Abstract
Patients with cancer and concomitant cardiovascular disease frequently need various imaging investigations to arrive at a correct diagnosis.
In this chapter we provide some illustrative cases and briefly discuss the pertinent imaging findings in patients with myocarditis, myocardial infarction, cardiac mass and cardiomyopathy.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
Case 1: What Is the Reason for the Elevated Troponin?
-
(a)
A 48 year old man with limited stage small cell lung cancer, receiving chemotherapy presented with chest pain. The 12 lead ECG was normal but the troponin was elevated to 13.0 ng/L Left ventricular (LV) systolic function was normal with an LV ejection fraction (EF) of 55%. Due to normal ECG and clinical presentation the possibility of myocarditis was raised. A cardiac magnetic resonance (CMR) imaging was done, which showed distal left anterior descending (LAD) artery territory infarction without significant ischemia in the remaining LAD territory (Figs. 4.1 and 4.2). A diagnosis of myocardial infarction was made and patient discharged on aspirin, statin and ACE inhibitors. A coronary angiogram was not done given the inability to continue uninterrupted dual antiplatelet therapy.
-
(b)
A 21 year old male, with symptoms suggestive of an upper respiratory illness presented with acute chest pain and was found to have troponin elevation to 32 ng/mL. Coronary CT angiography (CTA) was done which confirmed normal origin of coronary arteries and no evidence of coronary artery disease. CMR was done to assess for myocarditis, which showed findings consistent with myocarditis (Fig. 4.3). Below are short axis images which showed subepicardial scar involving the inferolateral and anterolateral segments. Both these segments also had markedly elevated T2 values suggesting this is an acute process (T2 values of 70 ms, normal 45–50 ms).
Teaching points
-
1.
CMR is valuable in identifying infarct pattern from myocarditis. This has therapeutic implications particularly in cancer patients who have high bleeding risks.
-
2.
Myocardial infarction produces a pattern which is either subendocardial or transmural scar. Due to the wave front phenomenon of myocardial injury, the subendocardium is most sensitive to ischemia and is susceptible to infarction. In contrast other myocardial processes that do not involve coronary involvement lead to “subepicardial” scar patterns and furthermore not confined to a coronary territory. T2 mapping has been established as a method to identify acute myocarditis and has been validated in studies that have also used endomyocardial biopsy to establish diagnoses of myocarditis [1, 2].
Case 2: What Is This Mass?
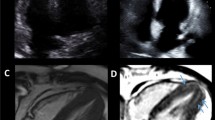
A 59 year old female, with history of atrial fibrillation, mitral regurgitation and a rapidly growing mass in the left forearm presented with worsening fatigue. In the emergency department a CT angiogram was done which revealed two left atrial masses. A transthoracic echocardiogram (TTE) showed a hypoechoic mobile mass in the left atrium, which was 2.8 × 2.5 cm in size (Fig. 4.4). A cardiac MRI was done to differentiate between tumor and thrombus. The cardiac MRI showed two masses, one in the left atrial appendage and the other in the left atrium (Figs. 4.5 and 4.6). The left atrial mass was bright on T2 weighted images, bright on fat saturation, with no evidence of perfusion. Also on post contrast delayed enhancement imaging, it was hypointense. Further confirmation of thrombus was noted, with T1 time of 600 ms. Given the appearance of the bright mass on steady state free precession images (SSFP) images, cystic transformation of a thrombus was the most likely explanation. Further workup including a biopsy of the lesion in her forearm was recommended, but the patient declined.
Teaching points
-
1.
CMR is valuable in identifying whether a mass is a tumor or a thrombus. It can also easily distinguish benign tumors such as lipomas. Using a variety of techniques from T1 and T2 characterization, perfusion and dynamic changes after contrast administration along with T1 and T2 mapping, we can accurately characterize a mass.
Using contrast echocardiography, a filling defect confirms a mass. A mass in the setting of adjacent myocardial akinesis is most likely to be a thrombus. However using MRI, the tissue characteristics of the mass i.e. fat, cyst or thrombus can be accurately assessed using T1 and T2 weighted sequences and its change after gadolinium administration provides clues regarding perfusion of the mass [3]. MRI can demonstrate the lack of vascular perfusion of a thrombus using first pass perfusion sequences. In addition, T2 and T1 mapping can identify thrombi based on tissue characterization. For example, an inversion time, to null thrombus specifically, i.e. 600 ms, in a delayed enhancement image, can accurately differentiate thrombus from other masses i.e. myxoma [4].
