Abstract
Current electrophysiological monitoring is based on invasive electrodes or surface electrodes. Here, a surface electromyography (sEMG) electrode with self-similar serpentine configuration is designed to monitor biological signal. Such electrode can bear rather large deformation (such as >30%) under an appropriate areal coverage. And the electrode conformally attached on the skin surface via van del Waal interaction could furthest reduce the motion artifacts from the motion of skin. The capacitive electrodes that isolates the electrodes from the body also provide an effective way to minimize the leakage current. The sEMG electrodes have been used to record physiological signals from different parts of the body with sharp curvature, such as index finger, back neck and face, and they exhibit great potential in application of human-machine interface in the fields of robots and healthcare. Integrating wireless data transmission capabilities into the wearable sEMG electrodes would be studied in future for intelligent could healthcare platform.
W. Dong and C. Zhu contributed equally to this work.
Access provided by CONRICYT-eBooks. Download conference paper PDF
Similar content being viewed by others
Keywords
1 Introduction
Surface electromyography (sEMG) is a non-invasive technique for measuring electrical signals generated by the contraction of skeletal muscles [1, 2]. sEMG electrode that involves sensors placed on the skin, is exploited for many clinical and research purposes, ranging from diagnosing neuromuscular disorders, studying muscle pain [3, 4], and controlling prosthetic or orthotic devices [5,6,7,8,9]. The sEMG electrodes laminated onto the skin surface should satisfy large deformations and conformally contact with the skin surface for continuous electrophysiological (EP) monitoring.
Conventional capacitive sensors with rigid electrodes affixed on the skin with adhesive tapes for biological signal monitoring which limits mounting locations to large, relatively flat regions of the body, such as wrist and chest [10]. It also causes noise in the biopotential measurement as the motion artifacts due to the relative slippage at electrode-skin interface [1, 11]. Recently, epidermal electronic system with ultrathin, low-modulus, lightweight, stretchable substrate that conformably laminates onto the skin surface has been designed to overcome such problems [11,12,13,14]. And tremendous affords are developed to achieving stretchability for biological monitoring via designing proper materials and structures [12, 15]. Multifunctional epidermal electronics system conformally contact with the skin surface has also been proposed to record physiological signals, such as, temperature, pulse beating, strain, and sEMG, electrocardiograms (ECG) [16]. It is considered that an optimal parameters of the filamentary serpentine structure can significantly improve the performance of the sEMG electrode [12, 17]. sEMG electrode with self-similar serpentine interconnect is designed to satisfy larger deformation for the motion of skin as an EP sensor [18, 19]. However, beyond the deformation, the conformability of the capacitive electrode should be taken into consideration for the biological signals monitoring in the parts of the body with sharp curvature.
The capacitive electrode with self-similar serpentine structure is designed to monitor the biological signals, which could satisfy large deformability (>30%) and high areal coverage. The design of sEMG includes three parts: stretchability, conformability, and capacitive epidermal sensing system. The stretchability is analyzed by experiments and finite elements modelling (FEM), and the conformability is measured by the interfacial peeling experiment. The capacitive sensing electrodes conformally contact with the skin surface via van der Waals interaction alone. The capacitive electrodes are used to record physiological signals from different parts of human body with sharp curvature, such as index finger, back neck and face. It is also used to record the ECG signal from the chest.
2 Design and Fabrication Process
2.1 Principle of Capacitive Sensing Electrodes
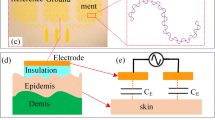
Figure 1(a) shows the optical image of the capacitive electrode conformally laminated onto the skin surface. The cross-section of sEMG electrode is shown in Fig. 1(b) with multi-layers. The sEMG electrode with the PDMS/PI/Au/PI multilayer structure guarantees the Au layer at the neutral plane of the sEMG for improving the stretchability of the sEMG electrodes. Figure 1(c) depicts the equal electrical model of capacitive electrode with a voltage follower. Higher signal gains are realized with higher input impedance and low-input capacitance to improve the signal quality of the sEMG electrodes. In the skin-electrode interface with PI dielectric layer, the capacitance is represented as C in = εS effective/h PI = εαS sEMG/h PI. SsEMG is the area of the electrode, α is the areal coverage of the electrode, h PI is the thickness of the dielectric layer PI. R B is a bias resistance between the ground and the input of the operational amplifier (op amp). R in and C in are the resistance and capacitance of the voltage follower. The resistance and capacitance circuit is used to depict the skin-electrode interface property. Both the stratum corneum (SC) and insulation layers are equivalent to a RC parallel component and therefore the transducing function are calculated by the equivalent skin/electrode model.
