Abstract
Blood-based biomarkers are valuable tools for the diagnosis, estimation of prognosis, and monitoring of therapy responsiveness and disease course in patients with neuroendocrine tumors (NETs). Prior to their clinical use, the methodical and quality soundness of biomarker assays, robustness against potentially influencing preanalytical factors, and their utility for the clinical question of interest have to be investigated thoroughly. In gastroenteropancreatic NETs, chromogranin A (CgA) has proven to be the most informative monoanalyte, while in lung NETs progastrin-related peptide (ProGRP) and neuron-specific enolase (NSE) provide the best diagnostic performance and are valuable for disease monitoring. New approaches include multimarker analysis such as the 51 gene-based NETest that shows an excellent sensitivity and specificity profile for the detection of gastroenteropancreatic NETs and may improve the biomarker-based diagnostic in the future.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
- Therapy Responsiveness
- Gastroenteropancreatic NETs
- Neuroendocrine Tumors (NETs)
- Neuron-specific Enolase
- Small Cell Lung Cancer (SCLC)
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
1 Biological Basis and Use of Biomarkers
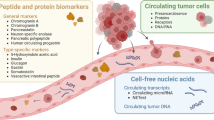
Biological markers are alterations on the cellular, biochemical, or molecular level that can be objectively measured in the tissue, blood, or other bodily fluids and that indicate a physiological or pathophysiological condition or a response to a therapeutic intervention [1, 2]. Biomarkers are frequently used for multiple indications such as risk assessment or prediction of a disease, diagnosis, estimating prognosis, or monitoring the disease course during or after therapy. Thereby they complement other diagnostic approaches such as imaging or clinical exams. Blood or body fluid biomarkers comprise cells, cellular particles, and diverse molecules such as proteins, peptides, amino acids, carbohydrates, lipids, nucleic acids, drugs, and others. In cancer disease, cell surface and secreted proteins and peptides are most frequently used. Newer approaches include circulating nucleic acids that are released from cancer cells into plasma and serum (CNAPS) such as cell-free tumor DNA (ctDNA) with its genetic or epigenetic characteristics, gene expression fingerprints, as well as patterns of regulative noncoding RNAs (miRNAs and lncRNAs). These markers can also be extracted from circulating cancer cells and exosomes that constitute an enrichment compartment for cancer-specific markers [3, 4]. To detect and quantify biomarkers reliably, highly sensitive and specific techniques are needed, and rigorous quality controls have to be performed in laboratories dedicated to patient diagnostics.
This chapter focusses on blood-based biomarkers that are in use for the diagnosis and management of patients with neuroendocrine tumors. While definitive diagnosis still requires imaging and tissue exams, this approach has several advantages as blood drawing is only minimal invasive and can be done serially in individuals. Furthermore, analyses are objective, quantitative, highly sensitive, robust, cost-effective, and highly quality controlled. During course of cancer disease, biomarkers that circulate in the blood can be employed to answer many questions that are highly relevant for the management of health and disease in a specific person. In detail they are applied for the following indications (Fig. 3.1):
-
The screening of presumably healthy persons (without any symptoms)
-
The monitoring of persons at risk for cancer disease (but without symptoms)
-
The risk estimation of a person with suspicious symptoms or signs
-
The definitive estimation of differential diagnosis in persons with specific symptoms
-
The estimation of severeness (and staging) of a cancer disease
-
The estimation of prognosis in patients with a defined cancer diagnosis
-
The stratification of cancer patients for a specific therapy
-
The monitoring of the response to anticancer therapy
-
The early estimation of therapy response as a special application
-
The monitoring of a patient after the primary therapy
-
The early detection of recurrent disease
Blood-based biomarkers can be used for many indications during the course of cancer disease, for (1) cancer detection and differential diagnosis, (2) estimation of prognosis, (3) prediction and monitoring of therapy response, (4) early detection of therapy resistance and of recurrent disease. Biomarker changes in relation to individual baseline values often sensitively mirror the course of disease. Cancer screening is the most challenging indication for circulating biomarkers (Adapted from [5]; with permission from Springer)
In cancer patients, one-time biomarker determinations (often a combination of several markers) are performed for (1) screening purposes, (2) supporting differential diagnosis, and (3) estimating prognosis. In contrast, the monitoring of serial biomarker testings is mostly applied for (1) the screening of patients who are at risk for cancer disease, (2) the monitoring response to local or systemic therapies, and (3) the early detection of disease recurrence after the primary therapy has been finished. Newer biomarkers such as CNAPS markers are highly meaningful as companion diagnostics to stratify patients for a newly developed targeted therapy and to monitor the responsiveness of this therapy as well as for the detection of drug resistance and biochemical recurrence in order to enable an early and specific therapy adaptation on an individual basis [5].
