Abstract
The development of left ventricular (LV) hypertrophy and the progression to LV failure have been extensively studied and have provided valuable insight into the mechanisms of disease progression. However, there is minimal data on the right ventricular (RV) adaptation to pressure and volume loading. These hemodynamic stressors are commonly seen in children and adults after surgery for congenital heart disease (CHD), placing the RV at risk for progression to heart failure. Here, we will highlight some of similarities and differences in the molecular remodeling between the right and left ventricles when subjected to abnormal loading conditions.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
Introduction
Congenital heart disease (CHD) is the single most common class of birth defects and is a leading cause of infant mortality in developing countries [1, 2]. CHD often leads to abnormal loading conditions starting in the neonatal period both before and after surgical palliation leading to a lifetime of chronic pressure and/or volume loading. While numerous animal and human studies have led to valuable insight into the molecular mechanisms of left ventricular (LV) hypertrophy and the progression from a compensated state to one of overt heart failure, [3, 4] little is known about the right ventricular (RV) response to stress [5, 6]. The RV response to pressure overload secondary to pulmonary hypertension (PHTN) and work in animal models of CHD are only now being evaluated. In addition to RV pressure overload seen in CHD patients with single RVs, systemic RVs as in l-transposition or d-transposition following atrial switch and PHTN, RV volume overload is a much more common and unique sequelae after repair of many forms of CHD such as tetralogy of Fallot or pulmonary atresia following RV outflow tract reconstruction. There is even less data on RV remodeling following volume overload. With advances in medical and surgical care of these children, they are growing into adolescence and adulthood with the potential for a lifetime of abnormal loading conditions on the RV, predisposing them to early heart failure, arrhythmias, transplantation and death. Their quality of life will ultimately depend on our ability to understand the basic mechanism of disease progression, identify potential drug targets and develop RV-specific therapies. We will highlight our current knowledge of the RV adaptation to the unique hemodynamic stressors seen in the CHD patient while discussing the similarities and differences between right and left ventricular remodeling and addressing novel therapies currently in development.
The Right and Left Ventricles Respond Differently to Increasing Afterload
The RV is uniquely at risk when exposed to chronic afterload. This is best demonstrated in long-term studies of patients with single ventricle physiology, where the systemic RV progresses to heart failure sooner and more often than those with a systemic LV [7]. Similarly, patients with l-transposition of the great arteries or patients who have undergone an atrial switch operation for d-transposition of the great arteries, where the RV functions as the systemic ventricle, have an increased risk of RV failure, even in the absence of atrioventricular valve regurgitation or other lesions. These systemic RVs develop hypertrophy at a very early age or never regress, suggesting that increased wall stress alone cannot be the only factor predisposing these ventricles to failure. In addition, standard left heart failure drugs (β-blockers, ACE inhibitors, angiotensin II receptor blockers), have been shown to not improve function or survival in patients with systemic RV failure due to CHD in multiple clinical trials. Critically, β-blockers appeared to worsen outcomes in patients with a systemic RV in one trial [8,9,10,11] suggesting fundamental differences in the mechanisms of right vs. left ventricular failure, particularly when treating systemic RV failure in CHD.
In the past, these differences between the right and left ventricles were thought to be related to changes in shape, structure and loading conditions [12, 13]. We now know that these differences begin early in development, before afterload differences become operative. The primary and secondary heart fields, lead to the differentiation of left or right ventricular cardiomyocytes during early development, with subsequent chamber-specific differences in cell signaling and Ca2+ handling, all suggesting fundamental differences between the two ventricles at the cellular level as well [14].
Using animal models of pressure overload stress, we and others have shown that the two ventricles exhibit similar molecular alterations in genes regulating extracellular matrix and cytoskeletal remodeling. However, there are important differences in genes regulating reactive oxygen species (ROS) production and antioxidant protection, angiogenesis, energy production and mitochondrial function (Table 3.1 and Fig. 3.1) [15,16,17,18]. These results confirm differences at the cellular and molecular level in the mechanisms leading to heart failure between the two ventricles, which are discussed below.
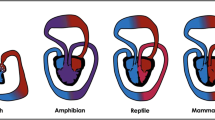
Right ventricular susceptibility to heart failure. Stable LVH is characterized by an increase in antioxidant enzymes and angiogenesis and decreased ROS generation, which protects the LV from early progression to heart failure. However in RVH, there is an early decrease in antioxidant enzymes and failure of angiogenesis along with increased ROS and cell death thereby making the RV more susceptible to early heart failure. LVH left ventricular hypertrophy, RVH right ventricular hypertrophy, PS pulmonary stenosis, ROS reactive oxygen species. Cardiac illustrations by Mingming Zhao. With permission from Reddy S, Bernstein D. The vulnerable right ventricle. Current opinion in pediatrics. 2015 Oct;27 (5):563–8 © Wolters Kluwer Health 2015 [18]
RV Molecular Remodeling: Adaptive and Maladaptive Responses
ROS Production and Antioxidant Defenses
Under conditions of afterload stress , both ventricles increase overall cellular ROS production ; however, the RV antioxidant defenses via superoxide dismutase (SOD) and glutathione peroxidase (GPX) fail early, whereas in the LV they remain intact until a more advanced stage of failure [19, 20]. This results in early ROS-related damage and apoptosis [21]. It is encouraging to note that the antioxidant EUK-134 (a superoxide dismutase and catalase mimetic) was shown to reduce oxidative stress and ROS production in the failing RV due to PHTN and to improve RV systolic function [22]. There are also differences between the two ventricles in the primary source of ROS production, with greater mitochondrial ROS generation by NADPH oxidase and mitochondrial complex II in RV failure [23].
