Abstract
Vanadium (V) is a transition metal that presents in multiple oxidation states and numerous inorganic compounds and is also an ultra-trace element considered to be essential for most living organisms. Despite being one of the lightest metals, V offers high structural strength and good corrosion resistance and thus has been widely adopted for high-strength steel manufacturing. High doses of V exposure are toxic, and inhalation exposure to V adversely affects the respiratory system. The neurotoxicological properties of V are just beginning to be identified. Recent studies by our group and others demonstrate the neurotoxic potential of this metal in the nigrostriatal system and other parts of the central nervous system (CNS). The neurotoxic effects of V have been mainly attributed to its ability to induce the generation of reactive oxygen species (ROS). It is noteworthy that the neurotoxicity induced by occupational V exposure commonly occurs with co-exposure to other metals, especially manganese (Mn). This review focuses on the chemistry, pharmacology, toxicology, and neurotoxicity of V.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
Introduction
Vanadium (V) is a transition metal which belongs to Group VB of the periodic table, with an atomic weight of 50.9415, an atomic number of 23, and oxidizing states ranging from −1 to +5. Vanadium has many industrial uses, and its contribution to environmental contamination has been steadily growing in recent years. Vanadium is widely distributed in the earth’s crust but occurs in low abundance. Vanadium is an essential trace element for normal cell growth but can be toxic when present at higher concentration. It can exist in many oxidation states with many oxyanions and oxycations, which form in solution. The multiple oxidation states, ready hydrolysis, and polymerization confer a level of complexity to the chemistry of vanadium well above that of many metals. Vanadium dissolves in natural waters as the vanadyl ion V(IV) and the vanadate ion V(V). Both species have different nutritional and toxic properties. Studies carried out on yeast cells, for example, have demonstrated that V(V) is a strong inhibitor of the enzyme Na and K-ATPase, while V(IV) appears to be a weaker inhibitor (Patel et al. 1990).
Vanadium is among the list of essential micronutrients required in small quantities for normal metabolism (Ray et al. 2006). It has therefore been incorporated in the formulations and preparations of many multinational pharmaceutical companies (Nutrition Dynamics Inc., Texas, USA; All Nature Pharmaceuticals Inc., USA; and Ranbaxy Pvt. Ltd., Mumbai, India) along with vitamins and other essential trace elements for maintenance of normal health. Although the micronutrients lack pharmacological potencies, they assume a repair function for the essential critical molecules of the cell, such as DNA and proteins (Fenech and Ferguson 2001).
Uses of Vanadium and Its Compounds
Vanadium is used widely in industrial processes, including the production of special steels, temperature-resistant alloys, glass, pigments, and paints, for lining arc welding electrodes and as a catalyst. Its use with nonferrous metals is of particular importance in aircraft construction, atomic energy industry, and space technology (ChemIDPlus, 2016). Vanadium is preferred in the rising production of special steels and temperature-resistant alloys, namely, HSL-A, which is a high-strength, lightweight, and low-cost micro-alloyed steel, because it is one of the lightest metals with an inherent high strength. The characterization of metals in welding fumes by ICP-MS revealed that the V concentration is about 2.5% of whole transition metal content.
Vanadium is a chemical intermediate principally for V alloys and compounds. Vanadium is used (1) as a catalyst for many organic reactions; (2) as an oxidation catalyst in many industrial synthesis processes like automobile catalytic converters; (3) as a catalyst in the production of phthalic anhydride from naphthalene or 2-xylene, maleic anhydride from benzene or n-butane/butene, adipic acid from cyclohexanol/cyclohexanone, and acrylic acid from propane; (4) as a catalyst (ferrovanadium) to oxidize sulfur dioxide in sulfuric acid manufacturing; (5) as a depolarizer to manufacture yellow glass; (6) to manufacture ceramic coloring material, vanadium salts (YVO4), and pesticides; (7) in the inhibition or absorbance of UV transmission in glass; (8) as a photographic developer; (9) in dyeing textiles; and (10) in manufacturing a high capacity battery, namely, the vanadium redox battery, that uses vanadium ions in different oxidation states to store chemical potential energy.
Minor amounts of V are used to produce oxalic acid from cellulose and anthraquinone from anthracene. V is also used to lower the melting point of enamel frits for the coating of aluminum substrates. Further uses are in the making of superconductor magnets, as a corrosion inhibitor in carbon dioxide scrubbing solutions of the Benfield and related processes for the production of hydrogen from hydrocarbons, and as the cathode in primary and secondary (rechargeable) lithium batteries (ChemIDPlus 2016).
Chemistry of Vanadium
Vanadium usually occurs in aqueous solution as vanadate ions, often as polymerized isopolyvanadates, with the exact composition dependent on the protonation and condensation equilibria (Greenwood and Earnshaw 1997). Monomeric V ions are found only in very dilute solutions, since increases in the concentrations of these ions lead to polymerization, especially if the solution is acidic, reducing their bioavailability and associated toxicity (Duffus 2007). In industrial processes catalyzed by vanadium pentoxide (V2O5), V is involved in the oxidation of many organic compounds, forming reactive intermediates, some of which are ROS and may be carcinogenic (Hussain et al. 2003; Valko et al. 2006).
Vanadium occurs in various oxidative states with the ability to participate in reactions involving the formation of free radicals (Crans et al. 2004). V is quickly reduced to V(IV) in plasma by enzymatic (e.g., NADPH) and nonenzymatic (ascorbic acid) plasmatic antioxidants and is then transported and bound to plasma proteins. The equations below show a few such reactions which may occur inside the cell (Liochev and Fridovich 1990), forming peroxovanadyl radicals {V(IV) – OO·} and vanadyl hydroperoxide {V(IV) – OH·} (Evangelou 2002).