2.2 Fabrication
Figure 2 shows the main fabrication process of the sEMG electrode with mutli-layer structure. Figure 2(a) depicts the sEMG electrode is fabricated by microelectromechanical system (MEMS) techniques on a carrier wafer with polymethyl methacrylate (PMMA) sacrificial layer. The PDMS substrate on the glass substrate is used to pick up the sEMG electrode after the PMMA sacrificial layer is dissolved in acetone bathing shown in Fig. 2(b). The whole structure (PI/Au/PI) is patterned in stretchable form, released from the carrier Si wafer and transferred to the PDMS substrate. Figure 2(c) depicts the sEMG electrode is delaminated from the glass substrate with help of the polyvinyl alcohol (PVA) film which reduces the interfacial energy at the electrode/glass interface. Figure 2(d) shows the sEMG electrode is directly printed onto the surface of the skin. The electrode adheres onto the surface conformally after dissolving the PVA film in the water for sEMG signal recordings.
The electrical performance of the sEMG electrode mainly depends on material selection and geometry layout. Second order serpentine structure is adopted to improve the areal coverage and stretchability compared to the first order serpentine structure. The sEMG electrode with self-similar configuration is designed to monitor the EP signals for satisfying larger stretchability (>30%). The sEMG electrode with ultrathin construction and neutral mechanical plane configuration improves the stretchability and contact behavior. Figure 3(a) depicts optical graph of the sEMG designed to quantify biological signal measurement with reference, ground, and measurement electrodes with self-similar serpentine structure. The node connection of the unit cells in the network sEMG electrode forms triangular lattices. The line width of electrode is 50 μm. Figure 3(b) shows the local enlarged graph of the network sEMG electrode with hexagon structure with self-similar interconnect. Figure 3(c) depicts optical image of the self-similar serpentine structure. Figure 3(d) depicts the local enlarged graph of the electrode with one node. The SEM image of cross section of the sEMG electrode with PI/Au/PI layers is shown in Fig. 3(e) where PMMA/PI (~1.8 μm), Au (~0.3 μm), and PI (~1.2 μm). Capacitive sEMG electrode is designed to measure the EP signals between the electrode-skin interfaces with PI polymer dielectric layer.
(a) Optical image of the capacitive EMG electrode; (b) Local graph of the electrode with one regular hexagon formed by self-similar second order serpentine structure; (c) Optical image of the self-similar serpentine structure; (d) Enlarged image of electrode with one node; (e) SEM image of cross section of the electrode with multi-layers structure onto the carrier wafer.
3 Mechanical and Electrical Performances
3.1 Conformability
Conformal contact at the electrode-skin interface can improve the accuracy of EP signal measurement. It reduces the motion artifact due to the movements of skin. The sEMG electrode with self-similar structure adheres onto the surface of the skin driven only by van der Waals interactions. The sEMG electrode mounts in this way exhibit excellent compliance and ability to follow skin motions without constraint or delamination, which satisfies large deformation of the skin. The total energy of the epidermal device adhered onto skin consists of bending energy and membrane energy of sEMG electrode, elastic energy of the skin and the device-skin interfacial adhesion energy. Figure 4(a) and (b) show the device adhered onto the surface of the skin in the compressed and stretched form respectively. Figure 4(c) depicts the sEMG electrode is delaminated from the surface of the skin for recycling and reusing the electrodes. The sEMG electrode is in the twisted form shown in Fig. 4(d). It shows that the sEMG electrode with low bending stiffness (thin, soft electrode/backing layer), smooth and soft skin, and strong adhesion all promote conformal contact.
