not. These parameters include the inherent antigenicity of the dying cell, activation or stress encountered by the cell before dying, the properties of the cell death-inducing entity, the specific cell death pathway engaged, and the availability of immune cells [3].
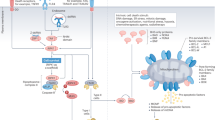
“Physiological” apoptosis evolved to be an immunologically silent event characterized by absence of “don’t eat me” signals (e.g., CD31 and CD47), exposure of “eat me” signals (such as phosphatidylserine [PS] upon the outer membrane leaf), and release of “find me” signals and chemokines (e.g., ATP) into the local environment [2] (see Table 5.1). Cancer cells can follow the same physiological pathway to die, or may even begin to mimic aspects of apoptosis (e.g., PS exposure [24]), which would result in concealment of immunostimulatory DAMPs and immune evasion [24]. Indeed (and often forgotten in tumor biology), tumor cell loss plays a significant component in tumor development, with higher levels of apoptosis linked to poorer prognoses [2]. Apoptosis may also actively recruit precursor myeloid cells into the tumor bed through “find me” signals (see Fig. 5.1). Following the uptake of apoptotic tumor material, recruited myeloid cells can differentiate and become polarized toward a tolerogenic (M2) phenotype. These cells are subsequently well positioned to inhibit immunosurveillance [25].
Tolerogenic cell death in the microenvironment of tumors. Apoptosis of cancer cells can induce a local state of tolerance that enables tumors to escape immunosurveillance. Antigen presenting cells (APCs) such as dendritic cells (DCs) are drawn to apoptotic cells in tumor beds via sensing their surface exposure of phosphatidylserine (PTS) or following their release of “find me” signals such as ATP. “Find me” signals cause mobile phagocytic cells to migrate as a whole (chemotaxis) or extend parts of the cell (chemotropism) toward the dying cell. APCs subsequently take up apoptotic debris and associated tumor antigens. Following migration to draining lymph nodes (dLN) DCs can present these antigens to CD8+ T lymphocytes, but in the absence of costimulation. This causes priming of “helpless” cytotoxic lymphocytes that are unable to successfully attack tumors, and which are highly susceptible to TRAIL-mediated activation-induced cell death. Apoptotic debris can also induce the differentiation of myeloid cells into tolerogenic M2 macrophages, which in turn can drive regulatory CD4+ T cell (Treg)-mediated immunosuppression through their production of the cytokines TGF-β and IL-10
Tumor cell apoptosis may similarly modulate dendritic cell (DCs) function in the tumor microenvironment. DCs are the sentinel APCs of the immune system and act as initiators of antigen-specific T cell responses. When positioned in the proximity of dying cancer cell, DCs can take up tumor-associated antigens (TAAs) and present these to generate T cell-mediated anti-tumor responses. Besides presenting TAAs to T cells, DCs must also receive stimuli such as DAMPs or PAMPs to stimulate their maturation. This in turn allows DCs to upregulate their surface expression of co-stimulatory molecules, required to prime efficient cytotoxic tumor-targeted T cells. However, engulfment of cells undergoing apoptosis may actively prevent this DC maturation [26], leaving DCs in a tolerogenic, immature state [27]. Furthermore, insufficiently activated DCs may cross-present antigens derived from apoptotic material to CD8+ T cells, which can result in immune suppression [28, 29]. This can occur in cases where there is an absence of CD4+ T cell help.
CD4+ T cells program DCs to correctly prime CD8+ cytotoxic T lymphocytes (CTLs) that are resistant to activation-induced cell death on antigen reencounter. This checkpoint against potential CTL-driven autoimmunity is mediated by CD40-CD40 ligand interaction between DCs and CD4+ T cells [3]. Activation-induced cell death is initiated by the death ligand TRAIL (TNF-related apoptosis-inducing ligand), which instigates apoptosis in sub-optimally primed CTLs and other activated T cells. Unlike DCs that engulf necrotic cells, DCs that engulf apoptotic cells have been shown to present antigen to CD8+ T cells, but not to CD4+ T cells [30]. These CD8+ T cells were seen to produce TRAIL on reencounter with the antigen, which blocked cell-mediated immune responses from occurring. Accordingly, TRAIL-deficient mice are resistant to tolerance following injection of apoptotic cells [30].
Apoptosis can also promote tolerance by modifying DAMPs. During apoptosis, naturally produced reactive oxygen species oxidize a key cysteine residue in high-mobility group box 1 (HMGB1), an immunogenic DAMP. This event renders HMGB1 ineffective in promoting immune responses [31]. It is likely that other immunostimulatory molecules are “silenced” by ROS, or by other means, as a safeguard against autoimmunity. However, these safety mechanisms could be potentially hijacked by tumor cells.
The release of immunosuppressive mediators by cells undergoing apoptosis, or by cells that have uptaken apoptotic material, is an additional mechanism to ensure a tolerogenic microenvironment. Intravenous infusion of apoptotic cells in vivo induces an expansion of Tregs that appears to be TGF-β-dependent [32, 33]. Lymphocytes dying by apoptosis can produce immunosuppressive cytokines such as IL-10 and TGF-β [34,35,36], although whether this is true for apoptotic tumor cells is less clear. Nonetheless, immunosuppressive cytokine production following apoptosis is observed from macrophages or DCs that have phagocytozed apoptotic cells. Macrophages have been shown to produce TGFβ, IL-10, and several lipid mediators when they come into contact with apoptotic cells [3, 37,38,39], whereas proinflammatory cytokine gene expression is inhibited [40]. Moreover, because production of immunosuppressive mediators can induce Foxp3+ regulatory T cells (Treg), positive reinforcement of intratumoral immunosuppression can occur [41,42,43]. Besides prominent immunosuppressive cytokines, ecto-ATPases exposed or secreted by tumor cells (such as CD39 or CD73) can similarly expand Treg populations. These enzymes consume immunogenic extracellular ATP, and can increase immunosuppressive adenosine levels in the microenvironment [44,45,46].
5.3 Immunogenic Death of Cancer Cells
Although apoptosis is considered an immunologically “silent” event, certain stimuli can induce non-classical subtypes of apoptosis that cause immune responses against components of the dying cell. Chemotherapeutic agents and radiotherapy are well-studied inducers of these cell death modalities (Table 5.2). Several studies have revealed responses to chemotherapy are often more efficient in immunocompetent than immunodeficient hosts, this being valid in both mouse [44, 47] and clinical [55] studies. Additionally, in vivo injection of murine cancer cells previously treated with anthracyclines, oxaliplatin, or UVC irradiation, confers long-term immune-mediated protection against challenge with live cancer cells of the same type [17, 48]. These findings suggested chemotherapy-induced tumor cell death engages immune cells to propagate anti-tumor immunity.