The generated superoxide undergoes further conversion by a dismutation reaction with SOD to H2O2. Some studies have shown that a one-electron reduction of V(V) to V(IV), which is mediated by nonenzymatic ascorbate and phosphate, may be an important V(V) reduction pathway in vivo (Ding et al. 1994). The resulting ROS formed by V(IV) from H2O2 and lipid hydroperoxide through the Fenton-like reaction might be critically significant in the mechanisms of V(V)-induced cellular injury during physiological conditions (Ding et al. 1994; Zhang et al. 2001):
Vanadium compounds even in signal transduction studies point to their ability to induce oxidative stress and mitochondrial permeability transition pore opening related to oxidative stress (Afeseh Ngwa et al. 2009; Zhao et al. 2010). Vanadium produces ROS-like hydroxyl free radicals by different ways (Cortizo et al. 2000; Gândara et al. 2005), initiating the peroxidative decomposition of cellular membrane phospholipids. This radical was also shown to damage the inner mitochondrial membrane, triggering a sequence of events leading to the loss of cell viability upon mitochondrial deenergization.
Vanadium Toxicology and Pharmacology
Vanadium has been reported in the blood, feces, and urine of workers following occupational exposure to V2O5 dust, demonstrating absorption as a consequence of V2O5 inhalation (Sjoberg 1954). Vanadium compounds released in large quantities, mainly by burning fossil fuels and also from various industrial processes, can be precipitated on the soil and drained by rain and groundwater which may be directly absorbed by plants (Pyrzyńska and Wierzbicki 2004), eventually reaching those who consume these plants. The major anthropogenic point sources of atmospheric emission are metallurgical plants (30 kg V per ton), followed by the burning of crude or residual oil and coal (0.2–2 kg V per 1000 tons and 30–300 kg V per 106 L). By-products containing V2O5 include dust, soot, boiler scale, and fly ash. The processing of V slag (about 120 g V2O5 per kg) is characterized by the formation of dust, with V concentrations ranging from 5 to 120 mg/m3 (IARC 2006b). Crude oil from Venezuela is believed to have the highest V concentrations of 1400 mg/kg. Elevated levels of airborne V (4.7 mg/m3) have been found in the breathing zone of steel industry workers (Kiviluoto et al. 1979). The toxicity of V compounds increases with its valency, making V2O5 the most toxic form and therefore warranting the full characterization of its toxicological properties. Studies have shown that inhaled V2O5 causes occupational lung diseases (bronchitis and airway fibrosis) commonly referred to as pneumoconiosis. The consequences of environmental exposure to lower levels of V2O5 on human health remain unclear, in part because air pollution particulates are a complex mixture of many organic and inorganic components, including a variety of metals [5].
The IARC classifies V2O5 as a Group 2B (possible) human carcinogen (IARC 2006a). Acute cases of V poisoning have been reported in man with sequelae of anemia, weakness, vomiting, anorexia, nausea, tinnitus, headache, dizziness, palpitations, transient coronary insufficiency, bradycardia with extra systoles, dermatitis, green discoloration of the tongue, leucopenia, leukocyte granulation, and lower cholesterol levels (Friberg et al. 1986). Epidemiological studies have reported an association between decreased birthweight and V exposure estimated from particulate matter (Jiang et al. 2016). Exposure to geogenic particulate matter (PM) comprising mineral particles has been linked to human health effects. Vanadium exposure in humans has been shown to induce motor deficits and neurobehavioral changes (Jiang et al. 2016; Li et al. 2013; Zhu et al. 2016). However, very little data exist on the neurological health effects associated with occupational dust exposure in natural settings. ICP-MS analyses of roadside dust samples revealed Al (55,090 μg/g), V (70 μg/g), Mn (511 μg/g), and Fe (21,600 μg/g). The ratio of V to Mn in inhaled dust during occupational exposures can vary from 1:1 to 1:8. People with V concentrations around 14.2 mg/L in their urine demonstrated neurobehavioral deficits, especially in visuospatial abilities and attention (Barth et al. 2002; SIMRAC 2000). Vanadium alters the viability of macrophages isolated from dogs, rabbits, and rats exposed to V2O5 in vitro for 20 h (Sheridan et al. 1978). The i.p. administration of V2O5 altered phospholipid content and induced significant increases in the levels of glucose-6-phosphate dehydrogenase and 6-phosphogluconate dehydrogenase in rats (Kacew et al. 1982). In animal studies, intranasal delivery of geogenic dust containing Mn and V (0.01–100 mg/kg dust) into adult mice via oropharyngeal aspiration induced a neuroinflammatory response (DeWitt et al. 2016; Keil et al. 2016). Very recently, Azeez et al. (2016) demonstrated that chronic postnatal V exposure in mice led to a functional deficit and region-dependent myelin damage associated with glial cell activation and proinflammatory cytokine induction. Rats experienced a significant spatial memory deficit in the Morris water maze (MWM) 3–12 months after V exposure (Folarin et al. 2016). Additional studies showed that exposure to dust containing elevated concentrations of metals can cause neuroinflammation and neurodegeneration (Calderon-Garciduenas et al. 2016; Jiang et al. 2016; Reis et al. 2014).
The cytotoxicity caused by compounds of V has been documented (Cortizo et al. 2000; Sabbioni et al. 1991). Various V compounds are known to impede the activities of ribonuclease (Lau et al. 1993), protein kinases (Bollen et al. 1990; Stankiewicz and Tracey 1995), ATPases (Sabbioni et al. 1991), and phosphatases (Tracey 2000). Some V compounds either inhibit or stimulate the activity of DNA or RNA enzymes eliciting mutagenic and genotoxic responses (Stemmler and Burrows 2001). Single-stranded DNA breaks in cells of mouse testes were observed 24 h after one intraperitoneal (i.p.) injection of V2O5 (5.75, 11.5, and 23 mg/kg) (Altamirano-Lozano et al. 1996), indicating an ability to cross the blood-testis barrier. Vanadium has also been reported to cross the blood-brain barrier, inducing neurochemical changes in the brain (Witkowska and Brzezinski 1979). Vanadium-containing substances alter blood levels of thyroid hormone with higher triiodothyronine plasma levels in V-treated rats (Badmaev et al. 1999; Mukherjee et al. 2004). Vanadium-containing compounds can also change the metabolism of sugars and lipids (Nakai et al. 1995). The ability of V-containing compounds to alter gene expression has generated interest among biological scientists for such compounds. In insulin receptor-overexpressing cells, greater levels of Ras, MAPK, p70s6k, and c-raf-1 have been observed following V exposure (Pandey et al. 1999). Increased levels of macrophage inflammatory protein (MIP)-2 mRNA triggered by vanadates are accompanied by increased NFκB DNA-binding activity in bronchoalveolar lavage (BAL) cells (Chong et al. 2000). Vanadate exposure has also been shown to induce gene expression of tumor necrosis factor-alpha (TNF-α), activator protein-1 (AP-1), and interleukin-8 (IL-8) (Ding et al. 1999; Jaspers et al. 1999; Ye et al. 1999). Mechanistically, vanadate activates TNF-α gene promoter through NFκB (Jaspers et al. 2000; Ouellet et al. 1999).
