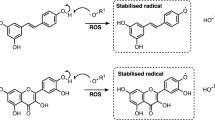
Abstract
Polyphenols are natural molecular entities exhibiting a wide variety of bioactivities including anticholinergic and/or antiamyloidogenic activities. Their low solubility is recognized as a key factor for bioavailability and their glycosylation is indeed relevant to improve the bioaccess to these molecules. In this chapter, chemical and enzymatic syntheses of glycosylated flavonoids, stilbenoids, phenylethanoids and phenylpropanoids are illustrated, covering examples that demonstrate the impact of coupling sugars to bioactive aglycones in their bioavailability and in their pharmacological activity. The chapter is focused particularly on glycosyl polyphenols with promising activities against neurodegenerative impairments, given their potential to intervene in biological processes that cause catastrophic diseases, namely the Alzheimer’s disease.
Catarina Dias and Ana M. Matos have both contributed equally to the chapter.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
- Glycosylated polyphenols
- Synthesis
- Flavonoids
- Stilbenoids, phenylethanoids
- Phenylpropanoids
- Neurodegenerative disorders
1 Introduction
Polyphenols are plant secondary metabolites present in the common human diet and known to play important roles in human health. They are poorly absorbed, resulting in a very low concentration in the circulatory streams [69]. The modification of their physicochemical properties such as solubility and partition coefficient by glycosylation seems to exert a positive influence on the entry of polyphenols into enterocytes [69]. The low solubility of most of the polyphenol aglycones may also result from their tendency to form aggregates via hydrophobic interactions with aromatic rings, and hydrogen bonding by the hydroxy groups [3]. In nature, polyphenols occur often as glycosylated derivatives. The sugar moiety of polyphenol glycosides plays a major role in their absorption [69] but polyphenol glycosylation may also exert other benefits by improving bioavailability or preventing oxidation by masking phenolic groups. In this chapter, synthetic strategies via chemical or enzymatic methodologies to access biologically active glycosyl polyphenols are illustrated, covering flavonoids, stilbenoids, phenylpropanoids and phenylethanoids. Natural occurrence and compound bioactivities are also reviewed for the promising polyphenol molecular entities described that exhibit neuroprotective activities.
2 Glycosylated Flavonoids
Flavonoids are polyphenolic secondary metabolites in the plant kingdom whose structural feature is based on derivatives of a phenyl-substituted 1-phenylpropane possessing a C15 skeleton. In this chapter, the given examples focus particularly on flavones, whose structure is that of a 1-benzopyran (chromene), in which the aromatic ring is designated as ring A and the pyran as ring C, along with the (substituted) phenyl group (ring B) on ring C at position 2 (flavone) or position 3 (isoflavone). Thousands of different scaffolds have been isolated and structurally identified over the past decades, and many have been reported due to their wide-range bioactive profiles often associated with very potent antioxidant and anti-inflammatory effects [70]. They may occur as aglycones or as the corresponding glycosylated forms, either as O-glycosides or C-glycosyl derivatives; yet, the advantages of glycosyl flavones over the corresponding aglycones have been highlighted in the context of Alzheimer’s disease with respect to their ability to remodel and inactivate neurotoxic amyloid β (Aβ) aggregates [36], again reinforcing the importance of the sugar moiety for optimized anti-neurodegenerative activity.
The growing interest in the therapeutic potential of glycosyl flavonoids has motivated organic and medicinal chemists to develop efficient synthetic and bioenzymatic routes involving a diverse collection of sugar coupling reactions. By describing the synthesis of some of the most promising molecular entities with neuroprotective activities, we will provide an overview of the most useful methodologies for the generation of flavones bearing in their structure O-linked or C-linked sugars, covering both chemical and enzymatic synthesis reported in the literature.
2.1 Flavone Glycosides
The 7-O-β-glucuronide of baicalein, baicalin (1), is one of the most abundant compounds in Scutellaria baicalensis Georgi, a plant extensively used in traditional Chinese medicine for the treatment of inflammatory disorders, bacterial infections, among others [6]. Baicalin (1) itself was recently found to improve Aβ-induced learning and memory deficits in rats by attenuating hippocampal injury and neuron apoptosis [11]. Its anti-inflammatory activity has actually been proposed as a paramount mechanism underlying these neuroprotective effects [6], namely by inhibiting microglial activation and inflammatory cytokine secretion [83]. Moreover, baicalin (1) could also upregulate antioxidant enzymes such as superoxide dismutase, catalase and glutathione peroxidase, thus contributing to decreased oxidative injury in the brain of diseased animals [11].
Based on its promising therapeutic potential, Li and co-workers described an efficient route for baicalin (Scheme 1) starting from the selectively acetylated aglycone 3, which was accessed after a series of simple protection–deprotection reactions [45], and using the first type of sugar donor ever applied in the synthesis of flavonoid glycosides: a glycosyl bromide [62]. In this method, 6-OTBDPS protected bromide 2 was coupled to the aglycone in an Ag2O-promoted reaction that afforded only the β-glucoside in 92% yield due to acyl neighbouring group participation, thus overpowering the otherwise dominant anomeric effect that would have given the α-anomer as the major product. After deprotection with TBAF, position 6″ was then submitted to Widlanski oxidation using TEMPO and BAIB to give the glucuronic acid derivative 4, followed by a deacylation reaction that led to the desired product, baicalin (1).
Reagents and conditions: a Ag2O, 4 ÅMS, quinoline, r.t. (92%); b TBAF, AcOH, THF, 4 h (86%); c TEMPO, BAIB, DCM/H2O, r.t. (87%), d Mg(OMe)2, MeOH, r.t. (85%) [45]
The 4′-hydroxy analogue of baicalin (1), scutellarin (5), is the major component of the Erigeron breviscapus Hand-Mazz flavonoid extract, also used in traditional Chinese medicine for the treatment of cerebral infarction and other cardiovascular diseases [54]. Similarly, this compound was found to attenuate neuroinflammation through the suppression of microglial activation [15], and was indeed associated with major improvements in neuronal injury and behaviour of rats with cerebral ischemia [14, 68]. Moreover, it is able to inhibit Aβ aggregation in vitro, while preventing Aβ-mediated neuronal cell death [91].
Nonetheless, pharmacokinetic studies have revealed that scutellarin (5) displays a rather poor bioavailability due to the action of endogenous β-glucuronidase enzymes that readily hydrolyze the glycosidic bond [5, 17, 23]. To surpass this problem, Li and co-workers designed and synthesized the scutellarin β-O-glucosyl analogue 6 with improved physicochemical properties and an even more pronounced attenuation of H2O2-induced neuronal damage when compared to scutellarin (5) [40]. Using scutellarin itself as the aglycone source, the authors coupled compound 7 to the glucosyl bromide 8 with Ag2O and CuSO4 as promotors; yet, the β-O-glucoside 6 was achieved in only moderate yield (40%) (Scheme 2).
Reagents and conditions: a 6 N HCl, EtOH, 120 °∁ (17%); b pyridine, Ac2O, DMAP, 25 °∁ (79%); c BnBr, K2CO3, KI, acetone, reflux (70%); d Pd/C, H2, DCM/EtOH, 25 °∁ (95%); e CuSO4, AgO, quinoline, 25 °∁ (40%); f NaOH, CHCl3, 0 °∁ (41%) [40]
Among the most promising O-glucosyl flavonoid leads against neurodegenerative diseases is quercetin 3-β-O-glucoside (10) (trivial name: isoquercetin), which has been isolated from a variety of sources, including mangos or medicinal plants such Serjania erecta Radlk (Sapindaceae) or Psidium guajava L. [18, 46, 48]. In addition to its antioxidant and anti-inflammatory activities, this compound is able to prevent hippocampal neuronal apoptosis after cerebral ischemia and reperfusion injury [76, 77], and displays protective effects against Aβ-induced cytotoxicity. Importantly, it was also found to inhibit both BACE-1 and AChE with IC50 values of 41.2 and 66.9 µM, respectively [26, 27]. Furthermore, a comparative study between polyphenolic glycosides and their respective aglycones has shown that whilst quercetin (11) acts by remodelling Aβ toxic oligomers into large aggregates, isoquercetin (10) rapidly disaggregates the amyloid structures into soluble polypeptides as a result of a synergistic action between the sugar and the aglycone [36].
Isoquercetin (10) can be obtained from quercetin (11) by the action of UGT78D1, a flavonoid-specific uridine diphosphate glycosyltransferase (Scheme 3) that catalyzes the in vitro regioselective transfer of a glucose or a rhamnose unit from UDP-glucose or UDP-rhamnose, respectively, to flavonoid glycosyl acceptors containing a hydroxyl group in position 3, as reported by Ren and co-workers [59]. This study was able to clarify the substrate specificity of this enzyme in detail, showing that only flavones hydroxylated in both rings A and B are recognized by UGT78D1, highlighting 2′-OH flavones as exceptions to this rule.
