Abstract
The right ventricle (RV) eventually fails in most patients with severe chronic pulmonary hypertension, however, the individual myocardial reserve and ability to cope with the increased afterload, inflammation and metabolic derangements are highly variable. Hypertrophy is required for the RV to successfully adapt to the chronically elevated pressure and shear stress in the lung vessels. RV hypertrophy associated with an appropriate myocardial capillary density and normal function of the capillary endothelial cells are hallmarks of successful adaptation. Neuroendocrine hyperactivity or overdrive may contribute and facilitate the transition from adaptive RV hypertrophy to RV failure. Therapeutic strategies that reduce cellular stress and modify damaging failure components such as inflammation, lipotoxicity and proteotoxicity need to be explored in order to evaluate whether they can preserve RV function and improve outcome, even when the afterload of the RV cannot be significantly reduced.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
Introduction
In the early days of pulmonary hypertension research two important observations were made: First, it was recognized that many, perhaps most, patients with severe pulmonary arterial hypertension (PAH) died from right heart failure, many from sudden death. Second, the medical records showed, at a time when there was no treatment for PAH, that there were a few patients with very high pulmonary artery pressures, yet they were none-the-less long-time survivors [1]. Interestingly, when intravenous prostacyclin treatment was established for the treatment of patients with “primary ” PAH, and shown to improve survival [2], it was not intuitive that improved survival was due to improved right ventricular (RV) function and reversal of right ventricular failure (RVF). An early assessment of inadequate attention to the all-important RV failure component of severe PAH can be found in Michael Bristow’s then state-of-the-art presentation at the Aspen Lung Conference in 1997.
There are excellent data implicating RV function as an important determinant of the natural history of PPH. However, in this disorder, RV dysfunction has not received the kind of investigative scrutiny that pulmonary vascular mechanisms have enjoyed. This is partly because subjects with PPH are cared for by pulmonologists, whose investigative interests are focused on the lung rather than the heart….one of the fundamentally important questions surrounding PPH is that subjects differ substantially in their tendency to develop RVF. This leads to a variable natural history….therefore mechanistic hypotheses for the development of RVF need to accommodate this biological variability [3].
This call to investigate mechanisms leading to RV failure has been answered in the past decade. The following paragraphs concern the main message of this chapter: In severe PAH, the lung vessels, the heart, the endocrine system and the immune system are involved, and therefore a systems-based approach and analysis are appropriate.
Is Right Ventricular Hypertrophy in Pulmonary Hypertension Bad?
Traditional teaching is that ‘all hypertrophy is detrimental’. However, the exception is the RV in the setting of chronic pressure overload. Although never formally investigated, it is safe to say that the successful adaptation to a chronically elevated pulmonary artery pressure, with or without increased blood flow related to a shunt, requires RV hypertrophy. Similarly, in patients with congenitally corrected transposition of the great arteries a sub-aortic RV supports the high resistance systemic circulation.
Experimentally, banding of the main pulmonary artery (PAB) in the rat results in a robust RV hypertrophic response, yet without evidence of RV failure, even when PAB animals are additionally exposed to chronic hypoxia [4].We postulate that a hypertrophied, but well vascularized RV, with development of an adequate capillary bed does not fail. However, RV hypertrophy not adequately supplied by a proportionately developed microcirculation is prone to fail. To some degree, severe PAH associated with congenital heart disease and characterized by Eisenmenger physiology is the clinical example of a well-adapted RV [5]. In these patients, RV wall thickness is greater than in patients with IPAH, and there is less diffuse myocardial fibrosis [6]. In theory, it would be beneficial if the RV in patients with IPAH could be transformed into an “Eisenmenger RV”. While cardiac myocytes make up 1/3 of the cells in the myocardium, they account for 80% of the mass; yet, hypertrophy is mainly due to KLF-5-driven up-regulation of IGF-1 in cardiac fibroblasts—a good example of cell-cell interaction [7]. Another characteristic of successful RV hypertrophic adaptation is enhanced fatty acid oxidation (FAO). A microvascular density that matches the increased muscle mass requires that the signaling chain that leads from transcription factor- and growth factor expression to an angiogenic response remains intact. The most important transcription factors involved in the capillary growth response are HIF-1alpha and PGC-1alpha.
Classic studies by Murray and Vatner in conscious dogs showed that blood flow in the hypertrophied RV approximately doubles [8], while the number of capillaries is maintained (Fig. 2.1). Thus, a muscular, well-perfused RV is required to handle an increased pulmonary vascular resistance.
