Abstract
The purpose of this chapter is to highlight the state of progress for phosphodiesterase-4 (PDE4) modulation as a potential therapeutic for psychiatric illness, and to draw attention to particular hurdles and obstacles that must be overcome in future studies to develop PDE4-mediated therapeutics. Pathological and non-pathological related memory loss will be the focus of the chapter; however, we will at times also touch upon other psychiatric illnesses like anxiety and depression. First, we will provide a brief background of PDE4, and the rationale for its extensive study in cognition. Second, we will explore fundamental differences in individual PDE4 subtypes, and then begin to address differences between pathological and non-pathological aging. Alterations of cAMP/PDE4 signaling that occur within normal vs. pathological aging, and the potential for PDE4 modulation to combat these alterations within each context will be described. Finally, we will finish the chapter with obstacles that have hindered the field, and future studies and alternative viewpoints that need to be addressed. Overall, we hope this chapter will demonstrate the incredible complexity of PDE4 signaling in the brain, and will be useful in forming a strategy to develop future PDE4-mediated therapeutics for psychiatric illnesses.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction and Background of PDE4
Phosphodiesterases (PDEs) are a collective group of 11 enzyme families that hydrolyze the second messengers cAMP and/or cGMP. The PDE families are differentiated from one another through unique protein structure, tissue expression, cyclic nucleotide specificities, and distinct genes located on different chromosomes (Zhang et al. 2002; Conti et al. 2003; Houslay and Adams 2003; Shepherd et al. 2003; O’Donnell and Zhang 2004). Among the 11 PDE families, PDE4 has received much focus in regards to psychiatric illness due to its strong expression in the brain, in particular, the brain areas involved in psychiatric illness such as the prefrontal cortex, striatum, hippocampus, and amygdala.
PDE4 was first characterized as an enzyme capable of cAMP phosphodiesterase activity from rat liver plasma membranes (Marchmont and Houslay 1980); but, its involvement in cognition and behavior was first identified through mutations in the Drosophila melanogaster dunce PDE4D gene, which led to deficient cAMP-specific PDE activity and abnormal memory (Kauvar 1982; Qui et al. 1991). PDE4 was later discovered in both rodents (Davis et al. 1989; Swinnen et al. 1989) and humans (Bolger et al. 1993) to consist of a small family of enzymes encoded through four different genes, which are now referred to as PDE4-A, B, C, and D (Reeves et al. 1987; Wang et al. 1997; Houslay and Adams 2003; O’Donnell and Zhang 2004). These PDE4 subtypes are not expressed equally throughout the body. PDE4C has been shown to be largely deficient in the brain (Cherry and Davis 1999; Perez-Torres et al. 2000; Lakics et al. 2010). In regards to cognitive research, PDE4-A, B, and D are the predominant subtypes which are focused upon (Zhang 2009).
The canonical protein structure of PDE4 is divided into several domains along the N′ to C′ termini that each play unique functions in the role of the enzyme. The individual PDE4 splice variants have unique N′ termini that are encoded through alternative promoters, and are involved with cellular localization and binding to unique binding partners (Beard et al. 1999, Beard et al. 2002; Jang et al. 2010). The PDE4 enzyme is contrasted from other PDEs in that it contains two upstream conserved regions (UCRs) termed UCR1 and UCR2 located immediately downstream of the N′-terminus region of the protein. These UCR regions play an important regulatory role, modulating both activity of the enzyme (Sette et al. 1996; Hoffmann et al. 1999; Houslay and Baillie 2003; Burgin et al. 2010) and dimerization with other PDE4 units (Richter and Conti 2002, 2004; Bolger et al. 2015). Within the C′-terminus lies the catalytic domain of the PDE4 enzyme which is responsible for the hydrolysis of cAMP to 5′-AMP (Saldou et al. 1998; Conti et al. 2003; Houslay and Adams 2003; Zhang 2009; Houslay 2010). From this canonical structure, there are then numerous PDE4 splice variants within each subtype that are produced through alternative promoters and splicing events. Twenty-five PDE4 splice variants have been identified to date, including 6 PDE4A splice variants, 5 PDE4B variants, 3 PDE4C variants, and 11 PDE4D variants (Bolger et al. 1996, Bolger et al. 1997, Bolger et al. 2003b; Huston et al. 1997; Sullivan et al. 1998; Rena et al. 2001; Miró et al. 2002; Shepherd et al. 2003; Wallace et al. 2005; Richter et al. 2005; Chandrasekaran et al. 2008; Mackenzie et al. 2008; Zhang 2009). This alternative splicing results in the formation of different length PDE4s that are classified as long form, short form, super-short form, and dead-short form. Long-form PDEs are full length and contain no truncation, short forms lack a UCR1 region, super-short forms lack a UCR1 and have a truncated UCR2, and dead-short lack both UCR regions and also contain a truncated catalytic domain that render the PDE4 functionally inactive (O’Donnell and Zhang 2004; Zhang 2009; Richter et al. 2013). Interestingly, this alternative splicing has been shown to be increased in mammals and humans in parallel with increasing levels of cognitive capacity (Johnson et al. 2010). The following sections will describe the expression and broad behavioral roles of the individual PDE4 subtypes.
1.1 PDE4A
The PDE4A subtype has six splice variants; PDE4A1 is short (Sullivan et al. 1998), PDE4A7 is the dead-short form (Horton et al. 1995; Johnston et al. 2004), and PDE4A5 (PDE4A4 is the human orthologue) (McPhee et al. 1995; Huston et al. 2000; Beard et al. 2002), PDE4A8 (Bolger et al. 1996), PDE4A10 (Rena et al. 2001), and PDE4A11 (Wallace et al. 2005) are long forms (Bolger et al. 1997; Owens et al. 1997; Conti et al. 2003; O’Donnell and Zhang 2004; Cheung et al. 2007; Houslay et al. 2007; Chandrasekaran et al. 2008; Lynex et al. 2008; Zhang 2009). The broad PDE4A subtype has been found to be strongly expressed in the olfactory bulbs, deep layers of the cortex, amygdala, hypothalamus, hippocampus, piriform cortex, and cerebellum (Engels et al. 1995; Cherry and Davis 1999; Perez-Torres et al. 2000; McPhee et al. 2001; D’Sa et al. 2005; Lakics et al. 2010; Johansson et al. 2012; Kelly et al. 2013).
PDE4A is one of the few PDE4 subtypes where differential splice-variant expression patterns have been looked at. McPhee and colleagues found that the long PDE4A5/10 isoforms were present throughout most of the cortex, CA1, and CA2 in overlapping patterns. However, PDE4A10 was present in the islands of Calleja while PDE4A1 and PDE4A5 were not. In addition, PDE4A5 and PDEA10 were present in the medial nucleus of the amygdala where PDE4A1 expression was absent. PDE4A1 had a different expression pattern than the longer isoforms, with very strong expression in the glomerular layer of the olfactory bulbs, CA3 of the hippocampus, the cerebellum, and low staining in the brain stem (McPhee et al. 2001). D’Sa and colleagues also looked at PDE4A expression, and observed slightly different patterns. Specifically, they saw that PDE4A1 was high in the medial septum, diagonal band, olfactory system, hippocampus and cerebellum; PDE4A5 was highly expressed in the olfactory nuclei, deep cortical layers, and the DG and CA1 of the hippocampus; PDE4A10 was highly expressed in the DG and CA1; and PDE4A8 expression was absent in the brain (D’Sa et al. 2005).
PDE4A1 has also been found to be unique, in that it is inserted into lipid bilayers through a TAPAS-1 (tryptophan anchoring phosphatidic acid selective-binding domain 1); these bilayers are typically found on golgi that traffic out to the membrane (Baillie et al. 2002). PDE4A5 is unique from the remaining subtypes in that it can interact with SH3 domains on proteins through its N′ Terminal region (O’Connell et al. 1996). This SH3 binding plays a significant role in the targeting of PDE4A5, which has been found to be expressed in perinuclear regions, and also within membrane ruffles (Beard et al. 2002). This expression pattern of PDE4A5 has been shown to be disrupted in apoptotic cells by cleavage of caspase 3 at its SH3 binding sites, and overexpression of PDE4A5 protects against apoptosis (Huston et al. 2000). Another unique trait of PDE4A5 is that it can bind to an immunophilin known as XAP2 which partially inhibits the enzyme up to 60%, and increases its sensitivity to the broad PDE4 inhibitor rolipram (Bolger et al. 2003b). Interestingly, PDE4A can also be activated by the P70S6 kinase in adipocytes during adipogenesis, which suggests it could play a role in obesity (MacKenzie et al. 1998). Lastly, PDE4A5 is the only member of the PDE4A family which can be phosphorylated by MAPK and subsequently attenuate its activation by PKA (MacKenzie et al. 2011). The PDE4A7 and PDE4A8 splice variants are exclusively found in the testis in rodents, however in humans PDE4A8 has undergone an evolutionary change which causes it to be expressed in the brain (Mackenzie et al. 2008).
The role of PDE4A in behavior is at a very preliminary level, however recent studies have shown that PDE4A knock out (KO) mice display increased anxiety-like behavior and enhancement in fear memory (Hansen et al. 2014). Other studies have also shown that PDE4A may play a role in memory deficits caused by sleep deprivation (Vecsey et al. 2009). To date, there are no known PDE4A mutations implicated in the etiology of any human disease, although PDE4A expression has been shown to be altered in the brains of autistic individuals (Fatemi et al. 2010). At this time, there are still no PDE4A selective pharmacological modulators.
1.2 PDE4B
PDE4B has five splice variants identified PDE4B1–5; PDE4B1, 3, and 4 are long isoforms, PDE4B2 is short, and PDE4B5 is super short (Bolger et al. 1997; Conti et al. 2003; O’Donnell and Zhang 2004; Cheung et al. 2007; Houslay et al. 2007; Chandrasekaran et al. 2008; Lynex et al. 2008; Zhang 2009).
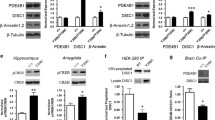
Most of the PDE4 subtype expression patterns overlap a good bit and vary with different studies, but PDE4B is unique in the fact that it is consistently the highest PDE4 subtype expressed in the striatum, globus-pallidus, nucleus accumbens, hypothalamus, and amygdala (Engels et al. 1995; Cherry and Davis 1999; Perez-Torres et al. 2000; McPhee et al. 2001; D’Sa et al. 2005; Lakics et al. 2010; Johansson et al. 2012; Kelly et al. 2013). PDE4B is also unique in that mutations in PDE4B are strongly believed to play a role in schizophrenia through its interaction with disrupted in schizophrenia 1 protein (DISC1) (Millar et al. 2005; Fatemi et al. 2008; Numata et al. 2008, Numata et al. 2009; Kähler et al. 2010). Interestingly, it was observed that this PDE4B mutation results in a decrease of PDE4B expression (Fatemi et al. 2008).
PDE4B KO mice studies have shown that PDE4B is the main PDE4 subtype responsible for the release of tumor necrosis factor-α (TNF-α) in response to lipopolysaccharide injections (LPS). In particular, these studies found that PDE4B KO reduced the amount of TNF-α released in response to LPS and PDE4A/PDE4D did not play a role (Jin and Conti 2002; Jin et al. 2005a). PDE4B was also specifically found to be altered in the brain after systemic LPS infusions, while PDE4A and PDE4D displayed no changes (Johansson et al. 2011). Thus, it appears that PDE4B may be one of the main PDE4 subtypes involved with regulation of microglia (Pearse and Hughes 2016), as PDE4B has also been shown to be involved with Amyloid beta induced microglia activation (Sebastiani et al. 2006). Additional studies have shown that PDE4B KO is anxiogenic and increases corticosterone levels, and also decreases depressive-like behavior in mice (Zhang et al. 2008).
Traditionally, it was not possible to target individual PDE4 subtypes pharmacologically due to the lack of highly selective inhibitors of specific PDE4 subtypes; however, recent studies have demonstrated feasibility for specific inhibition of PDE4B by targeting the Leu674 residue in PDE4B region control region 3 (CR3) (Fox et al. 2014; Hagen et al. 2014).
1.3 PDE4D
PDE4D has 11 identified splice variants termed PDE4D1–11; PDE4D1/2 are short forms, PDE4D6 is super short, and the remaining splice variants are all full length PDE4 proteins (Bolger et al. 1997; Conti et al. 2003; O’Donnell and Zhang 2004; Richter et al. 2005; Cheung et al. 2007; Houslay et al. 2007; Chandrasekaran et al. 2008; Lynex et al. 2008; Zhang 2009; Maurice et al. 2014).
PDE4D has a diffuse pattern of expression throughout the brain, with noticeable expression in the hippocampus, cortex, thalamus, area postrema, periaqueductal grey, brain stem, and cerebellum (Engels et al. 1995; Cherry and Davis 1999; Perez-Torres et al. 2000; McPhee et al. 2001; D’Sa et al. 2005; Lakics et al. 2010; Johansson et al. 2012; Kelly et al. 2013). Initial studies have demonstrated some differential expression of PDE4D splice variants in the brain, with PDE4D2 displaying distinct expression in the dorsal and median raphe nuclei, PDE4D1 in white matter cells, and PDE4D1 and 2 in the area postrema (Miró et al. 2002). PDE4D8 is predominantly expressed outside of the central nervous system, in particular in cardiac myocytes (De Arcangelis et al. 2009; Raymond et al. 2009; Mika and Conti 2015).
In addition, PDE4D also has specific binding partners such as myomegalin protein (Verde et al. 2001), beta arrestins (Baillie et al. 2003, Baillie et al. 2007; Bolger et al. 2003a; Lynch et al. 2005, Lynch et al. 2007; Li et al. 2009), RACK1 (Conti et al. 2003), SH3-domain regions (Beard et al. 1999) and the A-Kinase anchoring proteins (AKAPS), Erk, and exchange protein directly activated by cAMP (EPAC) (Dodge et al. 2001; Dodge-Kafka et al. 2005).