Studies of the events following the immunogenic cell death (ICD) have identified subtle molecular and metabolic signals (e.g., DAMPs) that stimulate APCs to promote immune responses. DCs act as the central immune cell able to sense and transform cell death signals into anti-tumor T cell responses. A population of CD11c+CD11b+Ly6Chi myeloid precursor cells (characteristic of inflammatory monocytes) have been shown to be the precursors to intratumoral DCs following ICD-inducing chemotherapy. This precursor population accumulates in tumor beds following anthracycline treatment, and appears particularly efficient at engulfing dying tumor cells and presenting TAAs to CD8+ T cells [56]. Intratumoral accumulation of this myeloid population appears dependent upon the release of ATP from cells dying by ICD, as this process fails to occur in murine tumors engineered to overexpress the ecto-ATPase CD39. Subsequent studies have revealed that intratumoral accumulation of CD11c+CD11b+Ly6ChiMHCII+ myeloid cells post anthracycline chemotherapy also requires Ccl2 chemokine signaling, since neoplasms growing in mice deficient for Ccl2 fail to recruit this population post treatment [57]. Interestingly, the same study identified that draining lymph nodes are dispensable for the priming and proliferation of antigen-specific immunity post anthracycline chemotherapy [57]. Intratumoral accumulation of myeloid precursor cells has been corroborated in a taxane-based chemotherapy setting [58].
Antineoplastic chemotherapeutic agents can alter the immune infiltrate of tumors, the characteristics of which often determine therapeutic outcome. A good example is how certain anti-cancer agents increase the ratio of CTLs to Foxp3+ Tregs, which often correlates with favorable therapeutic responses [59]. Of the various T cell populations, CTLs and CD4+ T helper (Th)1 cells, prominent producers of the cytokine interferon (IFN)-γ, are key mediators of tumor eradication. The role that interleukin (IL)-17 producing T cells (e.g., Th17) play post chemotherapy is less well understood. IL-17A production by γδ T cells appears to be a key component for optimal anticancer responses following ICD-inducing chemotherapy or radiotherapy [60, 61]. Tumor infiltration by this innate lymphocyte population was demonstrated as a prerequisite for subsequent accumulation of tumor-killing CD8+ αβ T cells. Before triggering this immune response, the γδ T cells first required activation by the pro-inflammatory cytokine IL-1β [49, 60]. The indispensable role of the cytokines mediating this cascade of events following ICD has been confirmed in experimental settings using either neutralizing antibodies, or through knockout of the genes encoding the cytokines (Il1b, Il17a, Ifng) and their receptors (Il1r, Il17r, Ifngr) [17, 44, 49, 56, 60]. TNF-α signaling does not appear to contribute to the antineoplastic effects of anthracycline chemotherapies in murine tumor models [62].
Several subtle biochemical changes in the plasma membrane and microenvironment of dying cells drive anti-tumor immunity through PRRs of innate immune cells. These prominent hallmarks of ICD include ER stress and calreticulin (CRT) exposure, the release of HMGB1, the autophagy-dependent release of ATP, and viral mimicry that induces type I IFN signaling.
5.3.1 ER Stress and Calreticulin Exposure
An early event required for immune stimulation following ICD is the exposure of CRT on dying cells [17]. CRT is present in high concentrations in the endoplasmic reticulum (ER) lumen, as well as other subcellular compartments. Following exposure to certain ICD-inducing chemotherapies (e.g., anthracyclines), malignant cells undergo a rapid phase of intense ER stress. ER stress may be defined as a disturbance in the homeostatic protein processing function of the ER, caused by an imbalance between protein folding load and capacity [9]. To fully activate danger signaling, the overproduction of ROS likely synergizes with the cellular response to ER stress since optimal immunogenicity of ICD requires the combination of these two factors (indeed, ICD is reduced in the presence of antioxidants [54]) [9]. Downstream of these events occurs an increase in the cytoplasmic Ca2+ concentration and activation of the protein kinase PERK (protein kinase RNA-like ER kinase). Activated PERK phosphorylates the eukaryotic translation initiation factor 2α (eIF2α), which in turn results in a halt in protein translation. These processes are necessary for the successful propagation of ICD, since siRNA-mediated downregulation of PERK, knock-in of a non-phosphorylatable variant of eIF2α, or the use of intracellular Ca2+ chelators, each prevent the anthracycline-induced exposure of CRT and thus immunogenicity [63]. Subsequent to these events, downstream activation of caspase-8 along with the pro-apoptotic proteins BAX and BAK occurs, these latter two proteins playing a central role in mitochondrial outer membrane permeabilization. Finally, anterograde transport of CRT from the ER to the Golgi apparatus takes place, which allows exocytosis of CRT-containing vesicles to the cell surface membrane [4, 54]. External cell membrane exposure of CRT appears only to occur with ICD-inducing anticancer therapies, although the exact intracellular molecules and signaling pathways required for CRT translocation appear to be heterogeneous and dependent on the particular ICD trigger [4]. Additionally, any inhibition of CRT exposure through blocking antibodies or CRT transcript knockdown abrogates anthracycline immunogenicity, highlighting the key requirement of this process to ICD [17].
Recent studies have shown that the chemokine CXCL8 (better known as IL-8), and its mouse ortholog CXCL2, are also involved in the translocation of CRT to the outer leaflet of the plasma membrane [64]. Treatment with the ICD-inducing agent mitoxantrone stimulates human cancer cells to produce CXCL8 in vitro, and murine tumors to produce CXCL2 in vivo. In addition, mitoxantrone-induced CRT exposure is diminished if the receptors of CXCL8 (CXCR1) or CXCL2 (CXCR1 and CXCR2) are knocked down in human or murine cancer cells, respectively. Knockdown of the receptors for CXCL2 was also observed to reduce the immunogenicity of mitoxantrone-treated dying tumor cells in vivo, which could be restored if exogenous CXCL2 was provided [64].
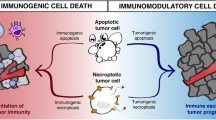
The exposure of CRT on the cell surface of dying tumor cells couples to induction of anti-tumor immune responses by acting as an “eat me” signal to APCs such as DCs (Fig. 5.2). Recognition of CRT and engulfment of CRT-exposing cells can occur through the transmembrane receptor CD91. On binding to CD91, CRT stimulates APCs to produce proinflammatory cytokines, such as TNF-α and IL-6, which facilitate antigen presentation and T cell responses. ICD-induced CRT exposure precedes PS exposure at the cell surface. Although both act as “eat me” signals, whereas CRT is required for immunogenicity of this death process, PS mediates the removal of apoptotic corpses without activating inflammatory or immune responses [17]. Also worth mentioning here is that in addition to CRT exposure, a co-translocation of the ER-sessile disulphide isomerase ERp57 occurs to the cell surface. Unlike CRT, ERp57 is however, per se, unable to exert pro-immunogenic effects [63].
