Abstract
The neoplastic proliferation of parafollicular thyroid cells (C cells), generically defined as C cell disease, may occur as either medullary thyroid carcinoma (MTC) or C cell hyperplasia (CCH).
The preoperative diagnosis of C cell disease is difficult. CCH, in fact, can only be recognized at surgical pathology. MTC, typically presenting as a thyroid nodule, may often be overlooked by traditional fine needle aspiration cytology (FNAC).
Because neoplastic C cells maintain calcitonin (CT) expression, the hormone constitutes a very sensitive pre- and postoperative marker of C cell disease, with high prognostic value.
MTC patients also present increased serum CEA levels. At variance with circulating CT, however, serum CEA displays a poor preoperative sensitivity for MTC diagnosis and a more limited efficacy as a prognostic factor.
In the preoperative settings, in fact, circulating CT is the most specific and sensitive disease marker for MTC, while CEA displays a lower diagnostic accuracy, and both are of prognostic utility, allowing accurate estimation of tumor burden.
During the postsurgical follow-up of MTC patients, the evolution of both CT and CEA levels allows a good estimate of MTC progression, and their increase reliably heralds persistence/relapse. Nevertheless, some patients may present discrepant results with disproportionately low circulating CT as compared to CEA levels. In such instance, the clinician should be alerted and consider the possibility of dedifferentiated, more aggressive MTC.
In recent years, CT and CEA doubling times (DT) have been proposed as prognostic factors, being CT-DT a more precise indicator of MTC progression than CEA-DT.
Circulating CT and CEA levels represent also accurate markers of response to local treatment in early and advanced/recurrent MTC, but their suitability for the evaluation of response to systemic therapy needs confirmation.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
1 Introduction
The parafollicular cells of the thyroid gland are also recognized as C cells because of their ability to secrete the calcitonin (CT) hormone [1]. Their neoplastic proliferation is generically defined C cell disease and may occur as either medullary thyroid carcinoma (MTC) or C cell hyperplasia (CCH) [2, 3].
MTC is a rare cancer, representing 4–10% of all thyroid malignancies [4]. Approximately 75% of MTCs are sporadic tumors [4], and 25% of them are hereditary forms [4], associated to germline mutations of the rearranged during transfection (RET) proto-oncogene [4,5,6]. Familial MTC may be isolated or develop in the context of multiple endocrine neoplasia (MEN) type 2A and type 2B [3, 4].
CCH with nuclear and/or cytoplasmic aspects of atypia was initially described in association with hereditary MTC. In this context, CCH represents a C cell carcinoma in situ [2]. C cells with morphometric characteristics similar to those present in familial CCH may occasionally be observed in individual cases of severe chronic lymphocytic thyroiditis [2], but also in thyroid glands from normal subjects [5]. The significance of this sporadic CCH is not exactly clear, and its progression to MTC has never been demonstrated.
Preoperative diagnosis of C cell disease is difficult. In familial forms, the introduction of genetic testing has greatly contributed to the early identification of the subjects at risk, allowing prompt radical surgery and great improvement of the outcome [3, 4, 6]. In the sporadic form, CCH can only be recognized at surgical pathology, and MTC typically presents as a thyroid nodule. Although some clinical findings (pain on palpation, location in the upper third of a lobe, presence of enlarged lymph nodes) and suspicious for malignancy US features (i.e., hypoechogenic nodule, microcalcifications, lymph node abnormalities) could suggest the possibility of MTC, its diagnosis is often a challenge. At variance with differentiated thyroid cancer of follicular derivation, the identification of C cell lineage malignancies has taken limited advantage from cytology by fine needle aspiration biopsy (FNAC). This technique, in fact, displays a relatively poor MTC detection rate [3, 7, 8], thus exposing to the risk of inadequate preoperative evaluation and late diagnosis.
Neoplastic C cells fully maintain CT expression and secretion [2,3,4]. Therefore, the hormone constitutes a very sensitive pre- and postoperative marker of C cell disease, with high prognostic value [3, 4].
Carcinoembryonic antigen (CEA) [9, 10] is one of the oldest and most widely used tumor markers. In addition to colorectal cancer (CRC) and other adenocarcinomas, MTC patients also present increased serum CEA levels [4]. At variance with circulating CT, however, circulating CEA displays a poor preoperative sensitivity for MTC diagnosis and a more limited efficacy as a prognostic factor [4].
2 Circulating Calcitonin in Healthy Subjects and in C Cell Disease
2.1 CT Assay
CT is the product of cleavage and posttranslational processing of procalcitonin, a precursor peptide derived from pre-procalcitonin. The term “mature” CT represents the bioactive hormone and defines a small monocatenar peptide (32 amino acids) with a disulfide bridge at its amino terminal end. Other “immature” CT forms may also be found in MTC tissue and in the serum [11, 12]. Additionally, procalcitonin may be released in the circulation during sepsis or other general inflammatory conditions by tissues that do not normally express the CT gene [12, 13].
Because of its molecular heterogeneity, serum CT levels measured by assays utilizing antisera directed against different epitopes of the hormone can yield discrepant results [3]. Two-site immunoassays combining monoclonal antibodies recognizing distinct portions of the unique bioactive monomer avoid the interference of procalcitonin or other calcitonin-related peptides/precursors and allow accurate determination of mature CT concentrations [3]. Such immunoassays, based on radioisotopic, enzymatic, or luminescent labeling, represent the most sensitive tool for measuring serum CT levels [11]. Importantly, CT measurements obtained with different commercial assays may widely vary [3, 11]. Therefore, the reference ranges of basal and stimulated serum CT measurements should be defined in each laboratory, making it imperative that comparison of CT values in individual patients should be performed using the same method [11].
Heterophilic antibodies (human antibodies induced against external antigens and displaying reactivity with antibodies of other animal species) can interfere with two-site immunoassays, causing either spuriously elevated or, with a lesser frequency, artificially lower serum CT levels [14]. A “hook effect” (i.e., falsely low results due to interference caused by extremely high analyte concentrations) may seldom occur also with the most recent immunochemiluminescent assays (ICMAs) [15]. This kind of interference should always be suspected when low CT levels are observed in MTC patients with a large tumor burden [3, 11].
2.2 Circulating Calcitonin Levels in Healthy Subjects
CT levels are slightly higher in men than in women, probably because men have a larger C cell mass than women [16, 17]. They are also weakly influenced by age, body mass index (particularly in males), and smoking [18]. Depending on the employed assay method, 90–97% adults have CT levels <10 pg/ml, and more than 50% of normal subjects have serum CT concentrations below the functional sensitivity of the assay [18]. Notably, the most recent MTC guidelines released by the American Thyroid Association [19] do not recommend definite reference ranges of basal or stimulated serum CT levels. Data from young children are limited. It is suggested that CT levels <40 pg/ml should be considered normal during the first 6 months of life, with a progressive decline thereafter [16]. After the third year of life, CT values reach levels indistinguishable from those observed in adults [16].
2.3 Calcitonin Levels in Diseases
In MTC patients, serum CT levels rise early and parallel tumor progression [3, 4], thus representing a sensitive disease marker [3, 4]. A more limited CT elevation is usually observed in CCH [3], even though the original report by Guyetant et al. [5] showed that the sporadic form could also occur in the absence of CT increase. In addition to C cell disease, a rise in serum CT levels has also been described in patients with other pathological conditions (Table 9.1) such as chronic renal failure, autoimmune thyroiditis, hypergastrinemia, sepsis, type 1A pseudohypoparathyroidism, and mastocytosis [3]. Concerning the influence of thyroid autoimmunity, the association with increased serum CT is still controversial [3]. Some studies, in fact, have reported decreased CT levels in smaller groups of Hashimoto’s patients, possibly due to atrophy and/or fibrosis associated to destruction of both follicular and C cells [3]. Moreover, a recent study [20] has shown that circulating CT levels in patients with positive anti-TPO antibodies were not higher than in controls (4.71 ± 6.46 vs. 4.84 ± 13.11 pg/ml; P > 0.05) and the frequency of “suspicious” (>10 pg/ml) CT values was not significantly different between the two groups of patients (3.9 vs. 3.0%) [20].
Increased circulating CT levels, up to values comparable to MTC, are often observed in patients with non-C cell-derived malignancies (Table 9.1), such as small cell and large cell lung cancers and in neuroendocrine tumors of the gastrointestinal tract [3]. In such instances, the correlation between CT increase and tumor burden is not so tight as it is in MTC patients [3].