PDE4D has also been extensively studied using PDE4D KO mice designed by homolgous recombination (Jin et al. 2005b) and through viral manipulation. PDE4D KO mice exhibit improved memory (Li et al. 2011), increased synaptic long-term potentiation (LTP) (Rutten et al. 2008), reduced levels of depressive-like behavior (Zhang et al. 2002), elevated neurogenesis (Li et al. 2011), and impaired growth and fertility (Jin et al. 1999). This is supported by studies showing that RNAi or inhibitors with relatively high selectivity for PDE4D also produce memory-enhancing effects (Burgin et al. 2010; Bruno et al. 2011; Li et al. 2011), reverse memory deficits induced by beta-amyloid peptide 1–42 (Zhang et al. 2014) and/or produce antidepressant activity (Wang et al. 2013). Furthermore, mice deficient in PDE4D have been shown to be less senitive to the antidepressant effect of rolipaim (Zhang et al. 2002). Together, these studies have strongly implicated PDE4D in memory as well as depressive-like behavior. Unfortunately, PDE4D KO also causes emetic-like behavior, the major side effect of broad PDE4 inhibitors such as rolipam (Robichaud et al. 2002a, b). Upon further investigation this data makes sense, as PDE4D has the highest expression of any of the PDE4 subtypes in the area postrema which is the emetic center of the brain (Cherry and Davis 1999; Miró et al. 2002; Mori et al. 2010).
PDE4D single nucleotide polymorphisms (SNP) have also been associated with stroke (He et al. 2013; Heyer et al. 2013; Liu et al. 2013a, b), however the results have been inconsistent (Shao et al. 2014). In a meta-analysis, Yan and colleagues found that PDE4D SNP83 is higher in Asian and Chinese populations, but not among Caucasians (Yan et al. 2014). PDE4D mutations have also been associated with skeletal dysplasia and intellectual disability (Lindstrand et al. 2014).
While it was previously very difficult to make a PDE4 subtype-specific inhibitor, x-ray structure of the PDE4 proteins (Wang et al. 2007a) revealed a phenylalanine-196 residue that was only present on PDE4D, which was able to be targeted by allosteric inhibitors (Burgin et al. 2010). These PDE4D allosteric inhibitors were able to reduce the emetic effects of PDE4D inhibition as measured through duration of anesthesia induced by ketamine/xylazine (Robichaud et al. 2002a, b) while maintaining the beneficial nootropic effects (Burgin et al. 2010). The memory enhancing effects of PDE4D dysruption have also been supported by other recently developed PDE4D selective inhibitors (Bruno et al. 2011, 2014; Ricciarelli et al. 2017; Sierksma et al. 2013).
2 Differences Between Normal Aging and Pathological Aging
Contrary to public belief, normal aging does not necessitate a general decline of overall cognitive ability. Indeed, normal aging usually affects specific cognitive abilities such as spatial memory, working memory, reaction time, and long-term memory (Podtelezhnikov et al. 2011; Hansen and Zhang 2013). Not all individuals are affected in normal aging, and certain forms of cognition such as short-term memory remain unchanged, while other forms of memory such as semantic memory can actually improve with age (Glisky 2007).
It was initially believed that with normal aging there is a natural loss of neurons in the brain (Brody 1955; Shefer 1973; Henderson et al. 1980); however, it is now believed there is no significant neuronal death that occurs with normal aging (Peters et al. 1998). The previous misconceptions were likely due to artifacts involved with the processing of aged brains (Haug 1985). Young brains shrink more during the fixation process, giving them the appearance of increased neuronal density (Peters et al. 1998). Once this confounding variable was accounted for through the use of unbiased stereological methods, the aged brain displays no differences in neuronal number (Giannaris and Rosene 2012).
One change normally observed in health aging is a decrease in brain volume (Skullerud 1985; Scahill et al. 2003; Driscoll et al. 2009) which may be due to decreases in neuronal size and somatic density (Terry et al. 1987). An alternative theory is that there is a loss of synapses in the aged brain (Geinisman 1999; Terry and Katzman 2001); however, others have observed no change of synapses in the aged brain (CA1 of the rat hippocampus), suggesting that memory deficits observed in senescence may be functional in nature (Geinisman et al. 2004). This decreased functional hypothesis is supported through observations that columns in the rhesus monkey brain display attenuated strength despite the fact that neuronal density and number are not changed (Cruz et al. 2004). These changes observed in normal aging are much different from what is seen in pathological aging.
Pathological aging or dementias such as Alzheimer’s Disease (AD) are a significant diversion from the aging process, and have many differences from normal aging. Unlike normal aging which displays no significant changes in neuronal death, AD is characterized by massive atrophy in the brain which results in cognitive deficits much more severe than normal aging (Scheltens et al. 1995; Jack et al. 1997; Drachman 2006; Glisky 2007; Herrup 2010; Podtelezhnikov et al. 2011). The exact mechanisms and etiology for AD are still unknown, but in sporadic or non-familial cases (which account for the majority of AD cases) age is the main risk factor. Individuals over the age of 85 have a 50% chance of contracting this disease, and after contracting the disease there is an average survival of 5–10 years (Drachman 2006; Herrup 2010; Podtelezhnikov et al. 2011).
It has been suggested that AD could represent an accelerated aging process of the brain (Podtelezhnikov et al. 2011) and that the neurons could be undergoing massive functional transformation. Indeed, AD brains display significant changes in genes involved with lipid metabolism, the stress response, and inflammation. Interestingly, they also have changes in genes involved with cell adhesion, migration, and morphogenesis that bear a striking resemblance to patterns of gene expression also observed in epithelial mesenchymal transition (EMT) tissues (Podtelezhnikov et al. 2011). These findings reveal a similarity between AD brains and EMT that suggest a major transformation in brain tissue physiology and receptor signaling is occurring in AD. When the data obtained from the normal aged patients was extrapolated, it was predicted that in non-pathological aging, normal subjects would not reach cognitive decline comparable to AD subjects until 130–140 years of age (Podtelezhnikov et al. 2011). Other studies have confirmed that massive gene changes take place in AD. While some of these gene changes also occur in normal aging, the unique changes only in AD reinforce that pathological and normal again processes are different (Miller et al. 2008). We will next focus on alterations that occur with cAMP and PDE4 with age, and discuss the correlations that this has with cognitive decline.
3 Alterations of cAMP/PDE4 Signaling in Normal Aging
There are many components to the cAMP pathway that could be altered with aging. The immediate downstream effector of cAMP is protein kinase A (PKA), which releases from autoinhibition upon cAMP binding, and then translocates to the nucleus where it phosphorylates cAMP response element binding protein (CREB). CREB is a 43 kDa transcription factor that binds to cAMP response element (CRE) promoter sites (Carlezon et al. 2005) located on specific CRE-mediated genes such as brain derived neurotrophic factor (BDNF) (Finkbeiner et al. 1997; Tao et al. 1998); however, thousands of other CREB targets have been identified (Conkright et al. 2003; Zhang et al. 2005). CREB has been highly implicated in learning and memory (Dash et al. 1990; Bourtchuladze et al. 1994) and long term potentiation (LTP), a putative mechanism for the formation of memory (Impey et al. 1996). Other downstream effectors for cAMP include exchange protein directly activated by cAMP (EPAC) (de Rooij et al. 1998; Kraemer et al. 2001; Bos 2006; Gloerich and Bos 2010); or hyperpolarization-activated cyclic nucleotide-gated (HCN) channels which are expressed throughout the brain (Moosmang et al. 1999) and can be activated by cAMP resulting in significant influences on neuronal activity strength and behavior (Ramos et al. 2006; Wang et al. 2007b; Arnsten et al. 2010). It is important to note that as a controller of cAMP, PDE4 and its subtypes therefore also regulate these downstream targets, and changes in PDE4 activity, expression, or even localization could all have significant effects downstream.
When addressing what effects normal aging has on cAMP levels there are many variables to take into consideration. First, it should not be expected that there would be a global increase or decrease of cAMP throughout the entire body or even within the brain itself; therefore, it’s possible nuanced changes might be occurring within discrete brain regions or nuclei where one region displays increased cAMP and another displays decreased cAMP. It is also important to differentiate between baseline and stimulated levels of cAMP. Although baseline levels may remain unchanged, stimulated levels which would occur through application of a neurotransmitter such as dopamine or norepinephrine may reveal a different finding. Also, one needs to take into consideration the model organism, and the method with which the tissue was collected and cAMP measured. It has been suggested that cAMP and cGMP in vivo are notoriously difficult to study and can change quickly after death; because of this, some would argue that the only “accurate” way to measure cAMP from a living animal would be to perform microwave fixation (Stavinoha 1993; Delaney and Geiger 1996; O’Callaghan and Sriram 2004; Murphy 2010). Microwave fixation is able to achieve near instantaneous denaturing of proteins (rendering them inactive) in the brain such as adenylyl cyclases (AC), proteases, phosphatases, and PDEs, all of which could contribute to confounding changes in cAMP levels after death in animals. Microwave fixation essentially allows the researcher to save a “snapshot” of the brain comparable to when the animal was alive, and avoids most of the confounds observed with various other methods of sacrificing (O’Callaghan and Sriram 2004).
With these variables in mind, there have been several studies which have sought to determine how cAMP changes with maturation and normal aging (Hansen and Zhang 2013). One of the first studies showed that in the cerebral cortex of aged rats there was a noticeable decrease in baseline cAMP from 26 to 2 pmol/mg at 2–6 months of age, respectively (Zimmerman and Berg 1974); however, levels were unchanged during the last 18 months of life. This is consistent with other studies that have shown decreased cAMP in aged rat cerebellum (Austin et al. 1978) and striatum (Sugawa and May 1994), senescent human lymphocytes (Birkenfeld and Ben-Zvi 1984), and the aged gerbil hippocampal CA1 region and cerebral cortex (Hara et al. 1992). Schmidt and Thornberry showed that in aged rats from 3–24 months of age, there was no decline of baseline cAMP levels in the hypothalamus, cortex, hippocampus, or brain stem; however, there was a 44% decrease of norepinephrine-stimulated cAMP levels in the cerebellum (Schmidt and Thornberry 1978). Others have also shown no change in baseline levels of cAMP with aging, yet observed attenuated dopamine activation of adenylyl cyclases (Puri and Volicer 1977; Sugawa and May 1994). Contradicting these results, elevations of cAMP have been observed in the striatum of aged rats (Sugawa and May 1993; Sugawa and May 1994). The data on how baseline levels of cAMP change with aging have yet to come to agreement; however, the effect of aging on activated levels of cAMP seems to be more consistent. It has been observed that adenylyl cyclase activity (Makman et al. 1980; O’Connor et al. 1981; Sugawa and May 1994) and expression (O’Connor et al. 1983; Araki et al. 1995; Ramos et al. 2003; Mons et al. 2004) decrease with age, suggesting that there may be a loss of hormonal sensitivity with aging (Boas et al. 1973). Baseline levels of adenylyl cyclase are actually increased in the aged caudate and cerebellum, while activated levels of AC are lower in all the brain regions tested (Boas et al. 1973). It is interesting that these two regions have altered baseline activity of adenylyl cyclase, as it was previously mentioned that both the striatum and cerebellum have been noted to have consistent changes in cAMP with aging. One possible explanation is that different brain regions age differently. While increased baseline adenylyl cyclase activity in the cerebellum could be a compensatory mechanism to account for a loss of cAMP (Austin et al. 1978; Schmidt and Thornberry 1978), increased baseline activity in the caudate could be the direct reason for elevated levels of cAMP observed in the aged striatum (Sugawa and May 1993; Sugawa and May 1994). Although these studies fail to converge on an overall unifying trend of how cAMP changes with aging in the brain, they at least suggest cAMP levels are altered, and these alterations may be specific to different brain regions.
Changes in cAMP observed with aging could be due to differences in neurotransmitter levels and altered PDE4 activity. Indeed, neurotransmitters such as dopamine, norepinephrine, acetylcholine, and glutamate have been observed to be decreased in the aged brain (Austin et al. 1978; Kaiser et al. 2005; Tomobe et al. 2007). Though there have been no consistent results on how PDE4 changes in the healthy aged brain, accumulating evidence suggests altered PDE4 activity and expression may contribute to changes in cAMP. PDE4 activity has been shown to be increased in the cortex, hypothalamus, and hippocampus of aged rats (Stancheva and Alova 1991); meanwhile decreases of PDE4 activity have been observed in the aged Macaca mulatta striatum and frontal cortex, and rat brain (Tohda et al. 1996). PDE4 expression was shown to be unchanged in the aged mouse hippocampus (Kelly et al. 2013), however it was decreased in the cerebral cortex which is in agreement with other studies (Tohda et al. 1996). Interestingly, PDE4A expression has also been shown to be decreased in the striatum (Kelly et al. 2013), which parallels the increase cAMP observed in this area (Sugawa and May 1993; Sugawa and May 1994). PDE4D is significantly decreased in the aged mouse cerebellum (Kelly et al. 2013), which has consistently shown age-related changes in cAMP levels (Austin et al. 1978; Schmidt and Thornberry 1978). One would expect decreased PDE4D expression in the cerebellum to result in increased cAMP levels. However, this decrease may be compensatory in nature to adapt to the falling levels of cAMP, which may be due to a loss of hormonal sensitivity or adenylyl cyclase activity. Other studies have not observed age-related changes of PDE4 in the brain (Puri and Volicer 1977; Tohda et al. 1996; Ramos et al. 2003). Overall, these findings highlight the incredible complexity of PDE4 signaling. Regulation of PDEs are elaborate, and the very molecule (cAMP) they hydrolyze also regulates their expression in a negative feedback loop (Liu et al. 2000; O’Donnell and Xu 2012). Interestingly, elevations of cAMP can increase PDE4 expression through a CREB dependent mechanism (D’Sa et al. 2002), thus downstream targets of cAMP also have further feedback regulation of its own activity.
Alterations of cAMP signaling should result in changes in downstream targets. Decreases of PKA activity have been seen in the aged fruit fly brain (Laviada et al. 1997) and hippocampus and frontal cortex of aged rats (Karege et al. 2001a, b). Decreases in PKA activity resulting from altered cAMP signaling should subsequently diminish phosphorylated activation of CREB (pCREB). Indeed, decreases of CREB signaling have been observed in the aged brain (Yamamoto-Sasaki et al. 1999; Hattiangady et al. 2005; Kudo et al. 2005; Porte et al. 2008; Xu et al. 2010). CREB is an important mediator of LTP and memory (Dash et al. 1990; Bourtchuladze et al. 1994; Yin et al. 1994; Impey et al. 1996; Johannessen et al. 2004; Carlezon et al. 2005; Brightwell et al. 2007) and enhancement of pCREB activation in the aged brain improves memory (Xu et al. 2010; Zhao et al. 2013). These results show that while cAMP signaling may be diminished in certain brain areas, restoration of downstream signaling may be able to rescue the behavioral deficits caused by this alteration in function. Together, this suggests that cognitive deficits which develop with normal aging are not permanent, and offers hope these deficits are functional in nature with the possibility of being treated.
