Abstract
Bacterial and fungal infections continue to be an unwelcome complication of the treatment of patients with hematological malignancies despite much progress in the oncologic management in recent years. Some new treatment advances and modalities have offered hope to entire new patient populations, but have also opened the door for opportunistic and invasive organisms. Developments in antimicrobial agents, diagnostic methods, and infection control practices have unfortunately not been able to keep up with rapid developments in oncologic therapies nor antimicrobial resistance. Understanding and control of risk factors for antineoplastic therapy-associated immune-suppression, antimicrobial mechanisms and effects, and changing microbial characteristics are key factors to prevention and treatment of bacterial and fungal infection in this complex patient population. This chapter aims to provide a solid understanding of all these principles and factors to enable educated decision-making, considering guidelines and evidence when these exist, and rational recommendations when evidence is lacking for a particular topic.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
- Febrile neutropenia
- Neutropenic fever
- Bacterial infection
- Fungal infection
- Bacteremia
- Fungemia
- Immune-compromised host
- Infections in hematological malignancy
Introduction
The treatment of hematological malignancies relies heavily on cytotoxic chemotherapy that most often places patients at risk for invasive bacterial and fungal infections due to disruptions in mucosal barrier integrity and impaired myelopoiesis. A weakened mucosal barrier permits translocation of fungi and bacteria present in the oropharynx, gastrointestinal tract, or skin to enter the body and, when this is combined with immune compromise from impaired myelopoiesis, severe infections are not uncommon. Antineoplastic therapy-associated neutropenia frequently results in a muted inflammatory response, with fever often being the sole presenting symptom. In addition to cytotoxic antineoplastic therapy, these patients often receive concomitant glucocorticoids which may altogether blunt even a febrile response [1]. This necessitates a high index of suspicion on the part of the clinician to effectively recognize infection in the neutropenic patient who might only present with hypotension or tachycardia with or without other nonspecific findings. Also known as neutropenic fever (these terms will be used interchangeably throughout this chapter), this clinical entity is defined as a single oral temperature measurement of 38.3 °C, or a temperature greater than 38.0 °C that is sustained for more than an hour in a neutropenic patient [2,3,4]. The generally accepted definition of neutropenia is an absolute neutrophil count (ANC) of <1500 cells/μL. Severe neutropenia is defined as an ANC of <500 cells/μL, or when there is an expected ANC nadir of <500 cells/μL within the next 48 h.
Bacterial Infections
The pathogenesis of bacterial and fungal infections in the patient with hematologic malignancy is a complex interplay between host factors, effects of antineoplastic therapy, and changes in the host caused by the underlying malignancy (Table 50.1). As mentioned in the introduction to this chapter, the impairment of mucosal barriers and immune system dysfunction create an environment favorable to invasive microbial infections that is often compounded by frequent healthcare exposure and patients’ inherent increased risk for exposure to organisms of increased virulence and drug resistance [2]. It is believed that the majority of infections in patients with hematologic malignancies are due to treatment-related mucositis and translocation of gastrointestinal tract flora and colonizing organisms that eventually reach the bloodstream. Less common mechanisms include disruption of respiratory, genitourinary, and lymphatic barriers due to the underlying malignancy or associated with medical procedures. Of particular concern are the presence of central venous catheters (CVCs) and other indwelling catheters [5].
Epidemiology
Bacterial infection is a common cause for febrile neutropenia in patients who have received antineoplastic chemotherapy , with approximately 23% of them presenting with fever or neutropenic fever [6]. Of patients who present with febrile neutropenia, an infectious etiology is only identified in roughly one-quarter of cases, and often only through isolation of an organism causing bacteremia, which is found in up to 25% of all patients with neutropenic fever [2, 7]. Of note, the vast majority of organisms causing infections in hematological cancer patients are bacteria, followed by fungi and viruses as distant second and third place culprits. Given the principal factors that predispose to febrile neutropenia (disruption of mucosal barriers and immune suppression), translocation and infection by saprophytic flora explain the overwhelming predominance of these organisms isolated from cultures [8, 9].
The most commonly isolated organisms tend to be Gram positive bacterial organisms, with a predominance of Staphylococcus epidermidis , followed by a variety of other streptococci and staphylococci including Staphylococcus aureus [10, 11]. There has been a significant shift in the patterns of causative bacterial pathogens over time due to a variety of factors including antibiotic pressure from prophylaxis and/or treatment, the increased use of long-term indwelling catheters, and novel antineoplastic regimens [12]. Prior to the early 1990s, the majority of organisms isolated during evaluation of episodes of febrile neutropenia were Gram negative bacteria, with Pseudomonas aeruginosa being especially notorious [13, 14]. This trend began to turn gradually, with a growing predominance of Gram positives being most commonly isolated in the mid-1990s and into the early 2000s, when Gram negative prophylaxis was common. Approximately 80% of all isolates were Gram positive bacterial organisms [15, 16]. Today a reversal in this trend is being noted, this time the cause for the trend starting to turn back to Gram negative bacterial organisms seems to be related to the emergence of multidrug-resistant organisms (MDROs). MDRO groupings commonly referred to in practice include Gram positive bacteria [methicillin-resistant Staphylococcus aureus (MRSA), vancomycin-resistant enterococci (VRE) ], as well as Gram negative bacteria [extended spectrum beta lactamase (ESBL) , Klebsiella pneumoniae carbapenemase (KPC), and carbapenem-resistant enterobacteriaceae (CRE)]. Currently the overall percentage of infections due Gram positive bacterial organisms has decreased to around 60% [17,18,19,20,21]. Another important point is that outside of bloodstream infections, where Gram positive bacterial organisms are predominant, Gram negative bacterial organisms are the most common pathogens of infections related to the urinary, gastrointestinal, and biliary tracts [22].
The severity of bacterial infections varies due to a number of factors specific to each organism. Among the Gram positive bacterial organisms, MRSA and VRE are some of the most severe [16]. Though infrequent, anaerobes can contribute to severe, life-threatening infections such as necrotizing fasciitis, typhlitis (neutropenic enterocolitis), and sinusitis. Interestingly, though still rare, polymicrobial infections also appear to be becoming more frequent than in prior decades [19, 23, 24].
Risk Factors
Cancer patients undergoing systemic chemotherapy for hematologic malignancies often suffer significant side effects, especially effects on mucosal integrity and myeloid production and function. These will predispose to the development of infections due to translocation of gastrointestinal tract colonizing bacteria and fungi. Concomitant neutropenia results in a blunted immune response to invasion by previously colonizing organisms [25]. A thorough evaluation of the host characteristics, risk for developing febrile neutropenia, and risk assessment for serious complications associated with febrile neutropenia will guide empiric antimicrobial therapy and dictate additional workup and need for hospital admission [2]. Risk factors associated with an increased risk of developing neutropenic fever are best thought of in terms of host factors, factors related to the underlying malignancy, and those related to the antineoplastic therapies (Table 50.1). Host factors include the patients’ underlying conditions and comorbidities that may alter their immune function (e.g., drug-induced neutropenia will predispose to bacterial and candidal infections, underlying cellular immunodeficiency will predispose to opportunistic viral infections, and underlying humoral immunodeficiency will predispose to mycobacterial infections), those that predispose to infection due to anatomic and or functional abnormalities (vesicoureteral reflux, nephrolithiasis, bronchiectasis, etc.) and those that alter patients’ flora (prolonged antibiotic use that causes selective pressure favoring more invasive or resistant organisms). Treatment-related factors include mucosal barrier disruption due to cytotoxic therapy’s effect on high turnover cells of the lining the gastrointestinal tract, impaired myelogenous production, and decreased neutrophil phagocytic and chemotactic activity.
Patients with hematologic malignancy presenting with neutropenic fever should be risk stratified according to their risk of developing serious complications (Table 50.2), which in turn guides the need for hospital admission, parenteral antimicrobial therapy, and prolonged hospitalization (Fig. 50.1).
Diagnosis
The clinical manifestations of bacterial infections in the patient with hematological malignancies can be very nonspecific due to impaired inflammatory processes. Though much emphasis so far in this chapter has been placed on febrile neutropenia, it is not uncommon for these patients to present with nonspecific findings and be infected despite the absence of fever, or conversely to have a fever as the sole presenting symptom. For the hematological malignancy patient presenting with neutropenic fever, or thought to be otherwise infected, it is imperative to conduct a rapid and thorough workup that focuses on the prompt collection of cultures, especially blood cultures, to enable rapid initiation of broad spectrum empiric antibiotic therapy without compromising their diagnostic yield. The sooner appropriate empiric antimicrobial therapy is initiated the better, as delays in administration correlate with mortality [26, 27].