2.4 Calcitonin Provocative Testing
C cells from MTC patients are able to release CT in response to several pharmacological agents, the most effective resulting Ca2+ and pentagastrin (PG) [21]. The same response is also observed in CCH, but to a lesser extent than in MTC [3]. Since patients with limited C cell disease may have normal serum CT levels, these provocative tests have been widely used to disclose C cell abnormalities [11, 22]. In addition, these tests constitute important tools for differentiating increased serum CT associated to C cell disease from those occurring in non-thyroid malignancies [3]. In fact, CT response to Ca2+ and PG stimulation is virtually absent in lung and gastrointestinal neuroendocrine tumors [4].
2.4.1 The Pentagastrin Stimulation Test
The most widely used method for stimulating CT secretion consists in the slow intravenous administration of a PG bolus (0.5 μg/kg body weight) and measurement of serum hormone levels before and 3 and 5 min after starting the infusion [11]. Because some discomfort or potentially dangerous side effects (e.g., tachycardia, bradycardia, nausea, vomiting, dizziness, flushing, substernal tightness) may occur, this test is contraindicated in patients with coronary artery disease and/or hypertension and is not recommended in subjects >60 years of age [11].
Similarly to basal CT, stimulated hormone levels are on average higher in men than in women [3]. In 80% normal subjects, the maximum CT increase remains <10 pg/ml, and the peak levels do not exceed 30 pg/ml in 95% of cases [3]. A stimulated CT response >100 pg/ml is usually considered suggestive of C cell disease [3, 4]. Milder CT elevations may also be found in adults with other thyroid abnormalities [11]. In patients with MTC and elevated basal serum CT levels, a fivefold to tenfold increase occurs after PG stimulation [11]. A very limited increase (0–2 times) is observed in the case of other neuroendocrine tumors [23].
2.4.2 The Calcium Stimulation Testing
CT secretion may also be stimulated by a short intravenous Ca2+ infusion [11, 24, 25]. This approach represents an alternative to PG in countries (e.g., the USA) where PG is not available. Ca2+ infusion may also be combined with PG stimulation to increase sensitivity [22, 26]. Serum CT levels measured after a 30-s infusion of calcium gluconate (2.5 mg/kg) are comparable to those stimulated by PG administration in both normal subjects and patients with C cell disease [11].
A recent report has accurately defined the procedure, the cutoffs of CT response, and the safety of the calcium gluconate testing [27]. A 25 mg calcium gluconate dose (i.e., 2.3 mg or 0.12 mEq of elemental calcium)/kg body weight resulted safe and well tolerated. To avoid an overdose in obese patients, an adjusted body weight calculation has been recommended (www.manuelsweb.com/IBW.htm, for ideal body weight and adjusted body weight calculator). The procedure should start with a basal CT determination. Calcium gluconate should be administered i.v., during a minimum 3 min infusion time (5 ml/min). Additional CT determinations at 2, 5, and 10 min after stopping the infusion should be performed [27]. The optimal CT threshold peaks for MTC diagnosis were reported >79 pg/ml for female and >544 pg/ml for male patients [27].
3 The Carcinoembryonic Antigen
Carcinoembryonic antigen (CEA) was one of the earliest tumor markers to be identified and characterized [9, 10]. It was first described in 1965 by Gold and Freeman who identified an antigen present in both fetal colon and colon adenocarcinoma, but absent in normal human colonic tissue; for this reason it was defined carcinoembryonic antigen [9].
3.1 Molecular Characteristics and Physiological Role
CEA and other CEA-related antigens are encoded by the CEA family genes, belonging to the immunoglobulin superfamily. The human CEA family has been fully characterized and is composed by 29 genes, 18 of which are expressed. Seven of them belong to the CEA subgroup and 11 to the pregnancy specific glycoprotein subgroup [28].
CEA molecule is a cell surface glycoprotein normally produced in gastrointestinal tissue during fetal development, its production ending before birth [29]. For this reason, it is present only in very small amounts in the blood of healthy adults and in different normal tissues. Its molecular weight ranges from 150 to 300 (average 185) kDa. The protein component consists of a 30-amino acid single polypeptide chain, with a lysine residue at the N-terminus. The carbohydrate moiety contains fucose, mannose, and galactose residues [29].
Functionally, CEA appears to play a role in cell adhesion, acting as a glycosyl phosphatidyl inositol (GPI) cell surface-anchored glycoprotein [29]. The sialofucosylated glycoforms serve as functional colon carcinoma L-selectin and E-selectin ligands, which may be critical for the metastatic dissemination of colon carcinoma cells [30].
3.2 Measurement
CEA can be utilized as tumor marker by immunohistochemical staining of tumor tissue and by immunoassay in serum.
Several techniques have been developed and compared over time for quantitative evaluations of circulating CEA, namely, radioimmunoassay, immunoradiometric assay, and luminescent oxygen channeling immunoassay [31, 32].
3.2.1 Reference Range of Circulating CEA Levels
Small amounts of CEA are normally present in the circulation of the great majority of normal subjects [33]. Its turnaround time is generally 1 day and the half-life in plasma approximates 3 days, varying from 1 to 5 days [29].
The upper limit of CEA in the healthy population is 2.5–3 μg/l for non-smokers and 5 μg/l for smokers, being slightly higher in men than in women [33].
Several nonneoplastic disorders (Table 9.2) such as liver diseases (cirrhosis, viral hepatitis, chronic active hepatitis, obstructive jaundice), digestive diseases (diverticulitis, inflammatory bowel disease, peptic ulcers, polyps, pancreatitis), chronic lung diseases, and renal failure may determine increased serum CEA concentrations. CEA levels are not elevated in maternal serum during pregnancy, since this glycoprotein does not cross the placental barrier [29].
3.2.2 CEA as Tumor Marker
Circulating CEA is one of the most widely used tumor markers worldwide, mainly in CRC. Disappointingly, it displays a poor sensitivity for early diagnosis of disease [29].
Since nonneoplastic disorders may determine increased serum CEA concentrations, its specificity is also poor [29]. In addition, increased CEA levels were reported in up to 19% of active smokers and in 3% of a healthy control population [33]. For all these reasons, circulating CEA measurement is not appropriated as a screening procedure.
Among malignancies (Table 9.2), most types of adenocarcinomas (breast, gastric, lung, esophageal, ovarian, and pancreatic tumors) may present increased serum CEA levels (35). Supranormal CEA concentrations may also occur in MTC, mesothelioma, melanoma, and lymphoma.
Irrespective of cancer type, CEA is rarely elevated in patients with localized disease [34, 35]. In addition, both CEA levels and the proportion of patients with elevated CEA values tend to increase with progressive disease stage [34]. In CRC patients, it has been suggested that CEA levels could have prognostic value [34], being positively correlated with stage and negatively correlated with disease-free survival [34]. Importantly, several studies have shown that CEA concentration per gram of total protein is remarkably higher in well-differentiated CRCs tissue samples as compared to poorly differentiated specimens [35].
In clinical practice, circulating CEA is mainly employed to document progressive disease, to monitor response to therapy, and to detect recurrence of gastrointestinal malignancies. In the case of CRC, several studies have shown that an intensive surveillance regimen which included irregular CEA measurements following curative surgery resulted in a significantly better patient outcome than a follow-up lacking CEA testing [34]. Most expert panels in Europe [34] and the USA [34], therefore, recommend serial measurements of CEA after curative surgery for CRC. Indeed, there is no agreement concerning the amplitude of serum CEA variation that should be considered of clinical relevance in terms of disease progression. In addition to surveillance following curative resection of CRC, the second main clinical application of circulating CEA is the monitoring of treatment in advanced CRC, particularly when the disease progression cannot be evaluated by standard criteria.
4 CT and CEA Measurement for the Preoperative Diagnosis of C Cell Disease
Preoperative identification of C cell disease may be arduous, and the diagnosis is often performed at an advanced stage [3, 4]. This section will mainly focus on the preoperative diagnostic procedures currently used for MTC diagnosis, with particular attention to the contribution of CT and CEA determination.