Comparison of the well adapted, hypertrophied right ventricle and the failing right ventricle. The exact sequence of events that initiates the right heart failure program are not well understood. The schematic also postulates that there are genetically determined mechanisms that regulate adaptation to mechanical and oxidative stress
Ischemia
The failing RV in patients with severe PAH is ischemic [9] and the reduced RV blood flow in these patients is likely, at least in part, explained by capillary rarefaction. The association of capillary rarefaction with ventricular failure had previously been elegantly demonstrated by Wolfgang Schaper’s group in the hypertrophied LV in patients with aortic valve stenosis [10]. It is of great interest that myocardial blood flow reserve in patients with severe PAH is significantly reduced not only in the pressure overloaded RV, but also in the underfilled LV [11]. Whether in this setting LV capillary rarefaction occurs is unknown. However, decreased LV preload correlates with clinical deterioration in patients with severe PAH.
Reduced RV Ejection Fraction in Severe PAH
It has long been appreciated that in patients with severe PAH a declining cardiac output heralds poor prognosis. The important cardiac magnetic resonance imaging (MRI) study by Van Veerdonk and coworkers [12] has now established that in PAH patients on‘targeted’ therapy a decline in the RV ejection fraction (RVEF) and not a decrease in the pulmonary vascular resistance (PVR) is a predictor of outcome. While PVR is calculated from hemodynamic variables, measurement of RVEF requires volume measurements by cMRI. The importance of this study is that a temporally treatment-associated decrease in PVR cannot be interpreted as a sign of clinical improvement unless the RVEF is also known. Put more succinctly, a decrease in RVEF while on targeted treatment for severe PAH is a predictor of short-term survival.
Why Does the RV Fail?
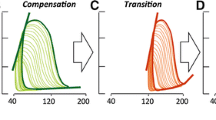
If pressure overload per se and RV hypertrophy are insufficient explanations for RV maladaptation and eventual failure, then what causes RV failure? Although RV failure is a clinical syndrome characterized by signs of congestion, there is increasing consensus that RV function can be assessed by measuring RVEF. While RV failure is characterized by RV volume overload, shift of the inter-ventricular septum and dilatation of the inferior vena cava, the factors which push the RV from the compensated state to failure differ perhaps between patients. There are signs of neurohormonal overdrive and of LV underfilling in most patients with progressive RV failure (Fig. 2.2) [13, 14]. Stress hormone production may be triggered by myocardial stress , ischemia and inflammation, all leading to an oxidant/antioxidant imbalance [15, 16] which predisposescardiomyocytes and capillary endothelial cells to succumb to apoptosis.
This schematic depicts and integrates various elements of myocardial stress such as ischemia, inflammation and apoptosis that lead to RV dilatation and failure. With permission from Bogaard HJ, Abe K, Vonk Noordegraaf A, Voelkel NF. The right ventricle under pressure: cellular and molecular mechanisms of right-heart failure in pulmonary hypertension. Chest 2009;135:794–804 © Elsevier 2009 [13]
There are several potential ways forward on the journey to investigate the causes of RV failure. Experimentally, one can apply specific strategies designed to provoke the RV into failure. An example of such a strategy has been to treat PAB rats with a pan histone deacetylase inhibitor [17]. These animals develop RV capillary rarefaction, fibrosis and failure. In the Sugen/chronic hypoxia rat model of severe PAH, inhibition of VEGF signaling prevents the proper capillarization of the RV and this circumstance is one explanation for the development of RV failure in this model.
Clinically, one can sample peripheral and pulmonary venous blood and measure circulating mediator molecules such as norepinephrine, endothelin, cortisol, renin, angiotensin and aldosterone [18].