A thorough history with particular emphasis on pertinent host, underlying malignancy, and antineoplastic therapy factors should be performed in addition to eliciting a listing of careful relevant exposures in the context of the aforementioned factors. The physical exam should be a meticulous head-to-toe examination focused on the skin and mucosal surfaces, oropharynx and teeth, sinuses, heart and lung systems; abdominal, genital and perineal exams should not be overlooked. Peripherally Inserted Central Catheters (PICCs), implanted ports, urinary catheters, old IV sites, procedural sites such as biopsy, aspirate and surgical sites also merit careful visual inspection and palpation. The blunted immune response can manifest in a paucity of inflammatory signs and requires a high index of suspicion.
Peripheral blood counts with white blood cell (WBC) differentials should be obtained as well as at least two sets of blood cultures from the outset when infection is suspected. The hematologic tests serve to both quantify and stratify the presence and severity of neutropenia, while the blood cultures aim to confirm an infection and identify the causative organism and subsequently determine its antimicrobial susceptibility patterns to tailor anti-infective therapy. When obtaining blood cultures, one should ideally obtain two sets of cultures of 20 mL each in the adult patient. These should be collected from two separate peripheral venipunctures in patients without indwelling venous catheters. In patients with indwelling venous catheters, one set should be from a peripheral venipuncture and one from the indwelling venous catheter. The frequency of subsequent cultures varies depending on the following variables: if fever persists after 24 h on appropriate broad spectrum anti-infective therapy, an additional 2 sets of blood cultures should be obtained and may be repeated a second time after 48 h in the presence of persistent fever. If the fever appears to have defervesced for greater than 48 h and recurs, it is reasonable to repeat blood cultures. Additionally, if initial blood cultures are positive, these should be repeated daily until bacteremia clears regardless of the fever pattern. A study published in 2013 has looked at an alternate single 40 mL sample method from central lines that seems promising, but needs further validation and cost analysis [28].
Treatment
As mentioned throughout this chapter, the prompt recognition and institution of appropriate empiric anti-infective therapy in the potentially infected neutropenic patient with hematological malignancy is life-saving. It is recommended that appropriately selected and dosed antimicrobials are received within an hour of medical contact at a maximum, with administration within 30 min being optimal [23, 26, 29, 30]. The concept of adequate antimicrobial therapy must be highlighted and contrasted with appropriate therapy, and though various somewhat different definitions have been published, we will frame the discussion in terms of the definitions set forth in the joint Infectious Disease Society of America (IDSA) and American Thoracic Society (ATS) guidelines on the management of hospital-acquired, ventilator-associated pneumonia and healthcare-associated pneumonia from 2005 and updated in 2016 [31, 32]. According to these definitions, appropriate antimicrobial therapy is the use of drugs with in vitro activity against the confirmed etiologic agent, whereas adequate treatment implies not only the use of the correct (appropriate) antimicrobial agent, but additionally administering the optimal dose as well as choosing a route for administration that allows for tissue penetration of the drug at the site of infection. In order to provide not just appropriate, but adequate, therapy to the neutropenic patient with hematologic malignancy, host factors as well those related to the underlying malignancy and antineoplastic therapy must be considered in order to determine the patient’s risk of developing serious complications of neutropenic fever. In addition to this risk stratification, the patient’s drug allergies, prior microbiologic culture data, antimicrobial agent exposures, and the local antibiogram need to be considered [33]. As discussed earlier in the Epidemiology section, the majority of causative organisms in neutropenic fever are Gram positive bacteria, especially skin flora; however, Gram negative bacteria tend to cause more serious clinical disease due to their virulence factors, and they are making a resurgence in terms of their frequency driven in part by the increase in MDROs. Table 50.3 summarizes key points in the treatment of bacterial infections in patients with hematological malignancy.
Those patients who are unable to tolerate oral antibiotics or who are risk stratified into the high-risk category should receive intravenous antibiotics (Fig. 50.1). A number of studies have attempted to find an ideal empiric antibiotic regimen, and no regimen has shown itself to be superior [34,35,36,37,38,39]. There are a number of acceptable monotherapy options that are as efficacious as combination regimens for empiric therapy in high-risk neutropenic fever syndromes: ceftazidime, cefepime, piperacillin-tazobactam (or other antipseudomonal beta lactam agents), imipenem-cilastatin, meropenem, etc. Combination regimens that use extended spectrum beta-lactams with fluoroquinolones, aminoglycosides, or double beta-lactams have not shown superiority and often increase toxicity [38, 39]. Furthermore, combination regimens that use a second agent to cover Gram positive bacterial organisms do not seem to confer clinical or mortality benefits and also are associated with greater toxicity and bacterial resistance [2, 7, 40,41,42,43]. The standard use of vancomycin, linezolid, and other drugs against Gram positive bacteria as part of empiric antibiotic regimens for neutropenic fever should be discouraged. These medications should be reserved for patients with presumed line infections, pneumonia, soft tissue infections, and septic shock. The importance of knowing the patient’s colonization status of resistant bacteria (MRSA, VRE, and other MDROs) and previous culture results cannot be overstated, as many patients with hematologic malignancies are colonized with resistant Gram positive organisms. Once appropriate empiric antibiotic therapy has been instituted in a timely fashion, close attention must be paid to clinical response and the progress of culture (with susceptibility data) to determine adequate therapy. Patients with a persistent unexplained neutropenic fever syndrome, who are clinically stable or improving, do not always require a change in antibiotic therapy for their ongoing fever (Fig. 50.2). Among oncology patients with solid tumors and tumor fever, neutropenic fever usually resolves within 48 h of empiric antibiotics; neutropenic fever among patients with hematologic malignancies can take up to an average of 5 days after initiation of treatment to defervesce [2].
Central line associated bloodstream infections (CLABSIs) are frequently the cause of neutropenic fever in hematologic malignancy patients, and adequate antibiotic administration alone is insufficient to treat these patients, with removal of the offending line being necessary. The exception to this rule is CLABSI due to coagulase negative staphylococci, in which case it may be reasonable to treat through the infection [2]. Catheter removal is recommended for infections with Pseudomonas aeruginosa , Staphylococcus aureus , fast growing atypical mycobacteria, and Candida spp. , and with less certainty for other fungal species [2, 44,45,46,47,48]. In these cases, antibiotics should be continued for a minimum of 2 weeks after clearance of bacteremia and removal of the CVC, whichever occurred last. Complicated CLABSIs such as those occurring with deep tissue infections (catheter tunnel tract or port pockets), septic thrombosis or embolisms, endocarditis, or persistent bacteremia (defined as greater than 72 h after initiation of adequate therapy), warrant extended courses of antibiotics in the 4- to 6-week duration range. See Fig. 50.3 for recommendations for management of infected long-term venous access catheters.
In general, the presence of infected material (such as an abscess, a CVC, or a urolith) necessitates both antibiotic penetration and activity to treat the infection. In cases where the drug activity and penetration are suboptimal, removal of the infected material is paramount to achieving control of the infection. Source control requires the removal of infected hardware, drainage of infected fluid collections, or removal of infected tissue or other material. The indications and risk benefit for the interventions already discussed are beyond the scope of this chapter and merit the consultation of infectious diseases specialists, surgeons, or interventional radiologists as clinically dictated.
Bacterial Prophylaxis
Several studies have demonstrated the utility of antimicrobial prophylaxis to prevent neutropenic fever and infectious complications, especially among high-risk patients [49, 50]. A Cochrane Review meta-analysis in 2012 showed that antibiotic prophylaxis was associated with lower all-cause mortality when compared to placebo or no treatment. Prophylaxis was also associated with significantly reduced occurrence of fever, fewer clinically documented and microbiologically documented infections, and lower risk of infection-related death [49]. Fluoroquinolones (levofloxacin and ciprofloxacin) have been extensively studied given their broad spectrum (covering Gram negative bacteria including Pseudomonas aeruginosa, as well as Gram positive bacteria) and good oral bioavailability. Fluoroquinolone prophylaxis reduces the risk for all-cause mortality as well as infection-related mortality [2]. A combination of a fluoroquinolone plus an antibiotic with enhanced Gram positive activity is not recommended [2]. Some studies have shown that this approach may reduce infections caused by Staphylococcus and Streptococcus spp., but they do not affect infection-related mortality and may increase the rated of resistant bacteria [51,52,53,54]. See Table 50.4 for suggested empiric antibiotic regimens for high-risk patients.
There are concerns about toxicities and antimicrobial resistance [52, 55], especially among patients concurrently using QT prolonging medications such as amiodarone or voriconazole. In 2016, the Food and Drug Administration (FDA) issued safety warnings about fluoroquinolones causing acute tendonitis, neuropathy, and central nervous system side effects, which has led to a revision of the package inserts for these medications.