2 Methods and Quality Requirements for Biomarkers
In order to give reliable and meaningful results that can be used for patient guidance, circulating biomarkers and the methods that are applied for their determination have to fulfill the highest methodical, preanalytical, and clinical quality criteria if they are to be implemented into patient care. There are several methodical preconditions biomarker assays have to meet [6, 7], among others:
-
A high analytical sensitivity (the analyte is detected at very low concentrations)
-
A high analytical specificity (only the analyte is measured)
-
A high accuracy including a high intra- and between-run imprecision
-
A high recovery and dilution linearity in the given matrix
-
A high robustness against potentially disturbing factors
Analytical performance of the assays has to be regularly controlled by internal and external quality controls.
Preanalytical aspects may greatly influence the results of biomarker measurements. Therefore, preanalytics should be standardized for routine diagnostics as well as for study settings. The following aspects have to be considered:
-
The conditions of the patient and the blood drawing
(time, fasting, position of the patient, tourniquet time, type of needle, etc.)
-
The conditions of the material
(type of blood matrix, i.e., serum or plasma, additives, tubes, volumes, etc.)
-
The conditions of the transport to the lab (time, temperature, pneumatic delivery, etc.)
-
The conditions of the centrifugation (time, temperature, speed, braking, etc.)
-
The conditions of the sample handling
(storage time, temperature, extraction, deep freezing, thawing frequency, etc.)
Potentially influencing preanalytical factors have to be considered prior to marker analysis as well as for the interpretation of marker results [5, 6].
3 Clinical Performance of Biomarkers
If biomarkers are applied to diverse clinical indications, some measures are informative about their clinical performance. For differential diagnosis of cancer disease, the clinical sensitivity and specificity of cancer biomarkers are greatly meaningful. The sensitivity indicates the percentage of positive results in the cancer patient group while the specificity is the percentage of negative results in the control group. Because for many cancer biomarkers the value ranges of cases and controls often overlap, it is hardly possible to define optimal cutoffs that enable cancer detection with 100% sensitivity and specificity. This is even more difficult if cancer patients are to be distinguished from the differential diagnostically relevant group of patients with organ-related nonmalignant diseases [8].
The diagnostic performance of a biomarker can best be demonstrated by receiver-operating characteristic (ROC) curves showing the complete profile of sensitivity and specificity. This graph gives the sensitivity and specificity at all possible cutoff points and is highly informative when the performances of different biomarkers are compared with each other. Meaningful measures are (1) the area under the curve (AUC), (2) the sensitivity at a defined specificity (e.g., 95%), or (3) an optimized sensitivity-specificity combination illustrated by the point closest to the left upper corner (Fig. 3.2). Most important is the choice of the groups that are compared by ROC curves. Best results are obtained if patients with advanced cancer disease are compared with young healthy individuals. However, in the clinical situation, it is more meaningful to distinguish coeval persons with suspicious symptoms who may suffer from an early cancer or a nonmalignant pathology. In these cases, the curves often will be less optimistic [8, 9].