PGC1α is a key regulator of both mitochondrial metabolism and mitochondrial biogenesis (the generation of “new” mitochondria) which has been shown to be decreased in animal models of RV failure, leading to impaired fatty acid oxidation, decreased mitochondrial mass and number, and reduced oxidative capacity. This represents another mechanism leading to increased ROS production, causing mitochondrial DNA damage and impairing mitochondrial biogenesis [20, 24]. These data were confirmed in patients by Karamanlidis et al., who demonstrated a progressive decline in mitochondrial DNA with the progression from RVH to RVF in children with CHD (including those with TOF, pulmonary stenosis, double outlet RV, double chambered RV and single RV). They also showed that mitochondrial DNA-encoded genes responsible for mitochondrial biogenesis fall before the onset of heart failure [24].
Blood Flow and Angiogenesis
The RV has a lower resting oxygen consumption and therefore lower resting coronary blood flow than the LV. In the normal RV, the majority of coronary flow occurs in systole, in contrast to the normal LV where coronary flow is mostly in diastole thereby making the RV more susceptible to ischemia in conditions of afterload stress. Animal data suggests that right coronary artery flow is increased in response to afterload stress. However, whether this is adequate to meet the increased oxygen demand of the hypertrophied RV remains to be determined [25, 26].
As a further insult to the vulnerable RV, there are differences in the RV’s ability to induce the formation of new blood vessels to compensate for myocardial hypertrophy compared to that of the LV [27, 28]. When the LV is exposed to afterload stress, it initially increases the production of new capillaries (angiogenesis), in order to keep up with the increased blood flow requirements of hypertrophied cardiomyocytes. This process is mediated by increased cardiomyocyte production of the pro-angiogenic factors HIF-1α and vascular endothelial growth factor (VEGF) Angiogenesis is only impaired when the LV begins to fail, which is mediated by an increase in p53, which then inhibits pro-angiogenic factors. In contrast, in the RV, even at the onset of pressure overload, VEGF falls and thus capillary density decreases, rendering the stressed RV more susceptible to micro-ischemic injury [23, 27,28,29,30,31]. In compensation, there is right coronary artery vasodilation via endogenous release of NO, thereby helping to improve oxygen demand-supply balance, at least temporarily. This is another feature not seen in the afterload stressed LV [32, 33]. Thus, whether differences in RV vs. LV oxygen delivery and microvascular remodeling are responsible for the differences in stress response between the two ventricles is still an area open to further investigation.
Response to Hypoxia
Hypoxia can be a component of the clinical picture in patients with RV failure, whether due to persistent right-to-left shunts in CHD, or in patients with PHTN. Hypoxia activates Hif1α/VEGF signaling in the RV in the SUGEN-hypoxia animal model of PHTN, but similar to isolated pressure overload, without increasing capillary density. This is particularly relevant in systemic RVs exposed to hypoxia such as in infants with hypoplastic left heart syndrome after a Norwood/Sano or Glenn palliation , and before their Fontan procedure, where the RV is hypertrophied, leading to increased metabolic demand, but fails to increase capillary density to enhance delivery of oxygen and nutrients. This combination of these stressors may be responsible for the increased rate of failure of these single RVs. Hypoxia also triggers glycolysis, which may correlate with the development of RV failure [15].
Neurohormonal Activation: Adrenergic Receptors
β-adrenergic receptor signal regulation is similar in the normal RV and LV, leading to a positive inotropic response in both ventricles. β-adrenergic receptor signal regulation is also similar in animal models of failing RV vs. the LV with downregulation of β1-, α1- and DA1 receptors, decreased cAMP levels and increased G protein-coupled receptor kinase-2 (GRK2) activity, leading to an impaired inotropic response. This downregulation of adrenergic signaling is greater in PHTN-induced than in PAB-induced RV hypertrophy and failure. Interestingly however, the clinical response of the two ventricles to β-adrenergic blockers is quite different [34]. Children with systemic RVs did not benefit from the use of β-blockers, and there is even a suggestion that this class of drugs can worsen heart failure symptoms [10]. Similarly, in adults with RV failure after repaired tetralogy of Fallot, β-blockade showed no improvement in peak VO2, RVEF, ventricular volume or NYHA class [35]. In contrast, one retrospective study of adults with a systemic RV and mild (NYHA class I and II) symptoms did show an improvement if they were taking a β-blocker for at least 4 months [36]. These patients showed an increase in β2-receptor mediated inotropy and lusitropy secondary to enhanced coupling of β2 receptors to Gs [37].