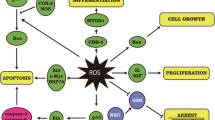
The ability of V compounds to be potent inhibitors of protein tyrosine phosphatases (PTPs) appears to be the underlying mechanism of some of its effects (Fig. 1). Examples include neurite outgrowth in human neuroblastoma SH-SY5Y cells and the differentiation and neurite outgrowth of rat pheochromocytoma cells (PC12 cells) after treatment with sodium orthovanadate (Rogers et al. 1994). This is distinct from the kind of differentiation signaling induced by nerve growth factor (Rogers et al. 1994). However, a reduced rate of proliferation in the presence of a couple of peroxovanadium complexes has been reported in neuroblastoma NB41 and glioma C6 (Faure et al. 1995). Faure et al. demonstrated that the mechanism responsible for this is the reversible block at the G2–M transition of the cell cycle and that removal of the peroxovanadium complex restored normalcy to cell cycling. Using apoptotic neuronal models, Farinelli and Greene (1996) have shown that substances which cause a cell-cycle block in the S, G2, and M phases don’t prevent cell death, whereas substances which block cell-cycle progression before the G1–S transition prevent apoptosis. The peroxovanadium-induced cell-cycle block at G2–M therefore might be linked to apoptotic cell death.
A postulated mechanism of inhibiting phosphatases by vanadate and peroxovanadium complexes (Morinville et al. 1998). Vanadate acts as a transition state analogue and forms a reversible bond, thus inhibiting protein tyrosine phosphatases (PTPs). On the other hand, peroxovanadium complexes oxidize the cysteine residue in the catalytic domain of PTPs to irreversibly inhibit PTPs
Vanadium compounds can show antineoplastic effects in vivo (Thompson et al. 1984). In vitro, sodium orthovanadate displayed a time- and dose-dependent cytotoxicity in proliferating primary cultures and tumor cell lines, while non-proliferating cells were found to be less susceptible to vanadate-induced cytotoxicity (Cruz et al. 1995).
Since V species like vanadates and peroxovanadium complexes alter intracellular phosphorylation levels in a nonselective manner through the inhibition of protein phosphatases, it is unsurprising that they have profound effects on intracellular signaling cascades. A good example is the MAPK cascade consisting of ERK, the c-Jun N-terminal protein kinases (JNKs), and p38 (Kyriakis and Avruch 1996; Marshall 1995; Whitmarsh and Davis 1996), which are implicated in the tight regulation of some intracellular pathways and connected to both cell survival and apoptotic responses (Kummer et al. 1997; Xia et al. 1995). Interference with the MAPK signaling cascade by V compounds could explain some of the observed insulin-mimicking effects of V compounds since they can activate the MAPK signaling cascade and since the insulin-mediated activation of IRK causes the activation of ERKs and the protein kinases p70s6k and p90rsk (Pandey et al. 1995). In addition, sodium orthovanadate, vanadyl sulfate, and sodium metavanadate stimulate ERK-1, ERK-2, p70s6k and p90rsk in CHO cells (Pandey et al. 1995), while peroxovanadates activate ERK in HeLa cells (Zhao et al. 1996). Given that MAPK links to cell survival and apoptosis, the ability of V to modulate the activity of its members is possibly responsible for V-induced toxicity, although the role of the MAPKs in mediating peroxovanadium complex-induced cell death has yet to be fully elucidated (Fig. 2) (Morinville et al. 1998).
A simplified diagram of the apoptotic signaling pathways modified by vanadium (V) compounds (Morinville et al. 1998). Vanadium has been shown to modulate multiple signaling pathways including the MAPK and NFκB signaling cascades that contribute to apoptotic cell death. The proteins that can be modulated by V are marked with an asterisk. Dotted lines indicate putative connections
The extent of V’s involvement in modulating various cell death pathways has yet to be explored. In one of the programmed cell death paradigms, extracellular ligands like TNF-α can bind to a death receptor (DR) spanning the cell membrane (Haunstetter and Izumo 1998). As previously mentioned, NFκB activity and JNK can be potentially altered by V complexes (Barbeau et al. 1997; Gopalbhai and Meloche 1998), with a real potential to modify cell death signal sites or routes (Morinville et al. 1998). The inhibition of PTPs can affect the transduction signals arising from DRs. Apoptotic signals from DRs involve caspase activation. The precise regulation of each caspase has yet to be fully elucidated even though it is known that these caspases are activated by cleavage at a specific aspartate residue. Also, peroxovanadium complexes can activate caspases (Morinville et al. 1998).
Vanadium compounds might not only cause apoptotic cell death but also death through necrosis. Despite evidence suggesting that the mechanism underlying V toxicity is independent of its ability to inhibit PTPs, the relationship between V exposure and necrosis needs to be extensively probed as very little is known of the players involved in this distinct necrotic cell death (Morinville et al. 1998).
