Abstract
Technological advances during the last years have contributed to the development of wireless and low-cost electroencephalography (EEG) acquisition systems and mobile brain-computer interface (mBCI) applications. The most popular applications are general-purpose (e.g., games, sports, daily-life, etc.). However, clinical usefulness of mBCIs is still an open question. In this paper we present a low-cost mobile BCI application and demonstrate its potential utility in clinical practice. In particular, we conducted a study in which visual evoked potentials (VEP) of two subjects were analyzed using our mBCI application, under different conditions: inside a laboratory, walking and traveling in a car. The results show that the features of our system (level of synchronization, robustness and signal quality) are acceptable for the demanding standard required for the electrophysiological evaluation of vision. In addition, the mobile recording and cloud computing of VEPs offers a number of advantages over traditional in-lab systems. The presented mobile application could be used for visual impairment screening, for ubiquitous, massive and low-cost evaluation of vision, and as ambulatory diagnostic tool in rural or undeveloped areas.
Access provided by CONRICYT-eBooks. Download conference paper PDF
Similar content being viewed by others
Keywords
1 Introduction
Mobile technology has become an essential part in people’s lives. Numerous technological advances have contributed to the development of this technology during the last years, with application in diverse fields. Apart from general-purpose applications such as telephone, mobile and low-cost clinical applications are increasingly becoming more and more frequent. For example, some smartphone-based approaches have been proposed for disease diagnosis [1, 2] and attention detection [3]. All this has led to introduce new technological concepts such as telemedicine [4], eHealth [5] and mHealth [6].
In the context of brain-computer interface (BCI) applications, the development of wireless and low-cost electroencephalography (EEG) acquisition systems, together with the advances in processing and classification algorithms, has favored the release of mobile BCI (mBCI) [7]. The most popular mBCI applications proposed to date are general-purpose, including mBCIs as part of body area networks (WBAN) [8, 9], games [10], sports [11], daily-life [12,13,14,15] and others [16]. However, the usefulness of wireless EEG acquisition systems and mBCIs in clinical practice is still an open question [17].
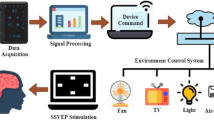
In this paper we present a low-cost mBCI application for clinical practice (see Fig. 1). In particular, the fully functional system (hardware and software) is able to perform the visual evoked potentials (VEP) test following the standard of the International Society for Clinical Evaluation of Vision (ISCEV) for stimulation and recording [18]. Visual evoked potentials are electrophysiological responses caused by visual stimuli. These responses are present in the EEG and can be recorded by placing one or more electrodes in the occipital area of the brain cortex. They are used in clinical practice to diagnose and monitor a broad list of diseases such as optic chiasm [19], Parkinson’s disease [20], multiple sclerosis [21], cataract [22], retinopathy [23], glaucoma [24], optic neuropathy [25] and stroke [26]. The proposed system consists of the RABio w8 low-cost device (developed by the University of Granada) for wireless EEG acquisition and a mobile device (e.g., smartphone, tablet, etc.) to perform the stimulation. After RABio w8 records the event-related responses (i.e., EEG data), they are sent to the cloud (i.e., a remote server) in charge of computing the VEPs in real-time. Once the VEPs have been extracted, the remote server sends the results to both the mobile device used for stimulation and the email addressed specified by the user. A comprehensive description of the mBCI application is reported in Sect. 2.3.
We conducted a study in order to prove the potential usefulness of our mBCI application for clinical practice. Those essential features in the VEP test such as level of synchronization, robustness and quality of VEPs (based on the amplitude, latency and morphology, that is, the quantifiable parameters of VEPs [27, 28]) were analyzed. The results show that they are acceptable for the standard requirements for the electrophysiological evaluation of vision, even under unfavorable environmental conditions (presence of severe artifacts).
Low-cost mobile applications have positive impact on society. The cost of many clinical test could be significantly reduced by using low-cost mobile technology. In addition, many clinical test could move from the lab (i.e., laboratories and hospitals) to everywhere and be performed at whenever time. In case of our mBCI application, it could be used as screening tool, as well as for massive studies in schools, local health centers, etc. by combining the mBCI application with big data in the cloud.
2 Methods
The aim of the conducted study was to test the performance of our mBCI application in the VEP clinical test, under different daily-life conditions, some of them with severe artifacts. The ISCEV standard for stimulation and recording of VEPs was followed. The details about the methodology are reported in this section.
2.1 Subjects and EEG Setup
Two healthy male volunteers participated in the study. They were not paid for their participation. All the subjects declared no visual pathologies or they used glasses to correct their vision during the experiment. They were informed about the experimental procedure and signed the informed consent prior to the beginning of the experiment.