In contrast, there is evidence that α1-adrenergic receptor signaling differs between the non-stressed RV and LV. Stimulation of α1-receptors results in a negative inotropic response in the RV and a positive inotropic response in the LV. This differential response is not mediated by differences in PKC activation, but instead by greater myofilament Ca2+ sensitivity through phosphorylation of myosin light chain (MLCK) in the LV versus the RV [38]. However, in a model of the failing RV induced by coronary artery ligation, MLCK increases, resulting in a greater myofilament Ca2+ sensitivity, and α1-signaling then switches from being a negative to being a positive inotrope [39]. The applicability of these findings in RV failure due to non-ischemic heart disease, such as in children with CHD, remains to be determined.
Neurohormonal Activation: Renin-Angiotensin-Aldosterone System (RAAS)
RAAS activation occurs in the setting of low LV cardiac output and or low systemic vascular resistance, causes vasoconstriction and increased tubular sodium reabsorption peripherally and is also a positive mediator of cardiomyocyte fibrosis. There is limited data on RAAS in RV failure other than a few studies in chronic obstructive pulmonary disease and systemic sclerosis causing PHTN [40,41,42]. RAAS activation is seen in patients with PHTN and treatment with losartan, an angiotensin II receptor antagonist decreases hypertrophy and restores normal RV-pulmonary arterial coupling [43]. Treatment with ACE inhibition in PHTN patients however has conflicting results, which is thought to be related to breakthrough aldosterone signaling from incomplete inhibition [43]. Similarly, whether RAAS is stimulated with RV failure in the setting of CHD remains to be determined, particularly important since ACE inhibitors (enalapril, ramipril) and angiotensin II receptor antagonists (losartan) have failed to improve right heart failure in CHD patients with a systemic RV [11, 44,45,46]. There are currently clinical trials evaluating the efficacy of losartan in adults with tetralogy of Fallot and RV failure, but a better understanding of the basic mechanisms of RAAS activation in the stressed RV needs to be concurrently undertaken.
Mitochondrial Remodeling
The mitochondrial protein profiles of the RV and LV are similar at rest, with equivalent cellular aerobic capacity, volume of mitochondria, mitochondrial enzyme content (cytochrome c oxidase, complexes 1, 3, 4 and 5, aconitase and Mn-SOD) and mitochondrial enzyme activities [47]. In the LV exposed to afterload stress, antioxidant enzymes such as Mn-SOD increase during the early stages, whereas in the RV Mn-SOD never increases, which may lead to an earlier increase in mitochondrial reactive oxygen species production and subsequent mitochondrial dysfunction and cell death when compared to the LV [21].
Mitochondrial membrane potential, a surrogate of overall mitochondrial function, is lower in the resting RV than in the LV. With RV afterload stress, there is an increase in mitochondrial membrane potential which may be related to activation of the NFAT pathway, and which is reversed by the PDK inhibitor dichloroacetate (discussed below). This difference in mitochondrial remodeling may represent another difference in the stress response between the RV and the LV, and represents another potential target for RV-specific therapy [48].
Metabolic Adaptations to Pressure Overload
The RV and LV differ in their work load, and hence in their energy needs. The LV workload is five times greater than the RV due to the higher systemic vascular resistance when compared to the low resistance pulmonary vascular bed. Due to this decreased workload on the resting RV, both oxygen consumption and metabolic stress are lower than in the LV [25, 49]. Despite this difference, the non-stressed RV and LV are largely similar in their energetic profiles [47].
The metabolic profile of both ventricles is profoundly altered with afterload stress. Both the RV and LV myocardium normally utilize free fatty acids for biosynthesis and energy production. With afterload stress, the myocardium shifts to a greater dependence on glucose for its energy source via increased glucose uptake and glycolysis. While this shift is beneficial during acute stress, chronic dependence on glycolysis for energy production is inadequate to meet the demands of the myocardium, leading to an energy starved state and subsequently to heart failure.
In RV failure secondary to pulmonary artery banding (PAB), this shift in glycolysis is associated with a 50% decrease in energy reserve [50]. Decreased glucose oxidation is also related to the activation of pyruvate dehydrogenase kinases (PDKs), preventing pyruvate from entering the Krebs cycle and decreasing ATP generation. Dichloroacetate inhibits PDKs and improves glucose oxidation and RV function in rat models of PHTN [15]. Partial inhibition of beta-oxidation by trimetazidine has been shown to improve both LV and RV function in patients with diabetic cardiomyopathy [51]. To date, these drugs have not been tested in animal models of CHD. Other mediators increasing glycolysis, Hif-1α and p38-MAPK are also activated when the RV begins to fail. In a murine model of acute, severe PAB-induced RV failure, we showed a downregulation of mitochondrial enzymes (acetyl-coenzyme A acyltransferase 2, NADH dehydrogenase, NADH-ubiquinone oxidoreductase, succinate dehydrogenase complex and ATP synthase). Whereas a decrease in mitochondrial enzymes-complex 1, III and IV are seen in children with tetralogy of Fallot; there is no data on systemic right ventricles as in l-TGA, d-TGA after atrial switch or single right ventricles [52]. There is suggestion that at least some components of RV electrical remodeling may be secondary to metabolic derangements such as the reduced Kv channel expression, prolonging the action potential duration and QT interval and increasing the risk of arrhythmias [15]. Interestingly, these can be reversed by restoration of glucose oxidation using dichloroacetate.
