Abstract
The selection and implementation of a successful aqueous two-phase system (ATPS) strategy requires an extensive amount of research to optimize the different system design parameters to obtain the desired product yields and purity. This procedure might become even more difficult depending on the complexity of the sample being processed. However, because of their characteristics, ATPS represent an interesting strategy for the recovery of different proteic products from a wide array of available sources.
The aim of this chapter is to highlight the different alternatives – from a practical point of view – in the selection of different ATPS to serve as a guide for the correct design and use of these operations for the recovery of protein molecules. In this context, our group has been working with these strategies for more than two decades. Our experience ranges from the ATPS extraction of proteins from simple sources to the extraction of these molecules from complex industrial wastes. In this chapter, we present a general and successful strategy that has served us as a first approach in implementing ATPS operations; we also present several of our most recent attempts in using ATPS in an intensive manner for the extraction of high added-value proteins from waste streams, chemical reactions, and the refolding of denatured proteins.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
- Aqueous two-phase system design parameters
- Operation design and implementation
- Waste streams
- PEGylated proteins
- Protein refolding
3.1 Introduction
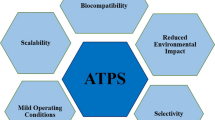
Because of their biocompatibility , aqueous two-phase systems (ATPS) have shown to be an interesting primary recovery operation of different biological products from which proteins are probably the most important (Rito-Palomares 2004). Nowadays, a wide range of proteic formulations ranging from household products to pharmaceutical therapies can be found in the market. In fact, it could be expected that the number of protein-based products will continue to show a steady increase during the following decades mainly because there are many functions that these molecules can perform. Even more, with the advancements in synthetic biology techniques , proteins with novel properties or functionalities can be easily designed transforming them into very versatile molecules which without doubt will find interesting applications in different aspects of human life (Benavides et al. 2008). The production and purification of proteins will continue to be an important engineering problem for the biotechnological industry and innovations in the way these biomolecules are prepared and processed will always represent advancements in the area. Certainly, in this context, ATPS purification strategies will continue to be an easy-to-implement and attractive alternative for these purposes.
However, the development of aqueous two-phase system strategies for the recovery of proteic products requires, first, a complete understanding of the different stream components that are going to be processed. Being a primary recovery operation, ATPS are usually implemented in the first steps of the downstream process , and therefore the number of molecules that is introduced in them is usually large and/or diverse. Ideally, the target product is sought to partition toward either phase, while the rest of the molecules in the sample or contaminants should partition to the opposite one (Benavides and Rito-Palomares 2011). This, however, is not a trivial task and extensive experimentation, and modeling is required since the different physicochemical properties involved in the partition of molecules within an ATPS are very complex. Furthermore, in most cases there is no complete partition of the target molecule or the contaminants to different phases, and concentrated mixtures of either the target biomolecule and some contaminants or vice versa are presented adding difficulties to the generalized implementation of this strategy. Nonetheless, there is now an extensive knowledge on the different partition behaviors of many molecules in different ATPS making it, to some extent, easier to predict and develop efficient strategies in different engineering contexts.
In our experience, the work with ATPS for the recovery of proteins has represented a very interesting topic with successful outcomes using diverse protein sources and mixtures . In this sense, besides working with protein extraction from crude extracts which is commonly one of the most usual procedures in ATPS, we have also worked with the recovery of these biomolecules from waste streams and different chemical reactions. Table 3.1 shows some of our works in the recovery of proteins using ATPS . This experience has allowed the devise of efficient ATPS implementation strategies where several common processing steps are always used. In this context, the objective of this chapter is to present a practical overview on the different procedures, challenges, and experiences we have encountered along the way, to lead and advice other people working with ATPS.
3.2 General Strategy in the Selection of ATPS
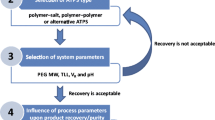
As it has been mentioned, one of the main advantages making ATPS an interesting primary recovery operation for the recovery of proteins is their highly aqueous environment and the possibility they provide to work at neutral pH and other non-denaturing conditions found in traditional liquid-liquid extraction procedures. Furthermore, ATPS exhibit other advantages such as economic attractiveness, scalability, short phase separation, and processing times besides mild-separating conditions that help maintain the biological activities of the molecules being processed (González-González et al. 2011; Ruiz-Ruiz et al. 2012). Although both polymer–salt and polymer–polymer systems have been mainly used for protein recovery, polyethylene glycol (PEG)–salt ATPS are the most commonly used. From this group, the most suitable phase-forming chemical combination for protein extraction is the use of PEG and phosphate salts due to processing advantages such as the low cost, the vast knowledge accumulated in the characterization of these systems, and the range of operating system pH values (from 6 to 9) under which these ATPS are stable (Rito-Palomares 2004). Besides, in these extraction systems, the product of interest is generally concentrated in the PEG-rich phase (top phase) and the contaminants in the salt-rich phase (bottom phase) in different extents. However, since there are several combinations of polymer–polymer or polymer–salt systems available, it can be difficult to decide on an option when working with a protein for which no previous systems have been reported. In such cases, the recommendation would be to start with the general strategy described in Fig. 3.1. This strategy considers four main stages : (1) the characterization of the protein or the extract, (2) partition screening using 16 PEG–phosphate systems, (3) analysis and optimization of the best systems, and (4) protein recovery.