4 Alterations of cAMP/PDE4 Signaling in Pathological Aging
Pathological aging such as AD also results in significant alterations of cAMP signaling. Alzheimer’s patients display increased cAMP in their cerebrospinal fluid (CSF) (Martınez et al. 1999) and microvessels of the brain, particularly the hippocampus (Hernandez et al. 2001). BACE1 is the enzyme responsible for the proteolytic cleavage of amyloid-β protein that results in the hallmark neuritic plaques of AD. Interestingly, in addition to its contribution to the formation of amyloid plaques, BACE1 directly interacts with adenylyl cyclase via its transmembrane domain, and overexpression of BACE1 results in the reduction of cAMP signaling and downstream targets such as PKA and pCREB (Chen et al. 2012). Adenylyl cyclase activity and expression are also decreased in AD brains (Ohm et al. 1989, 1991; Cowburn et al. 1992; O’Neill et al. 1994; Schnecko et al. 1994; Yamamoto et al. 2000).
Altered adenylyl cyclase activity would most likely immediately affect PKA activation. Administration of amyloid-β to hippocampal neurons decreases PKA activity; when given the selective PDE4 inhibitor rolipram, this effect was able to be reversed (Vitolo et al. 2002). Interestingly, in both human AD and animal models there is a loss of LTP and spatial memory before neuronal death and morphological changes occur (Vitolo et al. 2002). These observations suggest biochemical changes precede anatomical changes, and might represent a potential site for therapeutic intervention. Increased PKA-regulatory subunit expression has also been observed in AD subjects (Blalock et al. 2003). Increased expression of the PKA-regulatory subunit could be predicted to decrease PKA activity and downstream signaling.
In addition to altered adenylyl cyclase activity, altered PDE4 signaling could directly result in cAMP alterations. McLachlan and colleagues observed decreased expression of PDE4 isoforms in the hippocampus of AD subjects, however, the PDE4D1 isoform doubled in expression (McLachlan et al. 2013). PDE4 expression also changes dynamically with the severity of the disease. During stage 1 through 2 of the Braak scale, AD brains display increased expression of PDE4A and PDE4B mRNA in the entorhinal cortex, and an increase of PDE4A mRNA in the frontal cortex (Pérez-Torres and Mengod 2003). Progression to Braak stages 3–4, results in decreased PDE4A expression in the frontal cortex and CA2 region of the hippocampus, and increased PDE4D expression in the putamen (Pérez-Torres and Mengod 2003). It is possible that in the early stages of AD or Braak stages 1–2, increased levels of PDE4 are a direct effect of amyloid-β plaques or oligomers, causing a decrease in cAMP levels and subsequent decrease in the cAMP/CREB pathway. However, in the later stages of the disease, the decrease in PDE4A expression seen in the frontal cortex and CA2 could be a compensatory mechanism due to chronically reduced levels of cAMP (O’Donnell and Xu 2012). Application of amyloid-β to rat microglial cells increases PDE4B expression and TNF-α production (Sebastiani et al. 2006). It is interesting to note that inflammation may be a major mediator of Alzheimer’s disease (Rubio-Perez and Morillas-Ruiz 2012), and that PDE4B is highly implicated in inflammation processes (Jin and Conti 2002; Jin et al. 2005a; Pearse and Hughes 2016). Inhibition of PDE4B and PDE4D may represent novel therapeutic targets for Alzheimer’s disease (Gurney et al. 2015; Pearse and Hughes 2016).
Diminished cAMP signaling should manifest downstream through decreased phosphorylation of CREB. Phospho-CREB levels are reduced in the Alzheimer’s Tg2576 mouse model, and exposure of rat primary hippocampal neurons to amyloid-β decreases CREB promoter activity (Pugazhenthi et al. 2011). Application of amyloid-β to hippocampal cultures also reduces glutamate-invoked pCREB levels (Vitolo et al. 2002), and rats infused with amyloid-β into the hippocampus display decreased pCREB and memory (Wang et al. 2012). Alzheimer’s brains display an inverse correlation between amyloid-β levels and CREB (Pugazhenthi et al. 2011). Familial Alzheimer’s mutations in amyloid precursor protein (APP) negatively regulate CRE-mediated transcription (Ikezu et al. 1996; Giambarella et al. 1997). Indeed, diminished pCREB has been observed in Alzheimer’s brains (Yamamoto-Sasaki et al. 1999). However, other enzymes involved in the formation of amyloid-β such as APP and the presenilins do not always result in reduced pCREB and subsequent CRE mediated gene expression. Mutations in the presenilin gene which is observed in familial AD result in constitutive over-activation of CREB (Müller et al. 2011). The amount of amyloid-β present may also fundamentally affect how the CREB pathway is altered. For instance, moderate levels of intracellular amyloid-β increase CRE-mediated gene expression (Echeverria et al. 2005). However, when these levels are significantly raised beyond normal levels, or are aggregated into fibrils and plaques like in AD, there is a significant decrease in CRE-mediated gene expression (Echeverria et al. 2005; Arvanitis et al. 2007). This suggests the relationship between amyloid-β and CREB modulation may be more complicated than a simple linear relationship, and in fact may be more accurately represented by the “inverted U” model. Non-pathological levels of amyloid-β may increase CREB activity, while pathological levels decrease CREB activity. Both elevations and decreases in CREB-mediated transcription have been observed in AD brains, suggesting that CREB dysregulation as a whole is altered in AD pathology. CREB-mediated signaling is altered in the first stages of incipient AD, suggesting it could be one of the first pathways to change in the start and progression of the disease (Satoh et al. 2009). Taking into consideration the aforementioned studies of how amyloid-β is able to both enhance and impair CREB, these observations should not be surprising. CREB has been shown to regulate thousands of gene transcription products (Johannessen et al. 2004; Carlezon et al. 2005). This dysregulation could represent both direct pathological manifestations of the disease and compensatory mechanisms aimed at combatting it.
5 Evidence for PDE4 Modulation as a Therapeutic Target in Pathological and Non-Pathological Aging
The literature suggests that cAMP signaling is altered in both normal aging and pathological aging; however, the question remains whether restoration of this signaling can improve cognitive function. Recent preclinical studies have shown that modulation of the cAMP pathway may attenuate cognitive deficits.
Amyloid-β decreases PKA and CREB activity in neuronal culture, and this effect is reversed through pretreatment with rolipram (Vitolo et al. 2002). Rolipram is also able to attenuate amyloid-β induced elevations of TNF-α and PDE4B in rat microglial cells (Sebastiani et al. 2006). This highlights the potential effects of PDE4 inhibition on inflammation, which is thought to play a main role in the disease (Frankola et al. 2011; Galimberti and Scarpini 2011; Clark et al. 2012; Montgomery and Bowers 2012; Rubio-Perez and Morillas-Ruiz 2012), and that targeting PDE4B may represent a novel therapeutic target for Alzheimer’s disease (Pearse and Hughes 2016). In mouse models of AD, rolipram reverses deficits in LTP and memory, and pCREB levels in the hippocampus (Gong et al. 2004). These effects appear to be long lasting, as one course of chronic rolipram treatment was able to improve LTP and memory in AD mice 2 months after the end of their treatment (Gong et al. 2004). In other animal studies where amyloid-β was infused into the hippocampus, rolipram treatment was able to attenuate the effects of amyloid-β by improving memory and increasing pCREB levels (Cheng et al. 2010), and also reversed apoptotic responses (Wang et al. 2012). Reversal of apoptotic responses is particularly important in regards to pathological aging due to the large loss of neurons observed. PDE4 inhibition is also able to reverse morphological changes in AD models. Supporting this, Smith and colleagues found that treatment with rolipram reversed decreases in dendritic spine density in the hippocampus of transgenic AD-APP mice (Smith et al. 2009). The effects of rolipram have even been investigated against the deficits caused by iron loading in the brain, a process that occurs with aging and has been suggested to be responsible for age-induced cognitive impairments and neurodegeneration. Rolipram administration was able to reverse the effects of iron deposition on object recognition memory (de Lima et al. 2008). Rolipram and drugs that enhance cAMP signaling also rescue memory and LTP deficits in aged WT C57Bl/6J mice (Bach et al. 1999).
More recently there have been advances in the development of PDE4B and PDE4D subtype specific inhibitors (Burgin et al. 2010; Bruno et al. 2011, 2014; Azam and Tripuraneni 2014; Fox et al. 2014). The PDE4D specific inhibitor GEBR-7b enhances memory without the standard emetic side effects observed with rolipram (Miró et al. 2002; Mori et al. 2010; Bruno et al. 2011). GEBR-7b was also able to reverse the spatial memory deficits observed in AD APPswe/PS1dE9 mice, although pCREB levels were unchanged (Sierksma et al. 2013). Knock down of the long form PDE4D splice variants also reversed the memory deficits caused by amyloid-β (Zhang et al. 2014). These findings are in agreement with earlier studies suggesting PDE4D is the predominant subtype involved with memory processes (Burgin et al. 2010; Li et al. 2011).
Resveratrol is another compound that hints at the potential of PDE4 for aging related phenotypes; however it might work in a slightly different manner than rolipram. Resveratrol is a polyphenol compound typically found in red wine of which the nootropic neurotrophic effects have been known for some time (Tredici et al. 1999); however, it was recently discovered that resveratrol is a non-specific PDE inhibitor able to mimic the effects of caloric restriction, and reverse metabolic aging-like phenotypes (Park et al. 2012). Most recently, we found that resveratrol reversed Aβ1-42-induced cognitive deficits via PDE4 inhibition and its subsequent activation of cAMP-CREB-BDNF signaling (Wang et al. 2016). The other pathway whereby resveratrol benefits aging is through a downstream cAMP effector known as EPAC, which is a guanine nucleotide exchange factor (GEF) for the small GTPases RAP1/2 (de Rooij et al. 1998; Kraemer et al. 2001; Bos 2006; Roscioni et al. 2008; Gloerich and Bos 2010) that exhibits implications for memory enhancement (Ouyang et al. 2008) and nerve growth (Murray and Shewan 2008; Murray et al. 2009). Reversal of age-induced metabolic phenotypes by resveratrol and EPAC was further found to be through the Sirtuin (SIRT) family (Park et al. 2012), which has been highly implicated in longevity and aging (Kim et al. 2007; Cristòfol et al. 2012). The effects of resveratrol were further explored in normally aged mice, and it was found that resveratrol was able to improve both memory and LTP following infusions into the cerebral ventricles (Zhao et al. 2013). Interestingly, the effect of resveratrol was not seen in SIRT1 KO mice. Further examination into the mechanisms of resveratrol action suggest downregulation of microRNAs 134 and 124, which are thought to regulate CREB expression (Zhao et al. 2013). These findings once again implicate CREB as one of the major potential downstream targets that might benefit from PDE4 inhibition and increased cAMP signaling.
The discovery of EPAC in downstream cAMP signaling has opened up new doors for therapeutic intervention. Interestingly, in the brains of AD patients, EPAC expression is altered (Mcphee et al. 2005). Typically, the two forms of EPAC (EPAC1 and EPAC2) have contrasting expression in the brain with EPAC2 more highly expressed in the CNS, and EPAC1 in the periphery; however, in AD brains EPAC1 expression is elevated, while EPAC2 expression is decreased (Mcphee et al. 2005). Other studies have also implicated EPAC in AD; activation of EPAC increased production of sAPPα (Maillet et al. 2003; Zaldua et al. 2007). This is a small neurotrophic molecule formed from the breakdown of APP in the non-amyloidogenic pathway. Thus, it is possible that activation of EPAC could shift the breakdown of APP away from the amyloidogenic pathway and the production of pathogenic levels of amyloid-β, towards the non-amyloidogenic pathway and production of therapeutic levels of the neurotrophic sAPPα.
6 Pitfalls and Side Effects of PDE4 Inhibition
Although targeting PDE4 for age-related memory loss seems promising, there are many side effects and pitfalls which need to be considered. The idea of using PDE4 as a target for psychiatric illness is not a novel one. Since PDE4 was first discovered, it was found shortly thereafter that the broad PDE4 inhibitor rolipram significantly improved memory (Randt et al. 1982; Egawa et al. 1997; Imanishi et al. 1997; Barad et al. 1998), and reversed depressive-like behavior in rodents (Wachtel 1983; Wachtel and Schneider 1986). Because of these initial findings, rolipram made it to clinical trials as a potential antidepressant and showed both promising (Zeller et al. 1984; Guiot-Goffioul et al. 1987; Laux et al. 1988) and inconsistent results (Bertolino et al. 1988). However, it was soon discovered that rolipram had a major flaw; several studies began confirming that rolipram induced severe nausea and emesis (Hebenstreit et al. 1989; Scott et al. 1991). The failure of rolipram in clinical trials leads to an important question, why was rolipram destined to fail? It has since been discovered that PDE4 is highly expressed in the area postrema (Cherry and Davis 1999) which is the emetic center of the brain (Mori et al. 2010). Inhibition of the PDE4 in this region “activates” the area postrema, and produces the emetic response typical of rolipram (Heaslip and Evans 1995; Robichaud et al. 2001; Richter et al. 2013). This emesis is most likely mediated by PDE4D, which has the highest expression levels in the area postrema (Cherry and Davis 1999; Miró et al. 2002; Mori et al. 2010). Subtype-specific inhibition would be one strategy to avoid the side effects caused by broad PDE4 inhibition. Recently subtype-specific inhibitors for PDE4B (Fox et al. 2014; Hagen et al. 2014) and PDE4D (Burgin et al. 2010; Bruno et al. 2011) have been developed that may avoid the strong emetic effects plaguing previous PDE4 inhibitors.
The development of broad PDE4 inhibitors for treating cognitive disorders in the future will be a difficult venture and is most likely not the most appropriate strategy. PDE4 is distributed throughout the body (Johansson et al. 2012), so treatment of a cognitive disorder with a broad PDE4 inhibitor would most likely result in many off-target effects. In addition, the multiple PDE4 subtypes and splice variants display differential expression in various brain regions and within individual neurons themselves. Within individual neurons distinct N′ termini of the individual splice variants results in unique compartmentalization, which is important for the control of different transduction messages, and allows the neuron to retain specificity over different pathways (Catherine Jin et al. 1998; Houslay and Adams 2003; Martin and Cooper 2006; Baillie 2009; Houslay 2010; Oliveira et al. 2010; Blackman et al. 2011; Vincent et al. 2012). This suggests the PDE4 subtypes and splice variants have non-redundant functional roles, and further demonstrates that broad inhibition is not the most appropriate strategy.