In summary, the RV and LV both undergo major shifts in metabolism in response to acute and chronic afterload stress. However, the switch to glycolysis appears to occur earlier in the pressure loaded RV vs. the pressure loaded LV resulting in an earlier decrease in net ATP production in the RV. If energetic pathways are more at risk in the chronic pressure-loaded RV, then strategies that maintain favorable ATP production and oxygen consumption may be beneficial. Several drugs that increase glucose utilization (ranolazine, trimetazidine, perhexiline) have been tested in animal models of PHTN and in human clinical trials of LV failure and PHTN-induced RV failure, with variable success [53,54,55,56]. There is little data on the metabolic derangements in CHD and no data on the utility of drugs to modify these alterations. Cardiac metabolic imaging, e.g. MR spectroscopy, has the potential to shed more light on alterations in RV metabolism in patients with CHD [57]. While important differences in metabolism exist between animal models and patients, thereby limiting direct translation, the use of animal models is the first step toward understanding the mechanism of disease. Patients with right heart failure due to PHTN show a metabolic shift away from fatty acid metabolism to glycolysis. Abnormalities in mitochondrial complexes 1, III and IV have been described in children with tetralogy of Fallot. These results are similar to those demonstrated in animal models of PHTN and pulmonary artery banding, making it feasible to use animal models as the first step in studying the mechanism of RV failure, with subsequent confirmation in patients.
Metabolic Response to Chronic Volume Overload
Our knowledge of the RV response to volume load is limited despite this being common late sequelae after RV outflow tract reconstruction, in single right ventricles with an aortopulmonary shunt, or in l-TGA with atrioventricular valve regurgitation, a major contributor to morbidity and mortality in this population. To address this shortcoming, we developed a murine model of chronic RV volume overload, and used this as a platform to evaluate the transcriptional regulation of the volume loaded RV [58]. Early diastolic dysfunction and preserved systolic function were associated with downregulation of several metabolic pathway regulators, including phosphofructokinase, a rate-limiting enzyme in glycolysis, and aconitase, an upstream TCA cycle enzyme, both important for ATP production. There were also decreases in ATP-binding transporters, genes encoding transport of nutrients across the cell membrane. With the worsening of diastolic dysfunction and the onset of fibrosis, but with preserved systolic function, there is a shift away from β-oxidation with downregulation of fatty acid binding protein and upregulation of AMP kinases, and increased glycogenolysis with upregulation of GSK3β and glycogen phosphorylase. These adaptations are similar to those described during LV volume overload, however, additional research will be required to determine if more subtle differences exist.
MicroRNAs
miRs are small, non-coding RNAs of 18–25 nucleotides that regulate gene expression by degradation or translational suppression of mRNA. As master regulators of entire gene networks, miRs have received considerable attention in cardiovascular development and in LV hypertrophy and failure and are being developed as therapeutic targets and as biomarkers to monitor disease progression [59,60,61].
While miR expression is largely similar between the afterload stressed RV and LV, there are interesting differences. We profiled miR expression at various stages of adaptive RVH progressing to RV failure, and identified four RV-specific miRs: miRs 34a, 28, 93 and 148a, none of which are increased in LV hypertrophy and failure induced by transverse aortic constriction (TAC) [62,63,64,65]. These miRs cause cell cycle arrest, oxidant damage, impairment of DNA repair as well as inhibition of pro-angiogenic factors [66, 67]. An interesting feature of these four RV-specific miRs is that they are upregulated only in non-cardiomyocytes in the heart (endothelial cells and fibroblasts), but affect cardiomyocytes via paracrine signaling. These four RV-specific miRs may enhance the progression to RV failure by increasing oxidative stress, reducing oxidative defenses, decreasing angiogenesis and activating cell death pathways, the very pathways that differ most between the RV and LV, as shown above. Potus et al. suggest a systemic vascular defect in the miR-126/VEGF pathway in PHTN in pulmonary artery endothelial cells and in the RV. This pathway was upregulated during compensated RVH but transitioned to being downregulated with RV failure [68]. PHTN is also characterized by a downregulation in miR-208 followed by an upregulation of its target Mef2 in RVF [69, 70].
Novel Models of RV Failure Simulating Residual Lesions After Surgery for Right Sided Obstructive Lesions
In order to address the minimal data on the cellular and molecular mechanisms of RV hypertrophy and the progression to RV failure in CHD, we have created murine models of RV pressure overload, volume overload and combined pressure and volume overload to simulate some of the common residual lesions seen after RV outflow tract reconstruction, thereby enabling the assessment of genome-wide changes in the RV. These models reflect the clinical picture seen in children with progression from a compensated, adaptive stage with predominant diastolic dysfunction to decompensated systolic dysfunction with clinical heart failure.
