Abstract
The conventional biological nitrogen removal process combining the nitrification and denitrification steps is widely implemented worldwide for the treatment of wastewaters with low to moderate nitrogen concentrations. For highly nitrogen-concentrated streams, the conventional process become limited, mainly due to sizing and operation issues. In the 1990s, the paradigm that the only way to biologically convert ammonium into nitrogen gas was the complete oxidation of ammonium to nitrate (nitrification) followed by the reduction of nitrate to nitrogen gas (denitrification) became obsolete. Novel bacteria involved in the nitrogen cycle were identified and isolated from natural and engineered systems, which culminated in the discovery of new metabolic pathways and development of more sustainable processes for nitrogen removal. The idea of this chapter is to illustrate the most relevant innovative nitrogen removal processes and how they evolved over the years from bench-scale research to full-scale implementation.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
5.1 Introduction
The approach toward the biological treatment of wastewaters has changed several times over the years. In the 1960s and 1970s, the main concern revolved around the removal of organic material from wastewaters given its high polluting capacity in the environment. In this context, several aerobic and anaerobic systems were developed aiming at the degradation of organic compounds.
In the 1980s, it was observed that even in the absence of organic material in wastewaters, the polluting effect on the environment remained intense, notably when nitrogen compounds were present. The increase in the global population and the consequent increase in the quantity of human waste as well as the use of synthetic nitrogen fertilizers produced from atmospheric N2 by the Haber-Bosch process, intensified in the past 40 years, are two factors which have contributed significantly to enhance the pollution levels caused by nitrogen compounds.
Nitrogen removal is of crucial importance. When it is available, together with phosphorus, other important nutrient, enhanced primary production occurs, which is exemplified by the excessive growth of phytoplankton species (algal bloom). This phenomenon is referred to as eutrophication. During this process, the microbial population responsible for the degradation of organic matter originating from phytoplankton species grows exponentially, and as a consequence, the oxygen demand is substantially increased. The death of fish and other aquatic organisms by asphyxiation, an increase in the cost of treating eutrophicated waters, and even the inappropriateness of these waters for many uses are some of the consequences of eutrophication.
In this context, it became clear that the application of processes aimed at the removal of nitrogen and phosphorus was essential in order to preserve the quality of the receiving water bodies. Thus, the conventional processes for biological nutrient removal (involving nitrification and denitrification stages) began to be used extensively.
In the following decade (1990s), the increase in the installation costs of traditional technologies for wastewater treatment, the increased strictness of the limits imposed on effluent discharges (the treatment plants requiring significant modifications in order to comply with environmental regulations), the limitations of the conventional processes regarding the treatment of high-strength nitrogen wastewaters, and the appearance of innovative ideas motivated the development of new technologies for biological nitrogen removal.
During this period, novel bacteria involved in the nitrogen cycle were identified and isolated from various environments (natural or engineered bioreactors), which culminated in the development of new nitrogen removal processes. In fact, these new processes still have rather limited application compared with conventional process, particularly for operation in larger scales. However, as will be discussed in Sect. 5.2, most of them have enormous potential for use in wastewater treatment, as they were developed as an attempt to overcome the limitations of the conventional nitrification-denitrification processes encountered under certain conditions.
In comparison with physicochemical processes, such as ammonia stripping and precipitation with magnesium ammonium phosphate, the recently developed biological processes for nitrogen removal offer a considerable economic advantage. The physicochemical processes require significant quantities of chemical products and thus lead to the production of a greater amount of chemical sludge. Despite the argument that physicochemical processes can allow the recovery of ammonium, only a small quantity of the ammonium is recovered in comparison with its general use, for instance, as a fertilizer. Additionally, these techniques, in general, require a greater amount of energy than biological nitrification-denitrification processes. In view of all of these factors, biological nitrogen removal is the option most commonly recommended and used (VAN LOOSDRECHT 2008).
The new nitrogen removal processes are the subject of this chapter. Before they are presented, the new aspects related to the microbial transformation of nitrogen in the context of wastewater treatment are first discussed.
5.2 New Processes for Biological Nitrogen Removal
5.2.1 Introduction and Contextualization
Complex interactions occur between the different nitrogen species (such as ammonium, nitrite, and nitrate) and in the different transformation mechanisms. Organic nitrogen is made up of various compounds including amino acids, urea, uric acid, and nitrogen bases. By means way of hydrolysis and mineralization, organic nitrogen is converted into ammonium nitrogen. Ammonium is one of the most important nitrogen compounds in surface waters and other ecosystems for several reasons: (1) it is the preferred nutrient of various species of plants and autotrophic bacteria; (2) it is chemically reduced and thus can be easily oxidized in natural aquatic environments resulting in the consumption of dissolved oxygen; and (3) non-ionized ammonia (NH3) is toxic to several forms of aquatic life even at low concentrations (<0.2 mg/L) (KADLEC and KNIGHT 1996).
Under aerobic conditions, ammonium is oxidized to nitrite, which is further oxidized to nitrate. This process is called nitrification. Two main groups of bacteria are involved in nitrification: ammonium-oxidizing bacteria (AOB) and nitrite-oxidizing bacteria (NOB). Nitrification (Eq. 5.1) is the first step in the conventional nitrogen removal process. The complementary step is denitrification, in which the reduction of nitrate to nitrogen gas occurs (Eq. 5.2). Equation 5.3 represents the combined process involving the two previous steps. The conventional nitrification-denitrification process is the commonly used approach for nitrogen removal in modern wastewater treatment plants. It is very efficient and, when properly operated, is stable and reliable. The operation cost is moderate and the process can be controlled relatively easily (METCALF and EDDY 2003).
Despite its extensive application, the conventional nitrogen removal process occurs very slowly, a characteristic associated with the low microbial activity and the reduced yield of this process. It is used for the treatment of wastewaters containing low concentrations of ammonium, generally less than 100 mgN/L. For the treatment of high-strength nitrogen wastewaters, such as those from anaerobic digesters, pig waste, landfill leachates, and certain industrial effluents, conventional nitrogen removal processes become limited, mainly due to sizing and operation issues. The space limitation and economic restriction are some problems which hinder the achievement of the desired performance in the larger existing treatment plants, particularly when the nitrogen load to be treated is high. Table 5.1 shows some examples of wastewaters containing high concentrations of ammonium.
The water originating from the dewatering of digested sludge is generally returned to the beginning of the treatment and mixed with the influent wastewater. On carrying out a nitrogen mass balance at the Dokhaven sewage treatment plant (Rotterdam, the Netherlands), it was observed that although the reject water contributes very little to the total flow, it corresponded to 15% of the nitrogen load (VAN DONGEN et al. 2001a; MULDER et al. 2001). A separate (sidestream) treatment of this stream rich in ammonium nitrogen, as indicated in Fig. 5.1, could reduce the nitrogen load originating from the sludge digesters and could contribute significantly to reducing the nitrogen concentration of the treatment plant effluent and allow the discharge limits to be reached.
Thus, the application of sidestream processes is particularly important when the treatment plant requires upgrading due to stricter effluent discharge standards or due to an increase in the nitrogen load. With the addition of a relatively small reactor volume, the nitrogen concentration of the effluent can be reduced. An additional advantage is that this allows for the construction of a reactor which is independent from the main treatment process, which is clearly a much simpler approach compared with modifying and expanding the existing treatment plants. Moreover, if the ammonium is not totally converted in the main treatment process, each kg of ammonium removed in the sidestream process will result in 1 kg less in the treatment plant effluent.
Landfill leachates are highly polluting and must be captured and treated. In general, the leachate is returned to the upper layer of the landfill, which leads to a reduction in the concentration of organic material. Conversely, the nitrogen concentration gradually increases, since the landfill acts as an anaerobic bioreactor (CLABAUGH 2001). Similarly, to reject water originating from dewatered sludge, some landfill leachates are characterized by a high concentration of ammonium and a low organic content (ILIES and MAVINIC 2001).
Pig wastes can be separated into two fractions: coarse and fine. The coarse fraction can be used as manure for soils while the fine fraction is treated. The composition of the fine fraction can vary depending on the separation method and the composition of the animal feed. In many cases, besides nitrogen and phosphorus, high concentrations of organic matter can be present, which is not favorable for the application of autotrophic nitrogen removal processes.
Wastewaters coming from industrial processes may contain high concentrations of nitrogen, especially when they are firstly treated in anaerobic digesters. Examples of these are the wastewaters generated in pharmaceutical plants (CARRERA et al. 2003); tanneries (MURAT et al. 2003); slaughterhouses (KELLER et al. 1997); potato, alcohol, and starch processing industries (ABELING and SEYFRIED 1993); and formaldehyde production (CAMPOS et al. 2003).
In recent years, the paradigm that the only way to biologically convert ammonium into nitrogen gas was the complete oxidation of ammonium to nitrate (nitrification) followed by the reduction of nitrate to nitrogen gas (denitrification) became obsolete. With the discovery of new metabolic pathways, more sustainable processes for nitrogen removal were developed and these have undergone continual improvement as research in this area has advanced. Among the most relevant new processes for removal of nitrogen, the following can be mentioned: partial nitrification and denitrification (SHARON, single-reactor high-activity ammonia removal over nitrite), CANON (completely autotrophic nitrogen removal over nitrite), OLAND (oxygen-limited autotrophic nitrification-denitrification), and aerobic/anoxic deammonification (DEMON). The last three are based on partial nitritation and on the relatively recently discovered anammox (anaerobic ammonium oxidation) process. The SHARON technology may also be coupled to anammox, giving rise to the so-called SHARON-anammox process.
In general, the specific application of alternatives available for the removal of nitrogen needs to be evaluated in relation to the aspects involving costs, chemical and energy requirements, operation experience, and the reliability and environmental impact of the process. However, the selection of the best alternative is usually based on the cost criteria. The new processes meet the objective of reducing the operating cost. In most new processes, the aim is to remove nitrogen via nitrite (which is used as electron acceptor) and not via nitrate as in the conventional process.
The nitritation-denitritation process (Eqs. 5.4 and 5.5) is one example of an alternative process for nitrogen removal via nitrite. It consumes less oxygen in the nitrification (partial nitrification up to nitrite) and requires less organic carbon for the denitrification (nitrite and not nitrate should be reduced to nitrogen gas) (SCHMIDT et al. 2003). Another advantage is a lower production of sludge. The application of the combined process of partial nitritation-anammox (described later in this chapter) brings even more advantages. In this combined process, organic matter is not required, since the nitrogen removal is carried out by autotrophic bacteria. This process is especially recommended for the treatment of wastewaters with a low organic carbon-to-nitrogen (C/N) ratio (RUIZ et al. 2003) and which contain high ammonium concentrations (between 100 and 5000 mgN/L) (MULDER 2003). Within this range, the autotrophic removal of nitrogen is more advantageous in relation to the conventional nitrification and denitrification process, requiring a lower amount of energy and chemical products.
In comparison with the traditional nitrogen removal process, the oxygen consumption in the nitritation-denitritation and partial nitritation-anammox processes are 25 and 60% lower, respectively. When the denitritation is applied after the nitritation process, a 40% saving can be obtained in terms of organic carbon. With the application of the anammox process downstream of the nitritation process, the saving is even greater, since this process does not require an organic carbon source (AHN 2006). HAO et al. (2001) and NIELSEN et al. (2005) note that for the treatment of highly concentrated streams, the relatively high installation cost of the combined partial nitritation-anammox processes is compensated for by the lower operating costs and by the good nitrogen removal performance.
The three processes (conventional, nitritation-denitritation, and partial nitritation-anammox), despite being very distinct in terms of oxygen and organic matter requirements, require similar levels of alkalinity, bearing in mind that 1 mol H+ is produced per mol of nitrogen converted. Thus, these processes are not associated with high costs for the pH control when the wastewater to be treated has a good buffering capacity (1 mol HCO3 − per mol of NH4).
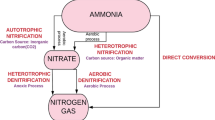
The main disadvantage of the autotrophic removal of nitrogen is the reduced growth rate of ammonium-oxidizing bacteria and anammox microorganisms. The performance of reactors in which slow-growing bacteria predominate can be improved through the application of high cell retention times. One alternative in this regard is the use of support media for the development of biofilms or the creation of conditions for the self-aggregation of the cells in the form of granules (VÁZQUEZ-PADÍN et al. 2009). Figure 5.2a, b show, in an illustrative and simplified manner, two different perspectives of the nitrogen cycle updated with the autotrophic removal of nitrogen. As can be observed, the detection of new microorganisms (such as anammox bacteria) increases substantially the complexity of the nitrogen cycle. The anammox process will be described in the next section. Further details about the new processes developed for nitrogen removal, many of them based on the anammox process, will be addressed further on in this chapter.
Schematic representation of the nitrogen cycle: (a) simplified comparison between the conventional nitrogen removal process and the anammox process; (b) main oxic and anoxic reactions involved in the nitrogen cycle. The different reactions are indicated in different colors: nitrogen fixation (dark gray), ammonification (orange), assimilation (purple), nitrification (black), denitrification (green), dissimilatory reduction of nitrate to ammonium (DNRA) (blue), and anammox (red). The dotted gray line indicates the formation of N2O by ammonium-oxidizing bacteria (adapted from Madigan et al. 2000)
5.2.2 Anammox Process
5.2.2.1 Brief History
In 1977, Engelbert Broda predicted, based on thermodynamic and evolutionary theories, that the oxidation of ammonium under anoxic conditions with nitrate or nitrite as the electron acceptor was possible (BRODA 1977). The oxidation of ammonium using an electron acceptor which is not oxygen had been previously predicted by researchers of marine environments based on mass balance studies (RICHARDS 1965) and later in combination with thermodynamic explanations (CLINE and RICHARDS 1972). These predictions were taken into consideration to some degree, although at the time few researchers were convinced that biological oxidation of ammonium could occur under anoxic conditions.
In 1985, the removal of ammonium was observed for the first time under anoxic conditions in a pilot-scale denitrifying reactor at a yeast powder production plant called Gist-Brocades, now part of the company DSM, in Delft, the Netherlands (HEIJNEN 1988; MULDER 1989; VAN DE GRAAF et al. 1990; MULDER et al. 1995). In this reactor, nitrate was added in order to obtain a combined process of sulfide oxidation and nitrate reduction. As an unexpected result, ammonium (considered not to be reactive under anoxic conditions) was also removed (MULDER et al. 1995). This new biological process was called the anammox process, an abbreviation for anoxic ammonium oxidation (MULDER 1989; VAN DE GRAAF et al. 1990) or anaerobic ammonium oxidation (MULDER 1989; VAN DE GRAAF et al. 1995), the name by which this process is most commonly known. A bench-scale reactor based on the pilot-scale denitrification reactor was run in the laboratory, and the enrichment of the organism responsible for the anammox process was achieved only after nitrite (and not nitrate) had been identified as the actual electron acceptor (VAN DE GRAAF et al. 1996).
Ten years after the first observations of the ammonium oxidation reaction under anoxic conditions in Delft (the Netherlands), in Germany (HIPPEN et al. 1996, 1997), and in Switzerland (BINSWANGER et al. 1997; SIEGRIST et al. 1998), the production of nitrogen gas instead of nitrate (leading to nitrogen loss from the liquid phase) was reported in rotating biological contactors (RBCs) treating ammonium-rich wastewaters originating from landfill leachate. Since the only electron donor present in significant quantities in these wastewaters was ammonium, it was expected that all of the nitrogen in the form of ammonium would be converted to nitrate, instead of being lost to the atmosphere in the form of N2. The conversion of soluble nitrogen compounds into N2 in these reactors was initially related to the denitrification carried out by nitrifying organisms, previously described by POTH and FOCHT (1985). Later on, it was attributed to a combination of nitrifying organisms and the bacteria responsible for the anammox process.
Anammox bacteria have been found not only in wastewater treatment plants but also in natural environments, such as in different marine ecosystems (e.g., the Black Sea) and rivers (KUYPERS et al. 2003), where they substantially influence the nitrogen cycle. Depending on the organic load, up to 70% of the N2 production in marine sediments can be attributed to the anammox process (DALSGAARD and THAMDRUP 2002).
Enriched cultures (purity of 50–90%) obtained by some researchers (SCHMID et al. 2000; EGLI et al. 2001; STROUS et al. 2006; TSUSHIMA et al. 2007a; LÓPEZ et al. 2008) were the only sources of information on the anammox process, since the numerous attempts to isolate the organisms responsible for this process had failed (STROUS et al. 1999a). In order to observe that the enriched organisms were in fact responsible for the anammox process, cells were physically purified up to 99.6% through density gradient centrifugation (Percoll method) (STROUS et al. 1999a). The fact that the purified cells were able to carry out the characteristic anammox conversion (ammonium to nitrite) at a high rate confirmed that the enriched microorganism was indeed responsible for the ammonium oxidation under anoxic conditions. The concentrated cell solution allowed the sequencing of the 16S rRNA gene, which provided evidence that the organism in question belonged to the Planctomycetes phylum (STROUS et al. 1999a) and was named Candidatus Brocadia anammoxidans.
5.2.2.2 Conversions Involved in the Anammox Process and Characteristics of the Organisms Responsible for this Process
The main substrates of the anammox process are ammonium, nitrite, and bicarbonate (HCO3 −) (VAN DE GRAAF et al. 1996). The coupling of the nitrogen atom of ammonium (electron donor) and the nitrogen atom of nitrite (electron acceptor) for the formation of nitrogen gas comprises the catabolic reaction (Eq. 5.6).
As an autotrophic biological process, HCO3 − is the carbon source for the production of biomass in the anabolic reactions (Eq. 5.7). The oxidation of nitrite to nitrate generates the electrons required for the HCO3 − reduction process (VAN DE GRAAF et al. 1996). The catabolic reaction is carried out 15 times for the fixation of one molecule of CO2 with nitrite acting as the electron donor, leading to the anaerobic production of nitrate in the anabolism. In autotrophic processes, the anoxic generation of nitrate can serve as a measure of the growth of anammox biomass and is a good indicator of the anammox activity (VAN LOOSDRECHT 2008).
The combination of catabolic (Eq. 5.6) and anabolic (Eq. 5.7) reactions using the biomass yield determined experimentally (0.066 mol C/mol NH4 +, STROUS et al. 1998) results in the following overall reaction (Eq. 5.8):
The stoichiometry of Eq. (5.8) is very close to that obtained experimentally (STROUS et al. 1998), represented in Eq. (5.9). As can be observed, ammonium and nitrite are consumed in almost equimolar proportions (1:1.3). The excess of nitrite (0.3 mol of nitrite per mol of ammonium) is oxidized anaerobically to nitrate. The electrons derived from this oxidation are probably used for the fixation of CO2 (VAN DE GRAAF et al. 1996). The main product of the anammox process is N2, although a small part of the nitrogen fed to the system is converted into nitrate. For the reduction of the nitrate produced through nitrification, organic carbon is needed, which does not present a problem since most real wastewaters contain at least a small quantity of biodegradable organic matter which can be used for this purpose.
The organisms responsible for the anammox process grow very slowly, as evidenced through the stoichiometry. The duplication time is several days under ideal operating conditions (STROUS et al. 1998; TSUSHIMA et al. 2007b). According to SCHMID et al. (2003), the duplication time is 11 days. Other authors, such as VAN DER STAR et al. (2008), have reported duplication times of between 5.5 and 7.5 days, values calculated based on the maximum conversion capacity. The same authors indicated the possibility of obtaining even shorter duplication times of around 3 days. Other researchers have reported the possibility of obtaining a duplication time of only 1.8 days under ideal operating conditions (ISAKA et al. 2006). One possible explanation for these divergent results is the method used for the determination of the growth rate. ISAKA et al. (2006) determined the growth rate based on the direct counting of the anammox bacteria, while in the other studies it was based on the biomass yield and nitrogen removal rate.