Levels of many cancer biomarkers overlap with those from healthy individuals. Receiver-operating characteristic (ROC) curves are an elegant tool to illustrate the diagnostic performance of a biomarker over the whole value range. To establish the ROC curve, the percentages of correctly negative controls (specificity) and correctly positive cancer patients (sensitivity) are delineated for all possible cutoff points (decreasing stepwise from 100% specificity) and transferred to the scheme. The area under the curve (AUC) and the sensitivity at a fixed specificity (e.g. 95%) are most informative measures for the comparison of diverse diagnostic biomarkers. As control groups, healthy individuals and patients with benign organ-related diseases that are relevant for differential diagnosis are considered (Adapted from [5]; with permission from Springer)
Beyond diagnostic applications, ROC curves are also used to illustrate the performance of a biomarker for the staging of disease (e.g., early stage cancer vs. metastatic cancer) or for the staging of therapy response (e.g., remission vs. non-remission). In the monitoring of disease, also kinetic information (increases or decreases of marker values) are used as marker variables.
It has to be pointed out that for screening purposes the positive and negative predictive values (PPV and NPV) are more important than the sensitivity and specificity. While PPV indicates the probability of disease if the value is positive, NPV gives the probability of being disease-free if the value is negative. This measure also takes the prevalence of a disease into account. Because the prevalence for cancer diseases in the normal population is quite low, PPV may be low even if the sensitivity and specificity are higher than 90%. Further, predictive values are informative if patients are stratified for specific therapies and responses are anticipated [5].
While prediction always relies to the response of a specific therapy, prognosis is related to the time of disease-free (DFS), progression-free (PFS), or overall survival (OS). Clinical and biomarker values can be obtained before or during a therapy. When monitoring therapy response, biomarker information that is available at the same time as the radiological staging can support the accurate estimation of the individual therapy response. If the information is available prior to the radiological staging, i.e., after one application of chemotherapy, the biomarker determination leads to a time advantage in terms of early estimation of therapy response that would enable an early and individual adaptation of the therapy strategy.
When a new cancer biomarker is evaluated on its clinical performance, a relevant number of patients with the target cancer disease have to be compared with healthy controls and patients with the organ-related benign diseases that are relevant for differential diagnosis [8, 10]. To get a whole picture of the usefulness of a biomarker, further cancer diseases and benign diseases that are involved in the marker catabolism such as renal and hepatic disorders have to be included as well. For therapy monitoring studies, a meaningful number of patients with a certain cancer that undergo a homogeneous type of therapy with favorable and non-favorable outcome have to be considered. Recently published guidelines support the professional validation of biomarkers for diagnostic and monitoring purposes [11, 12] as well as for the development and incorporation of biomarker studies in early clinical trials [7].
A new biomarker will only be implemented into patient care if it is superior to existing biomarkers or offers additive diagnostic, predictive, or monitoring information. Therefore, new biomarkers should always be compared with those that currently are used in clinical routine [8, 10]. Although only few single markers demonstrate a clear and reproducible advantage in these tough comparisons, the combination of multiple biomarkers could lead to a significant improvement of sensitivity and specificity. These combinations may result from a bottom-up approach that assembles biologically complementary markers or from a top-down approach that extracts meaningful markers out of a plentitude of markers. While the first approach is supported by logistic regression, supporter vector machine, or neuronal network models, the latter one often comprises cluster analysis or even more complex algorithms. In all cases a validation in an independent patient set is paramount to confirm the findings [5].
4 Monitoring Cancer Disease by Biomarkers
In order to monitor the state of cancer disease or response to anticancer therapy, biomarkers are frequently determined when clear clinical correlates are present, e.g., after tumor resection, at time of recurrent disease, before start of systemic therapies, and at time of radiological staging. Then biomarker levels are ideally assumed to be only influenced by disease activity or therapy response. However, it is necessary to develop rules which changes of biomarker levels are relevant for clinical decision making for the markers are implemented into clinical routine. For the individual interpretation of marker changes over time, several aspects have to be considered:
-
The biological variation of a biomarker in individual patients
-
The role of influencing factors
-
The disease state when the therapy is applied
-
The type of therapeutic interventions
-
The monitoring schedule for a biomarker and the data interpretation
-
The accuracy of biomarker monitoring and its consequences for patient management
For some biochemical markers, it is well known that their blood concentrations depend on age, gender, and ethnicity and can vary due to diurnal, mensal, annual, or other cycles. Further influencing factors are fasting; hydration; medication; the position at blood drawing; marker-specific factors such as stress, sports, etc.; and comorbidities or drug-related immune reactions. Although influencing factors cannot be ruled out completely, standardized procedures for blood collection are recommended [5, 7]. As heterogeneity among individuals is considerable for many markers, relative marker changes on an individual basis are preferred to absolute cutoff rules orientated at diseased patient groups.
