Abstract
Aqueous two-phase system is a liquid-liquid extraction technique that can be carried out in continuous mode. For this, equipment commonly employed for the chemical industry (columns and mixer settlers) has been used. However, given the characteristics of density, viscosity, and interfacial tension and the diverse nature of the phases, some of them have to be modified. The ideal equipment should contain the elements to perform all the unit operations for batch systems in an integrated system with automation possibilities. This would facilitate the integration of the continuous ATPS approaches to a more sophisticated purification train. So, in this chapter, a platform for selection, characterization, and scaling of continuous ATPS is presented. From microscale to pilot plant scale, continuous systems imply multiple advantages over batch platforms, such as reduction in buffer consumption, diminishing process time/costs, an increase in process yields, higher throughputs, and smaller footprints. Nonetheless, elements such as recirculation or phase recycling should be considered for a more appropriate equipment design, as well as mathematical modeling and fluid dynamic simulation of the system streams, which would be of great help for the design and development of this kind of technology.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
8.1 Introduction
Aqueous two-phase systems (ATPSs) are a clean alternative for traditional organic water solvent extraction systems (Gupta et al. 1999) that have been used for partitioning a great variety of biomolecules of interest from their contaminants (Haraguchi et al. 2004; Kamei et al. 2002; Andrews et al. 1996; Xu et al. 2001; Rosa et al. 2013; Breydo et al. 2013), exploiting their differences in molecular weight, hydrophobicity, isoelectric point, and affinity among other features. To employ this technique in downstream processing, the information about new systems and new applications is gaining spread (Nan et al. 2013; Liu et al. 2013). Although ATPSs are known to be a biocompatible, integrative, easily upscaling system, some of the drawbacks of this technology are related to limitations of phase separation of highly viscous systems, high cost of polymer components, limited predictive design, and lack of know-how in terms of installation, validation, and operation (Haraguchi et al. 2004; Soares et al. 2015). Furthermore, most of their applications have been developed at batch and bench scale.
Nowadays, the industry is moving from the large fed-batch/batch systems to produce biopharmaceuticals to more versatile continuous systems (Zydney 2016; Jungbauer 2013; Croughan et al. 2015; Jungbauer and Walch 2015). This involves efforts in the process integration in order to reduce buffer consumption, diminishing process time and costs, and increasing process yields (Jungbauer and Walch 2015; Igarashi et al. 2004a). Thus, if the drawbacks of this technology were fulfilled, ATPS as a continuous or semi-continuous operation would have clear competitive advantages in the biotechnology market. This chapter is dedicated to revise the development of ATPS implemented in continuous processes. First, the equipment devices that have been employed for this purpose are described. Then, the strategy for characterization of those continuous systems, including typical hydrodynamic parameters, is presented. The chapter also discussed selected examples of continuous ATPS processes, from micro- to pilot plant scale.
8.2 Devices Employed for Continuous ATPS Processes
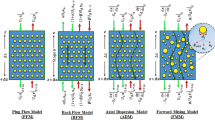
There is not specialized equipment to perform continuous ATPS processes. Devices that have been employed for this purpose are in the classification of column contactors, mixer-settler units, and other contactors that have been also employed in traditional liquid-liquid extractions (Espitia-Saloma et al. 2014). The geometry, the kinetics and the dynamics of the ATPS inside these prototypes can vary, but the same unit operations that are carried out in batch systems should be performed by any of those equipment (Fig. 8.1). The minimum number of operations to perform an ATPS extraction of biomolecules is described below:
-
Prepare the stocks of phase components. This step can be performed manually or as part of the continuous system. Stocks of single phase components can be stored in different containers or mixed to give various continuous phases in order to equal the viscosity and density of all the flows, regarding equipment restrains, such as power input in the pumping system. The sample can be pumped individually or diluted in one of the components streams.
-
Mix at the right phase composition. Put together the components and sample at their final dilution and mix thoroughly to let the molecule of interest to be in contact with the phase where it has preference. In batch systems, this stage is performed manually by using mechanical agitators. In continuous systems, pump-driven encountered flows can be used to perform this operation. Static mixers also are employed.
-
Phase coalescence or separation. It is important to allow phase separation after the phase emulsion formation, so the phase, where the product of interest is contained, could be recovered for further processing. This stage is accomplished by stopping the mixing in batch systems or by transference of the emulsion toward a separation tank or decanter. In continuous processes carried out in columns, top and bottom phases are continuously formed in the top and bottom sides of the column, respectively, because of gravity and density differences.
-
Recover the phase where the molecule of interest is present. After phases are separated, the phase with the contaminants should be removed. Whether it is top or bottom phase which contains the product of interest, the easiest way is to open a valve in the bottom part of the decanter to allow the bottom phase be pumped out from the vessel followed by the top phase. In continuous systems, the overflow of the top phase can drive this phase toward an opening in the respective column top part.
-
Discard the opposite phase or recycle it. By means of pumping, the phase that contains the contaminants can be discarded or conducted back to the mixer tank or to another mixer tank in order to partition residual product of interest. However, since the amount and characteristic of the contaminants can differ from the original sample, this can imply a change in the product of interest partitioning behavior that should be previously considered.
-
Multistep or back extraction. Alternatively, it would be possible to require a second round of ATPS extraction, using a different composition to allow back extraction or increase purity or recovery of the molecule of interest.
In sum the adequate equipment to perform all these tasks would be a closed system with the ability to change the flows of all the entrance streams, with three or more inlets, sampling ports at different stages of the extraction procedure, and final recovery of phases.
In this sense, the most common devices employed for continuous ATPS processes are columns (Table 8.1). Some designs in the set of column contactors employed for continuous ATPS are spray columns, perforated rotating disk contactors, pulsed cap columns, and other columns (packed, sieve plate and vane -agitated columns). The main variable among them is the mechanism by which the mass transfer between the phases is promoted (pulsed caps, rotating disks, rotating vanes, spray mechanism, static packing, and static mixer). Whether the dispersed phase is delivered as spray through a nozzle located in the bottom of the column or mixing elements located all around the column to mix the heavier and lighter phases as well as the product of interest which commonly is diluted in one of both phases, these columns commonly share two inlets and two outlets, while the mass transfer and phase coalescence occur inside the column. Phases are prepared in a previous step and let overnight to separate in order to pump them to their respective inlets.