Reagents and conditions: a Recombinant UGT78D1, 50 mM Tris-HCl pH 7.2, UDP-Glucose, 30 °∁ [59]
Tiliroside (12) is a kaempferol 3-β-O-glycoside that can be found in Agrimonia pilosa or Potentilla chinesis for instance, and displayed stronger AChE inhibitory activity when compared to isoquercetin (10), with an IC50 value of 25.5 μM [27, 57]. It was also found to inhibit neuroinflammation in activated microglial cells by modulating pro-inflammatory intracellular pathways, which was at least in part attributed to its antioxidant properties [71].
The synthesis of tiliroside (12) was described in 1981 by Vermes and co-workers (Scheme 4) [72]. In the first step of this route, glucosyl bromide 13 and 4’,7-O-dibenzyl kaempferol (14) were coupled in a reaction promoted by Ag2CO3 to afford the β-anomer in 54% yield. After debenzylation followed by acetylation and selective removal of the 6″-O-chloroacetyl protecting group, intermediate 15 was generated and subsequently esterified by p-coumaroyl chloride in pyridine. Further deprotection directly afforded tiliroside (12) in good yield. Many other phenylpropanoid glycosides with neuroprotective activities such as this one will be presented and their synthetic routes described in detail in Sect. 3.
Reagents and conditions: a Ag2CO3, pyridine, drierite, 0 °∁ (54%); b H2, Pd/C, EtOH, r.t. (90%); c Ac2O, pyridine, r.t. (88%); d MeOH, thiourea, r.t. (90%); e DCM, pyridine (62%); f CHCl3, NaOMe, MeOH (67%) [72]
2.2 C-Glycosyl Flavones and Isoflavones
In spite of the remarkable neuroprotective effects associated with the flavone O-glycosides described above, C-glycosyl flavonoids have been receiving growing attention for their insusceptibility to in vivo hydrolysis by glucosidases, allowing them to remain intact in the blood circulation following oral administration [9]. Even though the synthesis of the C–C bond usually requires stronger conditions when compared to the formation of the C–O bond in O-glycosides, a variety of methods have been reported in the literature over the past few decades, offering a wide range of options for regio- and stereoselective reactions using different glycosyl donors and acceptors when the time comes to design a synthetic route for the target compound [64].
Vitexin (17) and isovitexin (18), the 8-β-C- and 6-β-C-glucosyl derivatives of apigenin, respectively, are good examples of the potential of natural C-glycosyl flavonoid derivatives against neurodegenerative disorders. These compounds can be found in Serjania erecta Radlk (Sapindaceae) [18] or the flour from the Prosopis alba seed [4], for instance, and both were able to inhibit AChE and BChE with IC50 values ranging from 6.2 to 12.2 µM, although vitexin was substantially more effective as a BACE-1 inhibitor than isovitexin (IC50 = 51.1 µM vs. >100 µM), thus indicating a preference for the sugar moiety to be in position 8 for improved affinity towards the enzyme [7, 8]. Vitexin (17) has also been described to exert neuroprotective effects in cerebral ischemia and reperfusion injury by positively and negatively modulating cell proliferation and apoptosis pathways, respectively [79]. In addition, vitexin (17) was found to have a more pronounced impact in reversing Aβ-induced cytotoxicity not only when compared to isovitexin (18), but also when put alongside with the earlier presented isoquercetin (10) [18].
Back in 1995, Mahling and co-workers developed a synthetic route for both vitexin (17) and isovitexin (18) by taking advantage of the Fries-type rearrangement, described to occur in O-aryl glycosides with high regio- and stereoselectivity to afford the corresponding ortho-hydroxy C-glycosyl phenolic derivative [32, 47, 64]. Hence, in the first step of this synthesis (Scheme 5), the reaction of the glycosyl trichloroacetimidate 19 with the silyl-protected acetophenone 20 was catalyzed by TMSOTf at-30 °∁ and afforded the α-O-glycoside 21 in 85% yield. After cleavage of the remaining TBS group followed by regioselective benzylation in position 4, a Fries-type rearrangement took place in another TMSOTf-catalyzed reaction, this time at room temperature, to afford the corresponding β-C-glycosyl derivative in 57% yield. Subsequent acylation converted this derivative into intermediate 17 and, at this point, the Baker–Venkataraman rearrangement was carried out and resulted in a mixture of compounds 23 and 24, which were both cyclized and further deprotected after separation to give vitexin (17) and isovitexin (18).
Reagents and conditions: a TMSOTf, DCM, −30 °∁ (85%); b TBAF, THF (86%); c BnBr, NaH, DMF (89%); d TMSOTf, DCM, r.t. (57%); e WSC, DMAP, 4-(benzyloxy)benzoic acid, DCM (81%); f TBA·HSO4, K2CO3, H2O/Benzene, 60 °∁ (39%); g TMSOTf, DCM, r.t. (17%); h NaOMe, MeOH, 50 °∁ (62 and 100%, respectively); i H2, Pd/C, EtOAc/MeOH (88 and 89%, respectively) [47]
Vitexin (17) and isovitexin (18) were also obtained as protected intermediates in a more recent and concise synthesis developed by Furuta and co-workers [16] with the ultimate goal of accessing compound 25, an anti-inflammatory glycosyl flavone isolated from oolong tea extract [24]. In this route (Scheme 6), trichloroacetimidate 19 was directly coupled with the monobenzyl-protected acetophenone 26 to afford the desired β-C-glucosyl derivative in 69% yield, which was further acylated to give intermediate 27. In contrast with the previous work by Mahling et al., this procedure involves the initial formation of a glycoside at low temperature, which then undergoes, by warming up, the O → C Fries-type rearrangement in situ [64].
Reagents and conditions: a TMSOTf, DCM, 0 °∁ → r.t. (69%); b 4-(methoxymethoxy)benzoic acid, DCC, DMAP, DCM, r.t. (82%); c K2CO3, pyridine, reflux (17 and 15%, respectively); d Pd(OH)2, H2, EtOH, 35 °∁ (95%); e 1,1′-azobis(N,N-dimethylformamide), Bu3P, THF, 50 °∁; then HCl-dioxane, MeOH, r.t. (32%) [16]
In another one-pot reaction using potassium carbonate in pyridine under reflux, intermediate 27 was converted into both protected isovitexin (28) and protected vitexin (29); yet, to accomplish the synthesis of the target compound, only 28 proceeded in this route. After debenzylation, it was submitted to an intramolecular Mitsunobu reaction using modified experimental conditions in which inversion of the configuration of carbon 2″ led to the transformation of the gluco derivative into the desired manno derivative in tandem with the formation of a fused tetracyclic system with the aglycone. Further deprotection afforded the target compound 25, but it is still interesting to note that this was the major product of Mitsunobu reaction regardless of the presence of a primary alcohol and another phenolic group in its precursor, thus highlighting reaction regiospecificity.
Orientin (31) and isoorientin (32) are another pair of C-glucosyl flavonoid derivatives extensively studied for their potential against neurodegenerative processes and can both be found, for instance, in buckwheat bran [92], Glochidion hypoleucum (Miq.) Boerl leaves, or Stellaria holostea [1]. Orientin (31) was able to alleviate cognitive deficits in mice with Alzheimer’s disease, while attenuating mitochondrial dysfunction induced by Aβ [84]. Moreover, it exerted neuroprotective effects by inhibiting the activity of three members of the caspase family, including caspase 3, which is directly involved in synaptic loss and cognitive dysfunction in Alzheimer’s disease [37, 10]. Both orientin (31) and isoorientin (32) are BACE-1 inhibitors with IC50 values of 16.0 and 20.9 µM, respectively, showing that the presence of the additional hydroxy group in position 3′ when compared to vitexin (17) and isovitexin (18) positively affects the affinity towards the enzyme, especially in the case of 6-β-C-glucosyl derivatives. Furthermore, they are also AChE and BChE inhibitors and seem to be slightly selective towards the later, with similar IC50 values of roughly 11 µM [7, 8].
In contrast to the described synthetic approaches for vitexin (17) and isovitexin (18), each of these luteolin C-glucosyl derivatives has been accessed individually in more effective, regioselective routes. Kumazawa and co-workers reported, on the one hand, the synthesis of orientin (Scheme 7) in which the glucosyl fluoride 33 was coupled with acetophenone 34 in a BF3·Et2O-promoted reaction to afford the β-C-glucosyl derivative 35 in 96% yield [33]. Subsequently, an aldol condensation led to chalcone 37 which, after cyclization and deprotection, gave orientin (31) in very good yield.