The optimal timing for initiation and duration of antimicrobial prophylaxis has not been well studied. Some clinicians will start prophylaxis on the first day of cytotoxic chemotherapy even for those patients not yet neutropenic, while others will do so on the last day of chemotherapy. It is common to discontinue prophylaxis when the neutropenia has resolved.
Fungal Infections
Invasive fungal infections (IFIs) cause significant morbidity in patients with hematological malignancies and stem cell transplants. This section will elaborate on the epidemiology, clinical syndromes, diagnosis, and treatment of the most common IFIs in this population. IFIs are categorized into those caused by yeasts or molds, of which a basic microbiologic understanding is useful when we think about the syndromes and the term “mold active” antifungal agent. Yeasts are single cell organisms that reproduce by budding and include genera such as Candida or Cryptococcus, among others. Mold organisms have a filamentous growth stage that allows them to elongate by branching with longitudinal extension, and can be further categorized as opportunistic or endemic infections. The morphology of filamentous hyphae on pathology specimens plays an essential part in diagnosis and early management of mold infections. The most common opportunistic mold organisms include Aspergillus, Mucor, Rhizopus, and Fusarium species, while Histoplasma, Blastomyces, and Coccidioidomyces species comprise most of the common endemic fungi.
Candida
Candida yeasts are saprophytic organisms commonly found on the skin and mucosal surfaces of humans. Infections with these organisms can cause superficial (oral thrush, esophagitis, dermatitis, and vaginitis, etc.) and/or deep infections (candidemia, visceral organ abscesses, endophthalmitis, and many others). Herein we focus on the invasive forms of candidiasis (IC), which cause significant morbidity and mortality among hematologic cancer patients and stem cell transplant (SCT) recipients. Prior to the use of antifungal prophylaxis, the incidence of disseminated candidemia was approximately 17% among patients with hematologic malignancies [56, 57] and 11% after SCT [58, 59]. Mortality for patients with IC ranged from 39 to 73% [58, 59]. Autopsy studies in SCT patients from the 1980s suggest overall prevalence of Candida infections was 28% [60]. Table 50.5 summarizes key points in the management of Candida infections.
Risk Factors and Epidemiology
In addition to the risk factors for IC in the general population (CVCs, broad spectrum antibiotics, and total parenteral nutrition) [61], those with hematologic malignancies are at increased risk of disseminated disease secondary to prolonged neutropenia [62,63,64], use of antibacterial antibiotics [62, 64], Candida colonization [19, 52, 60, 65, 66], and mucosal damage from cytotoxic chemotherapy [62]. Specifically, neutropenia lasting over 15 days is a risk for hepatosplenic candidiasis [62].
Over time the incidence of infection by non-albicans Candida spp. is increasing [67, 68], and even surpassing the incidence of Candida albicans infections in the United States [68,69,70]. Hematology patients are at increased risk for specifically C. glabrata [63, 67], C. krusei [63, 71, 72], C. tropicalis [72, 73], C. guilliermondii [72], and emerging Candida spp. such as C. dubliniensis and C. kefyr [72]. This evolution of epidemiology is important as these species can be resistant to fluconazole and to a lesser extent voriconazole [72].
Clinical Presentation
The most common clinical presentation of IC in hematologic malignancy patients is fever. Acute disseminated candidiasis can result in severe sepsis and multiorgan failure. Early descriptions of IC from autopsy studies in the 1980s (prior to the use of antifungal prophylaxis in the 1990s) in subjects with acute leukemia report fever refractory to antibiotics or a second episode of fever. At autopsy, often multiple organs were involved [65, 74]. By the late 1990s fever was being reported in 99% patients with malignancies and was most often it was low grade [75].
Chronic disseminated candidiasis or hepatosplenic candidiasis deserves special attention as this entity occurs almost exclusively among patients with acute leukemia . Epithelial damage that occurs along the gastrointestinal mucosa from cytotoxic chemotherapy enables Candida to enter the hepatobiliary circulation, resulting in hepatic and splenic microabscesses. Symptoms of disease develop later into the course of infection, usually just after neutrophil recovery, when neutrophils can migrate to the sites of the microabscesses. As with acute IC, persistent fever is the most common manifestation, sometimes accompanied by right upper quadrant abdominal pain, tenderness, and an elevated alkaline phosphatase. An immune reconstitution-like syndrome has been hypothesized given the timing of symptoms following neutrophil recovery [76].
Diagnosis
There is no true gold standard for the diagnosis of IC, and limitations of culture and non-culture diagnostic tests make this challenging. While diagnosis often relies on positive blood cultures, and blood culture technology has improved over the decades, overall sensitivity of blood culture has been estimated to remain approximately 50% [77]. IC can be divided into three groups: candidemia in the absence of deep-seated infection, candidemia associated with deep-seated infection, and deep-seated candidiasis that is not associated with candidemia. Blood culture systems, while as sensitive as PCR in vitro, may capture 75% of group 1 and 2 above, leading to an overall sensitivity of 50% [77]. The 2- to 3-day turnaround time of blood cultures often delays diagnosis [78]. Of note, in hepatosplenic candidiasis (the third group described above), blood cultures may be positive in only 20% of cases, with tissue culture positivity in approximately 50%.
Non-culture-based techniques include antigen/antibody testing, β-D-glucan, and PCR, which can be used adjunctively with blood cultures. There are several limitations of these non-culture-based tests, some of which include the rapid clearance of antigen/antibody from the circulation and limitations in immunocompromised hosts. β-D-Glucan (a component of the fungal cell wall) is not specific for Candida, has poor specificity, and false positivity among immunocompromised patients who are at risk for a number of fungal pathogens. PCR is problematic in that there is a lack of standardization in methodologies and has similar limitations to β-D-glucan testing. Currently these tests are not considered standards in making the diagnosis of invasive candidiasis [77, 78]. There are no guidelines for interpreting these non-invasive diagnostic tests, because they provide adjunctive information to blood cultures and should not be ordered in place of blood cultures.
T2Candida is a newer diagnostic modality that is being implemented into some clinical practices. T2 diagnostic testing involves a miniaturized, magnetic resonance-based diagnostic approach that measures how water molecules react in the presence of magnetic fields. The T2Candida Panel test seems to be capable of improved sensitivity compared to blood culture, using automated systems with a turnaround time of 3–5 h [79]. In some instances, the T2Candida Panel test may be positive up to a week prior to blood culture [79].
Treatment
Treatment of ICs has been extensively reviewed and updated by the IDSA in 2016 [78]. Due to the increase in non-albicans infections, with the potential for fluconazole drug resistance, it has become standard practice that echinocandins (either anidulafungin, caspofungin, or micafungin) are initiated empirically upon microbiologic diagnosis of candidemia until speciation is known. Fluconazole is used in the majority of C. albicans infections given the >95% sensitivity of isolates to this drug. Treatment durations range from 2- to 4-weeks on average, depending on the underlying disease [78]. Dilated retinal exams are recommended on all patients with candidemia, but can be delayed until neutrophil recovery in hematologic patients. Removal of CVCs is also widely implemented in clinical practice.
Antifungal Prophylaxis
With the advent of a well-tolerated antifungal drug (fluconazole), initial studies from the 1990s showed that prophylactic fluconazole significantly reduced Candida colonization [56], reduced invasive Candida infections [80], and decreased the incidence of superficial and systemic IC among SCT recipients, with improvements in survival [81, 82]. A randomized placebo-controlled trial of fluconazole prophylaxis in neutropenic cancer patients identified that patients who benefited most from prophylaxis included those with acute myeloid leukemia undergoing induction with anthracycline-based regimens and those receiving autologous transplants not supported with hematopoietic growth factors [57]. Low dose (200 mg daily) of fluconazole was found to be as efficacious to high dose (400 mg daily) in preventing candidal infections among SCT recipients [83]. Since those pivotal studies, the use of fluconazole prophylaxis has become standard protocol throughout many hematologic and transplant centers and is supported by the National Comprehensive Cancer Network (NCCN) guidelines. Meta-analysis studies of antifungal prophylaxis in neutropenic chemotherapy recipients [84] and stem cell transplant recipients [85] report reduced morbidity, superficial and invasive fungal infections, and fungal infection-related mortality with the use of antifungal prophylaxis [84, 85].
Opportunistic Molds
The term invasive fungal disease (IFD) was coined by the European Organization for Research and Treatment of Cancer (EORTC) Invasive Fungal Infection Cooperative Group and the National Institute of Allergy and Infectious Diseases Mycoses Study Group (NIAID-MSG) in 2008 to describe any disease process dealing with fungi in high-risk patients [86]. The IDSA has now expanded their guidelines to include other groups of patients at risk such as solid organ transplant and those with primary immunodeficiencies [87]. For the purposes of this section we will refer to IFD as it relates to pathogenic mold fungal infections among hematologic patients, and the discussion will be limited to Aspergillus, the agents of Zygomycetes infections (Mucor, Rhizopus, and Rhizomucor) and Fusarium, the most common mold infections.