The Sick Lung Circulation Hypothesis
Given the pathological changes in a very large number of small pulmonary arterioles, and in some instances the pulmonary venules, which is the consequence of endothelial cell activation, endothelial cell apoptosis and phenotypic changes in the pulmonary vascular wall, functional changes in the lung circulation are not surprising. Most appreciated perhaps is endothelial cell dysfunction manifested by reduced endothelial cell nitric oxide (NO) and prostacyclin synthesis. We postulate that in addition, ‘bad humors’ are released from the sick lung circulation and predict that in chronic lung diseases where the lung vessels are involved, these ‘bad humors’ impact the heart [19,20,21,22]. A quantitative assessment of factors released by the sick lung circulation is possible by measuring the pulmonary arterio-venous gradient. To date only a few studies have reported on lung-tissue-dependent release of VEGF m, TGFbeta 1, PDGF-BB and PAI (plasminogen activator inhibitor). Release of MCP-1 and GDF-15 [23, 24] from the pulmonary hypertensive lung is likely, but has not been formally studied. Perros et al. [25] reported increased numbers of circulating cytotoxic cells and granulysin in patients with severe veno-occlusive PAH. In addition, free DNA and micro-RNA encapsulated in microspheres, emitted by the sick lung vessel cells, are likely to influence the structure and function of the myocardial micro-circulation. The postulated effects in the heart (both RV and LV) are activation and injury of the myocardial microvascular endothelial cells and stimulation of endothelial cell-mesenchymal transformation (enMT) , a process that can lead to myofibroblast formation and perivascular fibrosis.
The Molecular Gene Expression Signature of RV Failure
It is reasonable to postulate, based on what has been discussed so far, that the pattern of expressed genes and proteins differs between a compensated, hypertrophied RV and a failing RV. Mechanical wall stretch, ischemia and the altered myocardial milieu generated by the factors released by the sick lung circulation may all impact the RV and generate a gene expression signature of RV failure. Experiments were conducted and RNA was extracted from RV and LV tissue samples comparing normal rat hearts with those from PAB rats and rats that had undergone the Sugen/chronic hypoxia protocol [26]. The failing RV in Su/Hx rats was hypertrophied and dilated, TAPSE was significantly decreased and histologically the tissue was characterized by capillary rarefaction, apoptosis and fibrosis [27]. Remaining microvessels showed a lack of expression of the prostacyclin synthase protein. Microarray expression analysis of the four groups of animals elucidated patterns of expressed genes that reflected mechanisms underlying the compensated state versus the failure state. For example, as adaptive RV hypertrophy is driven by IGF-1 , it was highly expressed in the PAB RV, but decreased in the Su/Hx failing RV (Fig. 2.3). Phosphorylated Akt expression was decreased in the failing RV (Fig. 2.4), as were VEGFA and apelin [27]. Gene expression of key enzymes encoding fatty acid oxidation, including expression of the transcription factor PGC-1alpha (Figs. 2.5 and 2.6), were decreased and gene expression of enzymes encoding the glycolytic pathway were increased [26]. As expected, we found clear and categorical gene expression patterns, which allow mechanistic explanation of adapted and failing tissue at the level of cell growth/autophagy/apoptosis, inflammation, fibrosis and intact or impaired angiogenesis [26]. Because experimental RV failure in the Su/Hx rats could be reversed by treatment with carvedilol, this lead to testing of the hypothesis that components of the RV failure program or gene expression signature could be changed or normalized. It was found that carvedilol-induced reversal of RV failure was associated with a reduction of RV hypertrophy and return of a capillary density towards normal. Under the influence of chronic carvedilol treatment more than 400 genes were altered in their expression reflecting improved myocardial energy metabolism and reduced myocardial stress, even though RV afterload remained unchanged [27,28,29]. These experimental data provided the impetus to test carvedilol treatment as add-on therapy to established treatment of patients with severe PAH and assessment of the RVEF after 6 months of carvedilol therapy. The small pilot study results suggest that carvedilol treatment of PAH patients is safe and increases RVEF as measured by cMRI [30].