Reagents and conditions: a BF3·Et2O, MS4Å, DCM, −78 °∁ → r.t. (96%); b 1,4-dioxane, aq. NaOH 50%, r.t. (84%); c I2, DMSO, reflux (84%); d Pd/C, H2, EtOH, r.t. (quantitative yield) [33]
On the other hand, these authors were able to develop a synthetic path towards isoorientin (Scheme 8) using the same coupling methodology but taking advantage of differences in hydrogenolysis rates between benzyl and 2-methylbenzyl protecting groups [34]. Indeed, after a series of protection-deprotection reactions, the free hydroxy group was para to the sugar moiety in intermediate 40, and after aldol condensation, cyclization and deprotection, isoorientin (32) were successfully generated. It is noteworthy that, in this route, the C-glycosylation step was significantly less effective (75% yield) than the one described in the synthesis of orientin (31, 95% yield), even though the coupling method applied was the same in both cases. Given that the only difference between the two glycosyl acceptors was the 2-methylbenzyl group in compound 39, this result highlights the impact of protecting groups on the efficiency of this type of coupling reactions.
Reagents and conditions: a BF3·Et2O, MS4Å, DCM, −78 °∁ → r.t. (75%); b anhydrous K2CO3, 2-methylbenzyl chloride, DMF, 80 °∁ (quantitative yield); c Pd/C, H2, EtOAc, r.t. (40%); d 1,4-dioxane, aq. NaOH 50%, r.t. (91%); f I2, DMSO, 200 °∁ (76%); g Pd/C, H2, EtOAc/EtOH, r.t. (quantitative yield) [34]
More recently, the biosynthesis of vitexin (17), isovitexin (18), orientin (31) and isoorientin (32) was accomplished by Hao and co-workers using Desmodium incanum root proteins, starting from the corresponding 2-hydroxyflavanones, the required substrates of C-glycosyltransferases existent in Desmodium spp. [21, 22]. As clarified in a previous report [29], Wessely–Moser isomerization is responsible for the interconversion between the corresponding 8-β-C- (45 and 46) and 6-β-C-glucosyl derivatives (49 and 50), as 2-hydroxyflavanones may exist in solution in either open chain or cyclized structures (Scheme 9). In spite of the consequent lack of regioselectivity, these intermediates afforded the respective flavones in overall excellent yields after acid-promoted chemical dehydration.
Puerarin (51), the major component of Puerariae Lobatae Radix [82], is another C-glucosyl flavonoid with potential against neurodegenerative disorders and has received particular attention in regard to its ability to act against diabetes-induced cognitive dysfunction, complementing its known antidiabetic activity [44, 82, 84]. This is of particular importance due to the well-established relationship between type 2 diabetes and Alzheimer’s disease [31] and, in fact, puerarin (51) was found to have neuroprotective activity in STZ-induced diabetic rodents with learning and memory deficits by exerting antioxidant, anti-inflammatory and anti-apoptotic effects [42, 89]. In addition, this C-glucosyl isoflavone was able to attenuate Aβ-induced oxidative stress, cell injury and resulting cognitive impairment [39, 41, 78, 86, 90], and could also improve learning and memory functions in rats with vascular dementia by activating cellular antioxidant defense mechanisms [87].
The total synthesis of puerarin (51) was firstly reported by Lee and co-workers in 2003 [38] (Scheme 10). In this approach, the benzyl protected glycopyranolactone 52 was coupled to the lithiated glycosyl acceptor 53 at low temperature, followed by reduction with triethylsilane and BF3·Et2O to give the β-anomer in 56%. After a couple of protection-deprotection reactions, a Friedel-Crafts reaction catalyzed by AlCl3 and subsequent deacetylation gave intermediate 55, which then underwent aldol condensation with p-methoxybenzaldehyde (56) to afford chalcone 57. After acetylation, TTN-promoted oxidative rearrangement of ring B followed by closure of ring C and demethylation gave puerarin (51) in moderate overall yield.
Reagents and conditions: a THF, −78 °∁ → −10 °∁; then TESH, BF3·Et2O, DCM, −78 °∁ → r.t. (56%); b Pd/C, H2, MeOH, r.t. (quantitative yield); c pyridine, Ac2O, r.t. (96%); d AlCl3, AcCl, Et2O, r.t. (69%); e 1: Na, MeOH; 2: Dowex 50WX8-200 (97%); f NaOH, EtOH, r.t. (92%); g pyridine, Ac2O, r.t. (85%); h 1: Tl(NO3)3, MeOH/CH(OCH3)3; 2: 10% HCl, MeOH, reflux (84%); i TMSI, ACN (35%) [38]
The trihydroxyisoflavone analogue of puerarin (51) is the 8-β-d-glucosylgenistein (58), the main component of the ethyl acetate extract of Genista tenera, a plant found in Madeira island and used in folk medicine to treat diabetes [25]. In addition to its potent antidiabetic activity, 8-β-d-glucosylgenistein (58) was found to interact with Aβ1-42 polypeptides, suggesting potential neuroprotective effects as well. In this study, the binding epitope of 8-β-d-glucosylgenistein (58) with Aβ was disclosed, confirming the already expected key role of both aromatic rings in the resulting interaction, and reinforcing the importance of the sugar moiety in the antiamyloidogenic activity of this compound.
The synthesis of 8-β-D-glucosylgenistein (58) (Scheme 11) was accomplished by coupling the commercially available glucopyranoside 59 and acetophenone 60 catalyzed by TMSOTf, to give the desired C-glycosylation product in 56% yield, which was selectively benzylated to afford intermediate 61 [25]. Then aldol condensation with p-benzyloxybenzaldehyde followed by acetylation led to the formation of chalcone 63 and subsequent TTN-promoted oxidative rearrangement, ring closure and deprotection afforded the target compound, 58.
Reagents and conditions: a 1. TMSOTf, DCM/ACN, drierite, −40 °∁ → r.t. (56%); b BnBr, K2CO3, r.t. (74%), c 1: 1,4-dioxane, aq. NaOH 50%, reflux; 2: pyridine, Ac2O, DMAP, r.t. (60%); d 1: Tl(NO3)3, MeOH/CH(OCH3)3, 40 °∁; 2: THF/MeOH, aq. NaOH 50%, r.t. (63%); e Pd/C, H2, EtOAc/MeOH, r.t. (96%) [25]
In a nutshell, the coupling of sugars with polyphenols to generate bioactive glycosyl flavonoids may involve a variety of different strategies and experimental conditions which primarily depend upon the available starting materials, reaction promoters or catalysts, and the nature of the pursued C–C or C–O bond. Regio- and stereoselectivity can be achieved with the use of the appropriate sugar protecting groups and glycosyl acceptor, while temperature is a key factor in the formation of either O- or C-glycosyl derivatives, particularly when a Fries-type rearrangement is involved in the reaction mechanism. Also, by covering the synthesis of structurally complex compounds such as the presented bioactive glycosyl flavones and isoflavones, this section enclosed a number of useful protection–deprotection strategies, interesting rearrangement reactions and cyclization approaches, which may be convenient for the synthesis of new nature-inspired glycosylated molecules towards neurodegenerative disease prevention.
3 Stilbenoid Glycosides
Stilbenoids are natural compounds occurring in a number of plant families, particularly in grapevine [2]. Amongst them, the most well known is resveratrol (E)-3,4′,5-trihydroxystilbene, (64), possessing anti-inflammatory, antioxidant and chemopreventive activities. This powerful compound is present in wine and has been speculated to be responsible for so-called French paradox, where the saturated fat rich French diet correlates with a low mortality from coronary heart disease [60, 81]. Resveratrol also occurs ubiquitously in nature as resveratrol 3-β-glucoside (piceid, 67) (Fig. 1). Other stilbenes include pterostilbene (E)-4′-hydroxy-3,5-dimethoxystilbene, (65), piceantannol (E)-3,3′,4′,5-tetrahydroxystilbene, (66) (Fig. 1) and astringine (68) which biological activities have been reviewed [63].
Much attention has also been paid to stilbenes potential ability to protect from neurodegeneration. In fact, research points resveratrol as neuroprotective, not only due to the already mentioned antioxidant and anti-inflammatory activities, but also due to its ability to inhibit Aβ oligomeric cytotoxicity and to reduce neuronal cell death [58]. In a comparative study, the inhibitory activity of a series of stilbenes against Aβ (25–35) fibril formation was assessed. Both resveratrol 64 and piceid 67 effectively and dose dependently inhibited Aβ more extensively than curcumin [61].