Mixer settlers/mixer-settler columns are also widely employed. Among their advantages are cleaning and control easiness, multistep assembly, batch to continuous flow, extreme to unlimited phase ratio, and small footprint, co-countercurrent, or countercurrent fashion. Mixer-settler units were one of the first devices employed for continuous ATPS (Veide et al. 1984) and nowadays are being exploited at pilot plant scale to downstream recovery of antibodies. They pose as an important advantage to their inherent assembling easiness, suitable for individual stage screening (mixing, coalescence, and separation). However, a noteworthy gap is the hydrodynamics characterization, needed for a practical design platform implementation (Mistry et al. 1996; Salamanca et al. 1998). The two different modes of operation (static and dynamic) basically consist of a mixing stage in tanks or columns, coupled to a series of settling and separating units. Steady state is reached when the partition coefficient obtained in the continuous mode is similar to the one in the batch mode.
8.3 Problems Faced by Continuous ATPS Process Equipment
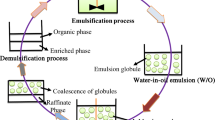
Having overviewed ATPS continuous devices, the main operational problems that can be identified are flooding, backmixing, emulsification, and poor phase separation degree (Fig. 8.2). They limit the selection of optimum operational parameters. Flooding-related problems may arise if a drastic agitation or countercurrent operation drives to small droplets of a dispersed phase into a continuous phase with high flow velocity, which leads to phase accumulation and increase in coalescence time (Cuhna and Aires-Barros 2002). However, it should be kept in mind that in some kind of contactors, optimal operation is near the flooding point, since the dispersion area is maximized and the mass transfer rate is the highest (Wachs et al. 1997).
Problems faced by continuous ATPS processes. (a) Flooding (flow rates are higher than should be, coalescence is not allowed, and contaminant phase level is above the limit). (b) Backmixing (coalesced phases are in contact with fresh feedings). (c) Emulsification (very small droplets are formed, increasing coalescence time beyond the needed, and contaminant phase invades the phase of interest at the outlets). (d) Separation efficiency of the device or holdup (short-length separation device does not allow phase coalescence, and both phases are obtained at the same outlet)
Whereas backmixing can be an advantage at the beginning of the ATPS extraction process, the two-phase behavior should exclude it at the end of the process. Most contactors have an agitation device that accelerates the interaction of phases allowing mass transfer among phases. While the physical presence of these devices decreases the contact of coalesced phases with the unreacted fresh feedings (backmixing), simultaneously the agitation stimulated by these devices increases it (Cuhna and Aires-Barros 2002; Martin 2000; Lounes and Thibault 1996; Stella and Clive 2006).
Emulsification is an issue that has not been extensively discussed when working with continuous ATPS. This may be due to the scarce analysis of ATPS other than polymer-salt systems and also for the larger-scale trials that would be needed for this purpose (Leng 2004; Selber et al. 2004). A high emulsification degree reduces mixing efficiency and increases coalescing time. Aggressive agitation should be avoided in order to restrain the problem, especially with systems that pose low interfacial tension. The mass transfer rate is inversely proportional to drop size, and, thus, an analysis of cost-benefit on these two parameters should be performed before deciding the mixing rate.
Separation efficiency can be measured by the holdup phenomena, which is the volume of the continuous phase divided by the total volume at the dispersed-phase outlet, and vice versa (Cavalcanti et al. 2008). It is directly proportional to the dispersed-phase flow rate and the drop inherent coalescence time and inversely proportional to the purification factor and the recovery efficiency (RE) (Igarashi et al. 2004b; Rostami and Alamshahi 2002). For any continuous ATPS process, a phase’s separation stage is fundamental. Even if the device promotes an excellent mixing and if the separation efficiency at the end of the process is reduced, the extraction performance in general is greatly diminished.
8.4 Characterization of Continuous Equipment
Because of the problems exposed previously, it is important to characterize the equipment that will be used for a continuous ATPS process. The evaluation of a continuous device to perform aqueous two-phase partitioning of biomolecules, with the aim to replicate the partition coefficients obtained from batch experiments, is often a hard work to do. One operational parameters studied in these devices is the physicochemical characteristics governed by phase composition and flow rates. ATPS composition and its related physical properties (viscosity, density, and interfacial tension) may limit the extraction and act as a resistance to mass transfer (Arsalani et al. 2005; Cuhna and Aires-Barros 2002; Igarashi et al. 2004a; Srinivas et al. 2002; Venancio and Teixeira 1995; Pawar et al. 1993, 1997).
Phase’s flow rate is another critical operational parameter in any contactor performance, since it plays a key role in mass transfer and in the column process related to operational issues as backmixing and flooding. In most of continuous ATPS processes, an increase in the dispersed-phase flow produces minor drop sizes that cause higher areas for mass transfer (Figuereido et al. 2004). However, flow rates cannot be increased unlimitedly, since they can hamper the separation efficiency.
Observing the partition behavior of any dye can help in the characterization of continuous equipment. The concentration of such dye can be measured at the outlet of each phase through time. The amount of colorant in each phase can be plotted and show residence time, which can be related to the total operation volume, given a determined total flow rate. If the distribution coefficient of the dye at the system equilibrium remains more or less close to unity, it means that the mixing time and intensity were not enough to mix thoroughly the phases, and thus the mixer efficiency should be improved. Intimate phase dispersion is necessary to improve mass transfer, and such solute interchange occurs immediately during dispersion. Different mixer configurations ensure the creation of an interfacial surface area that allow bulk homogenization ensuring that all flow components are distributed uniformly and exposed to similar levels of turbulence.
A high top-phase affine dye can be employed to characterize the length or height of the coalescer, since immediate transfer of color to the top phase is allowed using mixers. A compound that highly prefers one or other phase allows a considerable tubular settler length reduction and a significantly shorter residence time resulting in lower solvent and energy requirements. It is important to point that with a batch ATPS, every unit operation is realized in a discontinuous independent manner, with consequent loss of sample and time for analysis. With a continuous device, the manipulation of samples is diminished, and the partition time is decreased without a significant effect on partition coefficients.