3.2.1 Protein–Extract Characterization
In this first stage, which is generally performed prior to system construction, it is necessary to know the most important characteristics of the target protein such as molecular weight (MW), isoelectric point (pI), hydrophobicity, and concentration. If this molecule is an enzyme, it is very important to measure its activity and how the different contaminants or properties of the extract affect its measurement, the influence of pH on the activity, and the best technique to measure total protein concentration. In this sense, it is important to consider that protein monitoring throughout different quantification methodologies (i.e., UV spectrophotometry, fluorescence, colorimetric assays, etc.) is frequently hindered by the presence of high-polymer and salt concentrations (that commonly reach up to 30% w/w) in the systems (Barbosa et al. 2009; Dumetz et al. 2009; Gonzalez-Gonzalez 2011). Therefore, it is fundamental to determine the most favorable and reliable techniques for this purpose before starting with the operation. It should also be mentioned that another technique widely used to characterize the protein profile of the sample is SDS-PAGE. This technique allows the clear observation of protein profiles and concentration differences throughout the process. Therefore, it is widely recommended to perform it in each of the extraction stages of the ATPS operation. The selection of the different study parameters and necessary information or physicochemical properties related to the target molecule and the contaminants present in the sample or feedstock will depend on the objective for which the extraction is being carried out and the destination of the protein to be purified .
3.2.2 Partition Screening Using 16 PEG–Phosphate Systems
After determining the most relevant physicochemical characteristics of the sample according to the extraction purposes, the screening of the partition behavior of the different sample components can be initiated using different PEG–phosphate systems. Tables 3.2 and 3.3 show two different system sets that have been previously reported and used successfully for the recovery of proteins (Mayolo-Deloisa et al. 2009; González-Valdez et al. 2011; Sánchez-Trasviña et al. 2015; Bertrand et al. 2016). The idea in this stage is to evaluate the influences of polymer molecular weight (MW) and the tie-line length (TLL) in the partitioning of these components since it is well known that an increase in the polymer MW and its concentration can affect both the protein hydrophobicity (Franco et al. 1996) and the excluded phase volume (Benavides and Rito-Palomares 2004). The analysis of these systems has then the purpose of finding the preference of the target protein and the contaminants for each phase. The overall goal is to find those conditions in which the target molecule migrates toward the PEG-rich phase and the contaminants to the opposite phase (salt phase). Based on practical experience, it has been observed that the recovery of hydrophilic high molecular weight (>10,000 g/mol) compounds is favored when both low MW PEG (<4000 g/mol) and low or medium TLL values (<40% w/w) are selected. In contrast, the recovery of hydrophobic low molecular weight (<10,000 g/mol) products is favored when high MW PEG (>6000 g/mol) and medium or high TLL (>30% w/w) are used (Benavides and Rito-Palomares 2008). So, the use of these well-defined systems allows the characterization of how different polymer molecular weights and TLL values affect the behavior of the sample while performing a complete partition analysis. Regarding phase volume ratio (V R) and pH, initial values of 1.0 and 7.0 are recommended to avoid any potential concentration effects or induced electrostatic interactions. It is also important to point out that the systems must be constructed to the most adequate final volume considering the concentration and quantity of sample. For this reason, systems with a total weight of 2, 5, or 10 g with sample concentrations representing no more than 10% of the total system weight can be constructed to perform this screening. High-throughput screening (HTS) techniques can also be used when sample reduction is necessary (Benavides and Rito-Palomares 2008) .
3.2.3 Analysis and Optimization of the Best Systems
After obtaining the results from the screening analysis, the best systems can be selected to perform an optimization procedure. Different quantitative and practical criteria can be used to carry out this selection. Noticeably, the quantitative ones will be based on the obtained partition coefficient (K P), recovery percentages, selectivity, yield, specific activity, and/or purification factor. However, it is also important to take into consideration practical approaches such as the phase viscosity, the formation of an interface and/or a precipitate, and the interference of the phases in the activity or stability of the protein. Again, all these entirely depend on the final use of the product of interest.
If the selected combination of MW of PEG and TLL results in the required product recovery, the system parameters stage is complete (Benavides and Rito-Palomares 2008). Otherwise, different strategies can be used to increase the recovery such as (1) varying the V R to concentrate the protein in one of the phases, (2) enhance sample loading, and (3) addition of neutral salts. The systems presented in Tables 3.2 and 3.3 are designed to use a V R = 1.0. But if that is not enough to accomplish a suitable protein recovery percentage, it is recommended using lower or higher values than that recommended initial value (v.gr. 0.3 and 3). In this context, changes in V R modify the free volume available in the phases that might promote a change in partition. It has been suggested that as V R increases, the partition coefficient increases as more target protein is partitioned into the polymer-rich top phase (Ng et al. 2011). From practical experience, it has been concluded that an increment in V R usually causes an increment in both the recovery and purity of high-molecular-weight products (>100,000 g/mol), while low-molecular-weight products (<50,000 g/mol) observe an increment in recovery but not always in purity (Benavides and Rito-Palomares 2008). Additionally, sample loading can be modified until the system becomes saturated. Generally, the systems are designed for the incorporation of up to a 10% w/w of sample. But this concentration can be changed using water to reach the total weight of each system. Thus, sample concentration can be modified from 1 to 10% w/w. However, increases up to 40% w/w have been achieved in PEG–salt systems with positive results (Benavides and Rito-Palomares 2008). The increase in sample concentration should however be performed gradually because, in some cases, increasing the loaded mass of a sample into the ATPS may decrease the V R and alter the composition of the systems. Besides, the components contained in the crude load (when a crude extract is used) may also change the characteristics of an ATPS causing a selected system to no longer present the best conditions for partitioning as seen in screening experiments (Ng 2011). As a final step, the addition of neutral salts to ATPS can be exploited to enhance the recovery of the target protein. Changes in the salt type and its concentration often produce an electrical potential difference between the two phases caused by the preference of one of the ions to a particular phase (Johansson 1970). The supplementary ionic strength may favor the solubilization of compounds accumulated at the interface of the system into either the top or bottom phase. However, the addition of salts, such as NaCl, may also cause reversible or irreversible denaturalization of halo-sensitive biological compounds present in the system. Therefore, it is necessary to evaluate the effect of the addition of neutral salts upon product recovery, purity, and activity. In this sense, experiments using a progressive increase in the concentration of NaCl such as 0, 0.25, 0.50, 1.0, and 2.0 M are recommended (Benavides and Rito-Palomares 2008) .