Despite the promising activities of resveratrol and its glycoside piceid, their bioavailability in humans is quite poor [73, 81]. Indeed, the oral bioavailability of resveratrol is less than 1% as a consequence of quick and extensive metabolism, mainly through glucuronidation and sulfation, although it is not known whether resveratrol metabolites have a positive biological impact. The water-insolubility of stilbenes such as resveratrol, pterostilbene and piceatannol limits their further pharmacological exploitation. Literature shows a number of efforts to develop new stilbene analogues with higher solubility and bioavailability, and glycochemistry has definitely played a very relevant role. Glycosylation allows water-insoluble and unstable organic compounds to be converted into the corresponding water-soluble and stable compounds.
The synthesis of piceid itself was first described by Orsini and co-workers, in an attempt to obtain this natural product more efficiently (Scheme 12) [53]. The synthetic strategy aimed at building the stilbene skeleton first, by Wittig reaction of the aldehyde 69 and phosphonium ylide 70, followed by desilylation. Methyl protected intermediary 71 was then glycosylated in the aqueous base under the phase transfer catalyst benzyltriethylammonium bromide (BTEAB) which afforded glucoside 73 in 32%. The diglucoside was also formed and isolated in 13% yield. Further deprotection (2 steps) afforded piceid [66] in 60% yield (13% overall yield) [53]. This methodology was also employed to the synthesis of other stilbene glycosides such as combretastatin analogues.
Reagents and conditions: a BuLi, THF, −20 °∁ (98%, Z/E 2.3:1); b TBAF, THF, quant.; c BTEAB, NaOH, CHCl3, 60 °∁, 32%; d MeONa, MeOH, 25 °∁, quant.; e EtSNa, DMF (60%) [53]
More recent efforts towards glycosylation of resveratrol take advantage of biotransformation for a simpler and more efficient synthesis. Glucosyltransferase PaGT3 from Phytolacca americana expressed in Bacillus subtilis was used to convert resveratrol into its 3- and 4′-β-glucosides (67 and 74), as well as pterostilbene and piceatannol into their 4′-β-glucosides 75 and 76, respectively, (Scheme 13). Glucosylation reactions were performed at 37 °∁, in potassium phosphate buffer supplemented with UDP-glucose and enzyme. Although the procedure was not very effective towards piceid (12% yield), it afforded the 4′-β-glucosides in yields ranging from 50 to 76% [20].
Glycosylation of stilbenes was also performed using cultured cells from P. Americana and glucosyltransferase (PaGT). This biocatalytic glycosylation using cultured cells, in opposition to the direct use of the extracted enzyme, afforded resveratrol glucosides 67 and 74 in 35 and 22% yield, respectively, [65], favouring the formation of piceid, and 77% of piceatannol glucoside 76, which proved to be the best substrate for this enzyme. Pterostilbene was only slightly converted into 75.
In addition, resveratrol 3- and 4′-β-glucosides were further glycosylated using cyclodextrin glucanotransferase (CGTase) to afford resveratrol 3- and 4′-β-maltosides (77 and 78), respectively, with yields of 17 and 27%. The phosphodiesterase (PDE) inhibitory activity or resveratrol and pterostilbene was enhanced by glycosylation, since resveratrol 3- and 4′-β-glucosides, resveratrol 4′-β-maltoside and pterostilbene 4′-β-glucoside were better PDE inhibitors than their corresponding aglycone. This is particularly relevant as PDE inhibitors could be used in the treatment of neurodegenerative disorders such as Alzheimer’s disease as they show potential to exert a neuroprotective role. Interestingly, piceatannol 4′-β-glucoside revealed also potent histamine release inhibitory activity (anti-allergic activity) [20, 65].
Enzymatic synthesis has also been employed in further glycosylation of the natural piceid, generating more soluble piceid glycosides such as 79, which was obtained after incubation of piceid with maltosyltransferase from Caldicellulosiruptor bescii and maltotriose at 70 °∁, in 18% yield. The water solubility of maltosyl piceid 79 is 8540 and 1860 times greater than that of resveratrol and piceid, respectively [55]. Since the α-1,4-glycosidic linkages present in 79 can be easily hydrolyzed in vivo by α-glucosidase, this piceid glycoside could potentially be a resveratrol prodrug, with increased bioavailability and delayed metabolism [55]. Several piceid glucosides have also been obtained using cyclodextrin glucanotransferase from Bacillus macerans [49].
More recently, a sucrose phosphorylate from Thermoanaerobacterium thermosaccharolyticum (TtSPP) was engineered envisioning quantitative glycosylation of resveratrol in aqueous media (Scheme 14). Desmet and co-workers were able to identify a residue particularly important in the active site of TtSPP, which normally does not have a pocket deep enough for the binding resveratrol. Such residue, R134, was replaced by a smaller residue aiming at leaving an opening in the enzyme’s closed conformation, enabling the accommodation of larger substrates. Indeed, the variant R134A, where arginine 134 was replaced by alanine, proved to have a reasonable affinity for resveratrol and to be very effective in the glycosylation of resveratrol at gram scale, allowing the quantitative production of resveratrol 3-α-glucoside in an aqueous system, using sucrose as a cheap glycosyl donor [12].
Enzymatic synthesis of maltosyl piceid. a Maltosyltransferase (MTase), maltotriose [55]
Cyclodextrin glucanotransferase was also used to convert resveratrol and starch to α-glucosylated resveratrol products at 3-OH, at 4′-OH and at both 3-OH and 4′-OH, with increased water solubility when compared to that of resveratrol [69]. Interestingly, while the water solubility of piceid is 0.37 g/L, its alpha anomer presented solubility higher than 2 g/L [69]. Nevertheless, it would be interesting to compare the bioactivity of 82 with its anomer piceid, as configuration may play an important role in bioactivity and bioavailability, as demosntarted for the solubility. To the best of our knowledge, no bioactivity studies were conducted on resveratrol 3-α-glucoside so far.
Keeping in mind the challenge of resveratrol low water solubility, a new resveratrol analogue was developed, where glucosyl units were added to a resveratrol core with a succinate linker. It was speculated that the presence of glycosyl groups may also improve bioavailability by influencing phenomena taking place upstream of entry into erythrocytes, as occurs for quercetin 3-O-glucoside [3]. For the construction of the resveratrol analogue, a succinyl linker was firstly attached to the 3-hydroxy group of diacetoneglucose (83) (Scheme 15). The resulting succinyl ester (85) was used for the transesterification with resveratrol hydroxy groups using EDC. Hydrolysis of the isopropylidene protecting groups afforded the resveratrol derivative 87 in a 57% overall yield. This compound is relatively stable in acidic conditions but can be converted into resveratrol by blood esterases. Pharmacokinetics parameters were also improved, as its administration resulted in a blood concentration versus time curve shifted to longer times in comparison to resveratrol. This chemical transformation is particularly attractive as it may be employed in other bioactive polyphenols with poor water solubility. In addition, coating the hydroxy groups with sugar moieties can even make them more palatable [3]. Thus, it would be interesting to study this kind of modification in other polyphenols and, in addition to pharmacokinetics properties, study its influence in their organoleptic characteristics.
Synthesis of resveratrol 3-α-glucoside with R134A variant of sucrose phosphorylase [12]
In summary, selected examples of glycosyl stilbenoids chemical and enzymatic synthesis were presented and described the reported benefits of stilbene glycosylation for the usefulness of such biomolecular entities towards prevention of neurodegenerative impairments and related diseases.
4 Phenylethanoid and Phenylpropanoid Glycosides
A number of phenylethanoid and phenylpropanoid glycosides, either of natural or synthetic origin, have been described to possess neuroprotective activities, tackling both the amyloid cascade and the cholinergic system.
Acteoside (95) is a natural phenylpropanoid glycoside, also known as verbascoside, first isolated from the plant Verbascum sinuatum in the 1960s [28]. Meanwhile, a number of relevant bioactivities have been described, including its neuroprotective properties. Acteoside, isolated from Orobanche minor, strongly inhibits the aggregation of Aβ1-42, with an IC50 of 8.9 µM [35] and protects against Aβ-induced cell injury by attenuation of reactive oxygen species production, by modulation of the apoptotic signal pathway through Bcl-2 family [75] and by upregulation of heme oxygenase-1 [74]. However, even before the mechanisms of neuroprotection were unravelled, the unsatisfactory extraction of this natural product from plant sources, prompted Sakuno and co-workers to develop the total synthesis of acteoside [28]. The synthetic strategy involves reaction of glucosyl chloride 88 (which was prepared from the peracetylated corresponding sugar) with the phenyletyl derivative 89 by the Koenigs-Knorr method in the presence of silver carbonate (Scheme 16). The presence of an acetyl group at position 2 directs to the formation of the 1,2-trans glycosidic bond through a neighbouring group participation mechanism [28]. A series of protection and deprotection steps to afford glycoside 91 is followed by the introduction of the caffeoyl moiety by esterification. Oxidative cleavage of the 3-O-allyl group and rhamnosylation, performed with 2,3,4-tri-O-acetyl-α-l-rhamnopyranosyl trichloroacetimidate in the presence of boron trifluoride diethyl etherate gives the expected α-rhamnoside 94 in 73% yield. Finally, two considerable challenges lie on both the selective deacetylation over the cleavage of caffeoyl ester, and the selective removal of benzyl groups while keeping the double bond. Acetyl cleavage was consummate with methylamine in methanol (MeNH2–MeOH), after which catalytic transfer hydrogenation of benzyl ethers using 1,4-cyclohexadiene as a hydrogen source afforded acteoside (95) successfully, in an overall yield of 3.5% [28].
