Abstract
The right ventricle (RV) is exposed to numerous stressors in patients with congenital heart defects. The ability of the RV to adapt to stress is important in maintaining long term structure and function. Genetic factors play an important role in influencing RV adaptation. These include variations in genes involved in hypoxia signaling, metabolic regulation and neurohormonal regulation. Variants in these genes influence the RV response to hypoxia, pressure or volume overload and surgical injury. Knowledge of patient genotype may help identify those at highest risk for adverse RV remodeling and RV failure, and importantly in guiding choice and timing of interventions to preserve RV function.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
- Right ventricle
- Personalized medicine
- Polymorphisms
- Genes
- Hypoxia inducible factor
- Renin-angiotensin-aldosterone system
- Fibrosis
Introduction
The goal of personalized medicine is to improve prediction of risk, guide pre-emptive interventions aimed at prevention and/or progression of disease, and to improve the precision of interventions by using biology driven therapies targeted to molecular pathways dysregulated in disease. Genome-guided therapies remain the cornerstone of personalized medicine. Advances in genomics have expedited the discovery of the genetic and genomic basis of Mendelian and complex disorders including congenital heart disease (CHD) [1,2,3,4]. The ability of next-generation sequencing to screen the entire human genome has led to the identification of not only single gene defects but also multi-genic disorders as well as biological pathways enriched in pathogenic variants.
The discovery in the last decade that the RV has distinct embryologic origins from the second heart field has opened up a new field of inquiry focused on identifying the role of the second heart field genes in RV development and disease [5]. For example, studies have identified structural and sequence variation in patients with second heart field defects and tetralogy of Fallot (TOF) [6,7,8]. Here we review genetic variants that modify RV disease severity and outcomes including severity of cardiac and extra-cardiac phenotypes. The most widely studied amongst RV diseases in the context of CHD includes tetralogy of Fallot (TOF) and single ventricle lesions where the RV is the systemic RV e.g. hypoplastic left heart syndrome (HLHS).
The Right Ventricle in Tetralogy of Fallot
Environmental Influences
The RV in TOF faces extraordinary stresses throughout the lifetime of the patient. In the unrepaired state during infancy, the RV is exposed to hypoxia and pressure load, and following complete repair the RV primarily faces volume overload secondary to pulmonary insufficiency and/or other residual hemodynamic lesions. The classic phenotype is progressive RV dilation and dysfunction that, untreated, culminates in RV failure. The rate at which this adverse RV remodeling progresses varies from patient to patient despite similar degree of pulmonary insuffiency. Pulmonary valve replacement is usually performed for moderate to severe RV dilation, RV dysfunction, exercise intolerance, arrhythmias and/or symptomatic heart failure. However, the extent of improvement in RV function and in exercise tolerance following pulmonary valve replacement is somewhat unpredictable, with some patients showing restoration of normal RV function while persistent RV dysfunction occurs in others. This has made clinical determination of the optimal timing for pulmonary valve replacement challenging. The patient to patient variability suggests that there are individual differences in how the RV in TOF adapts to ongoing stresses and that these differences may be genetic in origin. In this regard, hypoxia signaling is an important adaptive pathway in the heart and genetic variation in hypoxia signaling has the potential to influence RV adaptation.
Genetic Influences
There are important adaptive signaling pathways that are activated during periods of stress to maintain cardiovascular homeostasis. These pathways have been widely studied in the context of LV disease and less so in RV disease. Since the pulmonary vascular bed is uniquely sensitive to changes in oxygen tension, there has been particular interest in responses of the RV and the pulmonary vascular bed to hypoxic (and hemodynamic) stress. Hypoxia-inducible factor-1α (HIF1A) is an oxygen-sensitive transcription factor that is a central mediator of the response to hypoxia (Fig. 4.1). During acute hypoxia, HIF1A translocates to the nucleus to activate angiogenic, glycolytic and antioxidant genes that help with myocardial adaptation to hypoxia [9]. However, chronic HIF1A upregulation can be detrimental by promoting persistent activation of pro-fibrotic genes like transforming growth factor β1 (TGFB1) and resultant fibrosis [10]. Therefore HIF1A plays a complex role in mediating the cardiac hypoxic response.