Protein expression of IGF-1 in the RV myocardium, assessed by Western blot is dramatically decreased in the failing RV from rats exposed to the Sugen/chronic hypoxia protocol (SuHx), when compared to rats exposed to chronic hypoxia alone or to the RV from rats weeks following pulmonary artery banding (PAB). With permission from Bogaard HJ, Natarajan R, Mizuno S, Abbate A, Chang PJ, Chau VQ, Hoke NN, Kraskauskas D, Kasper M, Salloum FN, Voelkel NF. Adrenergic receptor blockade reverses right heart remodeling and dysfunction in pulmonary hypertensive rats. Am J Respir Crit Care Med. 2010;182:652–60 © American Thoracic Society 2010 [27]
Protein expression of phoshorylated Akt (pAkt) , initiated downstream in the signaling cascade after binding of the angiogenic VEGF ligand to its receptors in the RV myocardium, assessed by Western blot, is dramatically decreased in the failing RV from rats exposed to the Sugen/chronic hypoxia protocol (SuHx) when compared to rats exposed to chronic hypoxia alone or to the RV from rats weeks following pulmonary artery banding (PAB). With permission from Bogaard HJ, Natarajan R, Mizuno S, Abbate A, Chang PJ, Chau VQ, Hoke NN, Kraskauskas D, Kasper M, Salloum FN, Voelkel NF. Adrenergic receptor blockade reverses right heart remodeling and dysfunction in pulmonary hypertensive rats. Am J Respir Crit Care Med. 2010;182:652–60 © American Thoracic Society 2010 [27]
Protein expression of the transcription factor PGC-1alpha , important for VEGF transcription and transcription of genes encoding enzymes of fatty acid oxidation, is reduced in the failing RV from SuHx animals. Treatment of rats with carvedilol after established RV failure reversed the decrease in PGC-1 alpha expression. With permission from Gomez-Arroyo J, Mizuno S, Szczepanek K, Van Tassel B, Natarajan R, dos Remedios C, Drake JI et al. Metabolic remodeling and mitochondrial dysfunction in failing right ventricular hypertrophy secondary to pulmonary arterial hypertension. Cir Heart Failure 2013;6:136–144 © Wolters Kluwer 2013 [29]
Decreased expression of PGC-1alpha is of functional importance as its expression correlates with TAPSE (tricuspid valve plane systolic excursion). With permission from Gomez-Arroyo J, Mizuno S, Szczepanek K, Van Tassel B, Natarajan R, dos Remedios C, Drake JI et al. Metabolic remodeling and mitochondrial dysfunction in failing right ventricular hypertrophy secondary to pulmonary arterial hypertension. Cir Heart Failure 2013;6:136–144 © Wolters Kluwer 2013 [29]
Conclusion and New Hypotheses
Hypertrophy is required for the RV to adapt successfully to a chronically elevated afterload and a chronically elevated RV afterload per se is insufficient to cause RV failure. One postulate that needs to be investigated is that activation of neuroendocrine pathways is one important mechanism that promotes the transition from a stressed but functional RV to frank RV failure. A second postulate is that a myocardial microangiopathy is critically involved and responsible for the “metabolic remodeling ” and an EnMT that leads to perivascular fibrosis. Further, it is intriguing to speculate that the health of the lung vessel endothelium is linked to the health of the myocardial microvascular endothelium and that the function of the microvessel endothelial cells in the heart (Fig. 2.7) is linked to the metabolic reprogramming of the cardiomyocytes. If so, an improvement of endothelial cell function may repair the mitochondriopathy, which likely underlies the myocardial oxidative stress and endoplasmic reticulum stress.
Right ventricle, immuno-histochemistry; staining for an antibody directed against prostacyclin synthase, the terminal enzyme required for prostacyclin synthesis. Right ventricular tissue from a rat exposed to the SuHx protocol with established RV failure. The inserts show capillary endothelial cells . The expression of prostacyclin synthase protein is lost in the capillary from the failing RV. This indicates that there is endothelial cell dysfunction in the remaining RV capillaries, not only loss of capillaries
The experimental studies demonstrating reversal of RV failure by carvedilol treatment are encouraging and hopefully will stimulate the search for additional therapeutic strategies to support the right ventricle under pressure [13]. As atrial septostomy does not in any way influence the behavior of the hypertensive lung circulation, but unloads the RV and improves exercise tolerance [31], treatment to reduce cellular stress and modify damaging failure components such as lipotoxicity [32] and proteotoxicity [33] may stabilize the RV under pressure, even when its afterload cannot be significantly reduced.
References
Voelkel NF, Reeves JT. Primary pulmonary hypertension. In: Moser KM, editor. Pulmonary vascular diseases. New York: Marcel Dekker; 1979. p. 573–628.
Barst RJ, Rubin LJ, Long WA, McGoon MD, Rich S, Badesch DB, Groves BM, et al. A comparison of intravenous epoprostenol (prostacyclin) with conventional therapy_for primary pulmonary hypertension. NEJM. 1996;334:296–301.
Bristow MR, Zisman LS, Lowes BD, Abraham WT, Badesch DB, et al. The pressure overloaded right ventricle in pulmonary hypertension. Chest. 1998;114:101S–6S.
Bogaard HJ, Natarajan R, Henderson C, Long CS, Kraskauskas D, Smithson L, Ockaili R, McCord JM, Voelkel NF. Chronic pulmonary artery pressure overload is insufficient to explain right heart failure. Circulation. 2009;120:1951–60.