3.2.4 Recovery of the Partitioned Product
The recovery of the target protein can be finally reached separating the phases in the system. Sometimes the phase containing the protein can be passed directly into the next purification step or unit operation . In most occasions, the phase-forming chemicals need to be separated using membrane operations ultrafiltration/diafiltration which also allows the concentration of the product. Another alternative is to scale the selected system to enhance its capacity making it very important to have an established objective since the beginning of the separation using ATPS. As mentioned, this general strategy can be used for the recovery of any biomolecule, but its use has been probed mostly efficient for the recovery of different proteins. Even when PEG–phosphate salt systems have been directly approached, this strategy can be adopted in a similar fashion to polymer–polymer systems or other types of ATPS .
3.3 Experiences in the Primary Recovery of Proteins from Waste Streams
Proteins are one of the fundamental building blocks for the sustainability of life (Gong et al. 2016). As mentioned before, the increasing need to bring new protein-based products to pharmaceutical and industrial markets using scalable and efficient bioprocessing technology has raised the need of establishing different methodologies for their recovery (Sánchez-Trasviña 2015). The major drawbacks associated with the exploitation and procurement of enzymes are, in many cases, the high production costs and the low production yields that are obtained (Makris 2015). In this manner, the possibility of revalorizing waste materials to obtain high added-value products (as is the case of proteins) from them is essential since these by-product sources represent an economical, extensive, and safe source of them (Bertrand et al. 2013).
In this context, it has been reported that roughly one-third of the edible parts of food produced for human consumption gets lost or wasted globally which account for approximately 1.3 billion tons per year (Gustavsson et al. 2011). This figure does not consider the wastes generated by the cultivation or treatment of such products . These streams are an incredible source of raw materials and/or high added-value compounds that need to be processed to take economical advantage from them while reducing their impact on the environment. In this line, there is an actual need to develop novel recovery and reuse technologies, along with the development of sustainable ideas, technologies, and processes to avoid the loss of all these compounds attached to these wastes (Reis et al. 2012). Because of this, we believe it is the duty of researchers to find alternatives for the reuse of such wastes.
For this reason, we have dedicated part of our efforts to the recovery of different enzymes such as laccase using aqueous two-phase systems. Laccases are oxidoreductases commonly secreted out to the medium extracellularly by several fungi (specially white-rot fungi) during their secondary metabolism (Morozova et al. 2007). Laccase can be used in bioremediation, beverage (wine, fruit juice, and beer) processing, ascorbic acid determination, sugar-beet pectin gelation, baking, and biosensors, among other uses. Due to its environmental, industrial, and economic importance, laccase is widely studied, and its purification processes are not the exception (Minussi et al. 2002).
It has been reported that during the growth of edible fungi such as Agaricus bisporus and Pleurotus ostreatus on composted wheat straw, large amounts of laccase are produced. After fruit-body harvesting , a considerable amount of residual compost is discarded as by-product. The residue is also a potential source of other ligninolytic enzymes besides laccase (Trejo-Hernandez et al. 2001).
The potential of ATPS for the recovery of laccase from fungi has been widely reported (Lladosa et al. 2012; Moreira et al. 2013; Silvério et al. 2013; Prinz et al. 2014a, b; Schwienheer et al. 2015; Rajagopalu et al. 2016). However, most published works extract the enzyme from liquid fermentations and/or use the pure enzyme to show its partition in the systems under studies. The conditions of these experiments are very different because the complexity of the extract from which the enzyme is obtained is greater when residual compost is used.
In the same line of using industrial waste streams for the recovery of valuable biomolecules, we have reported the recovery of invertase from wasted brewer’s yeast using ATPS (León-González et al. 2015). The beer industry generates a very large amount of biomass since the utilized yeast is generally discarded after a certain number of fermentations to assure product quality and consistence. After this life-span, wasted yeast is usually used as animal feed, being a very good source of proteins for ruminants and swine. However, a large amount of valuable proteins and enzymes such as invertase, amylases, and proteases present in these microorganisms could represent an attractive side business or opportunity for the same beer companies since these enzymes are also usually supplemented by separate to their fermentation broths.
3.3.1 Recovery of Laccase from Residual Compost of Agaricus Bisporus Using ATPS
The complexity of the components of residual compost and the lack of information about them is one of the main problems when trying to obtain proteins from compost crude extracts. In fact, this is one of the general problems limiting the use of residues for the recovery of proteins in ATPS. However, by monitoring the enzymatic activity and the total protein concentration throughout the ATPS operation, these strategies have been found to be effective as it has been shown for laccase. To our knowledge, the recovery of laccase from residual compost of Agaricus bisporus (Mayolo-Deloisa 2009) was the first work where this protein was partially purified using ATPS. This strategy was chosen since there were no previous reports regarding the topic. As it has been mentioned and suggested as a general strategy, the 16 PEG–phosphate systems with a V R of 1.0 were used (Table 3.3), varying the TLL, the molecular weight of PEG, and the crude extract concentration. The general strategy consisted first in demonstrating the partition of laccase in one phase and second in trying to increase the recovery percentage of the enzyme in that phase.
In practice, the preparation of each system with a reported binodal curve is relatively easy. Generally, the systems can be prepared by weighing concentrate solutions of each phase component until reaching the exact composition, but in the work mentioned before, each component was weighted individually. To facilitate the reproducibility of the systems, the exact composition of each phase-forming chemical is shown in Table 3.3. It should be mentioned that these systems are robust and the formation of the two phases is not broken when the sample is added. However, it is important to assure that the sample weight does not exceed 10% w/w of the total weight of the system. Overall, laccase was partitioned toward the PEG-rich phase (top phase), and enzymatic activity was not detected in the salt-rich phase (bottom phase). Furthermore, it was clearly observed that the top-phase recovery of laccase decreased with increments in TLL and PEG MW .