Vanadium-Induced Neurotoxicity and its Relevance to Neurodegeneration
Parkinson’s disease (PD) is one of the most common neurodegenerative disorders, affecting over one million Americans and about 2% worldwide of those over the age of 60 years (Bergareche et al. 2004; Elbaz et al. 2002). Since most PD cases are sporadic, enormous interest has emerged in understanding the role of environmental factors in various Parkinsonian disorders (Hanna et al. 1999; Kumar et al. 2004). Many occupational exposures have been linked to the etiology and progression of PD, including farming, steel/alloy manufacturing, mining, wood/pulp processing, carpentry, planer milling, cleaning, forestry/logging, orchard farming, as well as certain occupations comprising body and fender repair, working in oil and gas fields, auto painting, and railroad and auto mechanics. These occupational exposures are often related to environmental exposure to certain metals, fuel oil, pesticides, herbicides, well water, magnetic fields, and rural living (Fall et al. 1999; Gorell et al. 2004; Jankovic 2005; Noonan et al. 2002). Some reports have suggested that welders may be at a higher risk of developing Parkinsonism and welding is a risk factor for PD pathogenesis (Racette et al. 2001, 2005). In a mortality study of occupational information reported on US death certificates, a significantly higher proportion of PD fatality cases correlated with likely exposure to Mn through welding-related jobs (Park et al. Park et al. 2005a, b). On the other hand, some occupational studies did not find any statistically significant association between PD and welding (Gorell et al. 1999, 2004).
Sundin (1998) estimated that more than one million people were employed as welders worldwide, and millions around the world are exposed to welding aerosols on a daily basis (Antonini et al. 2009a; Antonini et al. 2009b). The American welding association (http://www.aws.org/w/a/research/outlook.html), McInerny et al. 2009) expects that the number of welders will continue to grow to meet the increasing demand for steel and metal products around the world as developing countries continue to modernize. In 1991, it was also reported that more than three million people performed welding as a part of their work at least intermittently (Sferlazza and Beckett 1991). Welding fumes comprise a complex mixture of gases with fine and ultrafine particles of different metals and their oxides, which form during welding by metal vaporization and oxidation (McNeilly et al. 2004; Yu et al. 2000). The fumes from welding have also been found to contain silicates and fluorides of metals like chromium, Mn, V, titanium, molybdenum, cobalt, nickel, copper, and zinc (Sanderson 1968). These fumes, which also produce gases such as hydrogen fluoride, carbon monoxide, nitrogen oxide, fluorine, and ozone, can adversely affect the health of welders as well as the health of those in the immediate area (American society of safety engineers, http://www.asse.org/practicespecialties/articles/weldingfumes.php). The exact nature of the welding fumes is largely dependent on the composition of the electrode, the filler wire, and the type of welding being performed (Antonini et al. 1996; Sferlazza and Beckett 1991). More than 90% of V goes into steelmaking, and the dominant market driver of V production in recent years has been the rapid growth in worldwide steel production (Bunting 2006).
Individuals exposed to V have manifested neurological symptoms like tremor and CNS depression (Done 1979). Inhaled V2O5 has induced significant dopaminergic neuronal loss in the substantia nigra of mice, accompanied by morphological alterations of striatal medium spiny neurons (Avila-Costa et al. 2004). The same group also reported in their animal studies that V inhalation produced necrotic-like cell death, a loss of dendritic spines, and notorious alterations in the neuropile, resulting in the impairment of spatial memory as evaluated by the Morris water maze (Avila-Costa et al. 2004, 2006).
Our laboratory recently probed the cell death signaling mechanisms leading to the loss of dopaminergic neuronal cells following exposure to V (Afeseh Ngwa et al. 2009). Vanadium (V2O5) was found to be neurotoxic to rat dopaminergic neuronal (N27) cells, with an EC50 of 37 μM. ICP-MS analysis determined that a time-dependent uptake of V into the cells accompanied the neurotoxic effects. Also, the metal transporter proteins transferrin (Tf) and divalent metal transporter 1 (DMT1) were upregulated. We further showed that V exposure generated up to a threefold increase in ROS, which was accompanied by the release of mitochondrial cytochrome c into the cytoplasm with consequential activation of initiator caspase-9 and activator caspase-3. Interestingly, we also observed that V exposure further induced the caspase-mediated proteolytic cleavage of a pro-apoptotic kinase, protein kinase C delta (PKCδ), resulting in persistently increased kinase activity. Co-treating V with the pan-caspase inhibitor Z-VAD-FMK significantly blocked V-induced PKCδ proteolytic activation and increases in DNA fragmentation, hence supporting the role of caspase-mediated PKCδ cleavage in V-induced neurotoxicity. Importantly, V was also highly neurotoxic to murine primary mesencephalic dopaminergic neurons.
In another animal model study (Ngwa et al. 2014), we examined the neurotoxic effects of V on the olfactory bulb since anosmia is considered an early symptom of neurological diseases, including PD. C57 black mice were exposed intranasally to an environmentally relevant exposure dose of 182 μg V2O5 three times a week for up to 1 month. Behavioral, neurochemical, and histological studies were performed following the intranasal exposure. When compared to controls, the treatment group experienced dramatic decreases in olfactory bulb weights, tyrosine hydroxylase levels, as well as dopamine and DOPAC levels. The severe neurotoxic effect of V in the olfactory system had a neuroinflammatory component, as evidenced by the accumulation of astroglia in the glomerular layer of the olfactory bulb where dopaminergic neurons were degenerating. Neurobiological changes in response to intranasal V exposure were severe enough to be manifested at the behavioral level as impaired olfaction and significant locomotor deficits. These results suggest exposure to V is toxic to dopaminergic neurons and impairs olfaction in mouse models. However, more evidence is needed to prove a cause-and-effect relationship between PD and V exposure.
