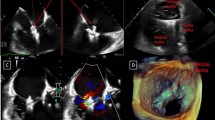
Abstract
Tricuspid valve disease is common but often has less attention than in the left side of the heart. Functional tricuspid regurgitation if left untreated is associated with unfavorable outcome. Moreover, tricuspid valve surgery is often associated with higher complications than for any other surgical valve intervention. Transthoracic echocardiography (TTE) is the gold standard imaging of choice in the assessment of tricuspid valve disease. TTE has many sub modalities such as the M-mode, 2D and 3D modes, the bi-plane mode and the recently introduced iRotate mode. Furthermore, Doppler interrogation of the tricuspid valve as well as blood flow over other cardiac valves, pulmonary, aortic, hepatic venous flow is the mainstay in hemodynamic assessment of patients with tricuspid valve disease. TTE role begins with screening for the presence or absence and type of tricuspid valve disease. Moreover, determination of the etiology, severity of the tricuspid lesion, associated other valvular problems and chamber quantification are part of an imaging protocol/approach, which required for full diagnosis of tricuspid valve disease. Pre-operative and pre-catheter interventional planning can be performed by TTE. Finally, post interventional outcome as well as follow-up is often determined via TTE. In this chapter, we will provide a comprehensive and state-of-the art overview of the use of TTE for the assessment of TV disease. Advantages as well as limitations of each TTE sub modality will be outlined. A perspective on the value of TTE in the era of percutaneous transcatheter TV interventions will also be highlighted.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
- Echocardiography
- Transthoracic
- M-mode
- Doppler
- iRotate
- 2 Dimensional
- 3 Dimensional
- Tricuspid
- Stenosis
- Regurgitation
- Severity
- Surgery
- Interventions
- Outcome
Introduction
Transthoracic echocardiography (TTE) has been the mainstream imaging modality of choice for the assessment of tricuspid valve (TV) disease. TTE is versatile , low cost, is available at bedside and can be easily repeated without risks of ionizing radiation such as in computed tomography or device incompatibility such as in magnetic resonance imaging. Tricuspid regurgitation (TR) is the most common form of TV disease. TTE provides functional information, grading of TR severity as well as detailed morphologic assessment of TV leaflets that are not easily seen on other imaging modalities. These TTE modalities are M-mode, Doppler, multiplane imaging and three-dimensional (3D) imaging. 3D–TTE provides a full perspective of right- as well as left-sided valves and chambers. Moreover, 3D colour-Doppler can provide quantification of TR severity. The multiplane iRotate mode which has been recently introduced into clinical practice, provides not only high frame rate imaging that is comparable to 2D echocardiography, but also a multiplane perspective as in 3D echocardiography.
Tricuspid Valve Apparatus
Detailed cardiac anatomy and embryology are provided in Chap. 1 of this book. In brief, the TV is the atrioventricular valve that separates the right atrium (RA) from the right ventricle (RV). It is the largest and most apical of all cardiac valves. Its main function is to prevent blood returning to the RA. The TV apparatus is composed of a fibrous annulus, three leaflets (anterior, posterior and septal), chordae tendinae, three papillary muscles, and right ventricular myocardium . Papillary muscles are relatively smaller and more widely spaced as compared to the left side of the heart. The septal leaflet is more fixed than the other two leaflets and the posterior leaflet is the smallest. Anterior and posterior leaflets have several secondary chordae which are attached to the moderator band and the highly trabeculated free wall.
The TV has a complex saddle-shaped structure, which results in the attachment of the three leaflets not being aligned. Therefore, imaging of the TV annulus together with all three leaflets requires a 3D modality. Anwar et al. have shown that only two leaflets can be seen simultaneously in one 2D view. Moreover, identification of individual leaflets and hence localization of TV pathology can be challenging, particularly in disease conditions [1]. However, manipulation of the ultrasound transducer in the apical 4-chamber view using an anterior to posterior sweep or preferably a well performed subcostal view acquisition could, in some instances, allow for simultaneous display of all three TV leaflets. These views require an experienced echographer as well as a 3D interpretation of the individual leaflet orientation in space. Successful TV function depends on integrated and well-coordinated components of the whole TV apparatus.
TTE Examination of the Tricuspid Valve
TV morphology can be evaluated using 2D-TTE , which is considered the first modality for screening. Generally, TTE assessment of the TV is preferred to transesophageal echocardiography (TEE) as the right heart is situated in the near field. TTE scanning of the TV is rather challenging because of the complex non-planner position of the annulus and its highly variable anatomy [1]. It is very difficult to visualize all three leaflets simultaneously in one 2D-TTE view. Therefore, other echocardiographic modalities such as the multiplane iRotate, 3D-TTE or transesophageal echocardiography may be needed to complete TV evaluation.
TTE Modes
Table 5.1 lists the TTE modes that could be used for screening and assessment of tricuspid valve disease.
2D–TTE
Standard 2D-TTE for the assessment of TV morphology and function can be performed from multiple acoustic windows. Essential 2D–TTE acoustic windows include the standard parasternal long-axis (RV inflow), the parasternal short-axis view, the apical 4-chamber view, and the subcostal view (Table 5.2 and Figs. 5.1, 5.2, and 5.3). A comprehensive TTE exam of the TV requires the use of all feasible 2D views alongside Doppler Interrogation to obtain full assessment of the TV apparatus, right heart chambers as well as venous and pulmonary circulation. A localized pathology such as a flail leaflet could be missed if all the TTE views are not attempted. Detailed transesophageal assessment of TV is provided in Chap. 6.