Reagents and conditions: a Pyr. DMAP, rt (78%); b Pyr, DMAP, EDC, rt. (74%); c TFA, rt (98%) [3]
More recently, an alternative and more efficient route towards phenylethanoid glycosides, such as acteoside, has been described [51], using a low substrate concentration and N-formylmorpholine modulated glycosylation for the construction of β- and α-glycosidic bonds. Interestingly, contrary to what was reported by Kawada and co-workers, the coupling of the β-glucoside 98 with the protected caffeic acid furnished not only the (E)-isomer of 99, but also trace amounts of the (Z)-isomer. Nevertheless, acteoside was obtained in an overall yield of 10.8% (E/Z 12:1) (Scheme 17) [51].
Synthesis of acteoside by Kawada et al. Reagents and conditions: a Ag2CO3, DCE (72%); b NaOMe, MeOH (94%); c TrCl, C5H5N (86%); d Ac2O, C5H5N (92%); e AcOH, dioxane (79%); f DCC, DMAP, DMAP·HCl, DCM (79%); g SeO2, AcOH-dioxane (66%); h BF3·Et2O, DCM (73%); i MeNH2, MeOH-DCM (49%); j 1,4-ciclohexadiene, 5% Pd/C, DMF-EtOH (44%) [28]
It is well-established that enhancing cholinergic transmission by blocking the activity of acetylcholinesterase (AChE) slows down the AD-associated decline in behaviour and cognition. The natural phenylpropanoid diglycoside rosavin (107) and its analogues (E)-3-phenylprop-2-en-1-yl β-d-xylopyranosyl-(1→6)-β-d-glucopyranoside (115), (E)-3-(4-methoxyphenyl)prop-2-en-1-yl α-l-arabinopyranosyl-(1→6)-β-d-glucopyranoside (116) and (E)-3-phenylprop-2-en-1-yl α-l-rhamnopyranosyl-(1→6)-β-d-glucopyranoside (117) (Scheme 19) displayed a remarkable anti-AChE with IC50 values of 1.72, 3.71, 4.23, 2.05 µM, respectively [43]. Indeed, rosavin displayed the most potent AChE inhibition out of the natural compounds described so far. This natural product was firstly synthesized as shown in Scheme 18. The disaccharide 104 was first constructed by reaction of the glycosyl bromide 102 with the isopropylidene protected glucose 103. After cleavage of the isopropylidene groups, acetylation and the introduction of the anomeric sulfanyl group, the glycosyl donor 105 was obtained. Activated by iodine, this donor reacted with the cinnamyl alcohol to afford the acetylated precursor 106, which further deprotection gave rosavin (107).
Synthesis of acteoside by Mulani et al. a TMSOTf, −60 °∁, 1:2:1 DCM-ACN-EtCN (63%); b NIS, TMSOTf, −40 °∁, 1:2:1 DCM-ACN-EtCN (60%); c PdCl2, NaOAc, AcOH, acetone (70%); d i. NFM; ii. NIS, TMSOTf, DCM (60%); e i. DDQ, 3:1 MeOH, CH2Cl2; ii. TREAT-HF, ET3N, pyr. (two steps 68%) [51]
Rosavin, along with its natural analogues 115–117, was also synthesized by an alternative methodology, where the phenylpropanoid monoglycosides 113a and 113b were first synthesized, and then coupled with the appropriate glycosyl trichloroacetimidate (118, 119 or 120), promoted by TMSOTf. Further deprotection afforded the natural rosavin analogues (Scheme 20). The same procedure was employed for the synthesis of a small library of phenylpropanoid glycosides, with derivatives incorporating substituted phenyl groups with F, Cl and Br, and varying the methoxy and hydroxy substitution patterns. However, none of the synthesized derivatives was as active as the natural diglycosides 107, 115–117 [43]. Other methodologies for the synthesis of rosavin and its counterparts can be found in the literature, including the use of Mizoroki–Heck type reaction, involving the coupling of phenylboronic acid and allyl glycosides [30].
a AgClO4, CaCO3, acetone (18%); b AcOH, 60 °∁; c Ac2O, HClO4; d HSCH3, BF3-Et2O, CHCl3 (64%); e I2, CHCl3 (30%); f MeONa, MeOH (95%) [43]
Other examples of powerful anti-AChE glycosides are the derivatives of the natural antidepressant helicid, synthesized starting from 4-hydroxybenzaldehyde, followed by glycosylation, deprotection and condensation with amines, as depicted in Scheme 21 [80]. These transformations afforded noteworthy AChE inhibitors with IC50 under 10 µM, three of them even under 0.55 µM. The synthetic approach was based on the reaction of glycosyl bromides with 4-hydroxybenzaldehyde in the presence of TBAB to afford the corresponding protected phenyl glycosides. Subsequent Zemplén deacetylation yielded the sugar-linked helicid analogues 122 and 124. Schiff base derivative 127 was synthesized by reaction of 126 with methoxyamine (Scheme 21) [80]. Although an extensive library of helicid derivatives was obtained by this method, only the most active ones are depicted in Scheme 21. Interestingly, while helicid was not active up to 500 µM, its epimer at C-3 (122) presented an IC50 of 0.45 µM. However, the most potent inhibitor is the 4-formylphenyl β-d-ribopyranoside (124). It exhibits the same configuration of carbons 2, 3 and 4 as helicid but its hydroxymethyl group is replaced by a hydrogen atom, presenting an IC50 value of 0.20 µM on electric eel AChE, twice more active than galantamine. Also the Shiff base 127 has an IC50 value of 0.49 µM. These results highlight the close correlation of the bioactivity with the sugar structure [80].
Reagents and conditions: a BzCl/Py, rt; b HBr-AcOH/Ac2O, rt; c NaI/H2O/acetone, 30 °∁ (85% three steps); d DBU/CCl3CN, rt (80%); e PhMe 80°C; f DIBAlH, PhMe, −20 °∁; g TMSOTf, DCM, −20 °∁; h NaOCH3, MeOH, 0 °∁ (41, 61%, two steps); i TrCl, DMAP, TEA, DMF, MS 4 Å (56, 64%); j BzCl, Py (87, 90%); k 90% TFA, DCM (84, 86%); l TMSOTf, DCM, −20 °∁; m NaOMe, MeOH (78–83%, two steps). (Yields in parentheses separated by comma are givenfor compounds type a and b, respectively) [43]
Structurally similar to the compounds discussed so far is also salidroside (132) (Scheme 22), a phenylpropanoid glycoside isolated from Rhodiola species that is one of the active principles responsible for plant antidepressant and anxiolytic activities. The low content of salidroside in Rhodiola sachalinensis, the unsustainable overexploitation of this species, and the need to fully exploit its potential clinical applications, have encouraged chemists to the develop a synthetic approach towards 2-(4-hydroxyphenyl)ethyl β-glucopyranoside. Various examples in the literature show the preference for the silver carbonate promoted glycosylation of tyrosol (128), which aromatic hydroxy group can be protected or unprotected, using peracetylated glucosyl bromide as a glycosyl donor [19, 66, 67]. In 2011, a multi-kilogram scale-up of salidroside was reported, featuring the selective acetylation of tyrosol aromatic hydroxy group in aqueous media, and affording the target natural glycoside in 72% yield (Scheme 22) [66].