Autotrophic growth is a very costly process in terms of energy, and thus it is always associated with low growth rates when compared with heterotrophic growth. Consequently, the start-up period of the anammox process is very long and it takes a considerable time to achieve appreciable reaction rates. The use of reactors with efficient biomass retention is crucial to obtaining the enrichment of the anammox culture (JETTEN et al. 2001). The low growth rate of the anammox bacteria and the difficulty associated with obtaining enriched cultures of these microorganisms may hinder research involving the anammox process (STROUS et al. 1998). However, the low growth rate does not represent a limitation to the nitrogen removal capacity, which can reach values of 5–10 mgN/(m3 day) due to the fact that the anammox microorganisms form compact biofilms or granules, enabling high concentrations of biomass to be reached in the bioreactor.
It should be noted that the extremely slow growth of anammox bacteria cannot be explained simply by autotrophy. The energy obtained from the catabolism (calculated per mol of electrons) is comparable with that of the autotrophic nitrification process, although the growth rate is much lower. Other plausible explanations for the slow growth may be related to the fact that the anammox bacteria have a low intrinsic conversion rate (of ammonium to nitrite) or that the enrichment of the culture is carried out under non-ideal growth conditions.
There are many uncertainties regarding the reaction intermediates in the catabolism of anammox bacteria. However, there is a general consensus that hydrazine (N2H4) is an intermediate. The production of hydrazine from hydroxylamine can be used as a method to detect active anammox biomass. The oxidation of this compound to N2 is an energy-generating stage. Nitrite is not converted directly to hydrazine, but via hydroxylamine and/or nitric oxide (VAN DE GRAAF et al. 1997; STROUS et al. 2006). A schematic representation of the three possible metabolisms is illustrated in Fig. 5.3. Important enzymes involved in the process are hydroxylamine oxidoreductase (HAO), purified by SCHALK et al. (2000); hydrazine oxidase (HZO), purified by SHIMAMURA et al. (2007); and the nitrite reductases ccNir, partially purified by SCHALK (2000), and cd1Nir, found in the genome of Kuenenia (STROUS et al., 2006). Since all of these enzymes are able to carry out several reactions involving the conversion of nitrogen, it is still not clear which enzyme is responsible for a certain reaction.
Catabolic reactions of the anammox process with hydrazine acting as the main intermediate. Other potential intermediates are hydroxylamine (a), nitric oxide (b), or hydroxylamine and nitric oxide (c) (adapted from VAN DER STAR 2008)
According to VAN DONGEN et al. (2001a), the enzyme hydrazinase converts hydroxylamine into hydrazine. The hydrazine formed is oxidized by hydroxylamine oxidoreductase (HAO) to nitrogen gas, a stage in which four protons and four electrons are released. When nitrite is present in the system, the four electrons released allow the conversion of nitrite into hydroxylamine by the enzyme nitrite reductase. When nitrite is not present in the system and the anammox process is operated under limited nitrite conditions, the electrons leave the system in a different way. This process generally occurs through the reaction of hydrazine disproportionation to ammonium and nitrogen gas according to reaction 5.10.
The disintegration of hydrazine occurs more slowly than the formation of hydroxylamine. As a consequence, hydrazine accumulates in the system. Since the disintegration of hydrazine into ammonium and nitrogen gas occurs, ammonium is expected to accumulate in the system.
It should be noted that while N2O is usually the intermediate compound associated with denitrifying bacteria, this compound is not part of the physiology of anammox bacteria. This means that this powerful greenhouse gas is not produced by the anammox organisms.
The main compartment of anammox bacteria is anammoxosome. Anammoxosome is surrounded by riboplasm (where the ribosomes and chromosomes are located), which in turn are surround by the paryphoplasm (LINDSAY et al. 2001; VAN NIFTRIK et al. 2008), as shown in Fig. 5.4. Considering that there is no consensus regarding the characteristics of the membrane between the paryphoplasm and the riboplasm, the classification of the paryphoplasm (whether as a true internal compartment or as a region which is similar to the periplasm in Gram-negative bacteria) is still a matter under debate.
Schematic representation of the different compartments of anammox bacteria (adapted from LINDSAY et al. (2001) and FUERST (2005)). The cytoplasm is divided into the paryphoplasm (external compartment), the riboplasm (where ribosomes and chromosomes are found), and the anammoxosome (where most or all of the cytochrome c is present and catabolism probably occurs)
Microscopic observations suggest that the anammox bacteria, as in the case of other Planctomycetes, do not have peptidoglycans, although they exhibit a protein cell membrane. The lipids of the anammox bacteria contain a combination of fatty acids bound to esters (a typical characteristic of bacteria and eukaryotic cells) and ethers (typically found in Archaea). Lipid membranes are essential for the establishment of gradients of ions and metabolites. The anammox bacteria contain a variety of membrane lipids which are quite special and unique in nature (SINNINGHE DAMSTÉ et al. 2002, 2005; KUYPERS et al. 2003). The anammox cells are spherical shaped (coccus) and have a diameter of less than 1 μm. The anammox biomass presents a reddish brown color (Fig. 5.5), which is probably due to the high content of cytochromes (JETTEN et al. 1999).
The anammoxosome has been considered as the locus of catabolism, with the function of generating energy, in a way analogous to the function of mitochondria in eukaryotic cells (LINDSAY et al. 2001; VAN NIFTRIK et al. 2004). This hypothesis implies that the proton-motive force is created through the anammoxosome membrane for the coupling of energy generation and anabolism. The presence of important enzymes (hydrazine/hydroxylamine oxidoreductase) in the anammoxosome indicates that the anammox catabolism occurs in this compartment.
A biochemical model (Fig. 5.6) has been proposed in which the anaerobic oxidation of ammonium is catalyzed by various c-type cytochromes and proteins (STROUS et al. 2006). In this model, nitrite is firstly reduced to nitric oxide by a c-type cytochrome and d1-type cytochrome containing the enzyme nitrite reductase (NirS). It is assumed that the nitric oxide and ammonium are combined, forming hydrazine through the action of hydrazine hydrolase, and this compound is finally oxidized to nitrogen gas by a c-type cytochrome protein called hydrazine/hydroxylamine oxidoreductase (SCHALK et al. 2000; SHIMAMURA et al. 2007). The four electrons derived from this oxidation are transferred to the cytochrome c electron carriers (CIRPUS et al. 2005; HUSTON et al. 2007), to ubiquinone, to cytochrome bc1 complex, to cytochrome c electron carriers, and finally to nitrite reductase and hydrazine hydrolase.
Biochemical model representing anaerobic oxidation of ammonium coupled with the anammoxosome membrane in anammox bacteria, resulting from the proton-motive force and subsequent synthesis of ATP via ATPases bound to the membrane. bc1 cytochrome bc1 complex, cyt cytochrome, hao hydrazine/hydroxylamine oxidoreductase, Q coenzyme Q, a anammoxosome compartment, r riboplasm compartment (adapted from STROUS et al. 2006)
In this model, the anammox reaction establishes a proton gradient through the translocation of protons from the riboplasm to the anammoxosome, which results in an electrochemical proton gradient directly from the anammoxosome to the riboplasm. This gradient contains chemical potential energy (chemical proton gradient in the form of a difference in the pH in which the riboplasm is more alkaline compared with the anammoxosome) and electrical potential energy (electrical proton gradient in the form of a difference in the charge considering that the riboplasm is negatively charged in relation to the anammoxosome). The differences in both the pH and charge cause a proton displacement from outside to inside the anammoxosome, providing the proton-motive force. This mechanism can be used for the synthesis of ATP catalyzed by adenosine triphosphatases (ATPases) located in the anammoxosome membrane, as shown in Fig. 5.6. The protons passively move back to the riboplasm (due to the electrochemical proton gradient) through the pores formed by the ATPases. The globular and hydrophobic domain of the ATPases where ATP is synthesized will be located in the riboplasm, and its hydrophobic domain where protons are translocated will be located in the anammoxosome membrane. The ATP synthesized will be released into the riboplasm.
The anammox bacteria are dependent on the electrochemical ion gradient through the membrane for ATP synthesis. Since the anammox catabolism is slow, only a few protons are translocated within a certain time, while the dissipation of the electrochemical gradient resulting from the passive diffusion is independent of the growth rate and proceeds at the normal rate. Thus, the passive diffusion of protons through the membrane is relatively important and leads to a greater energy expenditure in the case of anammox bacteria. To give an idea, the expenditure due to the passive diffusion of protons in mitochondria corresponds to 10% (HAINES 2001). Thus, it is clear that the presence of a special, less permeable membrane is essential for the metabolism of anammox cells. Additionally, the intermediates of the anammox reaction, such as hydrazine, easily diffuse through the membrane.
From a bioenergy perspective, the loss of energy associated with the loss of one hydrazine molecule from the anammox cell is equivalent to 15 catabolic cycles. The explanation for this is as follows: when one molecule of hydrazine is lost, the hydrazine reserve needs to be reestablished. Presumably, hydrazine is formed via the reduction of nitrite to nitric oxide and the subsequent combination of nitric oxide with ammonium. The equivalents required (four electrons) must come from the oxidation of the reserve material, such as glycogen, which is derived from CO2. Considering that only one molecule of CO2 is fixed for every 15 mol of ammonium oxidized (15 catabolic cycles), a 10% loss of hydrazine would cause a complete loss of cell viability. Thus, the limitation of the diffusion of both protons and anammox intermediates is extremely important for the metabolism of anammox bacteria. Since anammox catabolism occurs in the anammoxosome, the lipid membrane of this compartment (described previously) needs to have a rigid structure in order to limit the diffusion of intermediates which are important to the process outside the anammoxosome. This is actually a specific adaptation of anammox bacteria for their unusual metabolism.
Due to the dense structure of the anammoxosome membrane, specific transporters are required to regulate the transport of ammonium and nitrite. The genome of Candidatus Kuenenia stuttgartiensis (one of the species of anammox bacteria, as will be described below) encodes four ammonium transporters (Amt), four formate/nitrite transporters (FocA), and two nitrate/nitrite transporters (NarK) of unknown location (STROUS et al. 2006).
While the anammoxosome membrane must be rigid, the cytoplasmic membrane must be flexible and permeable for the maintenance of homeostasis, controlling the intracellular ion concentrations and transport processes. Thus, with a rigid anammoxosome membrane and a flexible cytoplasmic membrane, the cell can overcome a problem associated with the presence of only one membrane, which would have to be impermeable and permeable at the same time. Moreover, the use of an intracytoplasmic compartment (anammoxosome) for the synthesis of ATP through the proton-motive force results in complete control of this force, allowing efficient energy transduction.
Until now, four genera of anammox bacteria have been described. The similarity of the 16S rRNA gene sequences of the species varies between 87 and 99% (SCHMID et al. 2007). Despite this relatively large phylogenetic distance, all of the anammox organisms belong to the same family, called Anammoxaceae (JETTEN et al. 2008), forming a monophyletic group belonging to the phylum Planctomycetes (STROUS et al. 1999a). One characteristic of Planctomycetes is its unusually high level of cellular organization, each cell consisting of one or more internal compartments bound by membranes with variable and unknown functions (LINDSAY et al. 2001; FUERST 2005).
The different anammox bacteria include those which have been enriched from activated sludge reactors, such as Candidatus Kuenenia stuttgartiensis (SCHMID et al. 2000), Candidatus Brocadia anammoxidans (STROUS et al. 1999a), Candidatus Brocadia fulgida (KARTAL et al. 2004), and Candidatus Anammoxoglobus propionicus (KARTAL et al. 2007), as well as those detected in marine environments, particularly in sediments and zones with minimal oxygen content, such as Scalindua brodae, Scalindua wagneri, and Scalindua sorokinii (SCHMID et al. 2003).
Since none of the anammox bacteria have been obtained as a pure culture, all of the species take the name Candidatus. Brocadia and Kuenenia have been generally enriched in laboratory experiments using a synthetic medium similar to that employed by VAN DE GRAAF et al. (1996) regardless of the inoculum used (SCHMID et al. 2000; VAN DONGEN et al. 2001b; CHAMCHOI and NITISORAVUT 2007). The addition of fatty acids was reported to lead to the enrichment of Anammoxoglobus (KARTAL et al. 2007) and Brocadia fulgida (KARTAL et al. 2004, 2008). Despite the considerable diversity in different enrichment systems, Scalindua sorokinii is the dominant species in marine environments.
Some studies have indicated that besides the conversion of ammonium and nitrite, anammox bacteria belonging to the genera Brocadia, Anammoxoglobus, and Kuenenia are also able to metabolize fatty acids such as propionate, acetate, and formate (GÜVEN et al. 2005; KARTAL et al. 2008). The oxidation of these fatty acids to CO2 is coupled with the reduction of nitrate (via nitrite) to ammonium. Thus, the anammox bacteria are able to produce their own substrate (ammonium and nitrite) for their catabolism (Fig. 5.7).
Metabolic versatility of anammox bacteria. Besides the conversion of ammonium and nitrite (solid lines), anammox bacteria can use short-chain fatty acids as electron donors for ammonification (dashed lines) (adapted from VAN DER STAR 2008)
It is still unknown whether the conversion of nitrate to ammonium represents an additional catabolic reaction, which would constitute an energy source. Surprisingly, studies have indicated that the fatty acids are not incorporated into the biomass, being completely converted into CO2 (KARTAL et al. 2008). Bearing in mind that the anammox bacteria also carry out the fixation of CO2 via acetate (the reverse reaction in relation to the previously described acetate oxidation), this characteristic is rather surprising, although already established for anammox bacteria.
In addition to the conversion of fatty acids, Kuenenia stuttgartiensis has been shown to be able to oxidize Fe2+ to Fe3+ using nitrate as the electron acceptor, as well as to reduce Fe3+ to Fe2+ and Mn4+ to Mn2+ using formate as an electron donor (STROUS et al. 2006). As in the case of the fatty acids conversion, the metabolism and the growth on these substrates are unknown.
5.2.2.3 Factors Influencing Anammox Bacteria Activity
5.2.2.3.1 Substrates and Products
The concentration of nitrite is an important parameter that needs to be controlled in the anammox process. However, a certain concentration of nitrite is required during the start-up of the anammox system. If the concentration of nitrite is very low, substrate limitation may lead to a low growth rate. On the other hand, a very high concentration can lead to inhibition. Several ranges of nitrite concentration which can cause inhibition have been reported in the literature, and thus there is no consensus observed in the previous studies.
STROUS et al. (1999b) observed that the anammox process (using Candidatus Brocadia anammoxidans) was completely inhibited when the nitrite concentration was higher than 100 mgN/L. DAPENA-MORA et al. (2007) observed that 350 mgNO2-N/L caused 50% inhibition of the anammox process. FUX (2003) maintained the nitrite concentration at around 40 mgN/L for several days and observed an irreversible inactivation of anammox bacteria. To restore the anammox activity due to inhibition by nitrite, trace amounts of intermediates of the anammox process, such as hydroxylamine and hydrazine, were added, even after long periods of exposure to high nitrite concentrations (STROUS et al. 1999b).
In this regard, studies have shown that the nitrite tolerance differs according to the genera of the anammox bacteria. EGLI et al. (2001) carried out experiments with Candidatus Kuenenia stuttgartiensis and observed that the anammox process was only inhibited when submitted to nitrite concentrations higher than 182 mgN/L. The experiments carried out by STROUS et al. (1999b) showed that with an increase in the nitrite concentration, the stoichiometry of the process changed. The stoichiometry of the ammonium and nitrite consumption increased from 1.3 g of nitrite per gram of ammonium (when the nitrite concentration was 0.14 gN/L) to almost 4 g of nitrite per gram of ammonium (when the nitrite concentration was 0.7 gN/L).
The significant change in the stoichiometry at high nitrite concentrations suggests that the microorganisms, when submitted to these conditions, do not use only ammonium as the electron donor, but they must also generate an internal electron donor to reduce the nitrite. A change in the stoichiometry has also been observed at high temperatures. DOSTA et al. (2008) reported nitrite-to-ammonium consumption ratios of 1.38:1 at 30 °C and 1.05:1 at 18 °C.
STROUS et al. (1999b) reported that the anammox process is not inhibited by ammonium or by nitrate at concentrations below 1 gN/L. However, DAPENA-MORA et al. (2007) observed a 50% drop in activity at high concentrations of ammonium and nitrate (770 and 630 mgN/L, respectively). Considering that chemolithoautotrophic organisms essentially use inorganic carbon as a carbon source, the influent concentration of bicarbonate is an important factor which can affect the enrichment of the anammox culture.
DEXIANG et al. (2007) observed low anammox activity at low bicarbonate-to-ammonium ratios (2.3:1). The reduction in the activity under these conditions may be due to the limitation of CO2. On the other hand, a high bicarbonate concentration (bicarbonate-to-ammonium ratio of 4.7:1) can also lead to inhibition. In this case, the inhibition is related to the formation of significant quantities of free ammonia due to the increase in the pH to values above 8.
5.2.2.3.2 Oxygen
Anammox bacteria are strictly anaerobic and are inhibited by the presence of dissolved oxygen in the medium. The inhibition caused by low oxygen concentrations has been described in several reports in the literature as being reversible. Based on experiments in which oxygen was provided intermittently, STROUS et al. (1997) concluded that the anammox process was reversibly inhibited by oxygen, which would make it possible to obtain partial nitritation and anammox in a single reactor. EGLI et al. (2001) affirmed that the anammox metabolism is reversibly inhibited at low oxygen levels (0.25–2% of air saturation) although it is probably irreversible at high levels (>18% of air saturation).
5.2.2.3.3 Organic Carbon
The treatment of landfill leachates and effluents from sludge digesters, which contain a high concentration of nitrogen, can be carried out through the new nitrogen removal processes, such as the combined partial nitritation-anammox. However, besides the substantial amount of nitrogen, these wastewaters may also contain a high concentration of organic matter. Nevertheless, they are considered to be potentially treated in an anammox system. During anaerobic digestion, organic matter is easily biodegraded and converted into biogas. Thus, only the portion with low biodegradability remains in the digester effluent.
RUSCALLEDA et al. (2008) observed that anammox bacteria and denitrifying organisms can coexist and are important for the treatment of wastewaters with high concentrations of slowly biodegradable organic matter, such as digester effluent and leachates. In these wastewaters, in particular, the growth of denitrifying heterotrophic organisms is limited by the low availability of easily biodegraded organic carbon. As a consequence, these microorganisms cannot become dominant and do not hamper the growth of anammox organisms.
However, in several studies reported in the literature, a negative effect of the presence of organic matter on the growth of anammox bacteria was observed (JETTEN et al. 1999; MOLINUEVO et al. 2009; TANG et al. 2010). In the presence of certain quantities of organic matter, the slow-growing anammox bacteria are no longer able to compete with the denitrifying organisms for nitrite, given the higher growth rate of the latter. Furthermore, the denitrification reaction is thermodynamically more favorable than the anaerobic oxidation of ammonia, since the Gibbs free energy of anammox and denitrification reactions are of the order of −355 kJ/mol (JETTEN et al. 1999) and −427 kJ/mol (RITTMANN and MCCARTY 2001), respectively. Thus, the denitrifying heterotrophs will grow more rapidly when organic carbon is present in combination with ammonium and nitrite, hindering the development of anammox microorganisms.