The parasternal 2D-TTE views: standard right ventricular inflow tract view from a normal subject (a) and after further angulation (b) to exclude left ventricle from the scan sector and a standard parasternal short-axis view at base of the heart (aortic valve) level (c). Corresponding views from a patient with an abnormal TV (d, e and f). LV left ventricle, RV right ventricle, RA right atrium, RVOT right ventricular outflow tract, LA Left atrium, AO aorta
The 2D-TTE apical views from a normal right heart (a–e): focused (a); modified (b); aortic (c); coronary sinus (d) and subcostal (e). Corresponding views from a subject with an abnormal TV (f–j). Note that in “h” LV cavity is minimally visualized due to the prominent RV (see*). Abbreviations are similar to Fig. 5.1, CS coronary sinus
3D-TTE
3D-TTE is often used in experienced centers to provide a comprehensive interrogation of the TV leaflets, annulus, subvalvular apparatus and RV size and function quantification.
Acquisition
3D-TTE acquisition can be performed from any of the standard 2D acoustic windows (parasternal, apical and subcostal). Using current technology cardiac structures in the axial dimension (y, azimuthal) have the best resolution (0.5 mm), in the lateral dimension (x) (2.5 mm), and in the elevation dimension (3.0 mm) the least resolution. Therefore, the parasternal short-axis window should provide the best quality for an “enface surgical” view. Consequently, TV display on 3D-TTE has an intermediate quality in the parasternal long-axis window and has the least quality in the apical window acquisition. The two most commonly used 3D-TTE modes are the real-time and the full-volume modes. The former has a relatively narrow angle (smaller scan sector) but it provides a higher resolution than the latter. Furthermore, a real-time scan is not limited to the number of heart cycles nor it is limited by respiration or arrhythmia. Moreover, it allows for focusing on a specific region of interest. On the other hand, the full volume mode allows for a wider angle (larger scan sector) that can include more structures in a single acquisition. However, structures on the full-volume mode have much lower resolution and could be limited by artifacts due to respiration or arrhythmia. The trade-offs between the two modes should be taken into consideration as per patient and according to the objectives of the 3D-TTE scan.
Analysis and Display
Similar to other 3D imaging modalities such as magnetic resonance imaging or computed tomography, real-time 3D-TTE datasets in the multiplanar reconstruction mode, display the full TV apparatus in 3D as well as right sided chambers in the 2D long-axis (coronal and sagittal) and short-axis (axial) planes. This allows for a single image displaying the TV in an enface surgical view simultaneously with a full assessment of the TV leaflets (Fig. 5.4). For uniformity it has been proposed that display of the 3D-TTE enface view of the TV should preferably be performed with the septal leaflet in the 6 o’clock position [2].
Multiple 3D-TTE views of an open TV as seen from RV aspect (a) and from RA aspect (b) and the corresponding views with the valve closed (d and e); RV inflow view (c) and a 2D multiplane reconstruction from 3D dataset (f). Note that asterisk refers to the place of TV annulus. ATL anterior leaflet, PTL posterior leaflet, STL septal leaflet, MV mitral valve, Ao aorta, RV right ventricle, RA right atrium
Incremental Value of 3D-TTE over 2D-TTE of the TV
Compared with left sided structures, echocardiographic assessment of right-sided structures is more difficult due to the anterior location of the right heart and the variable position of the TV leaflets. Since 3DE is dependent on 2DE quality, 3DE acquisition of the right heart is still challenging. However, there is accumulating evidence that 3DE has incremental value in the assessment of TV disease compared with 2D-TTE.
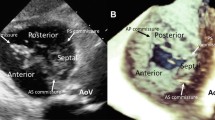
Visualization of TV leaflets: Using 2D-TTE, it is extremely difficult to identify all leaflets in the parasternal short-axis view due to variability in their position. Anwar et al. have shown that by using a 3D mental reconstruction, both septal and anterior leaflets can be identified in all cases from the parasternal long-axis (RV inflow) and the apical 4-chamber views (Fig. 5.5). The use of an enface view from a right atrial or right ventricular aspect, “surgical view” provides a simultaneous display of the TV leaflets and their attachment to the TV annulus as well as it provides accurate assessment of leaflet morphology, thickness, leaflet defect, prolapse and fusion of commissures.
Visualization of TV leaflets on three standard 2D-TTE views. Percentages of the leaflet identification have been reported below each 2D view. From Anwar et al. with permission [1]
Quantification of TV annulus: Another clear advantage of 3D-TTE over 2D-TTE is the ability of the former to accurately quantify the non-planner TV annulus [1, 3]. Detailed measurements of leaflets size, intercommisural distance, and cyclic variation can be made [1].
Assessment of TR: Moreover, real-time 3D-TTE has an increasing role in the assessment of TR (Fig. 5.6). Assessment of TV leaflets and commissures morphology, annulus size and non-coaptation distance provide important clues to etiology and mechanism of TR. Furthermore, although practically difficult, quantification of vena contracta area of TR jet on real-time colour Doppler 3D-TTE has been accomplished. The authors proposed new cut-off values for TR severity: 0.5 cm2 for mild, 0.5–0.75 cm2 for moderate, and >0.75 cm2 for severe TR [4]. However, these values need to be prospectively validated in a larger external population. Respiratory variation of TV jet regurgitant orifice area has been shown on colour Doppler 3D-TTE, as well as other TR parameters on 2D-TTE [5, 6].