Preparation of helicid derivatives. Reagents and conditions: a Preparation of helicid derivatives TBAB, NaOH, CHCl3/H2O, 45 °∁. b NaOMe, rt, 3 h; c amine, EtOH, reflux or 45 °∁, 2–6 h [80]
Outstandingly, this natural glucoside protects neurons from glutamate-induced oxidative stress and apoptosis and was shown to be therapeutically effective against cognitive decline during ageing. Salidroside also intervenes in the amyloid cascade events, as it protects against Aβ25–35-induced oxidative stress. In fact, pretreatment with salidroside noticeably attenuated Aβ25–35-induced loss of cell viability and apoptosis in a dose-dependent manner [88]. A fairly recent study also supports these findings and further attests the activity of this tyrosol glycoside by showing that it protects four different Drosophila models of AD against Aβ-induced neurotoxicity. The study also reveals that salidroside decreased Aβ levels and Aβ deposition in the fly’s brain and ameliorated toxicity in Aβ-treated primary neuronal culture [85].
However, perhaps one of the most well-known phenylpropanoid derivatives with well-documented neuroprotective activities is curcumin (133, Scheme 22), an active ingredient in the spice turmeric consisting of two cinnamoyl units linked by a methylene group. Curcumin has been reported to act on several biochemical pathways associated with the onset and progression of AD. It disrupts amyloid-β and tau peptide aggregation, inhibits inflammation and protects against oxidative stress [50, 52]. However, its pharmaceutical use is restricted due to its poor water and plasma solubility and consequent low bioavailability [52, 56]. Considering that the addition of a sugar moiety would significantly increase the water/plasma solubility of the molecule while retaining all the characteristics of the curcumin pharmacophore, a clicked galactose–curcumin conjugate was developed using click-chemistry [13].
Synthesis of salidroside. Reagents and conditions: a Ac2O, NaOH, H2O (90%); b Ag2CO3, DCM, molecular sieves (4 Å); c NaOMe, MeOH (80%, two steps) [66]
Such soluble “clicked” sugar conjugate of curcumin (SC) was synthesized as depicted in Scheme 22. The curcumin monoalkyne 134 was coupled with an acetyl-protected galactoside bearing an azide and, after removal of acetyl groups, a galactose-curcumin conjugate possessing a triazole-based linker was obtained. This non-toxic curcumin derivative is ca. 1000 times more soluble than curcumin in water, and exhibits enhanced ability to inhibit both amyloid-β and tau aggregation, at concentrations as low as 8 and 0.1 nM, respectively [13] (Scheme 23).
a K2CO3, DMF, r.t; b i. CuSO ·4 5H2O, sodium ascorbate, t-BuOH/THF/H2O, r.t (76%); c NaOMe, MeOH, r.t (70%) [13]
5 Conclusion
This chapter is devoted to highlight the biological importance of linking sugars to polyphenols and to the methodologies described for this purpose. Examples of polyphenol glycosides, that exhibit an increased neurodegenerative protective effect when compared to their aglycones, are given in this chapter. The role of sugar binding to improve polyphenol solubility and ameliorating its bioavailability is also clearly illustrated with examples. Chemical and enzymatic approaches to glycoside synthesis are described for various families of polyphenols, namely stilbenoids that include the well-known resveratrol, phenylethanoids and propanoids covering also the “dimeric analogue” curcumin and the flavonoids, covering only flavone glycosides. To flavone and isoflavone C-glycosylation is given a particular attention, given the relevance of the C–C bond, that is not hydrolytically cleaved, allowing C-glycosyl flavonoids to remain intact in the blood circulation following oral administration.
We really hope that this chapter will encourage chemists and biochemists to further investigate the role of sugar binding to polyphenols, not only to diversify and optimize coupling conditions but also to discover new biomolecular entities to effectively prevent neurodegenerative impairments with clinical applications.
References
Ancheeva E, Daletos G, Muharini R, Lin W, Teslov L, Proksch P (2015) Flavonoids from Stellaria nemorum and Stellaria holostea. Nat Prod Commun 10(3):437–440
Bavaresco L, Fregoni C, Cantù E, Trevisan M (1999) Stilbene compounds: from the grapevine to wine. Drugs Exp Clin Res 25(2–3):57–63
Biasutto L, Marotta E, Bradaschia A, Fallica M, Mattarei A, Garbisa S, Zoratti M, Paradisi C (2009) Soluble polyphenols: Synthesis and bioavailability of 3,4′,5-tri(α-D-glucose-3-O-succinyl) resveratrol. Bioorg Med Chem Lett 19(23):6721–6724
Cattaneo F, Costamagna M, Zampini I, Sayago J, Alberto M, Chamorro V, Pazos A, Thomas-Valdés S, Schmeda-Hirschmann G, Isla M (2016) Flour from Prosopis alba cotyledons: a natural source of nutrient and bioactive phytochemicals. Food Chem 208:89–96
Chen X, Cui L, Duan X, Ma B, Zhong D (2006) Pharmacokinetics and metabolism of the flavonoid scutellarin in humans after a single oral administration. Drug Metab Dispos 34(8):1345–1352
Chen C, Li X, Gao P, Tu Y, Zhao M, Li J, Zhang S, Liang H (2015) Baicalin attenuates Alzheimer-like pathological changes and memory deficits induced by amyloid β1-42 protein. Metab Brain Dis 30(2):537–544
Choi J, Islam M, Ali M, Kim E, Kim Y, Jung H (2014) Effects of C-glycosylation on anti-diabetic, anti-Alzheimer’s disease and anti-inflammatory potential of apigenin. Food Chem Toxicol 64:27–33
Choi J, Islam M, Ali M, Kim Y, Park H, Sohn H, Jung H (2014) The effects of C-glycosylation of luteolin on its antioxidant, anti-Alzheimer’s disease, anti-diabetic, and anti-inflammatory activities. Arch Pharm Res 37(10):1354–1363
Courts F, Williamson G (2015) The occurrence, fate and biological activities of C-glycosyl flavonoids in the human diet. Crit Rev Food Sci Nutr 55(10):1352–1367
D’Amelio M, Cavallucci V, Middei S, Marchetti C, Pacioni S, Ferri A, Diamantini A, De Zio D, Carrara P, Battistini L, Moreno S, Bacci A, Ammassari-Teule M, Marie H, Cecconi F (2011) Caspase-3 triggers early synaptic dysfunction in a mouse model of Alzheimer’s disease. Nat Neurosci 14(1):69–76
Ding H, Wang H, Zhao Y, Sun D, Zhai X (2015) Protective effects of baicalin on Aβ1-42-induced learning and memory deficit, oxidative stress, and apoptosis in rat. Cell Mol Neurobiol 35(5):623–632
Dirks-Hofmeister ME, Verhaeghe T, De Winter K, Desmet T (2015) Creating space for large acceptors: rational biocatalyst design for resveratrol glycosylation in an aqueous system. Angew Chem Int Ed 54:9289–9292
Dolai S, Shi W, Corbo C, Sun C, Averick S, Obeysekera D, Farid M, Alonso A, Banerjee P, Raja K (2011) “Clicked” sugar − curcumin conjugate: modulator of amyloid-β and tau peptide aggregation at ultralow concentrations. Chem Neurosci 2:694–699
Fang M, Yuan Y, Lu J, Li H, Zhao M, Ling E, Wu C (2016) Scutellarin promotes microglia-mediated astrogliosis coupled with improved behavioral function in cerebral ischemia. Neurochem Int [epub ahead of print]
Fang M, Yuan Y, Rangarajan P, Lu J, Wu Y, Wang H, Wu C, Ling E (2015) Scutellarin regulates microglia-mediated TNC1 astrocytic reaction and astrogliosis in cerebral ischemia in the adult rats. BMC Neurosci 16:84
Furuta T, Kimura T, Kondo S, Mihara H, Wakimoto T, Nukaya H, Tsuji K, Tanaka K (2004) Concise total synthesis of flavone C-glycoside having potent anti-inflammatory activity. Tetrahedron 60:9375–9379