Disease states of cancer patients may be very different including (1) local manifestations, (2) dissemination to distant lymph nodes or other sites in the body, (3) recurrences, or (4) continuous progressions. All these states have in common the presence of malignant masses that should be reduced by the therapy.
Treatment options comprise the local tumor eradication such as by surgery, external or internal radiotherapy, or local application of cytotoxic drugs and further systemic approaches if the cancer disease is already in an advanced stage, such as endocrine therapies, cytotoxic chemo- or radiotherapies, biological (targeted) therapies, and immune, gene, vaccine, or other therapies. All these therapies are assumed to reduce the tumor mass with different velocities suggesting a differentiated monitoring plan for each situation. This applies also to the different types of treatment strategies like neoadjuvant therapy before surgery, as well as primary, recurrent, or palliative therapy without surgery.
Sometimes no direct evidence of cancer disease is present, e.g., when monitoring is applied in (1) individuals at risk of developing cancer disease and (2) in patients after successful tumor eradication. Although biomarker monitoring has not been widely established in routine patient management, a sensitive detection of micrometastases could trigger early intervention trials that lead to improved tumor control and better outcomes in recurrent or advanced tumor stages [8].
To guide the individual patient management by biomarkers, a prospective scenario of appropriate determination intervals has to be defined that allows the sensitive and accurate estimation of therapy response or tumor (re)occurrence. These intervals depend on the one hand side on the efficiency of the therapy and on the other hand side on the expected half-life of the biomarker response.
It is recommended that biomarker assessments are not only done at the regular stagings with imaging exams but do also cover the initial phase of the therapy, e.g., the first hours or days after the initial treatment application but at least prior to every new therapy cycle, to enable a very early estimation of the biochemical response. Then they offer a real-time advantage over conventional strategies and may trigger an early adaptation of the therapeutic plan. This may be beneficial for the patient in terms of more efficient therapies, less toxic side effects and comorbidities, and considerable cost reduction [5].
Generally, there are three major indications for the early estimation of therapy response:
-
Monitoring the completeness of surgical tumor eradication and potentially suggesting adjuvant therapies
-
Monitoring response to systemic therapies (neoadjuvant, primary, palliative) and potentially suggesting alternative or additional therapies
-
Monitoring resistance to a part of the (targeted) therapies and potentially suggesting an alternative approach
For patients presenting with no evidence of disease (NED) who are monitored to early detect micrometastases or recurrence of cancer disease, the intervals will depend on the reoccurrence probability of the tumor and the regular follow-up program [13]. Nevertheless, the intervals should be close enough not to miss incidental recurrences and to offer a real-time advantage to regular radiological exams. However, biomarker monitoring will only be implemented into standardized patient guidance programs if it leads to earlier therapeutic interventions and to a clear benefit in terms of better overall survival and life quality [5].