3.2 Stretchability Analysis
The sEMG electrode with self-similar configuration satisfies large deformation of the skin surface. The stretchability of the sEMG electrode is evaluated quantitatively by FEM using the commercial package Abaqus 6.10. Figure 5(a) shows the 40% stretched state of the sEMG electrode by FEM simulation. The optical image of the electrode in the stretched format are shown in Fig. 5(b). Comparing the node parts Fig. 5(a1) and (b1), the deformation format of the optical image of the electrode is similar the FEM simulation results. Figure 5(a2) and (b2) show the distribution of maximum principal strain appear at the largest curvature of the microstructure. It is seen that the experimental results agree well with the FEM simulation results, which shows the patterned electrode structure satisfies 40% stretched deformation.
3.3 Electrical Performance
The sEMG electrode with self-similar structure laminated onto the skin surface forms a capacitance with PI dielectric layer [20]. The stretchable electrode reduces the contact impedance as it contact with skin surface conformally to increase the contact area. Capacitive electrodes with PI insulating layers exhibit higher stable gain over the frequency range 20–10000 Hz, due primarily to their relatively high CE. Figure 6(a) depicts the skin-electrode interface contact impedance decreases with the increasing scanning frequency. The blue line is measured with the area 40 mm2. Figure 6(b) depicts the leakage current from the capacitive electrodes is rather small compared to the conventional medical biological electrodes. It shows that the currents below 0.2 mA when 10 mA passes through the electrodes. It is helpful to protect the soft biological tissues during biological signal monitoring.
4 Biological Signal Recordings
The electromyography signals are measured by the capacitive sEMG electrode adhered onto the skin surface. To minimize the motion artifact during movement, the electrodes should maintain conformal contact with the skin in case that external load applied to the epidermal biological electrode. A customized PCB is used to collect the sEMG data from human body. It yields analog data, converted to digital signals for recording using LabView software (National Instruments, USA) and analysis using Matlab (The Mathworks, Inc., Natick, MA). Photograph of the electrode on the wrist appears with different actions in Fig. 7(a) clenched; (b) half clenched; (c) loosened. Enlarged photograph of the electrode on the wrist appears in Fig. 7(a). The corresponding biological signals are shown in Fig. 7(d). It has potential in clinics for diagnosis of neuromuscular disorders.
Figure 8 shows the physiological signals measurement of the capacitive electrode from different parts of human body, such as wrist, index finger and face with different motion of body. Capacitive electrode is even wrapped onto the finger, where bending creates high-quality sEMG signals, in spite of the relatively small area of the corresponding muscle Fig. 8(a) illustrates the stretchable capacitive electrodes for measuring sEMG signals on the forehead. The corresponding sEMG signals from the stretchable capacitive electrode are shown in Fig. 8(b) with frown and blink actions. Figure 8(c) shows the electrode laminated onto the index finger, as an example of a location where conventional gel-based electrode is not acceptable. And the corresponding sEMG signals when bending and unbending the finger in Fig. 8(d). The capacitive sEMG electrode laminated onto the surface of face shown in Fig. 8(e). Figure 8(f) depicts clenching the jaw, smiling, and moving the mouth create different types of sEMG signals, each of which is clearly distinguishable from the baseline noise. The biological signals from the parts are clearly distinguishable from the baseline noise, which demonstrated in these experiments suggests many possibilities in human-machine interface (HMI).
The measurements involve contact of the electrode laminated onto the chest for ECG signals recordings (Fig. 9(a)). The ECG signals are plotted in Fig. 9(b). Figure 9(c) shows the P wave, the QRS complex and the T wave are clearly defined from the capacitive electrode.
5 Conclusion
The work reported here illustrates advantages in EP measurements that follow from the stretchable capacitive electrode with self-similar serpentine structure, which could satisfy larger deformation (>30%) validated by FEM simulation and experiments. The resulting electrodes offer enhanced levels of wearability, expanded options in electrodes sterilization and reuse, and minimized artifacts from body motions compared to previously reported technologies. The sEMG electrode with thin, soft substrate is conformal contact with the surface of the skin via van del Waal interaction alone, and it reduced the motion artifacts caused by the motion of the skin. The EP measurements from different parts of body were gotten by the capacitive sEMG electrodes. Exploring these options biological signal monitoring and human machine interface capabilities into the capacitive sEMG electrode represents promising directions for future research in intelligent cloud healthcare.