3.3.2 Recovery of Laccase from Residual Compost of Pleurotus Ostreatus Using ATPS
For many reasons, Pleurotus genus , commonly known as oyster fungus , has been intensively studied in many different parts in the world: it presents a high gastronomic value, it can colonize and degrade a large variety of lignocellulosic residues, it requires shorter growth times when compared to other edible mushrooms, and it can be cultivated in a simple and cheap way (Jwanny et al. 1995; Bonatti et al. 2004). Pleurotus ostreatus belongs to a subclass of lignin-degrading microorganisms that produce laccases (Palmieri et al. 1997). The industrial production of Pleurotus ostreatus can be carried out using a variety of agricultural lignocellulosic residues as wheat straw. As with the Agaricus bisporus compost, this residue may contain a high-laccase concentration. It is also known that the catalytic activity of laccase depends on its source of production. Therefore, and because of the ease of obtaining this residual compost, it was decided to study the recovery of this enzyme from P. ostreatus using ATPS. This time, however, the background of the work with the crude extract of A. bisporus already existed, and some reports also demonstrated that polymers such as UCON (a random copolymer of ethylene oxide and propylene oxide) and PEG contributed to laccase stabilization (Silvério 2013). It was then decided to repeat the previously used strategy (Fig. 3.1) to corroborate the behavior of the enzyme with PEG–phosphate ATPS and other polymers as UCON, dextran, and ficoll.
As mentioned, the exact composition of the compost was not known, but it was clearly observed that the crude extract of P. ostreatus was cleaner, and the brown coloration was less intense than that of the crude extract of A. bisporus. When the PEG–phosphate systems were evaluated, no partition of laccase to the bottom phase was observed, as in the extract of A. bisporus. In general, the difference in the results obtained in both works was the recovery percentage. But the behavior of the partition and the effect of the molecular weight of PEG were the same. In this work, it was concluded that the PEG 1000 g/mol-phosphate ATPS were the most suitable for the primary recovery of laccase from both extracts.
After evaluating the behavior of PEG–phosphate systems, other previously reported systems for the recovery of laccase composed by UCON and different salts were tested. In all systems, laccase partitioned to the bottom phase (salt phase). For these experiments, the pH influence in the partition was analyzed. For the laccase of A. bisporus, a pre-analysis was made to determine the optimum pH for the recovery, since it is known that the laccase activity is better at acidic pH values between 4 and 5. A difference between both laccases was clear while studying the pH effect. For laccase from A. bisporus, pH 7 was the optimum for the recovery, and ATPS were prepared under those conditions. On the other hand, laccase from P. ostreatus presented higher recoveries in the UCON-NaH2PO4 systems with a pH value of 4 and a pH of 5 in the UCON-(NH4)2SO4 and UCON-Na2SO4 systems. Apparently, the high hydrophobicity of UCON causes the enzyme to be partitioned to the salt phase. Other polymer–polymer ATPS (i.e., ficoll and dextran systems) were also analyzed for the recovery of laccase from P. ostreatus, but the obtained yields were lower. The most important difference between these other polymer–polymer systems and the original polymer–salt systems was the fact that the enzyme was partitioned in both phases at different degrees.
The SDS-PAGE results show that the molecular weight of laccase from P. ostreatus is approximately 30 kDa and 60 kDa for that from A. bisporus. The general behavior of the enzymes is very similar in PEG-salt ATPS, but their recovery percentage is different. This may be directly related to the molecular weight of the enzymes, the source of production, and the complexity of the residual compost. Nonetheless, these results show that ATPS are an excellent alternative for the recovery of valuable enzymes from these compost waste streams .
3.3.3 Recovery of Invertase from Wasted Brewer’s Yeast
It must be noted that industrially waste streams are regarded as disposable and invaluable materials, so to make their processing or “reuse” attractive, economically speaking, easy-to-implement and relatively inexpensive procedures should be devised to obtain profitable strategies from them. In this sense, ATPS are a very attractive operation since their implementation requires a low investment when compared to other strategies such as chromatography. Therefore, in the case of the recovery of invertase from wasted brewer’s yeast, ATPS could be regarded as an interesting alternative to promote the extraction of said biomolecule.
In this context, our work was centered in characterizing the partition behavior of this enzyme in the aforementioned 16 PEG–phosphate ATPS and in intensifying the amount of biomass that could be processed in the operation. To do so, different amounts of total yeast extract were also loaded to each one of the tested systems. After screening and optimization, a PEG 400 g/mol ATPS with a TLL of 45% w/w, V R 1.0, and pH 7.0 loaded with 8% w/w of crude yeast extract proved to be suitable for the primary recovery of the enzyme in the salt-rich phase of the system. After this, the selected system was scaled to a total mass of 15 g allowing the recovery of the enzyme with a yield of 66%. Furthermore, this case is particularly interesting since the recovery invertase was achieved in a very low PEG MW system, making the later processing of the recovered phase even easier in other operations such as ultrafiltration if required .
3.4 ATPS Strategies for the Recovery of PEGylated Proteins
PEGylation is defined as the covalent attachment of at least one PEG chain to the structure of a protein or another molecule (Veronese 2001). This modification confers these molecules with multiple advantages related mostly to the increase in their molecular size after reaction. For example, in pharmaceutical compounds, this polymer attachment increases their circulating lives, makes them have a lower degradability, and allows their administration at lower and more spaced dosages, among other advantages (Harris and Chess 2003). However, common PEGylation reactions result in the attachment of different number of chains at different sites in the protein. This low selectivity results in a problem since usually, only one of the protein-polymer conjugates presents the adequate activity. Most frequently it is the mono-PEGylated conjugate that presents these characteristics, and even when the reactions have been optimized to promote higher yields of this conjugate, the appearance of other (i.e., di- and poly-PEGylated) conjugates is unavoidable. From an engineering perspective, this situation has generated an important challenge since after reaction, not only do the conjugates must be separated per their PEGylation degree but also by the positional isomerism of the grafted polymeric chains (Fee and Van Alstine 2006).