When using model proteins in batch systems, larger partition times are observed. This reflects the need for an increased processing time and an improved mass transfer and, at the same time, the need to increase the contact area between phases. With the use of a continuous system, protein partitioning could be significantly improved, allowing the continuous harvesting of product from both phases. A minor disadvantage of the use of in-line static mixers is the flow restriction and power adjustments needed to surpass the packing of the mixers; however, such restriction is necessary to maximize transfer of the desired product.
When implementing continuous devices to recover different protein fractions from a complex feedstock, it is reasonable to suggest that different protein profiles could be found in each phase. The use of constant and controllable flows enabled simple and reproducible generation of stable interfaces. Variability will be observed mainly due to manual sampling of the phases or pulses generated by the pumping system; however, once the system evolved, a quasi-steady state is reached with partition coefficient values similar to batch systems. An additional clear advantage of a continuous device is a significantly lower accumulation of protein at the system interface, due mainly to the dynamic nature of the interface of two moving liquids.
In general, any continuous device can be suggested and tailor-made for different industrial purposes, from the continuous extraction of dyes from textile wastewater effluents to treatment of whey from dairy plants and further processing and commercialization of crude fermentation broths. However, to have a full knowledge of the hydrodynamic parameters needed to carry out the process, the aforementioned simple steps can be conducted.
8.5 Platform for Continuous ATPS Processes: From Microdevices to Pilot Plant
Most of the continuous ATPS processes that have been carried out start with a known system for a given particle. This means that previously there should have been a complex design of experiments that was accomplished through many batch small systems, in order to obtain the best composition for a maximum partition coefficient or purification factor. And only when the batch system is corroborated to maximize yield that it is employed to run the continuous process, either with columns or mixer-settler equipment. Thus, a complete continuous platform in order to avoid such waste of materials, reagents, and time should allow selection and characterization of the system previous to a scaling-up.
This platform must include a versatile and easy to manage set of continuous systems that can be categorized in three main scales: micro, bench, and pilot plant (Fig. 8.3). The microscale devices have been designed in order to observe phase formation of many mixtures of different phase components in a relatively short amount of time. This information can be used to prepare binodal curves for new systems or even for known devices but whose composition or properties may vary in different laboratories because of reagent or environmental conditions. Furthermore, given that phase composition may be varied by fine-tuning the flow rates of the stocks of phase components, there is a whole new and bigger range of phase compositions that can be studied to select an appropriate system for a given biomolecule (Silva et al. 2017).
Bench-scale systems can be employed after the ATPS is selected by direct scaling-up of the modular device. Studies have demonstrated that even when other kind of equipment is employed, the partitioning behavior of that given molecule is maintained at this scale. At this stage, other parameters can be varied in order to improve yields of certain particle that want to be recovered in higher amounts. Most of the model proteins that have been recovered in these kinds of systems come from fermentation products that can be also scaled up to several liters. An important advantage in this scale is that different modalities of the equipment can be studied, such as multistage arrangements or phase recycling, or can be adapted to other phases of the biomolecule recovery process, directly, because of the flow streams that are constantly supplied.
Continuous systems have been scaled up to pilot plants. The most common application is the recovery of antibodies from cell cultures. The advantage of pilot plant ATPS continuous processes is that the operational variables of the system can be studied in order to further apply them at an industrial level, whether if the phases are recycled or if back extraction is being applied. Also, as most of the instruments for flow control and temperature and pressure sensing, for example, are more developed for this scale, the system can be better controlled for some of these parameters, as well as agitation and phase behavior given the material of the walls of the system, en route to industrial implementation of the system. In Fig. 8.4, there is a summary of the characteristics of each of these stages of the proposed platform and the data that can be obtained at each stage. The selection of the starting point in either case will depend on the information available from the DOE or previous literature regarding the recovery for a specific bioproduct. In general, the first two stages could allow the implementation of different factors (back extraction, recycling, multistage, etc.) aiming to improve performance at pilot plant scale shortening times and resources.
8.6 Microdevice-Assisted Approach for ATPS Characterization
The miniaturization of continuous ATPS processes highlights some characteristics of the ATPS previously unknown while studying the partition behavior of certain high valuable pharmaceutical biomolecules (Rosa et al. 2013; Hardt and Hahn 2012; Ingram et al. 2013). This allows the shortening of data acquisition for the optimal partition parameters and makes more efficient the use of resources.
Under certain flow conditions, ATPS could be formed continuously inside a microchannel, starting from stocks of phase components. Micromixers included within the device, sequentially and rapidly, prepare two-phase systems across an entire range of useful phase compositions. Two-phase diagrams (binodal curves) can easily be prepared using the cloud-point method for systems of different components. In-line agitation system gives the possibility of increasing the diffusion of molecules through the chaotic mixing generated on a laminar flow of adjacent streams. This may allow the collection of preliminary data to work with continuous devices at bench scale. It is important to work with laminar flows and avoid Rayleigh-Plateau instabilities (perturbation of a jet of fluid of a dispersed phase with lower viscosity, into droplets inside a continuous phase) when working with flows lower than 100 μL/min. This is because the laminar behavior allows for higher reproducibility and no axial dependence on partitioning.
In batch systems, the difference in the density of the phases is responsible of the time needed for two phases, coalescence and sedimentation (Kim et al. 2010); however, at microscopic level, this difference seems to be insignificant, compared to other properties of the phases such as viscosity and interfacial tension (Geschiere et al. 2012). This may be one possible reason for the instantaneous phase formation after the micromixer; thus, the microchannel length is not a limitation.
However, an important restriction in microdevice operation to create the binodal curves was that the total flow inside the microchannel should be enough in order to avoid Rayleigh-Plateau instabilities (Moon et al. 2015) but not so high to generate backflow in the inlet channels of the microdevice . These operational limits will vary according to the nature of the components used (i.e., component concentrations or polymer molecular weight). As explained by Kim et al. (2010), this behavior is owed to viscosity and hydrophilicity (contact angle and superficial tension) and can be overcome by decreasing the contact angle among flows and adding mechanisms of passive control such as internal valves or by capillary action.