Pressure overload alone was characterized by upregulation of genes regulating phosphate and other inorganic ion transport, cell adhesion and cell death pathways. Although most of these transcriptional changes were similar between the RV and LV, there were several genes that were uniquely upregulated in the pressure loaded RV, including genes involved in Wnt signaling (Dickkopf 3, Sfrp2, and Wif1), annexin A7, clusterin/apolipoprotein J, neuroblastoma suppression of tumorigenicity 1 (Nbl1), formin binding protein (Fnbp4), and LOX. Metabolic pathways dominated the downregulated gene pathways [16]. Whether these differences in the RV vs. LV are related to their different geometric structures, to markedly different afterloads, or to basic differences in cardiomyocyte biology will be the subject of future research.
The gene expression changes in the volume-loaded RV vs. LV are also largely similar [58]. While RV pressure and volume overload led to dysregulation of pathways involved in extracellular matrix remodeling, the actin cytoskeleton and metabolism, pressure overload led to a greater change in transcripts reflecting a more severe phenotype. Whereas RV diastolic dysfunction is well described in children with CHD with residual pressure and volume overload lesions, the mechanisms underlying diastolic dysfunction are poorly understood. Our novel animal models with chronic RV diastolic function may aid in better understanding the mechanism of diastolic dysfunction.
Development of animal models of chronic RV failure are critical, as they may better represent the clinical course of patients with CHD, as opposed to models where failure occurs within a few weeks. Improving energy efficiency and arresting cell death and fibrosis are areas to target for new therapeutics and utilizing chronic animal models in a stable and compensated state will be ideal for therapeutic trials.
Key Points
-
RV failure secondary to CHD does not respond to standard left heart failure medical therapies.
-
New models of RV failure highlight differences in the molecular response to stress between the two ventricles.
-
Pathways regulating reactive oxygen species production and antioxidant protection, angiogenesis and cell death differ between the two ventricles when exposed to pressure and/or volume loading.
Conclusions
As a testament to the vast advances in medical care and in surgical techniques, the vast majority of children with even complex CHD are growing into adolescence and adulthood. This has dramatically increased the burden of hemodynamic stressors, in particular on the RV, thereby increasing the incidence of RV failure in this population. While the vast number of studies on LV failure has advanced our knowledge of the mechanisms of disease progression from a compensated state to a state of overt heart failure, much less is known about the mechanisms of RV adaptation to pressure and volume load and the progression to RV failure. New animal models of RV failure simulating residual lesions seen after CHD and new models of PHTN are beginning to unravel the molecular mechanisms of disease and the RV’s increased susceptibility to failure. These studies will pave the way toward developing RV-specific heart failure therapies.
References
Hoffman JI, Kaplan S. The incidence of congenital heart disease. J Am Coll Cardiol. 2002;39(12):1890–900.
Belmont JW. Recent progress in the molecular genetics of congenital heart defects. Clin Genet. 1998;54(1):11–9.
Lund O, Kristensen LH, Baandrup U, Hansen OK, Nielsen TT, Emmertsen K, et al. Myocardial structure as a determinant of pre- and postoperative ventricular function and long-term prognosis after valve replacement for aortic stenosis. Eur Heart J. 1998;19(7):1099–108.
Douglas PS, Reichek N, Hackney K, Ioli A, Sutton MG. Contribution of afterload, hypertrophy and geometry to left ventricular ejection fraction in aortic valve stenosis, pure aortic regurgitation and idiopathic dilated cardiomyopathy. Am J Cardiol. 1987;59(15):1398–404.
Kaufman BD, Desai M, Reddy S, Osorio JC, Chen JM, Mosca RS, et al. Genomic profiling of left and right ventricular hypertrophy in congenital heart disease. J Card Fail. 2008;14(9):760–7.
Buermans HP, Redout EM, Schiel AE, Musters RJ, Zuidwijk M, Eijk PP, et al. Microarray analysis reveals pivotal divergent mRNA expression profiles early in the development of either compensated ventricular hypertrophy or heart failure. Physiol Genomics. 2005;21(3):314–23.
Gentles TL, Mayer JE Jr, Gauvreau K, Newburger JW, Lock JE, Kupferschmid JP, et al. Fontan operation in five hundred consecutive patients: factors influencing early and late outcome. J Thorac Cardiovasc Surg. 1997;4(3):376–91.
Winter MM, Bouma BJ, Groenink M, Konings TC, Tijssen JG, van Veldhuisen DJ, et al. Latest insights in therapeutic options for systemic right ventricular failure: a comparison with left ventricular failure. Heart. 2009;95(12):960–3.
Szymanski P, Klisiewicz A, Hoffman P. Therapeutic options for systemic right ventricular failure. Heart. 2009;95(23):1950–1. Author reply 1.
Shaddy RE, Boucek MM, Hsu DT, Boucek RJ, Canter CE, Mahony L, et al. Carvedilol for children and adolescents with heart failure: a randomized controlled trial. JAMA. 2007;298(10):1171–9.
Hsu DT, Zak V, Mahony L, Sleeper LA, Atz AM, Levine JC, et al. Enalapril in infants with single ventricle: results of a multicenter randomized trial. Circulation. 2010;122(4):333–40.