5 Biomarkers in Neuroendocrine Tumors (NETs)
Neuroendocrine tumors (NET) display a very heterogeneous group of neoplastic diseases with respect to their localization, morphology, histology, and biochemical and clinical characteristics. They are quite rare with an incidence of 2–5 cases per 100,000 population. They can be subdivided into well-differentiated grade 1 and 2 NETs and poorly differentiated grade 3 neuroendocrine cancers. Often clinical symptoms of NETs are non-specific or appear only late leading to their diagnosis in an advanced stage of disease [14]. Around two thirds of NETs are localized in the gastroenteropancreatic tract (GEP) such as carcinoids, gastrinoma, insulinoma, vipoma, or glucagenoma. Other types of NETs develop in the lung such as small cell lung cancer (SCLC) and some large cell lung cancer types and in other organs like the medullary C-cell cancer in the thyroid or neuroendocrine subtypes of prostate cancer. Some of them grow locally, while others show a disseminating growth pattern with multiple manifestations. One feature they have in common is the production of peptide hormones, prohormones, or neuropeptides with paracrine or endocrine effects. These can be measured as cancer-associated biomarkers in the tissue, blood, urine, or other bodily fluids and support the diagnosis and monitoring of neuroendocrine cancer disease [2, 14]. Among the monoanalytes that are used in NET diagnostics, there are more general neuroendocrine biomarkers such as chromogranin A (CgA), neuron-specific enolase (NSE), progastrin-releasing peptide (ProGRP), NT-pro-brain natriuretic peptide (NT-proBNP), and cytokine markers released from diverse NETs. In addition, there are markers with higher specificity for one NET subtype such as serotonin and urine 5-hydroxyindoleacetic acid (5-HIAA) for carcinoids (APUDoma), gastrin for gastrinoma, glucagon for glucagenoma, insulin and C-peptide for insulinoma, vasoactive intestinal peptide (VIP) for vipoma, pancreatic polypeptide (PP) for pancreatic NETs, and calcitonin for medullary C-cell carcinoma of the thyroid, as well as diverse markers in neuroendocrine tumors of the pituitary gland or ectopic manifestations thereof (Table 3.1). These markers can also be elevated in combination, particularly in the case of multiple endocrine neoplasias (MEN) [2, 14].
Most analytical and clinical evidence is available for the biomarkers CgA, NSE, and ProGRP. Chromogranin A is a 68 kDa acidic glycoprotein that is most frequently used for the diagnosis of GEP-NETs. It is expressed in secretory dense core granules of neuroendocrine cells and is released upon stimulation along with other peptide hormones and neuropeptides. As there are various forms of CgA, specificity and affinity of antibodies used in the immunoassays are essential for detection of CgA subtypes [14]. Sensitivity for NET detection ranges between 60 and 80% depending on primary site, grade, and status of the disease. It is mainly elevated in carcinoids and other ileal or pancreatic NETs and correlates with tumor burden, presence of metastases, recurrence, and prognosis. For interpretation of CgA results, it has to be considered that non-specific elevations are seen in patients with renal failure, cardiac diseases, inflammatory disorders, and other types of cancer as well as in patients treated with proton pump inhibitors [2, 14].
Neuron-specific enolase (NSE) is a 100 kDa glycolytic enzyme that is present in neurons and neuroendocrine cells. It is a sensitive biomarker for the diagnosis and therapy monitoring of small cell lung cancer. Moreover, it is used for diagnosis of other NETs as well as in neuroblastoma and Wilms tumors of pediatric patients [15]. Thereby NSE correlates with tumor burden, poor histological differentiation, and high cellular turnover. NSE is a cytoplasmic enzyme that is not actively secreted and has a lower sensitivity for the diagnosis of GEP-NETs (30–50%) as compared with CgA. Beyond these indications, NSE can also be elevated in various solid tumors particularly in metastatic stages. In addition, it is non-specifically increased in benign lung diseases, uremia, and neurodestructive diseases such as stroke, trauma, etc. Accurate preanalytic sample handling is essential for NSE interpretation as erythrocytes contain high concentrations of NSE and hemolysis may cause false-positive results [15].
ProGRP is a 16 kDa precursor protein of gastrin-releasing peptide (GRP). In contrast to 27 amino acidic GRP with a half time of 2 min in serum, recombinant ProGRP (31–98) with 125 amino acids is much more stable in the blood. ProGRP is the most specific and sensitive marker for the differential diagnosis of neuroendocrine lung cancers, particularly SCLC. It is one of the few tumor markers that is almost exclusively released from one tumor type in high concentrations and is considered as diagnostic marker of SCLC if values are >300 pg/mL [8, 16]. Solely patients with medullary C-cell cancer of the thyroid and other neuroendocrine cancers may achieve similar high value levels at times [17]. In other cancer types or in nonmalignant conditions, only occasionally slight elevations up to 100 pg/mL are observed. However, renal failure is a well-recognized source of false-positive results which has to be taken into consideration for interpretation of ProGRP values [8, 16]. Further, differences with regard to the preanalytic stability of serum samples are observed for some immunoassays [17].