In addition, it can’t be assumed that elevations of cAMP/CREB signaling is beneficial in all brain regions. Although elevation of cAMP and pCREB enhances memory in the hippocampus, increasing cAMP in the amygdala could promote anxiety-like behavior or thoughts. In vitro studies demonstrate the anxiogenic corticotrophin releasing factor (CRF) elevates cAMP signaling, whereas the anxiolytic compound neuropeptide Y (NPY) decreases cAMP signaling (Mulchahey et al. 1999; Sheriff et al. 2001). This parallels in vivo studies that show stress and anxiety are correlated with increases in pCREB (Adamec et al. 2011) and BDNF levels (Lakshminarasimhan and Chattarji 2012). Furthermore, overexpression of pCREB in the amygdala increases anxiety-like behavior (Wallace et al. 2004), and PDE4A and PDE4B KO mice display significant increases in anxiety-like behavior that correlate with an increase in cAMP signaling (Zhang et al. 2008; Hansen et al. 2014). Elevation of pCREB signaling in the nucleus accumbens can also have additional detrimental effects. Stress in rats produces an increase in CREB activation in the nucleus accumbens, and overexpression of CREB in this area leads to depressive-like anhedonia; dominant-negative infusion of CREB displays the opposite behavioral effect (Muschamp et al. 2011). Also, elevated cAMP/PKA activity in the prefrontal cortex impairs working memory (Taylor et al. 1999; Ramos et al. 2003), and aged rats have elevated levels of CREB and pCREB in the PFC as they age (Ramos et al. 2003; Vandesquille et al. 2013). It should come as no surprise that working memory and long-term memory (LTM) are affected differently by cAMP signaling as working memory “requires the continuous and dynamic updating of memory buffers, whereas long-term memory consolidation involves changes that are static and long lasting” (Arnsten et al. 2005).
One other pitfall is that the effects of altering cAMP levels on different pathological targets remain to be fully understood. While the majority of research shows amyloid-β decreases cAMP/CREB signaling, there are some studies showing the opposite (Echeverria et al. 2005; Satoh et al. 2009; Müller et al. 2011). CREB is one of the principal transcription factors affected in AD. A complete dysregulation of CREB signaling has been observed with some transcripts being up regulated and others down regulated (Blalock et al. 2003). Eliciting pathological changes from compensatory changes observed in this altered CREB signaling are a significant hurdle as cAMP/PDE4 signaling is notorious for being incredibly compensatory (O’Donnell and Xu 2012). Globally raising cAMP could have detrimental effects contributing to the pathology of AD. For instance, raising cAMP levels causes an increase in APP protein expression, and activation of both the amyloidogenic and non-amyloidogenic pathways (Canepa et al. 2013). Assuming the “amyloid hypothesis” of AD is correct, increasing APP could have detrimental effects on the amount of amyloid-β plaques produced and subsequent cognitive performance. Increased cAMP signaling also results in hyper-phosphorylation of tau protein, one of the hallmark pathologies of AD (Litersky and Johnson 1992; Jicha et al. 1999).
7 The Future of PDE4 for Therapeutic Intervention
Although much has been learned about PDE4 in cognition, there is still much to be discovered. First, the field needs to consistently characterize where individual PDE4 splice variants are expressed in the brain, and the roles these variants play in behavior and cognition. This is one of the most complicated challenges that need to be overcome due to the large number of splice variants in the PDE4 family, unique expression of these splice variants in different brain regions, and localized expression of these variants within individual neurons which leads to microdomains and compartmentalization of signaling. Progress has been made with the development of PDE4B and PDE4D inhibitors (Burgin et al. 2010; Bruno et al. 2011; Fox et al. 2014); however, there are still no PDE4A specific inhibitors, nor are there drugs capable of targeting specific PDE4 splice variants. An optional approach to using drugs to identify the functional roles of PDE4 specific splice variants would be to use genetic approaches such as viral-mediated knockdown (Li et al. 2011; Wang et al. 2013; Zhang et al. 2014). Due to the incredible number of PDE4 splice variants, this will take time and patience. Characterization of unique splice variant compartmentalzation is a fundamental issue that needs to be addressed in future research, as this may allow for targeted intervention of unique downstream targets for the individual splice variants that may not be able to be resolved through subtype inhibition.
Inhibition of PDE4 may also not be appropriate in all scenarios. One should recognize the incredibly delicate balance of cAMP signaling in the brain, and that global activation of cAMP through broad PDE4 inhibition might not be the best approach. In regards to working memory, it appears that decreases in cAMP levels in the PFC would actually improve memory. This also might hold true in the nucleus accumbens and amygdala, as overexpression of CREB in these regions has been shown to produce anhedonia (Muschamp et al. 2011) and anxiety, respectively (Wallace et al. 2004). There may need to be a paradigm shift in the way we think about PDE4 modulation as a therapeutic for cognitive disorders; in particular, researchers may need to begin thinking about PDE4 activation as a possible therapeutic strategy for the future. This makes sense in regards to anxiety or working memory. However, in regards to hippocampus-dependent memory PDE4 inhibition may still be the best approach.
Despite these drawbacks facing PDE4 as a therapeutic for aging and AD, PDE4 cognitive therapy remains promising. Many of the initial mechanisms that are disrupted in normal aging are also present in the early and later stages of AD. If animal models hold true, PDE4 inhibition may reverse morphological and functional behavioral changes that occur both in senescent memory and the initial pathological decline in AD. One of the main differences between AD and normal aging is the large number of neurons are killed as pathogical aging progresses, whereas no signficant neuronal loss occurs with normal aging. Thus, it seems that in normal aging the biological mechanisms may become stagnant, which shows great promise for PDE4 modulation as a therapeutic to “wake up” the cAMP signaling pathways. In regards to AD, the most promise for PDE4 modulation would be at the beginning of the disease before neuronal death begins to occur, and the signal transduction pathways are beginning to be changed. Once neuronal death begins to occur, the best hope for PDE4 therapies would be to stop further progression of the disease through increased neurogenesis or decreased apoptosis. It is unknown if PDE4 modulation would be able to restore lost cognitive ability derived from neuronal loss; however, elevations of cAMP signaling increases neurogenesis and enhances memory (Li et al. 2011), indicating a role of PDE4 in cognitive deficits caused by neuronal loss. Overall, PDE4 modulation could represent a prophylactic therapeutic for AD and other CNS diseases with cognitive deficits. However, more research is needed into the field to characterize the consequences of altering cAMP levels in a disease as severe as AD.
References
Adamec R, Hebert M, Blundell J. Long lasting effects of predator stress on pCREB expression in brains regions involved in fearful and anxious behavior. Behav Brain Res. 2011;221:118–33.
Araki T, Kato H, Fujiwara T, Itoyama Y. Age-related changes in bindings of second messengers in the rat brain. Brain Res. 1995;704:227–32.
Arnsten AFT, Ramos BP, Birnbaum SG, Taylor JR. Protein kinase A as a therapeutic target for memory disorders: rationale and challenges. Trends Mol Med. 2005;11:121–8.
Arnsten AFT, Paspalas CD, Gamo NJ, Yang Y, Wang M. Dynamic network connectivity: a new form of neuroplasticity. Trends Cogn Sci. 2010;14:365–75.
Arvanitis DN, Ducatenzeiler A, JN O, Grodstein E, Andrews SD, Tendulkar SR, Ribeiro-da-Silva A, Szyf M, Cuello AC. High intracellular concentrations of amyloid-beta block nuclear translocation of phosphorylated CREB. J Neurochem. 2007;103:216–28.
Austin J, Connole E, Kett D, Collins J. Studies in aging of the brain. V. Reduced norepinephrine, dopamine, and cyclic AMP in rat brain with advancing age. Age. 1978;1:121–4.
Azam MA, Tripuraneni NS. Selective phosphodiesterase 4b inhibitors: a review. Sci Pharm. 2014;82:453–81.
Bach ME, Barad M, Son H, Zhuo M, Lu YF, Shih R, Mansuy I, Hawkins RD, Kandel ER. Age-related defects in spatial memory are correlated with defects in the late phase of hippocampal long-term potentiation in vitro and are attenuated by drugs that enhance the cAMP signaling pathway. Proc Natl Acad Sci U S A. 1999;96:5280–5.
Baillie GS. Compartmentalized signalling: spatial regulation of cAMP by the action of compartmentalized phosphodiesterases. FEBS J. 2009;276:1790–9.
Baillie GS, Huston E, Scotland G, Hodgkin M, Gall I, Peden AH, MacKenzie C, Houslay ES, Currie R, Pettitt TR, Walmsley AR, Wakelam MJO, Warwicker J, Houslay MD. TAPAS-1, a novel microdomain within the unique N-terminal region of the PDE4A1 cAMP-specific phosphodiesterase that allows rapid, Ca2+−triggered membrane association with selectivity for interaction with phosphatidic acid. J Biol Chem. 2002;277:28298–309.
Baillie GS, Sood A, Mcphee I, Gall I, Perry SJ, Lefkowitz RJ, Houslay MD. Beta-Arrestin-mediated PDE4 cAMP phosphodiesterase recrutiment regulates beta-adrenoceptor switching from Gs to Gi. Proc Natl Acad Sci. 2003;100:940–5.
Baillie GS, Adams DR, Bhari N, Houslay TM, Vadrevu S, Meng D, Li X, Dunlop A, Milligan G, Bolger GB, Klussmann E, Houslay MD. Mapping binding sites for the PDE4D5 cAMP-specific phosphodiesterase to the N- and C-domains of beta-arrestin using spot-immobilized peptide arrays. Biochem J. 2007;404:71–80.
Barad M, Bourtchouladze R, Winder DG, Golan H, Kandel E. Rolipram, a type IV-specific phosphodiesterase inhibitor, facilitates the establishment of long-lasting long-term potentiation and improves memory. Proc Natl Acad Sci U S A. 1998;95:15020–5.
Beard M, O’Connell J, Bolger G, Houslay M. The unique N-terminal domain of the cAMP phosphodiesterase PDE4D4 allows for interaction with specific SH3 domains. FEBS Lett. 1999;460:173–7.
Beard MB, Huston E, Campbell L, Gall I, McPhee I, Yarwood S, Scotland G, Houslay MD. In addition to the SH3 binding region, multiple regions within the N-terminal noncatalytic portion of the cAMP-specific phosphodiesterase, PDE4A5, contribute to its intracellular targeting. Cell Signal. 2002;14:453–65.
Bertolino A, Crippa D, di Dio S, Fichte K, Musmeci G, Porro V, Rapisarda V, Sastre-y-Hernández M, Schratzer M. Rolipram versus imipramine in inpatients with major, “minor” or atypical depressive disorder: a double-blind double-dummy study aimed at testing a novel therapeutic approach. Int Clin Psychopharmacol. 1988;3:245–53.
Birkenfeld A, Ben-Zvi A. Age associated changes in intracellular cyclic adenosine monophosphate. Clin Exp Immunol. 1984;55:651–4.
Blackman B, Horner K, Heidmann J, Wang D, Richter W, Rich TC, Conti M. PDE4D and PDE4B function in distinct subcellular compartments in mouse embryonic fibroblasts. J Biol Chem. 2011;286:12590–601.
Blalock EM, Geddes JW, Chen KC, Porter NM, Markesbery WR, Landfield PW. Incipient Alzheimer ’ s disease : microarray correlation analyses reveal major transcriptional and tumor suppressor responses. PNAS. 2003;101:2173–8.
Boas J, Ano W, Paul J. Properties of adenylate cyclase from senescent rat brain. Brain Res. 1973;54:391–6.
Bolger G, Michaeli T, Martins T, St John T, Steiner B, Rodgers L, Riggs M, Wigler M, Ferguson K. A family of human phosphodiesterases homologous to the dunce learning and memory gene product of Drosophila melanogaster are potential targets for antidepressant drugs. Mol Cell Biol. 1993;13:6558–71.
Bolger GB, McPhee I, Houslay MD. Alternative splicing of cAMP-specific phosphodiesterase mRNA transcripts, characterization of a a novel tissue-specific isoform. J Biol Chem. 1996;271:1065–71.
Bolger GB, Erdogan S, Jones RE, Loughney K, Scotland G, Hoffmann R, Wilkinson I, Farrell C, Houslay MD. Characterization of five different mRNAs from the human cAMP-specific phosphodiesterase PDE4D gene. Biochem J. 1997;328:539–48.
Bolger GB, McCahill A, Huston E, Cheung Y-F, McSorley T, Baillie GS, Houslay MD. The unique amino-terminal region of the PDE4D5 cAMP phosphodiesterase isoform confers preferential interaction with beta-arrestins. J Biol Chem. 2003a;278:49230–8.
Bolger GB, Peden AH, Steele MR, MacKenzie C, McEwan DG, Wallace DA, Huston E, Baillie GS, Houslay MD. Attenuation of the activity of the cAMP-specific phosphodiesterase PDE4A5 by interaction with the immunophilin XAP2. J Biol Chem. 2003b;278:33351–63.
Bolger GB, Dunlop AJ, Meng D, Day JP, Klussmann E, Baillie GS, Adams DR, Houslay MD. Dimerization of cAMP phosphodiesterase-4 (PDE4) in living cells requires interfaces located in both the UCR1 and catalytic unit domains. Cell Signal. 2015;27:756–69.
Bos JL. Epac proteins: multi-purpose cAMP targets. Trends Biochem Sci. 2006;31:680–6.
Bourtchuladze R, Frenguelli B, Blendy J, Cioffi D, Schutz G, Silva A. Deficient long-term memory in mice with a targeted mutation of the cAMP-responsive element-binding protein. Cell. 1994;79:59–68.
Brightwell JJ, Smith CA, Neve RL, Colombo PJ. Long-term memory for place learning is facilitated by expression of cAMP response element-binding protein in the dorsal hippocampus. Learn Mem. 2007;14:195–9.
Brody H. Organization of the cerebral cortex. III. A study of aging in the human cerebral cortex. J Comp Neurol. 1955;102:511–6.