These fungi are saprophytic environmental molds that propagate on decaying soil. Concomitant environmental exposure and host immune deficiency are necessary for invasive disease, of which inhalation of fungal spores is the most common portal of entry. These molds grow by longitudinal extension and branching of filamentous hyphae and are angioinvasive. The morphologic appearance on pathology specimens is pivotal in diagnosing these infections (i.e., Aspergillus are seen as branching acute angle septate hyphae, while Zygomycetes are seen as non-septate, broad, ribbon-like hyphae).
Common Features of Invasive Fungal Diseases
There is significant overlap in the clinical and radiographic features of Aspergillus, Zygomycetes, and Fusarium. Early descriptions of invasive aspergillosis (IA) included persistent fever with pulmonary infiltrates in patients with acute leukemia [74, 88, 89], which also commonly occurs in Zygomycetes [90] and Fusarium [91, 92]. The range of pulmonary symptoms includes cough, hypoxemia, shortness of breath, pleuritic chest pain, and (given the angioinvasive character of these fungi) hemoptysis. Risk factors for infection have been extensively studied and include prolonged neutropenia, corticosteroids, and acute leukemia [89, 93,94,95,96]. In addition, those patients with active malignancy, persistent neutropenia at the end of treatment, and delayed initiation of treatment are associated with poor survival [97,98,99]. Delayed therapy for ≥10 days and 6 days has been associated with worse survival in Aspergillus [100] and Zygomycetes [99] infections, respectively. Utilization of high-resolution chest computed tomography (CT) scans has allowed earlier diagnosis of IFDs and hence improvement in outcomes with initiation of antifungal therapy.
After entry into the lungs, these mold infections can spread by either direct extension or systemic dissemination via the blood system and angioinvasion [101]. Pulmonary disease is the most common manifestation of infection [102,103,104], with lung involvement occurring in 90% of cases in one single center study [105]. IA does have a predilection to cause central nervous system disease, either through hematogenous dissemination or contiguous spread from the sinuses, however dissemination into any organ can occur [101]. Table 50.6 summarizes key points regarding invasive mold infections.
Epidemiology
Aspergillus
Invasive aspergillosis is the most common of the opportunistic mold infections occurring in patients with hematologic malignancies and SCT. The actual overall incidence of IA is difficult to estimate; however, the use of non-culture-based diagnostic tests has expanded the number of patients who will be defined as “possible” cases [106]. Among all causes of IFDs among immunocompromised and immunocompetent patients, IA represents approximately 70% of mold infections, with 85% of those occurring in patients with hematologic malignancies and SCT recipients [107]. The majority of IA occurs during the first course of induction chemotherapy for those with AML [108], when mold-active prophylaxis is not routinely employed. A bimodal distribution of IA has been described after SCT, with early disease being related to underlying myeloid deficiencies and late disease (after day 100) related to graft-versus-host disease, steroids, and immunosuppression [108]. Aspergillus fumigatus is the most common species isolated [102, 104, 107, 109], followed by A. flavus, A. niger, and A. terreus [102]. A history of aspergillosis during chemotherapy for AML does not preclude progression to transplantation, but the infection should be well controlled unless activity of the underlying transplant condition is the reason why aspergillosis cannot be controlled.
Survival and mortality vary significantly depending on the study and have been reported as 59% survival after hematologic malignances at 12 weeks [102], to 43–62% with hematologic malignancy or after SCT [102, 105, 110]. Factors for poor survival include SCT, progression of underlying disease [111, 112], steroid use [110, 113], neutropenia [112, 113], disseminated disease [113], and extent of pulmonary lesions [105].
Zygomycetes
Common Zygomycetes molds include organisms in the genera Mucor, Rhizopus, Rhizomucor, Lichtheimia (formerly Absidia), and Cunninghamella. Among hematologic malignancy patients, the most common presentation mimics IA; however, Zygomycetes infections do have a greater propensity to have sinus or rhinocerebral involvement [114,115,116]. The presence of sinus disease favors Zygomycetes, but does not rule out IA. In the two largest prospective studies on Zygomycetes, the incidence of pulmonary disease ranged from 30 to 46%, with rhinocerebral disease occurring in 27–29% [115, 116]. Single center studies prospectively comparing IA to Zygomycetes infections in those with hematologic malignancies report that sinusitis and rhinocerebral manifestations were significantly more common in those with Zygomycetes infections compared to Aspergillus [96, 99]. Other independent predictors for Zygomycetes also included prior use of voriconazole [96, 114], the presence of multiple pulmonary nodules (≥10 nodules), and pleural effusions [114]. Taken together, the two most significant distinguishing factors for Zygomycetes infections include sinus disease and breakthrough IFD on voriconazole prophylaxis.
Worse outcomes have been associated with active malignancy [97, 99], delay in therapy [99], and pulmonary and disseminated disease [93, 116]. A single center retrospective study showed that delay in initiation of an amphotericin product resulted in a twofold increased mortality at 12 weeks [99], which has been confirmed in other studies [115]. Use of posaconazole [99] and neutrophil recovery are associated with favorable outcomes [90, 99]. Mortality ranges from 52% in those with hematologic malignancies to 76% after stem cell transplant [116]. Interestingly, in one small pilot study, radiographic improvement was not predictive of 90-day survival, and 50% who survived did not have improved CT or magnetic resonance imaging at the end of treatment. However, clinical response 30 days after the end of treatment was predictive of survival at 90 days after treatment [97].
Fusarium
Notable characteristics that distinguish Fusarium from the above infections is the propensity for disseminated disease (not just pulmonary involvement), skin manifestations that occur in approximately 60% of infections, and the ability to grow in blood cultures in 40–55% of cases [91, 98]. The pulmonary nodules that occur in Fusarium infections tend to have more peripheral involvement compared to Zygomycetes or Aspergillus infections [117].
Fusarium solani is the most commonly reported isolate representing >50% of the Fusarium cases reported in the literature [92, 95, 98]. The most common skin manifestation is multiple painful erythematous papular or nodular lesions with or without central necrosis, and can also manifest as ulcerations, bullae, or ecthyma gangrenosum [118]. Skin lesions have been described to precede fungemia by 5 days [95, 119]. Early descriptions of Fusarium in hematologic patients reported fever refractory to antibacterial therapy with painful skin lesions in 91% of infections, with presumed pneumonia and sinusitis occurring in 84% and 26%, respectively [92].
Diagnosis
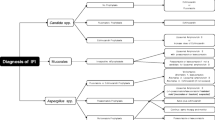
The EORTC/MSG and IDSA all support a composite definition for the diagnosis of IFD that includes host factors, clinical manifestations, and mycologic evidence of infection. Utilizing these criteria, patients are grouped as either proven, possible or probable IFD, with proven infection necessitating tissue biopsy with pathologic evidence of fungi invading tissue. Probable is the most common category in clinical practice, which includes a host factor (such as allogenic SCT, corticosteroids, T cell immune-suppression, or recent history of neutropenia >10 days), clinical features (such compatible CT imaging), and mycologic evidence (direct or indirect mycologic testing) be present [86].
Use of high-resolution CT imaging and non-culture-based diagnostic testing improves early diagnosis of IFD in hematologic patients, which in turn impacts outcome. There are several classic patterns of IFD on high-resolution CT of the lung, including the halo, air-crescent, or reverse halo sign [120]. The macronodule is the most common manifestation of IA [121]. A macronodule surrounded by ground glass (the halo sign) pathologically represents an area of fungal tissue invasion surrounded by alveolar hemorrhage [87, 120, 122]. The halo sign occurs early in the course of IFD, and while not specific for IA, it commonly occurs among hematologic patients with invasive aspergillosis (92–95% of patients in small series) [120, 123, 124]. The reverse halo sign, a focal area of ground glass opacity surrounded by a ring of consolidation, also occurs early in the course of IFD, but is more associated with infections caused by the agents of Zygomycetes rather than Aspergillus [120]. As neutrophils recover, cavitation and gas formation may occur, denoting the air-crescent sign.