By connecting tubing at the outlet streams, the design can be used to recover samples of the phases and to obtain the partition coefficients. The time frame needed to collect samples will depend on the flow rate of the phase component streams. The time needed to recover the necessary amount of sample could be decreased if a direct technique to measure proteins is implemented (i.e., NanoDrop, PDA flow cell, etc.) or in-line systems for biomolecule detection are employed.
The employment of two highly viscous fluids to form an ATPS, such as polyethylene glycol and dextran, implies higher times to reach equilibrium. With the use of a micromixer such as the one proposed in this work, that issue could be minimized, and given polymer concentrations can lead to phase formation.
In using microdevices to run ATPS, different biomolecules have been recovered, for example, cells, bovine serum albumin, proteins such as invertase from yeast, antibodies, etc. However, the differences observed may be explained due to a more efficient mixing at the microscale and the effect of the gravity that drives phase separation at the bench scale and not at the microscale, as pointed by Tsukamoto et al. (2009). However, the microdevice can be employed to select a given kind of phase components for further scale-up. The advantage of this approach dwells in the employment of a micrometric amount of phase components and sample to select and optimize the ATPS in minutes, previous to a scaled application to meet larger productivities. The partition coefficient will be consistent between the bench-scale device and the microscale, as long as the geometry of equipment is preserved. This could boost the use of ATPS at larger scales, in order to work with new systems and increase the database that nowadays exists in the field, which will contribute to integrate ATPS as a viable easy-to-setup unit operation for the primary recovery of biomolecules at pilot and industrial scale.
8.7 Multistage, Bench-Scale Systems
Multistage systems are typically more efficient in terms of selectivity, enrichment, and throughput than a single stage (Rosa et al. 2009a; Luo et al. 2013). With this mode of operation, more solute is transferred from raffinate to the extractive phase when raffinate enters into contact with fresh new solvent, increasing impurities removal. Additionally, with repeated equilibrations using a small amount of solvent more material is removed than when using a single extraction with large volume, avoiding the waste of solvent and dilution of the extract (Geankoplis 1993). There are substantial examples of multistage applications on ATPS. However, all of them are best suited for the commonly liquid-liquid extraction procedures carried out with typical solvents in the chemical industry. Mixing and settling can occur in the same vessel, allowing higher throughputs and smaller footprint.
In polymer-salt systems, it is preferred that enzyme and impurities partition to opposite phases and enrichment of the final product preferred in the bottom-phase outlet, since removal of the phase-forming components would be easier. To calculate the number of stages of countercurrent approach, some methods, such as the mass transfer unit method, have been employed. The calculated number of transfer units is not exactly the number of stages in most of the cases (using this would be costly), but is a useful parameter to have an idea of the efficiency of the system.
8.7.1 Continuous ATPS at Bench Scale for Antibody Purification
It has been demonstrated that immunoglobulin G (IgG ) can be successfully extracted with ATPS (Silva et al. 2012). Some continuous operation has been studied and documented in literature (Vazquez-Villegas et al. 2011; Cavalcanti et al. 2008; Porto et al. 2010; Rosa et al. 2013; Espitia-Saloma et al. 2014). The growing market for the monoclonal antibodies and the constant need for more economically attractive large-scale production processes make the studies of novel efficient continuous recovery processes more appealing (Rosa et al. 2013).
Silva and collaborators (2012) have shown that IgG can be effectively partitioned within microfluidic platforms. Microfluidic approaches allow the use of minimal reagent quantities and a quick evaluation of a larger number of ATPS compositions, just adjusting the phase component’s flow ratios (Hardt and Hahn 2012). The microfluidic approach proposed here effectively used to perform a quick and general screening of the main partition behavior of a molecule of interest could be a trustworthy tool to accelerate the bioprocess design.
Examples of multistage, bench-scale approach can be found in the literature. The main studied devices include multiplate column contactors and mixer-settler configurations demonstrating potential for an industrial scale process (Rosa et al. 2009a, b; Vazquez-Villegas et al. 2015; Prinz et al. 2014; Eggersgluess et al. 2014). The recovery of monoclonal antibodies has been carried out by using 3.45 L/h for outlet top phase and 2.01 L/h for outlet bottom phase in a countercurrent column extraction (2043 mm2 of expanded cross-sectional area) using a polyethylene glycol-phosphate system (Rosa et al. 2012). A steady state was reached in 4–5 h according to the authors. Eggersgluess et al. (2014) suggested a mixer-settler battery (40 mL mixers and 80 mL settlers) of ten stages for a polyethylene glycol-phosphate system (12% PEG , 18% PO4), using 6 and 18 g/min of light and heavy phases, respectively, with 4 h of residence time. Prinz and collaborators (2014) recovered laccase from fermented broths using a mixer-settler unit with three stages (pump mixer of 65 mL and settler of 135 mL) reaching a steady state after 7 h with 0.3 L/h. Rosa et al. (2009b) simulated a five-stage multistage batch process to recover IgG in a polyethylene glycol-phosphate ATPS with 10% w/w of sodium chloride, predicting an 89% recovery. Diffusive mass transfer has been demonstrated to be superior in countercurrent flow (Eggersgluess et al. 2014) and with the increment of mixing stages resulting in more IgG transferred to the bottom phase (Espitia-Saloma et al. 2016).
Rito-Palomares and Lydiatt (1996) carried out a partial recycling of top phase demonstrating a useful approach to reduce raw material consumption with minor effects on the extraction performance of enzymes. Espitia-Saloma and coworkers (2016) investigated the effect of recycling in a continuous, multistage ATPS on the yield and purity of IgG and observed improved performance in recovery.
There are still concerns on the application of ATPS at industrial scale related to the high consumption of phosphate and polyethylene glycol (PEG ) and, consequently, to their impact on water treatment. Although PEG is biodegradable and nontoxic, phosphate disposal is problematic. These bottlenecks may however be minimized if the recycling of both PEG and phosphate is considered (Mündges et al. 2015). In general, ATPSs have demonstrated to be a potential industrially suited primary recovery operation for human IgG ; furthermore, it has the versatility to be adapted to several platforms and products in order to achieve a generic and practical usage.