4.1 The Contribution of FNAC
All historical MTC series showed that the great majority of patients identified during the clinical work-up of thyroid nodules already presented lymph node involvement and/or distant metastases, with an unfavorable prognosis in most cases [4]. FNAC, in fact, displays a remarkably low sensitivity for MTC diagnosis [3, 8]. Overall, approximately 50% of MTC cases included in most studies would have been missed based on FNAC alone [3, 8], with only two studies reporting a reasonably good sensitivity of cytology for MTC [36, 37]. Also in recent years, a multicenter study conducted among 12 centers in the USA and Europe [7] and a meta-analysis [8] have confirmed that preoperative investigation for MTC cannot rely on FNAC only, because of an unacceptably high false negative rate.
4.2 Circulating CT for the Preoperative Diagnosis of MTC
Since early diagnosis and radical surgical treatment are necessary requirements to improve MTC morbidity and mortality [4], several efforts have been made over the last two decades to identify the most convenient approach for an early diagnosis of this malignancy.
The available evidence shows that the sensitivity of serum CT measurement for preoperative MTC identification approximates 100% [3]. However, prospective, randomized, large-scale, long-term studies are lacking, and the reported results were obtained using assays with great differences in both analytical sensitivity and normal reference values (Table 9.3) [3]. A 100% positive predictive value (PPV) for MTC, comprising also some incidentally discovered microscopic tumors (whose progression to clinically manifest neoplasms had never been proven), has been demonstrated only for basal CT elevation >100 pg/ml [38]. Moreover, increased basal CT levels in most series of unselected thyroid nodular disease patients (Table 9.3) included also a relevant proportion of false positives [3]. By consequence, the PPVs resulted rather low (ranging from 10% to 40%), except for two studies from the same group reporting a PPV >90% [39, 40]. To increase specificity, a two-step approach using also a provocative test was necessary in most studies [3]. Disappointingly, even after a positive CT response to PG or Ca2+ stimulation, CCH could not easily be preoperatively distinguished from MTC [3]. In fact, employing this two-step procedure, a rather elevated frequency (30–75%) of CCH in the absence of MTC was reported by most groups, particularly in patients with basal CT levels between 20 pg/ml and 100 pg/ml and a positive response to PG stimulation [3].
To avoid unnecessary surgery for sporadic CCH, it would be important to distinguish this condition from MTC before surgery. To this purpose, the amplitude of the stimulated CT peak might be of help (Fig. 9.1), though the proposed thresholds varied among different studies [3]. Particularly, one study reported that a CT response to PG stimulation >1000 pg/ml had a 100% PPV for MTC, while a CT peak >100 < 1000 pg/ml exhibited an 80% PPV for CCH [38]. In another report, a CT increase to 275 pg/ml after PG displayed a 100% PPV for MTC diagnosis, while a positive response below this threshold had a PPV of 89% for CCH [41]. These results seem encouraging, but further studies are required for a more precise definition of the appropriate threshold windows of CT response capable of discriminating CCH from MTC.
Preoperative calcitonin levels in patients with either medullary thyroid carcinoma or C cell hyperplasia. (a) Basal calcitonin values in patients submitted to prophylactic thyroidectomy for hereditary MTC (familial MTC or MEN type 2). (b) Basal calcitonin levels in patients with surgical histology indicative of sporadic C cell disease (CCH and MTC). (c) Stimulated calcitonin levels in patients with surgical histology indicative of sporadic C cell disease (CCH and MTC). From Costante et al., Nat Clin Pract Endocrinol Metab. 2009;5:35–44
For all the reasons illustrated above, the usefulness of universal CT screening in thyroid nodular disease patients remains controversial, and no straightforward recommendations have eventually been disclosed from the different scientific societies [42]. In fact, the latest guidelines released by the American Association of Clinical Endocrinologists (AACE) in conjunction with the European Thyroid Association (ETA) and the Italian Associazione Medici Endocrinologi (AME) [43] advocated CT screening only in the presence of clinical risk factors for MTC, while those from the American Thyroid Association (ATA) declined to recommend for or against such a procedure, while recognizing that a basal CT level > 100 pg/ml is highly suspicious for MTC [44, 45].
4.3 CT Measurement in the Washout Fluid of FNAC
During the last decade, the determination of CT in the washout fluid of FNAC samples (FNAC-CT) has been proposed as an ancillary method for the confirmation of preoperative MTC diagnosis in both primary lesions presenting as thyroid nodules and suspicious lymph nodes [8]. In all cases, the reported sensitivities of FNAC-CT were remarkably improved as compared to cytology alone, varying from 80% to 100% [8]. Importantly, a relevant proportion of these MTCs were identified in subjects with basal circulating CT <100 pg/ml. On the other hand, none of the published studies found undetectable CT levels in the washout fluids from patients eventually confirmed negative for MTC at surgical pathology, possibly due to the presence of nonneoplastic C cells in the FNAC aspirates [46,47,48]. Consequently, “arbitrarily” defined cutoff values of FNAC-CT have been proposed, not validated for the most frequent non-C cell-derived thyroid disorders such as autoimmune thyroiditis and colloid goiters [49] and with a wide variation (from 7.4 pg/ml pg to 67 pg/ml) among studies [8]. In addition, the lack of established results demonstrating the suitability of commercial assays for CT measurements on samples different from serum or plasma and the heterogeneity of the sample preparation procedures represent important limiting factors, making it difficult to make comparisons and to draw univocal conclusions. Finally, the cost to benefit effectiveness of CT-FNAC has never been appropriately addressed.
The latest ATA guidelines [44] recommend FNAC-CT in case of cytology suggestive for MTC or inconclusive FNAC results.
4.4 CEA for MTC Diagnosis
Analogously to CT [2, 3], CEA is also expressed in hyperplastic and malignant C cells [29]. In fact, both CT and CEA positive immunostaining in the absence of thyroglobulin represent a standard procedure for MTC diagnosis [2, 19]. Notably, CT immunostaining may vary in intensity and extent and is often reduced in undifferentiated tumors, whereas staining for CEA is almost always strongly positive [19, 50].
4.4.1 CEA Serum Levels for MTC Diagnosis
Although generally expressed by neuroendocrine tissues [29], CEA is not a specific biomarker for MTC diagnosis, also because its levels do not increase following calcium or PG stimulation [4, 19]. For these reasons, CEA measurement is considered to have lower diagnostic accuracy than CT [19]. Therefore, its determination is not suitable for MTC screening and of little use for the preoperative tumor diagnosis. Indeed, CEA is frequently increased at diagnosis, and, as in the case of circulating CT, the serum CEA levels might be of help for the risk stratification of MTC patients [51,52,53].
Although very limited data are available concerning the role of serum CEA as an alternative marker for the diagnosis of CT-negative MTCs [54], it can be used as a marker for the follow-up of MTCs that do not secrete CT [42, 50, 55]. In this respect, one study found CEA and CT almost uniformly expressed in CCH, micro-MTCs, and tumors with intra-thyroid extension [50]. Conversely, the patients with more aggressive MTCs presented an immunohistochemistry pattern characterized by an indistinct or absent CT and an intense CEA staining. Based on these data, it was suggested that CEA expression would be retained as a marker of early epithelial differentiation, while CT expression would more rapidly decline, representing a late differentiation phenomenon in C cell differentiation [50].
Rarely, patients with advanced MTC present normal or low serum levels of both CT and CEA [4, 19]. In such instances, either a misdiagnosis or an advanced MTC dedifferentiation, associated to a poor prognosis [56, 57], should always be taken into account. In this respect, a study [55] conducted on a large series showed that <1% of advanced sporadic MTC patients did not present increased circulating levels of both CT and CEA. In such instance, the occurrence of features such as poorly differentiated histology, high Ki-67 proliferation index, and high proportion of RET codon M918T mutations was consistent with a more aggressive behavior of these MTCs [55].
4.5 Screening for Familial C Cell Disease
Approximately 25% of MTCs are observed as autosomal dominant syndromes in the context of MEN type 2A or 2B familial cancer syndromes, which include MTCs and other neuroendocrine tumors [4, 6]. Prior to the advent of the molecular biology approach, all members of the affected families were periodically screened for MTC by both basal and PG-stimulated serum CT measurement [3], with all difficulties arising from the elevated false positive rate, borderline results, and inconveniences due to repeated PG testing. The discovery that germline mutations of the RET proto-oncogene are responsible for these syndromes allowed a much simpler and more effective management of these families [3, 6, 19]. Genetic screening can, in fact, identify subjects at risk before cancer development, with excellent sensitivity and specificity (virtually 100%). In this context, preoperative circulating CT measurement is recommended in subjects at risk, to help planning in terms of both optimal timing and extent of thyroidectomy [19]. In the absence of RET mutations, the risk for MEN-related cancers for nonaffected members is not greater than that of the general population, and they can be excluded from further surveillance with CT screening [19].