The illustration shows the left ventricular adaptive response to hypoxia. Hypoxia activates HIF1α. This leads to the transcription of target genes that increase erythropoiesis (erythropoietin), angiogenesis (VEGF), glycolysis and anti-oxidant effects (glutathione peroxidase) resulting in improved oxygen delivery and energy production and reduced reoxygenation injury. HIF1α hypoxia inducible factor 1α, VEGF vascular endothelial growth factor. Reproduced with permission from Reddy S, Osorio JC, Duque AM, Kaufman BD, Phillips AB, Chen JM, et al. Failure of Right Ventricular Adaptation in Children With Tetralogy of Fallot. Circulation. 2006 July 4, 2006;114(1 suppl):I-37–I-42 © Wolters Kluwer 2006 [12]
Hypoxia Response Genes and RV Adaptation
In a mouse study, Kim et al. identified the importance of HIF1a in maintaining low pulmonary vascular tone by decreasing myosin light chain phosphorylation in pulmonary artery smooth muscle cells with, conversely, development of pulmonary hypertension in HIF1a null mice [11]. Reddy et al. investigated the role of hypoxia response genes in RV adaptation in patients with TOF [12]. They found that cyanotic patients had a significantly lower expression of hypoxia response genes regulated by HIF1a including vascular endothelial growth factor, glycolytic enzymes, and glutathione peroxidase, and a higher expression of collagen compared with acyanotic patients (Fig. 4.2). Therefore, the hypoxic response of the RV in TOF differed from the LV and was associated with higher postoperative lactate levels suggesting a maladaptive RV response to chronic hypoxia.
Gene expression in RV of cyanotic vs acyanotic TOF. The bars represent the fold-difference in the gene expression in the RV myocardium of cyanotic compared with acyanotic TOF patients. (a) There was lower expression of angiogenic (VEGF) and metabolic (ALDO A, ADK3) genes in the cyanotic RV. (b) There was lower expression of antioxidant genes (GPX) and higher expression of collagen in the cyanotic RV. Expression of HIF1α and titin (TTN) was not different. *P < 0.05; **P < 0.01 between cyanotic vs acyanotic TOF. HIF1 hypoxia-inducible factor 1α, VEGF vascular endothelial growth factor, ALDO A aldolase A, ADK3 adenylate kinase 3, GPX glutathione peroxidase, Col collagen, TTN titin. Reproduced with permission from Reddy S, Osorio JC, Duque AM, Kaufman BD, Phillips AB, Chen JM, et al. Failure of Right Ventricular Adaptation in Children With Tetralogy of Fallot. Circulation. 2006 July 4, 2006;114(1 suppl):I-37–I-42 © Wolters Kluwer 2006 [12]
HIF1A Genetic Variants and RV Adaptation
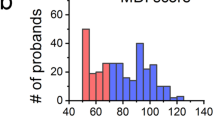
We explored the role of genetic variation in this highly conserved HIF1A signaling pathway in RV phenotype in TOF. Jeewa et al. studied the influence of three common single nucleotide polymorphisms (SNPs) in HIF1A on early and late RV remodeling in 180 TOF patients [13]. They found that patients with a higher number of functioning HIF1A alleles had higher TGFB1 expression and more fibrosis at initial repair when compared to patients with <4 functioning HIF1A alleles. However, during follow-up, patients with a higher number of functioning HIF1A alleles showed less RV dilation, better preservation of RV function (Fig. 4.3), and greater freedom from RV re-interventions (Fig. 4.4). This paper highlighted the importance of the hypoxia response genes in mediating RV adaptation to both hypoxia and hemodynamic stress. In particular, it raised the intriguing possibility that early RV fibrosis in the context of HIF1A upregulation was an adaptive response and may have allowed the RV to better handle long term hemodynamic stress while preserving RV function. The study also demonstrates the interaction of genetic and “environmental” factors in influencing clinical phenotype (Fig. 4.5) [14].