Induced immunogenic cell death in the tumor microenvironment. Exposure of tumor cells to certain forms of chemotherapy or γ-ray irradiation induces a pattern of cell death that stimulates anti-tumor host immune responses. This immunogenic cell death (ICD) is characterized by an ER stress response that results in the exposure of calreticulin (CRT) on the cell surface membrane of the dying cell, the release from dying cells of HMGB1 and ATP. These molecules interact with CD91, TLR4, and P2RX7 receptors, respectively, on antigen-presenting cells such as dendritic cells (DC), which can derive from myeloid precursors recruited by CCL2 and ATP signaling post treatment. This results in maturation of DCs and their secretion of pro-inflammatory cytokines such as IL-6 and TNF-α. This inflammatory environment, alongside the uptake by mature DCs of tumor antigens, helps to drive anti-tumor T cell responses. The resulting T cells can be attracted into tumor beds via tumor cell secretion of the chemokine CXCL10, which results from autocrine and paracrine type I IFN signaling among tumor cells post chemotherapy
Opposing the “eat me” signals that occur on ICD, other membrane molecules externally co-expressed on cancer cells can inhibit phagocytosis by APCs. An example here is CD47 that acts as a “don’t eat me” signal. Indeed, antibody blockade of CD47 increases phagocytosis of tumor cells by APCs, and helps initiate anti-tumor CTL responses [65]. CRT exposure (and the balance between surface expression of CRT and CD47) appears also to correlate favorably with the control of various human cancers, including acute myeloid leukemia [66], non-Hodgkin lymphoma [67], and colorectal cancer [68].
5.3.2 HMGB1 and TLR4
A second hallmark contributor to ICD is the release of HMGB1 from dying cells, and its sensing by TLR4 on APCs (Fig. 5.2). TLR4 is best characterized as a member of the TLR family of PRRs evolutionarily conserved for the detection of lipopolysaccharide (LPS) constructs present in Gram-negative bacteria [5, 10]. TLR4 pathway activation results in a potent immune cell production of proinflammatory cytokines. Three lines of evidence have demonstrated the important role of TLR4 in ICD: (a) expression of TLR4 (and its adaptor MyD88) is required by DCs for immune responses against dying cells in vivo following treatment with certain chemotherapies or radiotherapy; (b) the nonhistone chromatin protein HMGB1 released by dying tumor cells prompts DC processing and cross-presentation of TAAs through its ligating and triggering of TLR4; (c) node-positive breast cancer patients carrying a TLR4 loss-of-function allele relapse faster post radiotherapy or chemotherapy compared to patients carrying the normal TLR4 allele [47].
In support of these findings, a high-potency and exclusive TLR4 agonist has been shown to improve the immunogenicity and efficacy of chemotherapy against tumors with low expression of HMGB1, or in tumors where HMGB1 is depleted by RNA interference [69]. Furthermore, the natural TLR4 ligands deriving from gut commensals can determine the efficacy of chemotherapy, since eliminating this source of bacterial TLR4 ligands through treating mice with antibiotics (or in germ-free settings) inhibits the anti-tumoral efficacy of oxaliplatin as well as the activation and responses of tumor-infiltrating myeloid-derived cells [70]. Finally, some immunotherapies may rely on HMGB1/TLR4 signaling for their efficacy; an example being the therapeutic effect of anti-HER2/neu antibodies, which have been described to occur through host Myd88 and tumor-derived HMGB1 in vivo [71].
5.3.3 Release of ATP by Dying Cells and Autophagy
Besides TLR4 signaling, another PRR family, the inflammasomes, also contribute to ICD. Inflammasomes are an intracellular assembly of activated proteins, enzymes, and adaptor molecules that form a danger-sensing apparatus that may be actuated by PAMPs, or DAMPs such as uric acid and changes in K+ ion concentration [6, 72]. The outcome of inflammasome assembly and triggering following such stimuli is the downstream activation of caspase-1, which in turn proteolytically matures pro-IL-1β to active IL-1β. Release of IL-1β following its generation enables it to act its role as a potent pro-inflammatory cytokine, and a critical mediator of ICD.
NLRP3 inflammasome activation has been shown to be a key factor in ICD, since mice deficient for the inflammasome component genes Nlrp3 and Casp1 fail to generate ICD post oxaliplatin chemotherapy [49]. The mechanism behind this component of ICD was identified to be the release of ATP from dying or stressed cells, which activates the inflammasome within DCs indirectly through P2RX7 receptors present on DC surface membranes. This enabled DC release of IL-1β, necessary for the priming of IFN-γ-producing tumor-specific CD8+ T cells. Notably, it appears probable that activation of other PRRs (e.g., TLR4 activation by HMGB1) is a co-requirement to establish this immune response.
ATP release in response to ICD-inducing chemotherapy was later found to be under the control of autophagy during the dying process [44]. Unlike autophagy-competent cancer cells, those made autophagy-deficient were seen to have reduced release of ATP when undergoing cell death and, when implanted into mice, the resulting tumors failed to attract T lymphocytes and APCs into the tumor bed [44]. These deficiencies could however be reversed by pharmacological inhibition of extracellular ATP-degrading enzymes, which helped to boost ATP concentrations in the tumor microenvironment.
ATP is a potent chemoattractant for DCs and scavenging macrophages, stimulating these myeloid immune cells via their membrane-expressed P2RY2 and P2RX7 purinergic receptors. Indeed, the early tumor infiltration of myeloid precursor cells observed following anthracycline chemotherapy is abolished in the presence of a broad-spectrum purinergic receptor inhibitor or if local extracellular concentrations of ATP are decreased by overexpression of the ecto-ATPase CD39 [56]. Such events following ICD may determine the ensuing microenvironment of tumors, since ATP concentration (and presumably purinergic receptor signaling) might dictate whether myeloid precursors preferentially differentiate towards DCs as opposed to granulocytes (which are hypothesized under some situations to be detrimental to tumor control) [56]. These studies also suggest a potential immunosurveillance-escape strategy for cancer cells, where they may be able to negatively regulate intrinsic autophagic processes (and thus potentially ATP production if this precedes cell death). In accord with this, autophagy is often disabled during early oncogenesis [73], and the expression of ecto-ATPases by triple negative breast cancers promotes poor prognosis [74].
5.3.4 Viral Mimicry and the Release of Type I IFN
The ICD activity of anthracycline and oxaliplatin chemotherapies may also rely on type I IFN signaling. It has been identified that type I IFN signaling takes place in neoplastic cells rather than host cells following ICD-inducing chemotherapy [75]. Anthracycline and oxaliplatin were each seen to stimulate a rapid production of type I IFNs from malignant cells, an effect that was dependent on stimulation of the endosomal PRR TLR3. The precise anthracycline-elicited ligand(s) responsible for TLR3 stimulation in this scenario remains to be determined, though one could postulate that this is a dysregulated structure of self RNA released from stressed or dying cancer cells. Indeed, other DNA-damaging agents have been shown to generate double-stranded RNA molecules that trigger TLR3-dependent cytokine secretion [76]. Subsequent autocrine and paracrine signaling of type I IFNs appears to induce the production of the chemokine CXCL10, a potent chemoattractant that recruits T lymphocytes into the tumor bed. This cascade of events post anthracycline chemotherapy is supported by findings that tumors deficient for the genes encoding TLR3 or the type I IFN receptor are not controlled unless the subsequent steps in the cascade, type I IFN and CXCL10 respectively, are artificially provided [75]. In line with this experimental setting, the expression of MX1 by tumor cells (a prominent signature gene downstream of type I IFN signaling) predicts metastasis-free survival in neoadjuvant chemotherapy in breast carcinoma patients with poor prognosis [75].