Because approximately 7% of apparently sporadic MTCs harbor germline RET mutations (generally involving exon 13, 14, or 15), genetic testing should be performed in all such patients [3, 6, 19].
5 Role of CT and CEA Measurement for Postoperative Management of MTC
At the time of diagnosis, locoregional metastases can be observed in up to 70% of MTCs presenting as palpable thyroid nodules, and up to 7–23% of such patients display already distant metastases [3, 4]. After initial treatment, repeated surgery, external beam radiation therapy, or other local treatment modalities may effectively be employed for cervical/mediastinal recurrence and for limited distant metastatic disease [4]. Systemic treatment should be deserved to patients with significant tumor burden or rapidly progressive MTC, defined according to the Response Evaluation Criteria in Solid Tumor (RECIST) [58]. Unfortunately, the survival rate is approximately 25% at 5 years and 10% at 10 years after the discovery of MTC distant metastases [45]. For all these reasons, accurate postsurgical follow-up for early detection of persistent/recurrent disease is necessary, for improving the outcome of MTC patients.
5.1 Postoperative Detection of MTC Persistence/Relapse
MTC persistence/recurrence can be suspected based on biochemical evidence and should subsequently be identified with imaging procedures [19, 59, 60]. In this context, serum CT and CEA determinations play a pivotal role [19, 59, 60].
5.1.1 The Role of Postoperative CT
After initial surgery, the normalization of serum CT may require up to 4 weeks, depending on preoperative hormone levels [61]. Therefore, postoperative CT assessments should be postponed after the second month following thyroidectomy. In patients with postoperative basal CT <10 pg/ml, a provocative PG or calcium stimulation testing may be performed, to confirm the absence of small residual tumor tissue foci. Patients with normal basal and stimulated CT levels on two consecutive follow-up evaluations are probably disease-free [4]. Since a tiny proportion of these patients (generally <5%) can experience MTC recurrence, a long-term follow-up should be recommended, with neck ultrasound examination and periodic CT determination on a regular basis [19, 60]. The same protocol may apply to patients with normal basal CT levels and mild to moderate elevations after provocative stimulation.
5.1.2 The Role of Postoperative CEA
Similarly to CT, serum CEA levels also decline after surgery. Nevertheless, the kinetics of CEA decline are not so well defined as in the case of CT. Usually also serum CEA levels increase at the time of relapse in patients with initially undetectable marker and parallel disease progression [3, 19]. From a clinical standpoint, it is currently accepted that either a marked elevation in the serum CEA level out of proportion to a lower serum CT level or normal/low levels of both serum CT and CEA are indicative of poorly differentiated MTC [3, 19].
Notably, postoperative CEA levels were found normal also in some patients with occult metastatic disease [62,63,64,65,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88,89,66].
6 Prognostic Value of CT and CEA in MTC Patients
The prognosis of any type of cancer is related to both the tumor burden as determined by imaging procedures and the cancer progression rate, estimated according the RECIST criteria [58]. These estimates can be complicated in MTC patients, where metastatic disease often involves multiple lesions in different organs, some of which (e.g., liver metastases) may be difficult to visualize [4]. For these reasons, several attempts have been made to identify simple surrogate markers. Indeed, none of the candidates (mitotic rate, the Ki67 labeling index, and 18F-fluorodeoxyglucose uptake on PET scan) appeared reliable enough for use in clinical practice [19, 60].
At present, CT and CEA represent the only available markers that have been proven of some prognostic utility [19, 60].
6.1 Prognostic Value of Preoperative CT
In a large series, preoperative CT levels exhibited a significant correlation with the maximum MTC diameter and with the postoperative hormone levels [67]. Only 2% of MTC patients with preoperative levels <50 pg/ml presented postoperative CT elevations [67], while increased hormone levels after surgery were reported in 17% of patients presenting preoperative levels <100 pg/ml [38] and in 37% of those with CT levels >500 pg/ml [68].
The magnitude of the CT increase after PG stimulation may also reflect the extent of disease. A strong relationship has, in fact, been demonstrated between a less than tenfold CT response to PG stimulation and lymph node involvement, distant metastases, extrathyroidal extension, and normalization of postoperative CT levels [69].
6.2 Prognostic Value of Preoperative CEA
The contribution of abnormal preoperative CEA levels on the recognition of more invasive MTCs, requiring aggressive surgery, remains presently unclear. Indeed, limited data seem to indicate that serum CEA concentrations are increased at diagnosis in up to 50% of the patients and its preoperative levels can be correlated to disease progression and prognosis [53]. Based on the hypothesis that increased CEA levels might herald advanced disease, a retrospective analysis conducted on a large MTC series addressed the question of the relationship between preoperative CEA levels and tumor progression [53]. On multivariate analysis, abnormal preoperative CEA concentrations were significantly associated with the initial surgery rather than re-intervention, larger primary tumors, lymph node, and distant metastases. Data analysis limited to the patients with increased CEA levels before primary surgery showed a significant association between progressively increasing CEA levels, lymph node, and distant metastases [53].
As reported earlier, patients with poorly differentiated and more aggressive MTCs frequently show a disproportionately high CEA/CT ratio [3, 19].
7 CT and CEA for the Postoperative Management of MTC Patients
As mentioned above, the delay necessary for reaching nadir levels of serum CT levels after MTC surgical treatment is still controversial [3]. Some studies have suggested that 3 months after surgery is the optimal time to reach nadir levels of serum CT in MTC patients [70, 71]. Due to a more protracted half-life [29], CEA serum levels may require a longer interval to reach the postoperative nadir. Therefore, both the ETA and the ATA guidelines have recommended that serum levels of CT and CEA should be measured 3 months after surgery [19, 60]. In patients with undetectable or normal postoperative levels, both markers should be measured every 6 months for the first year and then yearly [19, 60].
Even though the determination of CEA and CT serum levels is important for postoperative surveillance, there is no definitive evidence showing that they are clearly related to progressive or stable status of the disease. In current practice, persistently elevated postsurgical CT levels >10 pg/ml indicate residual MTC tissue [11]. In a relevant proportion of such patients, no other evidence of disease may be demonstrated. Indeed, this finding is compatible with long-term survival [72, 73], with one to two thirds of such patients not developing symptomatic disease for several years after surgery and presenting a 10-year recurrence rate approximating 40% [73]. Importantly, the extent of circulating CT elevation allows a reliable prediction of disease extension (Fig. 9.2, Table 9.4), being the probability of local or distant metastases strongly related to its levels [3, 4]. In particular, lower concentrations (e.g., <150 pg/ml) are more likely associated to disease confined to the neck, whereas higher levels are more suggestive of distant metastases [3, 4]. The likelihood of structural disease (e.g., tumor tissue identified by imaging studies) is also related to circulating CT increase [3, 4]. In this respect, it is recommended that patients with serum CT >150 pg/ml should undergo appropriate imaging procedures for the localization of distant metastatic lesions [19].
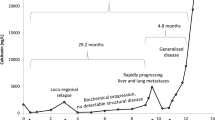
Correlation between serum calcitonin levels and disease progression in MTC patients. Basal plasma calcitonin levels are shown for a patient with advanced MTC followed for 20 years. The boxes highlight disease evolution during the follow-up period. After the primary treatment (total thyroidectomy, lymph node dissection, and radiation therapy), the patient displayed only biochemical evidence of persistent disease (calcitonin levels 134 ng/l). The calcitonin doubling time was approximately 3 years. Distant metastases in the liver appeared 7 years later, with disease progression despite chemotherapy. Death occurred after a 20-year period of follow-up. Abbreviations: M0 no distant metastases, MTC medullary thyroid carcinoma, N1 regional lymph node metastases, T4 primary tumor extension beyond the thyroid capsule and invading the subcutaneous soft tissues, larynx, trachea, esophagus, or recurrent laryngeal nerve. From Costante et al., Nat Clin Pract Endocrinol Metab. 2009;5:35–44
The relationship between tumor burden, levels of CT and CEA, and progression status according to RECIST criteria was analyzed in MTC patients presenting elevated CT levels and submitted to sequential imaging procedures [74]. That study indicated that CT and CEA levels were actually correlated with tumor burden. Nevertheless, no correlation could be demonstrated between CT and CEA levels and survival [74].