As in the case of nitrite, there is no consensus in the literature regarding the concentration of organic matter at which denitrifying microorganisms hamper the growth of anammox bacteria. CHAMCHOI et al. (2008) reported that concentrations of organic matter above 300 mg/L (in terms of COD) or COD/N ratios greater than 2 caused the inactivation of anammox organisms in a UASB reactor fed with milk with a high fat content as a source of organic carbon. TANG et al. (2010) reported that the denitrifying microorganisms began to dominate the system when a high COD/NO2-N ratio (2.9:1) was applied. MOLINUEVO et al. (2009) observed complete inhibition of the anammox process when the COD was 292 mg/L.
Since the anammox process removes only 90% of the nitrogen present in the form of ammonium/nitrite and 10% of the nitrogen remains in the effluent in the form of nitrate, the coexistence of anammox and denitrifying organisms is favorable. Under anoxic conditions, nitrate can be reduced to nitrite by denitrifiers, and nitrite can then be used by anammox bacteria for the anaerobic oxidation of ammonium (KUMAR and LIN 2010).
It should be noted that not all types of organic matter can be used in processes in which anammox and denitrifying bacteria coexist. As reported by GÜVEN et al. (2005), the anammox activity is completely and irreversibly inhibited by methanol and ethanol. This aspect needs to be taken into consideration, given that methanol is commonly used to remove nitrate in post-denitrification systems. Inhibition by methanol may be caused by the formation of formaldehyde by the enzyme hydroxylamine oxidoreductase (PAREDES et al. 2007).
On the other hand, some carbon sources do not have an inhibitory effect on the anammox activity. Therefore, they can be used by anammox bacteria. As mentioned in Sect. 5.2.2.2, where the different types of anammox metabolisms were described, some genera of anammox bacteria are able to oxidize acetate and propionate.
Studies regarding the adaptation of anammox bacteria to wastewaters containing toxic components have also been described in the literature. TOH and ASHBOLT (2002) and TOH et al. (2002) observed the acclimation of anammox organisms to a synthetic medium simulating a coke oven effluent, which contained not only a high concentration of organic compounds (COD of 2000–2500 mg/L) but also some chemical compounds such as phenol (300–800 mg/L), cyanides (10–90 mg/L), and thiocyanates (300–500 mg/L). The initial attempt to enrich the anammox bacteria failed, although the gradual addition of 50–500 mg/L of phenol allowed the adaptation of these organisms.
5.2.2.3.4 Temperature and pH
A pH range of 6.7–8.3 is considered to be ideal for anammox bacteria, with an optimum value of 8.0 (STROUS et al. 1999b). The ideal temperature is between 30 and 40 °C (STROUS et al. 1999b; EGLI et al. 2001). Experiments carried out by DOSTA et al. (2008) to evaluate the short-term effect of temperature on the anammox activity showed that the maximum non-adapted anammox biomass activity was obtained at between 35 and 40 °C, while a temperature of 45 °C caused an irreversible decrease in the anammox activity due to cell lysis. Small differences in the optimum temperature were found for K. stuttgartiensis (40 °C) and B. anammoxidans (37 °C) (STROUS et al. 1999b; EGLI et al. 2001).
Although the optimum temperatures for the anammox process are relatively high, CEMA et al. (2007) and ISAKA et al. (2006) managed to operate the anammox process in a rotating biological contactor (RBC) and an anaerobic biofilter, respectively, at a temperature of 20 °C. The gradual adaptation of the biomass appears to be a key factor in successfully operating the anammox process at temperatures lower than those considered ideal for the process (SZATKOWSKA and PLAZA 2006).
In order to start up the anammox system at low temperatures, one strategy is to produce the desired quantity of biomass in a separate reactor, which should be operated at temperatures close to those considered ideal. Later, the biomass can be gradually adapted to lower temperatures in the same reactor, and finally the adapted biomass can be inoculated into the reactor maintained under low temperature conditions (DOSTA et al. 2008).
Some researchers who have carried out studies on samples of anammox bacteria originating from sediments have reported anammox activity at low temperatures, suggesting that local environmental factors influence the characteristics of these bacteria. RYSGAARD et al. (2004) observed anammox activity in artic sediments at a temperature ranging from −1.3 to 30 °C. The optimum temperature was found to be 12 °C. Similar results were obtained by DALSGAARD and THAMDRUP (2002), who observed an optimum temperature of 15 °C for marine sediments from the Baltic Sea.
It should be noted that, in contrast to anammox bacteria in wastewater treatment systems, anammox bacteria in marine environments are dependent on another process to obtain the nitrite required in the process. In marine environments, nitrate is much more abundant than nitrite, and thus the anammox process requires an additional step for the reduction of nitrate to nitrite. Since the dissolved oxygen concentration decreases progressively through the sediment, in the deeper layers, nitrate reducers can cause the accumulation of nitrite, allowing the occurrence of the anammox process (DALSGAARD et al. 2005).
Although the physical properties of sludge and the bacterial populations can remain constant during the reactor operation at lower temperatures, the nitrogen conversion rate is substantially reduced. This drawback can be minimized by applying a strategy described by ISAKA et al. (2006), who achieved a high nitrogen conversion (8.1 kgN/(m3 day)) through reducing the hydraulic retention time (HRT) and adding appropriate and non-inhibiting concentrations of nitrite to the influent.
5.2.2.3.5 Biomass Concentration
The anammox activity is highly influenced by the biomass concentration. According to STROUS et al. (1999b), anammox bacteria are only active when the cell concentrations are greater than 1010–1011 cells/mL, even in highly enriched cultures. It is possible that the presence of contaminant cells, 1 in 200–500, is required to sustain the growth, since these cells could ensure vitamin supplementation and the removal of toxic components (KUENEN and JETTEN 2001).
PYNAERT et al. (2004) described a hypothesis in which the presence of ammonium-oxidizing bacteria is required for the reactivation of anammox organisms after the biological system has undergone some disturbances. Through the production or accumulation of hydroxylamine or hydrazine by the bacteria responsible for the oxidation of ammonium, anammox bacteria can reactive their metabolism. Once the process is reestablished, the tendency is that the ammonium-oxidizing bacteria do not participate in the anammox process. This supposition has also been described by STROUS (2000) based on the fact that the addition of the intermediates hydroxylamide and hydrazine was needed in order to restart the anammox process after its inhibition.
5.2.2.3.6 Suspended Solids
Flocculating agents are generally used to remove organic and inorganic colloidal substances from wastewaters prior to the anammox process. The effect of these flocculants on the anammox process was the focus of a study carried out by DAPENA-MORA et al. (2007). Concentrations of up to 1 g/L of a positively charged polymeric compound used as a flocculant did not have a negative effect on the anammox activity.
In a study carried out by YAMAMOTO et al. (2008), a large amount of influent suspended solids present in partially nitrified digested liquid adhered to the material covering the anammox biomass, which was growing on a support material. Consequently, the anammox activity decreased and the performance was significantly adversely affected. The use of a flocculant improved the settleability of the influent suspended solids and reduced its accumulation in the reactor. However, the flocculant was also retained on the surface of the support media, leading to a reduction in the anammox activity.
The precipitation of salts can also lead to the unstable operation of anammox reactors. TRIGO et al. (2006) operated an anammox reactor with membranes, which functioned as a barrier to retain inorganic salts which precipitated and accumulated in the biomass. The precipitation of these salts on the biomass surface led to a reduction in the nitrogen removal from 100 to 10 mg/(L day).
5.2.2.3.7 Light and Reactor Mixing Velocity
A study carried out by VAN DE GRAAF et al. (1996) indicated that anammox bacteria are sensitive to visible light. These authors observed a decrease in the anammox activity from 30 to 50%. The results influenced the operating conditions and thus the reactors were covered to avoid the negative effect of light. The effect of shear stress on the anammox process was evaluated by ARROJO et al. (2006), who observed that stirring velocity of up to 180 rpm did not have a negative effect on the performance of the anammox process. However, when the stirring speed was increase to 250 rpm, the anammox activity and the average diameter of the flocs were reduced by 40% and 45%, respectively. In addition, an accumulation of nitrite was observed under these conditions.
5.2.2.4 Application of Anammox Process
The anammox process offers several advantages for the removal of ammonium from wastewaters. In conventional systems, ammonium is removed through nitrification (oxidation of ammonium to nitrate) followed by denitrification (reduction of nitrate to nitrogen gas). The anammox process should always be combined with a partial nitritation process, in which half of the ammonium is oxidized to nitrite (and not to nitrate). The nitrite produced reacts with the remaining ammonium to form nitrogen gas. The main advantages of the combined autotrophic process of partial nitritation + anammox are:
-
Lower energy requirement considering the lower aeration costs.
-
An organic carbon source (external oxidant) is not required, which leads to a lower production of sludge with a consequent reduction in the sludge disposal costs.
-
Absence of CO2 emissions due to the autotrophic nature of the nitritation and anammox processes (CO2 is consumed and not produced).
The anammox process is especially appropriate for the treatment of wastewaters containing high concentrations of ammonium but with low organic content. Given that the anammox bacteria are characterized by their interaction with other bacteria, since they require nitrite generated by another microbial group, two main reactor configurations are possible for the removal of ammonium through the anammox process:
-
1.
A system comprised of two reactors (Fig. 5.8a) in which part of the ammonium is firstly oxidized to nitrite (partial nitritation) in the reactor maintained under aeration. Subsequently, the ammonium/nitrite mixture is sent to the second reactor, maintained under anoxic conditions, where it is subjected to the anammox process (VAN DONGEN et al. 2001b). The partial nitritation process can be carried out in a SHARON (single-reactor high-activity ammonia removal over nitrite) system, which will be described in detail below.
Fig. 5.8 Removal of ammonium through the anammox process carried out with two-reactor configurations: (a) two-reactor configuration, nitritation occurs in the first reactor (aerated) and the anammox process in the second reactor (anoxic); (b) both processes occur in the same aerated reactor (adapted from VAN DONGEN et al. 2001b)
-
2.
A configuration comprising only one reactor (Fig. 5.8b), in which partial nitritation and the anammox process occur in the same reactor, maintained under aeration. In this configuration the nitrification occurs in the aerobic region of the floc or granule, while the anammox reaction occurs in the deepest zone of the biofilm, maintained under anoxic conditions (HIPPEN et al. 1997; KUAI and VERSTRAETE 1998). The alternation of aerobic (aeration on) and anoxic (aeration off) periods is another approach to achieving nitritation and anammox reactions in a single reactor.
Currently, the anammox process is employed in both configurations for the removal of ammonium from sludge digester effluents, leachates, and different industrial wastewaters. Besides the application of the anammox process for the removal of ammonium, the combined removal of ammonium and nitrate has also been employed in laboratory scale (KALYUZHNYI et al. 2006; PATHAK et al. 2007). In this process, partial denitrification (reduction of nitrate to nitrite) is coupled with the anammox process. In cases where the electron donors for the denitrification consist of fatty acids, the anammox bacteria are able to carry out the reduction of nitrate (GÜVEN et al. 2005). In the next section, the two systems (two reactors and single reactor) aimed at partial nitritation and anammox will be described, with emphasis on the different types of reactors used and the practical implementation of these systems.
5.2.2.4.1 Partial Nitritation and Anammox in Two Separate Reactors (Two Stages)
The combined process of partial nitritation and anammox in two separate reactors makes use of the advantages of the first process to carry out the conversion of half of the ammonium only up to nitrite (and not up to nitrate), providing the substrates required for the anammox process (ammonium and nitrite) in proportions suitable for the generation of nitrogen gas.
One of the challenges during the operation of the first reactor is obtaining an effluent with an ammonium-to-nitrite ratio similar to the stoichiometric ratio of 1:1.32, proposed by STROUS et al. (1998) to represent the anammox reaction. In practice, however, this ratio should be close to 1:1 in order to prevent inhibition by nitrite and provide an excess of ammonium. Given that there is no need for organic compounds or anoxic periods, a partial nitritation reactor can provide the desired ammonium/nitrite mixture, without the need to control the refeeding. One factor which makes this conversion possible is that after 50% of the ammonium has been oxidized the decrease in the pH hinders the oxidation of the residual ammonium.
By limiting the supply of oxygen in a nitrifying reactor with sludge retention, the same results can be obtained, although control of the refeeding may be required (STROUS et al. 1997). It is important that the composition of the anammox reactor influent remains constant, considering the toxicity of nitrite, regardless of the strategy used to obtain adequate proportions of ammonium and nitrite in the first reactor (partial nitritation).
The application of the two-reactor configuration is particularly appropriate when biodegradable organic compounds and toxic compounds are present, since these are degraded in the step prior to partial nitritation and do not reach the anammox reactor (VÁZQUEZ-PADÍN et al. 2009; LACKNER et al. 2008).
As previously mentioned, nitrogen removal based on partial nitritation with the anammox process offers many advantages. Besides the fact that the external addition of carbon is not required, it generates a low quantity of sludge and requires 40% less oxygen than the conventional process, which leads to energy savings (AHN 2006). Furthermore, the operation of a system comprised of two separate reactors (one for partial nitritation and one for the anammox process) is more flexible in comparison with the configuration in which both processes occur in a single reactor. Additionally, since the two processes occur in different units, the process performance is more stable (WYFFELS et al. 2004). Detailed description of the operating conditions required to achieve partial nitritation and anammox conversions is provided below.
5.2.2.4.1.1 Partial Nitritation
In this section, firstly the factors which affect the nitrification process will be presented, some of which, or their combination, represent the basis of the development of partial nitritation technologies. Several studies on this subject will then be described.
In practice, all of the factors involved in achieving partial nitritation are related to the inhibition or limitation of the second stage of nitrification (nitratation or the formation of nitrate). The crucial point in the control of partial nitrification is to obtain a nitrifying reactor with the stable accumulation of nitrite. In order to force biological processes to follow the nitrite route, different strategies have been used (BERNET et al. 2005), which include controlling the temperature, hydraulic retention time, pH, dissolved oxygen, and presence of free ammonia. Table 5.2 details how these factors influence the growth and activity of the microorganisms responsible for nitrification.
One approach to achieving partial nitrification is based on the difference in the activation energies of ammonium (68 kJ/mol) and nitrite (44 kJ/mol) oxidation. The high activation energy of the ammonium oxidation reaction leads to the velocity of this process having a greater degree of dependence on the temperature in comparison with the nitrite oxidation reaction. Only at temperatures above 25 °C is it possible for ammonium-oxidizing bacteria to become dominant to the detriment of nitrite-oxidizing bacteria (VAN DONGEN et al. 2001a; BROUWER et al. 1996). If this condition is combined with a low hydraulic retention time and low cell retention time, the bacteria which oxidize nitrite can be selectively washed out of the system (HELLINGA et al. 1998).
The pH has a strong influence on the system due to the fact that at low values of this parameter the nitrite-oxidizing bacteria grow more rapidly than the ammonium-oxidizing bacteria. Thus, the hydraulic retention times (or dilution rate) required to maintain the ammonium oxidizers and wash out the nitrite oxidizers are more flexible at higher pH values (HELLINGA et al. 1998).
In relation to the pH ranges considered ideal for nitrification, some main effects of this parameter on nitrifying bacteria have been identified: activation/deactivation of nitrifying bacteria, nutritional effects associated with the alkalinity and inorganic carbon species, and inhibition by ammonium and nitrous acid (VILLAVERDE et al. 1997). The activation/deactivation of nitrifying bacteria is related to the binding of H+ or OH− ions to enzyme groups, blocking the active sites in a reversible manner (QUINLAN 1984). Nutritional effects are mainly associated with the availability of inorganic carbon, which is essential for autotrophic nitrifying microorganisms. At low pH values, the CO2 species predominate which can be easily removed from water by way of stripping. On the other hand, at high pH values, inorganic carbon is present mainly in the form of carbonate, which is rarely assimilated.
The presence of free ammonia and nitrous acid is strongly associated with the pH value of the medium. The pH affects the substrate concentration in both stages of nitrification, modifying the acid-base equilibrium. With an increase in the pH, for instance, greater concentrations of free ammonia are present in the medium, which can inhibit both the ammonium-oxidizing bacteria and the nitrite-oxidizing bacteria. A reduction in the pH, however, favors the presence of nitrous acid. Both the free ammonia and the nitrous acid can inhibit ammonium-oxidizing and nitrite-oxidizing microorganisms, although the latter are more sensitive than the former, especially in the presence of free ammonia.
Another strategy aimed at avoiding the development of nitrite-oxidizing bacteria and promoting the accumulation of nitrite is to reduce the concentration of dissolved oxygen in the medium. This approach is based on the fact that nitrite-oxidizing bacteria are more sensitive to low dissolved oxygen concentrations in the medium than ammonium-oxidizing bacteria, which have less affinity for oxygen. The oxygen saturation coefficients (Monod kinetics) for the oxidation of ammonium and nitrite are 0.3 and 1.1 mg/L, respectively (WEISMANN 1994).
A possible mechanism for the inhibition of nitrite oxidation caused by low oxygen concentrations is based on the accumulation of hydroxylamine, an intermediate product in ammonium oxidation. In general, ammonium-oxidizing bacteria obtain energy by way of ammonium oxidation to nitrite in a two-step reaction, with hydroxylamine (NH2OH) as an intermediate. The first step is the oxidation of ammonium, catalyzed by the enzyme ammonia monooxygenase, while the second step involves the oxidation of hydroxylamine, catalyzed by the enzyme hydroxylamine oxidoreductase. At low oxygen concentrations and high ammonium concentrations, the accumulation of hydroxylamine can occur, which can inhibit the nitrite-oxidizing microorganisms starting from concentrations of 250 μM. Concentrations above 2000 μM can also lead to the inhibition of ammonium-oxidizing bacteria. Equations 5.11–5.13 represent the accumulation of hydroxylamine under oxygen-limiting conditions (YANG 1990).
From these equations it can be observed that the four electrons generated from the oxidation of hydroxylamine can be transferred to the oxidation of ammonium when the reduction of the terminal oxygen is interrupted, due to the oxygen deficiency aimed at balancing the number of electrons of this redox reaction (YANG 1990). In a study carried out by HU (1990), the hydroxylamine caused a severe inhibition of Nitrobacter, suggesting that nitrite may be accumulated in nitrifying systems. However, hydroxylamine has been practically ignored in terms of nitrification processes, since its concentration is considered to be insignificant.
YANG and ALLEMAN (1992) studied the possibility of hydroxylamine accumulation and its relation to the accumulation of nitrite in a batch system containing a nitrifier-enriched culture. The results obtained by these authors indicated that the quantity of nitrite accumulated increased with an increase in pH, and this was directly related to an increase in the non-ionized hydroxylamine. Hydroxylamine was considered to be the main cause of nitrite accumulation in a nitrifying system maintained under conditions of low dissolved oxygen concentrations and high pH.
Several studies aimed at obtaining nitrification through controlling the dissolved oxygen have been carried out, both in suspended biomass and biofilm systems (Table 5.3). In the case of systems with suspended biomass operated under oxygen-limiting conditions, the complete and stable conversion of ammonium into nitrite was obtained, regardless of the sludge age. However, the sludge age became a critical parameter for obtaining partial nitrification when the operation was not carried out under oxygen-limiting conditions. YANG and ALLEMANN (1992) concluded that a combination of parameters, such as the concentrations of free ammonia, dissolved oxygen, and hydroxylamine, comprise the main factors involved in the accumulation of nitrite in a batch system containing a nitrifier-enriched culture.