It has similarly been shown that ionizing radiation-mediated tumor regression depends upon type I IFN signaling [77], this setting requiring the adaptor protein STING, but not MyD88 [78]. STING signaling was required for type I IFN production by DCs following their sensing of irradiated-tumor cells, which occurred through the cytosolic DNA sensor cyclic GMP-AMP synthase (cGAS). Addition of exogenous type I IFN was able to rescue DC cross-presentation of TAAs in settings where cGAS- or STING-deficient DCs were used. This signaling cascade in DCs was essential for radiation-induced adaptive immune responses, which could be further enhanced by activating STING with a second messenger cyclic GMP-AMP during radiotherapy [78]. Programmed death ligand-1 (PD-L1) blockade may synergize with radiotherapy in this context to control both local and distant tumors, an effect mediated by CD8+ T cells and a reduced myeloid-derived suppressor cell (MDSC) numbers within the tumor microenvironment [79, 80].
Induced expression of IFN-stimulated genes, and sensing through TLR3, are characteristic of the cellular response to viral infection [81]. Such “viral mimicry” appears to constitute an important attribute of successful chemotherapy and radiotherapy. Indeed, viruses also trigger ER stress and autophagy [73], which, as we have already mentioned, are important for chemotherapy-induced ICD.
5.4 Manipulating Cell Death for Therapeutic Control of Cancer
ICD can be visualized as initiating a cascade of defined biochemical changes and immune/inflammatory signaling pathways (as summarized in Fig. 5.2). However, not many chemotherapeutic treatments are able to induce ICD, instead promoting other cell death modalities such as apoptosis. Screening of 24 distinct cytotoxic chemotherapies revealed that only 4 of these induce protective anti-cancer immune responses in vivo, whereas all agents resulted in equivalent apoptosis of target cells [17]. These immunogenic agents included the three anthracyclines doxorubicin, idarubicin, and mitoxantrone, and the platinum compound oxaliplatin (of note, the structurally-related platinum compound cisplatin does not induce CRT exposure; Table 5.2). Each of these four anti-neoplastic agents induces the key hallmarks of ICD following exposure to tumor cells (i.e., CRT exposure, and release of ATP and HMGB1 during the dying process). Interestingly, by running numerous FDA-approved drugs through a screening platform able to detect ICD-induced biochemical changes, novel compounds have been identified that may prove to be promising adjunctive therapies in cases where standard cancer treatments are inadequately immunogenic [51]. Notably, cardiac glycosides (e.g. digoxin, digitoxin) were found to be a drug class particularly efficient at inducing ICD. Cardiac glycosides may induce ICD by inhibiting surface membrane Na+,K+-ATPase pumps, which results in a Ca2+ influx into cancer cells that can have proapoptotic and proimmunogenic effects [51].
Theoretically then, it would seem possible, in cases where ICD is absent, to design strategies that make therapeutic tumor cell death immunogenic—for example through administration of adjunctive treatments. Conceivable compensatory strategies might include the intratumoral administration of agents that induce ER stress. Treatment with thapsigargin (an inhibitor of the sarco/ER Ca2+ -ATPase), or enforced reduction of ER Ca2+ levels through overexpression of the Ca2+ channel reticulon 1C, can each restore CRT translocation in cases where CRT is absent following chemotherapy [53, 82]. In addition, inhibitors of the GADD34/PP1 complex (which forms a phosphatase of eIF2α) increase the rate of PERK-dependent eIF2α phosphorylation, thus promoting CRT surface exposure [83]. The proteasome inhibitor bortezomib can also facilitate immunogenic death of human tumors. Bortezomib induces premortem stress in cancer cells and surface exposure of CRT and Hsp90, which enables phagocytosis via CD91 and potential propagation of anti-tumor immune responses by DCs [84, 85].
Similarly, administration of TLR4 agonists may prove to be a useful adjunctive compensation therapy. Tumors exhibiting weak expression of nuclear HMGB1 respond to chemotherapy more effectively if combined with a local or systemic administration of highly purified TLR4 agonists [69]. Strategies to increase local concentrations of ATP (e.g., through use of ectonucleotidase inhibitors), or replacing this signal through purinergic receptor agonists, might also promote immunogenicity of a non-ICD-inducing agents [44]. Intratumoral therapies of recombinant cytokines, for example IL-1β or IL-17, may be effective if their production by immune cells within the tumor is low or absent. Similarly, patients with molecular defects in any of the molecules involved or downstream of TLR3-induced type I IFN-dependent signaling may benefit from targeted delivery of type I IFN or CXCL10 alongside anthracycline treatment [75, 86]. Finally, with the aforementioned discovery that cardiac glycosides induce ICD, adjunctive administration of cardiac glycosides could prove to be the most reachable future strategy [51].
Blocking the immunosuppressive tumor microenvironment could provide an alternative strategy. Targeting potent immunosuppressive cytokines is likely to be the most effective and tangible approach here, perhaps with intratumoral injection of IL-10- or TGF-β-neutralizing antibodies should these become clinically available. Since the rebooting of anti-cancer T cell responses following ICD induction, combination of ICD inducers with immune checkpoint inhibitors (e.g., blockade of CTLA-4 and the PD-1/PD-L1 axis) may provide another promising intervention.
5.5 Conclusions
The evolution of the immune system to discriminate between physiological and pathological instances of cell death, and to perceive cellular demise as immunogenic or tolerogenic, is pivotal to homeostasis and host defense. In this chapter, we have described how cancer cells can manipulate apoptosis to induce tolerance and evade immunosurveillance. On the other hand, we have discussed how ICD can be used therapeutically to induce durable immune responses that target and eradicate tumors. ICD is defined by set spatiotemporal combinations of DAMPs that are decoded by PRRs on immune cells to (re)activate an antitumor immune response, and to avoid further induction of tolerance. For this process to operate efficiently, certain prerequisites must be met. These include: (i) that cancer cells emit all the signals required for cell death to be interpreted as immunogenic, (ii) that immune cells have or maintain the capacity to properly recognize and decode such signals, and (iii) that the host immune system is able to translate these signals into a robust cell-mediated immune response. The identification and clinical development of agents and strategies that fulfill these criteria could revolutionize how we treat cancer.
References
Reed JC. Drug insight: cancer therapy strategies based on restoration of endogenous cell death mechanisms. Nat Clin Pract Oncol. 2006;3(7):388–98.
Gregory CD, Pound JD. Cell death in the neighbourhood: direct microenvironmental effects of apoptosis in normal and neoplastic tissues. J Pathol. 2011;223(2):177–94.