HIFA genotype and RV phenotype in TOF. (a) HIF1A genotype and RVOT dilation: RVOT z-score increased progressively after TOF repair but was lowe r in patients with a higher number of functioning HIF1Aalleles (P = 0.002) (n = 180). (b) HIF1A genotype and RV function: RV systolic function assessed using fractional area of change decreased with time, but the rate of decline of systolic function was lower in TOF patients with a higher number of functioning HIF1A alleles (P = 0.04). Red triangles, one to three functioning HIF1A alleles; black squares, 4 or 5 functioning HIF1A alleles; blue circles, six functioning HIF1A alleles. The red, black, and blue lines are regression lines showing the relationship between RVOT z-score or fractional area change and number of functioning HIF1A alleles. RVOT right ventricular outflow tract. Reproduced with permission from Jeewa A MA, Mertens L, Manlhiot C, Kinnear C, Mondal. T, Smythe J, Rosenberg H, Lougheed J, McCrindle BW, van Arsdell G, Redington AN, Mital S. Genetic determinants of right-ventricular remodeling after tetralogy of Fallot repair. Pediatr Res. 2012;72(4):407–13 © Nature Publishing Group 2012 [13]
Kaplan–Meier survival curve showing freedom from RV re-interventions (pulmonary valve or conduit replacement) during follow-up. Freedom from re-interventions was significantly lower in patients with four or more functioning HIF1A alleles as compared with those with fewer than four alleles. The hazard ratio for re-intervention decreased by 0.78 for every additional functioning allele (P = 0.04). HIF hypoxia-inducible factor 1α. Reproduced with permission from Jeewa A MA, Mertens L, Manlhiot C, Kinnear C, Mondal T, Smythe J, Rosenberg H, Lougheed J, McCrindle BW, van Arsdell G, Redington AN, Mital S. Genetic determinants of right-ventricular remodeling after tetralogy of Fallot repair. Pediatr Res. 2012;72(4):407–13 © Nature Publishing Group 2012 [13]
Interaction of “environmental” and genetic factors in determining clinical outcomes. Genetic and environmental factors interact to cause disease and to determine the biologic response to the condition and its treatment. HIF1A genetic variants affecting its expression influence the response in tetralogy of Fallot (TOF) to the environmental stresses of hypoxia, pressure overload, and surgical repair. Establishing a chain of evidence from underlying genetic variation to observed clinical outcome relies on the integration of biorepository and clinical database resources as demonstrated by this study. RV right ventricular. Reproduced with permission from Russell MW WN. Getting personal: understanding how genetic variation affects clinical outcomes in patients with tetralogy of Fallot. Pediatr Res. 2012;72(4):334–6.© Nature Publishing Group [14]
Interestingly, these findings were at odds with data obtained in patients with hypertrophic cardiomyopathy (HCM) . Alkon et al. found that pediatric HCM patients with a higher number of HIF1A functioning alleles had more myocardial TGFB1 upregulation and endothelial-mesenchymal transition in the LV [15]. These patients also had more severe septal hypertrophy, more diastolic dysfunction and lower freedom from myectomy compared to patients with fewer functioning HIF1A alleles. Thus, although chronic HIF1A upregulation was associated with pro-fibrotic TGFB1 upregulation in both TOF and HCM patients, this was associated with less adverse remodeling and dysfunction in the RV in TOF, but with more hypertrophy and dysfunction in the LV in HCM. This suggests that the impact of HIF1A genetic variation is dependent on the type of environmental insult and/or ventricular chamber type affected.
Genetic Variants and Bypass-mediated Injury
The RV encounters not only hemodynamic stresses but also surgical and bypass mediated injury which is compounded in patients requiring multiple re-operations. Bypass mediated injury is predominantly related to hypoxia-reoxygenation or ischemia-reperfusion that generates oxidative stress that can adversely impact ventricular function postoperatively. The extent of bypass-mediated injury varies considerably from one patient to the next. About 40% of East Asians carry an aldehyde dehydrogenase-2*2 (ALDH2*2) allele, an important anti-oxidant gene and metabolic gene. Zhang et al. studied ALDH2*2 in patients undergoing surgery for TOF repair and identified patients with increased bypass-mediated injury i.e. higher postoperative troponin I elevation, higher inotrope scores, and longer intensive care unit and/or hospital length of stay [16]. In their study, carriers of the ALDH2*2 allele demonstrated cardioprotection during surgery for TOF repair as seen by lower postoperative troponin I, inotrope score, and shorter postoperative length of intensive care and hospital stay. Thus, knowledge of ALDH2 genotype can identify patients at higher risk for bypass mediated injury amongst those undergoing TOF repair.