(a and b) Cardiac MRI images of the left atrium in a sagittal plane are shown. Figure (a) shows the cystic mass in a cine image. Figure (b) shows the lack of perfusion during first pass gadolinium administration. (c and d) Cardiac MRI images of the left atrium in a sagittal plane are shown. Figure (c) shows the bright mass on a T2 weighted image, suggestive of a thrombus. Figure (d) shows the hypointense mass on a post contrast delayed enhancement image. T1 times assessed by T1 mapping were 600 ms consistent with thrombus (not shown)
(a–c) Cardiac MRI images of the left atrium in an axial plane are shown. (a) Shows the bright mass, large mass inside the left atrium and a smaller mass within the left atrial appendage, on a cine image. (b) Shows the lack of perfusion during first pass gadolinium administration of both the left atrial and the appendage masses. (c) Shows the hypointense masses on a post contrast delayed enhancement image
Case 3: What Is the Reason for the Persistent Lower Extremity Edema?
A 34 year old man with 6 months of abdominal bloating, diarrhea initially diagnosed as irritable bowel syndrome but eventually found to have stage 4 neuroendocrine tumor of the pancreas with liver metastasis and presented to the clinic with progressive lower extremity swelling. Examination revealed a holosystolic murmur in the tricuspid area, which increased with inspiration. A TTE showed moderate tricuspid regurgitation and normal RV size and function, with significant increase in the tricuspid regurgitation relative to a TTE done 6 months ago (Figs. 4.7, 4.8 and 4.9). He also had moderate pulmonic regurgitation. He had a history of markedly elevated 5 HIAA, >5000 and was receiving somatostatin. Although he initially responded to diuresis, he developed refractory lower extremity swelling. Hypoalbuminemia and IVC compression were ruled out. He developed progressive severe tricuspid regurgitation and required tricuspid valve replacement.
Teaching points
-
1.
Carcinoid heart disease is a rare entity. It can however cause debilitating symptoms of peripheral venous congestion and edema. Prompt identification is critical and symptomatic management is the norm. However in selected patients where primary tumor is well controlled, tricuspid valve replacement is done.
Case 4: Unusual Etiology of Cardiomyopathy While Receiving Chemotherapy
A 79 year old female, with inflammatory breast cancer, and normal LV systolic function at baseline had finished receiving two cycles of Adriamycin. She had profound fatigue, was found to be anemic with a Hgb of 6 for which she received 2 units of PRBCs. Within 4 h of transfusion, she returned to the emergency department (ED) with acute dyspnea, orthopnea and chest pain. She was in florid heart failure with fluid overload. A 12 lead ECG showed a new LBBB with elevated troponin of 4.0 ng/L. A TTE demonstrated basal hyperkinesis with mid to distal akinesis (Figs. 4.10 and 4.11). A clinical diagnosis of cardiomyopathy/heart failure due to adrimaycin and concomitant NSTEMI was made and a coronary angiogram was done, which did not show any significant disease. A TTE demonstrated basal hyperkinesis with mid to distal akinesis. A diagnosis of stress (Takotsubo) cardiomyopathy was made and medical therapy was instituted which included a low dose beta blocker. On follow up in the clinic the patient was symptomatically markedly improved with no heart failure symptoms and a repeat TTE 2 weeks later revealed, normal LV systolic function with normal wall motion (Figs. 4.12 and 4.13). Diagnosis was stress cardiomyopathy and not related to anthracycline use.
Characteristic LV gram and global longitudinal strain polar map (from other patients with stress (Takotsubo) cardiomyopathy) are shown in Figs. 4.14 and 4.15 respectively.
Teaching points
-
1.
While anthracycline mediated cardiomyopathy is the most common etiology of cardiomyopathy in cancer patients exposed to this drug, the specific pattern of stress cardiomyopathy should warrant suspicion of stress cardiomyopathy. In patients not receiving cardiotoxic chemotherapy, stress cardiomyopathy should be entertained as a differential when patients have an abrupt presentation similar to the one outlined above.