8.8 Processes at Pilot Plant Scale
The first description of a pilot-scale continuous process of protein purification using ATPS was carried out for the extraction of enzymes from animal tissue. It employed a system with 20% (w/w) of biomass in a PEG -salt system and was successful using a disk separator rather than a decanter. In this study, a computer control was employed for the mixing step, and the obtained protein (fourfold purification factor, 83% recovery yield) was contained in a clarified solution suitable for further chromatographic methods (Boland et al. 1991). The disadvantage of this source of proteins is that it is expensive and animal proteins are increasingly being replaced by recombinant proteins produced in microbes (Boland 2002).
Containers with mechanically rotated stirrer for mixing of phase components and disk stack centrifuge for phase separation at pilot plant scale have been employed to recover enzymes from fermentation broths. The disadvantage of such equipment is that the centrifuge should be regularly desludged. The frequency of this will be a function of the biomass loading, the fineness of the homogenate, and the flow rate. The interval can only be found by trial and error (Boland 2002). As an example, membrane-bound cholesterol oxidase from unclarified culture broth of N. rhodochrous has been separated using an ethoxylated nonionic detergent-based ATPS in a stack centrifugal separator. Phase purity (i.e., only the studied phase, without contaminant phase) was close to 100%, and enzyme recovery yielded 87–93% with fourfold product concentration (Minuth et al. 1997). This method, as stated by the authors, is time-consuming (aprox. 20 h) but not labor-intensive and requires little investment (Minuth et al. 1997).
A 6.25 L pilot-scale centrifugal chromatographic column has also been widely employed for protein separation in ATPS using polymer-salt systems. Rotor speed, mobile-phase flow rate, and sample loading are optimizable parameters that have been studied at lab scale in order to scale up the process. Potential throughputs using this alternative have been over the range of 40 g of products per day (Sutherland et al. 2008). The most important advances in centrifugal partition chromatography technology have come from research into the hydrodynamics and kinetics of mixing (Sutherland et al. 2008).
A pilot-scale packed differential contactor was evaluated for the continuous countercurrent aqueous two-phase extraction (ATPE) of human IgG from a Chinese hamster ovary cell supernatant enriched with pure protein by Rosa et al. (2012). An experimental setup combining the packed column with a pump mixer-settler stage showed to have the best performance and to be advantageous when compared to the IgG batch extraction. An IgG recovery yield of 85% could be obtained with about 50% of total contaminants and more than 85% of contaminant protein removal. Mass transfer studies have revealed that the mass transfer was controlled by the PEG -rich phase. A higher efficiency could be obtained when using an extra pump mixer-settler stage and higher flow rates. Cunha and Aires-Barros (2002), from the same group, have written an extensive review of equipment employed for ATPS in continuous fashion.
In fact, the poor understanding of the responsible mechanisms for the partitioning of biomolecules in ATPS and the usually used batch equipment assembly (agitated vessel + centrifuge) leads to a certain reluctance from industry to embrace this unit operation as part of their own processes (Rito-Palomares 2004).
8.9 Current Challenges and Future Trends
The recovery of the bioproduct from fermentation broths and biological feedstock is one of the major bottlenecks in the bioprocessing industries. ATPS has shown a meaningful potential to be an alternative solution for contributing to downstream bottlenecks solution. Compared to batch systems, the continuous process involves shorter stabilization times and generally avoids centrifugation steps suggested in batch processes, allowing process integration. Only a few companies in the world, such as Genentech, have reported to use batch-mode ATPS for product recovery (Builder et al. 1993; Asenjo and Andrews 2012). Thus, there are still some issues that should be addressed in order to make continuous ATPS better adopted for other companies.
For instance, the lack of practical rules for an effective implementation has limited their generic application at commercial scale. Column contactors and mixer-settler devices have been the common choice for continuous ATPS processes, but some problems such as flooding, backmixing, emulsification, and efficiency of phase separation are found when working with those designs. This has raised some areas of opportunity identified for the practical implementation of continuous ATPS.
Mathematic modeling of scaling procedures that correlate the recovery of biomolecules with respect to flows and their characteristics is an opportunity area that has not been considered yet and that is expected to be characterized in the future. Different construction materials can be explored, as in the case of stainless steel for the static mixers or cheap acrylic configurations in order to lower initial inversion costs. Computational fluid dynamic simulation programs can be a valuable tool in order to select the best configuration. These can be further translated into theories about phase formation kinetics, taking in consideration the mixing energy and the droplet sizes (depending on the liquid characteristics).
The kind of system and their hydrodynamic characteristics are another opportunity area. Although traditional polymer-salt systems are yet expected to dominate applications, new kinds of ATPS, such as ionic liquids, are the tracking trend. These systems are considered a great option for the replacement of volatile organic solvents in LLE (Novak et al. 2012) and are more easily reusable and recycled (Li et al. 2010). New compounds such as carbohydrates and thermoseparating polymers are also under study (De Brito Cardoso et al. 2013; Show et al. 2012; Li et al. 2002).
One of the great challenges of ATPS is to surpass the apparent unattractive economical image. Studies comparing, in detail, the costs involved in ATPS implementation with alternative technologies are not common (Aguilar et al. 2006; Huenupi et al. 1999; Torres-Acosta et al. 2016). A pre-evaluation of ATPS recuperation costs for each biomolecule of interest should be made in order to determine the viability of an ATPS process.
Another important challenge is the determination of an equipment design platform. The tendency toward the miniaturization of continuous ATPS partition may shorten data acquisition for the optimal partition parameters of valuable pharmaceutical biomolecules and makes more efficient the use of resources (Rosa et al. 2013; Hardt and Hahn 2012; Ingram et al. 2013). Meanwhile, column contactors and mixer-settler devices have the more solid design guidelines, including its hydrodynamic characterization and verification of its performance capacity. Phase recycling is a missing gap that should be considered for continuous operation in column contactors as well as in static mixer units, given the environmental impact of phase-forming compounds. Studies about phase recycling and the employing of new phase components is a work than can be accomplished as part of an integral view of the process before and after ATPS, including the recovery of the product of interest from the phase-forming components. To date ion-exchange chromatography, precipitation, ultrafiltration, dialysis, and supercritical CO2 extraction have been considered (Li et al. 2010).