Bruno O, Fedele E, Prickaerts J, Parker LA, Canepa E, Brullo C, Cavallero A, Gardella E, Balbi A, Domenicotti C, Bollen E, HJM G, Vanmierlo T, Erb K, Limebeer CL, Argellati F, Marinari UM, Pronzato MA, Ricciarelli R. GEBR-7b, a novel PDE4D selective inhibitor that improves memory in rodents at non-emetic doses. Br J Pharmacol. 2011;164:2054–63.
Brullo C, Massa M, Rocca M, Rotolo C, Guariento S, Rivera D, et al. Synthesis, biological evaluation, and molecular modeling of new 3-(cyclopentyloxy)-4-methoxybenzaldehyde -(2-(2,6-dimethylmorpholino)-2-oxoethyl) oxime (GEBR-7b) related phosphodiesterase 4D (PDE4D) inhibitors. J Med Chem. 2014;57(16):7061–72.
Burgin AB, Magnusson OT, Singh J, Witte P, Staker BL, Bjornsson JM, Thorsteinsdottir M, Hrafnsdottir S, Hagen T, Kiselyov AS, Stewart LJ, Gurney ME. Design of phosphodiesterase 4D (PDE4D) allosteric modulators for enhancing cognition with improved safety. Nat Biotechnol. 2010;28:63–70.
Canepa E, Domenicott IC, Marengo B, Passalacqua M, Marinari U, Pronzato M, Fedele E, Ricciarelli R. Cyclic adenosine monophosphate as an endogenous modulator of the amyloid-β precursor protein metabolism. IUBMB Life. 2013;65:127–33.
Carlezon WA, Duman RS, Nestler EJ. The many faces of CREB. Trends Neurosci. 2005;28:436–45.
Catherine Jin S-L, Bushnik T, Lan L, Conti M. Subcellular localization of rolipram-sensitive, cAMP-specific phosphodiesterases. Differential targeting and activation of the splicing variants derived from The PDE4D gene. J Biol Chem. 1998;273:19672–8.
Chandrasekaran A, Toh KY, Low SH, Tay SKH, Brenner S, Goh DLM. Identification and characterization of novel mouse PDE4D isoforms: molecular cloning, subcellular distribution and detection of isoform-specific intracellular localization signals. Cell Signal. 2008;20:139–53.
Chen Y, Huang X, Zhang Y, Rockenstein E, Bu G, Golde TE, Masliah E, Xu H. Alzheimer’s β-secretase (BACE1) regulates the cAMP/PKA/CREB pathway independently of β-amyloid. J Neurosci. 2012;32:11390–5.
Cheng Y-F, Wang C, Lin H-B, Li Y-F, Huang Y, J-P X, Zhang H-T. Inhibition of phosphodiesterase-4 reverses memory deficits produced by Aβ25-35 or Aβ1-40 peptide in rats. Psychopharmacology (Berl). 2010;212:181–91.
Cherry JA, Davis RL. Cyclic AMP phosphodiesterases are localized in regions of the mouse brain associated with reinforcement, movement, and affect. J Comp Neurol. 1999;301:287–301.
Cheung Y, Kan Z, Garrett-engele P, Gall I, Murdoch H, Baillie GS, Camargo LM, Johnson JM, Houslay MD, Castle JC. PDE4B5, a novel, super-short, brain-specific cAMP phosphodiesterase-4 variant whose isoform-specifying N-terminal region is identical to that of cAMP. J Pharmacol Exp Ther. 2007;322:600–9.
Clark I, Atwood C, Bowen R, Paz-Filho G, Vissel B. Tumor necrosis factor-induced cerebral insulin resistance in Alzheimer’s disease links numerous treatment rationales. Pharmacol Rev. 2012;64:1004–26.
Conkright MD, Guzman E, Flechner L, Su AI, Hogenesch JB, Montminy M. Genome-wide analysis of CREB target genes reveals a core promoter requirement for cAMP responsiveness. Mol Cell. 2003;11:1101–8.
Conti M, Richter W, Mehats C, Livera G, Park J-Y, Jin C. Cyclic AMP-specific PDE4 phosphodiesterases as critical components of cyclic AMP signaling. J Biol Chem. 2003;278:5493–6.
Cowburn R, O’Neill C, Ravid R, Alafuzoff I, Winblad B, Fowler C. Adenylyl cyclase activity in postmortem human brain: evidence of altered G protein mediation in Alzheimer’s disease. J Neurochem. 1992;58:1409–19.
Cristòfol R, Porquet D, Corpas R, Coto-Montes A, Serret J, Camins A, Pallàs M, Sanfeliu C. Neurons from senescence-accelerated SAMP8 mice are protected against frailty by the sirtuin 1 promoting agents melatonin and resveratrol. J Pineal Res. 2012;52:271–81.
Cruz L, Roe DL, Urbanc B, Cabral H, Stanley HE, Rosene DL. Age-related reduction in microcolumnar structure in area 46 of the rhesus monkey correlates with behavioral decline. Proc Natl Acad Sci U S A. 2004;101:15846–51.
D’Sa C, Tolbert LM, Conti M, Duman RS. Regulation of cAMP-specific phosphodiesterases type 4B and 4D (PDE4) splice variants by cAMP signaling in primary cortical neurons. J Neurochem. 2002;81:745–57.
D’Sa C, Eisch AJ, Bolger GB, Duman RS. Differential expression and regulation of the cAMP-selective phosphodiesterase type 4A splice variants in rat brain by chronic antidepressant administration. Eur J Neurosci. 2005;22:1463–75.
Dash P, Hochner B, Kandel E. Injection of the cAMP-responsive element into the nucleus of Aplysia sensory neurons blocks long-term facilitation. Nature. 1990;345:718–21.
Davis RL, Takayasu H, Eberwine M, Myres J. Cloning and characterization of mammalian homologs of the Drosophila dunce+ gene. Proc Natl Acad Sci U S A. 1989;86:3604–8.
De Arcangelis V, Liu R, Soto D, Xiang Y. Differential association of phosphodiesterase 4D isoforms with beta2-adrenoceptor in cardiac myocytes. J Biol Chem. 2009;284:33824–32.
Delaney SM, Geiger JD. Brain regional levels of adenosine and adenosine nucleotides in rats killed by high-energy focused microwave irradiation. J Neurosci Methods. 1996;64:151–6.
Dodge KL, Khouangsathiene S, Kapiloff MS, Mouton R, Hill EV, Houslay MD, Langeberg LK, Scott JD. mAKAP assembles a protein kinase A/PDE4 phosphodiesterase cAMP signaling module. EMBO J. 2001;20:1921–30.
Dodge-Kafka KL, Soughayer J, Pare GC, Carlisle Michel JJ, Langeberg LK, Kapiloff MS, Scott JD. The protein kinase A anchoring protein mAKAP coordinates two integrated cAMP effector pathways. Nature. 2005;437:574–8.
Drachman DA. Aging of the brain, entropy, and Alzheimer disease. Neurology. 2006;67:1340–52.
Driscoll I, Davatzikos C, An Y, Wu X, Shen D, Kraut M, Resnick SM. Longitudinal pattern of regional brain volume change differentiates normal aging from MCI. Neurology. 2009;72:1906–13.
Echeverria V, Ducatenzeiler A, Chen CH, Cuello AC. Endogenous beta-amyloid peptide synthesis modulates cAMP response element-regulated gene expression in PC12 cells. Neuroscience. 2005;135:1193–202.
Egawa T, Mishima K, Matsumoto Y, Iwasaki K, Fujiwara M. Rolipram and its optical isomers, phosphodiesterase 4 inhibitors, attenuated the scopolamine-induced impairments of learning and memory in rats. Jpn J Pharmacol. 1997;75:275–81.
Engels P, Abdel’Al S, Hulley P, Lübbert H. Brain distribution of four rat homologues of the Drosophila dunce cAMP phosphodiesterase. J Neurosci Res. 1995;41:169–78.
Fatemi SH, King DP, Reutiman TJ, Folsom TD, Laurence JA, Lee S, Fan Y-T, Paciga SA, Conti M, Menniti FS. PDE4B polymorphisms and decreased PDE4B expression are associated with schizophrenia. Schizophr Res. 2008;101:36–49.
Fatemi SH, Folsom TD, Reutiman TJ, Braun NN, Lavergne LG. Levels of phosphodiesterase 4A and 4B are altered by chronic treatment with psychotropic medications in rat frontal cortex. Synapse. 2010;64:550–5.
Finkbeiner S, Tavazoie SF, Maloratsky A, Jacobs KM, Harris KM, Greenberg ME. CREB: a major mediator of neuronal neurotrophin responses. Neuron. 1997;19:1031–47.
Fox D, Burgin AB, Gurney ME. Structural basis for the design of selective phosphodiesterase 4B inhibitors. Cell Signal. 2014;26:657–63.
Frankola KA, Greig NH, Luo W, Tweedie D. Targeting TNF-α to elucidate and ameliorate neuroinflammation in neurodegenerative diseases. CNS Neurol Disord Drug Targets. 2011;10:391–403.
Galimberti D, Scarpini E. Inflammation and oxidative damage in Alzheimer’s disease: friend or foe? Front Biosci (Schol Ed). 2011;3:252–66.
Geinisman Y. Age-related decline in memory function: is it associated with a loss of synapses? Neurobiol Aging. 1999;20:353–6. discussion 359–360
Geinisman Y, Ganeshina O, Yoshida R, Berry RW, Disterhoft JF, Gallagher M. Aging, spatial learning, and total synapse number in the rat CA1 stratum radiatum. Neurobiol Aging. 2004;25:407–16.
Giambarella U, Murayama Y, Ikezu T, Fujita T, Nishimoto I. Potential CRE suppression by familial Alzheimer’s mutants of APP independent of adenylyl cyclase regulation. FEBS Lett. 1997;412:97–101.
Giannaris EL, Rosene DL. A stereological study of the numbers of neurons and glia in the primary visual cortex across the lifespan of male and female rhesus monkeys. J Comp Neurol. 2012;520:3492–508.
Glisky E. Changes in cognitive function in human aging. In: Riddle DR, editor. Brain aging model methods, mechanism. Boca Raton: CRC Press; 2007.
Gloerich M, Bos JL. Epac: defining a new mechanism for cAMP action. Annu Rev Pharmacol Toxicol. 2010;50:355–75.
Gong B, Vitolo OV, Trinchese F, Liu S, Shelanski M, Arancio O. Persistent improvement in synaptic and cognitive functions in an Alzheimer mouse model after rolipram treatment. J Clin Invest. 2004;114:1624–34.
Guiot-Goffioul F, Gerard-Vandenhove MA, Troisfontaines B, Breulet M, von Frenckell R, Bobon D. Preliminary results of a double-blind study between rolipram and desipramine in hospitalized patients with major depressive symptoms. Acta Psychiatr Belg. 1987;87:230–5.
Gurney ME, D’Amato EC, Burgin AB. Phosphodiesterase-4 (PDE4) molecular pharmacology and Alzheimer’s disease. Neurotherapeutics. 2015;12:49–56.
Hagen TJ, Mo X, Burgin AB, Fox D, Zhang Z, Gurney ME. Discovery of triazines as selective PDE4B versus PDE4D inhibitors. Bioorg Med Chem Lett. 2014;24:4031–4.
Hansen IIIRT, Zhang H-T. Senescent-induced dysregulation of cAMP/CREB signaling and correlations with cognitive decline. Brain Res. 2013;1516:93–109.
Hansen IIIRT, Conti M, Zhang H-T. Mice deficient in phosphodiesterase-4A display anxiogenic-like behavior. Psychopharmacology (Berl). 2014;231:2941–54.
Hara H, Onodera H, Kato H, Koqure K. Effects of aging on signal transmission and transduction systems in the gerbil brain: morphological and autoradiographic study. Neuroscience. 1992;46:475–88.
Hattiangady B, Rao MS, Shetty GA, Shetty AK. Brain-derived neurotrophic factor, phosphorylated cyclic AMP response element binding protein and neuropeptide Y decline as early as middle age in the dentate gyrus and CA1 and CA3 subfields of the hippocampus. Exp Neurol. 2005;195:353–71.
Haug H. Are neurons of the human cerebral cortex really lost during aging? A morphometric examination. Adv Appl Neurol Sci. 1985;2:150–63.
He Y, Yang DZ, Yu H, Li MY, Feng QC, Zheng H. Genetic variants of phosphodiesterase 4D gene are associated with an enhanced risk for ischemic stroke in young Chinese population. Neurol India. 2013;61:21–5.
Heaslip RJ, Evans DY. Emetic, central nervous system, and pulmonary activities of rolipram in the dog. Eur J Pharmacol. 1995;286:281–90.
Hebenstreit GF, Fellerer K, Fichte K, Fischer G, Geyer N, Meya U, Sastre-y-Hernández M, Schöny W, Schratzer M, Soukop W. Rolipram in major depressive disorder: results of a double-blind comparative study with imipramine. Pharmacopsychiatry. 1989;22:156–60.
Henderson G, Tomlinson B, Gibson P. Cell counts in human cerebral cortex in normal adults throughout life, using an image analysis computer. J Neurol Sci. 1980;46:113–36.
Hernandez AI, Martınez M, Hernanz A. Increased cAMP immunostaining in cerebral vessels in Alzheimer ’ s disease. Brain Res. 2001;922:148–52.
Herrup K. Reimagining Alzheimer’s disease--an age-based hypothesis. J Neurosci. 2010;30:16755–62.
Heyer EJ, Mergeche JL, Ward JT, Malone HR, Kellner C, Bruce SS, Connolly ES. Phosphodiesterase 4D single-nucleotide polymorphism 83 and cognitive dysfunction in carotid endarterectomy patients. Neurosurgery. 2013;73:791–796; discussion 796.
Hoffmann R, Baillie GS, MacKenzie SJ, Yarwood SJ, Houslay MD. The MAP kinase ERK2 inhibits the cyclic AMP-specific phosphodiesterase HSPDE4D3 by phosphorylating it at ser579. EMBO J. 1999;18:893–903.
Horton YM, Sullivan M, Houslay MD. Molecular cloning of a novel splice variant of human type IVA (PDE-IVA) cyclic AMP phosphodiesterase and localization of the gene to the p13.2-q12 region of human chromosome 19 [corrected]. Biochem J. 1995;308(Pt 2):683–91.
Houslay MD. Underpinning compartmentalised cAMP signalling through targeted cAMP breakdown. Trends Biochem Sci. 2010;35:91–100.