Visualization of different etiologies and mechanisms of TR on real-time 3D-TTE : (a) a long-axis and (b) a short-axis enface TV view as seen from the RV aspect, a pacemaker lead (arrow) restricts TV leaflets from closing. (c) and (d) are from a patient with a prolapse of the posterior leaflet of the TV with elongated chordae as seen from RV aspect with TV open (c) and RA aspect TV closed (d). The arrow points to incomplete TV coaptation due to prolapsed posterior leaflet. Image (e) shows an enface view of TV, as seen from the RV aspect, with a tear in the TV leaflets (arrow) after repeated biopsies in a patient with a heart transplant. Image (f) shows lack of TV leaflets coaptation (arrow) due to carcinoid disease in enface RV view. ATL anterior leaflet, PTL posterior leaflet, STL septal leaflet, PM pacemaker lead, LV left ventricle
Tricuspid annular dilatation in patients with functional TR can be a more accurate indicator of TR severity than color Doppler of TR alone [7,8,9]. Therefore; the ability to accurately quantify TV annulus with 3D-TTE is important in the assessment of TR severity [10, 11].
Display and assessment of TV enface area, similar to mitral enface area; in patients with suspected TV stenosis is required for accurate diagnosis of TV pathology, and of course only feasible on 3D-TTE [12, 13].
Simultaneous Multiplane Imaging
With the introduction of the MATRIX transducer, simultaneous multiplane imaging has become available. This new modality permits the use of a full electronic rotation of 360° (adjustable by 5° steps) of the 2D image (iRotate) and a simultaneously adjustable bi-plane 2D image (xPlane).
Bi-plane TTE
In the bi-plane mode an orthogonal view can be obtained through the midline of the primary image, such as the tricuspid valve, and displayed as a secondary image. If necessary, from the midline additional secondary images can be visualized by a lateral tilt of up to maximal +30° to −30° (Fig. 5.7).
2D-TTE bi-plane mode assessment of the TV in two different views: (a) from a normal subject where the reference line transects the tricuspid valve annulus in a focused RV apical view allowing measurements of two axes diameters; (b) from a patient with TV disease where the reference line, transects the tricuspid valve annulus in the RV inflow view allowing measurements of two axes diameters. All measurements can be performed in the same heartbeat. RV right ventricle, RA right atrium, RVOT right ventricular outflow tract
iRotate Mode
2D- iRotate echocardiography is a relatively new echo modality aiming at maximizing the benefits of using 2D- and 3D-TTE in routine clinical practice by combing the advantages of the two echo modalities. It allows for full assessment of a cardiac structure such as the right ventricle from a single transducer position. The iRotate images retain the advantages of a better quality and a higher frame rate than 3D-TTE. The feasibility of 2D-TTE iRotate has been examined by our group for the assessment of right ventricular function using fixed anatomic landmarks [14] as well as right ventricular strain assessment [15].
McGhie et al. proposed a novel 13-segment model to assess right ventricular function from a single apical acquisition using the iRotate mode . The proposed protocol uses four anatomic landmarks to identify the different right ventricular walls, namely: mitral valve for RV lateral free wall, coronary sinus for RV anterior wall, aortic valve for RV inferior wall and RVOT for RVOT anterior and inferior wall. From the apical window, a standard apical 4-Chamber view can be adjusted to acquire a focused non-foreshortened RV view with the tricuspid valve centered along, or as near as possible, to the midline of the sector. With the iRotate mode a full electronic rotation can be performed. Using the anatomic landmarks, as defined above, four standard TV annulus views can be acquired (Figs. 5.8 and 5.9).
The 2D-iRotate image acquisitions of the four RV views from a normal TV valve, left, and right the corresponding views from a patient with Ebstein’s anomaly (a, e) the mitral view: visualizing the RV lateral wall; (b, f) the coronary sinus view: visualizing the RV anterior wall; (c, g) the aortic view: visualizing the RV inferior wall; (d, g) the coronal view: visualizing the RV inferior wall and RVOT anterior wall. Note the position of the chordal attachments, towards the RVOT, of the Ebstein TV in (h)
The 2D-TTE iRotate mode provides a standard methodology for serial assessment of RV function [16]. High quality image acquisition using the 2D-TTE iRotate mode could be achieved after a relatively short learning curve of 20 cases. The feasibility of segmental RV wall analysis approached 95% in subjects with normal- and patients with dilated-RVs. Furthermore, quantification of RV function using tricuspid annular plane systolic excursion (TAPSE) and Doppler tissue velocities were feasible in more than 90% of subjects with normal RV size and in all patients with dilated RVs. Likewise, assessment of RV strain in subjects with normal and dilated RV has been shown feasible and reproducible using the 2D-TTE iRotate mode [15].
Translating those initial feasibility studies of the 2D-TTE iRotate mode into direct TV assessment could be speculated in several aspects. It could be used for more robust serial follow up of disease progression before- and reverse remodeling after percutaneous interventions or surgery involving the TV. Likewise, recovery of RV function after TV repair or replacement could be more accurately quantified. The 2D-TTE iRotate mode could be used as well for comprehensive and robust serial assessment of TV annulus size and function.
TTE Approach to Tricuspid Valve Disease
Tricuspid pathology can be broadly described as stenotic, regurgitant or both. TV disease varies widely from asymptomatic lesions to advanced cases with generalized anasarca due to severe TR. Congenital TV disease is beyond the scope of this book chapter.
A diagnostic approach begins with morphologic assessment and localization of the underlying TV pathology. Once the etiology of TV lesion is established, assessment of disease severity is the next step. Impact of TV disease on right sided chambers size and function as well as pulmonary and hepatic circulation should follow. Finally, associated valvular lesions and assessment of left sided chambers should be performed. Table 5.3 lists the comprehensive assessment of the TV with 2D-TTE.