7.1 CT and CEA Doubling Times as Prognostic Factors
In the case of sporadic MTC patients presenting persistently elevated CT and CEA levels, the assessment of the progression status according to RECIST may often be complicated, even using standardized imaging procedures, particularly in case of slowly progressive tumors [58]. Moreover, CT levels can vary substantially (30% or more) within short periods of time [7, 13]. In these MTC patients, the dynamics of elevated CT and CEA levels can be more precisely quantified by calculating their doubling times (DT) [75] and compared to tumor growth rate in relation to RECIST evaluation [76, 77]. Notably, evaluation of DTs can be a long procedure [75], requiring measurements at multiple time points (at least four consecutive determinations over a minimum of 2 years). In case of DTs <6 months, the calculations should be performed based on CT and CEA levels over 1 year postoperatively [19].
A study of 45 MTC patients showed strong correlation between CT-DT and CEA-DT and RECIST findings in over 80% of patients. Additionally, 94% of those with CT-DT and CEA-DT <24 months had RECIST findings indicative of progressive disease, while 86% of the patients with longer DTs (>24 months) had disease classified as stable based on RECIST criteria [75]. Based on these results, the 2012 ETA guidelines recommended to determine CT-DT for the assessment of MTC progression rate and to eventually confirm it by imaging procedures according to RECIST criteria [58]. Analogously, the most recent ATA guidelines for MTC management [19] recommended the determination of CT-DT and CEA-DT by measuring their serum levels at least every 6 months.
In this respect, a retrospective study evaluated 65 patients treated by total thyroidectomy and bilateral lymph node dissection, from 2.9 to 29.5 years after surgery [75]. In patients with serum CT-DT <6 months, the survival rate was 25% at 5 and 8% at 10 years, while it increased to 92% and 37%, respectively, for DTs between 6 and 24 months. Additionally, it has been reported that a DT of circulating CT <6 months carried a 75% risk of cancer-related death within the following 5 years, while DTs of >2 years were associated with no tumor-related death [74].
In rare cases, an MTC patient with a large tumor burden may present with low CT levels. If the possibility of a “hook effect” can be excluded [16], this finding may reflect a poorly differentiated tumor [3]. In cases of this type, CEA levels are usually increased, and the CEA-DT is likely to be reduced [4]. Such a discrepancy could herald aggressive disease, particularly if the CEA-DT is shorter as compared to the CT-DT [74].
In contrast to CT, only few studies have investigated the relationship between CEA-DT and survival outcomes. The available evidence suggests that CT-DT could be a more effective predictor of survival than the CEA-DT (7, 78). Nevertheless, a study [74] reported an 80% frequency of concordant CT and CEA DTs, with an average DTs of both CEA and CT of 12 months in patients with progressive disease, while stable disease was observed for DTs of 48 months for CEA-DT and 58 months for CT-DT. Importantly, progressive disease was evident in 94% of patients with DTs of both markers <24 months, while no evidence of progression could be demonstrated in 86% of patients with CT-DT and CEA-DT >24 months. For discordant DTs, progressive disease was observed in more than 50% of patients presenting a DT <25 months for either CT or CEA.
In a meta-analysis from Meijer et al., CEA-DT displayed a more pronounced impact on the prognosis [78]. This study showed that both CT-DT and CEA-DT were significant risk factors for recurrence and death caused by MTC for the cutoff value of 1 year. In the subgroup of patients for whom both CT and CEA were available, the model with CEA-DT <1 year had a higher predictive value for survival as compared to CT-DT. To explain the higher predictive value of CEA-DT, the authors speculated that CT and CEA production per individual MTC cell may not be constant during the course of the disease, particularly during the process of dedifferentiation, when CT production can be relatively normal [57, 79] or even decreased, while an increase in CEA production may occur [80].
In clinical practice, therefore, the DTs of both CT and CEA could be important for an adequate risk stratification of MTC patients. In this respect, both the ATA and ETA guidelines [19, 60] agreed that the CT-DT and CEA-DT of less than 2 years should be considered negative prognostic factors for MTC in patients with persistent or recurrent disease.
8 CT and CEA Measurement for the Assessment of Treatment Response
Because circulating CT and CEA levels are directly correlated to MTC tumor burden, their variations not only represent a useful parameter for estimating tumor progression but can also provide important information on the response to treatment. Indeed, their clinical utility for the decision-making process and for the evaluation of response to local (i.e., surgery, radiotherapy, interventional procedures) treatment in early and advanced/recurrent MTC is well known since many years [4].
Recently, tyrosine kinase inhibitors (TKIs) vandetanib and cabozantinib demonstrated improved objective response rates as compared to cytotoxic chemotherapy, with a significant increase in progression-free survival (PFS) [81,82,83], and have eventually been approved by both FDA and EMA for use in rapidly progressive recurrent/metastatic MTCs. Sequential determinations of serum CT levels have been used as potential marker for assessing MTC response to these drugs, based on the assumption that decreased CT concentrations during these treatments could effectively reflect targeting of tumor cells [59] and consequent tumor shrinkage or at least inhibition of either or both CT synthesis/secretion. Unfortunately, all trials evaluating RET inhibitors were unable to confirm the reliability of such a paradigm. No clear correlation could, in fact, be demonstrated between the variation in either CT or CEA levels and the response to TKI in terms of degree or duration of the observed objective responses [81, 84, 85]. Moreover, fluctuations in CEA and CT serum levels were observed in MTC patients irrespective of either response to treatment or tumor progression on TKI treatment [59]. Additionally, paradoxical increases in both biomarkers occurred in some responders [82, 86]. Eventually, most MTC patients presenting tremendously decreased CT and CEA levels after receiving TKIs frequently exhibited rebounds and oscillations up to 30% magnitude, without any correlation in terms of RECIST criteria [81,82,83,84, 87]. Dissociated effect of RET inhibitors on the proliferative and the secretory pathways of thyroid C cells has been suggested to explain such results [88], at least for CT. No direct effect of RET inhibition on CEA expression has been observed [89].
Recently, a retrospective study has addressed the issue of CEA and CT variations as predictive factors for tumor progression in MTC patients treated with vandetanib [89], showing that objective progressive disease (PD) could be predicted with 82% accuracy in case of 40% increase in serum CT levels. In contrast, no value for prediction of structural progression during TKI treatment could be demonstrated for variations of CEA levels.
A retrospective review [90] aimed at evaluating CT or CEA levels as surrogate markers of cell toxicity induced by traditional cytotoxic agents in MTC patients reported no correlation between early circulating CT or CEA variation and overall survival (OS) during the first 3 months of chemotherapy. No significant relationship between changes in CT levels and either tumor response or progression-free survival (PFS) could be demonstrated, though an increase in CEA levels was associated to a significantly shorter PFS. Based on this observation, the authors suggested that discontinuation of cytotoxic chemotherapy should be considered in case of CEA progression after 3 months of treatment. A prospective study is necessary for definitive validation of these results.
9 Conclusions and Key Points
CT and CEA are useful tools for the diagnosis and the management of MTCs.
-
1.
In the preoperative settings, circulating CT is the most specific and sensitive disease marker for MTC, while CEA displays a lower diagnostic accuracy, and both are of prognostic utility, allowing accurate estimation of tumor burden.
-
2.
During the postsurgical follow-up of MTC patients, the evolution of both CT and CEA levels reflects MTC progression, and their increase reliably heralds persistence/relapse.
-
3.
Discrepant results with disproportionately low circulating CT as compared to CEA levels should alert the clinician suggesting dedifferentiated, more aggressive MTCs.
-
4.
CT and CEA doubling times (DT) can be used as prognostic factors, representing CT-DT a more precise indicator of MTC progression than CEA-DT.
-
5.
Circulating CT and CEA levels are accurate markers of response to local treatment in early and advanced/recurrent MTC, but their suitability for the evaluation of response to systemic therapy needs confirmation.
References
Hazard JB. The C cells (parafollicular cells) of the thyroid gland and medullary thyroid carcinoma. A review. Am J Pathol. 1977;88:213–50.
LiVolsi VA. C cell hyperplasia/neoplasia. J Clin Endocrinol Metab. 1997;82:39–41.
Costante G, Durante C, Francis Z, Schlumberger M, Filetti S. Determination of calcitonin levels in C-cell disease: clinical interest and potential pitfalls. Nat Clin Pract Endocrinol Metab. 2009;5:35–44.