References
Sun, Y., Yu, X.B.: Capacitive biopotential measurement for electrophysiological signal acquisition: a review. IEEE Sens. J. 16(9), 2832–2853 (2016)
Lopez, A., Richardson, P.C.: Capacitive electrocardiographic and bioelectric electrodes. IEEE Trans. Biomed. Eng. 16(1), 99 (1969)
Liao, L.D., et al.: Design, fabrication and experimental validation of a novel dry-contact sensor for measuring electroencephalography signals without skin preparation. Sensors (Basel) 11(6), 5819–5834 (2011)
Al-Ajam, Y., et al.: The use of a bone-anchored device as a hard-wired conduit for transmitting EMG signals from implanted muscle electrodes. IEEE Trans. Biomed. Eng. 60(6), 1654–1659 (2013)
Jeong, J.W., et al.: Materials and optimized designs for human-machine interfaces via epidermal electronics. Adv. Mater. 25(47), 6839–6846 (2013)
Lim, S., et al.: Transparent and stretchable interactive human machine interface based on patterned graphene heterostructures. Adv. Funct. Mater. 25(3), 375–383 (2015)
Xu, B., et al.: An epidermal stimulation and sensing platform for sensorimotor prosthetic control, management of lower back exertion, and electrical muscle activation. Adv. Mater. 28(22), 4462–4471 (2015)
Pan, L., et al.: Improving myoelectric control for amputees through transcranial direct current stimulation. IEEE Trans. Biomed. Eng. 62(8), 1927–1936 (2015)
He, J., et al.: Invariant surface EMG feature against varying contraction level for myoelectric control based on muscle coordination. IEEE J. Biomed. Health Inf. 19(3), 874–882 (2015)
Mercer, J.A., et al.: EMG sensor location: does it influence the ability to detect differences in muscle contraction conditions? J. Electromyogr. Kinesiol. 16(2), 198–204 (2006)
Jeong, J.W., et al.: Capacitive epidermal electronics for electrically safe, long-term electrophysiological measurements. Adv. Healthc. Mater. 3(5), 642–648 (2014)
Kim, D.H., et al.: Epidermal electronics. Science 333(6044), 838–843 (2011)
Kim, J., et al.: Next-generation flexible neural and cardiac electrode arrays. Biomed. Eng. Lett. 4(2), 95–108 (2014)
Huang, X., et al.: Epidermal impedance sensing sheets for precision hydration assessment and spatial mapping. IEEE Trans. Biomed. Eng. 60(10), 2848–2857 (2013)
Hammock, M.L., et al.: 25th anniversary article: the evolution of electronic skin (e-skin): a brief history, design considerations, and recent progress. Adv. Mater. 25(42), 5997–6038 (2013)
Yeo, W.H., et al.: Multifunctional epidermal electronics printed directly onto the skin. Adv. Mater. 25(20), 2773–2778 (2013)
Viventi, J., et al.: A conformal, bio-interfaced class of silicon electronics for mapping cardiac electrophysiology. Sci. Trans. Med. 2(24), 24ra22 (2010)
Jang, K.I., et al.: Soft network composite materials with deterministic and bio-inspired designs. Nat. Commun. 6, 6566 (2015)
Fan, J.A., et al.: Fractal design concepts for stretchable electronics. Nat. Commun. 5(2), 3266 (2014)
Wang, L.-F., et al.: PDMS-based low cost flexible dry electrode for long-term EEG measurement. IEEE Sens. J. 12(9), 2898–2904 (2012)
Acknowledgement
The authors acknowledge supports from the National Natural Science Foundation of China (51322507, 51635007).
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 Springer International Publishing AG
About this paper
Cite this paper
Dong, W., Zhu, C., Wang, Y., Xiao, L., Ye, D., Huang, Y. (2017). Stretchable sEMG Electrodes Conformally Laminated on Skin for Continuous Electrophysiological Monitoring. In: Huang, Y., Wu, H., Liu, H., Yin, Z. (eds) Intelligent Robotics and Applications. ICIRA 2017. Lecture Notes in Computer Science(), vol 10464. Springer, Cham. https://doi.org/10.1007/978-3-319-65298-6_8
Download citation
DOI: https://doi.org/10.1007/978-3-319-65298-6_8
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-65297-9
Online ISBN: 978-3-319-65298-6
eBook Packages: Computer ScienceComputer Science (R0)