6 Diagnostic Performance of Monoanalytes in NETs
In a comprehensive study on patients with GEP-NETs, CgA has shown superior diagnostic performance in grade 1 and 2 NET and large cell neuroendocrine cancer (LCNEC) with AUCs of 0.86, 0.91, and 0.90 when compared with healthy controls followed by cytokeratin fragments (AUC 0.76, 0.86, 0.88) and NSE (AUC 0.54, 0.80, 0.83). CgA was strongly elevated in all three NET stages, while cytokeratins and NSE mainly increased in G2 NET and LCNEC; in consequence cytokeratins were prognostic in all stages, NSE only in LCNECs in multivariate analyses. ProGRP had no diagnostic relevance in GEP-NETs. However, in patients with small cell neuroendocrine cancer mainly in the lung, ProGRP was the most sensitive marker (AUC 0.86) particularly at high specificities (73% sensitivity at 95% specificity) followed by cytokeratins (AUC 0.87), NSE (AUC 0.79) and CgA (0.77). Once again cytokeratins and NSE were prognostically relevant [18]. Best differentiation of lung NETs from non-lung NETs as well as between grade 1 and 2 NETs was found for ProGRP, too. Regarding survival, additive prognostic value of ProGRP and CgA was reported [19].
Recently, a large multicentric trial across Europe and China with more than 2500 patients confirmed earlier results regarding the excellent methodical, preanalytical, and diagnostic performance of ProGRP for small cell lung cancer (SCLC) [17]. Thereby strong elevations were only observed in SCLC patients while healthy individuals, patients with NSCLC, benign lung diseases, other benign or cancer disease had no or only slightly increased, and patients with renal failure moderately elevated values. Most remarkably, ProGRP discriminated with high sensitivity and specificity not only between SCLC and benign lung diseases but also between SCLC and NSCLC (AUC 0.89 in Europe and 0.94 in China, respectively) underlining its high clinical utility for histological subtyping in case of unclear lung masses [17]. Earlier, a multiparametric score involving ProGRP, NSE, and CYFRA 21-1 achieved higher AUC for differentiation of SCLC and NSCLC than single markers did [20]. Molina et al. included ProGRP, NSE, CEA, CYFRA 21-1, SCC, and CA 15-3 in an algorithm that supported the diagnosis and histological subtyping of lung cancer [21]. In addition, ProGRP, NSE, and cytokeratin fragments have shown to be valuable markers for the monitoring and early prediction of response to systemic therapy in SCLC patients [22].
A consensus paper on the use of biomarkers for NET disease outlined the need for circulating biomarkers for diagnosis, prognosis, monitoring therapy response, identifying minimal residual disease, and detection of recurrent disease. While the limitation of monoanalytes in sensitivity and specificity for GEP and lung NETs was recognized, more accurate diagnostic tools were looked for. Current research approaches address circulating DNA, mRNA, microRNA, and metabolomic biomarkers as well as circulating tumor cells; however their clinical utility still has to be proven [23].
7 Perspective: Multianalyte Approaches
Great potential is seen in multianalyte approaches such as a multi-transcript molecular signature for PCR-based blood analysis with algorithmic evaluation that was specifically developed for GEP-NETs. The so-called NETest includes 51 genes involved in transcription, DNA repair, antigen processing and presentation, apoptosis, cell adhesion, cell division, immune response, and several metabolic processes. When investigating the assay in three independent blood sets, the gene-based classifier reached high sensitivities (85–98%) and specificities (93–97%), as well as positive (95–96%) and negative (87–98%) predictive values for NET diagnosis clearly outperforming CgA. In particular, the classifier indicated NET in more than 90% of patients with low CgA levels [24]. While superior performance of NETest over CgA was confirmed in a subsequent study, it showed to be elevated in all grades of NET, in both local and disseminated disease, and was not normalized by somatostatin analog therapy [25]. Importantly, it was unaffected by proton pump inhibitors (PPI), while CgA levels were increased in 83% of PPI-treated patients as well as in 26% of controls revealing a high rate of false-positive results [25]. These findings demonstrate that NETest meets the qualitative expectations in a sensitive and accurate diagnostic biomarker [23]. Unmet questions, however, are preanalytical and analytical quality control issues including standardization and harmonization, the high workload and hands-on time, as well as cost-efficiency and reimbursement issues. In relation with benefits from early NET diagnosis and improved quality of life for the patients, considerable cost savings of the society are expected [25]. However, these aspects have to be acknowledged from health insurances if such high-performance biomarker assay is to be implemented in future NET patient’s guidance.