Leboulleux S, Baudin E, Travagli JP, Schlumberger M. Medullary thyroid carcinoma. Clin Endocrinol. 2004;61:229–31.
Guyétant S, Rousselet MC, Durigon M, Chappard D, Franc B, Guerin O, Saint-André JP. Sex-related C cell hyperplasia in the normal human thyroid: a quantitative autopsy study. J Clin Endocrinol Metab. 1997;82:42–7.
Wells SA Jr, Pacini F, Robinson BG, Santoro M. Multiple endocrine neoplasia type 2 and familial medullary thyroid carcinoma: an update. J Clin Endocrinol Metab. 2013;98:3149–64. https://doi.org/10.1210/jc.2013-1204.
Essig GF Jr, Porter K, Schneider D, Debora A, Lindsey SC, Busonero G, et al. Fine needle aspiration and medullary thyroid carcinoma: the risk of inadequate preoperative evaluation and initial surgery when relying upon FNAB cytology alone. Endocr Pract. 2013;19:920–7.
Trimboli P, Treglia G, Guidobaldi L, Romanelli F, Nigri G, Valabrega S, Sadeghi R, Crescenzi A, Faquin WC, Bongiovanni M, Giovanella L. Detection rate of FNA cytology in medullary thyroid carcinoma: a meta-analysis. Clin Endocrinol. 2015;82:280–5. https://doi.org/10.1111/cen.12563. Epub 2014, Aug 14
Gold P, Freedman SO. Specific carcinoembryonic antigens of the human digestive system. J Exp Med. 1965 Sep 1;122:467–81.
Krupey J, Gold P, Freedman SO. Purification and characterization of carcinoembryonic antigens of the human digestive system. Nature. 1967 Jul 1;215:67–8.
Baloch Z, Carayon P, Conte-Devolx B, Demers LM, Feldt-Rasmussen U, Henry JF, LiVosli VA, Niccoli-Sire P, John R, Ruf J, Smyth PP, Spencer CA, Stockigt JR, Guidelines Committee, National Academy of Clinical Biochemistry. Laboratory medicine practice guidelines. Laboratory support for the diagnosis and monitoring of thyroid disease. Thyroid. 2003;13:68–79.
Becker KL, Nylén ES, White JC, Müller B, Snider RH Jr. Clinical review 167: Procalcitonin and the calcitonin gene family of peptides in inflammation, infection, and sepsis: a journey from calcitonin back to its precursors. J Clin Endocrinol Metab. 2004;89:1512–25.
Whang KT, Steinwald PM, White JC, Nylen ES, Snider RH, Simon GL, Goldberg RL, Becker KL. Serum calcitonin precursors in sepsis and systemic inflammation. J Clin Endocrinol Metab. 1998;83:3296–301.
Preissner CM, Dodge LA, O’Kane DJ, Singh RJ, Grebe SK. Prevalence of heterophilic antibody interference in eight automated tumor marker immunoassays. Clin Chem. 2005;51:208–10.
Leboeuf R, Langlois MF, Martin M, Ahnadi CE, Fink GD. “Hook effect” in calcitonin immunoradiometric assay in patients with metastatic medullary thyroid carcinoma: case report and review of the literature. J Clin Endocrinol Metab. 2006;91:361–4.
Basuyau JP, Mallet E, Leroy M, Brunelle P. Reference intervals for serum calcitonin in men, women, and children. Clin Chem. 2004;50:1828–30.
Guyetant S, Rousselet MC, Durigon M, Chappard D, Franc B, Guerin O, Saint-Andre JP. Sex-related C cell hyperplasia in the normal human thyroid: a quantitative autopsy study. J Clin Endocrinol Metab. 1997;82:42–7.
d’Herbomez M, Caron P, Bauters C, Do Cao C, Schlienger JL, Sapin R, Baldet L, Carnaille B, Wémeau JL, French Group GTE (Groupe des Tumeurs Endocrines). Reference range of serum calcitonin levels in humans: influence of calcitonin assays, sex, age, and cigarette smoking. Eur J Endocrinol. 2007;157:749–55.
Wells SA Jr, Asa SL, Dralle H, Elisei R, Evans DB, Gagel RF, Lee N, Machens A, Moley JF, Pacini F, Raue F, Frank-Raue K, Robinson B, Rosenthal MS, Santoro M, Schlumberger M, Shah M, Waguespack SG. American Thyroid Association Guidelines Task Force on Medullary Thyroid Carcinoma. Revised American Thyroid Association guidelines for the management of medullary thyroid carcinoma. Thyroid. 2015 Jun;25(6):567–610. https://doi.org/10.1089/thy.2014.0335.
Grani G, Nesca A, Del Sordo M, Calvanese A, Carbotta G, Bianchini M. Interpretation of serum calcitonin in patients with chronic autoimmune thyroiditis. Endocr Relat Cancer. 2012;19:345–9.
Hennessy JF, Wells SA Jr, Ontjes DA, Cooper CW. A comparison of pentagastrin injection and calcium infusion as provocative agents for the detection of medullary carcinoma of the thyroid. J Clin Endocrinol Metab. 1974;39:487–95.
Wells SA Jr, Baylin SB, Linehan WM, Farrell RE, Cox EB, Cooper CW. Provocative agents and the diagnosis of medullary carcinoma of the thyroid gland. Ann Surg. 1978;188:139–41.
Leboulleux S, Baudin E, Young J, Caillou B, Lazar V, Pellegriti G, Ducreux M, Schaison G, Schlumberger M. Gastroenteropancreatic neuroendocrine tumor metastases to the thyroid gland: differential diagnosis with medullary thyroid carcinoma. Eur J Endocrinol. 1999;140:187–91.
Verdy M, Cholette JP, Cantin J, Lacroix A, Sturtridge WC. Calcium infusion and pentagastrin injection in diagnosis of medullary thyroid carcinoma. Can Med Assoc J. 1978;119:29–35.
Gharib H, Kao PC, Heath H 3rd. Determination of silica-purified plasma calcitonin for the detection and management of medullary thyroid carcinoma: comparison of two provocative tests. Mayo Clin Proc. 1987;62:373–8.
O’Connell JE, Dominiczak AF, Isles CG, McLellan AR, Davidson G, Gray CE, Connell JM. A comparison of calcium pentagastrin and TRH tests in screening for medullary carcinoma of the thyroid in MEN IIA. Clin Endocrinol. 1990;32:417–21.
Mian C, Perrino M, Colombo C, Cavedon E, Pennelli G, Ferrero S, et al. Refining calcium test for the diagnosis of medullary thyroid cancer: cutoffs, procedures, and safety. J Clin Endocrinol Metab. 2014;99:1656–64.
Hammarström S. The carcinoembryonic antigen (CEA) family: structures, suggested functions and expression in normal and malignant tissues. Semin Cancer Biol. 1999;9:67–81.
Chatterjea MN, Shinde R. Textbook of medical biochemistry. 8th ed. New Delhi: Jaypee Brothers Medical Publishers Ltd; 2012. p. 790–814.
Thomas SN, Tong Z, Stebe KJ, Konstantopoulos K. Identification, characterization and utilization of tumor cell selectin ligands in the design of colon cancer diagnostics. Biorheology. 2009;46:207–25. https://doi.org/10.3233/BIR-2009-0534.
Valdes R, Stein KE. Comparison of RIA and IRMA methods for measurement of carcinoembryonic antigen (CEA). Clin Biochem. 1982;15:241–7.
Zur B, Holdenrieder S, Walgenbach-Brünagel G, Albers E, Stoffel-Wagner B. Method comparison for determination of the tumor markers AFP, CEA, PSA and free PSA between Immulite 2000 XPI and Dimension Vista 1500. Clin Lab. 2012;58:97–105.
Herbeth B, Bagrel A. A study of factors influencing plasma CEA levels in an unselected population. Oncodev Biol Med. 1980;1:191–8.
Renehan AG, Egger M, Saunders MP, O’Dwyer ST. Impact on survival of intensive follow up after curative resection for colorectal cancer: systematic review and meta-analysis of randomised trials. BMJ. 2002;324:813.
Wanebo HJ, Rao B, Pinsky CM, Hoffman RG, Stearns M, Schwartz MK, Oettgen HF. Preoperative carcinoembryonic antigen level as a prognostic indicator in colorectal cancer. N Engl J Med. 1978;299:448–51.