One EEG electrode was placed at Oz position of the 10–20 International System. The reference and the ground were placed at Fz and the left ear lobe, respectively. The impedance of the electrodes was below 5 K\({\Omega }\).
2.2 Materials
Stimulation was performed by using a mobile device with 13.3-inch screen and Matlab software (The Mathworks, MA, USA). EEG signals were recorded at 500 Hz with the RABio w8 device. This device is a wireless 8-channel EEG acquisition system based on a 24-bit analog/digital converter with programmable amplifiers, a microcontroller and a Bluetooth module. Server used for cloud-computing of VEPs was a desktop PC with Matlab software.
2.3 Generation, Mobile Recording and Cloud-Computing of VEPs
Visual evoked potentials were elicited by pattern-reversal stimuli with checkerboard. Square size was 1 degree of visual field with the subject placed at 50 cm from the screen. Stimulation rate was 2 stimuli per second. The VEP test consisted in 4 trials of 80 stimuli (i.e., a total of 320 stimuli) with an inter-trial rest time of 10 s.
The subjects performed the VEP test in three different conditions with an inter-condition rest time of 5 min (see Fig. 2): sitting in a chair within a laboratory (condition I), walking along a corridor (condition II) and sitting in the passenger seat of a moving car (condition III). Condition I is considered as the gold standard since it is the closest implementation of the ISCEV standard. Although lighting conditions were stable, severe motion artifacts are expected in condition II due to the walking movement. In condition III, severe artifacts caused by environmental lighting changes are expected. For the sake of simplicity, in this paper subjects and conditions are denoted as (subject, condition). For instance (S2, III) denotes subject 2, condition III.
During the VEP test, as mentioned before, RABio w8 device is in charge of recording the EEG. Stimulus onset information is also recorded by RABio w8. It sends the EEG data (including stimulus onset information), sample by sample, to the mobile stimulation device via Bluetooth (IEEE 802.15.1 standard). This device collects the EEG data corresponding to one trial and sends them to the cloud by using TCP/IP communication through a wireless access point and router. In condition III, a smartphone was used as gateway to connect to the 4G network. Once the one-trial EEG data have been received by the server, a bandpass filtering (1–100 Hz) is performed. Afterwards, the epoch corresponding to every stimulus is extracted by using the stimulus onset information. No epoch was discarded. All the epochs are averaged to obtain the mean. This mean is the stimulus response. Negative (N1) and positive (P1) VEPs are automatically identified and marked in the resulting plot (see Fig. 3). Finally, this plot, raw and processed EEG data are sent, through a simple mail transfer protocol (SMTP) server, to both the stimulation device and the email addresses specified by the user. All this is performed in real-time. The whole system is displayed in Fig. 1.
3 Results
The results of the study are reported in this section (see Fig. 3, Tables 1 and 2).
4 Discussion
In this paper we have proposed a low-cost mBCI for clinical applications. Our implementation is based on a RABio w8 device and the performance was validated by means of a clinical test. The results have shown that the level of synchronization, robustness and signal quality (amplitude, latency and morphology) are sound enough for the demanding standard required for the electrophysiological evaluation of vision. Moreover, the mobile recording and cloud computing of VEPs offers a number of advantages over traditional in-lab systems. Our low-cost mBCI application can be accommodated for ubiquitous and massive evaluation of vision, for visual impairment screening, and used as ambulatory diagnostic tool in rural or undeveloped areas.
Figure 3 shows the VEP of two subjects under the three conditions of the experiment. VEPs under condition I correspond to the closest implementation of the ISCEV standard and we considered it in this experiment as the gold standard for conditions II and III. For both subject we observe that under conditions II and III, VEPs are contaminated with noise (i.e., it is not as smooth as the condition I). This was expected since the ISCEV standard describe simply filters for EEG signals recorded in an isolated room and with small number of artifacts. The noise could be easily mitigated with standard procedures for artifacts reduction (e.g., by reducing the bandwidth low-band filter or by eliminating trials with disparate amplitudes). Despite that, VEPs under conditions II and III exhibits and high correlation level with condition I (90.38%, 93.00%, 90.57% and 82.21% in cases (S1, II), (S1, III), (S2, II) and (S2, III) respectively, see Table 1) and the error latency is just small except for (S2, III) (2 ms, 6 ms, 2 ms and 24 ms in cases (S1 ,II), (S1 ,III), (S2, II) and (S2, III) respectively, see Table 1). The last row of Fig. 3 shows the three conditions overlapping. From visual inspection we can conclude that there is not relevant difference between the three VEPs and our mBCI implementation could be used with some limitations for the rapid and low-cost implementation of this clinical protocol. The only exception (S2, III).