Traditionally, this problem has been addressed by different chromatographic approaches (Mayolo-Deloisa et al. 2011); however, in the search of more cost-effective and intensified operations, we have studied the use of ATPS strategies to do so with promising results. In this manner, the ATPS partition of the products of the PEGylation reactions of ribonuclease A (RNase A) and α-lactalbumin (α-Lac) was studied (González-Valdez et al. 2011). However, the quantification method used to establish the partition behavior of these proteins had to be tailored since the UV absorbance of the proteins became hindered by both the grafted polymeric chains and the ATPS environment, arising the need of obtaining correction factors and special calibration curves for each of the conjugates (González-Valdez 2011). After establishing the quantification method, the previously described 16 different PEG–phosphate ATPS were tested in the behavior studies of native, mono-, and di-PEGylated RNase A and α-Lac as a first approach. This initial screening showed that the native (unreacted) species were in all cases recovered in the bottom salt-rich phase of high PEG MW systems, while the PEGylated conjugates presented a preference of partition toward the polymeric phase. It had been previously established that PEGylated conjugates presented in general a preferable partition to the polymeric phase which increases per the PEGylation degree of the proteins (Delgado et al. 1994). After obtaining the initial results, the TLL and V R values were variated to promote a better partition and recovery of the conjugates. By taking the best V R 1.0 systems, variations were made in this value, and mono- and di-PEGylated RNase A conjugates were recovered with yields of 98% and 88%, respectively, in the top phase of a PEG 8000 g/mol, TLL 25% w/w VR 1.0, and pH 7.0 system, while mono- and di-PEGylated α-Lac were recovered with yields of 77% and 76%, respectively, in the polymeric phase of a PEG 8000 g/mol, TLL 35% w/w, VR 3.0, and pH 7.0 system (González-Valdez 2011). This study described the potential of PEG–phosphate ATPS for the selective fractionation of native unreacted proteins from their PEGylated conjugates after the reaction .
3.5 Protein Refolding Explorations in ATPS
Expression of genetically engineered proteins in bacteria often results in the accumulation of the product in inactive insoluble deposits inside the cells, called inclusion bodies (Basri et al. 1995). The refolding of proteins to their native conformation is still the limiting step during active protein recovery from these inclusion bodies (Sridhar 1996). In this context, traditional methods for protein reactivation or refolding consist primarily in the solubilization of such inclusion bodies in denaturing concentrations of guanidine hydrochloride or urea, followed by the removal of the denaturant, and refolding assistance by small molecule additives (García-Arellano et al. 2002). Refolding is commonly addressed by a practical approach using several methods that include dialysis, diafiltration, and size exclusion chromatography (Narain 2006). But such methods are time-consuming and, often, recovery yields of active proteins are low, and a trial-and-error process development is required to achieve success (Nagasaki et al. 2007). Additionally, most of the methods involve the use of additives to assist the correct refolding of the obtained proteins.
In this manner, it has been demonstrated that PEG (the most common polymer used in ATPS strategies) inhibits aggregation during the refolding process (as in the case of bovine carbonic anhydrase B) through the formation of a non-associating PEG-intermediate complex, preventing self-association and promoting correct refolding (Lu et al. 2008). And as it has been shown, ATPS are able to provide good and affordable physicochemical conditions to refold several proteins (Narain 2006).
Kuboi et al. developed a protein-refolding process using ATPS modified with stimuli-responsive polymers, which had a chaperone-like function (Kuboi et al. 2000). PEG bound to a thermos-reactive hydrophobic head (poly (propylene oxide)-phenyl group (PPO-Ph)) was used as the functional ligand to modify the PEG phase of ATPS. In general, the principal contribution of this work was the use of PP-Ph-PEG as chaperone agent. In another work, the usage of UCON and dextran T-500 systems was reported for the refolding of chymotrypsin inhibitor 2 (CI2) (Maruyama et al. 2002). The study stated that the partitioning behavior of CI2 in UCON-dextran and UCON-water systems was due to conformational changes between the native and the unfolded states of the protein; the unfolded CI2 could be refolded to the native form with high yields in the UCON solution. The partition and refolding of chymosin using PEG–phosphate and PEO-maltodextrin systems have been also reported (Reh et al. 2007). In this case, inclusion bodies contained prochymosin, and their refolding was carried out at pH 2.0 to facilitate the auto-conversion of prochymosin to recombinant chymosin. This finding agrees with the known PEG capacity to avoid the contact between protein molecules, thus preventing protein aggregation while enhancing correct refolding and increasing in this way the thermodynamic stability of a protein. Furthermore, another study reported an experimental design to determine the optimal conditions for the refolding of a recombinant thermostable and alkaline-active xylanase from Bacillus halodurans in PEG–phosphate systems (Sridhar 1996). Results showed that the recovery of active enzyme in the top phase increased with decrements in PEG molecular weight and decreased with increasing enzyme load. The model based on response surface methodology suggested that exposure of the hydrophobic residues and the increased surface of the proteins or denaturation with urea influences their partition in ATPS and predicted that the activity recovery decreased with increasing PEG molecular weight. This is attributed to the increasing hydrophobicity of the polymer, which causes more aggregation during the refolding process. In the same way, the partition behavior of native and denatured invertase in different PEG–phosphate systems has been evaluated (Narain 2006). The authors observed that recovered protein in the top phase presented in most systems refolding percentages of 100% that increased only in systems formed with PEG 8000 g/mol.
