Abstract
We have developed an alternative cold-adapted (4, 8 °C), Arctic/Alpine inoculum to overcome the obstacle of limited hydrolysis and methanogenesis that is common in low temperature anaerobic wastewater (WW) treatment systems. This special WW-fuelled inoculum was employed here to study its activity and growth at low temperatures (4 and 15 °C) with real wastewater as substrate. Cell specific methanogenic activities (4–15 °C) were comparable with those observed for mesophiles at higher temperatures (37 °C) (≈2.0 gCODmethane.gVSS−1.day−1). Low temperature (4 °C) acclimation forms methanogenic communities that perform robustly at 4 °C and better than those pre-acclimated to higher temperatures (8 °C) when incubated at 15 °C. An evaluation of the inoculum resistance to migration of ‘outsiders’ at low (4–8 °C) and high (15 °C) temperatures showed that the lower the temperature the higher the probability for migration (archaeal migration rate at 4–8 °C 1.69 × 10−5 death−1 > 6.44 × 10−6 death−1 at 15 °C). Limited cell growth at low temperature treatment systems is one of the main reasons. This limitation can be overcome as growth kinetics at low temperatures (4 °C) are comparable to those observed in mesophilic reactors (≈20 days – growth coef.: 0.05 day−1) depending on the substrate availability. Thus, continuous, rich in COD feeding regimes may assist cold adapted biomasses to not only grow at low temperatures but also to decrease the probability to be outpaced by of WW-originated ‘invaders’.
Access provided by CONRICYT-eBooks. Download conference paper PDF
Similar content being viewed by others
Keywords
1 Introduction
Most attempts to acclimatize mesophilic sludge to lower temperatures for anaerobic wastewater treatment purposes failing to accomplish both hydrolysis and methanogenesis at <8 °C with real WW as substrate. A recent study by Petropoulos et al. (2017) has shown that the development of a cold-adapted biomass using inocula obtained from cold environments is a promising approach for successful anaerobic domestic WW treatment at low temperatures (≥4 °C). The specific, WW-fuelled, methanogenic activity of this inoculum is re-evaluated here (from/to 4 and 15 °C) and compared with activities obtained by mesophilic inocula at various temperatures.
It is under question whether the microbial structure of the seed would remain unchanged if and when exposed to non-sterile wastewater. A way to estimate the status of the biomass in such scenario is by estimating the ‘migration rates’, the probability of an individual from the ‘inside’ die and be replaced by an individual from the ‘outside’. In this study the ‘inside’ is the sample out of the remaining reactor volume that stands for the ‘outside’. The cells from the former represent the local community that needs to remain stable in the face of the latter, the potential invaders. A high probability of outsiders outpacing the insiders (migration rate) is likely at low temperatures due to low growth and consequently a taxon in the local community is more likely to be replaced by individuals from a source community (e.g. actual WW). Hence an inoculum at low temperatures may be more prone to migration than an inoculum at higher temperatures, where growth is more evident. Migration can be tackled by high growth (Curtis and Sloan 2004). Following a continuous feeding regime and by adding a UF membrane to minimize cell losses we show that growth at low temperatures is attainable and subsequently migration can be lowered.
2 Materials and Methods
Inocula.
Eight reactors were seeded volumetrically with an equal mixture of putatively cold-adapted biomasses from reactors that were previously treating wastewater at low temperatures (4 and 8 °C). The nature and origin of this inoculum is sediments from Lake Geneva and soils from Svalbard, in the high Arctic (Petropoulos 2015).
Wastewater.
Wastewater was collected from the Tudhoe Mill (County Durham, UK) wastewater treatment plant (WWTP), which treats domestic wastewater. Primary settled influent was used to ensure the absence of large solids that might block the pumps that were employed to feed the reactors. During the operation no pH adjustments were made (pH: 7.0 on day 1). The wastewater COD varied from 150 to 600 mg.L−1.
Methanogenic activity tests.
Methanogenic activity of inocula pre-acclimated to 4 and 8 °C was measured at 4 and 15 °C (operational temperatures, average high and average low for the region of Newcastle UK). The selected setup was similar with the one described by Bowen et al. 2014. The seed: total. vol. ratio was 1:4 based on Petropoulos, 2015. All mini-reactors (Wheaton vials) were prepared in duplicates. Controls with only inoculum and only wastewater were also prepared. The results display the activity of the inoculum after abstracting the activity from the two controls. The activity is expressed per methanogenic cell, the cell enumeration was carried out on day 18 and 27 via McrA gene qPCR. The qPCR was carried out according to Steinberg and Regan (2009). The conversion of the activity cell−1 to VSS−1 was based on Rittman and McCrarty 2001 (1 cell = 1 × 10−12 gVSS).