Table 2 shows the amplitudes and latencies of negative (N1) and positive (P1) visual potentials. The amplitude and latency of these potentials help for the diagnosis of optic nerve impairment (WEB). Specifically, the N70 and P100 peak latencies and N70-P100 amplitude difference have been reported as sensitive measures of resolved optic neuritis [29]. For instance, people with resolved optic neuritis (ON) may have of 24 ms of mean delay of P1 in comparison with normal people [29]. Absolute and relative N1-P1 differences in amplitude and latency (compared with condition I), with the sole exception of (S2, III), are small (see Table 2). Therefore, it seems unlikely that the use of our mBCI system could give rise to a false positive of ON.
In conclusion, only (S2, III) shows amplitudes and latencies significantly different from those of the gold standard. This was expected because of the environmental lighting changes that could affect the vision of the subject. Our study needs to be extended in order to achieve reliable and comprehensive conclusions.
References
Woods, A.M., Nowostawski, M., Franz, E.A., Purvis, M.: Parkinson’s disease and essential tremor classification on mobile device. Pervasive Mob. Comput. 13, 1–12 (2014)
Maxhuni, A., Munoz-Melendez, A., Osmani, V., Perez, H., Mayora, O., Morales, E.F.: Classification of bipolar disorder episodes based on analysis of voice and motor activity of patients. Pervasive Mob. Comput. 31, 50–66 (2016)
Okoshi, T., Nozaki, H., Nakazawa, J., Tokuda, H., Ramos, J., Dey, A.K.: Towards attention-aware adaptive notification on smart phones. Pervasive Mob. Comput. 26, 17–34 (2016)
Ubeyli, E.D., Güler, N.F.: Theory and applications of telemedicine. J. Med. Syst. 26(3), 199–220 (2002)
Black, A.D., Car, J., Pagliari, C., Anandan, C., Cresswell, K., Bokun, T., McKinstry, B., Procter, R., Majeed, A., Sheikh, A.: The impact of ehealth on the quality and safety of health care: a systematic overview. PLoS Med. 8(1), 1–16 (2011)
Lorenz, A., Oppermann, R.: Mobile health monitoring for the elderly: designing for diversity. Pervasive Mob. Comput. 5(5), 478–495 (2009)
Minguillon, J., Lopez-Gordo, M.A., Pelayo, F.: Trends in EEG-BCI for daily-life: requirements for artifact removal. Biomed. Sig. Process. Control 31, 407–418 (2017)
Valenzuela-valdés, J.F., López, M.A., Padilla, P., Padilla, J.L., Minguillon, J.: Human neuro-activity for securing body area networks: application of brain-computer interfaces to people-centric internet of things. IEEE Commun. Mag. 55(2), 62–67 (2017)
Lopez-Gordo, M.A., Pelayo Valle, F.: Brain-computer interface as networking entity in body area networks. In: Aguayo-Torres, M.C., Gómez, G., Poncela, J. (eds.) WWIC 2015. LNCS, vol. 9071, pp. 274–285. Springer, Cham (2015). doi:10.1007/978-3-319-22572-2_20
Liao, L., Chen, C., Wang, I.: Gaming control using a wearable and wireless EEG-based brain-computer interface device with novel dry foam-based sensors. J. NeuroEng. Rehabil. 9(5) (2012)
Park, J.L., Fairweather, M.M., Donaldson, D.I.: Making the case for mobile cognition: EEG and sports performance. Neurosci. Biobehav. Rev. 52, 117–130 (2015)
Wang, Y., Wang, Y., Jung, T.: A cell-phone-based braincomputer interface for communication in daily life. J. Neural Eng. (2011)
Brennan, C., McCullagh, P., Lightbody, G., Galway, L., Feuser, D., González, J.L., Martin, S.: Accessing tele-services using a hybrid BCI approach. In: Rojas, I., Joya, G., Catala, A. (eds.) IWANN 2015. LNCS, vol. 9094, pp. 110–123. Springer, Cham (2015). doi:10.1007/978-3-319-19258-1_10
Mihajlovi, V., Grundlehner, B., Vullers, R., Penders, J.: Wearable, wireless EEG solutions in daily life applications: what are we missing? IEEE J. Biomed. Health Inf. 19(1), 6–21 (2015)
Lin, C., Lin, F., Chen, S.: EEG-based brain-computer interface for smart living environmental auto-adjustment. J. Med. Biol. Eng. 30(4), 237–245 (2010)