Conclusions
This review has covered the evidence supporting the idea that V and its compounds may interfere with various cellular functions including neuronal functions, leading to changes through the generation of ROS and interactions with protein tyrosine phosphatases (PTP) that affect cell signaling pathways, which may in turn produce or inhibit cell death depending on V’s oxidation state and the type of V compound. Much research on V and its compounds has been on its respiratory effects, as well as some on its effects on the kidney and liver, whereas comparatively little has been done on its possible neurotoxic effects. Vanadium and its compounds, often in synergy with other neurotoxic compounds like Mn that co-occur in occupational fumes, are likely neurotoxic. Manganese, which has been linked with Parkinson-like symptoms, has garnered almost all the attention for its association with welding fumes and neurotoxicity, while V and its compounds have thus far been largely neglected. Based on this review, much more work is warranted to explore how mixed metals, and their individual components like V, potentiate the neurotoxic effects caused by welding fumes.
References
Afeseh Ngwa H, Kanthasamy A, Anantharam V, Song C, Witte T, Houk R, Kanthasamy AG. Vanadium induces dopaminergic neurotoxicity via protein kinase Cdelta dependent oxidative signaling mechanisms: relevance to etiopathogenesis of Parkinson’s disease. Toxicol Appl Pharmacol. 2009;240(2):273–85.
Altamirano-Lozano M, Alvarez-Barrera L, Basurto-Alcantara F, Valverde M, Rojas E. Reprotoxic and genotoxic studies of vanadium pentoxide in male mice. Teratog Carcinog Mutagen. 1996;16(1):7–17.
Antonini JM, Murthy GGK, Rogers RA, Albert R, Ulrich GD, Brain JD. Pneumotoxicity and pulmonary clearance of different welding fumes after Intratracheal instillation in the rat. Toxicol Appl Pharmacol. 1996;140(1):188–99.
Antonini JM, Roberts JR, Stone S, Chen BT, Schwegler-Berry D, Frazer DG. Short-term inhalation exposure to mild steel welding fume had no effect on lung inflammation and injury but did alter defense responses to bacteria in rats. Inhal Toxicol. 2009a;21(3):182–92.
Antonini JM, Sriram K, Benkovic SA, Roberts JR, Stone S, Chen BT, Schwegler-Berry D, Jefferson AM, Billig BK, Felton CM, Hammer MA, Ma F, Frazer DG, O’Callaghan JP, Miller DB. Mild steel welding fume causes manganese accumulation and subtle neuroinflammatory changes but not overt neuronal damage in discrete brain regions of rats after short-term inhalation exposure. Neurotoxicology. 2009b;30(6):915–25.
Avila-Costa MR, Flores EM, Colin-Barenque L, Ordoñez JL, Gutiérrez AL, Niño-Cabrera HG, Mussali-Galante P, Fortoul TI. Nigrostriatal modifications after vanadium inhalation: an Immunocytochemical and cytological approach. Neurochem Res. 2004;29(7):1365–9.
Avila-Costa MR, Fortoul TI, Niño-Cabrera G, Colín-Barenque L, Bizarro-Nevares P, Gutiérrez-Valdez AL, Ordóñez-Librado JL, Rodríguez-Lara V, Mussali-Galante P, Díaz-Bech P, Anaya-Martínez V. Hippocampal cell alterations induced by the inhalation of vanadium pentoxide (V2O5) promote memory deterioration. Neurotoxicology. 2006;27(6):1007–12.
Azeez IA, Olopade F, Laperchia C, Andrioli A, Scambi I, Onwuka SK, Bentivoglio M, Olopade JO. Regional myelin and axon damage and Neuroinflammation in the adult mouse brain after long-term postnatal vanadium exposure. J Neuropathol Exp Neurol. 2016;75(9):843–54.
Badmaev V, Prakash S, Majeed M. Vanadium: a review of its potential role in the fight against diabetes. J Altern Complement Med. 1999;5(3):273–91.
Barbeau B, Bernier R, Dumais N, Briand G, Olivier M, Faure R, Posner BI, Tremblay M. Activation of HIV-1 long terminal repeat transcription and virus replication via NF-kappaB-dependent and -independent pathways by potent phosphotyrosine phosphatase inhibitors, the peroxovanadium compounds. J Biol Chem. 1997;272(20):12968–77.
Barth A, Schaffer AW, Konnaris C, Blauensteiner R, Winker R, Osterode W, Rudiger HW. Neurobehavioral effects of vanadium. J Toxicol Environ Health A. 2002;65(9):677–83.
Bergareche A, De la Puente E, López deMunain A, Sarasqueta C, de Arce A, Poza JJ, Martí-Massó JF. Prevalence of Parkinson’s disease and other types of Parkinsonism. J Neurol. 2004;251(3):340–5.
Bollen M, Miralpeix M, Ventura F, Toth B, Bartrons R, Stalmans W. Oral administration of vanadate to streptozotocin-diabetic rats restores the glucose-induced activation of liver glycogen synthase. Biochem J. 1990;267(1):269–71.
Bunting RM. Vanadium: how market developments affect the titanium industry. Strategic minerals corporation, Titanium 2006, International Titanium Association Conference. San Diego, California. 2006.
Calderon-Garciduenas L, Leray E, Heydarpour P, Torres-Jardon R, Reis J. Air pollution, a rising environmental risk factor for cognition, neuroinflammation and neurodegeneration: the clinical impact on children and beyond. Rev Neurol. 2016;172(1):69–80.
ChemIDPlus. Hazardous Substance Database. 2016.
Chong IW, Lin SR, Hwang JJ, Huang MS, Wang TH, Tsai MS, Hou JJ, Paulauskis JD. Expression and regulation of macrophage inflammatory protein-2 gene by vanadium in mouse macrophages. Inflammation. 2000;24(2):127–39.
Cortizo AMA, Bruzzone L, Molinuevo S, Etcheverry SB. A possible role of oxidative stress in the vanadium-induced cytotoxicity in the MC3T3E1 osteoblast and UMR106 osteosarcoma cell lines. Toxicology. 2000;147(2):89–99.
Crans DC, Smee JJ, Gaidamauskas E, Yang L. The chemistry and biochemistry of vanadium and the biological activities exerted by vanadium compounds. Chem Rev. 2004;104(2):849–902.
Cruz TF, Morgan A, Min W. In vitro and in vivo antineoplastic effects of orthovanadate. Mol Cell Biochem. 1995;153(1-2):161–6.