Buckberg GD, RESTORE Group. The ventricular septum: the lion of right ventricular function, and its impact on right ventricular restoration. Eur J Cardiothorac Surg. 2006;29(Suppl 1):S272–8.
Friedberg MK, Redington AN. Right versus left ventricular failure: differences, similarities, and interactions. Circulation. 2014;129(9):1033–44.
Kondo RP, Dederko DA, Teutsch C, Chrast J, Catalucci D, Chien KR, et al. Comparison of contraction and calcium handling between right and left ventricular myocytes from adult mouse heart: a role for repolarization waveform. J Physiol. 2006;571(Pt 1):131–46.
Piao L, Marsboom G, Archer SL. Mitochondrial metabolic adaptation in right ventricular hypertrophy and failure. J Mol Med. 2010;88(10):1011–20.
Urashima T, Zhao M, Wagner R, Fajardo G, Farahani S, Quertermous T, et al. Molecular and physiological characterization of RV remodeling in a murine model of pulmonary stenosis. Am J Physiol Heart Circ Physiol. 2008;295(3):H1351–H68.
Reddy S, Bernstein D. Molecular Mechanisms of Right Ventricular Failure. Circulation. 2015;132(18):1734–42.
Reddy S, Bernstein D. The vulnerable right ventricle. Curr Opin Pediatr. 2015;27(5):563–8.
Tsutsui H, Ide T, Hayashidani S, Suematsu N, Utsumi H, Nakamura R, et al. Greater susceptibility of failing cardiac myocytes to oxygen free radical-mediated injury. Cardiovasc Res. 2001;49(1):103–9.
Gomez-Arroyo J, Mizuno S, Szczepanek K, Van Tassell B, Natarajan R, dos Remedios CG, et al. Metabolic gene remodeling and mitochondrial dysfunction in failing right ventricular hypertrophy secondary to pulmonary arterial hypertension. Circ Heart Fail. 2013;6(1):136–44.
Ecarnot-Laubriet A, Rochette L, Vergely C, Sicard P, Teyssier JR. The activation pattern of the antioxidant enzymes in the right ventricle of rat in response to pressure overload is of heart failure type. Heart Dis. 2003;5(5):308–12.
Redout EM, van der Toorn A, Zuidwijk MJ, van de Kolk CW, van Echteld CJ, Musters RJ, et al. Antioxidant treatment attenuates pulmonary arterial hypertension-induced heart failure. Am J Physiol Heart Circ Physiol. 2010;298(3):H1038–47.
Redout EM, Wagner MJ, Zuidwijk MJ, Boer C, Musters RJ, van Hardeveld C, et al. Right-ventricular failure is associated with increased mitochondrial complex II activity and production of reactive oxygen species. Cardiovasc Res. 2007;75(4):770–81.
Karamanlidis G, Bautista-Hernandez V, Fynn-Thompson F, Del Nido P, Tian R. Impaired mitochondrial biogenesis precedes heart failure in right ventricular hypertrophy in congenital heart disease. Circ Heart Fail. 2011;4(6):707–13.
Zong P, Tune JD, Downey HF. Mechanisms of oxygen demand/supply balance in the right ventricle. Exp Biol Med. 2005;230(8):507–19.
Saito D, Tani H, Kusachi S, Uchida S, Ohbayashi N, Marutani M, et al. Oxygen metabolism of the hypertrophic right ventricle in open chest dogs. Cardiovasc Res. 1991;25(9):731–9.
Choi YH, Cowan DB, Nathan M, Poutias D, Stamm C, del Nido PJ, et al. Myocardial hypertrophy overrides the angiogenic response to hypoxia. PLoS One. 2008;3(12):e4042.
Bogaard HJ, Natarajan R, Henderson SC, Long CS, Kraskauskas D, Smithson L, et al. Chronic pulmonary artery pressure elevation is insufficient to explain right heart failure. Circulation. 2009;120(20):1951–60.
Zamir M. The physics of coronary blood flow. New York: Springer; 2005.
Sano M, Minamino T, Toko H, Miyauchi H, Orimo M, Qin Y, et al. p53-induced inhibition of Hif-1 causes cardiac dysfunction during pressure overload. Nature. 2007;446(7134):444–8.
Partovian C, Adnot S, Eddahibi S, Teiger E, Levame M, Dreyfus P, et al. Heart and lung VEGF mRNA expression in rats with monocrotaline- or hypoxia-induced pulmonary hypertension. Am J Phys. 1998;275(6 Pt 2):H1948–56.
Setty S, Tune JD, Downey HF. Nitric oxide contributes to oxygen demand-supply balance in hypoperfused right ventricle. Cardiovasc Res. 2004;64(3):431–6.
Tune JD, Richmond KN, Gorman MW, Feigl EO. Role of nitric oxide and adenosine in control of coronary blood flow in exercising dogs. Circulation. 2000;101(25):2942–8.