Migration rates.
The rates were calculated based on OTU tables generated after pyro-sequencing DNA (5 μl DNA sample from 100 μl extracted) from 1 ml mixed liquor of 1L batch reactors (Petropoulos et al. 2017 setup;).The OTU tables between 4 and 8 °C were merged as low temperatures (n = 4), the rates from 15 °C were estimated separately (n = 4), warm temperature. The assumption that the 4 and 8 °C count as one was made since the calculation requires at least 3 replicates (n = 2 each) and previous MDS plots (Petropoulos et al. 2017) showed that the difference between the two community structures is minimal. The estimation involved neutral models (Sloan et al. 2006) on the sequencing pattern of long retention time batch reactors (4–8 and 15 °C after 1100 days).
Continuous reactor setup.
Eight 1L reactors were seeded with a mixture of 4 and 8 °C (1:1 v/v) acclimated inocula (from par. 2.1), fed with UV sterilised primary settled domestic WW at a 1:1 v/v ratio and set at 4 and 15 °C (n = 4). The reactors were equipped with hollow fibre membrane units to prevent biomass washout, allowing an operational flux (LMH) of 2.3 L.m2.hr (HRT = 7 daysday34-present). Daily operation included a daily 4 h relaxation plus a 3 h backwash (after day 58). The operational OLR and SLR were 0.08 kgCOD.m−3.day−1 and \( 6.13\pm1.4\, {\text{kgCOD.kgVSS}_\text{methamogens}}^{-1}.\text{day}^{-1} \) (based on mcrA qPCR enumeration and conversion of the cells to VSS assuming that 1 cell weighs 1 × 10−12 grams (Rittman 2001)).
Chemical analysis.
COD, sCOD, TSS, VSS were measured based on APHA, 2005. VFAs were measured on DIONEX ICS-1000, Anions were measured on Dionex Anion Micro Membrane Suppressor (AMMS-ICE II); methane gas was measured onto a Carlo Erba HRGC S160 GC fitted with an FID detector and HP-PLOTQ column.
Methane production.
The methane production rate is a sum of the gaseous, and dissolved in the aqueous phase (mixed liquor and effluent) CH4 that was formed per HRT.
Cell enumeration and growth.
Methane formation combined with average per temperature specific methanogenic activity was used to give an indication of the number of methanogens that are present in the reactor (Eq. 1). Knowing the cell abundance (N) at Δt for t1 (No) and t2 (Nt) and by using 1st order kinetics (Eq. 2) we can calculate the k (growth coefficient).
3 Results and Discussion
3.1 Batch Setup
Methanogenic Activity.
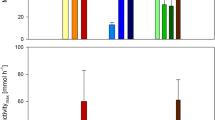
Activity tests (Bowen et al. 2014 setup) with inocula from permanently cold environments (Petropoulos 2015), that had been grown with UV-sterilized WW at 4 and 8 °C for >1100 days, showed that a biomass acclimated at low temperature (4oC) performs robustly at both cold and warmer temperature (15 °C) compared to a biomass that had been acclimated at higher temperature (8 °C) (Fig. 1a). Cold adapted cells can convert COD: CH4 at rates similar with those achieved by mesophilic inocula at warmer conditions (37 °C) (Fig. 1b). The activity at this study was found similar to the activity that was measured by previous putative psychrophilic populations in anaerobic reactors (Petropoulos et al. 2017; Collins et al. 2005 (Fig. 1b)). The exponential trend on Fig. 1b points the different methanogenic capacity between psychrophilic/cold-adapted and mesophilic methanogens at various temperatures.
(a) Specific methanogenic activity per methanogenic cell (‘Seed WW’ stands for arctic inoculum fed with domestic WW; 1st and 2nd number stands for the acclimation and the operational temperature respectively; (b) specific methanogenic activity by psychrophilic/cold-adapted and mesophilic inocula at various temperatures (Dolfing and Mulder 1985; Bowen et al. 2014; Keating et al. 2016; Luostarinen and Rintala 2005, 2007; McHugh et al. 2006 etc.)) (1 cell = 1 × 10−12 grams VSS (Rittman, 2001))
Microbial community stability.