Hopkins WE, Ochoa LL, Richardson GW, Trulock EP. Comparison of the hemodynamics and survival of adults with severe primary pulmonary hypertension or Eisenmenger syndrome. J Heart Lung Transplant. 1996;15:100–5.
Gomez-Arroyo JG, Santos-Martinez LE, Aranda A, Pullido T, Beltran M, et al. Differences in right ventricular remodeling secondary to pressure overload in patients with pulmonary hypertension. Am J Resp Crit Care Med. 2014;189:603–6.
Takeda N, Manabe I, Uchino Y, Eguchi K, Matsumoto S, Nishimura S, Shindo T, Sano M, Otsu K, Snider P, Conway SJ, Nagai R. Cardiac fibroblasts are essential for the adaptive response of the murine heart to pressure overload. J Clin Invest. 2010;120(1):254–65.
Murray PA, Baig H, Fishbein MC, Vatner SF. Effects of exerimental right ventricular hypertrophy on myocardial blood flow in conscious dogs. J Clin Invest. 1979;64:421–7.
Gómez A, Bialostozky D, Zajarias A, Santos E, Palomar A, Martínez ML, Sandoval J. Right ventricular ischemia in patients with primary pulmonary hypertension. J Am Coll Cardiol. 2001;38:1137–42.
Elsässer A, Decker E, Kostin S, Hein S, Skwara W, Müller KD, Greiber S, Schaper W, Klövekorn WP, Schaper JA. self-perpetuating vicious cycle of tissue damage in human hibernating myocardium. Mol. Cell Biochem. 2000;213:17–28.
Vogel-Claussen J, Skrok J, Shehata ML, Singh S, Sibley CT, Boyce DM, Lechtzin N, Girgis RE, Mathai SC, Goldstein TA, Zheng J, Lima JA, Bluemke DA, Hassoun PM. Right and left ventricular myocardial perfusion reserves correlate with right ventricular function and pulmonary hemodynamics in patients with pulmonary arterial hypertension. Radiology. 2011;258:119–27.
van de Veerdonk MC, Kind T, Marcus JT, Mauritz GJ, Heymans MW, Bogaard HJ, Boonstra A, Marques KM, Westerhof N, Vonk-Noordegraaf A. Progressive right ventricular dysfunction in patients with pulmonary arterial hypertension responding to therapy. J Am Coll Cardiol. 2011;58:2511–9.
Bogaard HJ, Abe K, Vonk Noordegraaf A, Voelkel NF. The right ventricle under pressure: cellular and molecular mechanisms of right-heart failure in pulmonary hypertension. Chest. 2009;135:794–804.
de Man FS, Handoko ML, Guignabert C, Bogaard HJ, Vonk-Noordegraaf A. Neurohormonal axis in patients with pulmonary arterial hypertension: friend or foe? Am J Respir Crit Care Med. 2013;187:14–9.
Voelkel NF, Natarajan R, Drake JI, Bogaard HJ. Right ventricle in pulmonary hypertension. Compr Physiol. 2011;1:525–40.
Purnomo Y, Piccart Y, Coenen T, Prihadi JS, Lijnen PJ. Oxidative stress and transforming growth factor beta 1-induced cardiac fibrosis. Cardiovasc Hematol Disord Drug Targets. 2013;13:165–72.
Bogaard HJ, Mizuno S, Hussaini AA, Toldo S, Abbate A, Kraskauskas D, Kasper M, Natarajan R, Voelkel NF. Suppression of histone deacetylases worsens right ventricular dysfunction after pulmonary artery banding in rats. Am J Respir Crit Care Med. 2011;183:1402–10.
Nolly MB, Caldiz CI, Yeves AM, Villa-Abrille MC, Morgan PE, et al. The signaling pathway for aldosterone-induced mitochondrial production of superoxide anion in the myocardium. J Mol Cell Cardiol. 2014;67:60–8.
Voelkel NF, Gomez-Arroyo J, Abbate A, Bogaard HJ, Nicolls MR. Pathobiology of pulmonary arterial hypertension and right ventricular failure. Eur Respir J. 2012;40:1555–65.
Voelkel NF. The sick lung circulation and the failing right ventricle. In: Voelkel NF, Schranz D, editors. The right ventricle in health and disease. New York: Springer; 2015. p. 303–13.