Treatment
Azole antifungal agents (fluconazole, itraconazole, voriconazole, posaconazole, and isavuconazole) are the most commonly used class of agents used to treat IFD infection, given their improved tolerability profile over amphotericin products. Table 50.7 expands upon the different antifungal agents, with expanded coverage of the higher generation azole antifungal agents. Echinocandins (micafungin, caspofungin, and anidulafungin) have good Aspergillus coverage, but lack activity against the agents of Zygomycetes and Fusarium. These are often used along with an azole for dual drug or salvage therapy for Aspergillus. Finally, amphotericin products do have a broad spectrum, but are most commonly used to treat Zygomycetes infections. Nephrotoxicity and infusion-related reactions are the most common limiting factors in using these agents. Knowledge of the antifungal spectrum that is used for prophylaxis is essential in making early treatment choices for suspected IFDs and breakthrough fungal infections.
Therapy for Aspergillus : In 2002 a randomized clinical control trial showed better treatment responses and improved survival with voriconazole as compared to amphotericin-B [109], which has been confirmed by two superseding studies [105, 113]. Given the high mortality of IA in immune-compromised patients, combination antifungal therapy is often considered for patients with a high risk of death and for critically ill patients. This usually consists of voriconazole plus an echinocandin. A randomized, controlled trial of voriconazole monotherapy versus combination therapy was published in 2015 [125]. There was a trend toward improved mortality at 6 weeks with combination therapy in patients with hematological malignancy or SCT, although statistical significance was not obtained.
Therapy for Zygomycetes : Mold active agents with activity against Zygomycetes include amphotericin, posaconazole, and isavuconazole. Double coverage of amphotericin and posaconazole is the most common regimen used early in the course of therapy. Surgical debridement in those with sino-cerebral disease is an important part of therapy and improves outcomes [90, 93, 126]. Hyperbaric oxygen and granulocyte transfusions are often considered in those with severe disease.
Therapy for Fusarium : The most common regimens for therapy include voriconazole with or without amphotericin, and therapy should be based on the isolate’s sensitivities. Neutrophil recovery is essential for recovery from Fusarium infections [63, 91], with some series reporting 0% survival without recovery from myelosuppression [92]. Granulocyte transfusions can be considered as an adjunct to antifungal therapy, working as a bridge until recovery from myelosuppression. Steroid use is also associated with poor outcome [91].
Summary
The evaluation of potential bacterial and fungal infections in patients with hematological malignancies necessitates a careful understanding of host, underlying disease, and antineoplastic therapy factors. The likelihood of infections that one is likely to encounter will influence the types of diagnostic testing and empiric treatment that should be ordered. Additionally, empiric and targeted treatments are influenced by the current knowledge of local microbial prevalence and antimicrobial resistance patterns. Providers managing these patients need to have a good understanding of these concepts and are encouraged to involve infectious diseases experts in the management of these complex patients, especially when facing poor clinical response to anti-infective therapy, MDROs, and fungal infections. To be certain, this is a constantly changing field, if nothing else due to microorganisms’ ability to constantly evolve and adapt; therefore, we must remain flexible, observant, and willing to adapt as well.
References
Egi M, Morita K. Fever in non-neurological critically ill patients: a systematic review of observational studies. J Crit Care. 2012;27(5):428–33.
Freifeld AG, Bow EJ, Sepkowitz KA, Boeckh MJ, Ito JI, Mullen CA, et al. Clinical practice guideline for the use of antimicrobial agents in neutropenic patients with cancer: 2010 update by the infectious diseases society of america. Clin Infect Dis. 2011;52(4):e56–93.
Jun HX, Zhixiang S, Chun W, Reksodiputro AH, Ranuhardy D, Tamura K, et al. Clinical guidelines for the management of cancer patients with neutropenia and unexplained fever. Int J Antimicrob Agents. 2005;26(Suppl 2):S128–32; discussion S33–40.
Link H, Bohme A, Cornely OA, Hoffken K, Kellner O, Kern WV, et al. Antimicrobial therapy of unexplained fever in neutropenic patients—guidelines of the Infectious Diseases Working Party (AGIHO) of the German Society of Hematology and Oncology (DGHO), Study Group Interventional Therapy of Unexplained Fever, Arbeitsgemeinschaft Supportivmassnahmen in der Onkologie (ASO) of the Deutsche Krebsgesellschaft (DKG-German Cancer Society). Ann Hematol. 2003;82(Suppl 2):S105–17.
Hanna H, Afif C, Alakech B, Boktour M, Tarrand J, Hachem R, et al. Central venous catheter-related bacteremia due to gram-negative bacilli: significance of catheter removal in preventing relapse. Infect Control Hosp Epidemiol. 2004;25(8):646–9.
McKenzie H, Hayes L, White K, Cox K, Fethney J, Boughton M, et al. Chemotherapy outpatients’ unplanned presentations to hospital: a retrospective study. Support Care Cancer. 2011;19(7):963–9.
Pizzo PA. Management of fever in patients with cancer and treatment-induced neutropenia. N Engl J Med. 1993;328(18):1323–32.
Sickles EA, Young VM, Greene WH, Wiernik PH. Pneumonia in acute leukemia. Ann Intern Med. 1973;79(4):528–34.
Kang CI, Chung DR, Ko KS, Peck KR, Song JH, Korean Network for Study of Infectious Diseases. Risk factors for infection and treatment outcome of extended-spectrum beta-lactamase-producing Escherichia Coli and Klebsiella Pneumoniae bacteremia in patients with hematologic malignancy. Ann Hematol. 2012;91(1):115–21.
Vidal L, Paul M, Ben dor I, Soares-Weiser K, Leibovici L. Oral versus intravenous antibiotic treatment for febrile neutropenia in cancer patients: a systematic review and meta-analysis of randomized trials. J Antimicrob Chemother. 2004;54(1):29–37.
Vidal L, Ben Dor I, Paul M, Eliakim-Raz N, Pokroy E, Soares-Weiser K, et al. Oral versus intravenous antibiotic treatment for febrile neutropenia in cancer patients. Cochrane Database Syst Rev. 2013;10:CD003992.
Schwaber MJ, Carmeli Y. Mortality and delay in effective therapy associated with extended-spectrum beta-lactamase production in Enterobacteriaceae bacteraemia: a systematic review and meta-analysis. J Antimicrob Chemother. 2007;60(5):913–20.
Elting LS, Rubenstein EB, Rolston KV, Bodey GP. Outcomes of bacteremia in patients with cancer and neutropenia: observations from two decades of epidemiological and clinical trials. Clin Infect Dis. 1997;25(2):247–59.
Bodey GP, Jadeja L, Elting L. Pseudomonas bacteremia. Retrospective analysis of 410 episodes. Arch Intern Med. 1985;145(9):1621–9.
Wisplinghoff H, Seifert H, Wenzel RP, Edmond MB. Current trends in the epidemiology of nosocomial bloodstream infections in patients with hematological malignancies and solid neoplasms in hospitals in the United States. Clin Infect Dis. 2003;36(9):1103–10.
Holland T, Fowler VG Jr, Shelburne SA 3rd. Invasive gram-positive bacterial infection in cancer patients. Clin Infect Dis. 2014;59(Suppl 5):S331–4.
Sipsas NV, Bodey GP, Kontoyiannis DP. Perspectives for the management of febrile neutropenic patients with cancer in the 21st century. Cancer. 2005;103(6):1103–13.
Gudiol C, Bodro M, Simonetti A, Tubau F, Gonzalez-Barca E, Cisnal M, et al. Changing aetiology, clinical features, antimicrobial resistance, and outcomes of bloodstream infection in neutropenic cancer patients. Clin Microbiol Infect. 2013;19(5):474–9.
Pagano L, Caira M, Nosari A, Rossi G, Viale P, Aversa F, et al. Etiology of febrile episodes in patients with acute myeloid leukemia: results from the Hema e-Chart Registry. Arch Intern Med. 2011;171(16):1502–3.
Perez F, Adachi J, Bonomo RA. Antibiotic-resistant gram-negative bacterial infections in patients with cancer. Clin Infect Dis. 2014;59(Suppl 5):S335–9.
Mikulska M, Viscoli C, Orasch C, Livermore DM, Averbuch D, Cordonnier C, et al. Aetiology and resistance in bacteraemias among adult and paediatric haematology and cancer patients. J Infect. 2014;68(4):321–31.
Viscoli C, Varnier O, Machetti M. Infections in patients with febrile neutropenia: epidemiology, microbiology, and risk stratification. Clin Infect Dis. 2005;40(Suppl 4):S240–5.
Rolston KV. Challenges in the treatment of infections caused by gram-positive and gram-negative bacteria in patients with cancer and neutropenia. Clin Infect Dis. 2005;40(Suppl 4):S246–52.
Viscoli C, Castagnola E. Planned progressive antimicrobial therapy in neutropenic patients. Br J Haematol. 1998;102(4):879–88.
Sickles EA, Greene WH, Wiernik PH. Clinical presentation of infection in granulocytopenic patients. Arch Intern Med. 1975;135(5):715–9.