Inoculation of a bioreactor with cold-adapted cells is a strategy for the promotion of WW treatment at low temperature. Curtis and Sloan (2004) suggested that ‘invasion’ of WW cells may challenge an established microbial diversity since the community develops through a continuous cycle of immigration, births and death (Sloan et al. 2006). After pyro-sequencing DNA samples from the mixed liquor of 1L batch reactors (Petropoulos et al. 2017) the migration rates of the inoculum were estimated. The estimation involved application of neutral models (Sloan et al. 2006) on the sequencing pattern of long retention time batch reactors (4–8 and 15 °C after 1100 days). Whilst bacterial migration rates were similar at all temperatures (2.84 × 10−5 death−1), the archaeal rates showed that the cells are more prone to migration at low temperatures (migration rate4-8oC of 1.69 × 10−5 death−1 > 6.44 × 10−6 death−1 at 15 °C). QPCRMcrA showed that this is attributed to low growth at low temperatures caused by slow hydrolysis/limited feed. Continuous feed may promote growth and lower migration (Curtis and Sloan 2004).
3.2 Continuous Setup
Continuous feeding regime.
During the continuous operation methane production was separated into two phases regardless the temperature, the lag phase (d.0-65) and the ‘vigorous’ phase (d.66-present) (Fig. 2).
Methane production.
Methane production was poor over the lag phase with the methane at 15 °C only increasing linearly. At 4 °C a curve in the methane production rate was formed with the minimum reached on day 58 (Fig. 2). The curve may signify the acclimation screening mechanism (mixture of biomasses adapted at 4 and 8 °C as inoculum) where only the resilient/adapted cells would thrive at these operational conditions, most likely the pre-acclimated to 8 °C cells were outpaced by those pre-acclimated to 4 °C when both at 4 °C. Once the community is screened (d.65) growth becomes apparent. Methane production rate at 4 °C increased reaching an average of 30 ± 9.6% CH4:COD (d.79-present) of the theoretical conversion; at the same period the conversion at 15 °C reached the 38 ± 14.6%.
COD sinks.
High WW sulphate concentration led to low COD to methane conversions (average SO4 influent concentration of 134 mg.L−1) at both temperatures (43 and 97% sulphate reduction at 4 and 15 °C respectively). Hence, sulphate reduction played an important role in COD removal, especially at 15 °C. This explains why the methane rate at higher temperature was lower to at 4 °C. The sulphate reduction Q10 was estimated as 2.1. Sulphate reduction is unlikely to be observed using simulated wastewater as substrate. Plethora of research endeavours that use synthetic wastewater aiming to the degradation of the substrate mainly by methanogens. With actual wastewater this is partly the case.
Enumeration and Growth.
The growth exponential trendline of the growth coefficients as a function of sCOD at Δt51-107 show that methanogenic cells from low and high temperature may achieve similar doubling times (Fig. 3a). This states that growth (k ~ 0.05 day−1, doubling time ~ 20 days) at low temperatures (≥4 °C) is attainable but requires high available COD (> 200 mg.L−1 for the cells to uptake (Fig. 3b). The positive growth coefficients were estimated and correlated with the sCOD in the mixed liquor. Negative coefficients were neglected since they do not signify growth. All negative coefficients appeared at sCOD lower to 116 and 38 mg.L−1 for 4 and 15 °C respectively. The 4 °C trend (Fig. 3b) is a rough estimation of the relationship between sCOD and growth since the R2 is low and the data points limited. Further experimentation would give more information at the Relationship between growth and carbon availability at extremely low temperatures (4 °C). Regardless the weak correlation growth at 4 °C is attainable and subsequently migration at low temperatures can be reduced to levels that would possibly allow the application of such inocula to operate in the presence of WW-originated cells.
(a) Abundance of the methanogenic cells based on the average specific activity at 4 and 15 °C; (b) methanogenic growth coefficient k as a function of the available sCOD present in the mixed liquor at 4 and 15 °C (negative coefficients based on Fig. 3a were excluded as they signify decay)
Wastewater Treatment Efficiency.
Between the 1st and the 2nd phase no significant differences in the CODeffluent pattern were observed at 15 °C. At 4 °C robust hydrolysis activity appeared on day 72 with a sCODML peak followed by a high effluent COD concentration that afterwards decreased to meet the UWWTD directive standard (COD < 125 mg/L) after day 121. The hypothesis of accumulation of organic material in the mixed liquor due to slow hydrolysis as a removal mechanism, especially at 4 °C, (Petropoulos et al., 2017) is plausible since sCODML > sCODinf (d. 93 – Fig. 4). At the 1st phase the membrane biofilm contributed to treatment predominantly at 15 °C (further COD removal between the mixed liquor and the effluent of 22 ± 4% and 32 ± 5% at 4 and 15 °C respectively (Fig. 4)). From day 65 the membrane was a key component at low temperatures contributing to the sCOD removal by up to 45%. This may be attributed to the promotion of syntrophic interactions that is required at lower temperatures (Smith et al. 2015).