As indicated in Table 5.3, the results obtained in biofilm systems are similar to those obtained in reactors with suspended biomass. In general, low oxygen concentrations lead to an accumulation of nitrite. However, in biofilm systems, the nitrite-oxidizing bacteria can be further adversely affected by the actual stratification of the biofilm. In most cases, ammonium-oxidizing bacteria are located in the most external regions of the biofilm, while the nitrite oxidizers are found in a deeper layer (KIM et al. 2003). This spatial distribution means that the nitrite-oxidizing bacteria are more exposed to oxygen-limiting conditions compared with the ammonium oxidizers.
The main reactors used to date to obtain partial nitritation are the continuous stirred-tank reactor (CSTR), membrane bioreactors (MBRs), and sequencing batch reactors (SBRs). In the MBR and SBR, high cell retention times (50–75 days) can be obtained (STROUS et al. 1997). In MBR systems and in other biofilm systems, the cell retention time is difficult to control, in contrast with reactors in which the biomass grows in suspension. Thus, it is difficult to force the washout of nitrite-oxidizing bacteria even under oxygen-limiting conditions (XUE et al. 2009) and the production of nitrite without the accumulation of nitrate may not be achieved (FUX et al. 2004).
In some cases, even applying criteria which favor the selection of ammonium-oxidizing bacteria over nitrite-oxidizing bacteria, such as a high concentration of free ammonia, low oxygen concentration, and a high ammonium load, the suppression of nitrite oxidizers is difficult. Thus, the use of reactor configurations such as CSTR and SBR with suspended biomass is recommended, particularly for operation in real scale. In reactors operated in continuous mode (e.g., CSTR), the criterion for the selection of ammonium-oxidizing bacteria is the hydraulic retention time. In batch reactors (e.g., SBR), the sludge age is the controlling factor regarding the nature of the dominant microbial populations.
The possibility of obtaining an effluent from the partial nitritation process which is ideal for subsequent treatment in an anammox reactor was initially tested by VAN DONGEN et al. (2001a), in a process known as SHARON (single-reactor high-activity ammonia removal over nitrite).
Although this process is not adequate for the treatment of all types of wastewaters, due to its strong dependence on temperature, it is ideal for the removal of nitrogen from high-strength nitrogen wastewater (effluent from sludge digesters, landfill leachates, wastewater from composting processes, and liquid from the sludge drying process), which would consume enormous quantities of dissolved oxygen in the conventional nitrification process (AHN 2006).
In many cases the SHARON process can function as a pretreatment, applied to substantially reduce the ammonium concentration, thus allowing the application of a subsequent conventional system for the final polishing of the wastewater (HELLINGA et al. 1998; MULDER and KEMPEN 1997). In this case, the application of the SHARON system as a sidestream process can be evaluated in terms of load removed and not in terms of effluent quality, since the effluent of the SHARON reactor will later be discharged to the main treatment plant (VAN LOOSDRECHT 2008).
The SHARON process makes use of the different growth rates of ammonium-oxidizing and nitrite-oxidizing bacteria at sufficiently high temperatures (above 26 °C). Thus, it is associated with the selection of ammonium-oxidizing bacteria (from an inoculum originating from a system in which nitrification occurs) in a continuous reactor operating with high specific feed flows. The process conditions are unfavorable for the bacteria responsible for nitrite oxidation and can promote their washout (SCHMIDT et al. 2003; MULDER and VAN KEMPEN 1997).
In the original proposal, operation of a single-stage system using intermittent aeration was envisioned. During the periods in which the reactor is aerated, reduction in the pH with the generation of nitrite (nitritation) is observed. On the other hand, in the periods without aeration, anoxic conditions are established, an external organic carbon source is supplied, and nitrite can be converted to nitrogen gas (denitritation). The latter step would cause an increase in the pH and the production of alkalinity, compensating for the acidifying effect of nitrification. Moreover, the denitritation step is responsible to prevent accumulation of nitrite, which is inhibitory to ammonium oxidizers. Thus, the sequential aerated and anoxic periods were defined as a function of the pH limit values stipulated a priori. Alternatively to the single-stage configuration, partial nitrification and denitritation may be carried out in two separate tanks (two-stage configuration) to decrease the aeration capacity. The nitritation-denitritation (SHARON) process is represented in Eqs. 5.4 and 5.5.
The SHARON process can also be followed by the anammox process, a combination referred to as SHARON-anammox process. In this case, only partial nitritation should be achieved in the SHARON reactor in order to obtain an effluent with NH4 +/NO2 − ratio close to 1, which is suitable for the anammox process. In this case, no organic carbon is required while sludge production is low. To reach the desired NH4 +/NO2 − ratio of 1, the alkalinity of the wastewater is an important factor to be controlled. Depending on this parameter, the SHARON reaction can convert a fraction or the entire ammonium load to nitrite. Considering that the oxidation of 1 mol of ammonium to nitrite consumes 2 mol of bicarbonate and given that this process is practically interrupted at pH values below 6.5, with an ammonium-to-bicarbonate molar ratio of 1:1, approximately 50% of the ammonium is converted to nitrite, the rest remaining in the form of ammonium.
Conversely, the accurate control of alkalinity does not have great importance in cases where a SHARON system achieves complete ammonium oxidation to nitrite to be followed by heterotrophic denitrification via nitrite (denitritation) (VAN DONGEN et al. 2001a; HELLINGA et al. 1998).
The SHARON process is operated with hydraulic retention times which are higher than the growth rate of the nitrite oxidizers, although not as long as that of the ammonium oxidizers. In general, nitritation is carried out without sludge retention, with a hydraulic retention time (HRT) of 1 day, within a temperature range of 30–40 °C and with pH values of 6.6–7. Under these conditions the nitrification process is stable, with nitrite being the final product (AHN 2006).
Since this process is conducted mainly in continuous systems and consequently without sludge retention (hydraulic retention time = cell retention time), the dilution rate (specific feed flow) must be determined in such a way that the ammonium-oxidizing organisms are able to grow sufficiently to remain in the reactor, while the nitrite oxidizers are washed out.
The SHARON process can also be operated with sludge retention. In this case the aeration time will be the limiting factor for the reactor design due to the greater quantity of oxygen required. The economic balance, taking into consideration the reactor volume and the sludge retention equipment, determines the appropriate choice for the application of the SHARON system with sludge retention. In practice, when the nitrogen concentration is above 0.4–0.5 gN/L, a system without biomass retention is cheaper. Also, a system operated without sludge retention requires less maintenance. The sludge produced in the SHARON reactor will leave with the effluent, which does not represent a problem since the effluent of this process will be sent to the influent of the main treatment plant.
As briefly noted, the SHARON process is not suitable for all types of wastewater since it is dependent on high temperatures. However, for the treatment of sludge digester effluents, the SHARON process is ideal, since the temperature of these effluents varies between 20 and 35 °C, permitting reactor operation at reduced sludge retention times. The absence of sludge retention and the fixed hydraulic residence time, common characteristics of the SHARON process, mean that the volumetric nitrogen load applied is dependent on the ammonium concentration of the influent. Therefore, the process cost is also dependent on the ammonium influent concentration, with the increase in the operation cost being directly proportional to the decrease in this concentration. The composition of the effluent of the SHARON process is also dependent on the rate of bacterial growth involved, which, in turn, varies according to the ammonium concentration of the influent (VAN DONGEN et al. 2001a).
Aeration is required not only to provide oxygen but also for the stripping of CO2 in the reactor and to control the pH. Nitrite can be reduced to nitrogen gas through denitritation using organic compounds (e.g., methanol) as electron donors, added periodically while the aeration is deactivated (SCHMIDT et al. 2003), or by applying the anammox process.
VAN HULLE et al. ( 2005 ) described the start-up of a laboratory-scale SHARON reactor operated at 35 °C without pH control. The effluent of the SHARON reactor was found to be suitable for feeding to the anammox process when the influent of the process consisted of synthetic wastewater containing an ammonium load of 1.5 kgN/m3 day.
FUX et al. (2002) operated a 2.1 m3 CSTR reactor in Zurich (Switzerland), which was submitted to an HRT of 1.1 days at 30 °C, without pH control. The reactor was fed with sludge digester effluents from two different treatment plants, and it was possible to obtain an ammonium-to-nitrite ratio of 1:1.32 at pH values of between pH 6.6 and 7.2.
The SHARON technology is currently used successfully in real scale to treat effluents from sludge digesters, removing high amounts of ammonium from those streams. Working large-scale SHARON reactors can be found in many wastewater treatment plants, such as those located in Rotterdam and Utrecht (the Netherlands), both representing the first full-scale demonstrations of the process (MULDER et al. 2001). Many other plants have been installed and are in operation in the Netherlands and other countries. The applied nitrogen load usually varies between 400 and 2500 kgN/day (VAN LOOSDRECHT and SALEM, 2005). Due to the high temperature requirement, spread of the technology in tropical countries is foreseen.
Despite its successful application in real scale, there are some disadvantages associated with the SHARON process. The HRT of sludge digesters is high, which guarantees the stable composition of their effluents to be subsequently fed to a SHARON reactor. The digester effluent is characterized by a high concentration of nitrogen and low content of biodegradable organic compounds. When the HRT of the digesters is shorter than normal or when industrial wastewaters are treated, fluctuations in the composition of the effluent fed to the SHARON process are likely to occur. Thus, some parameters of this process, such as the dissolved oxygen and pH, need to be controlled in the SHARON process in order to obtain the ideal ammonium-to-nitrite ratio for the subsequent anammox process (VOLCKE et al., 2006).
Another disadvantage of the SHARON process is the fact that the maximum volumetric capacity is limited, since the biomass is constantly removed from the system. In order to ensure stable conditions, the minimum HRT of a chemostat is limited to 1.0–1.2 days. In an MBR, SBR, or biofilm systems, the biomass is retained more easily, allowing the HRT to be independent of the cell retention time. In these systems HRT of less than 1 day can be applied, which results in greater volumetric capacities (WYFFELS et al. 2004).
As mentioned above, the temperature is crucial to obtaining good performance in a SHARON reactor. In general, this process is operated at relatively high temperatures (above 26 °C) and low sludge retention time (less than 2 days), a condition under which AOB are at an advantage over nitrite oxidizers and remain inside the reactor system. On the other hand, NOB are potentially washed out. However, when the influent of the SHARON process (generally effluent from sludge digesters) is below 24 °C, the maximum growth rate of the organisms which oxidize ammonium becomes lower than that of the nitrite oxidizers, and thus the nitrite formed is converted to nitrate (FUX et al. 2002).
Taking into consideration that the use of temperature as a selection criterion for the dominant microbial populations is not very favorable in economic terms, and given the difficulty associated with modifying and controlling this parameter in large-scale reactors, other strategies are needed in order to achieve partial nitritation at temperatures of less than 24 °C. One such approach is the inhibition of nitrite-oxidizing bacteria using free ammonia or nitrous acid and another is maintaining the reactor under oxygen-limiting conditions.
Free ammonia and nitrous acid are two potential inhibitors of the nitrifying process. Nitrite-oxidizing bacteria are the most strongly affected since relatively low concentrations are sufficient to promote their inhibition. ABELING and SEYFRIED (1992) reported that free ammonia concentrations can inhibit nitratation without affecting nitritation. The maximum specific rate of nitrite generation and minimum generation of nitrate are obtained with a free ammonia concentration of 5 mg/L, under pH and temperature conditions of 8.5 and 20 °C, respectively.
MAURET et al. (1996) reported that the inhibition of nitrite-oxidizing bacteria can be achieved with free ammonia concentrations in the range of 6.6–8.9 mgN/L. BALMELLE et al. (1992) observed that even at concentrations of only 1 mgN/L free ammonia can inhibit the nitratation stage. GANIGUÉ et al. (2007) showed that it is possible to obtain stable influent for the anammox process during leachate treatment in a sequencing batch reactor. At low pH values, the microbial activity is decreased due to the inhibitory effect of nitrous acid, resulting from the lack of alkalinity (bicarbonate). High pH values indicated a decrease in the oxygen consumption rate due to inhibition by free ammonia. The authors concluded that the pH controlled the partial nitritation process.
On applying partial nitritation followed by the anammox process for the treatment of effluent from an anaerobic digester, YAMAMOTO et al. (2008) observed stable conversion of ammonium to nitrite equivalent to 58%. The authors attributed this result to the inhibition promoted by free ammonia and nitrous acid. It should be noted that the strategy to control the action of bacteria only through the pH and the presence of free ammonia may not provide the desired success (RUIZ et al. 2003; SCHMIDELL and REGINATTO 2005), since the limit of the free ammonia concentration able to promote inhibition of the nitrite oxidation step may increase over time.
Some researchers have reported the possibility for the adaptation of nitrite-oxidizing bacteria to occur even at high free ammonia concentrations. WONG-CHONG and LOEHR (1978) observed that pure cultures of Nitrobacter acclimatized to free ammonia were able to tolerate up to 40 mgNH3-N-L, while non-acclimatized cultures were inhibited at concentrations equivalent to 3.5 mgNH3-N/L. Other studies on systems with biofilm or biomass in suspension have shown that nitrite-oxidizing organisms can adapt to high concentrations of free ammonia, observing that within a certain period (from 6 to 12 months) the nitrite accumulation decreases and the nitrate concentration increases (FUX et al. 2004; VILLAVERDE et al. 2000).
In order to investigate the accumulation of nitrite using the strategy of maintaining low oxygen levels, WYFFELS et al. (2003), operating a continuous reactor using membranes for total recycling of cells, reached 50% conversion of ammonium to nitrite when the system was submitted to a dissolved oxygen concentration of 0.1 mg/L, at 35 °C and pH 7.9.The complete conversion of ammonium to nitrite was observed when the concentration of oxygen was approximately 0.25 mg/L, a situation in which around 800 mgNO2 −-N/L was reached. TURK and MAVINIC (1987) observed that cells acclimatized under anoxic conditions were able to provide long periods of nitrite accumulation (up to hours), even under aeration conditions. The results obtained by these authors highlighted the possibility of intercalating periods of aeration with periods without aeration, obtaining the desired accumulation of nitrite, without generating considerable quantities of nitrate. This strategy provides greater simplicity in comparison with the use of refined systems to control the oxygen concentration at very low values. In addition, the use of alternating periods with and without aeration to limit the ammonium oxidation only up to nitrite favors the application of simultaneous ammonium and nitrite removal processes, such as the anammox process (MULDER et al. 1995; KUAI and VERSTRAETE 1998).
RUIZ et al. (2003) determined the best conditions for partial nitrification, with the accumulation with nitrite, in a synthetic wastewater with a high ammonium concentration, aimed at decreasing the total amount of oxygen required for the nitrification stage. On operating an activated sludge reactor in laboratory scale, they selected the pH and DO as the operation parameters to evaluate the possibility for nitrite accumulation, without affecting the overall ammonium removal. According to the authors, the pH was not an ideal parameter for promoting nitrite accumulation, considering that when this parameter had values in the range of 6.45–8.95 complete nitrification up to nitrate occurred in the system and at pH values below 6.45 and above 8.85 complete inhibition of the nitrification process occurred. In contrast, when the DO concentration in the reactor was maintained at 0.7 mg/L, it was possible to accumulate over 65% of the ammonium in the form of nitrite with an ammonium conversion of 98%. At DO concentrations below 0.5 mg/L, there was the accumulation of ammonium and at concentrations above 1.7 mg/L, complete nitrification up to nitrate was reached (RUIZ et al. 2003).
WYFFELS et al. (2004) used a membrane bioreactor as the first stage of the autotrophic nitrogen removal process, which was submitted to dissolved oxygen concentrations below 0.1 mg/L. The pH was maintained at 7.9 and the temperature at 35 °C. A reduction in the temperature did not have a significant effect on the ammonium-to-nitrite ratio obtained. In addition, a reduction in the ammonium concentration (which probably contributed to a decrease in the inhibitory effect on the nitrite oxidizers) did not alter the ammonium-to-nitrite ratio obtained. These results indicated that the oxygen limitation was the main operational factor which determined the ammonium-to-nitrite ratio obtained.
In general, the different strategies used in the studies described above to obtain the desired partial nitritation and produce an influent which is ideal for the anammox process can be summarized as follows:
-
Operation of the reactor at low dissolved oxygen concentrations (less than 0.5 mg/L)
-
Operation of the reactor at high pH values (7.5–8.5), resulting in an increase in the free ammonia concentration and a decrease in the nitrous acid concentration
-
Operation of the reactor at high temperature (above 25 °C)
-
Operation of the reactor with limited nitrification, which stops the ammonium oxidation before its complete conversion
5.2.2.4.1.2 Anammox
The application of the anammox process is still limited, which is mainly due to the long start-up of the process (up to one year), which is associated with the very low growth rates and low cellular yield of the anammox organisms. The frequent loss of biomass with the effluent increases the start-up period of anammox reactors. Consequently, the use of systems with a good biomass retention capacity is crucial to the success of the process. The cultivation of slow-growing bacteria, such as anammox organisms, is based on the ability of the biomass to form biofilms or aggregates such as flocs or granules (VAN DER STAR 2008).
Several different reactor configurations have been used for the enrichment of anammox bacteria. These include fixed-bed reactors, fluidized-bed reactors, UASB reactors, sequencing batch reactors (SBRs), and air-lift reactors (WYFFELS et al. 2004; STROUS et al. 2002). Of these, the SBRs have been the preferred choice due to their operational simplicity and because of their efficient biomass retention, homogeneous mixture inside the reactor, stability and reliability during long periods of operation, stability under limited-substrate conditions, and high nitrogen conversions (JETTEN et al. 1999; STROUS et al. 1998).
STROUS et al. (1997) initiated the operation of the anammox process in a fixed-bed reactor and in a fluidized-bed reactor with glass and sand particles acting as support material. The authors did not manage to avoid the biomass loss due to sludge flotation caused by gas bubbles. The same situation was observed by DAPENA-MORA et al. (2004) in an air-lift reactor. These authors observed that mechanical mixing in an SBR can be more effective for the elimination of the gas which penetrates the granules compared with the air-lift reactor.
In order to obtain the complete retention of the biomass in anammox systems, membrane bioreactors can be used. In contrast to reactors with granular biomass, the MBR allows the cultivation of slow-growing bacteria with very good biomass retention and does not require biomass with good settling characteristics. Therefore, this represents a good option to obtain anammox cells in suspension.
WANG et al. (2009) used a mixer in an MBR to promote the formation of free anammox cells and obtained a more homogeneous distribution of the substrate and biomass. However, for real-scale applications, reactors with biofilm or granular sludge are preferred in comparison with MBRs since the anammox bacteria easily form granules or biofilms, allowing obtaining a high biomass concentration in a simple and economic manner. Also, the fouling of the membrane is one of the disadvantages of MBR systems. The operating costs associated with the cleaning of the membrane (backwashing or the addition of chemical products) reduce the economic feasibility of the process (TRIGO et al. 2006). Another important point is the fact that wastewaters generally contain a certain quantity of solids, which will also be retained by the membrane. The accumulation of this material also promotes a reduction in the activity of the anammox organisms in the MBR system (YAMAMOTO et al. 2008).
As previously mentioned, the start-up period of anammox systems is one of the disadvantages of the process. Ensuring conditions in which oxygen is absent is essential during the start-up period. In general, this period is characterized by a gradual increase in the nitrite concentration. Although the ideal ammonium-to-nitrite ratio is around 1:1, an excess of ammonium is generally used, leading to a reduced efficiency in terms of the overall nitrogen removal, although ensuring greater process stability.