Dellinger RP, Levy MM, Rhodes A, Annane D, Gerlach H, Opal SM, et al. Surviving sepsis campaign: international guidelines for management of severe sepsis and septic shock, 2012. Intensive Care Med. 2013;39(2):165–228.
Shelton BK, Stanik-Hutt J, Kane J, Jones RJ. Implementing the surviving sepsis campaign in an ambulatory clinic for patients with hematologic malignancies. Clin J Oncol Nurs. 2016;20(3):281–8.
Pautas C, Sbidian E, Hicheri Y, Bastuji-Garin S, Bretagne S, Corbel C, et al. A new workflow for the microbiological diagnosis of febrile neutropenia in patients with a central venous catheter. J Antimicrob Chemother. 2013;68(4):943–6.
Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, et al. The third international consensus definitions for sepsis and septic shock (sepsis-3). JAMA. 2016;315(8):801–10.
Rosa RG, Goldani LZ. Cohort study of the impact of time to antibiotic administration on mortality in patients with febrile neutropenia. Antimicrob Agents Chemother. 2014;58(7):3799–803.
American Thoracic S. Infectious diseases Society of A. Guidelines for the management of adults with hospital-acquired, ventilator-associated, and healthcare-associated pneumonia. Am J Respir Crit Care Med. 2005;171(4):388–416.
Kalil AC, Metersky ML, Klompas M, Muscedere J, Sweeney DA, Palmer LB, et al. Management of Adults with Hospital-Acquired and Ventilator-Associated Pneumonia: 2016 Clinical Practice Guidelines by the Infectious Diseases Society of America and the American Thoracic Society. Clin Infect Dis. 2016;63(5):e61–e111.
Sepkowitz KA. Treatment of patients with hematologic neoplasm, fever, and neutropenia. Clin Infect Dis. 2005;40(Suppl 4):S253–6.
Pizzo PA, Hathorn JW, Hiemenz J, Browne M, Commers J, Cotton D, et al. A randomized trial comparing ceftazidime alone with combination antibiotic therapy in cancer patients with fever and neutropenia. N Engl J Med. 1986;315(9):552–8.
Cometta A, Calandra T, Gaya H, Zinner SH, de Bock R, Del Favero A, et al. Monotherapy with meropenem versus combination therapy with ceftazidime plus amikacin as empiric therapy for fever in granulocytopenic patients with cancer. The International Antimicrobial Therapy Cooperative Group of the European Organization for research and Treatment of Cancer and the Gruppo Italiano Malattie Ematologiche Maligne dell’Adulto Infection Program. Antimicrob Agents Chemother. 1996;40(5):1108–15.
Leyland MJ, Bayston KF, Cohen J, Warren R, Newland AC, Bint AJ, et al. A comparative study of imipenem versus piperacillin plus gentamicin in the initial management of febrile neutropenic patients with haematological malignancies. J Antimicrob Chemother. 1992;30(6):843–54.
Paul M, Dickstein Y, Schlesinger A, Grozinsky-Glasberg S, Soares-Weiser K, Leibovici L. Beta-Lactam versus Beta-lactam-aminoglycoside combination therapy in cancer patients with neutropenia. Cochrane Database Syst Rev. 2013;6:CD003038.
Peacock JE, Herrington DA, Wade JC, Lazarus HM, Reed MD, Sinclair JW, et al. Ciprofloxacin plus piperacillin compared with tobramycin plus piperacillin as empirical therapy in febrile neutropenic patients. A randomized, double-blind trial. Ann Intern Med. 2002;137(2):77–87.
Bliziotis IA, Michalopoulos A, Kasiakou SK, Samonis G, Christodoulou C, Chrysanthopoulou S, et al. Ciprofloxacin vs an aminoglycoside in combination with a beta-lactam for the treatment of febrile neutropenia: a meta-analysis of randomized controlled trials. Mayo Clin Proc. 2005;80(9):1146–56.
Vardakas KZ, Samonis G, Chrysanthopoulou SA, Bliziotis IA, Falagas ME. Role of glycopeptides as part of initial empirical treatment of febrile neutropenic patients: a meta-analysis of randomised controlled trials. Lancet Infect Dis. 2005;5(7):431–9.
Paul M, Dickstein Y, Borok S, Vidal L, Leibovici L. Empirical antibiotics targeting Gram-positive bacteria for the treatment of febrile neutropenic patients with cancer. Cochrane Database Syst Rev. 2014;1:CD003914.
Freifeld AG, Razonable RR. Viridans group streptococci in febrile neutropenic cancer patients: what should we fear? Clin Infect Dis. 2014;59(2):231–3.
Dompeling EC, Donnelly JP, Deresinski SC, Feld R, Lane-Allman EF, De Pauw BE. Early identification of neutropenic patients at risk of grampositive bacteraemia and the impact of empirical administration of vancomycin. Eur J Cancer. 1996;32A(8):1332–9.
Fowler VG Jr, Sanders LL, Sexton DJ, Kong L, Marr KA, Gopal AK, et al. Outcome of Staphylococcus Aureus bacteremia according to compliance with recommendations of infectious diseases specialists: experience with 244 patients. Clin Infect Dis. 1998;27(3):478–86.
Dugdale DC, Ramsey PG. Staphylococcus Aureus bacteremia in patients with Hickman catheters. Am J Med. 1990;89(2):137–41.
Raad I, Hanna H, Boktour M, Girgawy E, Danawi H, Mardani M, et al. Management of central venous catheters in patients with cancer and candidemia. Clin Infect Dis. 2004;38(8):1119–27.
Legrand M, Max A, Peigne V, Mariotte E, Canet E, Debrumetz A, et al. Survival in neutropenic patients with severe sepsis or septic shock. Crit Care Med. 2012;40(1):43–9.
El Helou G, Hachem R, Viola GM, El Zakhem A, Chaftari AM, Jiang Y, et al. Management of rapidly growing mycobacterial bacteremia in cancer patients. Clin Infect Dis. 2013;56(6):843–6.
Gafter-Gvili A, Fraser A, Paul M, Vidal L, Lawrie TA, van de Wetering MD, et al. Antibiotic prophylaxis for bacterial infections in afebrile neutropenic patients following chemotherapy. Cochrane Database Syst Rev. 2012;1:Cd004386.
Leibovici L, Paul M, Cullen M, Bucaneve G, Gafter-Gvili A, Fraser A, et al. Antibiotic prophylaxis in neutropenic patients: new evidence, practical decisions. Cancer. 2006;107(8):1743–51.
Wingard JR, Eldjerou L, Leather H. Use of antibacterial prophylaxis in patients with chemotherapy-induced neutropenia. Curr Opin Hematol. 2012;19(1):21–6.
Bow EJ. Fluoroquinolones, antimicrobial resistance and neutropenic cancer patients. Curr Opin Infect Dis. 2011;24(6):545–53.
Reduction of fever and streptococcal bacteremia in granulocytopenic patients with cancer. A trial of oral penicillin V or placebo combined with pefloxacin. International Antimicrobial Therapy Cooperative Group of the European Organization for Research and Treatment of Cancer. JAMA. 1994;272(15):1183–9.
Rangaraj G, Granwehr BP, Jiang Y, Hachem R, Raad I. Perils of quinolone exposure in cancer patients: breakthrough bacteremia with multidrug-resistant organisms. Cancer. 2010;116(4):967–73.
Baden LR. Prophylactic antimicrobial agents and the importance of fitness. N Engl J Med. 2005;353(10):1052–4.
Winston DJ, Chandrasekar PH, Lazarus HM, Goodman JL, Silber JL, Horowitz H, et al. Fluconazole prophylaxis of fungal infections in patients with acute leukemia. Results of a randomized placebo-controlled, double-blind, multicenter trial. Ann Intern Med. 1993;118(7):495–503.
Rotstein C, Bow EJ, Laverdiere M, Ioannou S, Carr D, Moghaddam N. Randomized placebo-controlled trial of fluconazole prophylaxis for neutropenic cancer patients: benefit based on purpose and intensity of cytotoxic therapy. The Canadian Fluconazole Prophylaxis Study Group. Clin Infect Dis. 1999;28(2):331–40.
Meyers JD. Fungal infections in bone marrow transplant patients. Semin Oncol. 1990;17(3 Suppl 6):10–3.
Goodrich JM, Reed EC, Mori M, Fisher LD, Skerrett S, Dandliker PS, et al. Clinical features and analysis of risk factors for invasive candidal infection after marrow transplantation. J Infect Dis. 1991;164(4):731–40.
Rossetti F, Brawner DL, Bowden R, Meyer WG, Schoch HG, Fisher L, et al. Fungal liver infection in marrow transplant recipients: prevalence at autopsy, predisposing factors, and clinical features. Clin Infect Dis. 1995;20(4):801–11.