Clinical Significance
Although additional validation studies are needed, these studies emphasize the important gene-environment interactions that influence disease phenotypes and the potential for genetic guided approaches to treatment in TOF patients. For example, physicians may be able to use targeted genetic testing to identify patients at risk for progressive RV dilation or dysfunction and treat them according to risk. This may include better cardio-protective strategies in those at risk for bypass mediated injury i.e. ALDH2*2 carriers to avoid perioperative injury to the RV, early complete repair to minimize duration of hypoxia exposure during infancy in those with fewer HIF1A functioning alleles, and lower threshold for pulmonary valve replacement during follow-up in those with fewer HIF1A functioning alleles to preserve long term RV function and avoid irreversible injury.
The Right Ventricle as the “Single” Ventricle
Environmental Influences
Hypoplastic left heart syndrome (HLHS) is one of the commonest forms of single ventricle lesions where the RV serves as the systemic RV. Similar to TOF, the systemic RV in single ventricle lesions is exposed to multiple stressors throughout a patient’s lifetime. The RV is exposed to significant volume overload during infancy following stage 1 palliation (Norwood procedure, Sano procedure, hybrid procedure) since the RV supports both the systemic and pulmonary circulation. The RV is unloaded (at least in terms of volume load) after superior cavopulmonary connection (SCPC) and the Fontan procedure) but is still required to support the systemic circulation even after complete palliation. While relatively rare in the first decade or two after successful Fontan palliation, the RV may fail during follow-up resulting in heart failure, death or need for transplant. Which patient will develop RV dysfunction, and how fast, may be related to genetic variation in ability for ventricular adaptation. Early identification of patients who are genetically predisposed to RV failure in single ventricle lesions may guide type and timing of intervention before end-stage heart failure.
Genetic Influences
The renin-angiotensin-aldosterone system (RAAS) is one of the most important neuro-hormonal systems responsible for cardiovascular homeostasis. Similar to hypoxia signaling, RAAS activation in response to acute stress is adaptive or compensatory; however chronic activation can result in adverse effects secondary to persistent elevation in systemic vascular resistance, increased myocardial oxygen consumption, myocyte injury and death, and myocardial fibrosis. The adverse impact of SNPs that were associated with RAAS upregulation on severity of LV hypertrophy was first reported in patients with HCM [17]. These five SNPs in the RAAS genes were subsequently evaluated in single ventricle infants participating in a Pediatric Heart Network multicenter clinical trial of enalapril (55% with systemic RVs) [18]. In this trial, 230 infants with single ventricle lesions were randomized after stage 1 palliation to receive placebo or enalapril for 14 months. Somatic growth and ventricular function were assessed before stage 2 palliation (usually around 6 months) and at 14 months follow-up. The study revealed that single ventricle patients had significant elevations in ventricular mass and volume prior to SCPC and while this improved after SCPC, it did not normalize in all patients at 14 months follow-up. Importantly, there was no benefit of enalapril therapy on either somatic growth or ventricular function and heart failure severity during 14 mos follow-up. However, a genetic sub-study in 154 trial participants revealed intriguing genetic associations with reverse ventricular remodeling. In the genetic substudy, the same five SNPs associated with RAAS upregulation were evaluated for response to ventricular unloading at the time of stage 2 palliation. While patients with fewer (<2) RAAS upregulation genotypes showed favorable reverse remodeling after stage 2 palliation, this reverse remodeling was not seen in patients with ≥2 RAAS upregulation genotypes. These patients demonstrated persistent elevation in ventricular mass and volume at 14 months follow-up (Fig. 4.6) [19]. This effect was independent of ventricular morphology i.e. systemic LV or RV, and of enalapril treatment. This study highlighted the important influence of RAAS genotype in influencing response to ventricular volume unloading surgery.