Gao C, Chen X, Zhong D (2011) Absorption and disposition of scutellarin in rats: a pharmacokinetic explanation for the high exposure of its isomeric metabolite. Drug Metab Dispos 39(11):2034–2044
Guimarães C, Oliveira D, Valdevite M, Saltoratto A, Pereira S, França S, Pereira A, Pereira P (2015) The glycosylated flavonoids vitexin, isovitexin, and quercetrin isolated from Serjania erecta Radlk (Sapindaceae) leaves protect PC12 cells against amyloid-β25-35 peptide-induced toxicity. Food Chem Toxicol 86:88–94
Guo Y, Zhao Y, Zheng C, Meng Y, Yang Y (2010) Synthesis, biological activity of salidroside and its analogues. Chem Pharm Bull 58(12):1627–1629
Hamada H, Shimoda K, Shimizy N, Akagi M (2014) Synthesis of glycosides of resveratrol, pterostilbene, and piceatannol by glucosyltransferase from phytolacca americana expressed in bacillus subtilis and their chemopreventive activity against cancer, allergic, and alzheimer’s diseases. Glycobiol Insights 4:1–6
Hamilton M, Caulfield J, Pickett J, Hooper A (2009) C-Glucosylflavonoid biosynthesis from 2-hydroxynaringenin by Desmodium uncinatum (Jacq.)(Fabaceae). Tetrahedron Lett 50:5656–5659
Hao B, Caulfield J, Hamilton M, Pickett J, Midega C, Khan Z, Wang J, Hooper A (2016) Biosynthesis of natural and novel C-glycosylflavones utilising recombinant Orzya sativa C-glycosyltransferase (OsCGT) and Desmodium incanum root proteins. Phytochemistry 125:73–87
Huang J, Weng W, Huang X, Ji Y, Chen E (2005) Pharmacokinetics of scutellarin and its aglycone conjugated metabolites in rats. Eur J Drug Metab Pharmacokinet 30(3):165–170
Ishikura Y, Tsuji K, Nukaya H (2002) Novel derivative of flavone C-glycoside and composition containing the same. WO 2004005296
Jesus A, Dias C, Matos A, Almeida R, Viana A, Marcelo F, Ribeiro R, Macedo M, Airoldi C, Nicotra F, Martins A, Cabrita E, Jiménez-Barbero J, Rauter A (2014) Exploiting the therapeutic potential of 8-β-d-glucopyranosylgenistein: synthesis, antidiabetic activity, and molecular interaction with islet amyloid polypeptide and amyloid β-peptide (1–42). J Med Chem 57(22):9463–9476
Jung H, Karki S, Kim J, Choi J (2015) BACE1 and cholinesterase inhibitory activities of Nelumbo nucifera embryos. Acta Pharm Res 38(6):1178–1187
Jung M, Park M (2007) Acetylcholinesterase inhibition by flavonoids from Agrimonia pilosa. Molecules 12(9):2130–2139
Kawada T, Asano R, Hayashida S, Sakuno T (1999) Total synthesis of the phenylpropanoid glycoside, acteoside. J Org Chem 64:9268–9271
Kerscher F, Franz G (1986) Biosynthesis of vitexin and isovitexin: enzymatic synthesis of the C-glucosylflavones vitexin and isovitexin with an enzyme preparation from Fagopyrum esculentum M. seedlings. Z. Naturforsch. 42c:519–524
Kishida M, Akita H (2005) Synthesis of rosavin and its analogues based on a mizoroki-heck type reaction. Tetrahedron Asymmetry 16:2625–2630
Koekkoek P, Kappelle L, Van den Berg E, Rutten G, Biessels G (2015) Cognitive function in patients with diabetes mellitus: guidance for daily care. Lancet Neurol 14(3):329–340
Komentani T, Kondo H, Fujimori Y (1988) Boron trifluoride-catalyzed rearrangement of 2-aryloxytetrahydropyrans: a new entry to C-arylglycosidation. Synthesis 1005–1007
Kumazawa T, Kimura T, Matsuba S, Sato S, Onodera J (2011) Synthesis of 8-C-glucosylflavones. Carbohydr Res 334(3):183–193
Kumazawa T, Minatogawa T, Matsuba S, Sato S, Onodera J (2000) An effective synthesis of isoorientin: the regioselective synthesis of a 6-C-glucosylflavone. Carbohydr Res 329(3):507–513
Kurisu M, Miyamae Y, Murakami K, Han J, Isoda H, Irie K, Shigemori H (2013) Inhibition of amyloid β aggregation by acteoside, a phenylethanoid glycoside. Biosci Biotechnol Biochem 77(6):1329–1332
Ladiwala A, Mora-Pale M, Lin J, Bale S, Fishman Z, Dordick J, Tessier P (2011) Polyphenolic glycosides and aglycones utilize opposing pathways to selectively remodel and inactivate toxic oligomers of amyloid β. ChemBioChem 12(11):1749–1758
Law B, Ling A, Koh R, Chye S, Wong Y (2014) Neuroprotective effects of orientin on hydrogen peroxide–induced apoptosis in SH-SY5Y cells. Mol Med Rep 9(3):947–954
Lee D, Zhang W, Karnati V (2003) Total synthesis of puerarin, an isoflavone C-glycoside. Tetrahedron Lett 44(36):6857–6859
Li J, Wang G, Liu J, Zhou L, Dong M, Wang R, Li X, Li X, Lin C, Niu Y (2010) Puerarin attenuates amyloid-beta-induced cognitive impairment through suppression of apoptosis in rat hippocampus in vivo. Eur J Pharmacol 649(1–3):195–201
Li N, Shen M, Wang Z, Tang Y, Shi Z, Fu Y, Shi Q, Tang H, Duan J (2013) Design, synthesis and biological evaluation of glucose-containing scutellarein derivatives as neuroprotective agents based on metabolic mechanism of scutellarin in vivo. Bioorg Med Chem Lett 23(1):102–106
Lin F, Xie B, Cai F, Wu G (2012) Protective effect of Puerarin on β-amyloid-induced neurotoxicity in rat hippocampal neurons. Arzneimittelforschung 62(4):187–193
Liu X, Mo Y, Gong J, Li Z, Peng H, Chen J, Wang Q, Ke Z, Xie J (2016) Puerarin ameliorates cognitive deficits in streptozotocin-induced diabetic rats. Metab Brain Dis 31(2):417–423
Li XD, Kang ST, Li X, Wang JH (2011) Synthesis of some Phenylpropanoid Glycosides (PPGs) and their acetylcholinesterase/xanthine oxidase inhibitory activities. Molecules 16:3580–3596
Li Z, Shangguan Z, Liu Y, Wang J, Li X, Yang S, Liu S (2014) Puerarin protects pancreatic β-cell survival via PI3 K/Akt signaling pathway. J Mol Endocrinol 53(1):71–79
Li Y, Biao Y, Sun J, Wang R (2015) Efficient synthesis of baicalin and its analogs. Tetrahedron Lett 56:3816–3819
Lozoya X, Meckes M, Abou-Zaid M, Tortoriello J, Nozzolillo C, Arnason J (1994) Quercetin glycosides in Psidium guajava L. leaves and determination of a spasmolytic principle. Arch Med Res 25(1):11–15
Mahling J, Jung K, Schmidt R (1995) Synthesis of flavone C-glycosides vitexin, isovitexin and isoembigenin. Leibigs Ann Chem 461–466
Masibo M, He Q (2008) Major mango polyphenols and their potential significance to human health. CRFSFS 7(4):309–319
Mathew S, Hedström M, Adlercreutz P (2012) Enzymatic synthesis of piceid glycosides by cyclodextrin glucanotransferase. Process Biochem 47:528–532
Mishra S, Palanivelu K (2006) The effect of curcumin (turmeric) on Alzheimer’s disease: An overview. Life Sci 78(18):2081–2087
Mulani SK, Guh JH, Mong KKT (2014) general synthetic strategy and the anti-proliferation properties on prostate cancer cell lines for natural phenylethanoid glycosides. Org Biomol Chem 12:2926–2937
Noorafshan A, Ashkani-Esfahani S (2013) A review of therapeutic effects of curcumin. Curr Pharm Des 19(11):2032–2046
Orsini F, Pelozzoni F, Bellini B, Miglierini G (1997) Synthesis of biologically active polyphenolic glycosides (combretastatin and resveratrol series). Carb Res 301:95–109
Pan Z, Feng T, Shan L, Cai B, Chu W, Niu H, Lu Y, Yang B (2008) Scutellarin-induced endothelium-independent relaxation in rat aorta. Phytother Res 22(11):1428–1433
Park H, Kim J, Choi KH, Hwand S, Yang SJ, Baek NI, Cha J (2012) Enzymatic synthesis of piceid glucosides using maltosyltransferase from caldicellulosiruptor bescii DSM 6725. J Agric Food Chem 60:8183–8189
Prasad S, Tyagi AK, Aggarwal BB (2014) Recent developments in delivery, bioavailability, absorption and metabolism of curcumin: the golden pigment from golden spice. Cancer Res Treat 46(1):2–18