In order to decrease the start-up time of the anammox process, the system can be inoculated with biomass originating from another anammox reactor. The start-up period of an SBR reactor operated by SLIEKERS et al. (2003) was only 1 day, since the reactor was inoculated with highly active anammox biomass. The continuous addition of pre-enriched anammox biomass was used in the Netherlands to start up a real-scale reactor of 70 m3. The start-up period of the reactor was around 3.5 years, and currently it provides stable operation with a nitrogen removal rate of 9.5 kgN/(m3 day) (VAN DER STAR et al. 2007).
A variation of the anammox process named DEAMOX (denitrifying ammonium oxidation) has been recently tested in laboratory scale. This process is based on the combination of an anammox reaction and autotrophic denitrification using sulfide as the electron donor for the production of nitrite from nitrate in an anaerobic biofilm (KALYUZHNYI et al. 2006). This process will be detailed further.
Table 5.4 details several studies described in the literature involving the autotrophic removal of nitrogen in two systems, including the anammox process. As can be observed, different reactor configurations were employed. The removal of nitrogen varies between systems, which is also related to the different nitrogen loads applied. The abovementioned systems promote efficient biomass retention aimed at counterbalancing the low cellular yield of anammox bacteria. The highest nitrogen removal rate (8.9 kgN/m3 day) was reported by SLIEKERS et al. (2002), who operated a laboratory-scale air-lift reactor containing granular sludge.
5.2.2.4.2 Partial Nitritation and Anammox in a Single Reactor (One Stage)
In theory, the combination of ammonium oxidation and denitrification can be carried out in biofilm systems submitted to low oxygen concentrations. However, in the conventional denitrification process, the organic carbon source can become a limiting factor since the electron donor for denitrification is more rapidly oxidized than the ammonium.
When ammonium acts as the electron donor (as in the case of the anammox process), this problem does not occur (VAN LOOSDRECHT et al. 2004). The combination of partial nitritation and the anammox reaction (Eqs. 5.4 and 5.8) in a single reactor implies that the aerobic autotrophic microorganisms responsible for the partial nitritation (ammonium-oxidizing bacteria) and the anammox microorganisms act in cooperation throughout the process, allowing sequencing reactions to occur simultaneously (AHN 2006). Although the operational controls needed for one-stage partial-nitritation-anammox systems are similar to those required by two-stage processes, the former require more sensitive controls in terms of dissolved oxygen, nitrogen load, temperature, and biofilm thickness. The oxygen concentration, in particular, is a crucial parameter which needs to be controlled.
The nitrifying microorganisms are responsible for the oxidation of ammonium to nitrite, consuming a large part of the oxygen and creating the anoxic conditions essential for the anammox process to occur, in which ammonium and nitrite (generated in the partial nitritation) are converted to nitrogen gas. So, the cooperation of AOB and anammox organisms is critical for successful operation of single-stage partial nitritation and anammox processes.
In general, the oxygen concentration needs to be low for two main reasons: (1) to avoid the inhibition of anammox bacteria, which are reversibly inhibited by oxygen, and (2) to obtain operating conditions under which it is possible to carry out partial nitritation (STROUS et al. 1997), hindering the growth of nitrite-oxidizing bacteria. NOB have a lower affinity for oxygen than AOB and also a lower affinity for nitrite than anammox bacteria (HANAKI et al. 1990). Therefore, under limiting oxygen conditions, both AOB and anammox are favored over NOB, and partial nitritation and anammox reactions can be achieved in single-stage units.
Specific operating conditions aimed at partial nitritation have been previously presented. The strategy for obtaining washout of the nitrite-oxidizing bacteria through the application of specific feed flows cannot be applied in partial nitritation and anammox systems in a single reactor. A representation of the autotrophic removal of nitrogen in biofilm systems is given in Fig. 5.9.
Schematic diagram of the autotrophic nitrogen removal in a biofilm process (adapted from VAN LOOSDRECHT 2008)
It should be noted that the dissolved oxygen concentration, a key parameter of the combined processes of partial nitritation and anammox in a single reactor, is related to the biofilm thickness. For a certain surface load of ammonium and under low temperature conditions, a thick biofilm is required. Consequently, the concentration of dissolved oxygen needs to be higher. On the other hand, a thin biofilm requires less dissolved oxygen and, in this case, high oxygen concentrations will lead to complete nitrification and lower nitrogen removal (HAO et al. 2002; KOCH et al. 2000).
The lower the applied nitrogen load, the lower the dissolved oxygen concentration needed to obtain partial nitrification will be. In this context, it is clear that considering only the dissolved oxygen in order to obtain the accumulation of nitrite is not sufficient, since other factors are involved, such as the biofilm thickness and nitrogen load applied. Figure 5.10 shows the dynamics of the nitrite/nitrate formation in the presence of different dissolved oxygen concentrations in a biofilm system, observed experimentally by GARRIDO et al. (1997) and theoretically explained by PICIOREANU et al. (1997).
Effect of dissolved oxygen concentration on nitrite accumulation in a biofilm system (adapted from VAN LOOSDRECHT 2008)
Two main strategies can be used to start up a system aimed at the autotrophic removal of nitrogen in a single reactor. The first consists of inoculating a nitrifying biomass into an anammox reactor which is operating in a satisfactory manner and supplying aeration to the reactor in order to maintain micro-aerobic conditions. The second strategy is based on the operation of a reactor aimed at partial nitritation under limited-oxygen conditions in order to obtain an ammonium-to-nitrite ratio of 1:1. Later anammox biomass is inoculated (PYNAERT et al. 2004; GONG et al. 2007).
The high nitrifying activity can protect anammox bacteria from oxygen, besides providing nitrite. Also, on inoculating the partial nitritation reactor with a biomass enriched with anammox bacteria, the start-up process for autotrophic removal of nitrogen in a single reactor is accelerated, and considerable nitrogen removal can be obtained within 1 or 2 months. If the reactor is not inoculated with an anammox biomass, a period of several months or even years may be required in order to achieve significant results for nitrogen removal.
The second strategy based on a single reactor for the autotrophic removal of nitrogen is known as the CANON (completely autotrophic nitrogen removal over nitrite) process, and this appears to be more suitable given the considerable reduction in the anammox activity when the first strategy is applied (SLIEKERS et al. 2002, 2003; LIU et al. 2008). Moreover, only a small amount of anammox biomass is required for the start-up of the CANON process. Further details about the CANON process (e.g., representative equation) can be found below.
The single-stage process is generally associated with a higher volumetric nitrogen removal rate and lower investment cost when compared with the two-stage configuration, since an additional reactor for the partial nitritation is not needed (WYFFELS et al. 2004). Nevertheless, the difficulty related to regulating the dissolved oxygen concentration and the incomplete removal of nitrogen during the treatment of highly concentrated wastewaters are some of the problems encountered when using a single-reactor configuration (HAO et al. 2001; NIELSEN et al. 2005).
Some mathematical models have been developed to understand and predict the behavior of systems under different operating conditions and the effect of the ammonium surface load. The main results obtained have shown that the ammonium load is associated with the biofilm thickness, a thin biofilm having a limited anammox activity, and the stable formation of nitrite is a limiting factor. On the other hand, the anammox process can occur in biofilm systems, although the time rather than the nitrogen load is the key factor in the process. In these systems, it has been predicted that a period of between 5 and 10 years is required to achieve an anammox population which allows the maximum conversion rates to be reached (VAN LOOSDRECHT et al. 2004).
Several types of reactors have been employed to carry out the combined partial nitritation and anammox process in a single reactor. These include sequencing batch reactors, rotating biological contactors, moving bed biofilm reactors, and air-lift reactors. Table 5.5 details the results of some studies with these reactors.
Initially, the best configuration for obtaining an efficient retention time for the slow-growing autotrophic biomass appears to be the sequencing batch reactors, which allow the biomass to be maintained in the reactor through applying alternating reaction/settling phases. However, high nitrogen removal rates were also obtained in other types of reactors, such as rotating biological contactors (PYNAERT et al. 2003, 2004) and air-lift reactors (SLIEKERS et al. 2003).
In biofilm reactors or granular sludge reactors, the microorganisms which oxidize ammonium are active in the external regions of the biofilm (or granule), producing an appropriate quantity of nitrite for the anammox organisms, which are located in inner layers. Thus, the anammox bacteria are protected from the oxygen, which is consumed by AOB in the external biofilm (granule) layers (WYFFELS et al. 2004).
A variation of the conventional biofilm reactors is the membrane reactors (GONG et al. 2007), in which hydrophobic membranes, permeable to gases, are used for the transfer of oxygen. In the region close to the membranes, dissolved oxygen is present, and this is where the ammonium-oxidizing bacteria convert ammonium to nitrite. On the other hand, anammox bacteria are active in the region rich in ammonium, close to the liquid phase.
When systems with biofilm or granules are used in the partial nitritation-anammox process, the resistance to mass transfer is generally the limiting stage. When the ammonium concentration in the external region of the biofilm is much higher than the oxygen or nitrite concentration, the diffusion of ammonium to the interior of the biofilm will not limit the speed of the process. If the nitrite produced in the external region is mainly consumed in the internal region, oxygen will become the main limiting factor of the overall process.
SZATKOWSKA et al. (2007) reported that the oxygen transfer was the limiting factor of the process in a pilot-scale moving bed reactor. The same finding was reported by SLIEKERS et al. (2003) for a laboratory-scale air-lift reactor. The oxygen limitation can be attributed to slow oxygen diffusion to the interior of the biofilm/granule or inefficient transfer at the gas-liquid interface.
As mentioned in Sect. 5.2.2.3, nitrite is a potential inhibitor of the anammox process. If it is consumed in the same proportion in which it is produced, its inhibitory effect is not significant. In addition, in situations in which the nitrite concentration is high, a negative effect on the anammox bacteria is not always observed. This observation was noted by VÁZQUEZ-PADÍN et al. (2009), who recorded nitrite concentrations of the order of 25 mgN/L. The presence of a concentration gradient inside the granules, where the anammox bacteria are located, probably results in lower nitrite concentrations in these regions, hindering their inhibitory effect.
Several names have been used for systems in which nitritation-anammox is carried out in a single reactor: CANON (completely autotrophic nitrogen removal over nitrite) process (THIRD et al. 2001), OLAND (oxygen-limited autotrophic nitrification and denitrification) process (KUAI and VERSTRAETE 1998), aerobic/anoxic DEMON (deammonification) process (HIPPEN et al. 1997; WETT 2007), and SNAP (single-stage nitrogen removal using anammox and partial nitritation) (FURUKAWA et al. 2006).
The different ways of referring to the same process arose from the fact that different research groups primarily attributed the anaerobic oxidation of ammonium to different microorganisms. For instance, when the OLAND process was developed, the organisms considered to be responsible for the anaerobic oxidation of ammonium under micro-aerobic conditions were the nitrifying bacteria (KUAI and VERSTRAETE 1998; HELMER et al. 1999). In the case of the CANON process, the anammox bacteria were assumed to be responsible for this conversion. Studies using the fluorescent in situ hybridization (FISH) technique have confirmed that the anaerobic oxidation of ammonium, in all reactors, was carried out by anammox organisms (PYNAERT et al. 2003; HELMER-MADHOK et al. 2002), although PYNAERT et al. (2003) do not exclude a specific function associated with the ammonium-oxidizing microorganisms.
5.2.2.4.2.1 CANON Process
The CANON process is essentially an integration of the combined SHARON-anammox process into a single reactor and can be represented by Eq. 5.14 (SLIEKERS et al. 2003). The scheme of the process is displayed in Fig. 5.12d.
In most of the studies on the CANON process reported in the literature, the system was operated in the temperature range of 30–35 °C, with a maximum nitrogen removal rate in the range of 0.075–1.5 kgN/m3 day (SLIEKERS et al. 2002, 2003). In this temperature range, the bacteria which oxidize ammonium grow more rapidly than those responsible for the nitrite oxidation. In addition, the growth of the anammox microorganisms is stimulated under these temperature conditions.
However, there are studies reported in the literature in which a significant removal of nitrogen (0.5 kgN/m3 day) was obtained at temperatures of between 20 and 24 °C, such as that carried out by VÁZQUEZ-PADÍN et al. (2009) in a sequencing batch reactor. The difference is that in this system low activity of the nitrite-oxidizing bacteria was observed. The possibility of obtaining a short start-up period and high nitrogen removal rate in autotrophic nitrogen removal systems at temperatures of around 20 °C has been reported by PYNAERT et al. (2004) in a single-stage system and by DOSTA et al. (2008) and ISAKA et al. (2006) in two-stage systems.
SLIEKERS et al. (2002) applied a specific strategy to the start-up of CANON reactors. These authors used a biomass enriched with anammox bacteria as the inoculum (80% of bacterial population), this stage being following by the supply of oxygen for the development of nitrifying microorganisms. The operation was carried out at 30 °C, and pH 7.8, with mixing at 100 rpm and a specific air flow of 0.04 vvm. The reactor was fed with synthetic medium containing NH4 + and NO2 −, with a total nitrogen load of 457 mg/L. Helium gas was used in the anaerobic stage. Ammonium oxidation activity tests were carried out under aerobic conditions during the first 2 weeks of operation and no activity was detected. The results for the FISH analysis indicated a large quantity of anammox bacteria, although the presence of ammonium-oxidizing or nitrite-oxidizing bacteria was not detected.
After 5 weeks of operation, the helium gas was replaced with atmospheric air and the nitrogen load was reduced to 131 mgN/L day. The nitrogen was added only in the form of ammonium. Based on the FISH technique, a substantial increase (to 45%) in the aerobic ammonium-oxidizing bacteria was observed, while the anammox bacteria decreased from 80 to 40%. The nitrogen removal rate of the reactor was found to be slow, both in the anoxic stage (anammox) and in the stage with oxygen limitation (CANON), for which the values were 0.315 and 0.064 kgN/m3 day, respectively.
The same authors also noted that under oxygen-limiting conditions anammox bacteria consume nitrite, while the nitrite-oxidizing bacteria are not active. This suggests that these latter organisms are present only when oxygen is not limiting. When oxygen is deficient, these bacteria, which are inhibited by free ammonia (present in a high concentration in this study), have to compete with ammonium-oxidizing bacteria for the low amount of oxygen available and also with anammox bacteria for nitrite.
The model developed by HAO et al. (2001) to describe the CANON process indicated that the maximum nitrogen removal rate would be reached only when the dissolved oxygen concentration is proportional to the surface load of ammonium. For variable ammonium loads, the dissolved oxygen should be regulated by way of controlled refeeding. The model developed by the same authors showed that the ideal dissolved oxygen concentration in a CANON reactor was around 1 mg/L, although this ideal value was dependent on the thickness and density of the biofilm, the organic matter concentration of the influent, and the temperature.
5.2.2.4.2.2 OLAND Process
The term OLAND was initially introduced by KUAI and VERSTRAETE (1998). The stoichiometry of the OLAND process considers the removal of ammonium in two stages: in the first ammonium is partially oxidized to nitrite and in the second a reaction between ammonium and nitrite occurs with the formation of nitrogen gas. This stoichiometry is very similar to that involved in the CANON process. The OLAND process can be represented by Eq. 5.15 (VERSTRAETE and PHILIPS 1998).
The key to this process is the supply of oxygen, to ensure that the nitrification occurs only up to nitrite. Subsequently, due to the low dissolved oxygen concentrations (final electron acceptor), nitrite is consumed in the oxidation of ammonium. Initially, the process was considered to depend only on aerobic AOB. However, further studies revealed that at low dissolved oxygen levels and absence of organic electron donors, AOB and anammox coexist in the OLAND reactor, as occurs in CANON systems. Indeed, the representative reactions of both processes are very similar.
In research carried out by DE CLIPPELEIR et al. (2009), high nitrogen removal rates were observed in granular sludge reactors. On operating an OLAND system, PYNAERT et al. (2003) obtained nitrogen removal at a rate of 1.8 kgN/m3 day, 100 days after the inoculation of the reactor (rotating biological contactor) with an aerobic granular sludge. In an air-lift reactor, SLIEKERS et al. (2003) observed a nitrogen conversion rate of 1.5 kgN/m3 day.
5.2.2.4.2.3 Aerobic/Anoxic Deammonification or DEMON
The term aerobic/anoxic deammonification or DEMON was firstly used to describe the significant loss of inorganic nitrogen (up to 90%) in the nitrifying stage of a rotating biological contactor used for leachate treatment, operating with high concentrations of ammonium and submitted to low oxygen concentrations (HIPPEN et al. 1997). In fact, aerobic deammonification is based on the principle of the CANON process (illustrated in Fig. 5.12d), in which there is cooperation between nitrifying and anammox bacteria under oxygen-limiting conditions. Both partial nitritation and anammox reactions occur in a single-reactor system.
The DEMON process is operated similarly to a conventional sequencing batch reactor (SBR). The SBR is subjected to intermittent aeration governed by three control mechanisms: time, pH, and dissolved oxygen control. The operating cycles (of around 8 h, as commonly reported in the literature) comprise a simultaneous filling/reaction phase, settling phase, and effluent withdrawal period. During the reaction phase, both partial nitritation and anaerobic ammonium oxidation occur. These two successive reactions affect the pH: the partial nitritation decreases the pH, while the anammox process increases the pH. So, the duration of the aerated and non-aerated intervals is controlled by the pH value, which characterizes the current state of the conversions (WETT 2007).
When oxygen is provided, partial nitrification is promoted, yielding H+ which causes a gradual pH drop. At the moment when the minimum pH threshold value (lower set-point) is reached, aeration is turned off. When oxygen becomes depleted, nitrite formed during partial nitritation is used to oxidize ammonium through the anammox reaction. This process recovers part of the alkalinity used in the aerated step, leading to pH increase. Additionally alkalinity is provided by the influent reject water, which is continuously supplied to the SBR until pH reaches the maximum threshold value (upper set point). Then aeration is switched on again. The alternating aerated and non-aerated periods continue until the settling phase begins. Dissolved oxygen levels are kept within a narrow and low range, close to 0.3 mg/L. Such accurate control is made to avoid nitrite buildup and repress nitrite oxidation to nitrate (INNEREBNER et al. 2007; WETT 2007).
Besides the aforementioned control mechanisms, monitoring of redox potential and electrical conductivity along with programmed safeguards may prevent unfavorable operating conditions, such as overaeration, which will potentially lead to a reduction in the process performance (WETT 2007).
Full-scale single sludge deammonification systems located in Strass (Austria) and Zurich (Switzerland) were described in the literature. The plant at Strass has a 500 m3 sequencing batch reactor for the deammonification of reject water from digested sludge dewatering (INNEREBNER et al. 2007). 2.5 years were necessary for the start-up of the DEMON process and biomass enrichment. Long-term results obtained in the pH-controlled deammonification system revealed that it reached the capacity of removing around 300 kg of nitrogen per day (WETT 2006) with annual ammonium removal above 90% (WETT 2007). Furthermore, the specific air demand decreased from 109 to 29 m3/kgN (WETT 2006). The sidestream deammonification step requires 1.16 kWh/kg of ammonium nitrogen removal, while the energy required for this conversion is around 6.5 kWh in the mainstream treatment (WETT, 2007). In Zurich a 1400 m3 reactor was reported to treat 500 gN/m3 day, with nitrogen conversions of over 90% (JOSS et al. 2009).
The main characteristics of some of aforementioned nitrogen removal processes are detailed in Table 5.6.