Piao L, Fang YH, Parikh KS, Ryan JJ, D’Souza KM, Theccanat T, et al. GRK2-mediated inhibition of adrenergic and dopaminergic signaling in right ventricular hypertrophy: therapeutic implications in pulmonary hypertension. Circulation. 2012;126(24):2859–69.
Norozi K, Bahlmann J, Raab B, Alpers V, Arnhold JO, Kuehne T, et al. A prospective, randomized, double-blind, placebo controlled trial of beta-blockade in patients who have undergone surgical correction of tetralogy of Fallot. Cardiol Young. 2007;17(4):372–9.
Doughan AR, McConnell ME, Book WM. Effect of beta blockers (carvedilol or metoprolol XL) in patients with transposition of great arteries and dysfunction of the systemic right ventricle. Am J Cardiol. 2007;99(5):704–6.
Molenaar P, Bartel S, Cochrane A, Vetter D, Jalali H, Pohlner P, et al. Both beta(2)- and beta(1)-adrenergic receptors mediate hastened relaxation and phosphorylation of phospholamban and troponin I in ventricular myocardium of Fallot infants, consistent with selective coupling of beta(2)-adrenergic receptors to G(s)-protein. Circulation. 2000;102(15):1814–21.
Wang GY, McCloskey DT, Turcato S, Swigart PM, Simpson PC, Baker AJ. Contrasting inotropic responses to alpha1-adrenergic receptor stimulation in left versus right ventricular myocardium. Am J Physiol Heart Circ Physiol. 2006;291(4):H2013–7.
Wang GY, Yeh CC, Jensen BC, Mann MJ, Simpson PC, Baker AJ. Heart failure switches the RV alpha1-adrenergic inotropic response from negative to positive. Am J Physiol Heart Circ Physiol. 2010;298(3):H913–20.
Anand IS, Chandrashekhar Y, Ferrari R, Sarma R, Guleria R, Jindal SK, et al. Pathogenesis of congestive state in chronic obstructive pulmonary disease. Studies of body water and sodium, renal function, hemodynamics, and plasma hormones during edema and after recovery. Circulation. 1992;86(1):12–21.
Farber MO, Roberts LR, Weinberger MH, Robertson GL, Fineberg NS, Manfredi F. Abnormalities of sodium and H2O handling in chronic obstructive lung disease. Arch Intern Med. 1982;142(7):1326–30.
Schrier RW, Bansal S. Pulmonary hypertension, right ventricular failure, and kidney: different from left ventricular failure? Clin J Am Soc Nephrol. 2008;3(5):1232–7.
Maron BA, Leopold JA. The role of the renin-angiotensin-aldosterone system in the pathobiology of pulmonary arterial hypertension (2013 Grover Conference series). Pulm Circ. 2014;4(2):200–10.
van der Bom T, Winter MM, Bouma BJ, Groenink M, Vliegen HW, Pieper PG, et al. Effect of valsartan on systemic right ventricular function: a double-blind, randomized, placebo-controlled pilot trial. Circulation. 2013;127(3):322–30.
Dore A, Houde C, Chan KL, Ducharme A, Khairy P, Juneau M, et al. Angiotensin receptor blockade and exercise capacity in adults with systemic right ventricles: a multicenter, randomized, placebo-controlled clinical trial. Circulation. 2005;112(16):2411–6.
Robinson B, Heise CT, Moore JW, Anella J, Sokoloski M, Eshaghpour E. Afterload reduction therapy in patients following intraatrial baffle operation for transposition of the great arteries. Pediatr Cardiol. 2002;23(6):618–23.
Phillips D, Aponte AM, Covian R, Neufeld E, ZX Y, Balaban RS. Homogenous protein programming in the mammalian left and right ventricle free walls. Physiol Genomics. 2011;43(21):1198–206.
Nagendran J, Gurtu V, Fu DZ, Dyck JR, Haromy A, Ross DB, et al. A dynamic and chamber-specific mitochondrial remodeling in right ventricular hypertrophy can be therapeutically targeted. J Thorac Cardiovasc Surg. 2008;136(1):168–78. 78 e1-3.
Kusachi S, Nishiyama O, Yasuhara K, Saito D, Haraoka S, Nagashima H. Right and left ventricular oxygen metabolism in open-chest dogs. Am J Phys. 1982;243(5):H761–6.
Do E, Baudet S, Verdys M, Touzeau C, Bailly F, Lucas-Heron B, et al. Energy metabolism in normal and hypertrophied right ventricle of the ferret heart. J Mol Cell Cardiol. 1997;29(7):1903–13.
Gunes Y, Guntekin U, Tuncer M, Sahin M. Improved left and right ventricular functions with trimetazidine in patients with heart failure: a tissue Doppler study. Heart Vessel. 2009;24(4):277–82.
Gu Q, Chen XT, Xiao YB, Chen L, Wang XF, Fang J, et al. Identification of differently expressed genes and small molecule drugs for Tetralogy of Fallot by bioinformatics strategy. Pediatr Cardiol. 2014;35(5):863–9.
Rastogi S, Sharov VG, Mishra S, Gupta RC, Blackburn B, Belardinelli L, et al. Ranolazine combined with enalapril or metoprolol prevents progressive LV dysfunction and remodeling in dogs with moderate heart failure. Am J Physiol Heart Circ Physiol. 2008;295(5):H2149–55.