Difference (and 95% confidence intervals) in ventricular mass z-scores at (a) pre-SCPC and (b) 14 months study visit between individual high risk versus no high risk RAAS genotypes using recessive model (*P < 0.05 versus low-risk genotype) showing a trend toward higher mass z-scores at 14 months in patients with high-risk genotypes with strongest association with AGTR1. (c) Linear regression model (mean ± 95% confidence limits) showing incremental effect of increasing number of RAAS-upregulation genotypes on ventricular mass z-score at 14 months. SCPC superior cavopulmonary connection, AGT angiotensinogen, ACE angiotensin-converting enzyme, AGTR1 angiotensin II type 1 receptor, CYP11B2 aldosterone synthase, CMA1 chymase, nRAAS total number of high-risk renin-angiotensin-aldosterone system genotypes, CI confidence interval. Reproduced with permission from Mital S, Chung WK, Colan SD, Sleeper LA, Manlhiot C, Arrington CB, et al. Renin-Angiotensin-Aldosterone Genotype Influences Ventricular Remodeling in Infants With Single Ventricle. Circulation. 2011;123(21):2353–62. © Wolters Kluwer 2011 [19]
Clinical Significance
Similar to patients with TOF, this study highlights the gene-environment interactions that influence cardiac response to environmental stressors like hemodynamic load. While these findings require further validation, it suggests that physicians may be able to do targeted genetic testing in single ventricle patients to identify those that are at risk for progressive and irreversible ventricular remodeling. These patients may benefit from earlier volume unloading surgery before the injury becomes irreversible. This is particularly important in single ventricle patients since high ventricular mass at the time of Fontan can adversely impact Fontan outcomes by reducing ventricular compliance and forward flow through the Fontan circulation.
Genetic Variation and Non-Cardiac Phenotype in Right Ventricular Lesions
Genetic variation can impact not only cardiac but also non-cardiac phenotype in children with heart disease. Children with CHD are at risk for adverse neurodevelopmental outcomes related not only to structural abnormalities but also to cerebral perfusion abnormalities and brain injury related to cardiopulmonary bypass and deep hypothermic circulatory arrest [20]. Gaynor et al. found that genetic variation had a significant impact on neurocognitive outcomes in infants who had cardiac surgery before 6 months of age [21]. Infants carrying the apolipoprotein E epsilon 2 allele (ApoE €2) had lower neurocognitive performance at 1 year of age measured using Bayley Scales of Infant Development (BSID) . This impairment persisted at 4 years of age [22]. Importantly, Zeltser et al. evaluated the influence of this genotype on neurodevelopmental outcome in 60 children after TOF repair [23]. The presence of a genetic syndrome and of the ApoE €2 allele was predictive of lower BSID scores at 1 year of age after TOF repair. The influence of this genotype on neurodevelopmental outcomes was replicated in infants with hypoplastic left heart syndrome enrolled in a Pediatric Heart Network trial—the Single Ventricle Reconstruction trial [24]. This suggests that genetic risk stratification may have utility in infants undergoing cardiac surgery to identify those at highest risk for neurological injury. These infants may benefit from neuro-protective strategies perioperatively and more importantly, these infants may need to be monitored closely during follow up to provide early intervention strategies in a timely manner.
Future Directions
While the candidate SNP association studies described here provide exciting insights into the importance of genetic factors in CHD outcomes, the search for genomic variants needs to be broadened from candidate SNP association studies to genome wide approaches. The use of next-generation sequencing allows an unbiased search of the patient genome or exome and allows capture of not only common but also rare and novel variants of potential clinical significance. It also allows identification of biological pathways enriched for variants. This approach is rapidly gaining traction with the reduction in sequencing costs and improvements in bioinformatics platforms and pipelines [25]. These discoveries will not only improve the precision of personalized risk stratification and targeted therapies but also help in the discovery of new therapies, particularly for diseases of the RV for which no therapies currently exist, by identifying potentially “druggable” targets in biologically relevant pathways. Genetic-guided approaches therefore have the potential to improve outcomes through personalized management of patients including tailoring type and timing of medical and surgical management based on predicted genetic risk and prediction of response.