Green DR, Ferguson T, Zitvogel L, Kroemer G. Immunogenic and tolerogenic cell death. Nat Rev Immunol. 2009;9(5):353–63.
Kroemer G, Galluzzi L, Kepp O, Zitvogel L. Immunogenic cell death in cancer therapy. Annu Rev Immunol. 2013;31:51–72.
Janeway CA Jr, Medzhitov R. Innate immune recognition. Annu Rev Immunol. 2002;20:197–216.
Martinon F, Tschopp J. Inflammatory caspases and inflammasomes: master switches of inflammation. Cell Death Differ. 2007;14(1):10–22.
Medzhitov R, Janeway CA Jr. Decoding the patterns of self and nonself by the innate immune system. Science. 2002;296(5566):298–300.
Matzinger P. Tolerance, danger, and the extended family. Annu Rev Immunol. 1994;12:991–1045.
Krysko DV, Garg AD, Kaczmarek A, Krysko O, Agostinis P, Vandenabeele P. Immunogenic cell death and DAMPs in cancer therapy. Nat Rev Cancer. 2012;12(12):860–75.
Akira S, Uematsu S, Takeuchi O. Pathogen recognition and innate immunity. Cell. 2006;124(4):783–801.
Galluzzi L, Vitale I, Abrams JM, Alnemri ES, Baehrecke EH, Blagosklonny MV, Dawson TM, Dawson VL, El-Deiry WS, Fulda S, Gottlieb E, Green DR, Hengartner MO, Kepp O, Knight RA, Kumar S, Lipton SA, Lu X, Madeo F, Malorni W, Mehlen P, Nunez G, Peter ME, Piacentini M, Rubinsztein DC, Shi Y, Simon HU, Vandenabeele P, White E, Yuan J, Zhivotovsky B, Melino G, Kroemer G. Molecular definitions of cell death subroutines: recommendations of the nomenclature committee on cell death 2012. Cell Death Differ. 2012;19(1):107–20.
Elliott MR, Chekeni FB, Trampont PC, Lazarowski ER, Kadl A, Walk SF, Park D, Woodson RI, Ostankovich M, Sharma P, Lysiak JJ, Harden TK, Leitinger N, Ravichandran KS. Nucleotides released by apoptotic cells act as a find-me signal to promote phagocytic clearance. Nature. 2009;461(7261):282–6.
Lauber K, Bohn E, Krober SM, Xiao YJ, Blumenthal SG, Lindemann RK, Marini P, Wiedig C, Zobywalski A, Baksh S, Xu Y, Autenrieth IB, Schulze-Osthoff K, Belka C, Stuhler G, Wesselborg S. Apoptotic cells induce migration of phagocytes via caspase-3-mediated release of a lipid attraction signal. Cell. 2003;113(6):717–30.
Truman LA, Ford CA, Pasikowska M, Pound JD, Wilkinson SJ, Dumitriu IE, Melville L, Melrose LA, Ogden CA, Nibbs R, Graham G, Combadiere C, Gregory CD. CX3CL1/fractalkine is released from apoptotic lymphocytes to stimulate macrophage chemotaxis. Blood. 2008;112(13):5026–36.
Fadok VA, Voelker DR, Campbell PA, Cohen JJ, Bratton DL, Henson PM. Exposure of phosphatidylserine on the surface of apoptotic lymphocytes triggers specific recognition and removal by macrophages. J Immunol. 1992;148(7):2207–16.
Gardai SJ, McPhillips KA, Frasch SC, Janssen WJ, Starefeldt A, Murphy-Ullrich JE, Bratton DL, Oldenborg PA, Michalak M, Henson PM. Cell-surface calreticulin initiates clearance of viable or apoptotic cells through trans-activation of LRP on the phagocyte. Cell. 2005;123(2):321–34.
Obeid M, Tesniere A, Ghiringhelli F, Fimia GM, Apetoh L, Perfettini JL, Castedo M, Mignot G, Panaretakis T, Casares N, Metivier D, Larochette N, van Endert P, Ciccosanti F, Piacentini M, Zitvogel L, Kroemer G. Calreticulin exposure dictates the immunogenicity of cancer cell death. Nat Med. 2007;13(1):54–61.
Arur S, Uche UE, Rezaul K, Fong M, Scranton V, Cowan AE, Mohler W, Han DK. Annexin I is an endogenous ligand that mediates apoptotic cell engulfment. Dev Cell. 2003;4(4):587–98.
Nakai Y, Shiratsuchi A, Manaka J, Nakayama H, Takio K, Zhang JT, Suganuma T, Nakanishi Y. Externalization and recognition by macrophages of large subunit of eukaryotic translation initiation factor 3 in apoptotic cells. Exp Cell Res. 2005;309(1):137–48.
Brown S, Heinisch I, Ross E, Shaw K, Buckley CD, Savill J. Apoptosis disables CD31-mediated cell detachment from phagocytes promoting binding and engulfment. Nature. 2002;418(6894):200–3.
Lv Z, Bian Z, Shi L, Niu S, Ha B, Tremblay A, Li L, Zhang X, Paluszynski J, Liu M, Zen K, Liu Y. Loss of cell surface CD47 clustering formation and binding avidity to SIRPalpha facilitate apoptotic cell clearance by macrophages. J Immunol. 2015;195(2):661–71.
Rovere P, Peri G, Fazzini F, Bottazzi B, Doni A, Bondanza A, Zimmermann VS, Garlanda C, Fascio U, Sabbadini MG, Rugarli C, Mantovani A, Manfredi AA. The long pentraxin PTX3 binds to apoptotic cells and regulates their clearance by antigen-presenting dendritic cells. Blood. 2000;96(13):4300–6.
Jaillon S, Jeannin P, Hamon Y, Fremaux I, Doni A, Bottazzi B, Blanchard S, Subra JF, Chevailler A, Mantovani A, Delneste Y. Endogenous PTX3 translocates at the membrane of late apoptotic human neutrophils and is involved in their engulfment by macrophages. Cell Death Differ. 2009;16(3):465–74.
Garg AD, Martin S, Golab J, Agostinis P. Danger signalling during cancer cell death: origins, plasticity and regulation. Cell Death Differ. 2014;21(1):26–38.
Weigert A, Tzieply N, von Knethen A, Johann AM, Schmidt H, Geisslinger G, Brune B. Tumor cell apoptosis polarizes macrophages role of sphingosine-1-phosphate. Mol Biol Cell. 2007;18(10):3810–9.
Sauter B, Albert ML, Francisco L, Larsson M, Somersan S, Bhardwaj N. Consequences of cell death: exposure to necrotic tumor cells, but not primary tissue cells or apoptotic cells, induces the maturation of immunostimulatory dendritic cells. J Exp Med. 2000;191(3):423–34.
Steinman RM, Hawiger D, Nussenzweig MC. Tolerogenic dendritic cells. Annu Rev Immunol. 2003;21:685–711.
Ferguson TA, Herndon J, Elzey B, Griffith TS, Schoenberger S, Green DR. Uptake of apoptotic antigen-coupled cells by lymphoid dendritic cells and cross-priming of CD8(+) T cells produce active immune unresponsiveness. J Immunol. 2002;168(11):5589–95.