Phan TT, Shivu GN, Choudhury A, Abozguia K, Davies C, Naidoo U, et al. Multi-centre experience on the use of perhexiline in chronic heart failure and refractory angina: old drug, new hope. Eur J Heart Fail. 2009;11(9):881–6.
Halbirk M, Norrelund H, Moller N, Schmitz O, Gotzsche L, Nielsen R, et al. Suppression of circulating free fatty acids with acipimox in chronic heart failure patients changes whole body metabolism but does not affect cardiac function. Am J Physiol Heart Circ Physiol. 2010;299(4):H1220–5.
Nikolaidis LA, Mankad S, Sokos GG, Miske G, Shah A, Elahi D, et al. Effects of glucagon-like peptide-1 in patients with acute myocardial infarction and left ventricular dysfunction after successful reperfusion. Circulation. 2004;109(8):962–5.
O’Connor RD, Xu J, Ewald GA, Ackerman JJ, Peterson LR, Gropler RJ, et al. Intramyocardial triglyceride quantification by magnetic resonance spectroscopy: In vivo and ex vivo correlation in human subjects. Magn Reson Med. 2011;65(5):1234–8.
Reddy S, Zhao M, Hu DQ, Fajardo G, Katznelson E, Punn R, et al. Physiologic and molecular characterization of a murine model of right ventricular volume overload. Am J Physiol Heart Circ Physiol. 2013;304(10):H1314–27.
Cordes KR, Srivastava D. MicroRNA regulation of cardiovascular development. Circ Res. 2009;104(6):724–32.
Callis TE, Wang DZ. Taking microRNAs to heart. Trends Mol Med. 2008;14(6):254–60.
El-Armouche A, Schwoerer AP, Neuber C, Emmons J, Biermann D, Christalla T, et al. Common microRNA signatures in cardiac hypertrophic and atrophic remodeling induced by changes in hemodynamic load. PLoS One. 2010;5(12):e14263.
Yang M, Yao Y, Eades G, Zhang Y, Zhou Q. MiR-28 regulates Nrf2 expression through a Keap1-independent mechanism. Breast Cancer Res Treat. 2011;129(3):983–91.
Smith-Vikos T, Slack FJ. MicroRNAs and their roles in aging. J Cell Sci. 2012;125(Pt 1):7–17.
Yu J, Li Q, Xu Q, Liu L, Jiang B. MiR-148a inhibits angiogenesis by targeting ERBB3. J Biomed Res. 2011;25(3):170–7.
Reddy S, Zhao M, Hu DQ, Fajardo G, Hu S, Ghosh Z, et al. Dynamic microRNA expression during the transition from right ventricular hypertrophy to failure. Physiol Genomics. 2012;44(10):562–75.
Tabuchi T, Satoh M, Itoh T, Nakamura M. MicroRNA-34a regulates the longevity-associated protein SIRT1 in coronary artery disease: effect of statins on SIRT1 and microRNA-34a expression. Clin Sci (Lond). 2012;123(3):161–71.
Boon RA, Iekushi K, Lechner S, Seeger T, Fischer A, Heydt S, et al. MicroRNA-34a regulates cardiac ageing and function. Nature. 2013;495(7439):107–10.
Potus F, Malenfant S, Graydon C, Mainguy V, Tremblay E, Breuils-Bonnet S, et al. Impaired angiogenesis and peripheral muscle microcirculation loss contribute to exercise intolerance in pulmonary arterial hypertension. Am J Respir Crit Care Med. 2014;190(3):318–28.
Thum T, Batkai S. MicroRNAs in right ventricular (dys)function (2013 Grover Conference series). Pulm Circ. 2014;4(2):185–90.
Paulin R, Sutendra G, Gurtu V, Dromparis P, Haromy A, Provencher S, et al. A miR-208-Mef2 axis drives the decompensation of right ventricular function in pulmonary hypertension. Circ Res. 2015;116(1):56–69.
Acknowledgements
Mingming Zhao, Dong-Qing Hu, and Giovanni Fajardo. NIH/NHLBI grant HL061535 (DB); Children’s Heart Foundation grant (DB and SR); Packard Children’s Hospital Pediatric Research Fund, Heart Center Research Fund and Reddy Foundation grant (SR); NIH/NHLBI 1K08HL127277 (SR). Department of Defense CMDRP in Congenital Heart Disease (DB and SR).
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 Springer International Publishing AG
About this chapter
Cite this chapter
Reddy, S., Bernstein, D. (2018). Molecular Aspects of Right Ventricular Adaptation to Stress. In: Friedberg, M., Redington, A. (eds) Right Ventricular Physiology, Adaptation and Failure in Congenital and Acquired Heart Disease. Springer, Cham. https://doi.org/10.1007/978-3-319-67096-6_3
Download citation
DOI: https://doi.org/10.1007/978-3-319-67096-6_3
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-67094-2
Online ISBN: 978-3-319-67096-6
eBook Packages: MedicineMedicine (R0)