References
Hulka B. Overview of biological markers. In: Hulka B, Griffith J, Wilcoska T, editors. Biologial markers in epidemiology. New York: Oxford University Press; 1990. p. 3–15.
Modlin IM, Oberg K, Taylor A, Drozdov I, Bodei L, Kidd M. Neuroendocrine tumor biomarkers: current status and perspectives. Neuroendocrinology. 2014;100:265–77.
Crowley E, Di Nicolantonio F, Loupakis F, Bardelli A. Liquid biopsy: monitoring cancer-genetics in the blood. Nat Rev Clin Oncol. 2013;10:472–84.
Schwarzenbach H, Hoon DS, Pantel K. Cell-free nucleic acids as biomarkers in cancer patients. Nat Rev Cancer. 2011;11:426–37.
Holdenrieder S. Circulating nucleic acids in therapy monitoring. In P.B. Gahan (ed.), Circulating nucleic acids in early diagnosis, prognosis and treatment monitoring, advances in predictive, preventive and personalised medicine, 5 2014: 309–348. Springer
Sturgeon CM, Hoffman BR, Chan DW, Ch'ng SL, Hammond E, Hayes DF, Liotta LA, Petricoin EF, Schmitt M, Semmes OJ, Söletormos G, van der Merwe E, Diamandis EP, National Academy of Clinical Biochemistry. National Academy of Clinical Biochemistry Laboratory medicine practice guidelines for use of tumor markers in clinical practice: quality requirements. Clin Chem. 2008;54:e1–e10.
Dancey JE, Dobbin KK, Groshen S, Jessup JM, Hruszkewycz AH, Koehler M, Parchment R, Ratain MJ, Shankar LK, Stadler WM, True LD, Gravell A, Grever MR, Biomarkers Task Force of the NCI Investigational Drug Steering Committee. Guidelines for the development and incorporation of biomarker studies in early clinical trials of novel agents. Clin Cancer Res. 2010;16:1745–55.
Stieber P, Heinemann V. Sensible use of tumor markers. J Lab Med. 2008;32:339–60.
Holdenrieder S. Trends in the application of tumor markers in the clinical routine. Med Welt. 2013;64:12–9.
Anonymous. European Group on Tumour Markers (EGTM): Consensus recommendations. Anticancer Res. 1999;19:2785–820.
Sturgeon CM, Duffy MJ, Stenman UH, Lilja H, Brünner N, Chan DW, Babaian R, Bast RC Jr, Dowell B, Esteva FJ, Haglund C, Harbeck N, Hayes DF, Holten-Andersen M, Klee GG, Lamerz R, Looijenga LH, Molina R, Nielsen HJ, Rittenhouse H, Semjonow A, IeM S, Sibley P, Sölétormos G, Stephan C, Sokoll L, Hoffman BR, Diamandis EP, National Academy of Clinical Biochemistry. National Academy of Clinical Biochemistry laboratory medicine practice guidelines for use of tumor markers in testicular, prostate, colorectal, breast, and ovarian cancers. Clin Chem. 2008;54:e11–79.
Sölétormos G, Duffy MJ, Hayes DF, Sturgeon CM, Barak V, Bossuyt PM, Diamandis EP, Gion M, Hyltoft-Petersen P, Lamerz RM, Nielsen DL, Sibley P, Tholander B, Tuxen MK, Bonfrer JM. Design of tumor biomarker-monitoring trials: a proposal by the European Group on Tumor Markers. Clin Chem. 2013;59:52–9.