Figure 5.11 illustrates some full-scale anammox-based reactors for nitrogen removal from several types of wastewaters.
Examples of full-scale implementation of anammox-based system for treatment of nitrogen-rich wastewaters. Information on each plant are given below each picture (provided by Paques). (a) Rotterdam, the Netherlands. Treatment of sludge digester effluent. Load: 500 kgN/day. Removal of nitrogen by two-step SHARON-anammox process. Operation since 2002. (b) Olburgen, the Netherlands. Treatment of digester reject water combined with wastewater from potato processing plant (pretreated in a UASB reactor). Load: 1200 kN/day and 245 kgP/day. Removal of nitrogen by one-stage anammox and removal of phosphorus by Phospaq and struvite production. Operation since 2006. (c) Niederglatt, Switzerland. Treatment of sludge digester effluent. Load: 60 kN/day. Removal of nitrogen by one-stage anammox. Operation since 2008. (d) China. Treatment of wastewater from monosodium glutamate plant. Load: 11,000 kgN/day. Removal of nitrogen by one-step anammox process. Operation since 2009. (e) Santa Catarina, Brazil. Treatment of wastewater from food industry. Load: 720 kgN/day. Removal of nitrogen by one-stage anammox. Operation since 2014. (f) Noord-Brabant, the Netherlands. Removal of nitrogen from the effluent of an UASB reactor treating animal waste in one-stage anammox. Load: 6000 kgN/day. Operation since 2013
5.2.3 Denitrifying Ammonium Oxidation (DEAMOX) Process
The DEAMOX process was developed to minimize the difficulties found in achieving partial nitritation of ammonium to nitrite, as required in one- or two-stage partial nitritation-anammox processes (e.g., SHARON + anammox and CANON). According to KALYUZHNYI et al. (2006), obtaining proper nitrite concentrations from partial nitritation requires advanced process control which may hamper large-scale industrial applications. Moreover, problems associated with nitrite inhibition are also overcome.
In this sense, the DEAMOX process does not require a separate production of nitrite, but consists of a combination of the anammox reaction with autotrophic denitrification using sulfide as electron donor, yielding the formation of nitrite from nitrate in an anoxic biofilm reactor. The main process conversions taking place in the different reactors are shown in Eqs. 5.16–5.19, while the scheme of the DEAMOX process is displayed in Fig. 5.12e. Interestingly, the DEAMOX process scheme is quite similar to the autotrophic denitrifying fluidized-bed pilot reactor in which the anammox process was first discovered (Mulder et al. 1995).
Flow diagrams representing the processes of (a) conventional nitrification-denitrification, (b) partial nitrification-denitritation, (c) partial nitritation-anammox, (d) CANON process, (e) DEAMOX process, and (f) NOx processes. The numbers in parenthesis represent the quantity of nitrogen in %, which can vary depending on the process; NO2 (g), gaseous NO2 a. In the presence of oxygen, the NO2 (g) acts as a regulatory signal and not as a substrate, inducing the denitrifying activity of aerobic ammonium-oxidizing bacteria (adapted from SCHMIDT et al. (2003) and KALYUZHNYI et al. (2006))
The technology involves the combination of three different reactors. First an anaerobic reactor (e.g., UASB) is used as a pretreatment step to generate sulfide and ammonium (Eq. 5.16). The effluent of the anaerobic system is split and partially fed to a nitrifying reactor where the main product generated is nitrate (nitrite is a minor product) (Eq. 5.17). The remaining is fed to the DEAMOX biofilm reactor where it is mixed with the nitrified effluent to enable autotrophic partial denitrification with sulfide (Eq. 5.18), generating nitrite. Anammox bacteria present in the DEAMOX reactor can then oxidize ammonium using nitrite as electron acceptor (Eq. 5.9). The distribution of the anaerobic/aerobic flows is chosen based on the wastewater composition, especially by the electron donor (sulfide and ammonium) concentrations (KALYUZHNYI et al. 2006, 2007).
KALYUZHNYI et al. (2006) reported stable process performance in the long term (after 400 days) with nitrogen removal of around 90% for incoming nitrogen loads above 1000 mg/(L day) to the DEAMOX reactor. In order to ensure stable operation, the authors recommend that the NOX-N/NH4-N ratio should be higher than 1.2 (taking into account the anammox reaction stoichiometry) and influent H2S/NO3 − ratio of 1:4 or H2S/NO3-N ratio of 0.57 mgH2S-S/mgNO3-N (taking into account the stoichiometry of the sulphide-based denitrification) (KALYUZHNYI et al. 2006, 2007; MASŁOŃ and TOMASZEK 2009).
Some key characteristics of the DEAMOX process are enumerated as follows (Kalyuzhnyi et al. 2006):
-
The production of nitrite does not require complex control of the process.
-
The maintenance of the DEAMOX reactor under denitrifying conditions enhances the growth of granules and therefore favors the anammox process.
-
High nitrite concentrations (possibly toxic) are avoided and the emission of greenhouse gases is reduced.
However, the inhibition of ammonium-oxidizing bacteria in the nitrifying reactor and anammox organisms in the DEAMOX tank by hydrogen sulfide in the treatment of wastewaters with high sulfate concentrations is one of the disadvantages of the process. Furthermore, the applicability of the DEAMOX concept depends on the presence of sulfate in the influent wastewater and on the sulfate-to-nitrogen ratio given that the process is directly dependent on the amount of sulfide in the DEAMOX reactor (Kalyuzhnyi et al. 2006, 2007; MASŁOŃ and TOMASZEK 2009).
5.2.4 NOX Processes
The control and stimulation of the denitrifying activity of microorganisms of the genus Nitrosomonas through the use of nitrogen oxides allows new possibilities for the treatment of wastewater. In the presence of NOx, these autotrophic microorganisms are able to carry out nitrification and denitrification simultaneously, even under completely aerobic conditions, with N2 as the main product.
In this process, only around 40% of the ammonium load is converted to nitrite. The oxygen demand is 50% lower than in the conventional nitrification process. Since nitrite is used as the terminal electron acceptor in denitrification, this step consumes less organic matter (COD) (SCHMIDT et al. 2003).
The NOx compounds (NO/NO2), added only in trace quantities (NH4 +/NO2 ratio varies from 1000/1 to 500/1), act as a regulatory signal which induces the denitrifying activity of the ammonium-oxidizing bacteria (SCHMIDT et al. 2001). Consequently, around 50% of the reducing equivalents [H] are transferred to nitrite, which acts as the terminal electron acceptor instead of oxygen. Thus, the consumption of oxygen in this process is reduced.
The flow diagrams in Fig. 5.12a–f illustrate some of the main processes employed for autotrophic removal of nitrogen described above. Based on our current understanding, the most important conversions of the nitrogen cycle occur during these processes. The traditional nitrogen removal process (nitrification and denitrification) and the combined partial nitrification and denitrification of nitrite (denitritation) are also represented for comparison with the recently developed technologies.
5.3 Final Considerations
New processes based on autotrophic nitrogen removal represent an alternative to the conventional processes, particularly for the treatment of wastewaters containing high concentrations of nitrogen and with a low organic carbon-to-nitrogen (C/N) ratio, which is unfavorable for the application of the conventional nitrification-denitrification process.
The new technologies based on partial nitrification coupled with the reduction of the nitrite formed, through the addition of a carbon source (denitritation) or even through reduction with ammonium (anaerobic oxidation of ammonium), are economically favorable in terms of nutrition requirements and operating costs.
As illustrated in this chapter, the autotrophic removal processes have been extensively studied by various research groups. Not only laboratory-scale tests but also pilot and industrial-scale studies have been described in the literature.
It is true that initially the autotrophic removal of nitrogen appears to be a difficult process to operate due to the need for strict control of substrate concentrations, pH, temperature, and dissolved oxygen. These are among the decisive factors which will determine the success of the treatment, since they influence the presence or absence of inhibitor compounds, which in many cases are used for the selection of specific bacterial populations.
Although the application of systems in which partial nitrification and anammox reaction occur in the same reactor has been carried out with success in many cases, the long-term stability of this process is still difficult to achieve and remains a challenge for future research.
Further research is needed in laboratory scale to gain a better and more detailed understanding of processes such as anammox. This will potentially leads to an increase in the number of real-scale treatment systems in the future.
In order to improve the development and intensify the application of autotrophic nitrogen removal processes, studies should be directed toward finding strategies to minimize long reactor start-up, which represent one of the main drawbacks regarding the application of these processes. In addition, continuous research aimed at understanding the metabolism of anammox bacteria should be carried out, seeking better ways to control the process.
Studies which explore the possibility of making the reactors aimed at the autotrophic removal of nitrogen part of the main treatment process rather than sidestream processes should be stimulated. This research topic referred to as mainstream anammox. In this case the reactors designed for the autotrophic removal of nitrogen (e.g., anammox reactors) need to be adapted to operate at the normal temperature of the treatment plant, which is often much lower than that considered to be optimal for the microorganisms involved.
Regardless of the main focus of the study, the fact that the metabolic pathways of the nitrogen transformation process are complex needs to be taken into account. The way in which different environmental factors affect these processes still needs to be better understood. Knowledge regarding the basic microbial processes involved in these systems is fundamental to increasing the nitrogen removal rate, obtaining satisfactory results for treatment in full scale and expanding the application of reliable treatment strategies.
References
ABELING, U., SEYFRIED, C.F. Anaerobic-aerobic treatment of high strength ammonium wastewater nitrogen removal via nitrite. Water Science and Technology, v. 26, p. 1007–1015, 1992.
ABELING, U., SEYFRIED, C.F. Anaerobic-aerobic treatment of potato-starch wastewater. Water Science and Technology, 28, p. 165–176, 1993.
ABELIOVICH, A. Transformations of ammonia and the environmental impact of nitrifying bacteria. Biodegradation, v. 3, p. 255–264, 1992.
AHN, Y.H. Sustainable nitrogen elimination biotechnologies: a review. Process Biochemistry, v. 41, p. 1709–1721, 2006.
AHN, Y.H., HWANG, I.S., MIN, K.S. Anammox and partial denitrification in anaerobic nitrogen removal from piggery waste. Water Science and Technology, v. 49, p. 145–153, 2004.
ALLEMAN, J.E. Elevated nitrite occurrence in biological wastewater treatment systems. Water Science and Technology, v. 17, p. 409–419, 1984.
ANTHONISEN, A.C., LOEHR, R.C., PRAKASAM, T.B.S., SRINATH, E.G. Inhibition of nitrification by ammonia and nitrous-acid. Journal Water Pollution Control Federation, v. 48, p. 835–852, 1976.
ANTONIOU, P., HAMILTON, J., KOOPMAN, B., JAIN, R., HOLLOWAY, B., LYBERATOS, G., SVORONOS, S.A. Effect of temperature and pH on the effective maximum specific growth rate of nitrifying bacteria. Water Research, v. 24, p. 97–101, 1990.
ARROJO, B., MOSQUERA-CORRAL, A., CAMPOS, J.L., MÉNDEZ, R. Effects of mechanical stress on Anammox granules in a sequencing batch reactor (SBR). Journal of Biotechnology, p. 453–463, 2006.
AUSTERMANN-HAUN, U., Meyer, H., Seyfried, C., Rosenwinkel, K.-H. Full scale experiences with anaerobic/aerobic treatment plants in the food and beverage industry. Water Science and Technology, v. 40, p. 305–312, 1999.
BALMELLE, B., NGUYEN, K.M., CAPDEVILLE, B., CORNIER, J.C., DEGUIN, A. Study of factors controlling nitrite build-up in biological processes for water nitrification. Water Science and Technology, v. 26, p. 1017–1025, 1992.
BERNET, N., DANGCONG, P., DELGENE’S, J.-P., MOLETTA, R. Nitrification at low oxygen concentration in biofilm reactor. Journal of Environmental Engineering, v. 127, p. 266–271, 2001.
BERNET, N., SANCHEZ, O., CESBRON, D., STEYER, J.P., DELGENES, J.P. Modeling and control of nitrite accumulation in a nitrifying biofilm reactor. Biochemical Engineering Journal., v. 24, p. 173–183, 2005.
BINSWANGER, S., SIEGRIST, H., LAIS, P. Simultane Nitrifikation/Denitrifikation von stark ammoniumbelasteten Abwassern ohne organische Koblenstoffquellen. Korresp. Abwasser. v. 44, p. 1573–1580, 1997.
BRODA, E. Two kinds of lithotrophs missing in nature. Z Allg Mikrobiol., v. 17, p. 491–493, 1977.
BROUWER, M., VAN LOOSDRECHT, M.C.M., HEIJNEN, J.J. One reactor system for ammonium removal via nitrite. STOWA report 96-01. STOWA, Utrecht, 1996.
CAMPOS, J.L., SÁNCHEZ, M., MOSQUERA, A., MÉNDEZ, R., LEMA, J.M. Coupled BAS and anoxic USB system to remove urea and formaldehyde from wastewater. Water Research, v. 37, p. 3445–3451, 2003.
CARRERA, J., BAEZA, J.A., VICENT, T., LAFUENTE, J. Biological nitrogen removal of high-strength ammonium industrial wastewater with two-sludge system. Water Research, v. 37, p. 4211–4221, 2003.
CEMA, G., WISZNIOWSKI, J., ZABCZYNSKI, S., ZABLOCKA-GODLEWSKA, E., RASZKA, A., SURMACZ-GÓRSKA, J. Biological nitrogen removal from landfill leachate by deammonification assisted by heterotrophic denitrification in a rotating biological contactor (RBC). Water Science and Technology, v. 55, p. 35–41, 2007.
CHAMCHOI, N., NITISORAVUT, S. Anammox enrichment from different conventional sludges. Chemosphere., v. 66, p. 2225–2232, 2007.
CHAMCHOI, N., NITISORAVUT, S., SCHMIDT, J.E. Inactivation of Anammox communities under concurrent operational of anaerobic ammonium oxidation (Anammox) and denitrification. Bioresource Technology, v. 99, p. 3331–3336, 2008.
CHEN, M., KIM, J.H., KISHIDA, N., NISHIMURA, O., SUDO, R. Enhanced nitrogen removal using C/N load adjustment and real-time control strategy in sequencing batch reactors for swine wastewater treatment. Water Science and Technology, v. 49, p. 309–314, 2004.
CHUNG, J., BAE, W., LEE, Y., KO, G., LEE, S., PARK, S. Investigation of the effect of free ammonia concentration upon leachate treatment by shortcut biological nitrogen removal process. In: IWA Speciality Symposium on Strong Nitrogenous and Agro-Wastewater, v. 1, Seoul, Korea, p. 93–104, 2003.
CIRPUS, I.E.Y., DE BEEN, M., OP DEN CAMP, H.J.M., STRUS, M., LE PASLIER, D., KUENEN, G.J., JETTEN, M.S.M. A new soluble 10 kDa monoheme cytochrome c-552 from the anammox bacterium Candidatus “Kuenenia stuttgartiensis”. FEMS Microbiology Letters, v. 252, p. 273–278, 2005.
CIUDAD, G., RUBILAR, O., MUÑOZ, P., RUIZ, G., CHAMY, R., VERGARA, C., JEISON, D. Partial nitrification of high ammonia concentration wastewater as a part of a shortcut biological nitrogen removal process. Process Biochemistry, v. 40, p. 1715–1719, 2005.
CLABAUGH, M.M. Nitrification of landfill leachate by biofilm columns. MSc thesis, Virginia Polytechnic Institute and State University, USA, 43p, 2001.
CLINE, J.D., RICHARDS, F.A. Oxygen deficient conditions and nitrate reduction in the eastern tropical North Pacific Ocean. Limnology and Oceanography, v. 17, p. 885–900, 1972.
DALSGAARD, T., THAMDRUP, B. Factors controlling anaerobic ammonium oxidation with nitrite in marine sediments. Applied and Environmental Microbiology, v. 68, p. 3802–3808, 2002.
DALSGAARD, T., THAMDRUP, B., CANFIELD, D.E. Anaerobic ammonium oxidation (anammox) in the marine environment. Research in Microbiology, v. 156, p. 457–464, 2005.
DAPENA-MORA, A., CAMPOS, J.L., MOSQUERA-CORRAL, A., JETTEN, M.S.M., MÉNDEZ, R. Stability of the Anammox process in a gas-lift reactor and a SBR. Journal of Biotechnology, v. 110, p. 159–170, 2004.
DAPENA-MORA, A., FERNANDEZ, I., CAMPOS, J.L., MOSQUERA-CORRAL, A., MENDEZ, R., JETTEN, M.S.M Evaluation of activity and inhibition effects on Anammox process by batch tests on the nitrogen gas production. Enzyme and Microbial Technology, v. 40, p. 859–865, 2007.
DE CLIPPELEIR, H., VLAEMINCK, S.E., CARBALLA, M., VERSTRAETE, W. A low volumetric exchange ratio allows high autotrophic nitrogen removal in a sequencing batch reactor. Bioresource Technology, p. 5010–5015, 2009.
DENG PETERSEN, P., JENSEN, K., LYNGSIE, P., HENDRIK JOHANSEN, N. Nitrogen removal in industrial wastewater by nitration and denitration - 3 years of experience. Water Science and Technology, v. 47, 181–188, 2003.
DEXIANG, L., XIAOMING, L., QI, Y., GUANGMING, Z., LIANG, G., XIU, Y. Effect of inorganic carbon on anaerobic ammonium oxidation enriched in sequencing batch reactor. Journal of Environmental Sciences, v. 20, p. 940–944, 2007.
DOSTA, J., FERNÁNDEZ, I., VÁZQUEZ-PADÍN, J.R., MOSQUERA-CORRAL, A., CAMPOS, J.L., MATA-ÁLVAREZ, J., MÉNDEZ, R. Short- and long-term effects of temperature on the Anammox process. Journal of Hazardous Materials, v. 154, p. 688–693, 2008.
EGLI, K., FANGER, U., ALVAREZ, P.J.J., SIEGRIST, H., VAN DER MEER, J.R., ZEHNDER, A.J.B. Enrichment and characterization of an anammox bacterium from a rotating biological contact treating ammonium-rich leachate. Archives of Microbiology, v. 175, p. 198–207, 2001.
FUERST, J.A. Intracellular compartmentation in Planctomycetes. Annual Review of Microbiology, v. 59, p. 299–328, 2005.
FURUKAWA, K., LIEU, P.K., TOKITOH, H., FUJII, T. Development of single-stage nitrogen removal using anammox and partial nitritation (SNAP) and its treatment performances. Water Science and Technology, v. 53, p. 83–90, 2006.
FUX, C. Biological nitrogen elimination of ammonium-rich sludge digester liquids. PhD thesis, ETH-Zürich, Switzerland, 2003.
FUX, C., BOEHLER, M., HUBER, P., BRUNNER, I., SIEGRIST, H. Biological treatment of ammonium-rich wastewater by partial nitritation and subsequent anaerobic ammonium oxidation (Anammox) in a pilot-plant. Journal of Biotechnology, v. 99, p. 295–306, 2002.
FUX, C., HUANG, D., MONTI, A., SIEGRIST, H. Difficulties in maintaining long-term partial nitritation of ammonium-rich sludge digester liquids in a moving-bed biofilm reactor (MBBR). Water Science and Technology, v. 49, p. 53–60, 2004.
GANIGUÉ, R., LÓPEZ, H., BALAGUER, M.D., COLPRIM, J. Partial ammonium oxidation to nitrite of high ammonium content urban landfill leachates. Water Research, v. 41, p. 3317–3326, 2007.