Heath WR, Carbone FR. Cross-presentation, dendritic cells, tolerance and immunity. Annu Rev Immunol. 2001;19:47–64.
Griffith TS, Kazama H, Van Oosten RL, Earle JK Jr, Herndon JM, Green DR, Ferguson TA. Apoptotic cells induce tolerance by generating helpless CD8+ T cells that produce TRAIL. J Immunol. 2007;178(5):2679–87.
Kazama H, Ricci JE, Herndon JM, Hoppe G, Green DR, Ferguson TA. Induction of immunological tolerance by apoptotic cells requires caspase-dependent oxidation of high-mobility group box-1 protein. Immunity. 2008;29(1):21–32.
Kleinclauss F, Perruche S, Masson E, de Carvalho Bittencourt M, Biichle S, Remy-Martin JP, Ferrand C, Martin M, Bittard H, Chalopin JM, Seilles E, Tiberghien P, Saas P. Intravenous apoptotic spleen cell infusion induces a TGF-beta-dependent regulatory T-cell expansion. Cell Death Differ. 2006;13(1):41–52.
Maeda A, Schwarz A, Kernebeck K, Gross N, Aragane Y, Peritt D, Schwarz T. Intravenous infusion of syngeneic apoptotic cells by photopheresis induces antigen-specific regulatory T cells. J Immunol. 2005;174(10):5968–76.
Chen W, Frank ME, Jin W, Wahl SM. TGF-beta released by apoptotic T cells contributes to an immunosuppressive milieu. Immunity. 2001;14(6):715–25.
Gao Y, Herndon JM, Zhang H, Griffith TS, Ferguson TA. Antiinflammatory effects of CD95 ligand (FasL)-induced apoptosis. J Exp Med. 1998;188(5):887–96.
Tomimori Y, Ikawa Y, Oyaizu N. Ultraviolet-irradiated apoptotic lymphocytes produce interleukin-10 by themselves. Immunol Lett. 2000;71(1):49–54.
Chung EY, Liu J, Homma Y, Zhang Y, Brendolan A, Saggese M, Han J, Silverstein R, Selleri L, Ma X. Interleukin-10 expression in macrophages during phagocytosis of apoptotic cells is mediated by homeodomain proteins Pbx1 and Prep-1. Immunity. 2007;27(6):952–64.
Fadok VA, Bratton DL, Konowal A, Freed PW, Westcott JY, Henson PM. Macrophages that have ingested apoptotic cells in vitro inhibit proinflammatory cytokine production through autocrine/paracrine mechanisms involving TGF-beta, PGE2, and PAF. J Clin Invest. 1998;101(4):890–8.
Voll RE, Herrmann M, Roth EA, Stach C, Kalden JR, Girkontaite I. Immunosuppressive effects of apoptotic cells. Nature. 1997;390(6658):350–1.
Kim S, Elkon KB, Ma X. Transcriptional suppression of interleukin-12 gene expression following phagocytosis of apoptotic cells. Immunity. 2004;21(5):643–53.
Ghiringhelli F, Menard C, Terme M, Flament C, Taieb J, Chaput N, Puig PE, Novault S, Escudier B, Vivier E, Lecesne A, Robert C, Blay JY, Bernard J, Caillat-Zucman S, Freitas A, Tursz T, Wagner-Ballon O, Capron C, Vainchencker W, Martin F, Zitvogel L. CD4+CD25+ regulatory T cells inhibit natural killer cell functions in a transforming growth factor-beta-dependent manner. J Exp Med. 2005;202(8):1075–85.
Ghiringhelli F, Puig PE, Roux S, Parcellier A, Schmitt E, Solary E, Kroemer G, Martin F, Chauffert B, Zitvogel L. Tumor cells convert immature myeloid dendritic cells into TGF-beta-secreting cells inducing CD4+CD25+ regulatory T cell proliferation. J Exp Med. 2005;202(7):919–29.
Wan YY, Flavell RA. The roles for cytokines in the generation and maintenance of regulatory T cells. Immunol Rev. 2006;212:114–30.
Michaud M, Martins I, Sukkurwala AQ, Adjemian S, Ma Y, Pellegatti P, Shen S, Kepp O, Scoazec M, Mignot G, Rello-Varona S, Tailler M, Menger L, Vacchelli E, Galluzzi L, Ghiringhelli F, di Virgilio F, Zitvogel L, Kroemer G. Autophagy-dependent anticancer immune responses induced by chemotherapeutic agents in mice. Science. 2011;334(6062):1573–7.
Stagg J, Divisekera U, McLaughlin N, Sharkey J, Pommey S, Denoyer D, Dwyer KM, Smyth MJ. Anti-CD73 antibody therapy inhibits breast tumor growth and metastasis. Proc Natl Acad Sci U S A. 2010;107(4):1547–52.
Stagg J, Smyth MJ. Extracellular adenosine triphosphate and adenosine in cancer. Oncogene. 2010;29(39):5346–58.
Apetoh L, Ghiringhelli F, Tesniere A, Obeid M, Ortiz C, Criollo A, Mignot G, Maiuri MC, Ullrich E, Saulnier P, Yang H, Amigorena S, Ryffel B, Barrat FJ, Saftig P, Levi F, Lidereau R, Nogues C, Mira JP, Chompret A, Joulin V, Clavel-Chapelon F, Bourhis J, Andre F, Delaloge S, Tursz T, Kroemer G, Zitvogel L. Toll-like receptor 4-dependent contribution of the immune system to anticancer chemotherapy and radiotherapy. Nat Med. 2007;13(9):1050–9.
Casares N, Pequignot MO, Tesniere A, Ghiringhelli F, Roux S, Chaput N, Schmitt E, Hamai A, Hervas-Stubbs S, Obeid M, Coutant F, Metivier D, Pichard E, Aucouturier P, Pierron G, Garrido C, Zitvogel L, Kroemer G. Caspase-dependent immunogenicity of doxorubicin-induced tumor cell death. J Exp Med. 2005;202(12):1691–701.
Ghiringhelli F, Apetoh L, Tesniere A, Aymeric L, Ma Y, Ortiz C, Vermaelen K, Panaretakis T, Mignot G, Ullrich E, Perfettini JL, Schlemmer F, Tasdemir E, Uhl M, Genin P, Civas A, Ryffel B, Kanellopoulos J, Tschopp J, Andre F, Lidereau R, McLaughlin NM, Haynes NM, Smyth MJ, Kroemer G, Zitvogel L. Activation of the NLRP3 inflammasome in dendritic cells induces IL-1beta-dependent adaptive immunity against tumors. Nat Med. 2009;15(10):1170–8.
Tesniere A, Schlemmer F, Boige V, Kepp O, Martins I, Ghiringhelli F, Aymeric L, Michaud M, Apetoh L, Barault L, Mendiboure J, Pignon JP, Jooste V, van Endert P, Ducreux M, Zitvogel L, Piard F, Kroemer G. Immunogenic death of colon cancer cells treated with oxaliplatin. Oncogene. 2010;29(4):482–91.