Abbreviations
- CHD:
-
Congenital heart disease
- HCM:
-
Hypertrophic cardiomyopathy
- HIF1A:
-
Hypoxia inducible factor 1α
- LV:
-
Left ventricle
- RAAS:
-
Renin-angiotensin-aldosterone system
- RV:
-
Right ventricle
- SNP:
-
Single nucleotide polymorphism
- TGFB1:
-
Transforming growth factor β1
- TOF:
-
Tetralogy of Fallot
References
Purcell SM, Moran JL, Fromer M, Ruderfer D, Solovieff N, Roussos P, et al. A polygenic burden of rare disruptive mutations in schizophrenia. Nature. 2014;506(7487):185–90.
Michaelson JJ, Shi Y, Gujral M, Zheng H, Malhotra D, Jin X, et al. Whole-genome sequencing in autism identifies hot spots for de novo germline mutation. Cell. 2012;151(7):1431–42.
Zaidi S, Choi M, Wakimoto H, Ma L, Jiang J, Overton JD, et al. De novo mutations in histone-modifying genes in congenital heart disease. Nature. 2013;498(7453):220–3.
Al Turki S, Manickaraj AK, Mercer CL, Gerety SS, Hitz MP, Lindsay S, et al. Rare variants in NR2F2 cause congenital heart defects in humans. Am J Hum Genet. 2014;94(4):574–85.
Buckingham M, Meilhac S, Zaffran S. Building the mammalian heart from two sources of myocardial cells. Nat Rev Genet. 2005;6(11):826–35.
Stevens KN, Hakonarson H, Kim CE, Doevendans PA, Koeleman BP, Mital S, et al. Common variation in ISL1 confers genetic susceptibility for human congenital heart disease. PLoS One. 2010;5(5):e10855.
Greenway SC, Pereira AC, Lin JC, DePalma SR, Israel SJ, Mesquita SM, et al. De novo copy number variants identify new genes and loci in isolated sporadic tetralogy of Fallot. Nat Genet. 2009;41(8):931–5.
Silversides CK, Lionel AC, Costain G, Merico D, Migita O, Liu B, et al. Rare copy number variations in adults with tetralogy of Fallot implicate novel risk gene pathways. PLoS Genet. 2012;8(8):e1002843.
Semenza GL, Agani F, Feldser D, Iyer N, Kotch L, Laughner E, et al. Hypoxia, HIF-1, and the pathophysiology of common human diseases. Adv Exp Med Biol. 2000;475:123–30.
Sun S, Ning X, Zhang Y, Lu Y, Nie Y, Han S, et al. Hypoxia-inducible factor-1alpha induces Twist expression in tubular epithelial cells subjected to hypoxia, leading to epithelial-to-mesenchymal transition. Kidney Int. 2009;75(12):1278–87.
Kim Y-M, Barnes EA, Alvira CM, Ying L, Reddy S, Cornfield DN. Hypoxia-inducible factor-1α in pulmonary artery smooth muscle cells lowers vascular tone by decreasing myosin light chain phosphorylation. Circ Res. 2013;112(9):1230–3.
Reddy S, Osorio JC, Duque AM, Kaufman BD, Phillips AB, Chen JM, et al. Failure of right ventricular adaptation in children with tetralogy of fallot. Circulation. 2006;114(1 Suppl):I37–42.
Jeewa A, Manickaraj AK, Mertens L, Manlhiot C, Kinnear C, Mondal T, Smythe J, Rosenberg H, Lougheed J, McCrindle BW, van Arsdell G, Redington AN, Mital S. Genetic determinants of right-ventricular remodeling after tetralogy of Fallot repair. Pediatr Res. 2012;72(4):407–13.
Russell MW, Wilder NS. Getting personal: understanding how genetic variation affects clinical outcomes in patients with tetralogy of Fallot. Pediatr Res. 2012;72(4):334–6.