GARRIDO, J.M., VAN BENTHUM, W.A.J., VAN LOOSDRECHT, M.C.M., HEIJNEN, J.J. Influence of dissolved oxygen concentration on nitrite accumulation in a biofilm airlift suspension reactor. Biotechnology and Bioengineering, v. 53, p. 168–178, 1997.
GIL, K.-I., CHOI, E. Nitrogen removal by recycle water nitritation as an attractive alternative for retrofit technologies in municipal wastewater treatment plants. Water Science and Technology, v. 49, p. 39–46, 2004.
GONG, Z., YANG, F.L., LIU, S.T., BAO, H., HU, S.W, FURUKAWA, K.J. Feasibility of a membrane-aerated biofilm reactor to achieve single-stage autotrophic nitrogen removal based on Anammox. Chemosphere, v. 69, p. 776–784, 2007.
GONZÁLEZ-MARTÍNEZ, A., POYATOS, J.M., HONTORIA, E., GONZALEZ-LOPEZ, J., Osorio, J. Treatment of Effluents Polluted by Nitrogen With New Biological Technologies Based on Autotrophic Nitrification-Denitrification Processes Recent Pat Biotechnol 5(2), 74–84. 2011.
GÜVEN, D., DAPENA, A., KARTAL, B., SCHMID, M.C., MAAS, B., VAN DE PAS-SCHOONEN, K., SÖZEN, S., MENDEZ, R., OP DEN CAMP, H.J.M., JETTEN, M.S.M., STROUS, M., SCHMIDT, I. Propionate oxidation and methanol inhibition of anaerobic ammonium-oxidizing bacteria. Applied and Environmental Microbiology, v. 71, p. 1066–1071, 2005.
HAINES, T.H. Do sterols reduce proton and sodium leaks through lipid bilayers. Progress in Lipid Research, v. 40, p. 299–324, 2001.
HANAKI, K., WANTAWIN, C., OHGAKI, S. Effects of the activity of heterotrophs on nitrification in a suspended-growth reactor. Water Research, v. 24, p. 289–296, 1990.
HAO, X., HEIJNEN, J.J., VAN LOOSDRECHT, M.C.M. Model-based evaluation of temperatura and inflow variations on a partial nitrification-Anammox biofilm process. Water Research, v. 36, p. 4839–4849, 2002.
HAO, X., HEIJNEN, J.J., VAN LOOSDRECHT, M.C.M. Sensitivity analysis of a biofilm model describing a one-stage completely autotrophic nitrogen removal (CANON) process. Biotechnology and Bioengineering, v. 77, p. 266–277, 2001.
HEIJNEN, J.J. Biologishe anaëroob-aërobe afvalwaterzuivering bij Gist-Brocades: eindrapport 1977–1986. ‘s-Gravenhage. N.L., Staatsuitgeverij/DOP, 1988.
HELLINGA, C., SCHELLEN, A.A.J.C., MULDER, J.W., VAN LOOSDRECHT, M.C.M., HEIJNEN, J.J. The Sharon process: an innovative method for nitrogen removal from ammonium-rich wastewater. Water Science and Technology, v. 37, p. 135–142, 1998.
HELMER, C., KUNST, S., JURETSCHKO, S., SCHMID, M.C., SCHLEIFER, K.-H., WAGNER, M. Nitrogen loss in a nitrifying biofilm system. Water Science and Technology, v. 39, p. 13–21, 1999.
HELMER-MADHOK, C., SCHMID, M., FILIPOV, E., GAUL, T., HIPPEN, A., ROSENWINKEL, K.H., SEYFRIED, C.F., WAGNER, M., KUNST, S. Deammonification in biofilm systems: population structure and function. Water Science and Technology, v. 46, p. 223–231, 2002.
HIPPEN, A., HELMER, C., KUNST, S., ROSENWINKEL, H., SEYFRIED, C.F. Six years’ practical experience with aerobic/anoxic deammonification in biofilm systems. Water Science and Technology, v. 44, p. 39–48, 2001.
HIPPEN, A., ROSENWINKEL, K.-H., BAUMGARTEN, G., SEYFRIED, C.F. Aerobic deammonification: a new experience in the treatment of wastewaters. Medelingen – Faculteit Landbouwkundige en Toegepaste Biologische Wetenschappen (Universiteit Gent), v. 61, p. 1967–1974, 1996.
HIPPEN, A., ROSENWINKEL, K.-H., BAUMGARTEN, G., SEYFRIED, C.F. Aerobic deammonification: a new experience in the treatment of wastewaters. Water Science and Technology, v. 35, p. 111–120, 1997.
HU, S.S. Acute substrate-intermediate-product related inhibition of nitrifiers. M.S. Thesis, School of Civil Engineering, Purdue University, West Lafalyette, Indiana, 1990.
HUSTON, W.M., HARHANGI, H.R., LEECH, A.P., BUTLER, C.S., JETTEN, M.S.M., OP DEN CAMP, H.J.M., MOIR, J.W.B. Expression and characterization of a major c-type cytochrome encoded by gene kustc0563 from Kuenenia stuttgartiensis as a recombinant protein in Escherichia coli. Protein Expression and Purification, v. 51, p. 28–33, 2007.
ILIES, P., MAVINIC, D. S. The effect of decreased ambient temperature on the biological nitrification and denitrification of a high ammonia landfill leachate. Water Research, v. 35, p. 2065–2072, 2001.
INNEREBNER, G., INSAM, H., FRANKE-WHITTLE, I., WETT, B. Identification of anammox bacteria in a full-scale deammonification plant making use of anaerobic ammonia oxidation. Systematic and Applied Microbiology, v. 30, p. 408–412, 2007.
ISAKA, K., DATE, Y., SUMINO, T., YOSHIE, S., TSUNEDA, S. Growth characteristic of anaerobic ammonium-oxidizing bacteria in an anaerobic biological filtrated reactor. Applied Microbiology and Biotechnology, v. 70, p. 47–52, 2006.
JENICEK, P., SVEHLA, P., ZABRANSKA, J., DOHANYOS, M. Factors affecting nitrogen removal by nitritation/denitritation. Water Science and Technology, v. 49, p. 73–79, 2004.
JETTEN, M.S.M., HOM, S.J., VAN LOOSDRECHT, M.C.M. Towards a more sustainable wastewater treatment system. Water Science and Technology, v. 35, p. 171–179, 1997.
JETTEN, M.S.M., OP DEN CAMP, J.M., KUENEN, J.G., STROUS, M. Taxonomic description of the family Anammoxaceae. IN: Hedlund, B., Krieg, N.R., Paster, B.J., K.H., Schleifer, Staley, J.T., Ward, N., Whitman, W.B. (eds). Bergey’s manual of systematic bacteriology (2 ed.), v. 4. New York: Springer, 2008.
JETTEN, M.S.M., STROUS, M., VAN DE PAS-SCHOONEN, K.T., SCHALK, J., VAN DONGEN, L.G.J.M., VAN DE GRAAF, A.A., LOGEMANN, S., MUYZER, G., VAN LOOSDRECHT, M.C.M., KUENEN, J.G. The anaerobic oxidation of ammonium. FEMS Microbiology Reviews, v. 22, p. 421–437, 1999.
JETTEN, M.S.M., WAGNER, M., FUERST, J., VAN LOOSDRECHT, M.C.M., KUENEN, G., STROUS, M. Microbiology and application of the anaerobic ammonium oxidation (‘anammox’) process. Current Opinion in Biotechnology, v. 12, p. 283–288, 2001.
JOKELA, J., KETTUNEN, R., SORMUNEN, K., RINTAL, J. Biological nitrogen removal from municipal landfill leachate: low-cost nitrification in biofilters and laboratory scale in-situ denitrification. Water Research, v. 36, p. 4079–4087, 2002.
JOO, S.-H., KIM, D.-J., YOO, I.-K., PARK, K., CHA, G.-C. Partial nitrification in an upflow biological aerated filter by O2 limitation. Biotechnol Lett., v. 22, p. 937–940, 2000.
JOSS, A., SALZGEBER, D., EUGSTER, J., KONIG, R., ROTTERMANN, K., BURGER, S., FABIJAN, P., LEUMANN, S., MOHN, J., SIEGRIST, H. Full-scale nitrogen removal from digester liquid with partial nitritation and anammox in one SBR. Environmental Science and Technology, v. 43, p. 5301–5306, 2009.
KADLEC, R.H., KNIGHT, R.L. Treatment Wetlands, Lewis Publishers, Boca Raton, 893pp, 1996.
KALYUZHNYI, S., GLADCHENKO, M. Sequenced anaerobic-aerobic treatment of high strength, strong nitrogenous landfill leachates. Water Science and Technology, v. 49, p. 301–312, 2004.
KALYUZHNYI, S., GLADCHENKO, M., MULDER, A., VERSPRILLE, B. DEAMOX – new biological nitrogen removal process based on anaerobic ammonia oxidation coupled to sulphide-driven conversion of nitrate into nitrite. Water Research, v. 40, p. 3637–3645, 2006.
KALYUZHNYI, S., GLADCHENKO, M., MULDER, A., VERSPRILLE, B. Comparison of quasisteady-state performance of the DEAMOX process under intermittent and continuous feeding and different nitrogen loading rates. Biotechnology Journal, v. 2, n. 7, p. 894–900, 2007.
KARTAL, B., RATTRAY, J., VAN NIFTRIK, L., VAN DE VOSSENBERG, J.L., SCHMID, M.C., FUERST, J.A., SINNINGHE DAMSTÉ, J., JETTEN, M.S.M., STROUS, M. Candidatus “Anammoxoglobus propionicus” gen. nov., sp. nov., a new propionate oxidizing species of anaerobic ammonium oxidizing bacteria. Systematic and Applied Microbiology, v. 30, p. 39–49, 2007.
KARTAL, B., VAN NIFTRIK, L.A., RATTRAY, J., VAN DE VOSSENBERG, J.L.C.M., SCHMID, M.C., SINNINGHE DAMSTÉ, J., JETTEN, M.S.M., STROUS, M. Candidatus ‘Brocadia fulgida’: an autofluorescent anaerobic ammonium oxidizing bacterium. FEMS Microbiology Ecology, v. 63, p. 46–55, 2008.
KARTAL, B., VAN NIFTRIK, L.A., SLIEKERS, A.O., SCHMID, M., SCHMIDT, I., VAN DE PAS-SCHOONEN, K., CIRPUS, I., VAN DER STAR, W.R.L., VAN LOOSDRECHT, M.C.M., ABMA, W., KUENEN, J., MULDER, J.W., JETTEN, M.S.M., OP DEN CAMP, H., STROUS, M., VAN DE VOSSENBERG, J. Application, eco-physiology and biodiversity of anaerobic ammonium-oxidizing bacteria. Reviews in Environmental Science and Biotechnology, v. 3, p. 255–264, 2004.
KELLER, J., SUBRAMANIAM, K., GÖSSWEIN, J., GREENFIELD, P. Nutrient removal from industrial wastewater using single tank sequencing batch reactors. Water Science and Technology, v. 35, p. 137–144, 1997.
KIM, D.J., CHANG, J.S., LEE, D.I., HAN, D.W., YOO, I.K., CHA, G.C. Nitrification of high strength ammonia wastewater and nitrite accumulation characteristics. Water Science and Technology, v. 47, p. 45–51, 2003.
KOCH, G., EGLI, K., VAN DER MEER, J.R., SIEGRIST, H. Mathematical modeling of autotrophic denitrification in a nitrifying biofilm of a rotating biological contactor. Water Science and Technology, v. 41, p. 191–198, 2000.
KUAI, L., VERSTRAETE, W. Ammonium removal by the oxygen-limited autotrophic nitrification-denitrification system. Applied and Environmental Microbiology, v. 64, p. 4500–4506, 1998.
KUENEN, J.G., JETTEN, M.S.M. Extraordinary anaerobic ammonia-oxidizing bacteria. ASM News, p. 456–463, 2001.
KUMAR, M., LIN, J. Co-existence of anammox and denitrification for simultaneous nitrogen and carbon removal – strategies and issues. Journal of Hazardous Materials, v. 178, p. 1–9, 2010.
KUYPERS, M.M.M., SLIEKERS, A.O., LAVIK, G., SCHMID, M., JORGENSEN, B.B., KUENEN, J.G., SINNINGHE DAMSTÉ, J.S., STROUS, M., JETTEN, M.S.M. Anaerobic ammonium oxidation by anammox bacteria in the Black Sea. Nature, v. 422, p. 608–611, 2003.
LACKNER, S., TERADA, A., SMETS, B.F. Heterotrophic activity compromises autotrophic nitrogen removal in membrane-aerated biofilms: results of a modelling study. Water Research, v. 42, p. 1102–1112, 2008.
LINDSAY, M.R., WEBB, R.I., STROUS, M., JETTEN, M.S.M., BUTLER, M.K., FORDE, R.J., FUERST, J.A. Cell compartmentalization in Planctomycetes: novel types of structural organisation for the bacterial cell. Archives in Microbiology, 175, p. 413–429, 2001.
LIU, S., YANG, F., GONG, Z., SU, Z. Assessment of the positive effect of salinity on the nitrogen removal performance and microbial composition during the start-up of CANON process. Applied Microbiology and Biotechnology, v. 80, p. 339–348, 2008.
LÓPEZ, H., PUIG, S., GANIGUÉ, R., RUSCALLEDA, M., BALAGUER, M.D., COLPRIM, J. Start-up and enrichment of a granular anammox SBR to treat high nitrogen load wastewaters. Journal of Chemical Technology & Biotechnology, v. 83, p. 233–241, 2008.
MADIGAN, M.T., MARTINO, J.M., PARKER, J. Brock Biology of Microorganisms. New-Jersey: Prentice-Hall, p 1175. 2000.
MASŁOŃ, A., TOMASZEK, J.A. Anaerobic ammonium nitrogen oxidation in DEAMOX process. Environment Protection Engineering, p. 123–130, 2009
MAURET, M., PAUL, E., PUECH-COSTES, E., MAURETTE, M.T., BAPTISTE, P. Application of experimental research methodology to the study of nitrification in mixed culture. Water Science and Technology., v. 34, p. 245–252, 1996.
METCALF and EDDY Inc. Wastewater engineering: treatment and reuse. New York: McGraw Hill (4 Ed.), 2003.
MOLINUEVO, B., GARCIA, M.C., KARAKASHEV, D., ANGELIDAKI, I. Anammox for ammonia removal from pig manure effluents: effect of organic matter content on process performance. Bioresource Technology, v. 100, p. 2171–2175, 2009.
MULDER, A. Anoxic ammonia oxidation of wastewater. European Patent Ep327184. Assignee: Gist-Brocades NV, NL, 1989.
MULDER, A. The quest for sustainable nitrogen removal technologies. Water Science and Technology, v. 48, p. 67–75, 2003.
MULDER, A., VAN DE GRAAF, A.A., ROBERTSON, L.A., KUENEN, J.G. Anaerobic ammonium oxidation discovered in a denitrifying fluidized bed reactor. FEMS Microbiology Ecology, v. 16, p. 177–184, 1995.
MULDER, J.W., VAN KEMPEN, R. N-removal by SHARON. Water Quality International, IAWQ (Edit.), p. 30–31, 1997.
MULDER, J.W., Van Loosdrecht, M.C.M., Hellinga, C. Van Kempen, R. Full-scale application of the SHARON process for treatment of rejection water of digested sludge dewatering. Water Science and Technology, 43, p. 127–134, 2001.
MURAT, S., INSEL, G., ARTAN, N., ORHON, D. Performance evaluation of SBR treatment for nitrogen removal from tannery wastewater. In: IWA Speciality Symposium on Strong Nitrogenous and Agro-Wastewater, v. 2. Seoul, Korea, p. 598–605, 2003.
NIELSEN M., BOLLMANN A., SLIEKERS O., JETTEN M., SCHMID M., STROUS M., SCHMIDT I., LARSEN L.H., NIELSEN L.P., REVSBECH N.P. Kinetics, diffusional limitation and microscale distribution of chemistry and organisms in a CANON reactor. FEMS Microbiology Ecology. v. 51, 247–256, 2005.
OBAJA, D., MACÉ, S., COSTA, J., SANS, C., MATA-ALVAREZ, J. Nitrification, denitrification and biological phosphorus removal in piggery wastewater using a sequencing batch reactor. Bioresource Technology, v. 87, p. 103–111, 2003.
PAINTER, H.A., LOVELESS, J.E. Effect of temperature and pH value on the growth-rate constants of nitrifying bacteria in the activated-sludge process. Water Research, v. 17, p. 237–248, 1983.
PAREDES, D., KUSCHK, P., MBWETTE, T.S.A., STANGE, F., MÜLLER, R.A., KÖSER, H. New aspects of microbial nitrogen transformation in the context of wastewater treatment – a review. Engineering Life Sciences, v. 7, p. 13–25, 2007.
PATHAK, B.K., KAZAMA, F., SAIKI, Y., SUMINO, T. Presence and activity of anammox and denitrification process in low ammonium-fed bioreactors. Bioresource Technology, v. 98, p. 2201–2206, 2007.
PICIOREANU, C., VAN LOOSDRECHT, M.C.M., HEIJNEN, J.J. Modelling the effect of oxygen concentration on nitrite accumulation in a biofilm airlift suspension reactor. Water Science and Technology, v. 36, p. 147–156, 1997.
PLAZA E., TRELA J., GUT L., LÖWÉN M., SZATKOWSKA B. Deammonification process for treatment of ammonium rich wastewater. In: Integration and optimisation of urban sanitation systems, Joint Polish-Swedish Reports, No 10. Royal Institute of Technology, Stockholm, p. 77–87, 2003.
POO, K., JUN, B., LEE, S., WOO, H., KIM, C. Treatment of strong nitrogen swine wastewater at full-scale sequencing-batch reactor. Water Science and Technology, v. 49, p. 315–323, 2004.
POTH, M., FOCHT, D.D. Nitrogen-15 kinetic analysis of nitrous oxide production by Nitrosomonas europaea: an examination of nitrifier denitrification. Applied and Environmental Microbiology, v. 49, p. 1134–1141, 1985.
PYNAERT, K., SMETS, B.F., BEHEYDT, D., VERSTRAETE, W. Start-up of autotrophic nitrogen removal reactors via sequential biocatalyst addition. Environmental Science and Technology, v. 38, p. 1228–1235, 2004.
PYNAERT, K., SMETS, B.F., WYFFELS, S., BEHEYDT, D., SICILIANO, S.D., VERSTRAETE, W. Characterization of an autotrophic nitrogen-removing biofilm from a highly loaded lab-scale rotating biological contactor. Applied and Environmental Microbiology, v. 69, p. 3626–3635, 2003.
PYNAERT, K., WYFFLES, S., SPRENGERS, R., BOECKX, P., VAN CLEEMPUT, O, VERSTRAETE, W Oxygen limited nitrogen removal in a lab scale rotating biological contactor treating an ammonium rich wastewater. Water Science and Technology, v. 45, p. 357–363, 2002.
QUINLAN, A.V. Prediction of the optimum pH for ammonia-N oxidation by Nitrosomonas europaea in well-aerated natural and domestic wastewater. Water Research, v. 18, p. 561, 586, 1984.
RICHARDS, F. Anoxic basins and fjords. In: Chemical Oceanography. Riley, J.P., Skirrown G. (eds). New York: Academic Press, v. 1, p. 611–645, 1965.
RITTMANN, B.E., MCCARTY, P.I. Environmental Biotechnology: Principles and Applications. McGraw-Hill, 2001.