Chang TC, Wu SL, Hsiao YL. Medullary thyroid carcinoma: pitfalls in diagnosis by fine needle aspiration cytology and relationship of cytomorphology to RET proto-oncogene mutations. Acta Cytol. 2005;49:477–82.
Papaparaskeva K, Nagel H, Droese M. Cytologic diagnosis of medullary carcinoma of the thyroid gland. Diagn Cytopathol. 2000;22:351–8.
Costante G, Meringolo D, Durante C, Bianchi D, Nocera M, Tumino S, et al. Predictive value of serum calcitonin levels for preoperative diagnosis of medullary thyroid carcinoma in a cohort of 5817 consecutive patients with thyroid nodules. J Clin Endocrinol Metab. 2007;92:450–5.
Pacini FF, Fontanelli M, Fugazzola L, Elisei R, Romei C, Di Coscio G, et al. Routine measurement of serum calcitonin in nodular thyroid diseases allows the preoperative diagnosis of unsuspected sporadic medullary thyroid carcinoma. J Clin Endocrinol Metab. 1994;78:826–9.
Elisei R, Bottici V, Luchetti F, Luchetti F, Di Coscio G, Romei C, Grasso L, et al. Impact of routine measurement of serum calcitonin on the diagnosis and outcome of medullary thyroid cancer: experience in 10,864 patients with nodular thyroid disorders. J Clin Endocrinol Metab. 2004;89(1):163–8.
Milone F, Ramundo V, Chiofalo MG, Severino R, Paciolla I, Pezzullo L, et al. Predictive value of pentagastrin test for preoperative differential diagnosis between C-cell hyperplasia and medullary thyroid carcinoma in patients with moderately elevated basal calcitonin levels. Clin Endocrinol. 2010;73:85–8.
Costante G, Filetti S. Early diagnosis of medullary thyroid carcinoma: is systematic calcitonin screening appropriate in patients with nodular thyroid disease? Oncologist. 2011;16:49–52. https://doi.org/10.1634/theoncologist.2010-0344.
Gharib H, Papini E, Paschke R, Duick DS, Valcavi R, Hegedüs L, et al. American Association of Clinical Endocrinologists, Associazione Medici Endocrinologi, and European Thyroid Association medical guidelines for clinical practice for the diagnosis and management of thyroid nodules: Executive Summary of recommendations. J Endocrinol Investig. 2010;33:1–50.
Haugen BR, Alexander EK, Bible KC, Doherty GM, Mandel SJ, Nikiforov YE, Pacini F, Randolph GW, Sawka AM, Schlumberger M, Schuff KG, Sherman SI, Sosa JA, Steward DL, Tuttle RM, Wartofsky L. 2015 American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid. 2016 Jan;26(1):1–133. https://doi.org/10.1089/thy.2015.0020.
American Thyroid Association (ATA) Guidelines Taskforce on Thyroid Nodules and Differentiated Thyroid Cancer, Cooper DS, Doherty GM, Haugen BR, Kloos RT, Lee SL, et al. Revised American Thyroid Association management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid. 2009;19:1167–214.
Trimboli P, Nigri G, Romanelli F, et al. Medullary thyroid nodulesby measurement of calcitonin (Ct) in aspiration needle washout in patients with multinodular goiter and moderately elevated serum Ct. Exp Clin Endocrinol Diabetes. 2012;120:234–7.
Trimboli P, Rossi F, Baldelli R, et al. Measuring calcitonin in washout of the needle in patients undergoing fine needle aspiration with suspicious medullary thyroid cancer. Diagn Cytopathol. 2012;40:394–8.
Trimboli P, Cremonini N, Ceriani L, et al. Calcitonin measurement in aspiration needle washout fluids has higher sensitivity than cytology in detecting medullary thyroid cancer: A retrospective multicentre study. Clin Endocrinol. 2014;80:135–40.
de Lima MA, Santos BM, Tiveron FS, de Abreu ME. C cells in normal thyroid aspirates. Acta Cytol. 1999;43:558–62.
Mendelsohn G, Wells SA Jr, Baylin SB. Relationship of tissue carcinoembryonic antigen and calcitonin to tumor virulence in medullary thyroid carcinoma. An immunohistochemical study in early, localized, and virulent disseminated stages of disease. Cancer. 1984;54:657–62.
Wells SA, Haagensen DE, Linehan WM, Farrell RE, Dilley WG. The detection of elevated plasma levels of carcinoembryonic antigen in patients with suspected or established medullary thyroid carcinoma. Cancer. 1978;42:1498–503.
Rougier P, Calmettes C, Laplanche A, Travagli JP, Lefevre M, Parmentier C, Milhaud G, Tubiana M. The values of calcitonin and carcinoembryonic antigen in the treatment and management of nonfamilial medullary thyroid carcinoma. Cancer. 1983;51:855–62.
Machens A, Ukkat J, Hauptmann S, Dralle H. Abnormal carcinoembryonic antigen levels and medullary thyroid cancer progression: a multivariate analysis. Arch Surg. 2007;142:289–93.
Giovanella L, Crippa S, Cariani L. Serum calcitonin-negative medullary thyroid carcinoma: role of CgA and CEA as complementary markers. Int J Biol Markers. 2008;23:129–31.
Frank-Raue K, Machens A, Leidig-Bruckner G, Rondot S, Haag C, Schulze E, Lorenz A, Kreissl MC, Dralle H, Raue F, Schmid KW. Prevalence and clinical spectrum of nonsecretory medullary thyroid carcinoma in a series of 839 patients with sporadic medullary thyroid carcinoma. Thyroid. 2013;23:294–300.
Bockhorn M, Frilling A, Rewerk S, et al. Lack of elevated serum carcinoembryonic antigen and calcitonin in medullary thyroid carcinoma. Thyroid. 2004;14:468–70.
Dora JM, Canalli MH, Capp C, et al. Normal perioperative serum calcitonin levels in patients with advanced medullary thyroid carcinoma: case report and review of the literature. Thyroid. 2008;18:895–9.
Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, Verweij J, Van Glabbeke M, van Oosterom AT, Christian MC, Gwyther SG. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–16.
Schlumberger M, Carlomagno F, Baudin E, Bidart JM, Santoro M. New therapeutic approaches to treat medullary thyroid carcinoma. Nat Clin Pract Endocrinol Metab. 2008;4:22–32.
Schlumberger M, Bastholt L, Dralle H, Jarzab B, Pacini F, Smit JW, European Thyroid Association Task Force. European thyroid association guidelines for metastatic medullary thyroid cancer. Eur Thyroid J. 2012;1:5–14. https://doi.org/10.1159/000336977.
Brauckhoff M, Gimm O, Brauckhoff K, Ukkat J, Thomusch O, Dralle H. Calcitonin kinetics in the early postoperative period of medullary thyroid carcinoma. Langenbeck’s Arch Surg. 2001;386:434–9.
Vuillez JP, Peltier P, Caravel JP, Chetanneau A, Saccavini JC, Chatal JF. Immunoscintigraphy using 111In-labeled F(ab’)2 fragments of anticarcinoembryonic antigen monoclonal antibody for detecting recurrences of medullary thyroid carcinoma. J Clin Endocrinol Metab. 1992;74:157–63.
Peltier P, Curtet C, Chatal JF, et al. Radioimmunodetection of medullary thyroid cancer using a bispecific anti-CEA ⁄ antiindium- DTPA antibody and an indium-111-labeled DTPA dimer. J Nucl Med. 1993;34:1267–73.
Behr TM, Gratz S, Markus PM, et al. Anti-carcinoembryonic antigen antibodies versus somatostatin analogs in the detection of metastatic medullary thyroid carcinoma. Cancer. 1997;80:2436–57.
Barbet J, Peltier P, Bardet S, et al. Radioimmunodetection of medullary thyroid carcinoma using indium-111 bivalent hapten and anti-CEA_anti-DTPA-indium bispecific antibody. J Nucl Med. 1998;39:1172–8.
Cohen R, Campos JM, Salaün C, Heshmati HM, Kraimps JL, Proye C, Sarfati E, Henry JF, Niccoli-Sire P, Modigliani E. Preoperative calcitonin levels are predictive of tumor size and postoperative calcitonin normalization in medullary thyroid carcinoma. Groupe d’Etudes des Tumeurs a Calcitonine (GETC). J Clin Endocrinol Metab. 2000;85:919–22.