Qiao W, Zhao C, Qin N, Zhai H, Duan H (2011) Identification of trans-tiliroside as active principle with anti-hyperglycemic, anti-hyperlipidemic and antioxidant effects from Potentilla chinesis. J Ethnopharmacol 135(2):515–521
Rege SD, Geetha T, Griffin GD, Broderick TL, Babu JR (2014) Neuroprotective effects of resveratrol in Alzheimer disease pathology. Front Aging Neurosci 6(1):1–12
Ren G, Hou J, Fang Q, Sun H, Liu X, Zhang L, Wang P (2012) Synthesis of flavonol 3-O-glycoside by UGT78D1. Glycoconj J 29(5–6):425–432
Renaud S, Lorgeril M (1992) Wine, alcohol, platelets, and the French paradox for coronary heart disease. Lancet 339(8808):1523–1526
Rivière C, Richard T, Quentin L, Krisa S, Mérillon JM, Monti JP (2007) Inhibitory activity of stilbenes on Alzheimer’s β-amyloid fibrils in vitro. Bioorg Med Chem 15:1160–1167
Robertson A, Robinson R (1926) Experiments on the synthesis of anthocyanins. Part I. J Chem Soc 129:1713–1720
Roupe KA, Remsberg CM, Yáñez JA, Davies NM (2006) Pharmacometrics of stilbenes: guing towards the Clinic. Curr Clin Pharmacol 1:81–101
Santos R, Jesus A, Caio J, Rauter A (2011) Fries-type reactions for the C-glycosylation of phenols. Curr Org Chem 15:128–148
Sato D, Shimizu N, Shimizu Y, Akagi M, Eshita Y, Ozaki S, Nakajima N, Ishihara K, Sato D, Shimizu N, Shimizu Y, Akagi M, Eshita Y, Ozaki S-I, Nakajima N, Ishihara K, Masuoka N, Hamada H, Shimoda K, Kubota N (2014) Synthesis of glycosides of resveratrol, pterostilbene, and piceatannol, and their anti-oxidant, anti-allergic, and neuroprotective activities. Biosci Biotech Biochem 78:1123–1128
Shi T, Chen H, Jing L, Liu X, Sun X, Jiang R (2011) Development of a kilogram-scale synthesis of salidroside and its analogs. Synth Commun 41:2594–2600
Shi LF, Cai Z, Yao B (2004) Preparation of salidroside derivatives as antioxidants. Faming Zhuanli Shenqing Gongkai Shuomingshu. CN Patent 1475492 A 20040218
Tang H, Tang Y, Li N, Shi Q, Guo J, Shang E, Duan J (2014) Neuroprotective effects of scutellarin and scutellarein on repeatedly cerebral ischemia-reperfusion in rats. Pharmacol Biochem Behav 118:51–59
Torres P, Poveda A, Jimenez-Barbero J, Parra JL, Comelles F, Ballesteros AO, Plou FJ (2011) Enzymatic Synthesis of α-Glucosides of Resveratrol with surfactant activity. Adv Synth Catal 353:1077–1086
Uriarte-Pueyo I, Calvo M (2011) Flavonoids as acetylcholinesterase inhibitors. Curr Med Chem 18(34):5289–5302
Velagapudi R, Aderogba M, Olajide O (2014) Tiliroside, a dietary glycosidic flavonoid, inhibits TRAF-6/NF-κB/p38-mediated neuroinflammation in activated BV2 microglia. Biochim Biophys Acta 1840(12):3311–3319
Vermes B, Chari V, Wagner H (1981) Structure elucidation and synthesis of flavonol acylglycosides. The synthesis of tiliroside. Helv Chim Acta 64(6):1964–1967
Walle T (2011) Bioavailability of resveratrol. Ann NY Acad Sci 1215:9–15
Wang HQ, Xu YX, Zhu CK (2012) Upregulation of heme oxygenase-1 by acteoside through ERK and PI3 K/Akt pathway confer neuroprotection against beta-amyloid-induced neurotoxicity. Neurotoxic Res 21:368–378
Wang HQ, Xu YX, Yan J, Zhao XY, Sun XB, Zhang YP, Guo JC, Zhu CK (2009) Acteoside protects human neuroblastoma SH-SY5Y cells against beta-amyloid-induced cell injury. Brain Res 1283:139–147
Wang C, Li J, Zhang L, Zhang X, Yu S, Liang X, Ding F, Wang Z (2013) Isoquercetin protects cortical neurons from oxygen-glucose deprivation-reperfusion induced injury via suppression of TLR4-NF-кB signal pathway. Neurochem Int 63(8):741–749
Wang C, Shi Y, Tang M, Zhang X, Gu Y, Liang X, Wang Z, Ding F (2016) Isoquercetin ameliorates cerebral impairment in focal ischemia through anti-oxidative, anti-inflammatory, and anti-apoptotic effects in primary culture of rat hippocampal neurons and hippocampal CA1 region of rats. Mol Neurobiol [epub ahead of print]
Wang C, Xie N, Zhang H, Li Y, Wang Y (2014) Puerarin protects against β-amyloid-induced microglia apoptosis via a PI3 K-dependent signalling pathway. Neurochem Res 39(11):2189–2196
Wang Y, Zhen Y, Wu X, Jiang Q, Li X, Chen Z, Zhang G, Dong L (2015) Vitexin protects brain against ischemia/reperfusion injury via modulating mitogen-activated protein kinase and apoptosis signalling in mice. Phytomedicine 22(3):379–384
Wen H, Lin C, Que L, Ge H, Ma L, Cao R, Wan Y, Peng W, Wang Z, Song H (2008) Synthesis and biological evaluation of helicid analogues as novel acetylcholinesterase inhibitors. Eur J Med Chem 43:166–173
Wenzel E, Somoza V (2005) Metabolism and bioavailability of trans-resveratrol. Mol Nutr Food Res 49:472–481
Wu K, Liang T, Duan X, Xu L, Zhang K, Li R (2013) Anti-diabetic effects of puerarin, isolated from Pueraria lobata (Willd.), on streptozotocin-diabetogenic mice through promoting insulin expression and ameliorating metabolic function. Food Chem Toxicol 60:341–347
Xiong J, Wang C, Chen H, Hu Y, Tian L, Pan J, Geng M (2014) Aβ-induced microglial cell activation is inhibited by baicalin through the JAK2/STAT3 signaling pathway. Int J Neurosci 124(8):609–620
Yu L, Wang S, Chen X, Yang H, Li X, Xu Y, Zhu X (2015) Orientin alleviates cognitive deficits and oxidative stress in Aβ1-42-induced mouse model of Alzheimer's disease. Life Sci 121:104–109
Zhang B, Wang Y, Li H, Xiong R, Zhao Z, Chu X, Li Q, Sun S, Chen S (2016) Neuroprotective effects of salidroside through P13 K/Akt pathway activation in Alzheimer’s disease models. Drug Des Dev Ther 10:1335–1343
Zhang H, Liu Y, Wang H, Xu J, Hu H (2008) Puerarin protects PC12 cells against beta-amyloid-induced cell injury. Cell Biol Int 32(10):1230–1237
Zhang J, Guo W, Tian B, Sun M, Li H, Zhou L, Liu X (2015) Puerarin attenuates cognitive dysfunction and oxidative stress in vascular dementia rats induced by chronic ischemia. Int J Clin Exp Pathol 8(5):4695–4704
Zhang L, Yu H, Zhao X, Lin X, Tan C, Cao G, Wang Z (2010) Neuroprotective effects of salidroside against beta-amyloid-induced oxidative stress in SH-SY5Y human neuroblastoma cells. Neurochem Int 57(5):547–555
Zhao S, Yang W, Jin H, Ma K, Feng G (2015) Puerarin attenuates learning and memory impairments and inhibits oxidative stress in STZ-induced SAD mice. Neurotoxicology 51:166–171
Zhou Y, Xie N, Li L2, Zou Y, Zhang X, Dong M (2014) Puerarin alleviates cognitive impairment and oxidative stress in APP/PS1 transgenic mice. Int J Neuropsychopharmacol 17(4):635–644
Zhu J, Choi R, Li J, Xie H, Bi C, Cheung A, Dong T, Jiang Z, Chen J, Tsim K (2009) Estrogenic and neuroprotective properties of scutellarin from Erigeron breviscapus: a drug against postmenopausal symptoms and Alzheimer's disease. Planta Med 75(14):1489–1493
Żmijewski M, Sokół-Łętowska A, Pejcz E, Orzeł D (2015) Antioxidant activity of rye bread enriched with milled buckwheat groats fractions. Rocz Panstw Zakl Hig 66(2):115–121
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 Springer International Publishing AG
About this chapter
Cite this chapter
Dias, C., Matos, A.M., Rauter, A.P. (2018). Chemical Approaches Towards Neurodegenerative Disease Prevention: The Role of Coupling Sugars to Phenolic Biomolecular Entities. In: Witczak, Z., Bielski, R. (eds) Coupling and Decoupling of Diverse Molecular Units in Glycosciences. Springer, Cham. https://doi.org/10.1007/978-3-319-65587-1_8
Download citation
DOI: https://doi.org/10.1007/978-3-319-65587-1_8
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-65586-4
Online ISBN: 978-3-319-65587-1
eBook Packages: Chemistry and Materials ScienceChemistry and Material Science (R0)