Finally, a method to work with ATPS as a primary recovery strategy, in a fast and secure way for scaling up a continuous process, should be developed. In this way, the batch application, with its correspondent waste of material and costs, can be neglected and the user-related experimental variance in ATPS formulation minimized. The demonstrated potential of ATPS-based continuous systems places them as a promising liquid-liquid extraction technology that can be successfully implemented at large scales and with great potential to solve needs of biotechnological industry of an economical, efficient, predictable, and reliable downstream operation for bench- and large-scale applications.
8.10 Concluding Remarks
ATPS strategies have been typically performed in continuous mode using equipment adapted from traditional LLE processes of the chemical industry. However, most of these apparatus present challenges for their application using highly viscous and dense phases from the different existing two-phase systems in biomolecules extraction. These challenges are mainly related to hydrodynamic problems such as flooding, backmixing, emulsification, and poor separation efficiency at the end of the continuous process. Thus, new platforms for continuous ATPS should be developed. Regarding this, microscale systems provide an excellent opportunity to change the actual practice of this purification technique from bench to continuous systems. Although this approach is already in early stages and further modeling and bottlenecks have to be solved, a substantial work is being done around the world in order to develop useful guidelines, so this liquid-liquid extraction technology could be successfully implemented at larger scales.
Abbreviations
- ATPE:
-
Aqueous two-phase extraction
- ATPS:
-
Aqueous two-phase system
- DOE:
-
Design of experiments
- IgG :
-
Immunoglobuline G
- LLE:
-
Liquid-liquid extraction
- PDA:
-
Photodiode array detector
- PEG :
-
Polyethylene glycol
- PO4 :
-
Phosphates
- RE:
-
Recovery efficiency
References
Aguilar O, Albiter V, Serrano-Carreón L, Rito-Palomares M. Direct comparison between ion-exchange chromatography and aqueous two-phase processes for the partial purification of penicillin acylase produced by E. coli. J Chromatogr B. 2006;835:77–83.
Andrews BA, Nielsen S, Asenjo JA. Partitioning and purification of monoclonal antibodies in aqueous two-phase systems. Bioseparation. 1996;6:303–13.
Arsalani V, Rostami K, Kheirolomoom A. Lipoxygenase-1 mass-transfer coefficient in aqueous two-phase system using spray extraction column. Ind Eng Chem Res. 2005;44:7469–73.
Asenjo JA, Andrews BA. Aqueous two-phase systems for protein separation: phase separation and applications. J Chromatogr A. 2012;1238:1–10.
Biazus JP, Santana JC, Souza RR, Jordao E, Tambourgi EB. Continuous extraction of α- and β-amylases from Zea mays malt in a PEG4000/CaCl2 ATPS. J Chromatogr B. 2007;85:227–33.
Bim MA, Teixeira FT. Extraction in aqueous two-phase systems of alkaline xylanase produced by Bacillus pumilus and its application in kraft pulp bleaching. J Chromatogr B. 2000;743:349–56.
Boland MJ. Aqueous two-phase extraction and purification of animal proteins. Mol Biotechnol. 2002;20:85–93.
Boland MJ, Heddelink PGM, Papamichael N, Hustedt H. Extractive purification of enzymes from animal tissue using aqueous two phase systems: pilot scale studies. J Biotechnol. 1991;19:19–33.
Breydo L, Mikheeva LM, Madeira PP, Zaslavsky BY, Uversky VN. Solvent interaction analysis of intrinsically disordered proteins in aqueous two-phase systems. Mol BioSyst. 2013;9:3068–79.
Builder S, Hart R, Lester P, Ogez J, Reifsnyder D. USA Patent no. 407,810; 1993.
Cavalcanti MTH, Carneiro-Da-Cuhna MG, Brandi IV, Porto TS, Converti A, Filho JLL, Porto ALF, Pessoa A. Continuous extraction of α toxin from a fermented broth of Clostridium perfringens Type A in perforated rotating disc contactor using aqueous two-phase PEG-phosphate system. Chem Eng Process. 2008;47:1771–6.
Coleby J. The RTL (formerly Graesser Raining-Bucket) contactor. In: Lo TC, Baird M, Hanson C, editors. Handbook of solvent extraction. New York: Wiley; 1983.
Croughan MS, Konstantinov KB, Cooney CL. The future of industrial bioprocessing: batch or continuous? Biotechnol Bioeng. 2015;112:648–51.
Cuhna T, Aires-Barros R. Large scale extraction of proteins. Mol Biotechnol. 2002;11:29–40.
De Brito Cardoso G, Mourao T, Menezes-Pereira F, Freire MG, Tinoco Fricks A, Faria Soares CM, Silva LA. Aqueous two-phase systems based on acetonitrile and carbohydrates and their application to the extraction of vanillin. Sep Purif Technol. 2013;140:106–13.
Dos-Reis CJ, Thömmes J, Kula MR. Continuous separation of whey proteins with aqueous two-phase systems in a Greasser contactor. J Chromatogr A. 1994;668:85–94.
Eggersgluess JK, Richet M, Dieterle M, Strube J. Multi stage aqueous two phase extraction for the purification of monoclonal antibodies. Chem Eng Technol. 2014;37:1–9.
Espitia-Saloma E, Vazquez-Villegas P, Aguilar O, Rito-Palomares M. Continuous aqueous two-phase systems devices for the recovery of biological products. Food Bioprod Process. 2014;92:101–12.
Espitia Saloma E, Vazquez-Villegas P, Rito-Palomares M, Aguilar O. An integrated practical implementation of continuous aqueous two-phase systems for the recovery of human IgG: from the microdevice to a multistage bench-scale mixer-settler device. Biotechnol J. 2016;11:708–16.
Figuereido PAL, Asfora SL, Aparecida MK, Farias MHJ, Lima-Filho JL, Campos-Takaki GM, Basile TE. Recovery of ascorbic oxidoreductase from crude extract with an aqueous two-phase system in a perforated rotating disc contactor. Braz Arch Biol Technol. 2004;47:821–6.