Munoz et al. have identified common stressors, i.e. surgery, radiation, chemotherapy, acute illness and emotional stressors as triggers for stress cardiomyopathy in cancer patient. Multiple studies have identified patients with malignancy as a vulnerable population for stress cardiomyopathy [5,6,7].
Global longitudinal strain polar map: Predominant apical segmental abnormality polar map pattern in a patient with classic apical ballooning syndrome variant of stress cardiomyopathy. Note the marked difference isolated to the five apical segments on the polar map LV representation, atypical for a specific coronary distribution. (Image courtesy of Dr. J. Banchs)
Case 5: Other Causes of Cardiomyopathy in Patients with Cancer
While evaluating the etiology of heart failure, CMR can be helpful to identify amyloidosis, hypertrophic cardiomyopathy or iron overload. For example in amyloidosis, the basal slices show diffuse myocardial scar with preservation of the apex, as shown in Fig. 4.16. In addition the presence of transmural myocardial scar and right ventricular scar as well helps differentiate the types of amyloidosis (Transthyretin related amyloidosis) from light chain amyloidosis [8]. CMR can also be used to identify hypertrophic cardiomyopathy especially the phenotypic variants, i.e. Apical variant in addition to the asymmetric septal hypertrophy as outlined in this review by Maron et al. [9].
Basal slices are represented in A and B while the apical slice is shown in C. Basal slices have diffuse myocardial fibrosis in a near transmural pattern, in contrast to the apical slices which have no fibrosis. This correlates with the preservation of apical strain, with severe reduction of basal strain using echocardiography (Fig. 4.17).
Figure 4.18 shows the typical ECG and echocardiogram findings in a patient with biopsy proven cardiac amyloidosis.
References
Thavendiranathan P, Walls M, Giri S, Verhaert D, Rajagopalan S, Moore S, Simonetti OP, Raman SV. Improved detection of myocardial involvement in acute inflammatory cardiomyopathies using T2 mapping. Circ Cardiovasc Imaging. 2012;5:102–10.
Bohnen S, et al. Performance of T1 and T2 mapping cardiovascular magnetic resonance to detect active myocarditis in patients with recent-onset heart failure. Circ Cardiovasc Imaging. 2015;8:e003073. https://doi.org/10.1161/CIRCIMAGING.114.003073.
Esposito A, De Cobelli F, Ironi G et al. CMR in the assessment of cardiac masses. JACC Cardiovasc Imaging. 2014;7:1057–61.
Weinsaft JW, et al. Detection of left ventricular thrombus by delayed-enhancement cardiovascular magnetic resonance prevalence and markers in patients with systolic dysfunction. J Am Coll Cardiol. 2008;52:148–57.
Munoz E, et al. Takotsubo stress cardiomyopathy: “Good News” in cancer patients? J Am Coll Cardiol. 2016;68(10):1143–4.
El-Sayed AM, et al. Demographic and co-morbid predictors of stress (Takotsubo) cardiomyopathy. Am J Cardiol. 2012;110(9):1368–72.
Tornvall P, et al. A case-control study of risk markers and mortality in Takotsubo stress cardiomyopathy. J Am Coll Cardiol. 2016;67(16):1931–6.
Dungu JN, et al. CMR-based differentiation of AL and ATTR cardiac amyloidosis. JACC Cardiovasc Imaging. 2014;7(2):133–42.
Maron MS, et al. Clinical utility of cardiovascular magnetic resonance in hypertrophic cardiomyopathy. J Cardiovasc Magn Reson. 2012;14:13.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 Springer International Publishing AG, part of Springer Nature
About this chapter
Cite this chapter
Vasu, S., Hundley, W.G. (2018). Imaging to Diagnose Myocarditis, Cardiomyopathy, Tumor and Thrombus. In: Yusuf, S., Banchs, J. (eds) Cancer and Cardiovascular Disease. Springer, Cham. https://doi.org/10.1007/978-3-319-62088-6_4
Download citation
DOI: https://doi.org/10.1007/978-3-319-62088-6_4
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-62086-2
Online ISBN: 978-3-319-62088-6
eBook Packages: MedicineMedicine (R0)