Menger L, Vacchelli E, Adjemian S, Martins I, Ma Y, Shen S, Yamazaki T, Sukkurwala AQ, Michaud M, Mignot G, Schlemmer F, Sulpice E, Locher C, Gidrol X, Ghiringhelli F, Modjtahedi N, Galluzzi L, Andre F, Zitvogel L, Kepp O, Kroemer G. Cardiac glycosides exert anticancer effects by inducing immunogenic cell death. Sci Transl Med. 2012;4(143):143ra99.
Schiavoni G, Sistigu A, Valentini M, Mattei F, Sestili P, Spadaro F, Sanchez M, Lorenzi S, D’Urso MT, Belardelli F, Gabriele L, Proietti E, Bracci L. Cyclophosphamide synergizes with type I interferons through systemic dendritic cell reactivation and induction of immunogenic tumor apoptosis. Cancer Res. 2011;71(3):768–78.
Martins I, Kepp O, Schlemmer F, Adjemian S, Tailler M, Shen S, Michaud M, Menger L, Gdoura A, Tajeddine N, Tesniere A, Zitvogel L, Kroemer G. Restoration of the immunogenicity of cisplatin-induced cancer cell death by endoplasmic reticulum stress. Oncogene. 2011;30(10):1147–58.
Panaretakis T, Kepp O, Brockmeier U, Tesniere A, Bjorklund AC, Chapman DC, Durchschlag M, Joza N, Pierron G, van Endert P, Yuan J, Zitvogel L, Madeo F, Williams DB, Kroemer G. Mechanisms of pre-apoptotic calreticulin exposure in immunogenic cell death. EMBO J. 2009;28(5):578–90.
Ray-Coquard I, Cropet C, Van Glabbeke M, Sebban C, Le Cesne A, Judson I, Tredan O, Verweij J, Biron P, Labidi I, Guastalla JP, Bachelot T, Perol D, Chabaud S, Hogendoorn PC, Cassier P, Dufresne A, Blay JY, European Organization for R., Treatment of Cancer Soft T., Bone Sarcoma G. Lymphopenia as a prognostic factor for overall survival in advanced carcinomas, sarcomas, and lymphomas. Cancer Res. 2009;69(13):5383–91.
Ma Y, Adjemian S, Mattarollo SR, Yamazaki T, Aymeric L, Yang H, Portela Catani JP, Hannani D, Duret H, Steegh K, Martins I, Schlemmer F, Michaud M, Kepp O, Sukkurwala AQ, Menger L, Vacchelli E, Droin N, Galluzzi L, Krzysiek R, Gordon S, Taylor PR, Van Endert P, Solary E, Smyth MJ, Zitvogel L, Kroemer G. Anticancer chemotherapy-induced intratumoral recruitment and differentiation of antigen-presenting cells. Immunity. 2013;38(4):729–41.
Ma Y, Mattarollo SR, Adjemian S, Yang H, Aymeric L, Hannani D, Portela Catani JP, Duret H, Teng MW, Kepp O, Wang Y, Sistigu A, Schultze JL, Stoll G, Galluzzi L, Zitvogel L, Smyth MJ, Kroemer G. CCL2/CCR2-dependent recruitment of functional antigen-presenting cells into tumors upon chemotherapy. Cancer Res. 2014;74(2):436–45.
Ruffell B, Chang-Strachan D, Chan V, Rosenbusch A, Ho CM, Pryer N, Daniel D, Hwang ES, Rugo HS, Coussens LM. Macrophage IL-10 blocks CD8+ T cell-dependent responses to chemotherapy by suppressing IL-12 expression in intratumoral dendritic cells. Cancer Cell. 2014;26(5):623–37.
Zitvogel L, Galluzzi L, Smyth MJ, Kroemer G. Mechanism of action of conventional and targeted anticancer therapies: reinstating immunosurveillance. Immunity. 2013;39(1):74–88.
Ma Y, Aymeric L, Locher C, Mattarollo SR, Delahaye NF, Pereira P, Boucontet L, Apetoh L, Ghiringhelli F, Casares N, Lasarte JJ, Matsuzaki G, Ikuta K, Ryffel B, Benlagha K, Tesniere A, Ibrahim N, Dechanet-Merville J, Chaput N, Smyth MJ, Kroemer G, Zitvogel L. Contribution of IL-17-producing gamma delta T cells to the efficacy of anticancer chemotherapy. J Exp Med. 2011;208(3):491–503.
Mattarollo SR, Loi S, Duret H, Ma Y, Zitvogel L, Smyth MJ. Pivotal role of innate and adaptive immunity in anthracycline chemotherapy of established tumors. Cancer Res. 2011;71(14):4809–20.
Ma Y, Yamazaki T, Yang H, Kepp O, Galluzzi L, Zitvogel L, Smyth MJ, Kroemer G. Tumor necrosis factor is dispensable for the success of immunogenic anticancer chemotherapy. Oncoimmunology. 2013;2(6):e24786.
Kepp O, Menger L, Vacchelli E, Locher C, Adjemian S, Yamazaki T, Martins I, Sukkurwala AQ, Michaud M, Senovilla L, Galluzzi L, Kroemer G, Zitvogel L. Crosstalk between ER stress and immunogenic cell death. Cytokine Growth Factor Rev. 2013;24(4):311–8.
Sukkurwala AQ, Martins I, Wang Y, Schlemmer F, Ruckenstuhl C, Durchschlag M, Michaud M, Senovilla L, Sistigu A, Ma Y, Vacchelli E, Sulpice E, Gidrol X, Zitvogel L, Madeo F, Galluzzi L, Kepp O, Kroemer G. Immunogenic calreticulin exposure occurs through a phylogenetically conserved stress pathway involving the chemokine CXCL8. Cell Death Differ. 2014;21(1):59–68.
Tseng D, Volkmer JP, Willingham SB, Contreras-Trujillo H, Fathman JW, Fernhoff NB, Seita J, Inlay MA, Weiskopf K, Miyanishi M, Weissman IL. Anti-CD47 antibody-mediated phagocytosis of cancer by macrophages primes an effective antitumor T-cell response. Proc Natl Acad Sci U S A. 2013;110(27):11103–8.
Wemeau M, Kepp O, Tesniere A, Panaretakis T, Flament C, De Botton S, Zitvogel L, Kroemer G, Chaput N. Calreticulin exposure on malignant blasts predicts a cellular anticancer immune response in patients with acute myeloid leukemia. Cell Death Dis. 2010;1:e104.
Zappasodi R, Pupa SM, Ghedini GC, Bongarzone I, Magni M, Cabras AD, Colombo MP, Carlo-Stella C, Gianni AM, Di Nicola M. Improved clinical outcome in indolent B-cell lymphoma patients vaccinated with autologous tumor cells experiencing immunogenic death. Cancer Res. 2010;70(22):9062–72.