Ostrosky-Zeichner L, Sable C, Sobel J, Alexander BD, Donowitz G, Kan V, et al. Multicenter retrospective development and validation of a clinical prediction rule for nosocomial invasive candidiasis in the intensive care setting. Eur J Clin Microbiol Infect Dis. 2007;26(4):271–6.
Bow EJ, Loewen R, Cheang MS, Schacter B. Invasive fungal disease in adults undergoing remission-induction therapy for acute myeloid leukemia: the pathogenetic role of the antileukemic regimen. Clin Infect Dis. 1995;21(2):361–9.
Hachem R, Hanna H, Kontoyiannis D, Jiang Y, Raad I. The changing epidemiology of invasive candidiasis: Candida Glabrata and Candida Krusei as the leading causes of candidemia in hematologic malignancy. Cancer. 2008;112(11):2493–9.
Lagunes L, Rello J. Invasive candidiasis: from mycobiome to infection, therapy, and prevention. Eur J Clin Microbiol Infect Dis. 2016;35(8):1221–6.
Schwartz RS, Mackintosh FR, Schrier SL, Greenberg PL. Multivariate analysis of factors associated with invasive fungal disease during remission induction therapy for acute myelogenous leukemia. Cancer. 1984;53(3):411–9.
Guiot HF, Fibbe WE, van’t Wout JW. Risk factors for fungal infection in patients with malignant hematologic disorders: implications for empirical therapy and prophylaxis. Clin Infect Dis. 1994;18(4):525–32.
Pfaller MA, Diekema DJ, Gibbs DL, Newell VA, Meis JF, Gould IM, et al. Results from the ARTEMIS DISK Global Antifungal Surveillance study, 1997 to 2005: an 8.5-year analysis of susceptibilities of Candida species and other yeast species to fluconazole and voriconazole determined by CLSI standardized disk diffusion testing. J Clin Microbiol. 2007;45(6):1735–45.
Pfaller MA, Jones RN, Castanheira M. Regional data analysis of Candida non-albicans strains collected in United States medical sites over a 6-year period, 2006–2011. Mycoses. 2014;57(10):602–11.
Pfaller M, Neofytos D, Diekema D, Azie N, Meier-Kriesche HU, Quan SP, et al. Epidemiology and outcomes of candidemia in 3648 patients: data from the Prospective Antifungal Therapy (PATH Alliance(R)) registry, 2004–2008. Diagn Microbiol Infect Dis. 2012;74(4):323–31.
Horn DL, Neofytos D, Anaissie EJ, Fishman JA, Steinbach WJ, Olyaei AJ, et al. Epidemiology and outcomes of candidemia in 2019 patients: data from the prospective antifungal therapy alliance registry. Clin Infect Dis. 2009;48(12):1695–703.
Pfaller MA, Diekema DJ, Gibbs DL, Newell VA, Ellis D, Tullio V, et al. Results from the ARTEMIS DISK Global Antifungal Surveillance Study, 1997 to 2007: a 10.5-year analysis of susceptibilities of Candida Species to fluconazole and voriconazole as determined by CLSI standardized disk diffusion. J Clin Microbiol. 2010;48(4):1366–77.
Pfaller MA, Andes DR, Diekema DJ, Horn DL, Reboli AC, Rotstein C, et al. Epidemiology and outcomes of invasive candidiasis due to non-albicans species of Candida in 2,496 patients: data from the Prospective Antifungal Therapy (PATH) registry 2004-2008. PLoS One. 2014;9(7):e101510.
Andes DR, Safdar N, Baddley JW, Playford G, Reboli AC, Rex JH, et al. Impact of treatment strategy on outcomes in patients with candidemia and other forms of invasive candidiasis: a patient-level quantitative review of randomized trials. Clin Infect Dis. 2012;54(8):1110–22.
DeGregorio MW, Lee WM, Linker CA, Jacobs RA, Ries CA. Fungal infections in patients with acute leukemia. Am J Med. 1982;73(4):543–8.
Viscoli C, Girmenia C, Marinus A, Collette L, Martino P, Vandercam B, et al. Candidemia in cancer patients: a prospective, multicenter surveillance study by the Invasive Fungal Infection Group (IFIG) of the European Organization for Research and Treatment of Cancer (EORTC). Clin Infect Dis. 1999;28(5):1071–9.
Rammaert B, Desjardins A, Lortholary O. New insights into hepatosplenic candidosis, a manifestation of chronic disseminated candidosis. Mycoses. 2012;55(3):e74–84.
Clancy CJ, Nguyen MH. Finding the “missing 50%” of invasive candidiasis: how nonculture diagnostics will improve understanding of disease spectrum and transform patient care. Clin Infect Dis. 2013;56(9):1284–92.
Pappas PG, Kauffman CA, Andes DR, Clancy CJ, Marr KA, Ostrosky-Zeichner L, et al. Clinical Practice Guideline for the Management of Candidiasis: 2016 Update by the Infectious Diseases Society of America. Clin Infect Dis. 2016;62(4):e1–50.
Pfaller MA, Wolk DM, Lowery TJ. T2MR and T2Candida: novel technology for the rapid diagnosis of candidemia and invasive candidiasis. Future Microbiol. 2016;11(1):103–17.
van Burik JH, Leisenring W, Myerson D, Hackman RC, Shulman HM, Sale GE, et al. The effect of prophylactic fluconazole on the clinical spectrum of fungal diseases in bone marrow transplant recipients with special attention to hepatic candidiasis. An autopsy study of 355 patients. Medicine. 1998;77(4):246–54.
Goodman JL, Winston DJ, Greenfield RA, Chandrasekar PH, Fox B, Kaizer H, et al. A controlled trial of fluconazole to prevent fungal infections in patients undergoing bone marrow transplantation. N Engl J Med. 1992;326(13):845–51.
Slavin MA, Osborne B, Adams R, Levenstein MJ, Schoch HG, Feldman AR, et al. Efficacy and safety of fluconazole prophylaxis for fungal infections after marrow transplantation—a prospective, randomized, double-blind study. J Infect Dis. 1995;171(6):1545–52.
MacMillan ML, Goodman JL, DeFor TE, Weisdorf DJ. Fluconazole to prevent yeast infections in bone marrow transplantation patients: a randomized trial of high versus reduced dose, and determination of the value of maintenance therapy. Am J Med. 2002;112(5):369–79.
Bow EJ, Laverdiere M, Lussier N, Rotstein C, Cheang MS, Ioannou S. Antifungal prophylaxis for severely neutropenic chemotherapy recipients: a meta analysis of randomized-controlled clinical trials. Cancer. 2002;94(12):3230–46.
Robenshtok E, Gafter-Gvili A, Goldberg E, Weinberger M, Yeshurun M, Leibovici L, et al. Antifungal prophylaxis in cancer patients after chemotherapy or hematopoietic stem-cell transplantation: systematic review and meta-analysis. J Clin Oncol. 2007;25(34):5471–89.
De Pauw B, Walsh TJ, Donnelly JP, Stevens DA, Edwards JE, Calandra T, et al. Revised definitions of invasive fungal disease from the European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and the National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG) Consensus Group. Clin Infect Dis. 2008;46(12):1813–21.
Patterson TF, Thompson GR 3rd, Denning DW, Fishman JA, Hadley S, Herbrecht R, et al. Practice Guidelines for the Diagnosis and Management of Aspergillosis: 2016 Update by the Infectious Diseases Society of America. Clin Infect Dis. 2016;63(4):e1–e60.
Meyer RD, Young LS, Armstrong D, Yu B. Aspergillosis complicating neoplastic disease. Am J Med. 1973;54(1):6–15.
Gerson SL, Talbot GH, Hurwitz S, Strom BL, Lusk EJ, Cassileth PA. Prolonged granulocytopenia: the major risk factor for invasive pulmonary aspergillosis in patients with acute leukemia. Ann Intern Med. 1984;100(3):345–51.
Kontoyiannis DP, Wessel VC, Bodey GP, Rolston KV. Zygomycosis in the 1990s in a tertiary-care cancer center. Clin Infect Dis. 2000;30(6):851–6.
Nucci M, Anaissie EJ, Queiroz-Telles F, Martins CA, Trabasso P, Solza C, et al. Outcome predictors of 84 patients with hematologic malignancies and Fusarium infection. Cancer. 2003;98(2):315–9.
Boutati EI, Anaissie EJ. Fusarium, a significant emerging pathogen in patients with hematologic malignancy: ten years’ experience at a cancer center and implications for management. Blood. 1997;90(3):999–1008.