RUIZ, G., JEISON, D., CHAMY, R. Nitrification with high nitrite accumulation for the treatment of wastewater with high ammonia concentration. Water Research, v. 37, p. 1371–1377, 2003.
RUSCALLEDA, M., LÓPEZ, H., GANIGUÉ, R., OUIG, S., BALAGUER, M., COLPRIM, J. Heterotrophic denitrification on granular anammox SBR treating urban landfill leachate. Water Science and Technology, v. 58, p. 1749–1755, 2008.
RYSGAARD, S., GLUD, R.N., RISGAARD-PETERSEN, N., DALSGAARD, D. Denitrification and anammox activity in arctic marine sediments. Limnology and Oceanography, p. 1493–1502, 2004.
Schalk, J. A study of the metabolic pathway of anaerobic ammonium oxidation. PhD thesis. Delft University of Technology, Delft, 107 p., 2000.
SCHALK, J., DE VRIES, S., KUENEN, J.G., JETTEN, M.S.M. Involvement of a novel hydroxylamine oxidoreductase in anaerobic ammonium oxidation. Biochemistry, v. 39, p. 5405–5412, 2000.
SCHMID, M., TWACHTMANN, U., KLEIN, M., STROUS, M., JURETSCHKO, S., JETTEN, M., METZGER, J.W., SCHLEIFER, K.-H., WAGNER, M. Molecular evidence for genus level diversity of bacteria capable of catalyzing anaerobic ammonium oxidation. Systematic and Applied Microbiology, v. 23, p. 93–106, 2000.
SCHMID, M.C., RISGAARD-PETERSEN, N., VAN DE VOSSENBERG, J., KUYPERS, M.M.M., LAVIK, G., PETERSEN, J., HULTH, S., THAMDRUP, B., CANFIELD, D., DALSGAARD, T., RYSGAARD, S., SEJR, M.K., STROUS, M., OP DEN CAMP, H.J.M., JETTEN, M.S.M. Anaerobic ammonium-oxidizing bacteria in marine environments: widespread occurrence but low diversity. Environmental Microbiology, v. 9, p. 1476–1484, 2007.
SCHMID, M., WALSH, K., WEBB, R., IRENE, W., RIJPSTRA, C., VAN DE PAS-SCHOONEN K., VERBRUGGEN, M.J., HILL, T., MOFFETT, B., FUERST, J., SCHOUTEN, S., SINNINGHE DAMSTÉ, J.S., HARRIS, J., SHAW, P., JETTEN, M., STROUS, M. Candidatus “Scalindua brodae”, sp. nov., Candidatus “Scalindua wagneri”, sp., nov., two new species of anaerobic ammonium oxidizing bacteria. Syst Appl Microbiol. v. 26, p. 529–538, 2003.
SCHMIDELL, W., REGINATTO, V.S. Remoção de Nitrogênio. In: V Curso de Tratamento Biológico de Resíduos. Florianópolis-SC, 2005.
SCHMIDT, I., BOCK, E., JETTEN, M.S.M. Ammonia oxidation by Nitrosomonas eutropha with NO2 as oxidant is not inhibited by acetylene. Microbiology, v. 147, 2247–2253, 2001.
SCHMIDT, I., SLIEKERS, O., SCHMID, M., BOCK, E., FUERST, J., KUENEN, J.G., JETTEN, M.S.M., STROUS, M. New concepts of microbial treatment processes for the nitrogen removal in wastewater. FEMS Microbiology, v. 27, p. 481–492, 2003.
SCHMIDT, J.E., BATSTONE, D.J., ANGELIDAKI, I. Improved nitrogen removal in upflow anaerobic sludge blanket (UASB) reactors by incorporation of Anammox bacteria into the granular sludge. Water Science and Technology, v. 49, p. 69–76, 2004.
SEYFRIED, C.F., HIPPEN, A., HELMER, C., KUNST, S., ROSENWINKEL, K.H. One-stage deammonification: nitrogen elimination at low costs. Water Science and Technology, v. 1, p. 71–80, 2001.
SHIMAMURA, M., NISHIYAMA, T., SHIGETOMO, H., TOYOMOTO, T., KAWAHARA, Y., FURUKAWA, K., FUJII, T. Isolation of a multiheme protein with features of a hydrazine-oxidizing enzyme from an anaerobic ammonium-oxidizing enrichment culture. Applied and Environmental Microbiology, v. 73, p. 1065–1072, 2007.
SIEGRIST, H., REITHAAR, S., KOCH, G., LAIS, P. Nitrogen loss in a nitrifying rotating contactor treating ammonium-rich wastewater without organic carbon. Water Science and Technology, v. 38, p. 241–248, 1998.
SINNINGHE DAMSTÉ, J.S., RIJPSTRA, W.I.C., GEENEVASEN, J.A.J., STROUS, M., JETTEN, M.S. Structural identification of ladderane and other membrane lipids of planctomycetes capable of anaerobic ammonium oxidation (anammox). FEBS Journal, v. 272, p. 4270–4283, 2005.
SINNINGHE DAMSTÉ, J.S., STROUS, M., RIJPSTRA, W.I.C., HOPMANS, E.C., GEENEVASEN, J.A.J., VAN DUIN, A.C.T., VAN NIFTRIK, L.A., JETTEN, M.S.M. Linearly concatenated cyclobutane lipids form a dense bacterial membrane. Nature, v. 419, p. 708–712, 2002.
SLIEKERS, A.O., DERWORT, N., CAMPOS-GOMEZ, J.L., STROUS, M., KUENEN, M., JETTEN, M.S.M. Completely autotrophic nitrogen removal over nitrite in a single reactor. Water Research, v. 36, p. 2475–2482, 2002.
SLIEKERS, A.O., THIRD, K.A., ABMA, W., KUENEN, J.G., JETTEN, M.S.M. CANON and Anammox in a gas-lift reactor. FEMS Microbiol. Lett., v. 218, p. 339–344, 2003.
STROUS, M. Microbiology and application of anaerobic ammonium oxidation. PhD Thesis, 144 p., 2000.
STROUS, M., FUERST, J.A., KRAMER, E.H.M., LOGEMANN, S., MUYZER, G., Van De Pas-Schoonen, K.T., Webb, R., Kuenen, J.G., Jetten, M.S.M. Missing lithotroph identified as new Planctomycete. Nature, v. 400, p. 446–449, 1999a.
STROUS, M., HEIJNEN, J.J., KUENEN, J.G., JETTEN, M.S.M. The sequencing batch reactor as a powerful tool for the study of slowly growing anaerobic ammonium-oxidizing microorganisms. Applied Microbiology and Biotechnology, v. 50, p. 589–596, 1998.
STROUS, M., KUENEN, J.G., FUERST, J.A., WAGNER, M., JETTEN, M.S.M. The Anammox case – a new experimental manifesto for microbiological eco-physiology. Antonie van Leeuwenhoek, v. 81, p. 693–702, 2002.
STROUS, M., KUENEN, J.G., JETTEN, M.S.M. Key physiology of anaerobic ammonium oxidation. Applied and Environmental Microbiology, v. 65, p. 3248–3250, 1999b.
STROUS, M., PELLETIER, E., MANGENOT, S., RATTEI, T., LEHNER, A., TAYLOR, M.W., HORN, M., DAIMS, H., BARTOL-MAVEL, D., WINCKER, P., BARBE, V., FONKNECHTEN, N., VALLENET, D., SEGURENS, B., SCHENOWITZ-TRUONG, C., MEDIGUE, C., COLLINGRO, A., SNEL, B., DUTILH, B.E., OP DEN CAMP, H.J.M., VAN DER DRIFT, C., CIRPUS, I., VAN DE PAS-SCHOONEN, K.T., HARHANGI, H.R., VAN NIFTRIK, L., SCHMID, M., KELTJENS, J., VAN DE VOSSENBERG, J., KARTAL, B., MEIER, H., FISHMAN, D., HUYNEN, M.A., MEWES, H.-W., WEISSENBACH, J., JETTEN, M.S.M., WAGNER, M., LE PASLIER, D. Deciphering the evolution of an Anammox bacterium from a community genome. Nature, v. 440, p. 790–794, 2006.
STROUS, M., VAN GERVEN, E., KUENEN, J.G., JETTEN, M.S.M. Effects of aerobic and microaerobic conditions on anaerobic ammonium-oxidizing (Anammox) sludge. Applied and Environmental Microbiology, v. 63, p. 2446–2448, 1997.
SZATKOWSKA, B., CEMA, G., PLAZA, E., TRELA, J., HULTMAN, B. A one-stage system with partial nitritation and Anammox processes in the moving-bed biofilm reactor. Water Science and Technology, v. 55, p. 19–26, 2007.
SZATKOWSKA, B., PLAZA, E. Temperature as a factor influencing the Anammox process performance. Water and Environmental Management Series. Young Researchers, p. 51–58, 2006.
TANG, C., ZHENG, P., WANG, C., MAHMOOD, Q. Suppression of anaerobic ammonium oxidizers under high organic content in high-rate Anammox UASB reactor. Bioresource Technology, v. 101, p. 1762–1768, 2010.
THIRD, K.A., SLIEKERS, O.A., KUENEN, J.G., JETTEN, M.S.M. The CANON System (Completely Autotrophic Nitrogen-removal Over Nitrite) under ammonium limitation: interaction and competition between three groups of bacteria. Systematic and Applied Microbiology, v. 24, p. 588–596, 2001.
TILCHE, A., BACILIERI, E., BORTONE, G., MALASPINA, F., PICCININI, S., STANTE, L. Biological phosphorus and nitrogen removal in a full scale sequencing batch reactor treating piggery wastewater. Water Science and Technology, v. 40, p. 199–206, 1999.
TOH, S.K., ASHBOLT, N.J. Adaptation of anaerobic ammonium-oxidising consortium to synthetic coke-ovens wastewater. Applied Microbiology and Biotechnology, v. 59, p. 344–352, 2002.
TOH, S.K., WEBB, R.I., ASHBOT, N.J. Enrichment of autotrophic anaerobic ammonium-oxidizing consortia from various wastewaters. Microbial Ecology., v. 43, p. 154–167, 2002.
TRIGO, C., CAMPOS, J.M., GARRIDO, J.M., MENDEZ, R. Start-up of the Anammox process in a membrane bioreactor. Journal of Biotechnology, v. 126, p. 475–487, 2006.
TSUSHIMA, I., KINDAICHI, T., OKABE, S. Quantification of anaerobic ammonium-oxidizing bacteria in enrichment cultures by real-time PCR. Water Research, v. 41, p. 785–794, 2007b.
TSUSHIMA, I., OGASAWARA, Y., KINDAICHI, T., SATOH, H., OKABE, S. Development of high-rate anaerobic ammonium-oxidizing (Anammox) biofilm reactors. Water Research, v. 41, p. 1623–1634, 2007a.
TURK, O., MAVINIK, D.S. Selective inhibition: a novel concept for removing nitrogen from highly nitrogenous wastes. Environmental Technology Letters, v. 8, 419–426, 1987.
VAN DE GRAAF, A.A., DE BRUIJN, P., ROBERTSON, L.A., JETTEN, M.S.M., KUENEN, J.G. Autotrophic growth of anaerobic ammonium-oxidizing microorganisms in a fluidized bed reactor. Microbiology, v. 142, p. 2187–2196, 1996.
VAN DE GRAAF, A.A., DE BRUIJN, P., ROBERTSON, L.A., JETTEN, M.S.M., KUENEN, J.G. Metabolic pathway of anaerobic ammonium oxidation on the basis of 15N studies in a fluidized bed reactor. Microbiology, v. 143, p. 2415–2421, 1997.
VAN DE GRAAF, A.A., MULDER, A., DE BRUIJN, P., JETTEN, M.S.M., ROBERTSON, L.A., KUENEN, J.G. Anaerobic oxidation of ammonium is a biologically mediated process. Applied and Environmental Microbiology, v. 61, p. 1246–1251, 1995.
VAN DE GRAAF, A.A., MULDER, A., SLIJKHUIS, H., ROBERTSON, L.A., KUENEN, J.G. Anoxic ammonium oxidation. Proceedings of Europeans Congress on Biotechnology, 5th, p. 388–391, 1990.
VAN DER STAR W., Growth and Metabolism of Anammox Bacteria. Ph.D Thesis. Delft University of Technology, 2008.
VAN DER STAR, W.R., ABMA, W.R., BLOMMERS, D., MULDER, J.W., TOKUTOMI, T., STROUS, M., PICIOREANU, C., VAN LOOSDRECHT, M.C.M. Startup of reactors for anoxic ammonium oxidation: experiences from the first full-scale anammox reactor in Rotterdam. Water Research, v. 41, p. 4149–4163, 2007.
VAN DER STAR, W.R.L., VAN DE GRAAF, M.J., KARTAL, B., PICIOREANU, C., JETTEN, M.S.M., VAN LOOSDRECHT, M.C.M. Response of anaerobic ammonium-oxidizing bacteria to hydroxylamine. Applied and Environmental Microbiology, v. 74, p. 4417–4426, 2008.
VAN DONGEN, L.G.J.M., JETTEN, M.S.M., VAN LOOSDRECHT, M.C.M. The combined SHARON/Anammox process. London: IWA Publishing, 2001a.
VAN DONGEN, U., JETTEN, M.S.M., VAN LOOSDRECHT, M.C.M. The Sharon-Anammox process for treatment of ammonium rich wastewater. Water Science and Technology, v. 44, p. 153–160, 2001b.
VAN HULLE, S.W.H., VAN DEN BROECK, S., MAERTENS, J., VILLEZ, K., SCHELSTRAETE, G., VOLCKE, E.I.P., VANROLLEGHEM, P.A. Construction, start-up and operation of a continuously aerated laboratory-scale SHARON reactor in view of coupling with an Anammox reactor. Water SA, v. 31, p. 327–334, 2005.
VAN LOOSDRECHT, M.C.M. Innovative nitrogen removal. In: Biological Wastewater Treatment. Principles, Modelling and Design. Henze, M., van Loosdrecht, M.C.M., Ekama, G., Brdjanovic, D. (eds). London: IWA, p. 139–153, 2008.
VAN LOOSDRECHT, M.C.M., HAO, X.-D., JETTEN, M.S.M., ABMA, W. Use of Anammox in urban wastewater treatment. Water Science and Technology: Water Supply, v. 4, p. 87–94, 2004.
VAN LOOSDRECHT, M.C.M., SALEM, S. Biological treatment of sludge digester liquid. Proceedings of IWA Specialist Conference Nutrient Management in Wastewater Treatment Process and Recycle Steams, p. 13–22. Krakow, Poland, 2005.
VAN NIFTRIK, L.A., FUERST, J.A., SINNINGUE DAMSTÉ, J.S., KUENEN, J.G., JETTEN, M.S.M., STROUS, M. The anammoxosome: an intracytoplasmic compartment in anammox bacteria. FEMS Microbiology Letters, v. 233, p. 7–13 2004.
VAN NIFTRIK, L.A., GEERTS, W.J.C., VAN DONSELAAR, E.G., HUMBEL, B.M., WEBB, R.I., FUERST, J.A., VERKLEIJ, A.J., JETTEN, M.S.M., STROUS, M. Linking ultrastructure and function in four genera of anaerobic ammonium-oxidizing bacteria: cell plan, glycogen storage and localization of cytochrome c proteins. The Journal of Bacteriology, v. 190, p. 708–717, 2008.
VÁZQUEZ-PADÍN, J., FERNÁDEZ, I. FIGUEROA, M., MOSQUERA-CORRAL, A., CAMPOS. J.L., MÉNDEZ, R. Applications of Anammox based processes to treat anaerobic digester supernatant at room temperature. Bioresource Technology, p. 2988–2994, 2009.
VERSTRAETE, W., PHILIPS, S. Nitrification-denitrification processes and technologies in new contexts. Environ. Poll., v. 102, p. 717–726, 1998.
VILLAVERDE, S., FDZ-POLANCO, F., GARCIA, P.A. Nitrifying biofilm acclimation to free ammonia in submerged biofilters. Start-up influence. Water Research, v. 34, p. 602–610, 2000.
VILLAVERDE, S., GARCÍA-ENCINA, P.A., FDZ-POLANCO, F. Influence of pH over nitrifying biofilm activity in submerged biofilters. Water Research, v. 31, p. 1180–1186, 1997.
VOLCKE, E.I.P., VAN LOOSDRECHT, M.C.M., VANROLLEGHEM, P.A. Controlling the nitrite:ammonium ratio in a SHARON reactor in view of its coupling with an Anammox process. Water Science and Technology, v. 53, p. 45–54, 2006.
WANG, T., ZHANG, H., YANG, F., LIU, S., FU, Z., CHEN, H. Start-up of the Anammox process from the conventional activated sludge in a membrane bioreactor. Bioresource Technology, v. 100, p. 2501–2506, 2009.
WETT, B. Development and implementation of a robust deammonification. Water Science and Technology, v. 56, p. 81–88, 2007.
WETT, B. Solved upscaling problems for implementing deammonification of rejection water. Water Science and Technology, v. 53, p. 121–128, 2006.
WIESMANN, U. Biological nitrogen removal from wastewater. In: Advanced in Biochemical Engineering/Biotechnology. Fiechter, A. (ed.)., v. 51, p. 113–154, 1994.
WONG-CHONG, G.M., LOEHR, R.C. Kinetics of microbial nitrification – nitrite-nitrogen oxidation. Water Research, v. 12, p. 605–609, 1978.
WYFFELS, S., BOECKX, P., PYNAERT, K., VERSTRAETE, W., VAN CLEEMPUT, O. Sustained nitrite accumulation in a membrane-assisted bioreactor (MBR) for the treatment of ammonium-rich wastewater. Journal of Chemical Technology and Biotechnology, v. 78, p. 412–419, 2003.
WYFFELS, S., BOECKX, P., PYNAERT, K., ZHANG, D., VAN CLEEMPUT, O., CHEN, G., VERSTRAETE, W. Nitrogen removal from sludge reject water by a two-stage oxygen limited autotrophic nitrification denitrification process. Water Science and Technology, v. 49, p. 57–64, 2004.
XUE, Y., YANG, F.L., LIU, S.T., FU, Z.M. The influence of controlling factors on the start-up and operation for partial nitrification in membrane bioreactor. Bioresource Technology, v. 100, p. 1055–1060, 2009.
YAMAMOTO, T., TAKAKI, K., KOYAMA, T., FURUKAWA, K. Long-term stability of partial nitritation of swine wastewater digester liquor and its subsequent treatment by Anammox. Bioresource Technology, v. 99, p. 6419–6425, 2008.
YANG, L. Investigation of nitrite build-up within an enriched nitrification process. PhD Dissertation, School of Civil Engineering, Purdue University, West Lafayette, Indiana, 1990.
YANG, L., ALLEMAN, J.E. Investigation of batchwise nitrite build-up by an enriched nitrification culture. Water Science and Technology, v. 26, p. 997–1005, 1992.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Copyright information
© 2018 Springer International Publishing AG
About this chapter
Cite this chapter
Bassin, J.P. (2018). New Processes for Biological Nitrogen Removal. In: Advanced Biological Processes for Wastewater Treatment. Springer, Cham. https://doi.org/10.1007/978-3-319-58835-3_5
Download citation
DOI: https://doi.org/10.1007/978-3-319-58835-3_5
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-58834-6
Online ISBN: 978-3-319-58835-3
eBook Packages: Earth and Environmental ScienceEarth and Environmental Science (R0)