Stieber P, Nagel D, Heinemann V. Tumor markers in metastatic breast cancer: high tumor specificity within the reference range. J Clin Oncol. 2006;24(18S):10554.
Verbeek WH, Korse CM, Tesselaar ME. GEP-NETs UPDATE: Secreting gastro-enteropancreatic neuroendocrine tumours and biomarkers. Eur J Endocrinol. 2016;174:R1–7.
Lamerz R. Neuron-specific enolase. In: Thomals L, editor. Labor und diagnose. Frankfurt: TH-Books; 2012. p. 1677–81.
Molina R, Holdenrieder S, Auge JM, Schalhorn A, Hatz RA, Stieber P. Diagnostic relevance of circulating biomarkers in patients with lung cancer. Cancer Biomark. 2010;6:163–78.
Korse CM, Holdenrieder S, Zhi X, Zhang X, Qiu L, Geistanger A, Lisy MR, Wehnl B, van den Broek D, Escudero JM, Standop J, Mu H, Molina R. Multicenter evaluation of a new progastrin-releasing peptide (ProGRP) immunoassay across Europe and China. Clin Chim Acta. 2015;438:388–95.
Korse CM, Taal BG, Vincent A, van Velthuysen ML, Baas P, Buning-Kager JC, Linders TC, Bonfrer JM. Choice of tumour markers in patients with neuroendocrine tumours is dependent on the histological grade. A marker study of Chromogranin A, neuron specific enolase, progastrin-releasing peptide and cytokeratin fragments. Eur J Cancer. 2012;48:662–71.
Korse CM, Taal BG, Bonfrer JM, Vincent A, van Velthuysen ML, Baas P. An elevated progastrin-releasing peptide level in patients with well-differentiated neuroendocrine tumours indicates a primary tumour in the lung and predicts a shorter survival. Ann Oncol. 2011;22:2625–30.
Gruber C, Hatz R, Reinmiedl J, Nagel D, Stieber P. CEA, CYFRA 21-1, NSE, and ProGRP in the diagnosis of lung cancer: a multivariate approach. J Lab Med. 2008;32:361–71.
Molina R, Marrades RM, Auge JM, et al. Assessment of a combined panel of six serum tumor markers for lung cancer. Am J Respir Crit Care Med. 2016;193:427–37.
Holdenrieder S, v Pawel J, Dankelmann E, Duell T, Faderl B, Markus A, Siakavara M, Wagner H, Feldmann K, Hoffmann H, Raith H, Nagel D, Stieber P. Nucleosomes, ProGRP, NSE, CYFRA 21-1 and CEA in the therapy monitoring of small-cell lung cancer during first-line chemotherapy. Clin Cancer Res. 2008;14:7813–21.
Oberg K, Modlin IM, De Herder W, Pavel M, Klimstra D, Frilling A, Metz DC, Heaney A, Kwekkeboom D, Strosberg J, Meyer T, Moss SF, Washington K, Wolin E, Liu E, Goldenring J. Consensus on biomarkers for neuroendocrine tumour disease. Lancet Oncol. 2015;16:e435–46.
Modlin IM, Drozdov I, Kidd M. The identification of gut neuroendocrine tumor disease by multiple synchronous transcript analysis in blood. PLoS One. 2013;8:e63364.
Modlin IM, Aslanian H, Bodei L, Drozdov I, Kidd M. A PCR blood test outperforms Chromogranin A in carcinoid detection and is unaffected by proton pump inhibitors. Endocr Connect. 2014;3:215–23.
Conflict of Interest Statement
The author declares to have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 Springer International Publishing AG
About this chapter
Cite this chapter
Holdenrieder, S. (2018). Circulating Biomarkers: Biological Basis, Methods, and Interpretation Criteria. In: Giovanella, L. (eds) Atlas of Thyroid and Neuroendocrine Tumor Markers. Springer, Cham. https://doi.org/10.1007/978-3-319-62506-5_3
Download citation
DOI: https://doi.org/10.1007/978-3-319-62506-5_3
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-62505-8
Online ISBN: 978-3-319-62506-5
eBook Packages: MedicineMedicine (R0)