Peng RQ, Chen YB, Ding Y, Zhang R, Zhang X, Yu XJ, Zhou ZW, Zeng YX, Zhang XS. Expression of calreticulin is associated with infiltration of T-cells in stage IIIB colon cancer. World J Gastroenterol. 2010;16(19):2428–34.
Yamazaki T, Hannani D, Poirier-Colame V, Ladoire S, Locher C, Sistigu A, Prada N, Adjemian S, Catani JP, Freudenberg M, Galanos C, Andre F, Kroemer G, Zitvogel L. Defective immunogenic cell death of HMGB1-deficient tumors: compensatory therapy with TLR4 agonists. Cell Death Differ. 2013;21(1):69–78.
Iida N, Dzutsev A, Stewart CA, Smith L, Bouladoux N, Weingarten RA, Molina DA, Salcedo R, Back T, Cramer S, Dai RM, Kiu H, Cardone M, Naik S, Patri AK, Wang E, Marincola FM, Frank KM, Belkaid Y, Trinchieri G, Goldszmid RS. Commensal bacteria control cancer response to therapy by modulating the tumor microenvironment. Science. 2013;342(6161):967–70.
Park S, Jiang Z, Mortenson ED, Deng L, Radkevich-Brown O, Yang X, Sattar H, Wang Y, Brown NK, Greene M, Liu Y, Tang J, Wang S, Fu YX. The therapeutic effect of anti-HER2/neu antibody depends on both innate and adaptive immunity. Cancer Cell. 2010;18(2):160–70.
Ogura Y, Sutterwala FS, Flavell RA. The inflammasome: first line of the immune response to cell stress. Cell. 2006;126(4):659–62.
Ma Y, Galluzzi L, Zitvogel L, Kroemer G. Autophagy and cellular immune responses. Immunity. 2013;39(2):211–27.
Loi S, Pommey S, Haibe-Kains B, Beavis PA, Darcy PK, Smyth MJ, Stagg J. CD73 promotes anthracycline resistance and poor prognosis in triple negative breast cancer. Proc Natl Acad Sci U S A. 2013;110(27):11091–6.
Sistigu A, Yamazaki T, Vacchelli E, Chaba K, Enot DP, Adam J, Vitale I, Goubar A, Baracco EE, Remedios C, Fend L, Hannani D, Aymeric L, Ma Y, Niso-Santano M, Kepp O, Schultze JL, Tuting T, Belardelli F, Bracci L, La Sorsa V, Ziccheddu G, Sestili P, Urbani F, Delorenzi M, Lacroix-Triki M, Quidville V, Conforti R, Spano JP, Pusztai L, Poirier-Colame V, Delaloge S, Penault-Llorca F, Ladoire S, Arnould L, Cyrta J, Dessoliers MC, Eggermont A, Bianchi ME, Pittet M, Engblom C, Pfirschke C, Preville X, Uze G, Schreiber RD, Chow MT, Smyth MJ, Proietti E, Andre F, Kroemer G, Zitvogel L. Cancer cell-autonomous contribution of type I interferon signaling to the efficacy of chemotherapy. Nat Med. 2014;20(11):1301–9.
Bernard JJ, Cowing-Zitron C, Nakatsuji T, Muehleisen B, Muto J, Borkowski AW, Martinez L, Greidinger EL, Yu BD, Gallo RL. Ultraviolet radiation damages self noncoding RNA and is detected by TLR3. Nat Med. 2012;18(8):1286–90.
Burnette BC, Liang H, Lee Y, Chlewicki L, Khodarev NN, Weichselbaum RR, Fu YX, Auh SL. The efficacy of radiotherapy relies upon induction of type i interferon-dependent innate and adaptive immunity. Cancer Res. 2011;71(7):2488–96.
Deng L, Liang H, Xu M, Yang X, Burnette B, Arina A, Li XD, Mauceri H, Beckett M, Darga T, Huang X, Gajewski TF, Chen ZJ, Fu YX, Weichselbaum RR. STING-dependent cytosolic DNA sensing promotes radiation-induced type I interferon-dependent antitumor immunity in immunogenic tumors. Immunity. 2014;41(5):843–52.
Deng L, Liang H, Burnette B, Beckett M, Darga T, Weichselbaum RR, Fu YX. Irradiation and anti-PD-L1 treatment synergistically promote antitumor immunity in mice. J Clin Invest. 2014;124(2):687–95.
Deng L, Liang H, Burnette B, Weicheslbaum RR, Fu YX. Radiation and anti-PD-L1 antibody combinatorial therapy induces T cell-mediated depletion of myeloid-derived suppressor cells and tumor regression. Oncoimmunology. 2014;3:e28499.
MacMicking JD. Interferon-inducible effector mechanisms in cell-autonomous immunity. Nat Rev Immunol. 2012;12(5):367–82.
Tufi R, Panaretakis T, Bianchi K, Criollo A, Fazi B, Di Sano F, Tesniere A, Kepp O, Paterlini-Brechot P, Zitvogel L, Piacentini M, Szabadkai G, Kroemer G. Reduction of endoplasmic reticulum Ca2+ levels favors plasma membrane surface exposure of calreticulin. Cell Death Differ. 2008;15(2):274–82.
Kepp O, Galluzzi L, Giordanetto F, Tesniere A, Vitale I, Martins I, Schlemmer F, Adjemian S, Zitvogel L, Kroemer G. Disruption of the PP1/GADD34 complex induces calreticulin exposure. Cell Cycle. 2009;8(23):3971–7.
Cirone M, Di Renzo L, Lotti LV, Conte V, Trivedi P, Santarelli R, Gonnella R, Frati L, Faggioni A. Primary effusion lymphoma cell death induced by bortezomib and AG 490 activates dendritic cells through CD91. PLoS One. 2012;7(3):e31732.
Spisek R, Charalambous A, Mazumder A, Vesole DH, Jagannath S, Dhodapkar MV. Bortezomib enhances dendritic cell (DC)-mediated induction of immunity to human myeloma via exposure of cell surface heat shock protein 90 on dying tumor cells: therapeutic implications. Blood. 2007;109(11):4839–45.
Yang X, Zhang X, Fu ML, Weichselbaum RR, Gajewski TF, Guo Y, Fu YX. Targeting the tumor microenvironment with interferon-beta bridges innate and adaptive immune responses. Cancer Cell. 2014;25(1):37–48.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 Springer International Publishing AG
About this chapter
Cite this chapter
Pitt, J.M., Kroemer, G., Zitvogel, L. (2017). Immunogenic and Non-immunogenic Cell Death in the Tumor Microenvironment. In: Kalinski, P. (eds) Tumor Immune Microenvironment in Cancer Progression and Cancer Therapy. Advances in Experimental Medicine and Biology, vol 1036. Springer, Cham. https://doi.org/10.1007/978-3-319-67577-0_5
Download citation
DOI: https://doi.org/10.1007/978-3-319-67577-0_5
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-67575-6
Online ISBN: 978-3-319-67577-0
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)