DeWitt J, Buck B, Goossens D, Hu Q, Chow R, David W, Young S, Teng Y, Leetham-Spencer M, Murphy L, Pollard J, McLaurin B, Gerads R, Keil D. Health effects following subacute exposure to geogenic dusts from arsenic-rich sediment at the Nellis dunes recreation area, Las Vegas, NV. Toxicol Appl Pharmacol. 2016;304:79–89.
Ding M, Gannett PM, Rojanasakul Y, Liu K, Shi X. One-electron reduction of vanadate by ascorbate and related free radical generation at physiological pH. J Inorg Biochem. 1994;55(2):101–12.
Ding M, Li JJ, Leonard SS, Ye JP, Shi X, Colburn NH, Castranova V, Vallyathan V. Vanadate-induced activation of activator protein-1: role of reactive oxygen species. Carcinogenesis. 1999;20(4):663–8.
Done AK. Of metals and chelation. AK: Done; 1979. p. 186–9.
Duffus JH. Carcinogenicity classification of vanadium pentoxide and inorganic vanadium compounds, the NTP study of carcinogenicity of inhaled vanadium pentoxide, and vanadium chemistry. Regul Toxicol Pharmacol. 2007;47(1):110–4.
Elbaz A, Bower JH, Maraganore DM, McDonnell SK, Peterson BJ, Ahlskog JE, Schaid DJ, Rocca WA. Risk tables for parkinsonism and Parkinson’s disease. J Clin Epidemiol. 2002;55(1):25–31.
Evangelou AM. Vanadium in cancer treatment. Crit Rev Oncol Hematol. 2002;42(3):249–65.
Fall PA, Fredrikson M, Axelson O, Granerus AK. Nutritional and occupational factors influencing the risk of Parkinson’s disease: a case-control study in southeastern Sweden. Mov Disord. 1999;14(1):28–37.
Farinelli SE, Greene LA. Cell cycle blockers mimosine, ciclopirox, and deferoxamine prevent the death of PC12 cells and postmitotic sympathetic neurons after removal of trophic support. J Neurosci. 1996;16(3):1150–62.
Faure R, Vincent M, Dufour M, Shaver A, Posner BI. Arrest at the G2/M transition of the cell cycle by protein-tyrosine phosphatase inhibition: studies on a neuronal and a glial cell line. J Cell Biochem. 1995;59(3):389–401.
Fenech M, Ferguson LR. Vitamins/minerals and genomic stability in humans. Mutat Res/Fundament Mol Mech Mutagen. 2001;475(1–2):1–6.
Folarin O, Olopade F, Onwuka S, Olopade J. Memory deficit recovery after chronic vanadium exposure in mice. Oxidative Med Cell Longev. 2016;2016:4860582.
Friberg L, Nordberg GF, Kessler E, Vouk VB. Handbook of the toxicology of metals. New York: Elsevier Science Publishers BV; 1986.
Gândara RMC, Soares SS, Martins H, Gutiérrez-Merino C, Aureliano M. Vanadate oligomers: in vivo effects in hepatic vanadium accumulation and stress markers. J Inorg Biochem. 2005;99(5):1238–44.
Gopalbhai K, Meloche S. Repression of mitogen-activated protein kinases ERK1/ERK2 activity by a protein tyrosine phosphatase in rat fibroblasts transformed by upstream oncoproteins. J Cell Physiol. 1998;174(1):35–47.
Gorell JM, Rybicki BA, Cole Johnson C, Peterson EL. Occupational metal exposures and the risk of Parkinson’s disease. Neuroepidemiology. 1999;18(6):303–8.
Gorell JM, Peterson EL, Rybicki BA, Johnson CC. Multiple risk factors for Parkinson’s disease. J Neurol Sci. 2004;217(2):169–74.
Greenwood NN, Earnshaw A. Chemistry of the elements (2nd edition): Butterworth-Heinemann; 1997.
Hanna PA, Jankovic J, Kirkpatrick JB. Multiple system atrophy: the putative causative role of environmental toxins. Arch Neurol. 1999;56(1):90–4.
Haunstetter A, Izumo S. Apoptosis: basic mechanisms and implications for cardiovascular disease. Circ Res. 1998;82(11):1111–29.
Hussain SP, Hofseth LJ, Harris CC. Radical causes of cancer. Nat Rev Cancer. 2003;3(4):276–85.
IARC. Cobalt in hard metals and cobalt sulfate, gallium arsenide, indium phosphide and vanadium pentoxide, IARC monographs on the evaluation of carcinogenic risks to humans. Lyon: International Agency for Research on Cancer; 2006a. p. 227–92.
IARC. Cobalt in hard metals and cobalt sulfate, gallium arsenide, indium phosphide and vanadium pentoxide, IARC monographs on the evaluation of carcinogenic risks to humans. International Agency for Research on Cancer, Lyon, France; 2006b. p. 227–92.
Jankovic J. Searching for a relationship between manganese and welding and Parkinson’s disease. Neurology. 2005;64(12):2021–8.
Jaspers I, Samet JM, Reed W. Arsenite exposure of cultured airway epithelial cells activates kappaB-dependent interleukin-8 gene expression in the absence of nuclear factor-kappaB nuclear translocation. J Biol Chem. 1999;274(43):31025–33.
Jaspers I, Samet JM, Erzurum S, Reed W. Vanadium-induced kappaB-dependent transcription depends upon peroxide-induced activation of the p38 mitogen-activated protein kinase. Am J Respir Cell Mol Biol. 2000;23(1):95–102.
Jiang M, Li Y, Zhang B, Zhou A, Zheng T, Qian Z, Du X, Zhou Y, Pan X, Hu J, Wu C, Peng Y, Liu W, Zhang C, Xia W, Xu S. A nested case-control study of prenatal vanadium exposure and low birthweight. Hum Reprod. 2016;31(9):2135–41.
Kacew S, Parulekar MR, Merali Z. Effects of parenteral vanadium administration on pulmonary metabolism of rats. Toxicol Lett. 1982;11(1):119–24.