TTE Approach to Tricuspid Valve Stenosis
TV stenosis can be due to rheumatic, infiltration such as in carcinoid disease, or rarely, due to compression by external structure such as tumor, thrombus or the aorta. When suspected, morphologic assessment with 2D-TTE and colour-Doppler, spectral pulsed- and continuous-wave Doppler is required (Fig. 5.10). Tracing of Doppler flow envelopes should be performed and averaged from three to five cardiac cycles. Velocity timed integral (VTI) and mean gradient can then calculated automatically. There are several methods for calculation of the TV cross sectional area (Tables 5.4 and 5.5).
Doppler estimation of TV cross-sectional area is based on the conservation of mass theory. Using the continuity equation, TV cross-sectional area can be calculated from the standard formula (TV cross sectional area in cm2 = [A1*V1/V2]) where A1 is the cross-sectional area from left or right ventricular outflow, V1 is the peak flow velocity from left or right ventricular outflow on pulsed-wave Doppler. V2 is the peak flow velocity from forward TV flow on continuous-wave Doppler. Another method for estimation of TV cross-sectional area can be derived from the formula TV area in cm2 = stroke volume/TV-VTI. Stroke volume, mentioned above, is derived from left or right ventricular outflow. Stroke volume can be estimated from the formula (stroke volume = cross sectional area * velocity time integral) based on Doppler interrogation of either left or right outflow tracts.
TV area can also be estimated from the formula 190/PHT, where PHT is the pressure half time from forward TV flow on continuous-wave Doppler. In the presence of mild or more TR, the derived area will be underestimated. Furthermore, the stroke volume calculation becomes inaccurate in cases of aortic or pulmonary regurgitation. Tricuspid inflow velocities are largely dependent on respiration, heart rate and rhythm. Therfore, all measurements must be averaged throughout the respiratory cycle or recorded at endexpiratory apnea. As a rule of thumb, Doppler measurements from a minimum of three (sinus rhythm) or five cardiac cycles (atrial fibrillation) should be averaged. Likewise, pressure half-time is not reliable in case of tachycardia.
TTE Approach to Tricuspid Valve Regurgitation
TR is the most frequently seen TV pathology. It can be organic due to leaflet pathology or functional secondary to annular dilatation and/or ventricular dysfunction. The latter is the predominant form mostly encountered in clinical practice. Trivial TR is considered physiologic and it is very often seen on routine echocardiography. Mild TR has been reported in 80–90% of echocardiograms. The prevalence of moderate or severe TR was 0.8% in the Framingham heart study with an increased prevalence with age and was fourfold more frequent in females than males [17]. One-third of patients with mitral valve stenosis have at least moderate TR [18]. Severe TR has been reported in 23–37% of patients who underwent mitral valve replacement for rheumatic mitral valve disease [19, 20]. This form of TR is defined as “functional” or “secondary” since no primary TV pathology could be seen in most of these patients. Likewise, functional TR is often seen in patients with advanced left heart disease [21].
Residual moderate or severe TR after correction of left sided lesions is not benign; it does not often regress and is associated with reduced cardiac output and right-sided heart failure. Redo surgery is associated with up to 10% mortality. More importantly, it has been associated with poor long-term outcome [22]. Therefore, surgical TV repair or replacement is indicated for patients with stages C and D functional severe TR undergoing left sided valve surgery [23]. Details on TR clinical spectrum are described in Chap. 2 of this book.
TTE Diagnostic Algorithm of TR
According to the current guidelines, TTE is the primary modality for TR assessment. Complete TR assessment requires; identification of etiology, severity and impact on right ventricle (and vice versa) size and function as well as right atrial size and assessment of inferior vena cava. Furthermore, pulmonary artery systolic pressure should be estimated and any associated left heart disease should be assessed [23, 24].
Etiology and Mechanisms of TR
TR can be due to a primary cause such as a structural abnormality of the TV or incomplete leaflet closure secondary to a dilated TV annulus or a dilated right ventricle or to mal-coaptation and/or tethering of the TV leaflets (Table 5.6).
Grading of TR Severity
Similar to all other valves, TR is first assessed by visual inspection of the return of blood from the right ventricle into right atrium using colour Doppler. As recommended by the ASE guidelines, the use of a multi-parametric approach is mandatory [26, 40]. TR severity is determined according to 2D chamber measurements and function and Doppler recordings of jet characters. There are a few colour Doppler jet characteristics including jet area, vena contracta (VC) width and area, proximal isovelocity area (PISA) radius and flow convergence. Continuous-wave Doppler parameters include TR Doppler jet envelope shape and density. Furthermore, Doppler interrogation of hepatic venous flow is an integral part of 2D echocardiographic estimation of TR severity. However, Doppler cut-off values, which are used for grading TR severity, are largely not well validated, nor in respect all aspects of TR severity, particularly functional TR (Table 5.7
).
When moderate or severe TR is suspected it is often associated with an abnormally dilated right atrium, right ventricle and inferior vena cava. Spectral Doppler profile of the hepatic veins assists in the diagnosis of TR severity: systolic flow blunting is often seen in patients with moderate TR and systolic flow reversal in severe TR.
Use of Colour Doppler in TR Severity Assessment
Colour flow Doppler is often used as the first modality to screen for regurgitant valvular lesions. It provides visualization of the jet origin, jet width and its spatial orientation in the receiving chamber. In case of severe regurgitation, flow convergence occurs into the regurgitant orifice. An optimally visualized TR jet is composed of three parts: jet head, seen inside the right ventricle, jet neck and jet body seen inside the right atrium (Figs. 5.11 and 5.12).