Voelkel NF, Gomez-Arroyo J, Abbate A, Bogaard HJ. Mechanisms of righheart failure-A work in progress and a plea for failure prevention. Pulm Circ. 2013;3:137–43.
Oka T, Hikoso S, Yamaguchi O, Taneike M, Takeda T, et al. Mitochondrial DNA that escapes from autophagy causes inflammation and heart failure. Nature. 2012;485:251–5.
Park JE, Lyon AR, Shao D, Hector LR, Xu H, O'Gara P, Pinhu L, Chambers RC, Wort SJ, Griffiths MJ. Pulmonary venous hypertension and mechanical strain stimulate monocyte chemoattractant protein-1 release and structural remodelling of the lung in human and rodent chronic heart failure models. Thorax. 2014;69:1120–7.
Nickel N, Kempf T, Tapken H, Tongers J, Laenger F, Lehmann U, Golpon H, Olsson K, Wilkins MR, Gibbs JS, Hoeper MM. Wollert KCGrowth differentiation factor-15 in idiopathic pulmonary arterial hypertension. Am J Respir Crit Care Med. 2008;178:534–41.
Perros F, Cohen-Kaminsky S, Gambaryan N, Girerd B, Raymond N, Klingelschmitt I, Huertas A, Mercier O, Fadel E, Simonneau G, Humbert M, Dorfmüller P, Montani D. Cytotoxic cells and granulysin in pulmonary arterial hypertension and pulmonary veno-occlusive disease. Am J Respir Crit Care Med. 2013;187(2):189–96.
Drake JI, Bogaard HJ, Mizuno S, Clifton B, Xie B, Gao Y, Dumur CI, Fawcett P, Voelkel NF, Natarajan R. Molecular signature of a right heart failure program in chronic severe pulmonary hypertension. Am J Respir Cell Mol Biol. 2011;45:1239–47.
Bogaard HJ, Natarajan R, Mizuno S, Abbate A, Chang PJ, Chau VQ, Hoke NN, Kraskauskas D, Kasper M, Salloum FN, Voelkel NF. Adrenergic receptor blockade reverses right heart remodeling and dysfunction in pulmonary hypertensive rats. Am J Respir Crit Care Med. 2010;182:652–60.
Drake JI, Gomez-Arroyo J, Dumur CI, Kraskauskas D, Natarajan R, Bogaard HJ, Fawcett P, Voelkel NF. Chronic carvedilol treatment partially reverses the right ventricular failure transcriptional profile in experimental pulmonary hypertension. Physiol Genomics. 2013;45:449–61.
Gomez-Arroyo J, Mizuno S, Szczepanek K, Van Tassel B, Natarajan R, dos Remedios C, Drake JI, et al. Metabolic remodeling and mitochondrial dysfunction in failing right ventricular hypertrophy secondary to pulmonary arterial hypertension. Cir Heart Fail. 2013;6:136–44.
Grinnan D, Bogaard HJ, Grizzard J, Van Tassell B, Abbate A, DeWilde C, Priday A, Voelkel NF. Treatment of group I pulmonary arterial hypertension with carvedilol is safe. Am J Respir Crit Care Med. 2014;189:1562–4.
Sandoval J, Torbicki A. Atrial septostomy. In: Voelkel NF, Schranz D, editors. The right ventricle in health and disease. New York: Springer; 2015. p. 419–37.
Hemnes AR, Brittain EL, Trammell AW, Fessel JP, Austin ED, Penner N, Maynard KB, Gleaves L, Talati M, Absi T, Disalvo T, West J. Evidence for right ventricular lipotoxicity in heritable pulmonary arterial hypertension. Am J Respir Crit Care Med. 2014;189:325–34.
McLendon PM, Robbins J. Proteotoxicity and cardiac dysfunction. Circ Res. 2015;116:1863–82.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 Springer International Publishing AG
About this chapter
Cite this chapter
Voelkel, N.F. (2018). How Does the Pressure-Overloaded Right Ventricle Adapt and Why Does It Fail? Macro-and Micro-Molecular Perspectives. In: Friedberg, M., Redington, A. (eds) Right Ventricular Physiology, Adaptation and Failure in Congenital and Acquired Heart Disease. Springer, Cham. https://doi.org/10.1007/978-3-319-67096-6_2
Download citation
DOI: https://doi.org/10.1007/978-3-319-67096-6_2
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-67094-2
Online ISBN: 978-3-319-67096-6
eBook Packages: MedicineMedicine (R0)