Houslay MD, Adams DR. PDE4 cAMP phosphodiesterases: modular enzymes that orchestrate signalling cross-talk, desensitization and compartmentalization. Biochem J. 2003;370:1–18.
Houslay MD, Baillie GS. The role of ERK2 docking and phosphorylation of PDE4 cAMP phosphodiesterase isoforms in mediating cross-talk between the cAMP and ERK signalling pathways. Biochem Soc Trans. 2003;31:1186–90.
Houslay MD, Baillie GS, Maurice DH. cAMP-Specific phosphodiesterase-4 enzymes in the cardiovascular system: a molecular toolbox for generating compartmentalized cAMP signaling. Circ Res. 2007;100:950–66.
Huston E, Lumb S, Russell A, Catterall C, Ross AH, Steele MR, Bolger GB, Perry MJ, Owens RJ, Houslay MD. Molecular cloning and transient expression in COS7 cells of a novel human PDE4B cAMP-specific phosphodiesterase, HSPDE4B3. Biochem J. 1997;328(Pt 2):549–58.
Huston E, Beard M, McCallum F, Pyne NJ, Vandenabeele P, Scotland G, Houslay MD. The cAMP-specific phosphodiesterase PDE4A5 is cleaved downstream of its SH3 interaction domain by caspase-3. Consequences for altered intracellular distribution. J Biol Chem. 2000;275:28063–74.
Ikezu T, Okamoto T, Komatsuzakil K, Matsui T, Martyn JAJ, Nishimoto I. Negative transactivation of cAMP response element by familial Alzheimer ’ s mutants of APP. EMBO J. 1996;15:2468–75.
Imanishi T, Sawa A, Ichimaru Y, Miyashiro M, Kato S, Yamamoto T, Ueki S. Ameliorating effects of rolipram on experimentally induced impairments of learning and memory in rodents. Eur J Pharmacol. 1997;321:273–8.
Impey S, Mark M, Villacres E, Poser S, Chavkin C, Storm D. Induction of CRE-mediated gene expression by stimuli that generate long-lasting LTP in area CA1 of the hippocampus. Neuron. 1996;16:973–82.
Jack CR, Petersen RC, YC X, Waring SC, O’Brien PC, Tangalos EG, Smith GE, Ivnik RJ, Kokmen E. Medial temporal atrophy on MRI in normal aging and very mild Alzheimer’s disease. Neurology. 1997;49:786–94.
Jang D-J, Park S-W, Lee J-A, Lee C, Chae Y-S, Park H, Kim M-J, Choi S-L, Lee N, Kim H, Kaang B-K. N termini of apPDE4 isoforms are responsible for targeting the isoforms to different cellular membranes. Learn Mem. 2010;17:469–79.
Jicha GA, Weaver C, Lane E, Vianna C, Kress Y, Rockwood J, Davies P. cAMP-dependent protein kinase phosphorylations on tau in Alzheimer’s disease. J Neurosci. 1999;19:7486–94.
Jin S-LC, Conti M. Induction of the cyclic nucleotide phosphodiesterase PDE4B is essential for LPS-activated TNF-alpha responses. Proc Natl Acad Sci U S A. 2002;99:7628–33.
Jin SL, Richard FJ, Kuo WP, D’Ercole AJ, Conti M. Impaired growth and fertility of cAMP-specific phosphodiesterase PDE4D-deficient mice. Proc Natl Acad Sci U S A. 1999;96:11998–2003.
Jin SL, Lan L, Zoudilova M, Conti M. Specific role of phosphodiesterase 4B in lipopolysaccharide-induced signaling in mouse macrophages. J Immunol. 2005a;175:1523–31.
Jin SL, Latour AM, Conti M. Generation of PDE4 knockout mice by gene targeting. Methods Mol Biol. 2005b;307:191–210.
Johannessen M, Delghandi MP, Moens U. What turns CREB on? Cell Signal. 2004;16:1211–27.
Johansson E, Sanabra C, Cortes R, Vilaro M, Mengod G. Lipopolysaccharide administration in vivo induces differential expression of cAMP-specific phosphodiesterase 4B mRNA splice variants in the mouse brain. J Neurosci Res. 2011;89:1761–2.
Johansson EM, Reyes-Irisarri E, Mengod G. Comparison of cAMP-specific phosphodiesterase mRNAs distribution in mouse and rat brain. Neurosci Lett. 2012;525:1–6.
Johnson KR, Nicodemus-Johnson J, Danziger RS. An evolutionary analysis of cAMP-specific Phosphodiesterase 4 alternative splicing. BMC Evol Biol. 2010;10:247.
Johnston LA, Erdogan S, Cheung YF, Sullivan M, Barber R, Lynch MJ, Baillie GS, Van Heeke G, Adams DR, Huston E, Houslay MD. Expression, intracellular distribution and basis for lack of catalytic activity of the PDE4A7 isoform encoded by the human PDE4A cAMP-specific phosphodiesterase gene. Biochem J. 2004;380:371–84.
Kähler AK, Otnaess MK, Wirgenes KV, Hansen T, Jönsson EG, Agartz I, Hall H, Werge T, Morken G, Mors O, Mellerup E, Dam H, Koefod P, Melle I, Steen VM, Andreassen OA, Djurovic S. Association study of PDE4B gene variants in Scandinavian schizophrenia and bipolar disorder multicenter case-control samples. Am J Med Genet B Neuropsychiatr Genet. 2010;153B:86–96.
Kaiser LG, Schuff N, Cashdollar N, Weiner MW. Age-related glutamate and glutamine concentration changes in normal human brain: 1H MR spectroscopy study at 4 T. Neurobiol Aging. 2005;26:665–72.
Karege F, Lambercy C, Schwald M, Steimer T, Cissé M. Differential changes of cAMP-dependent protein kinase activity and 3H-cAMP binding sites in rat hippocampus during maturation and aging. Neurosci Lett. 2001a;315:89–92.
Karege F, Schwald M, Lambercy C, Murama JJ, Cisse M, Malafosse A. A non-radioactive assay for the cAMP-dependent protein kinase activity in rat brain homogenates and age-related changes in hippocampus and cortex. Brain Res. 2001b;903:86–93.
Kauvar LM. Defective cyclic adenosine 3′:5-monophosphate phosphodiesterase in the Drosophila memory mutant dunce. J Neurosci. 1982;2:1347–58.
Kelly MP, Adamowicz W, Bove S, Hartman AJ, Mariga A, Pathak G, Reinhart V, Romegialli A, Kleiman RJ. Select 3′,5′-cyclic nucleotide phosphodiesterases exhibit altered expression in the aged rodent brain. Cell Signal. 2013;26:383–97.
Kim D, Nguyen MD, Dobbin MM, Fischer A, Sananbenesi F, Rodgers JT, Delalle I, Baur JA, Sui G, Armour SM, Puigserver P, Sinclair DA, Tsai L-H. SIRT1 deacetylase protects against neurodegeneration in models for Alzheimer’s disease and amyotrophic lateral sclerosis. EMBO J. 2007;26:3169–79.
Kraemer A, Rehmann HR, Cool RH, Theiss C, de Rooij J, Bos JL, Wittinghofer A. Dynamic interaction of cAMP with the Rap guanine-nucleotide exchange factor Epac1. J Mol Biol. 2001;306:1167–77.
Kudo K, Wati H, Qiao C, Arita J, Kanba S. Age-related disturbance of memory and CREB phosphorylation in CA1 area of hippocampus of rats. Brain Res. 2005;1054:30–7.
Lakics V, Karran EH, Boess FG. Quantitative comparison of phosphodiesterase mRNA distribution in human brain and peripheral tissues. Neuropharmacology. 2010;59:367–74.
Lakshminarasimhan H, Chattarji S. Stress leads to contrasting effects on the levels of brain derived neurotrophic factor in the hippocampus and amygdala. PLoS One. 2012;7:e30481.
Laux G, Becker T, Kühne G, Lesch KP, Riederer P, Beckmann H. Clinical and biochemical effects of the selective phosphodiesterase inhibitor rolipram in depressed inpatients controlled by determination of plasma level. Pharmacopsychiatry. 1988;21:378–9.
Laviada ID, Galve-Roperh I, Malpartida JM, Haro A. cAMP signalling mechanisms with aging in the Ceratitis capitata brain. Mech Ageing Dev. 1997;97:45–53.
Li X, Baillie GS, Houslay MD. Mdm2 directs the ubiquitination of beta-arrestin-sequestered cAMP phosphodiesterase-4D5. J Biol Chem. 2009;284:16170–82.
Li Y-F, Cheng Y-F, Huang Y, Conti M, Wilson SP, O’Donnell JM, Zhang H-T. Phosphodiesterase-4D knock-out and RNA interference-mediated knock-down enhance memory and increase hippocampal neurogenesis via increased cAMP signaling. J Neurosci. 2011;31:172–83.
de Lima MN, Presti-Torres J, Garcia VA, Guimarães MR, Scalco FS, Roesler R, Schröder N. Amelioration of recognition memory impairment associated with iron loading or aging by the type 4-specific phosphodiesterase inhibitor rolipram in rats. Neuropharmacology. 2008;55:788–92.
Lindstrand A, et al. Different mutations in PDE4D associated with developmental disorders with mirror phenotypes. J Med Genet. 2014;51:45–54.
Litersky JM, Johnson GV. Phosphorylation by cAMP-dependent protein kinase inhibits the degradation of tau by calpain. J Biol Chem. 1992;267:1563–8.
Liu H, Palmer D, Jimmo SL, Tilley DG, Dunkerley HA, Pang SC, Maurice DH. Expression of phosphodiesterase 4D (PDE4D) is regulated by both the cyclic AMP-dependent protein kinase and mitogen-activated protein kinase signaling pathways. A potential mechanism allowing for the coordinated regulation of PDE4D activity and expression. J Biol Chem. 2000;275:26615–24.
Liu K, Wang J, Yu Z, Qin X, Wu Y, Li N, Kui Y, Fang K, Wang X, Wu T, Chen D, Hu Y. Association study between PDE4D gene polymorphism and ischemic stroke. Beijing Da Xue Xue Bao. 2013a;45:359–63.
Liu X, Zhu R, Li L, Deng S, Li Q, He Z. Genetic polymorphism in PDE4D gene and risk of ischemic stroke in Chinese population: a meta-analysis. PLoS One. 2013b;8:e66374.
Lynch MJ, Baillie GS, Mohamed A, Li X, Maisonneuve C, Klussmann E, van Heeke G, Houslay MD. RNA silencing identifies PDE4D5 as the functionally relevant cAMP phosphodiesterase interacting with beta arrestin to control the protein kinase A/AKAP79-mediated switching of the beta2-adrenergic receptor to activation of ERK in HEK293B2 cells. J Biol Chem. 2005;280:33178–89.
Lynch MJ, Baillie GS, Houslay MD. cAMP-specific phosphodiesterase-4D5 (PDE4D5) provides a paradigm for understanding the unique non-redundant roles that PDE4 isoforms play in shaping compartmentalized cAMP cell signalling. Biochem Soc Trans. 2007;35:938–41.
Lynex CN, Li Z, Chen ML, Toh KY, Low RWC, Goh DLM, Tay SKH. Identification and molecular characterization of a novel PDE4D11 cAMP-specific phosphodiesterase isoform. Cell Signal. 2008;20:2247–55.
MacKenzie SJ, Yarwood SJ, Peden a H, Bolger GB, Vernon RG, Houslay MD. Stimulation of p70S6 kinase via a growth hormone-controlled phosphatidylinositol 3-kinase pathway leads to the activation of a PDE4A cyclic AMP-specific phosphodiesterase in 3T3-F442A preadipocytes. Proc Natl Acad Sci U S A. 1998;95:3549–54.
Mackenzie KF, Topping EC, Bugaj-Gaweda B, Deng C, Cheung Y-F, Olsen AE, Stockard CR, High Mitchell L, Baillie GS, Grizzle WE, De Vivo M, Houslay MD, Wang D, Bolger GB. Human PDE4A8, a novel brain-expressed PDE4 cAMP-specific phosphodiesterase that has undergone rapid evolutionary change. Biochem J. 2008;411:361–9.
MacKenzie KF, Wallace DA, Hill EV, Anthony DF, Henderson DJP, Houslay DM, Arthur JSC, Baillie GS, Houslay MD. Phosphorylation of cAMP-specific PDE4A5 (phosphodiesterase-4A5) by MK2 (MAPKAPK2) attenuates its activation through protein kinase A phosphorylation. Biochem J. 2011;435:755–69.
Maillet M, Robert SJ, Cacquevel M, Gastineau M, Vivien D, Bertoglio J, Zugaza JL, Fischmeister R, Lezoualc’h F. Crosstalk between Rap1 and Rac regulates secretion of sAPPalpha. Nat Cell Biol. 2003;5:633–9.
Makman M, Ahn H, Thal L, Sharpless N, Dvorkin B, Horowitz S, Rosenfeld M. Evidence for selective loss of brain dopamine- and histamine-stimulated adenylate cyclase activities in rabbits with aging. Brain Res. 1980;192:177–83.
Marchmont RJ, Houslay MD. A peripheral and an intrinsic enzyme constitute the cyclic AMP phosphodiesterase activity of rat liver plasma membranes. Biochem J. 1980;187:381–92.
Martin ACL, Cooper DMF. Layers of organization of cAMP microdomains in a simple cell. Biochem Soc Trans. 2006;34:480–3.
Martınez M, Fernandez E, Frank A, Guaza C, Hernanz A. Increased cerebrospinal fluid cAMP levels in Alzheimer’s disease. Brain Res. 1999;846:265–7.
Maurice DH, Ke H, Ahmad F, Wang Y, Chung J, Manganiello VC. Advances in targeting cyclic nucleotide phosphodiesterases. Nat Rev Drug Discov. 2014;13:290–314.
McLachlan C, Chen M, Lynex C, Goh D, Brenner S, Tay S, Pded AD. Changes in PDE4D isoforms in the hippocampus of a patient with advanced Alzheimer disease. Arch Neurol. 2013;64:456–7.
McPhee I, Pooley L, Lobban M, Bolger G, Houslay MD. Identification, characterization and regional distribution in brain of RPDE-6 (RNPDE4A5), a novel splice variant of the PDE4A cyclic AMP phosphodiesterase family. Biochem J. 1995;310(Pt 3):965–74.
McPhee I, Cochran S, Houslay MD. The novel long PDE4A10 cyclic AMP phosphodiesterase shows a pattern of expression within brain that is distinct from the long PDE4A5 and short PDE4A1 isoforms. Cell Signal. 2001;13:911–8.