TR Jet Area
While a large jet area on colour Doppler is associated with more significant TR, there are several determinants of the TR jet area on colour Doppler regarding severity: hemodynamic or loading conditions, etiology and shape of the jet as well as technical factors (Table 5.8). Optimal machine settings include a Nyquist limit (aliasing velocity) of ±50–70 cm/s, and a colour gain that is just enough to eliminate random colour speckles from non-moving tissue. Optimized and standardized machine settings are essential in serial follow-up studies to avoid measurement errors (Fig. 5.13) while assessing progression or regression of TR after pharmacological, percutaneous or surgical interventions. There are several caveats in using color jet area for assessing TR severity. TR jet area can show a considerable overlap in patients with mild versus moderate TR. Furthermore, eccentric and wall impringing jets appear smaller than central jets with similar regurgitation volume. Another extreme form of misleading color Doppler jet area could be seen in patients with severe TR and wide-open (no TV coaptation). In those patients the TR velocity could be quite low twithout aliasing or distinct jet pattern [40].
TR Jet Vena Contracta
The vena contracta (VC) , (Fig. 5.14), is the narrowest portion of a jet that occurs at or just downstream from the orifice. Visualization of TR VC is technically less demanding than the PISA method. For optimization of the TR jet VC width one should: use colour Doppler interrogation of the TR signal in the apical 4-chamber view and RV inflow parasternal view; set the Nyquist limit to +/−50–70 cm/s and use a narrow sector and zoom in to allow a frame rate of >20 frames per second. A jet VC of >7 mm has 89% sensitivity and 93% specificity for severe TR [25, 26].
Chen et al. [27] Have shown that VC area (A) measurement on 3D–TTE is feasible in the majority of patients with at least mild TR and in sinus rhythm but not in patients with atrial fibrillation, even with <20% cycle length variation (Fig. 5.15). Based on current evidence, a VC area > 0.4 cm2 is a reasonable cutoff value for severe TR [33, 40].
Three-dimensionally guided 2D direct planimetry of vena contracta area (VCA) in multiplanar reconstruction (MPR) mode in a patient with severe TR. Three-dimensional colour pyramidal full-volume data sets were reviewed in MPR mode in the mid-systolic phase, the red quadrate/sagittal plane (top right) and green quadrate/coronal plane (top left) were carefully adjusted to be parallel to the colour jet and moved into the center of TR, and then the blue quadrate/transverse plane (bottom left) was moved to reach the level of the VC, where direct planimetry of VCA (A1), long axis (D1), and short axis (D2) was performed. The white quadrate (bottom right) shows the MPR slice view of three orthogonal planes of the 3D pyramidal volume
Flow convergence method (proximal isovelocity surface area [PISA] method). Flow Acceleration and TR Jet Effective Regurgitant Orifice Area (EROA)
PISA can be assessed in the apical 4-chamber view . For optimal optimization of the TR jet PISA radius, set the Nyquist limit to an aliasing velocity of 28 cm/s. A PISA radius of >9 mm correlates with severe TR, 6–9 mm correlates with moderate TR, while a PISA radius of 5 mm or less is often associated with mild TR.
Method 1. PISA Quantification of TR
Step 1. Measure the radius of the PISA hemisphere to calculate the hemispheric area where area = 2πr2
Step 2. Measure the flow through the PISA using the formula: Q (flow in mL/min) = Area *Velocity or 2πr2 * VA (VA = Aliasing velocity based on Doppler scale).
Step 3. Measure flow velocity of the TR jet (VR) from the CW Doppler envelope.
Step 4. Based on the continuity equation, flow at PISA is equal to the flow at the regurgitant orifice area (ROA) . Therefore, ROA* VR = 2πr2 * VA or ROA = (2πr2 * VA)/VR.
Method 2. PISA Quantification of TR
There is another simplified one step approach for PISA quantification of TR severity based on the measurement of the PISA radius. The rationale behind this simplified approach is based on adjusting the colour Doppler aliasing velocity to 1/12 of the TR jet velocity on continuous-wave Doppler. This is shown in the following algorithm:
Begin with step 3 as above, and then adjust VA to 1/12 of VR on ultrasound machine.
-
ROA = (2πr2 * VA)/VR = (6.28 r2 * VA)/VR
-
Since VA/VR = 1/12, then ROA = 6.28 r2 * 1/12 = 0.5 r2
-
From the table below, you can estimate ROA from the PISA radius
However, it is important to correct for underestimation of the flow rate due to flattening of the isovelocity shells close to the orifice [28]. Therefore, correct for velocity by using the ratio (V/[V − VA]), where V is the peak TR velocity from continuous-wave Doppler and correct for the irregular funnel shaped TR orifice (Table 5.9).
Thus, the mid-systolic instantaneous is calculated as TR flow Q = (2π*r2 * VA)* (V/V − VA)*(alpha/180). Where alpha is the angle of the systolic inverted tricuspid valve funnel [25]. Therefore, ROA = Q/V.