Roden MM, Zaoutis TE, Buchanan WL, Knudsen TA, Sarkisova TA, Schaufele RL, et al. Epidemiology and outcome of zygomycosis: a review of 929 reported cases. Clin Infect Dis. 2005;41(5):634–53.
Campo M, Lewis RE, Kontoyiannis DP. Invasive fusariosis in patients with hematologic malignancies at a cancer center: 1998–2009. J Infect. 2010;60(5):331–7.
Nucci M, Anaissie E. Fusarium infections in immunocompromised patients. Clin Microbiol Rev. 2007;20(4):695–704.
Kontoyiannis DP, Lionakis MS, Lewis RE, Chamilos G, Healy M, Perego C, et al. Zygomycosis in a tertiary-care cancer center in the era of Aspergillus-active antifungal therapy: a case-control observational study of 27 recent cases. J Infect Dis. 2005;191(8):1350–60.
Spellberg B, Kontoyiannis DP, Fredricks D, Morris MI, Perfect JR, Chin-Hong PV, et al. Risk factors for mortality in patients with mucormycosis. Med Mycol. 2012;50(6):611–8.
Nucci M, Marr KA, Vehreschild MJ, de Souza CA, Velasco E, Cappellano P, et al. Improvement in the outcome of invasive fusariosis in the last decade. Clin Microbiol Infect. 2014;20(6):580–5.
Chamilos G, Lewis RE, Kontoyiannis DP. Delaying amphotericin B-based frontline therapy significantly increases mortality among patients with hematologic malignancy who have zygomycosis. Clin Infect Dis. 2008;47(4):503–9.
von Eiff M, Roos N, Schulten R, Hesse M, Zuhlsdorf M, van de Loo J. Pulmonary aspergillosis: early diagnosis improves survival. Respiration. 1995;62(6):341–7.
Patterson TF, Kirkpatrick WR, White M, Hiemenz JW, Wingard JR, Dupont B, et al. Invasive aspergillosis. Disease spectrum, treatment practices, and outcomes. I3 Aspergillus Study Group. Medicine. 2000;79(4):250–60.
Steinbach WJ, Marr KA, Anaissie EJ, Azie N, Quan SP, Meier-Kriesche HU, et al. Clinical epidemiology of 960 patients with invasive aspergillosis from the PATH Alliance registry. J Infect. 2012;65(5):453–64.
Morrison VA, Haake RJ, Weisdorf DJ. Non-Candida fungal infections after bone marrow transplantation: risk factors and outcome. Am J Med. 1994;96(6):497–503.
Marr KA, Carter RA, Crippa F, Wald A, Corey L. Epidemiology and outcome of mould infections in hematopoietic stem cell transplant recipients. Clin Infect Dis. 2002;34(7):909–17.
Nivoix Y, Velten M, Letscher-Bru V, Moghaddam A, Natarajan-Ame S, Fohrer C, et al. Factors associated with overall and attributable mortality in invasive aspergillosis. Clin Infect Dis. 2008;47(9):1176–84.
Neofytos D, Treadway S, Ostrander D, Alonso CD, Dierberg KL, Nussenblatt V, et al. Epidemiology, outcomes, and mortality predictors of invasive mold infections among transplant recipients: a 10-year, single-center experience. Transpl Infect Dis. 2013;15(3):233–42.
Azie N, Neofytos D, Pfaller M, Meier-Kriesche HU, Quan SP, Horn D. The PATH (Prospective Antifungal Therapy) Alliance(R) registry and invasive fungal infections: update 2012. Diagn Microbiol Infect Dis. 2012;73(4):293–300.
Wald A, Leisenring W, van Burik JA, Bowden RA. Epidemiology of aspergillus infections in a large cohort of patients undergoing bone marrow transplantation. J Infect Dis. 1997;175(6):1459–66.
Herbrecht R, Denning DW, Patterson TF, Bennett JE, Greene RE, Oestmann JW, et al. Voriconazole versus amphotericin B for primary therapy of invasive aspergillosis. N Engl J Med. 2002;347(6):408–15.
Baddley JW, Andes DR, Marr KA, Kontoyiannis DP, Alexander BD, Kauffman CA, et al. Factors associated with mortality in transplant patients with invasive aspergillosis. Clin Infect Dis. 2010;50(12):1559–67.
Ribrag V, Dreyfus F, Venot A, Leblong V, Lanore JJ, Varet B. Prognostic factors of invasive pulmonary aspergillosis in leukemic patients. Leuk Lymphoma. 1993;10(4–5):317–21.
Cornely OA, Maertens J, Bresnik M, Ebrahimi R, Ullmann AJ, Bouza E, et al. Liposomal amphotericin B as initial therapy for invasive mold infection: a randomized trial comparing a high-loading dose regimen with standard dosing (AmBiLoad trial). Clin Infect Dis. 2007;44(10):1289–97.
Upton A, Kirby KA, Carpenter P, Boeckh M, Marr KA. Invasive aspergillosis following hematopoietic cell transplantation: outcomes and prognostic factors associated with mortality. Clin Infect Dis. 2007;44(4):531–40.
Chamilos G, Marom EM, Lewis RE, Lionakis MS, Kontoyiannis DP. Predictors of pulmonary zygomycosis versus invasive pulmonary aspergillosis in patients with cancer. Clin Infect Dis. 2005;41(1):60–6.
Kontoyiannis DP, Azie N, Franks B, Horn DL. Prospective antifungal therapy (PATH) alliance((R)): focus on mucormycosis. Mycoses. 2014;57(4):240–6.
Skiada A, Pagano L, Groll A, Zimmerli S, Dupont B, Lagrou K, et al. Zygomycosis in Europe: analysis of 230 cases accrued by the registry of the European Confederation of Medical Mycology (ECMM) Working Group on Zygomycosis between 2005 and 2007. Clin Microbiol Infect. 2011;17(12):1859–67.
Farooq A, Alrabaa S, Quilitz R, Yacoub A, Maroon E, Fulp W, et al. Comparison of clinical and radiological features of aspergillus, zygomycosis, and fusarium pneumonia in neutropenic patients. Infect Dis Clin Pract. 2014;22(5):288–93.
Nucci M, Anaissie E. Cutaneous infection by fusarium species in healthy and immunocompromised hosts: implications for diagnosis and management. Clin Infect Dis. 2002;35(8):909–20.
Dignani MC, Anaissie E. Human fusariosis. Clin Microbiol Infect. 2004;10(Suppl 1):67–75.
Georgiadou SP, Sipsas NV, Marom EM, Kontoyiannis DP. The diagnostic value of halo and reversed halo signs for invasive mold infections in compromised hosts. Clin Infect Dis. 2011;52(9):1144–55.
Greene RE, Schlamm HT, Oestmann JW, Stark P, Durand C, Lortholary O, et al. Imaging findings in acute invasive pulmonary aspergillosis: clinical significance of the halo sign. Clin Infect Dis. 2007;44(3):373–9.
Lee YR, Choi YW, Lee KJ, Jeon SC, Park CK, Heo JN. CT halo sign: the spectrum of pulmonary diseases. Br J Radiol. 2005;78(933):862–5.
Hauggaard A, Ellis M, Ekelund L. Early chest radiography and CT in the diagnosis, management and outcome of invasive pulmonary aspergillosis. Acta Radiologica (Stockholm, Sweden: 1987). 2002;43(3):292–8.
Caillot D, Casasnovas O, Bernard A, Couaillier JF, Durand C, Cuisenier B, et al. Improved management of invasive pulmonary aspergillosis in neutropenic patients using early thoracic computed tomographic scan and surgery. J Clin Oncol. 1997;15(1):139–47.
Marr KA, Schlamm HT, Herbrecht R, Rottinghaus ST, Bow EJ, Cornely OA, et al. Combination antifungal therapy for invasive aspergillosis: a randomized trial. Ann Intern Med. 2015;162(2):81–9.
Gleissner B, Schilling A, Anagnostopolous I, Siehl I, Thiel E. Improved outcome of zygomycosis in patients with hematological diseases? Leuk Lymphoma. 2004;45(7):1351–60.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 Springer International Publishing AG, part of Springer Nature
About this chapter
Cite this chapter
Franco, M.P., Green, J.S., Young, JA.H. (2018). Evaluation and Management of Bacterial and Fungal Infections in Patients with a Hematological Malignancy: A 2018 Update. In: Wiernik, P., Dutcher, J., Gertz, M. (eds) Neoplastic Diseases of the Blood. Springer, Cham. https://doi.org/10.1007/978-3-319-64263-5_50
Download citation
DOI: https://doi.org/10.1007/978-3-319-64263-5_50
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-64262-8
Online ISBN: 978-3-319-64263-5
eBook Packages: MedicineMedicine (R0)