Lin, C., Chang, C., Lin, B.: A real-time wireless braincomputer interface system for drowsiness detection. Biomed. Circ. Syst. IEEE Trans. 4(4), 214–222 (2010)
Duvinage, M., Castermans, T., Petieau, M., Hoellinger, T., Cheron, G., Dutoit, T.: Performance of the Emotiv Epoc headset for P300-based applications. Biomed. Eng. Online 12(1), 56 (2013)
Odom, J.V., Bach, M., Brigell, M., Holder, G.E., McCulloch, D.L., Mizota, A., Tormene, A.P.: ISCEV standard for clinical visual evoked potentials: (2016 update). Doc. Ophthalmol. 133(1), 1–9 (2016)
Brecelj, J.: A VEP study of the visual pathway function in compressive lesions of the optic chiasm. Full-field versus half-field stimulation. Electroencephalogr. Clin. Neurophysiol./Evoked Potentials 84(3), 209–218 (1992)
Nightingale, S., Mitchell, K.W., Howe, J.W.: Visual evoked cortical potentials and pattern electroretinograms in Parkinson’s disease and control subjects. J. Neurol. Neurosurg. Psychiatry 49, 1280–1287 (1986)
Frederiksen, J.L., Larsson, H.B.W., OlesenI, J., Stigsby, B.: MRI, VEP, SEP and biothesiometry suggest monosymptomatic acute optic neuritis to be a first manifestation of multiple sclerosis. Acta Neurol. Scand. 83, 343–350 (1991)
McCulloch, D.L., Skarf, B.: Pattern reversal visual evoked potentials following early treatment of unilateral, congenital cataract. Arch. Ophthalmol. 112, 510 (1994)
Folk, J.C., Thompson, H.S., Han, D.P., Brown, C.K.: Visual function abnormalities in central serous retinopathy. Arch. Ophthalmol. 102, 1299–1302 (1984)
Hood, D.C., Greenstein, V.C.: Multifocal VEP and ganglion cell damage: applications and limitations for the study of glaucoma. Prog. Retinal Eye Res. 22(2), 201–251 (2003)
Carroll, W.M., Mastaglia, F.L.: Leber’s optic neuropathy: a clinical and visual evoked potential study of affected and asymptomatic members of a six generation family. Brain: J. Neurol. 102, 559–580 (1979)
Julkunen, L., Tenovuo, O., Vorobyev, V., Hiltunen, J., Teräs, M., Jääskeläinen, S.K., Hämäläinen, H.: Functional brain imaging, clinical and neurophysiological outcome of visual rehabilitation in a chronic stroke patient. Restorative Neurol. Neurosci. 24(2), 123–132 (2006)
Van den Bruel, A., Gailly, J., Hulstaert, F., Devriese, S., Eyssen, M.: The value of EEG, evoked potentials in clinical practice, Good Clinical Practice (GCP). Brussel: Belgian Health Care Knowledge Centre (KCE), KCE report 109C, pp. 1–138 (2009)
Evans, A.B.: Clinical utility of evoked potentials. Medscape, 1–28 (2014)
Brigell, M., Kaufman, D.I., Bobak, P., Beydoun, A.: The pattern visual evoked potential. A multicenter study using standardized techniques. Ophthalmic Lit. 48(4), 308 (1995)
Acknowledgments
This work was supported by Nicolo Association for the R+D in Neurotechnologies for disability, the Ministry of Economy and Competitiveness DPI2015-69098-REDT, the research project P11-TIC-7983 of Junta of Andalucia (Spain), and the Spanish National Grant TIN2015-67020-P, co-financed by the European Regional Development Fund (ERDF).
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 Springer International Publishing AG
About this paper
Cite this paper
Minguillon, J., Lopez-Gordo, M.A., Morillas, C., Pelayo, F. (2017). A Mobile Brain-Computer Interface for Clinical Applications: From the Lab to the Ubiquity. In: Ferrández Vicente, J., Álvarez-Sánchez, J., de la Paz López, F., Toledo Moreo, J., Adeli, H. (eds) Biomedical Applications Based on Natural and Artificial Computing. IWINAC 2017. Lecture Notes in Computer Science(), vol 10338. Springer, Cham. https://doi.org/10.1007/978-3-319-59773-7_8
Download citation
DOI: https://doi.org/10.1007/978-3-319-59773-7_8
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-59772-0
Online ISBN: 978-3-319-59773-7
eBook Packages: Computer ScienceComputer Science (R0)