Caveats in PISA Estimation of TR
Assessment of TR severity based on the PISA method can results in significant underestimation of the regurgitant orifice area (ROA) [29,30,31]. The shape of the tricuspid regurgitant orifice is often elliptical or very irregular, particularly in cases of severely tethered TV leaflets. Another important caveat in the PISA calculation is related to respiratory variations in the TR jet velocity and PISA radius. During inspiration, flow increases but TR velocity decreases and the opposite occurs during expiration [32]. Therefore, both measurements should be averaged when assessing the severity of TR. Topilsky et al. have shown that despite a reduction in the regurgitation gradient and consequently TR peak jet velocity during inspiration, there is a significant increase in tricuspid regurgitant volume due to a large increase in effective regurgitant orifice [32]. The latter is linked to an inspiratory widening of the RV cavity and thus TV annular enlargement, which means a decreased valvular coverage and increased valvular tenting [32] (Fig. 5.16).
Impact of inspiration and expiration on the measurements of TR flow using the flow convergence method (top) and peak velocity using continuous-wave Doppler (Vmax; bottom) with calculation of ERO in inspiration (left) and expiration (right). Respirometer curves ascend with inspiration and descend with expiration. During inspiration, Vmax decreases, regurgitant flow increases, and ERO increases considerably. Modified with permission from Topilsky et al. [32]
PISA Quantification on 3DE
It has been shown that the EROA as well as the regurgitation volume can be measured directly from 3DE [33]. The 3D method is quite simple, where a direct measurement of the flow convergence area is made possible on a 3D-TEE multiplanar reconstruction, thus no geometric assumption is made (Fig. 5.17). The method however, requires inclusion of the entire TR jet PISA in the 3D dataset and avoidance of rhythm or respiratory artifacts. Important caveats of the 3D PISA measurements are in general the low temporal and spatial resolution and the lack of wide availability.
A multiplanar reconstruction of 3D-TTE dataset obtained via a single beat acquisition. 3D PISA is automatically extracted and displayed as green overlay on a 3D colour Doppler image (top, the three reference planes: left, four-chamber view; center, two-chamber view; right, short-axis view). Bottom left: 3D rendered PISA in the volume-rendered image. ERO: Effective regurgitant orifice. Modified from de Agustin et al. with permission [33]
TR Jet on Continuous-Wave Doppler
Assessment of the severity of the TR jet on continuous-wave Doppler should include shape of Doppler profile, density of the TR jet, peak TR jet velocity and peak TV inflow velocity. The rationale behind continuous-wave Doppler is that it records the velocities of all the blood cells moving along the path of the sound beam. Therefore, the Doppler envelope contains the full envelope spectrum; the outer boundary represents the fastest moving blood cells [34]. An important caveat is the ultrasound beam being parallel with the blood flow, this is often difficult to achieve, particularly for eccentric TR jets (Fig. 5.18).
Hepatic Vein Flow in Patients with TR
Assessment of the hepatic venous flow is an integral part of 2D-TTE assessment of TR severity. The normal flow pattern of the hepatic vein on a pulsed-wave Doppler recording consists of four phasic components. The forward systolic velocity (S) is larger than the diastolic (D) velocity. Two relatively small reversal waves are seen in late systole (V-wave) and late diastole (A-wave).
Patients with mild TR often have normal hepatic vein flow patterns. Patients with moderate TR often display a flow pattern that consists of a blunted S-wave, a large D-wave, and an S/D ratio < 1. Inspiration results in increased velocity of the S and D waveforms, however, the S/D ratio < 1 persists independently of the phase of respiration. Patients with severe TR often display a flow pattern that consists of a prominent systolic reversal (SR) wave that replaces the forward S-wave. The SR peaks in late systole and the only forward flow is seen in diastole (Fig. 5.19).
There are several caveats, which are important in the interpretation of hepatic vein flow patterns for grading TR severity. Hepatic flow is dependent on several factors including hemodynamic loading, respiration, cardiac rhythm and RA and RV compliance [35]. Inspiration will increase both systolic and diastolic flow velocity, therefore the ratio will not be affected. Therefore, a blunted systolic flow would be present despite inspiratory increase in the flow velocities. A compliant right atrium would lessen the impact of severe TR leading to a blunted systolic flow instead of systolic reversal of flow (Table 5.10).
Other Surrogate Signs of TR Severity
There are several signs of severe TR on 2D-TTE such as a dilated inferior vena cava and hepatic veins (Fig. 5.20).
Optimal visualization of inferior vena cava measurements in patients with suspected TR: a dilated IVC on 2D-TTE measurement according to guidelines [40] (a) and colour flow reversal in hepatic vein due to severe TR (b)
Hemodynamically significant TR is often associated with dilated right-sided chambers. The latter is either the cause or the result of significant TR. Therefore, assessment of the RV and RA size and function and pulmonary artery pressure are essential components of the complete TR assessment. Visualization of interventricular septum is important to depict RV volume or pressure overload. When the position of the septum produces a D-shaped LV predominantly in diastole (RV volume overload pattern). On ther other hand, when septal flattening is present throughout the cardiac cycle, it reflects the diastolic and systolic overload of the RV (RV pressure overload pattern) commonly seen in TR is due to pulmonary hypertension. Current guidelines recommend the use of TAPSE, FAC for RV systolic function. A TAPSE <1.6 cm and an RV FAC <35% are suggestive of RV dysfunction. TAPSE may yield both false-positive and -negative results. Impaired RV functio in the presence of a morphologically normal TV is more likely the cause rather than the effect of TR [41]. Significant chronic TR also causes enlargement of the RA and inferior vena cava. Furthermore, a RA enlargement in patients with permanent atrial fibrillation and concomitant TV annular dilatation (>35 mm) may result in secondary TR. Advanced imaging using tissue Doppler and speckle tracking for RV deformation can also be attempted. However, volumetric assessment of the right heart chambers should preferably be based on a 3D modality such as 3D-TTE or magnetic resonance imaging due to the complex morphology of RV. On the other hand, estimated pulmonary artery pressure is commonly performed from the TR jet velocity profile based on the modified Bernoulli equation, detailed assessment of RV and pulmonary artery hemodynamics are provided in Chap. 11 of this book.