Alkon J, Friedberg MK, Manlhiot C, Manickaraj AK, Kinnear C, McCrindle BW, Benson LN, Addonizio LJ, Colan SD, Mital S. Genetic variations in hypoxia response genes influence hypertrophic cardiomyopathy phenotype. Pediatr Res. 2012;72(6):583–92.
Zhang H, Gong DX, Zhang YJ, Li SJ, Hu S. Effect of mitochondrial aldehyde dehydrogenase-2 genotype on cardioprotection in patients with congenital heart disease. Eur Heart J. 2012;33(13):1606–14.
Ortlepp JR, Vosberg HP, Reith S, Ohme F, Mahon NG, Schroder D, et al. Genetic polymorphisms in the renin-angiotensin-aldosterone system associated with expression of left ventricular hypertrophy in hypertrophic cardiomyopathy: a study of five polymorphic genes in a family with a disease causing mutation in the myosin binding protein C gene. Heart. 2002;87(3):270–5.
Hsu DT, Zak V, Mahony L, Sleeper LA, Atz AM, Levine JC, et al. Enalapril in infants with single ventricle: results of a multicenter randomized trial. Circulation. 2010;122(4):333–40.
Mital S, Chung WK, Colan SD, Sleeper LA, Manlhiot C, Arrington CB, et al. Renin-angiotensin-aldosterone genotype influences ventricular remodeling in infants with single ventricle. Circulation. 2011;123(21):2353–62.
Wypij D, Newburger JW, Rappaport LA, duPlessis AJ, Jonas RA, Wernovsky G, et al. The effect of duration of deep hypothermic circulatory arrest in infant heart surgery on late neurodevelopment: The Boston Circulatory Arrest Trial. J Thorac Cardiovasc Surg. 2003;126(5):1397–403.
Gaynor JW, Gerdes M, Zackai EH, Bernbaum J, Wernovsky G, Clancy RR, et al. Apolipoprotein E genotype and neurodevelopmental sequelae of infant cardiac surgery. J Thorac Cardiovasc Surg. 2003;126(6):1736–45.
Gaynor JW, Nord AS, Wernovsky G, Bernbaum J, Solot CB, Burnham N, et al. Apolipoprotein E genotype modifies the risk of behavior problems after infant cardiac surgery. Pediatrics. 2009;124(1):241–50.
Zeltser I, Jarvik GP, Bernbaum J, Wernovsky G, Nord AS, Gerdes M, Zackai E, Clancy R, Nicolson SC, Spray TL, Gaynor JW. Genetic factors are important determinants of neurodevelopmental outcome after repair of tetralogy of Fallot. J Thorac Cardiovasc Surg. 2008;135(1):91–7.
Gaynor JW, Kim DS, Arrington CB, Atz AM, Bellinger DC, Burt AA, Ghanayem NS, Jacobs JP, Lee TM, Lewis AB, Mahle WT, Marino BS, Miller SG, Newburger JW, Pizarro C, Ravishankar C, Stolle CA, Wilder NS, Jarvik GP, Mital S, Russell MW. Validation of association of the apo-lipoprotein E (APOE) ε2 allele with neurodevelopmental dysfunction after cardiac surgery in neonates and infants. J Thorac Cardiovasc Surg. 2014;148(6):2560–6.
Ashley EA, Hershberger RE, Caleshu C, Ellinor PT, Garcia JGN, Herrington DM, et al. Genetics and cardiovascular disease: a policy statement from the american heart association. Circulation. 2012;126(1):142–57.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 Springer International Publishing AG
About this chapter
Cite this chapter
Mital, S. (2018). Genetic Variation and Outcomes in Right Ventricular Congenital Heart Disease. In: Friedberg, M., Redington, A. (eds) Right Ventricular Physiology, Adaptation and Failure in Congenital and Acquired Heart Disease. Springer, Cham. https://doi.org/10.1007/978-3-319-67096-6_4
Download citation
DOI: https://doi.org/10.1007/978-3-319-67096-6_4
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-67094-2
Online ISBN: 978-3-319-67096-6
eBook Packages: MedicineMedicine (R0)