In our experience, we have conducted refolding procedures using as a first approach the 16 different ATPS in the search of an attractive system that promotes the refolding of denatured invertase (Sánchez-Trasviña et al. 2015). In this case, however, variations in V R using values of 0.33, 1.0, and 3.0 were tested since the beginning giving a total of 48 different systems. The partition behaviors of native and denatured invertase presented differences since the denatured species presented a predominant preference for the salt-rich phase. It was also observed that system design parameters (i.e., PEG MW, TLL, and V R) presented some sort of influence on both the partition and the refolding of the enzyme. A complete invertase refolding could be observed in the polymeric phases of the systems, but very low enzyme concentrations were achieved in this phase (0.5 mg/mL). On the other hand, refolding percentages in the bottom phase ranged between 50% and 75%. Therefore, a PEG 3350 g/mol, TLL 25% w/w, and V R 1.0 system was selected as the best option for refolded invertase recovery in the bottom phase with up to 0.73 mg of enzyme recovered from the original 2.0 mg of denatured enzyme loaded. In general, there are few studies about the use of ATPS for the refolding of proteins; however, their potential has been clearly stated. Some of the reports abovementioned used proteins denatured with different agents as guanidine hydrochloride before the refolding studies. So, there is an area of opportunity in the study of protein refolding from inclusion bodies produced in industrial processes and the use of new intelligent polymers as part of the ATPS to enhance the operation.
3.6 Concluding Remarks
Regardless of the biomolecule being processed or the source where it comes from, the successful implementation of aqueous two-phase system strategies can be addressed using a simple algorithm that has shown effectiveness in a large amount of cases. Shortly, this pathway requires the understanding of the physicochemical characteristics and concentrations of the target molecule and the contaminants present in the sample; an initial screening with well-defined ATPS; the optimization of those systems that give the best results regarding recovery yield and purity; and the recovery of the target molecule. In this chapter, we have presented a series of cases where this strategy has been used successfully and furthermore showing the tremendous capability of ATPS as an attractive primary recovery operation.
It should be noted that the industrial implementation of ATPS strategies is usually halted by regulatory situations (like in the case of pharmaceutical products) where a golden standard for processing operations has already been established and approved and little can be made. However, there is an important opportunity area in other fields such as the management of waste streams and the procurement of high added-value molecules from them. As it has been mentioned, to have a sustainable and profitable operation when handling wastes, it is important for the selected downstream processing operations to be as economic and robust as possible. ATPS operations possess both characteristics making them and interesting option to achieve this. Furthermore, and besides their implementation in the handling of wastes, ATPS present important advantages over other traditional techniques in the processing of different samples as is the case of chemical reactions or the refolding of proteins from inclusion bodies as it has been discussed. In this sense, it is very important for scientists and industrials to identify those niche opportunity areas where this type of operations can be used. With the aid of the available knowledge in the processing of a very large amount of proteins and the identification of the similarities between these processes, easy-to-implement and successful ATPS strategy should be available regardless of the complexity of the sample or matrix where the product is present.
Abbreviations
- ATPS:
-
Aqueous two-phase systems
- CI2:
-
Chymotrypsin inhibitor 2
- HTS:
-
High-throughput screening
- K P :
-
Partition coefficient
- MW:
-
Molecular weight
- PEG:
-
Polyethylene glycol
- pI:
-
Isoelectric point
- PPO-Ph:
-
Poly(propylene oxide)-phenyl
- RNase A:
-
Ribonuclease A
- TLL:
-
Tie-line length
- UCON:
-
Ethylene oxide and propylene oxide
- V R :
-
Volume ratio
- α-Lac:
-
α-lactalbumin
References
Aguilar O, Rito-Palomares M. Processing of soybean (Glycine max) extracts in aqueous two-phase systems as a first step for the potential recovery of recombinant proteins. J Chem Technol Biotechnol. 2008;83(3):286–93.
Barbosa H, Slater NKH, Marcos JC. Protein quantification in the presence of poly(ethylene glycol) and dextran using the Bradford method. Anal Biochem. 2009;395(1):108–10.
Basri M, Ampon K, Yunus WMZW, Razak CNA, Salleh AB. Synthesis of fatty esters by polyethylene glycol-modified lipase. J Chem Technol Biotechnol. 1995;64(1):10–6.
Benavides J, Rito-Palomares M. Bioprocess intensification: a potential aqueous two-phase process for the primary recovery of B-phycoerythrin from Porphyridium cruentum. J Chromatogr B. 2004;807(1):33–8.
Benavides J, Rito-Palomares M. Practical experiences from the development of aqueous two-phase processes for the recovery of high value biological products. J Chem Technol Biotechnol. 2008;83(2):133–42.
Benavides J, Rito-Palomares M. Simplified two-stage method to B-phycoerythrin recovery from Porphyridium cruentum. J Chromatogr B. 2006;844(1):39–44.
Benavides J, Rito-Palomares M, Asenjo JA. 2.49 – aqueous two-phase systems. In: Moo-Young M, editor. Comprehensive biotechnology. 2nd ed. Burlington: Academic Press; 2011. p. 697–713.
Bertrand B, Martínez-Morales F, Trejo-Hernández MR. Fungal laccases: induction and production. Re Mex Ing Quím. 2013;12:473–88.
Bertrand B, Mayolo-Deloisa K, González-González M, Tinoco-Valencia R, Serrano-Carreón L, Martínez-Morales F, Trejo-Hernández MR, Rito-Palomares M. Pleurotus ostreatus laccase recovery from residual compost using aqueous two-phase systems. J Chem Technol Biotechnol. 2016;91(8):2235–42.
Bonatti M, Karnopp P, Soares HM, Furlan SA. Evaluation of Pleurotus ostreatus and Pleurotus sajor-caju nutritional characteristics when cultivated in different lignocellulosic wastes. Food Chem. 2004;88(3):425–8.