Assessment of TR Severity in Functional TR (Special Considerations)
Functional TR pathology can be categorized into one of three stages according to the severity of TR, in the presence or absence of annular dilatation and leaflet coaptation (Table 5.11).
-
The severity of TR is based on a multiparametric echocardiographic approach as explained above.
Annular dilatation
-
Definition of significant annular dilatation is based on the 2D-TTE measurements of a TV annulus of >40 mm or >21 mm/m2 in end-diastolic diameter on the apical 4-chamber view. Based on the current ACC/AHA guidelines, significant annular dilatation is the main imaging criterion to indicate severe TR [23].
-
The assessment of TV leaflet coaptation is based on measurement of the surface of contact between the leaflets, which is defined as the coaptation height or coaptation length. Normal coaptation height is seen at the level of the annulus or just below it, and is considered normal if the coaptation length is of 5–10 mm. Therefore, leaflet tethering is considered present if the tenting distance is >8 mm and/or tenting area is >1.6 cm2 [36] on 2D echocardiography.
-
In stage 1, patients have no or mild TR without annular dilatation in the presence of a normal TV leaflets coaptation. Those patients do not often require surgical intervention. In stage 2, patients have mild or moderate TR in the setting of a dilated TV annulus and a limited so called edge-to-edge coaptation. TV annuloplasty could help to reduce TR. In the advanced stage 3, patients often have severe TR due to absent leaflets coaptation and dilated annulus. In the latter annuloplasty is not enough but leaflets augmentation is often required to treat TR.
TTE Assessment of the Tricuspid Valve Annulus
The TV annulus is nonplanar, saddle-shaped similar to mitral valve but more irregular, oval or elliptical. Tricuspid annular size varies during the cardiac cycle as it moves downwards during systole [1, 37, 38]. Because of the relatively fixed interventricular septum; atrial or ventricular dilatation causes annular dilatation, which occurs in the direction of the free wall of the right ventricle. The latter pulls on the leaflets and causes TR [39]. Furthermore; apical displacement causes leaflet tethering which causes more TR [31]. Tricuspid annular dilatation is an important indicator for surgery in the current American and European guidelines. Patients with even mild or moderate TR and a TV annulus ≥40 mm or 21 mm/m2 who are undergoing left sided valve surgery should also undergo a TV repair [23, 24]. More notably, in the most recent ACC/AHA guidelines, the presence of pulmonary hypertension without TV annulus dilation is an indication for TV repair in patients with functional mild or moderate TR undergoing left sided valve surgery [23] (Fig. 5.21).
Displays end-diastolic tricuspid annuls (TA) measurements on 2D-TTE in the RV inflow long-axis view (a), short-axis view at the aortic valve level (b), apical four-chamber (c) and subcostal long-axis view (d). Image (e) display a multiplane area and image (f) details direct area measurement on the 3D
Assessment of the TV annulus is an important step for complete understanding of the TV pathology as well as for preoperative planning. It provides as well prognostic information. Detailed assessment of the TV annulus is provided in Chap. 10 of this book.
Key Points
-
TTE is more suitable than TEE in the assessment of the TV due to its position in the near field. However, the transgastric TEE view has an important role in guiding TV interventions.
-
TR is a highly dynamic pathology in which TR severity is greatly affected by preload, afterload and RV contractility as well as respiration. Impact of loading conditions, respiratory variation and right heart hemodynamics should be considered with serial evaluation of TR for targeted therapy such as percutaneous TV repair.
-
Functional TR is the most common form of TR and its severity should not only be assessed via Doppler but rather via a comprehensive assessment of the entire TV apparatus.
-
3DE has several unique advantages over 2D-TTE in TV assessment including the enface view of the TV and accurate RV chamber quantification. Also 3DE colour Doppler measurements of the vena contracta.
-
3DE has several limitations related to the lower spatial and temporal resolution, which makes it difficult to assess thin structures such as valve leaflets. However, technological advances in transducer technologies could improve its capabilities in the near future.
-
Multiplane iRotate echocardiography is a relatively new 2D echo modality that could be an essential tool in the assessment of TV in routine clinical practice.
-
Quantification of the TV annulus should be based on a 3D technique or at least the iRotate mode; however the clinical utility of the latter is yet to be confirmed.
-
Leaflets coaptation as well as annulus dimensions are important for therapeutic decision in patients with functional tricuspid regurgitation
-
Appendix (Fig. 5.22): Suggested algorithm for the integration of multiple parameters of TR severity
Algorithm for the integration of multiple parameters of TR severity. Good-quality echocardiographic imaging and complete data acquisition are assumed. If imaging is technically difficult, consider TEE or CMR. TR severity may be indeterminate due to poor image quality, technical issues with data, internal inconsistency among echo findings, or discordance with clinical findings [40]
References
Anwar AM, Geleijnse ML, Soliman OI, McGhie JS, Frowijn R, Nemes A, van den Bosch AE, Galema TW, Ten Cate FJ. Assessment of normal tricuspid valve anatomy in adults by real-time three-dimensional echocardiography. Int J Cardiovasc Imaging. 2007;23:717–24.