Machens A, Schneyer U, Holzhausen HJ, Dralle H. Prospects of remission in medullary thyroid carcinoma according to basal calcitonin level. J Clin Endocrinol Metab. 2005;90:2029–34.
Machens A, Hauptmann S, Dralle H. Medullary thyroid cancer responsiveness to pentagastrin stimulation: an early surrogate parameter of tumor dissemination? J Clin Endocrinol Metab. 2008;93:2234–8.
Ismailov SI, Piulatova NR. Postoperative calcitonin study in medullary thyroid carcinoma. Endocr Relat Cancer. 2004;11:357–63.
Elisei R, Pinchera A. Advances in the follow-up of differentiated or medullary thyroid cancer. Nat Rev Endocrinol. 2012;8:466–75.
Van Heerden JA, Grant CS, Gharib H, Hay ID, Ilstrup DM. Long term course of patients with persistent hypercalcitoninemia after apparent curative primary surgery for medullary thyroid carcinoma. Ann Surg. 1990;212:395–401.
Pellegriti G, Leboulleux S, Baudin E, Bellon N, Scollo C, Travagli JP, Schlumberger M. Long-term outcome of medullary thyroid carcinoma in patients with normal postoperative medical imaging. Br J Cancer. 2003;88:1537–42.
Giraudet AL, Al Ghulzan A, Auperin A, Leboulleux S, Chehboun A, Troalen F, Dromain C, Lumbroso J, Baudin E, Schlumberger M. Progression of medullary thyroid carcinoma: assessment with calcitonin and carcinoembryonic antigen doubling times. Eur J Endocrinol. 2008;158:239–46.
Barbet J, Campion L, Kraeber-Bodéré F, Chatal JF, GTE Study Group. Prognostic impact of serum calcitonin and carcinoembryonic antigen doubling-times in patients with medullary thyroid carcinoma. J Clin Endocrinol Metab. 2005;90:6077–84.
Miyauchi A, Onishi T, Morimoto S, Takai S, Matsuzuka F, Kuma K, Maeda M, Kumahara Y. Relation of doubling time of plasma calcitonin levels to prognosis and recurrence of medullary thyroid carcinoma. Ann Surg. 1984;199:461–6.
Saad MF, Fritsche HA Jr, Samaan NA. Diagnostic and prognostic values of carcinoembryonic antigen in medullary carcinoma of the thyroid. J Clin Endocrinol Metab. 1984;58:889–94.
Meijer J, le Cessie S, van den Hout WB, Kievit J, Schoones JW, Romijn JA, Smit JW. Calcitonin and CEA doubling times as prognostic factors in medullary thyroid carcinoma, a structured meta-analysis. Clin Endocrinol. 2010;72:534–42.
Wang TS, Ocal IT, Sosa JA, Cox H, Roman S. Medullary thyroid carcinoma without marked elevation of calcitonin: a diagnostic and surveillance dilemma. Thyroid. 2008;18:889–94.
Osaka M, Soga J, Tamiya Y, Suzuki T. Dedifferentiation of neoplastic cells in medullary thyroid carcinoma: report of a case. Surg Today. 1999;29:1189–94.
Wells SA Jr, Gosnell JE, Gagel RF, Moley J, Pfister D, Sosa JA, Skinner M, Krebs A, Vasselli J, Schlumberger M. Vandetanib for the treatment of patients with locally advanced or metastatic hereditary medullary thyroid cancer. J Clin Oncol. 2010;28:767–72.
Wells SA Jr, Robinson BG, Gagel RF, Dralle H, Fagin JA, Santoro M, Baudin E, Elisei R, Jarzab B, Vasselli JR, Read J, Langmuir P, Ryan AJ, Schlumberger MJ. Vandetanib in patients with locally advanced or metastatic medullary thyroid cancer: a randomized, double-blind phase III trial. J Clin Oncol. 2012;30:134–41.
Kurzrock R, Sherman SI, Ball DW, Forastiere AA, Cohen RB, Mehra R, Pfister DG, Cohen EE, Janisch L, Nauling F, Hong DS, Ng CS, Ye L, Gagel RF, Frye J, Müller T, Ratain MJ, Salgia R. Activity of XL184 (Cabozantinib), an oral tyrosine kinase inhibitor, in patients with medullary thyroid cancer. J Clin Oncol. 2011;29:2660–6.
Schlumberger MJ, Elisei R, Bastholt L, Wirth LJ, Martins RG, Locati LD, Jarzab B, Pacini F, Daumerie C, Droz JP, Eschenberg MJ, Sun YN, Juan T, Stepan DE, Sherman SI. Phase II study of safety and efficacy of motesanib in patients with progressive or symptomatic, advanced or metastatic medullary thyroid cancer. J Clin Oncol. 2009;27:3794–801.
Hong DS, Sebti SM, Newman RA, Blaskovich MA, Ye L, Gagel RF, Moulder S, Wheler JJ, Naing A, Tannir NM, Ng CS, Sherman SI, El Naggar AK, Khan R, Trent J, Wright JJ, Kurzrock R. Phase I trial of a combination of the multikinase inhibitor sorafenib and the farnesyltransferase inhibitor tipifarnib in advanced malignancies. Clin Cancer Res. 2009;15:7061–8.
Hong DS, Cabanillas ME, Wheler J, Naing A, Tsimberidou AM, Ye L, Busaidy NL, Waguespack SG, Hernandez M, El Naggar AK, Bidyasar S, Wright J, Sherman SI, Kurzrock R. Inhibition of the Ras/Raf/MEK/ERK and RET kinase pathways with the combination of the multikinase inhibitor sorafenib and the farnesyltransferase inhibitor tipifarnib in medullary and differentiated thyroid malignancies. J Clin Endocrinol Metab. 2011;96:997–1005.
Lam ET, Ringel MD, Kloos RT, Prior TW, Knopp MV, Liang J, Sammet S, Hall NC, Wakely PE Jr, Vasko VV, Saji M, Snyder PJ, Wei L, Arbogast D, Collamore M, Wright JJ, Moley JF, Villalona-Calero MA, Shah MH, et al. Phase II clinical trial of sorafenib in metastatic medullary thyroid cancer. J Clin Oncol. 2010;28:2323–30.
Akeno-Stuart N, Croyle M, Knauf JA, Malaguarnera R, Vitagliano D, Santoro M, Stephan C, Grosios K, Wartmann M, Cozens R, Caravatti G, Fabbro D, Lane HA, Fagin JA. The RET kinase inhibitor NVP-AST487 blocks growth and calcitonin gene expression through distinct mechanisms in medullary thyroid cancer cells. Cancer Res. 2007;67:6956–64.
Werner RA, Schmid JS, Muegge DO, Lückerath K, Higuchi T, Hänscheid H, Grelle I, Reiners C, Herrmann K, Buck AK, Lapa C. Prognostic value of serum tumor markers in medullary thyroid cancer patients undergoing Vandetanib treatment. Medicine. 2015;94(45):1–5.
Hajje G, Borget I, Leboulleux S, Chougnet C, Al Ghuzlan A, Mirghani H, Caramella C, Hartl D, Schlumberger M, Baudin E. Early changes in carcinoembryonic antigen but not in calcitonin levels are correlated with the progression-free survival in medullary thyroid carcinoma patients treated with cytotoxic chemotherapy. Eur J Endocrinol. 2013;168:113–8.
Kurzrock R, Atkins J, Wheler J, Fu S, Naing A, Busaidy N, Hong D, Sherman S. Tumor marker and measurement fluctuations may not reflect treatment efficacy in patients with medullary thyroid carcinoma on longterm RET inhibitor therapy. Ann Oncol. 2013;24:2256–61. https://doi.org/10.1093/annonc/mdt177. Epub 2013 May 14.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 Springer International Publishing AG
About this chapter
Cite this chapter
Costante, G., Lalami, Y., Jungels, C., Awada, A. (2018). Calcitonin and Carcinoembryonic Antigen for the Diagnosis and Management of Medullary Thyroid Carcinoma. In: Giovanella, L. (eds) Atlas of Thyroid and Neuroendocrine Tumor Markers. Springer, Cham. https://doi.org/10.1007/978-3-319-62506-5_9
Download citation
DOI: https://doi.org/10.1007/978-3-319-62506-5_9
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-62505-8
Online ISBN: 978-3-319-62506-5
eBook Packages: MedicineMedicine (R0)