Keil D, Buck B, Goossens D, Teng Y, Leetham M, Murphy L, Pollard J, Eggers M, McLaurin B, Gerads R, DeWitt J. Immunotoxicological and neurotoxicological profile of health effects following subacute exposure to geogenic dust from sand dunes at the Nellis dunes recreation area, Las Vegas, NV. Toxicol Appl Pharmacol. 2016;291:1–12.
Kiviluoto M, Pyy L, Pakarinen A. Serum and urinary vanadium of vanadium-exposed workers. Scand J Work Environ Health. 1979;5(4):362–7.
Kumar A, Calne SM, Schulzer M, Mak E, Wszolek Z, Van Netten C, Tsui JK, Stoessl AJ, Calne DB. Clustering of Parkinson disease: shared cause or coincidence? Arch Neurol. 2004;61(7):1057–60.
Kummer JL, Rao PK, Heidenreich KA. Apoptosis induced by withdrawal of trophic factors is mediated by p38 mitogen-activated protein kinase. J Biol Chem. 1997;272(33):20490–4.
Kyriakis JM, Avruch J. Sounding the alarm: protein kinase cascades activated by stress and inflammation. J Biol Chem. 1996;271(40):24313–6.
Lau JY, Qian KP, Wu PC, Davis GL. Ribonucleotide vanadyl complexes inhibit polymerase chain reaction. Nucleic Acids Res. 1993;21(11):2777.
Li H, Zhou D, Zhang Q, Feng C, Zheng W, He K, Lan Y. Vanadium exposure-induced neurobehavioral alterations among Chinese workers. Neurotoxicology. 2013;36:49–54.
Liochev SI, Fridovich I. Vanadate-stimulated oxidation of NAD(P)H in the presence of biological membranes and other sources of O2−. Arch Biochem Biophys. 1990;279(1):1–7.
Marshall CJ. Specificity of receptor tyrosine kinase signaling: transient versus sustained extracellular signal-regulated kinase activation. Cell. 1995;80(2):179–85.
McInerny SC, Brown AL, Smith DW. Region-specific changes in mitochondrial D-loop in aged rat CNS. Mech Ageing Dev. 2009;130(5):343–9.
McNeilly JD, Heal MR, Beverland IJ, Howe A, Gibson MD, Hibbs LR, MacNee W, Donaldson K. Soluble transition metals cause the pro-inflammatory effects of welding fumes in vitro. Toxicol Appl Pharmacol. 2004;196(1):95–107.
Morinville A, Maysinger D, Shaver A. From Vanadis to Atropos: vanadium compounds as pharmacological tools in cell death signalling. Trends Pharmacol Sci. 1998;19(11):452–60.
Mukherjee B, Patra B, Mahapatra S, Banerjee P, Tiwari A, Chatterjee M. Vanadium—an element of atypical biological significance. Toxicol Lett. 2004;150(2):135–43.
Nakai M, Watanabe H, Fujiwara C, Kakegawa H, Satoh T, Takada J, Matsushita R, Sakurai H. Mechanism on insulin-like action of vanadyl sulfate: studies on interaction between rat adipocytes and vanadium compounds. Biol Pharm Bull. 1995;18(5):719–25.
Ngwa HA, Kanthasamy A, Jin H, Anantharam V, Kanthasamy AG. Vanadium exposure induces olfactory dysfunction in an animal model of metal neurotoxicity. Neurotoxicology. 2014;43:73–81.
Noonan CW, Reif JS, Yost M, Touchstone J. Occupational exposure to magnetic fields in case-referent studies of neurodegenerative diseases. Scand J Work Environ Health. 2002;28(1):42–8.
Ouellet M, Barbeau B, Tremblay MJ. p56(lck), ZAP-70, SLP-76, and calcium-regulated effectors are involved in NF-kappaB activation by bisperoxovanadium phosphotyrosyl phosphatase inhibitors in human T cells. J Biol Chem. 1999;274(49):35029–36.
Pandey SK, Chiasson JL, Srivastava AK. Vanadium salts stimulate mitogen-activated protein (MAP) kinases and ribosomal S6 kinases. Mol Cell Biochem. 1995;153(1-2):69–78.
Pandey SK, Theberge JF, Bernier M, Srivastava AK. Phosphatidylinositol 3-kinase requirement in activation of the ras/C-raf-1/MEK/ERK and p70(s6k) signaling cascade by the insulinomimetic agent vanadyl sulfate. Biochemistry. 1999;38(44):14667–75.
Park J, Yoo CI, Sim CS, Kim HK, Kim JW, Jeon BS, Kim KR, Bang OY, Lee WY, Yi Y, Jung KY, Chung SE, Kim Y. Occupations and Parkinson’s disease: a multi-center case-control study in South Korea. Neurotoxicology. 2005a;26(1):99–105.
Park RM, Schulte PA, Bowman JD, Walker JT, Bondy SC, Yost MG, Touchstone JA, Dosemeci M. Potential occupational risks for neurodegenerative diseases. Am J Ind Med. 2005b;48(1):63–77.
Patel B, Henderson GE, Haswell SJ, Grzeskowiak R. Speciation of vanadium present in a model yeast system. Analyst. 1990;115(8):1063–6.
Pyrzyńska K, Wierzbicki T. Determination of vanadium species in environmental samples. Talanta. 2004;64(4):823–9.
Racette BA, McGee-Minnich L, Moerlein SM, Mink JW, Videen TO, Perlmutter JS. Welding-related parkinsonism: clinical features, treatment, and pathophysiology. Neurology. 2001;56(1):8–13.
Racette BA, Tabbal SD, Jennings D, Good L, Perlmutter JS, Evanoff B. Prevalence of parkinsonism and relationship to exposure in a large sample of Alabama welders. Neurology. 2005;64(2):230–5.
Ray RS, Rana B, Swami B, Venu V, Chatterjee M. Vanadium mediated apoptosis and cell cycle arrest in MCF7 cell line. Chem Biol Interact. 2006;163(3):239–47.