Delgado C, Malik F, Selisko B, Fisher D, Francis GE. Quantitative analysis of polyethylene glycol (PEG) in PEG-modified proteins/cytokines by aqueous two-phase systems. J Biochem Biophys Methods. 1994;29(3–4):237–50.
Dumetz AC, Chockla AM, Kaler EW, Lenhoff AM. Comparative effects of salt, organic, and polymer precipitants on protein phase behavior and implications for vapor diffusion. Crys Growth Des. 2009;9(2):682–91. doi:10.1021/cg700956b.
Fee CJ, Van Alstine JM. PEG-proteins: reaction engineering and separation issues. Chem Eng Sci. 2006;61(3):924–39.
Franco TT, Andrews AT, Asenjo JA. Use of chemically modified proteins to study the effect of a single protein property on partitioning in aqueous two-phase systems: effect of surface hydrophobicity. Biotechnol Bioeng. 1996;49(3):300–8.
García-Arellano H, Valderrama B, Saab-Rincón G, Vazquez-Duhalt R. High temperature biocatalysis by chemically modified cytochrome C. Bioconjug Chem. 2002;13(6):1336–44.
Garza-Madrid M, Rito-Palomares M, Serna-Saldivar SO, Benavides J. Potential of aqueous two-phase systems constructed on flexible devices: human serum albumin as proof of concept. Process Biochem. 2010;45(7):1082–7.
Gong M, Aguirre AM, Bassi A. Chapter 5 – technical issues related to characterization, extraction, recovery, and purification of proteins from different waste sources A2. In: Dhillon GS, editor. Protein byproducts. London: Academic; 2016. p. 89–106.
González-González M, Mayolo-Deloisa K, Rito-Palomares M, Winkler R. Colorimetric protein quantification in aqueous two-phase systems. Process Biochem. 2011;46(1):413–7.
González-Valdez J, Cueto LF, Benavides J, Rito-Palomares M. Potential application of aqueous two-phase systems for the fractionation of RNase A and α-Lactalbumin from their PEGylated conjugates. J Chem Technol Biotechnol. 2011;86(1):26–33.
Gustavsson J, Cederberg C, Sonesson U, van Otterdijk R, Maybeck A. Global food losses and food waste – extent, causes an prevention. International Congress Save Food. Rome: Food and Agriculture Organization of the United Natios; 2011.
Harris JM, Chess RB. Effect of PEGylation on pharmaceuticals. Nat Rev Drug Discov. 2003;2(3):214–21.
Hernandez-Mireles T, Rito-Palomares M. New aqueous two-phase systems based on poly(ethylene oxide sulfide) (PEOS) and potassium phosphate for the potential recovery of proteins. J Chem Technol Biotechnol. 2006;81(6):997–1002.
Ibarra-Herrera CC, Torres-Acosta MA, Mendoza-Ochoa GI, Aguilar-Yañez JM, Rito-Palomares M. Recovery of major royal jelly protein 1 expressed in Pichia Pastoris in aqueous two-phase systems. J Chem Technol Biotechnol. 2014;89(7):941–7.
Johansson G. Partition of salts and their effects on partition of proteins in a dextran-poly(ethylene glycol)-water two-phase system. Biochim Biophys Acta Protein Struct. 1970;221(2):387–90.
Jwanny EW, Rashad MM, Abdu HM. Solid-state fermentation of agricultural wastes into food through Pleurotus cultivation. Appl Biochem Biotechnol. 1995;50(1):71–8.
Kuboi R, Morita S, Ota H, Umakoshi H. Protein refolding using stimuli-responsive polymer-modified aqueous two-phase systems. J Chromatogr B. 2000;743(1):215–23.
León-González G, González-Valdez J, Mayolo-Deloisa K, Rito-Palomares M. Intensified fractionation of brewery yeast waste for the recovery of invertase using aqueous two-phase systems. Biotechnol Appl Biochem. 2015;63(6):886–94.
Lladosa E, Silvério SC, Rodríguez O, Teixeira JA, Macedo EA. (Liquid + liquid) equilibria of polymer-salt aqueous two-phase systems for laccase partitioning: UCON 50-HB-5100 with potassium citrate and (sodium or potassium) formate at 23 °C. J Chem Thermodyn. 2012;55:166–71.
Lu Y, Harding SE, Turner A, Smith B, Athwal DS, Grossmann JG, Davis KG, Rowe AJ. Effect of PEGylation on the solution conformation of antibody fragments. J Pharm Sci. 2008;97(6):2062–79.
Makris DP. Chapter 16 – recovery and applications of enzymes from food wastes A2. In: Galanakis CM, editor. Food waste recovery. San Diego: Academic; 2015. p. 361–79.
Maruyama T, Nagasawa S, Goto M. Poly(ethylene glycol)-lipase complex that is catalytically active for alcoholysis reactions in ionic liquids. Biotechnol Lett. 2002;24(16):1341–5.
Mayolo-Deloisa K, Gonzalez-Valdez J, Guajardo-Flores D, Aguilar O, Benavides J, Rito-Palomares M. Current advances in the non-chromatographic fractionation and characterization of PEGylated proteins. J Chem Technol Biotechnol. 2011;86(1):18–25.
Mayolo-Deloisa K, Trejo-Hernandez MD, Rito-Palomares M. Recovery of laccase from the residual compost of Agaricus bisporus in aqueous two-phase systems. Process Biochem. 2009;44(4):435–9.
Minussi RC, Pastore GM, Durán N. Potential applications of laccase in the food industry. Trends Food Sci Technol. 2002;13(6–7):205–16.