Mcphee I, Gibson LC, Kewney J, Darroch C, Stevens PA, Spinks D, Cooreman A, Mackenzie SJ. Cyclic nucleotide signalling: a molecular approach to drug discovery for Alzheimer’s disease. Biochem Soc Trans. 2005;33:1330–2.
Mika D, Conti M. PDE4D phosphorylation: a coincidence detector integrating multiple signaling pathways. Cell Signal. 2015;28:719–24.
Millar JK, et al. DISC1 and PDE4B are interacting genetic factors in schizophrenia that regulate cAMP signaling. Science. 2005;310:1187–91.
Miller JA, Oldham MC, Geschwind DH. A systems level analysis of transcriptional changes in Alzheimer’s disease and normal aging. J Neurosci. 2008;28:1410–20.
Miró X, Pérez-Torres S, Puigdomènech P, Palacios JM, Mengod G. Differential distribution of PDE4D splice variant mRNAs in rat brain suggests association with specific pathways and presynaptical localization. Synapse. 2002;45:259–69.
Mons N, Segu L, Nogues X, Buhot M. Effects of age and spatial learning on adenylyl cyclase mRNA expression in the mouse hippocampus. Neurobiol Aging. 2004;25:1095–106.
Montgomery SL, Bowers WJ. Tumor necrosis factor-alpha and the roles it plays in homeostatic and degenerative processes within the central nervous system. J Neuroimmune Pharmacol. 2012;7:42–59.
Moosmang S, Biel M, Hofmann F, Ludwig A. Differential distribution of four hyperpolarization-activated cation channels in mouse brain. Biol Chem. 1999;380:975–80.
Mori F, Pérez-Torres S, De Caro R, Porzionato A, Macchi V, Beleta J, Gavaldà A, Palacios JM, Mengod G. The human area postrema and other nuclei related to the emetic reflex express cAMP phosphodiesterases 4B and 4D. J Chem Neuroanat. 2010;40:36–42.
Mulchahey JJ, Regmi A, Sheriff S, Balasubramaniam A, Kasckow JW. Coordinate and divergent regulation of corticotropin-releasing factor (CRF) and CRF-binding protein expression in an immortalized amygdalar neuronal cell line. Endocrinology. 1999;140:251–9.
Müller M, Cárdenas C, Mei L, Cheung K-H, Foskett JK. Constitutive cAMP response element binding protein (CREB) activation by Alzheimer’s disease presenilin-driven inositol trisphosphate receptor (InsP3R) Ca2+ signaling. Proc Natl Acad Sci U S A. 2011;108:13293–8.
Murphy EJ. Brain fixation for analysis of brain lipid-mediators of signal transduction and brain eicosanoids requires head-focused microwave irradiation: an historical perspective. Prostaglandins Other Lipid Mediat. 2010;91:63–7.
Murray AJ, Shewan DA. Epac mediates cyclic AMP-dependent axon growth, guidance and regeneration. Mol Cell Neurosci. 2008;38:578–88.
Murray AJ, Tucker SJ, Shewan DA. cAMP-dependent axon guidance is distinctly regulated by Epac and protein kinase A. J Neurosci. 2009;29:15434–44.
Muschamp JW, Van’t Veer A, Parsegian A, Gallo MS, Chen M, Neve RL, Meloni EG, Carlezon WA. Activation of CREB in the nucleus accumbens shell produces anhedonia and resistance to extinction of fear in rats. J Neurosci. 2011;31:3095–103.
Numata S, Ueno S-I, Iga J-I, Song H, Nakataki M, Tayoshi S, Sumitani S, Tomotake M, Itakura M, Sano A, Ohmori T. Positive association of the PDE4B (phosphodiesterase 4B) gene with schizophrenia in the Japanese population. J Psychiatr Res. 2008;43:7–12.
Numata S, Iga J-I, Nakataki M, Tayoshi S, Taniguchi K, Sumitani S, Tomotake M, Tanahashi T, Itakura M, Kamegaya Y, Tatsumi M, Sano A, Asada T, Kunugi H, Ueno S-I, Ohmori T. Gene expression and association analyses of the phosphodiesterase 4B (PDE4B) gene in major depressive disorder in the Japanese population. Am J Med Genet B Neuropsychiatr Genet. 2009;150B:527–34.
O’Callaghan JP, Sriram K. Focused microwave irradiation of the brain preserves in vivo protein phosphorylation: comparison with other methods of sacrifice and analysis of multiple phosphoproteins. J Neurosci Methods. 2004;135:159–68.
O’Connell JC, McCallum JF, McPhee I, Wakefield J, Houslay ES, Wishart W, Bolger G, Frame M, Houslay MD. The SH3 domain of Src tyrosyl protein kinase interacts with the N-terminal splice region of the PDE4A cAMP-specific phosphodiesterase RPDE-6 (RNPDE4A5). Biochem J. 1996;318(Pt 1):255–61.
O’Connor S, Scarpace P, Abrass I. Age-associated decrease of adenylate cyclase activity in rat myocardium. Mech Ageing Dev. 1981;16:91–5.
O’Connor S, Scarpace P, Abrass I. Age-associated decrease in the catalytic unit activity of rat myocardial adenylate cyclase. Mech Ageing Dev. 1983;21:357–63.
O’Donnell JM, Xu Y. Evidence for global reduction in brain cyclic adenosine monophosphate signaling in depression. Biol Psychiatry. 2012;72:524–5.
O’Donnell JM, Zhang H-T. Antidepressant effects of inhibitors of cAMP phosphodiesterase (PDE4). Trends Pharmacol Sci. 2004;25:158–63.
O’Neill C, Wiehager B, Fowler C, Ravid R, Winblad B, Cowburn R. Regionally selective alterations in G protein subunit levels in the Alzheimer’s disease brain. Brain Res. 1994;636:193–201.
Ohm T, Bohl J, Lemmer B. Reduced cAMP-signal transduction in postmortem hippocampus of demented old people. Prog Clin Biol Res. 1989;317:501–9.
Ohm T, Bohl J, Lemmer B. Reduced basal and stimulated (isoprenaline, Gpp(NH)p, forskolin) adenylate cyclase activity in Alzheimer’s disease correlated with histopathological changes. Brain Res. 1991;540:229–36.
Oliveira RF, Terrin A, Di Benedetto G, Cannon RC, Koh W, Kim M, Zaccolo M, Blackwell KT. The role of type 4 phosphodiesterases in generating microdomains of cAMP: large scale stochastic simulations. PLoS One. 2010;5:e11725.
Ouyang M, Zhang L, Zhu JJ, Schwede F, Thomas SA. Epac signaling is required for hippocampus-dependent memory retrieval. Proc Natl Acad Sci U S A. 2008;105:11993–7.
Owens RJ, Catterall C, Batty D, Jappy J, Russell A, Smith B, Connell JO, Perry MJ. Human phosphodiesterase 4A; characterization of full-length and truncated enzymes expressed in COS cells. Biochem J. 1997;326:53–60.
Park S-J, Ahmad F, Philp A, Baar K, Williams T, Luo H, Ke H, Rehmann H, Taussig R, Brown AL, Kim MK, Beaven MA, Burgin AB, Manganiello V, Chung JH. Resveratrol ameliorates aging-related metabolic phenotypes by inhibiting cAMP phosphodiesterases. Cell. 2012;148:421–33.
Pearse DD, Hughes ZA. PDE4B as a microglia target to reduce neuroinflammation. Glia. 2016;64:1698–709.
Pérez-Torres S, Mengod G. cAMP-specific phosphodiesterases expression in Alzheimer’s disease brains. Int Congr Ser. 2003;1251:127–38.
Perez-Torres S, Miro X, Palacios JM, Cortes R, Puigdomenech P, Mengod G. Phosphodiesterase type 4 isozymes expression in human brain examined by in situ hybridization histochemistry and[3 H]rolipram binding autoradiography. Comparison with monkey and rat brain. J Chem Neuroanat. 2000;20:349–74.
Peters A, Morrison JH, Rosene DL, Hyman BT. Are neurons lost from the primate cerebral cortex during normal aging? Cereb Cortex. 1998;8:295–300.
Podtelezhnikov AA, Tanis KQ, Nebozhyn M, Ray WJ, Stone DJ, Loboda AP. Molecular insights into the pathogenesis of Alzheimer’s disease and its relationship to normal aging. PLoS One. 2011;6:e29610.
Porte Y, Buhot M-C, Mons N. Alteration of CREB phosphorylation and spatial memory deficits in aged 129T2/Sv mice. Neurobiol Aging. 2008;29:1533–46.
Pugazhenthi S, Wang M, Pham S, Sze C, Eckman CB. Downregulation of CREB expression in Alzheimer ’ s brain and in A beta-treated rat hippocampal neurons. Mol Neurodegener. 2011;6:60.
Puri S, Volicer L. Effect of aging on cyclic amp levels and adenylate cylcase and phosphodiesterase activities in the rat corpus striatum. Mech Ageing Dev. 1977;2:53–8.
Qui Y, Chen C, Malone T, Richter L, Beckendorf S, Davis R. Characterization of the memory gene dunce of Drosophila melanogaster. J Mol Biol. 1991;222:553–65.
Ramos BP, Birnbaum SG, Lindenmayer I, Newton SS, Duman RS, Arnsten AFT. Dysregulation of protein kinase a signaling in the aged prefrontal cortex: new strategy for treating age-related cognitive decline. Neuron. 2003;40:835–45.
Ramos BP, Stark D, Verduzco L, Van DCH, Arnsten AFT, van Dyck CH. α2A-adrenoceptor stimulation improves prefrontal cortical regulation of behavior through inhibition of cAMP signaling in aging animals. Learn Mem. 2006;13:770–6.
Randt CT, Judge ME, Bonnet KA, Quartermain D. Brain cyclic AMP and memory in mice. Pharmacol Biochem Behav. 1982;17:677–80.
Raymond DR, Carter RL, Ward CA, Maurice DH. Distinct phosphodiesterase-4D variants integrate into protein kinase A-based signaling complexes in cardiac and vascular myocytes. Am J Physiol Heart Circ Physiol. 2009;296:H263–71.
Reeves ML, Leigh BK, England PJ. The identification of a new cyclic nucleotide phosphodiesterase activity in human and guinea-pig cardiac ventricle. Implications for the mechanism of action of selective phosphodiesterase inhibitors. Biochem J. 1987;241:535–41.
Rena G, Begg F, Ross A, MacKenzie C, McPhee I, Campbell L, Huston E, Sullivan M, Houslay MD. Molecular cloning, genomic positioning, promoter identification, and characterization of the novel cyclic amp-specific phosphodiesterase PDE4A10. Mol Pharmacol. 2001;59:996–1011.
Richter W, Conti M. Dimerization of the type 4 cAMP-specific phosphodiesterases is mediated by the upstream conserved regions (UCRs). J Biol Chem. 2002;277:40212–21.
Richter W, Conti M. The oligomerization state determines regulatory properties and inhibitor sensitivity of type 4 cAMP-specific phosphodiesterases. J Biol Chem. 2004;279:30338–48.
Richter W, Jin SL, Conti M. Splice variants of the cyclic nucleotide phosphodiesterase PDE4D are differentially expressed and regulated in rat tissue. Biochem J. 2005;388:803–11.
Richter W, Menniti F, Zhang H, Conti M. PDE4 as a target for cognition enhancement. Expert Opin Ther Targets. 2013;17:1011–27.
Ricciarelli R, Brullo C, Prickaerts J, Arancio O, Villa C, Rebosio C, et al. Memory-enhancing effects of GEBR-32a, a new PDE4D inhibitor holding promise for the treatment of Alzheimer’s disease. Sci Rep. 2017;7:46320.
Robichaud A, Savoie C, Stamatiou PB, Tattersall FD, Chan CC. PDE4 inhibitors induce emesis in ferrets via a noradrenergic pathway. Neuropharmacology. 2001;40:262–9.
Robichaud A, Savoie C, Stamatiou PB, Lachance N, Jolicoeur P, Rasori R, Chan CC. Assessing the emetic potential of PDE4 inhibitors in rats. Br J Pharmacol. 2002a;135:113–8.
Robichaud A, Stamatiou PB, Jin SL, Lachance N, MacDonald D, Laliberté F, Liu S, Huang Z, Conti M, Chan C-C. Deletion of phosphodiesterase 4D in mice shortens alpha(2)-adrenoceptor-mediated anesthesia, a behavioral correlate of emesis. J Clin Invest. 2002b;110:1045–52.
de Rooij J, Zwartkruis FJ, Verheijen MH, Cool RH, Nijman SM, Wittinghofer A, Bos JL, Dj R. Epac is a Rap1 guanine-nucleotide-exchange factor directly activated by cyclic AMP. Nature. 1998;396:474–7.
Roscioni SS, Elzinga CR, Schmidt M. Epac: effectors and biological functions. Naunyn Schmiedeberg's Arch Pharmacol. 2008;377:345–57.
Rubio-Perez JM, Morillas-Ruiz JM. A review: inflammatory process in Alzheimer’s disease, role of cytokines. ScientificWorldJournal. 2012;2012:756357.
Rutten K, Misner DL, Works M, Blokland A, Novak TJ, Santarelli L, Wallace TL. Enhanced long-term potentiation and impaired learning in phosphodiesterase 4D-knockout (PDE4D) mice. Eur J Neurosci. 2008;28:625–32.
Saldou N, Obernolte R, Huber A, Baecker PA, Wilhelm R, Alvarez R, Li B, Xia L, Callan O, Su C, Jarnagin K, Shelton ER. Comparison of recombinant human PDE4 isoforms: interaction with substrate and inhibitors. Cell Signal. 1998;10:427–40.
Satoh J, Tabunoki H, Arima K. Molecular network analysis suggests aberrant CREB-mediated gene regulation in the Alzheimer disease hippocampus. Dis Markers. 2009;27:239–52.
Scahill RI, Frost C, Jenkins R, Whitwell JL, Rossor MN, Fox NC. A longitudinal study of brain volume changes in normal aging using serial registered magnetic resonance imaging. Arch Neurol. 2003;60:989–94.
Scheltens P, Barkhof F, Leys D, Wolters EC. Histopathologic correlates of white-matter changes on MRI in Alzheimer ‘s disease and normal aging. Neurology. 1995;45:883–8.