4 Conclusions
Cold adapted inocula may facilitate anaerobic treatment wastewater at low temperatures with specific methanogenic rates comparable with those attained by mesophiles at higher temperatures. Acclimation to low temperature (4 °C) forms communities that perform robustly at 4 °C and better than biomasses that were acclimated to higher ones when both at high temperatures (e.g. 15 °C). Cold-adapted methanogens are able to grow at 4 °C with realistic doubling times in the presence of adequate organic material. Thus, migration rates can be lowered and stability of the cold-adapted microbial community structure can be promoted.
References
Bowen EJ, Dolfing J, Davenport RJ, Read FL, Curtis TP (2014) Low temperature limitation of bioreactor sludge in anaerobic treatment of domestic wastewater. Wat Sci Tech 69(5):1004–1013
Collins G, Mahony T, O’Flaherty V (2005) Stability and reproducibility of low-temperature anaerobic biological wastewater treatment. FEMS Microbiol Ecol 55:449–458
Curtis TP, Sloan WT (2004) Prokaryotic diversity and its limits: microbial community structure in nature and implications for microbial ecology. Cur Opin Microbiol 7:221–226
Dolfing J, Mulder J-W (1985) Comparison of methane production rate and coenzyme F420 content of methanogenic consortia in anaerobic granular sludge. Appl Environ Microbiol 49(5):1142–1145
Keating C, Chin JP, Hughes D, Manesiotis P, Cysneiros D, Mahony T, O’Flaherty V (2016) Biological phosphorus removal during high-rate, low-temperature, anaerobic digestion of wastewater. Front Microbiol 7:226–240
Luostarinen SA, Rintala JA (2005) Anaerobic on-site treatment of black water and dairy parlour wastewater in UASB-septic tanks at low temperatures. Wat Res 39:436–448
Luostarinen SA, Rintala JA (2007) Anaerobic on-site treatment of kitchen waste in combination with black water in UASB-septic tanks at low temperatures. Biores Technol 98(9):1734–1740
McHugh S, Collins G, O’Flaherty V (2006) Long-term, high-rate anaerobic biological treatment of whey wastewaters at psychrophilic temperatures. Biores Technol 97:1669–1678
Petropoulos E, Dolfing J, Bowen E, Davenport R, Curtis T (2017) Developing cold-adapted biomass for the anaerobic treatment of domestic wastewater at low temperatures (4, 8 and 15 °C) with inocula from cold environments. Wat Res 112:100–109. doi:10.1016/j.watres.2016.12.009
Petropoulos E (2015) Investigating the true limits of anaerobic wastewater treatment of wastewater at low temperature using a cold-adapted inoculum, PhD thesis, Newcastle University, Newcastle, UK
Rittman BE, McCarty PL (2001) Environmental Biotechnology. McGraw-Hill, Singapore
Smith AL, Skerlos SJ, Raskin L (2015) Anaerobic membrane bioreactor treatment of domestic wastewater at psychrophilic temperatures ranging from 15 °C to 3 °C. Environ Sci Wat Res Technol 1:56–64
Sloan WT, Lunn M, Woodcock S, Head IM, Nee S, Curtis TP (2006) Quantifying the roles of immigration and chance in shaping prokaryote community structure. Environ Microbiol 8:732–740
Steinberg LM, Regan JM (2009) mcrA-Targeted real-time quantitative PCR method to examine methanogen communities. Appl Environ Microbiol 75(13):4435–4442
Acknowledgements
This work was funded by the BBSRC (BB/K003240/1; Engineering synthetic microbial communities for biomethane production).
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 Springer International Publishing AG
About this paper
Cite this paper
Petropoulos, E., Dolfing, J., Curtis, T.P. (2017). Methanogenic Activity and Growth at Low Temperature Anaerobic Wastewater Treatment (4, 15 °C) Using Cold Adapted Inocula. In: Mannina, G. (eds) Frontiers in Wastewater Treatment and Modelling. FICWTM 2017. Lecture Notes in Civil Engineering , vol 4. Springer, Cham. https://doi.org/10.1007/978-3-319-58421-8_58
Download citation
DOI: https://doi.org/10.1007/978-3-319-58421-8_58
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-58420-1
Online ISBN: 978-3-319-58421-8
eBook Packages: EngineeringEngineering (R0)