Moreira S, Silvério SC, Macedo EA, Milagres AMF, Teixeira JA, Mussatto SI. Recovery of Peniophora cinerea laccase using aqueous two-phase systems composed by ethylene oxide/propylene oxide copolymer and potassium phosphate salts. J Chromatogr A. 2013;1321(0):14–20.
Morozova O, Shumakovich G, Gorbacheva M, Shleev S, Yaropolov A. “Blue” laccases. Biochem Mosc. 2007;72:1136–50.
Nagasaki Y, Yoshinaga K, Kurokawa K, Iijima M. Thermal- and dispersion-stable lipase-installed gold colloid: PEGylation of enzyme-installed gold colloid. Colloid Polym Sci. 2007;285(5):563–7.
Narain R. Tailor-made protein–glycopolymer bioconjugates. React Funct Polym. 2006;66(12):1589–95.
Ng HS, Tan CP, Chen SK, Mokhtar MN, Ariff A, Ling TC. Primary capture of cyclodextrin glycosyltransferase derived from Bacillus Cereus by aqueous two phase system. Sep Purif Technol. 2011;81(3):318–24.
Palmieri G, Giardina P, Bianco C, Scaloni A, Capasso A, Sannia G. A novel white laccase from Pleurotus ostreatus. J Biol Chem. 1997;272(50):31301–7.
Prinz A, Hönig J, Schüttmann I, Zorn H, Zeiner T. Separation and purification of laccases from two different fungi using aqueous two-phase extraction. Process Biochem. 2014a;49(2):335–46.
Prinz A, Koch K, Górak A, Zeiner T. Multi-stage laccase extraction and separation using aqueous two-phase systems: experiment and model. Process Biochem. 2014b;49(6):1020–31.
Rajagopalu D, Show PL, Tan YS, Muniandy S, Sabaratnam V, Ling TC. Recovery of laccase from processed Hericium erinaceus (Bull.:Fr) Pers. fruiting bodies in aqueous two-phase system. J Biosci Bioeng. 2016;122(3):301–6.
Reh G, Spelzini D, Tubio G, Pico G, Farruggia B. Partition features and renaturation enhancement of chymosin in aqueous two-phase systems. J Chromatogr B. 2007;860(1):98–105.
Reis IAO, Santos SB, Santos LA, Oliveira N, Freire MG, Pereira JFB, Ventura SPM, Coutinho JAP, Soares CMF, Lima ÁS. Increased significance of food wastes: selective recovery of added-value compounds. Food Chem. 2012;135(4):2453–61.
Rito-Palomares M, Middelberg APJ. Aqueous two-phase systems for the recovery of a recombinant viral coat protein from Escherichia coli. J Chem Technol Biotechnol. 2002;77(9):1025–9.
Rito-Palomares MA. Practical application of aqueous two-phase partition to process development for the recovery of biological products. J Chromatogr B. 2004;807(1):3–11.
Ruiz-Ruiz F, Benavides J, Aguilar O, Rito-Palomares M. Aqueous two-phase affinity partitioning systems: current applications and trends. J Chromatogr A. 2012;1244:1–13.
Schwienheer C, Prinz A, Zeiner T, Merz J. Separation of active laccases from Pleurotus sapidus culture supernatant using aqueous two-phase systems in centrifugal partition chromatography. J Chromatogr B. 2015;1002:1–7.
Silvério SC, Rodríguez O, Tavares APM, Teixeira JA, Macedo EA. Laccase recovery with aqueous two-phase systems: enzyme partitioning and stability. J Mol Catal B Enzym. 2013;87(0):37–43.
Simental-Martínez J, Rito-Palomares M, Benavides J. Potential application of aqueous two-phase systems and three-phase partitioning for the recovery of superoxide dismutase from a clarified homogenate of Kluyveromyces marxianus. Biotechnol Prog. 2014;30(6):1326–34.
Sridhar P. Modelling of affinity separation by batch and fixed bed adsorption – a comparative study. Chem Eng Technol. 1996;19(4):357–63.
Sánchez-Trasviña C, González-Valdez J, Mayolo-Deloisa K, Rito-Palomares M. Impact of aqueous two-phase system design parameters upon the in situ refolding and recovery of invertase. J Chem Technol Biotechnol. 2015;90(10):1765–72.
Trejo-Hernandez MR, Lopez-Munguia A, Quintero Ramirez R. Residual compost of Agaricus bisporus as a source of crude laccase for enzymic oxidation of phenolic compounds. Process Biochem. 2001;36(7):635–9.
Veronese FM. Peptide and protein PEGylation: a review of problems and solutions. Biomaterials. 2001;22(5):405–17.
Vázquez-Villegas P, Aguilar O, Rito-Palomares M. Continuous enzyme aqueous two-phase extraction using a novel tubular mixer-settler in multi-step counter-current arrangement. Sep Purif Technol. 2015;141(0):263–8.
Vázquez-Villegas P, Espitia-Saloma E, Rito-Palomares M, Aguilar O. Low-abundant protein extraction from complex protein sample using a novel continuous aqueous two-phase systems device. J Sep Sci. 2013;36(2):391–9.
Zaslavsky B. Aqueous two-phase partitioning: physical chemistry and bioanalytical applications. New York: Marcel Dekker Inc.; 1995.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 Springer International Publishing AG
About this chapter
Cite this chapter
González-Valdez, J., Mayolo-Deloisa, K. (2017). Practical Aspects for the Development of ATPS-Based Processes for Protein Recovery. In: Rito-Palomares, M., Benavides, J. (eds) Aqueous Two-Phase Systems for Bioprocess Development for the Recovery of Biological Products. Food Engineering Series. Springer, Cham. https://doi.org/10.1007/978-3-319-59309-8_3
Download citation
DOI: https://doi.org/10.1007/978-3-319-59309-8_3
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-59308-1
Online ISBN: 978-3-319-59309-8
eBook Packages: Chemistry and Materials ScienceChemistry and Material Science (R0)