Geankoplis CJ. Transport process and unit operation. London: Prentice Hall; 1993.
Geschiere AD, Ziemecka I, van Steijn V, Koper GJM, van Esch JH, Kreutzer MT. Slow growth of the Rayleigh-Plateau instability in aqueous two phase systems. Biomicrofluidics. 2012;6:1–11.
Giraldo-Zuniga AD, Coimbra JSR, Minim LA, Garcia REE. Dispersed phase hold-up in a Graesser raining bucket contactor using aqueous two-phase systems. J Food Eng. 2006;72:302–9.
Gupta R, Bradoo S, Saxena RK. Aqueous two-phase systems: an attractive technology for downstream processing of biomolecules. Curr Sci. 1999;77:520–3.
Haraguchi LH, Mohamed RS, Loh W, Pessoa Filho PA. Phase equilibrium and insulin partitioning in aqueous two-phase systems containing block copolymers and potassium phosphate. Fluid Phase Equilibr. 2004;215:1–15.
Hardt S, Hahn T. Microfluidics with aqueous two-phase systems. Lab Chip. 2012;12:434–42.
Huenupi E, Gomez A, Andrews BA, Asenjo JA. Optimization and design considerations of two-phase continuous protein separation. J Chem Technol Biot. 1999;74:256–63.
Igarashi L, Kieckbusch TG, Franco TT. Xylanase mass transfer studies in aqueous two-phase systems using spray and sieve plate columns. Bioprocess Biosyst Eng. 2004a;26:151–7.
Igarashi L, Kieckbusch TG, Franco TT. Mass transfer in aqueous two-phases system packed column. J Chromatogr B. 2004b;807:75–80.
Ingram T, Mehling T, Smirnova I. Partition coefficients of ionizable solutes in aqueous micellar two-phase systems. Chem Eng J. 2013;218:204–13.
Jarudilokkul S, Paulsen E, Stuckey DC. Lysozyme extraction from egg white using reverse micelles in a Graesser contactor: mass transfer characterization. Biotechnol Bioeng. 2000;69:618–26.
Jungbauer A. Continuous downstream processing of biopharmaceuticals. Trends Biotechnol. 2013;31:479–92.
Jungbauer A, Walch N. Buffer recycling in downstream processing of biologics. Curr Opin Chem Eng. 2015;10:1–7.
Kamei DT, King JA, Wang DI, Blankschtein D. Separating lysozyme from bacteriophage P22 in two-phase aqueous micellar systems. Biotechnol Bioeng. 2002;78:203–16.
Kim SJ, Lim YT, Yang H, Kim K, Kim YT. Passive regulation of volume-flow ratio for microfluidic streams with different hydrophilicity and viscosity. Electrophoresis. 2010;31:709–13.
Leng RB. From bench to plant: scale up specialty chemical processes directly. Chem Eng Prog. 2004;100:37–44.
Li M, Kim JW, Peeples TL. Amylase partitioning and extractive bioconversion of starch using thermoseparating aqueous two-phase systems. J Biotechnol. 2002;93:15–26.
Li Z, Pei Y, Wang H, Fan J, Wang J. Ionic liquid-based aqueous two-phase systems and their applications in green separation processes. TrAC. 2010;11:1336–46.
Liu Y, Feng YQ, Zhao Y. Liquid–liquid equilibrium of various aqueous two-phase systems: experiment and correlation. J Chem Eng Data. 2013;58:2775–84.
Lounes M, Thibault J. Axial dispersion in a reciprocating plate column. Can J Chem Eng. 1996;74:187–94.
Luo Q, Li S, Jing S. The study of fluid dynamics in countercurrent multi-stage micro-extraction system. Energy Procedia. 2013;39:275–82.
Martin AD. Interpretation of residence time distribution data. Chem Eng Sci. 2000;55:5907–17.
Minuth T, Pape GU, Raths HC, Thommes J, Kula MR. Pilot scale processing of detergent-based aqueous two-phase systems. Biotechnol Bioeng. 1997;55:339–47.
Mistry SL, Kaul A, Merchuk JC, Asenjo JA. Mathematical modeling and computer simulation of aqueous two-phase continuous protein extraction. J Chromatogr A. 1996;741:151–63.
Moon BU, Jones SG, Hwang DK, Tsai SS. Microfluidic generation of aqueous two-phase system (ATPS) droplets by controlled pulsating inlet pressures. Lab Chip. 2015;15:2437–44.
Mündges J, Zierow J, Zeiner T. Experiment and simulation of an aqueous two-phase extraction process for the purification of a monoclonal antibody. Chem Eng Process. 2015;95:31–42.
Nan E-L, Williams GR, Song H-H, Quan J, Nie H-L, Zhu LM. Liquid-liquid-solid triple-phase data for aqueous two-phase systems comprising ethanol-1-propanol-2-propanol-acetone and salts. J Chem Eng Data. 2013;58:3314–9.
Novak U, Pohar A, Plazl I, Žnidaršič-Plazl P. Ionic liquid-based aqueous two-phase extraction within a microchannel system. Sep Purif Technol. 2012;97:172–8.
Pawar PA, Rostami JK, Sawant SB, Joshi JB. Enzyme mass transfer coefficient in aqueous-two phase systems: spray extraction column. Chem Eng Commun. 1993;122:151–69.
Pawar P, Parasu U, Sawant S, Joshi J. Enzyme mass transfer coefficient in aqueous two-phase systems: modified spray extraction columns. Can J Chem Eng. 1997;75:751–8.
Porto PS, Marques TP, Porto CS, Moreira KA, Lima-Filho JL, Converti A, Pessoa A, Porto ALF. Extraction of ascorbate oxidase from Cucurbita maxima by continuous process in perforated rotating disc contactor using aqueous two-phase systems. Appl Biochem Biotechnol. 2010;160:1057–64.
Prinz A, Koch K, Górak A, Zeiner T. Multi-stage laccase extraction and separation using aqueous two-phase systems: experiment and model. Process Biochem. 2014;49:1020–31.
Rabelo APB, Tambourgi EB. Performance of a pulsed-cap microcolumn for protein extraction. Braz J Chem Eng. 2003;20:357–62.