Reis AP, Patinha C, Noack Y, Robert S, Dias AC. Assessing human exposure to aluminium, chromium and vanadium through outdoor dust ingestion in the Bassin Minier de Provence, France. Environ Geochem Health. 2014;36(2):303–17.
Rogers MV, Buensuceso C, Montague F, Mahadevan L. Vanadate stimulates differentiation and neurite outgrowth in rat pheochromocytoma PC12 cells and neurite extension in human neuroblastoma SH-SY5Y cells. Neuroscience. 1994;60(2):479–94.
Sabbioni E, Pozzi G, Pintar A, Casella L, Garattini S. Cellular retention, cytotoxicity and morphological transformation by vanadium(IV) and vanadium(V) in BALB/3T3 cell lines. Carcinogenesis. 1991;12(1):47–52.
Sanderson JT. Hazards of the arc-air gouging process. Ann Occup Hyg. 1968;11(2):123–33.
Sferlazza SJ, Beckett WS. The respiratory health of welders. Am Rev Respir Dis. 1991;143(5 Pt 1):1134–48.
Sheridan CJ, Pfleger RC, McClellan RO. Cytotoxicity of vanadium pentoxide on pulmonary alveolar macrophages from dog, rabbit, and rat: effect on viability and effect on lipid metabolism. Ann Resp Inhalation Toxicol; 1978. p. 294–8.
SIMRAC. Hazardous metals in mining processing plants in South Africa. The risk of occupational exposure Mine Health and Safety Council (Safety in Mines Research Advisory Committee Report). 2000.
Sjoberg S-G. Vanadium bronchitis from cleaning oil-fired boilers. Occup Med. 1954;4(1):31.
Stankiewicz PJ, Tracey AS. Stimulation of enzyme activity by oxovanadium complexes. Met Ions Biol Syst. 1995;31:249–85.
Stemmler AJ, Burrows CJ. Guanine versus deoxyribose damage in DNA oxidation mediated by vanadium(IV) and vanadium(V) complexes. J Biol Inorg Chem. 2001;6(1):100–6.
Sundin DS. National occupational exposure survey database 1981–1983. 1998.
Thompson HJ, Chasteen ND, Meeker LD. Dietary vanadyl(IV) sulfate inhibits chemically-induced mammary carcinogenesis. Carcinogenesis. 1984;5(6):849–51.
Tracey AS. Hydroxamido vanadates: aqueous chemistry and function in protein tyrosine phosphatases and cell cultures. J Inorg Biochem. 2000;80(1–2):11–6.
Valko M, Rhodes CJ, Moncol J, Izakovic M, Mazur M. Free radicals, metals and antioxidants in oxidative stress-induced cancer. Chem Biol Interact. 2006;160(1):1–40.
Whitmarsh AJ, Davis RJ. Transcription factor AP-1 regulation by mitogen-activated protein kinase signal transduction pathways. J Mol Med. 1996;74(10):589–607.
Witkowska D, Brzezinski J. Alteration of brain noradrenaline, dopamine and 5-hydroxytryptamine levels during vanadium poisoning. Pol J Pharmacol Pharm. 1979;31(4):393–8.
Xia Z, Dickens M, Raingeaud J, Davis RJ, Greenberg ME. Opposing effects of ERK and JNK-p38 MAP kinases on apoptosis. Science. 1995;270(5240):1326–31.
Ye J, Ding M, Zhang X, Rojanasakul Y, Nedospasov S, Vallyathan V, Castranova V, Shi X. Induction of TNFalpha in macrophages by vanadate is dependent on activation of transcription factor NF-kappaB and free radical reactions. Mol Cell Biochem. 1999;198(1-2):193–200.
Yu IJ, Kim KJ, Chang HK, Song KS, Han KT, Han JH, Maeng SH, Chung YH, Park SH, Chung KH, Han JS, Chung HK. Pattern of deposition of stainless steel welding fume particles inhaled into the respiratory systems of Sprague–Dawley rats exposed to a novel welding fume generating system. Toxicol Lett. 2000;116(1–2):103–11.
Zhang Z, Huang C, Li J, Leonard SS, Lanciotti R, Butterworth L, Shi X. Vanadate-induced cell growth regulation and the role of reactive oxygen species. Arch Biochem Biophys. 2001;392(2):311–20.
Zhao Z, Tan Z, Diltz CD, You M, Fischer EH. Activation of mitogen-activated protein (MAP) kinase pathway by pervanadate, a potent inhibitor of tyrosine phosphatases. J Biol Chem. 1996;271(36):22251–5.
Zhao Y, Ye L, Liu H, Xia Q, Zhang Y, Yang X, Wang K. Vanadium compounds induced mitochondria permeability transition pore (PTP) opening related to oxidative stress. J Inorg Biochem. 2010;104(4):371–8.
Zhu CW, Liu YX, Huang CJ, Gao W, Hu GL, Li J, Zhang Q, Lan YJ. Effect of vanadium exposure on neurobehavioral function in workers. Zhonghua lao dong wei sheng zhi ye bing za zhi = Zhonghua laodong weisheng zhiyebing zazhi Chin J Indust Hyg Occup Dis. 2016;34(2):103–6.
Acknowledgment
This chapter was supported by National Institutes of Health Grants ES10586 and ES26892. The W. Eugene and Linda Lloyd Endowed Chair for AGK is also acknowledged. We thank Gary Zenitsky for assistance in preparing this chapter.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 Springer International Publishing AG
About this chapter
Cite this chapter
Ngwa, H.A., Ay, M., Jin, H., Anantharam, V., Kanthasamy, A., Kanthasamy, A.G. (2017). Neurotoxicity of Vanadium. In: Aschner, M., Costa, L. (eds) Neurotoxicity of Metals. Advances in Neurobiology, vol 18. Springer, Cham. https://doi.org/10.1007/978-3-319-60189-2_14
Download citation
DOI: https://doi.org/10.1007/978-3-319-60189-2_14
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-60188-5
Online ISBN: 978-3-319-60189-2
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)