Schmidt MJ, Thornberry JF. Cyclic AMP and cyclic GMP accumulation in vitro in brain regions of young, old and, aged rats. Brain Res. 1978;139:169–77.
Schnecko A, Witte K, Bohl J, Ohm T, Lemmer B. Adenylyl cyclase activity in Alzheimer’s disease brain: stimulatory and inhibitory signal transduction pathways are differently affected. Brain Res. 1994;644:291–6.
Scott AI, Perini AF, Shering PA, Whalley LJ. In-patient major depression: is rolipram as effective as amitriptyline? Eur J Clin Pharmacol. 1991;40:127–9.
Sebastiani G, Morissette C, Lagacé C, Boulé M, Ouellette M-J, McLaughlin RW, Lacombe D, Gervais F, Tremblay P. The cAMP-specific phosphodiesterase 4B mediates Abeta-induced microglial activation. Neurobiol Aging. 2006;27:691–701.
Sette C, Conti M, Chem MJB. Phosphorylation and activation of a cAMP-specific phosphodiesterase by the cAMP-dependent protein kinase. J Biol Chem. 1996;271:16526–34.
Shao M, Yi X, Chi L, Lin J, Zhou Q, Huang R. Ischemic stroke risk in a southeastern Chinese population: insights from 5-lipoxygenase activating protein and phosphodiesterase 4D single-nucleotide polymorphisms. J Formos Med Assoc. 2014;114:422–9.
Shefer V. Absolute number of neurons and thickness of cerebral cortex during aging, senile and vascular dementia, and Pick’s and Alzheimer’s diseases. Neurosci Behav Physiol. 1973;6:319–24.
Shepherd M, McSorley T, Olsen AE, Johnston LA, Thomson NC, Baillie GS, Houslay MD, Bolger GB. Molecular cloning and subcellular distribution of the novel PDE4B4 cAMP-specific phosphodiesterase isoform. Biochem J. 2003;370:429–38.
Sheriff S, Dautzenberg FM, Mulchahey JJ, Pisarska M, Hauger RL, Chance WT, Balasubramaniam A, Kasckow JW. Interaction of neuropeptide Y and corticotropin-releasing factor signaling pathways in AR-5 amygdalar cells. Peptides. 2001;22:2083–9.
Sierksma AS, van den Hove DL, Pfau F, Philippens M, Bruno O, Fedele E, Ricciarelli R, Steinbusch HW, Vanmierlo T, Prickaerts J. Improvement of spatial memory function in APPswe/PS1dE9 mice after chronic inhibition of phosphodiesterase type 4D. Neuropharmacology. 2013;77:120–30.
Skullerud K. Variations in the size of the human brain. Influence of age, sex, body length, body mass index, alcoholism, Alzheimer changes, and cerebral atherosclerosis. Acta Neurol Scand. 1985;102:1–94.
Smith DL, Pozueta J, Gong B, Arancio O, Shelanski M. Reversal of long-term dendritic spine alterations in Alzheimer disease models. Proc Natl Acad Sci U S A. 2009;106:16877–82.
Stancheva S, Alova L. Age-related changes of cyclic AMP phosphodiesterase activity in rat brain regions and a new phosphodiesterase inhibitor--nootropic agent adafenoxate. Gen Pharmacol. 1991;22:955–8.
Stavinoha WB. Use of microwaves for rapid fixation of tissues in vivo. Scanning. 1993;15:115–7.
Sugawa M, May T. Age-related alteration in signal transduction: involvement of the cAMP cascade. Brain Res. 1993;618:57–62.
Sugawa M, May T. Signal transduction in aging. Arch Gerontol Geriatr. 1994;19:235–46.
Sullivan M, Rena G, Begg F, Gordon L, Olsen AS, Houslay MD. Identification and characterization of the human homologue of the short PDE4A cAMP-specific phosphodiesterase RD1 (PDE4A1) by analysis of the human HSPDE4A gene locus located at chromosome 19p13.2. Biochem J. 1998;333:693–703.
Swinnen JV, Joseph DR, Conti M. Molecular cloning of rat homologues of the Drosophila melanogaster dunce cAMP phosphodiesterase: evidence for a family of genes. Proc Natl Acad Sci U S A. 1989;86:5325–9.
Tao X, Finkbeiner S, Arnold DB, Shaywitz AJ, Greenberg ME. Ca2+ influx regulates BDNF transcription by a CREB family transcription factor-dependent mechanism. Neuron. 1998;20:709–26.
Taylor JR, Birnbaum S, Ubriani R, Arnsten a F. Activation of cAMP-dependent protein kinase A in prefrontal cortex impairs working memory performance. J Neurosci. 1999;19:RC23.
Terry RD, Katzman R. Life span and synapses: will there be a primary senile dementia? Neurobiol Aging. 2001;22:347–8.
Terry R, DeTeresa R, Hansen L. Neocortical cell counts in normal human adult aging. Ann Neurol. 1987;21:530–9.
Tohda M, Murayama T, Nogiri S, Nomura Y. Influence of aging on rolipram-sensitive phosphodiesterase activity and [3H]rolipram binding in the rat brain. Biol Pharm Bull. 1996;19:300–2.
Tomobe K, Okuma Y, Nomura Y. Impairment of CREB phosphorylation in the hippocampal CA1 region of the senescence-accelerated mouse (SAM) P8. Brain Res. 2007;1141:214–7.
Tredici G, Miloso M, Nicolini G, Galbiati S, Cavaletti G, Bertelli A. Resveratrol, map kinases and neuronal cells: might wine be a neuroprotectant? Drugs Exp Clin Res. 1999;25:99–103.
Vandesquille M, Baudonnat M, Decorte L, Louis C, Lestage P, Béracochéa D. Working memory deficits and related disinhibition of the cAMP/PKA/CREB are alleviated by prefrontal α4β2*-nAChRs stimulation in aged mice. Neurobiol Aging. 2013;34:1599–609.
Vecsey CG, Baillie GS, Jaganath D, Havekes R, Daniels A, Wimmer M, Huang T, Brown KM, Li X, Descalzi G, Kim SS, Chen T, Shang Y, Zhuo M, Houslay MD, Abel T. Sleep deprivation impairs cAMP signaling in the hippocampus. Nature. 2009;461:1122–5.
Verde I, Pahlke G, Salanova M, Zhang G, Wang S, Coletti D, Onuffer J, Jin SL, Conti M. Myomegalin is a novel protein of the golgi/centrosome that interacts with a cyclic nucleotide phosphodiesterase. J Biol Chem. 2001;276:11189–98.
Vincent P, Castro LR, Gervasi N, Guiot E, Brito M, Paupardin-Tritsch D. PDE4 control on cAMP/PKA compartmentation revealed by biosensor imaging in neurons. Horm Metab Res. 2012;44:786–9.
Vitolo OV, Sant’Angelo A, Costanzo V, Battaglia F, Arancio O, Shelanski M. Amyloid beta-peptide inhibition of the PKA/CREB pathway and long-term potentiation: reversibility by drugs that enhance cAMP signaling. Proc Natl Acad Sci U S A. 2002;99:13217–21.
Wachtel H. Potential antidepressant activity of rolipram and other selective cyclic adenosine 3′,5′-monophosphate phosphodiesterase inhibitors. Neuropharmacology. 1983;22:267–72.
Wachtel H, Schneider H. Rolipram, a novel antidepressant drug, reverses the hypothermia and hypokinesia of monoamine-depleted mice by an action beyond postsynaptic monoamine receptors. Neuropharmacology. 1986;25:1119–26.
Wallace TL, Stellitano KE, Neve RL, Duman RS. Effects of cyclic adenosine monophosphate response element binding protein overexpression in the basolateral amygdala on behavioral models of depression and anxiety. Biol Psychiatry. 2004;56:151–60.
Wallace DA, Johnston LA, Huston E, Macmaster D, Houslay TM, Cheung Y, Campbell L, Millen JE, Smith RA, Gall I, Knowles RG, Sullivan M, Houslay MD. Identification and characterization of PDE4A11, a novel, widely expressed long isoform encoded by the human PDE4A cAMP phosphodiesterase gene. Mol Pharmacol. 2005;67:1920–34.
Wang P, Myers JG, Wu P, Cheewatrakoolpong B, Egan RW, Billah MM. Expression, purification, and characterization of human subtypes A, B, C, and D. Biochem Biophys Res Commun. 1997;234:320–4.
Wang H, Peng M-S, Chen Y, Geng J, Robinson H, Houslay MD, Cai J, Ke H. Structures of the four subfamilies of phosphodiesterase-4 provide insight into the selectivity of their inhibitors. Biochem J. 2007a;408:193–201.
Wang M, Ramos BP, Paspalas CD, Shu Y, Simen A, Duque A, Vijayraghavan S, Brennan A, Dudley A, Nou E, Mazer JA, McCormick DA, Arnsten AFT. Alpha2A-adrenoceptors strengthen working memory networks by inhibiting cAMP-HCN channel signaling in prefrontal cortex. Cell. 2007b;129:397–410.
Wang C, Yang X-M, Zhuo Y-Y, Zhou H, Lin H-B, Cheng Y-F, Xu J-P, Zhang H-T. The phosphodiesterase-4 inhibitor rolipram reverses Aβ-induced cognitive impairment and neuroinflammatory and apoptotic responses in rats. Int J Neuropsychopharmacol. 2012;15:749–66.
Wang Z, Zhang Y, Liu Y, Zhao N, Zhang YZ, Yuan L, An L, Li J, Wang X, Qin J, Wilson S, O’Donnell J, Zhang H, Li Y. RNA interference-mediated phosphodiesterase 4D splice variants knock-down in the prefrontal cortex produces antidepressant-like and cognition-enhancing effects. Br J Pharmacol. 2013;168:1004–14.
Wang G, Chen L, Pan X, Chen J, Wang L, Wang W, Cheng R, Wu F, Feng X, Yu Y, Zhang HT, O'Donnell JM, Xu Y. The effect of resveratrol on beta amyloid-induced memory impairment involves inhibition of phosphodiesterase-4 related signaling. Oncotarget. 2016;7:17380–92.
Xu J, Rong S, Xie B, Sun Z, Deng Q, Wu H, Bao W, Wang D, Yao P, Huang F, Liu L. Memory impairment in cognitively impaired aged rats associated with decreased hippocampal CREB phosphorylation: reversal by procyanidins extracted from the lotus seedpod. J Gerontol A Biol Sci Med Sci. 2010;65:933–40.
Yamamoto M, Go ME, Ozawa H, Luckhaus C, Saito T, Rosler M, Riederer P. Hippocampal level of neural specific adenylyl cyclase type I is decreased in Alzheimer’s disease. Biochim Biophys Acta. 2000;1535:60–8.
Yamamoto-Sasaki M, Ozawa H, Saito T, Rösler M, Riederer P. Impaired phosphorylation of cyclic AMP response element binding protein in the hippocampus of dementia of the Alzheimer type. Brain Res. 1999;824:300–3.
Yan Y, Luo X, Zhang J, Su L, Liang W, Huang G, Wu G, Huang G, Gu L. Association between phosphodiesterase 4D polymorphism SNP83 and ischemic stroke. J Neurol Sci. 2014;338:3–11.
Yin J, Wallach J, Del Vecchio M, Wilder E, Zhou H, Quinn W, Tully T. Induction of a dominant negative CREB transgene specifically blocks long-term memory in Drosophila. Cell. 1994;79:49–58.
Zaldua N, Gastineau M, Hoshino M, Lezoualc’h F, Zugaza JL. Epac signaling pathway involves STEF, a guanine nucleotide exchange factor for Rac, to regulate APP processing. Sci Technol. 2007;581:5814–8.
Zeller E, Stief HJ, Pflug B, Sastre-y-Hernández M. Results of a phase II study of the antidepressant effect of rolipram. Pharmacopsychiatry. 1984;17:188–90.
Zhang H-T. Cyclic AMP-specific phosphodiesterase-4 as a target for the development of antidepressant drugs. Curr Pharm Des. 2009;15:1688–98.
Zhang H-T, Huang Y, Jin SL, Frith SA, Suvarna N, Conti M, O’Donnell JM. Antidepressant-like profile and reduced sensitivity to rolipram in mice deficient in the PDE4D phosphodiesterase enzyme. Neuropsychopharmacol Off Publ Am Coll Neuropsychopharmacol. 2002;27:587–95.
Zhang X, Odom DT, Koo S-H, Conkright MD, Canettieri G, Best J, Chen H, Jenner R, Herbolsheimer E, Jacobsen E, Kadam S, Ecker JR, Emerson B, Hogenesch JB, Unterman T, Young RA, Montminy M. Genome-wide analysis of cAMP-response element binding protein occupancy, phosphorylation, and target gene activation in human tissues. Proc Natl Acad Sci U S A. 2005;102:4459–64.
Zhang H-T, Huang Y, Masood A, Stolinski LR, Li Y, Zhang L, Dlaboga D, Jin SL, Conti M, O’Donnell JM. Anxiogenic-like behavioral phenotype of mice deficient in phosphodiesterase 4B (PDE4B). Neuropsychopharmacology. 2008;33:1611–23.
Zhang C, Cheng Y, Wang H, Wang C, Xu J, Zhang H. RNA interference-mediated knockdown of long-form phosphodiesterase-4D (PDE4D) enzyme reverses amyloid-β42-induced memory deficits in mice. J Alzheimers Dis. 2014;38:269–80.
Zhao Y, Li W, Li F, Zhang Z, Dai Y, Xu A, Qi C, Gao J. Resveratrol improves learning and memory in normally aged mice through microRNA-CREB pathway. Biochem Biophys Res Commun. 2013;435:597–602.
Zimmerman I, Berg A. Levels of adenosine 3′,5′-cyclic monophosphate in the cerebral cortex of aging rats. Mech Ageing Dev. 1974;3:33–6.
Conflict of Interest
The authors declare that they have no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 Springer International Publishing AG
About this chapter
Cite this chapter
Hansen, R.T., Zhang, HT. (2017). The Past, Present, and Future of Phosphodiesterase-4 Modulation for Age-Induced Memory Loss. In: Zhang, HT., Xu, Y., O'Donnell, J. (eds) Phosphodiesterases: CNS Functions and Diseases. Advances in Neurobiology, vol 17. Springer, Cham. https://doi.org/10.1007/978-3-319-58811-7_7
Download citation
DOI: https://doi.org/10.1007/978-3-319-58811-7_7
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-58809-4
Online ISBN: 978-3-319-58811-7
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)