Rito-Palomares M. Practical application of aqueous two-phase partition to process development for the recovery of biological products. J Chromatogr B. 2004;807:3–11.
Rito-Palomares M, Lyddiatt A. Impact of cell disruption and polymer recycling upon aqueous two-phase processes for protein recovery. J Chromatogr B. 1996;17:81–8.
Rosa PAJ, Azevedo AM, Ferreira IF, Sommerfeld S, Bäcker W, Aires-Barros MR. Downstream processing of antibodies: single-stage versus multi-stage aqueous two-phase extraction. J Chromatogr A. 2009a;1216:8741–9.
Rosa PAJ, Azevedo A, Sommerfeld S, Mutter M, et al. Application of aqueous two-phase systems to antibody purification: a multistage approach. J Biotechnol. 2009b;139:306–13.
Rosa PAJ, Azevedo AM, Sommerfield S, Backer W, Aires-Barros MR. Continuous aqueous two-phase extraction of human antibodies using a packed column. J Chromatogr B. 2012;880:148–56.
Rosa PAJ, Azevedo AM, Sommerfeld S, Mutter M, et al. Continuous purification of antibodies from cell culture supernatant with aqueous two-phase systems: from concept to process. Biotechnol J. 2013;8:352–62.
Rostami K, Alamshahi M. Enzyme mass-transfer coefficient in aqueous two-phase systems sing static mixer extraction column. Bioprocess Biosyst Eng. 2002;25:169–78.
Salamanca MH, Merchuk JC, Andrews BA, Asenjo JA. On the kinetics of phase separation in aqueous two phase systems. J Chromatogr B. 1998;711:319–29.
Selber K, Tjerneld F, Collén A, Hyytiä T, Nakari-Setälä T, Bailey M, et al. Large-scale separation and production of engineered proteins, designed for facilitated recovery in detergent-based aqueous two-phase extraction systems. Process Biochem. 2004;39:889–96.
Show LP, Tan CP, Anuar MS, Ariff A, Yusof YA, Chen SK, Ling TC. Extractive fermentation for improved production and recovery of lipase derived from Burkholderia cepacia using a thermoseparating polymer in aqueous two-phase systems. Bioresour Technol. 2012;116:226–33.
Silva DFC, Azevedo AM, Fernandes P, Chu V, et al. Design of a microfluidic platform for monoclonal antibody extraction using an aqueous two-phase system. J Chromatogr A. 2012;1249:1–7.
Silva DFC, Azevedo AM, Fernandes P, Chu V, et al. Determination of partition coefficients for biomolecules in a microfluidic aqueous two phase system platform using fluorescence microscopy. J Chromatogr A. 2017;1487:242–7. doi:10.1016/j.chroma.2016.12.036.
Soares RR, Azevedo AM, Van Alstine JM, Aires-Barros MR. Partitioning in aqueous two-phase systems: analysis of strengths, weaknesses, opportunities and threats. Biotechnol J. 2015;10:1158–69.
Srinivas ND, Narayan AV, Raghavarao KSMS. Mass transfer in a spray column during two-phase extraction of horse radish peroxidase. Process Biochem. 2002;38:387–91.
Stella A, Clive PHR. Backmixing in Karr reciprocating-plate extraction columns. Ind Eng Chem Res. 2006;45:6555–62.
Sutherland IA, Audo G, Burton E, Couillard F, Fisher D, Garrad I, Hewitson P, Intes O. Rapid linear scale-up of a protein separation by centrifugal partition chromatography. J Chromatogr A. 2008;1190:57–62.
Torres-Acosta MA, Aguilar-Yáñez JM, Rito-Palomares M, Titchener-Hooker NJ. Economic analysis of uricase production under uncertainty: contrast of chromatographic purification and aqueous two-phase extraction (with and without PEG recycle). Biotechnol Prog. 2016;32:126–33.
Tsukamoto M, Taira S, Yamamura S, Morita Y, Nagatani N, Takamura Y, Tamiya E. Cell separation by an aqueous two-phase system in a microfluidic device. Analyst. 2009;134:1994–8.
Vazquez-Villegas P, Aguilar O, Rito-Palomares M. Study of biomolecules partition coefficients on a novel continuous separator using polymer-salt aqueous two-phase systems. Sep Purif Technol. 2011;78:69–75.
Vazquez-Villegas P, Aguilar O, Rito-Palomares M. Continuous enzyme aqueous two-phase extraction using a novel tubular mixer settler in multi-step counter current arrangement. Sep Purif Technol. 2015;141:263–8.
Veide A, Lindbäck T, Enfors SO. Continuous extraction of β-D-galactosidase form Escherichia coli in an aqueous two-phase system: effects of biomass concentration on partitioning and mass transfer. Enzym Microb Technol. 1984;6:325–30.
Venancio A, Teixeira JA. Protein mass transfer studies on a spray column using the PEG-Reppal PES 100 aqueous two-phase system. Bioprocess Eng. 1995;13:251–5.
Wachs A, Benyamin J, Semiat R, Lewin DR. Control of a pilot-scale Karr liquid–liquid extraction column. Comput Chem Eng. 1997;21:601–6.
Xu Y, Souza MA, Ribeiro-Pontes MZ, Vitolo M, Pessoa-Jr A. Liquid-liquid extraction of pharmaceuticals by aqueous two-phase systems. Braz J Pharm Sci. 2001;37:306–20.
Zydney AL. Continuous downstream processing for high value biological products: a review. Biotechnol Bioeng. 2016;113:465–75.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 Springer International Publishing AG
About this chapter
Cite this chapter
Vázquez-Villegas, P., Aguilar, O. (2017). Continuous Aqueous Two-Phase System Processes. In: Rito-Palomares, M., Benavides, J. (eds) Aqueous Two-Phase Systems for Bioprocess Development for the Recovery of Biological Products. Food Engineering Series. Springer, Cham. https://doi.org/10.1007/978-3-319-59309-8_8
Download citation
DOI: https://doi.org/10.1007/978-3-319-59309-8_8
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-59308-1
Online ISBN: 978-3-319-59309-8
eBook Packages: Chemistry and Materials ScienceChemistry and Material Science (R0)