Lang RM, Badano LP, Tsang W, Adams DH, Agricola E, Buck T, Faletra FF, Franke A, Hung J, de Isla LP, Kamp O, Kasprzak JD, Lancellotti P, Marwick TH, McCulloch ML, Monaghan MJ, Nihoyannopoulos P, Pandian NG, Pellikka PA, Pepi M, Roberson DA, Shernan SK, Shirali GS, Sugeng L, Ten Cate FJ, Vannan MA, Zamorano JL, Zoghbi WA, American Society of Echocardiography, European Association of Echocardiography. EAE/ASE recommendations for image acquisition and display using three-dimensional echocardiography. J Am Soc Echocardiogr. 2012;25:3–46.
Anwar AM, Geleijnse ML, Ten Cate FJ, Meijboom FJ. Assessment of tricuspid valve annulus size, shape and function using real-time three-dimensional echocardiography. Interact Cardiovasc Thorac Surg. 2006;5:683–7.
Velayudhan DE, Brown TM, Nanda NC, Patel V, Miller AP, Mehmood F, Rajdev S, Fang L, Frans EE, Vengala S, Madadi P, Yelamanchili P, Baysan O. Quantification of tricuspid regurgitation by live three-dimensional transthoracic echocardiographic measurements of vena contracta area. Echocardiography. 2006;23:793–800.
Mutlak D, Carasso S, Lessick J, Aronson D, Reisner SA, Agmon Y. Excessive respiratory variation in tricuspid regurgitation systolic velocities in patients with severe tricuspid regurgitation. Eur Heart J Cardiovasc Imaging. 2013;14:957–62.
Agmon Y, Caspi O. Respiratory variation in tricuspid valve regurgitant orifice: a three-dimensional transthoracic echocardiographic perspective. Eur Heart J Cardiovasc Imaging. 2016;17(10):1188.
Dreyfus GD, Chan KM. Functional tricuspid regurgitation: a more complex entity than it appears. Heart. 2009;95:868–9.
Dreyfus GD, Martin RP, Chan KM, Dulguerov F, Alexandrescu C. Functional tricuspid regurgitation: a need to revise our understanding. J Am Coll Cardiol. 2015;65:2331–6.
Raja SG, Dreyfus GD. Basis for intervention on functional tricuspid regurgitation. Semin Thorac Cardiovasc Surg. 2010;22:79–83.
Dreyfus J, Durand-Viel G, Raffoul R, Alkhoder S, Hvass U, Radu C, Al-Attar N, Ghodbhane W, Attias D, Nataf P, Vahanian A, Messika-Zeitoun D. Comparison of 2-dimensional, 3-dimensional, and surgical measurements of the tricuspid annulus size: clinical implications. Circ Cardiovasc Imaging. 2015;8:e003241.
Anwar AM, Soliman OI, Nemes A, van Geuns RJ, Geleijnse ML, Ten Cate FJ. Value of assessment of tricuspid annulus: real-time three-dimensional echocardiography and magnetic resonance imaging. Int J Cardiovasc Imaging. 2007;23:701–5.
Anwar AM, Geleijnse ML, Soliman OI, McGhie JS, Nemes A, ten Cate FJ. Evaluation of rheumatic tricuspid valve stenosis by real-time three-dimensional echocardiography. Heart. 2007;93:363–4.
Faletra F, La Marchesina U, Bragato R, De Chiara F. Three dimensional transthoracic echocardiography images of tricuspid stenosis. Heart. 2005;91:499.
McGhie JS, Menting ME, Vletter WB, Frowijn R, Roos-Hesselink JW, Soliman OI, van der Zwaan HB, Geleijnse ML, van den Bosch AE. A novel 13-segment standardized model for assessment of right ventricular function using two-dimensional iRotate echocardiography. Echocardiography. 2016;33:353–61.
McGhie JS, Menting ME, Vletter WB, Frowijn R, Roos-Hesselink JW, van der Zwaan HB, Soliman OI, Geleijnse ML, van den Bosch AE. Quantitative assessment of the entire right ventricle from one acoustic window: an attractive approach. Eur Heart J Cardiovasc Imaging. 2016 Aug 7. pii: jew165. [Epub ahead of print].
Addetia K, Yamat M, Mediratta A, Medvedofsky D, Patel M, Ferrara P, Mor-Avi V, Lang RM. Comprehensive two-dimensional interrogation of the tricuspid valve using knowledge derived from three-dimensional echocardiography. J Am Soc Echocardiogr. 2016;29:74–82.
Singh JP, Evans JC, Levy D, Larson MG, Freed LA, Fuller DL, Lehman B, Benjamin EJ. Prevalence and clinical determinants of mitral, tricuspid, and aortic regurgitation (the Framingham Heart Study). Am J Cardiol. 1999;83:897–902.
Boyaci A, Gokce V, Topaloglu S, Korkmaz S, Goksel S. Outcome of significant functional tricuspid regurgitation late after mitral valve replacement for predominant rheumatic mitral stenosis. Angiology. 2007;58:336–42.
Porter A, Shapira Y, Wurzel M, Sulkes J, Vaturi M, Adler Y, Sahar G, Sagie A. Tricuspid regurgitation late after mitral valve replacement: clinical and echocardiographic evaluation. J Heart Valve Dis. 1999;8:57–62.
Izumi C, Iga K, Konishi T. Progression of isolated tricuspid regurgitation late after mitral valve surgery for rheumatic mitral valve disease. J Heart Valve Dis. 2002;11:353–6.
Bruce CJ, Connolly HM. Right-sided valve disease deserves a little more respect. Circulation. 2009;119:2726–34.
Nath J, Foster E, Heidenreich PA. Impact of tricuspid regurgitation on long-term survival. J Am Coll Cardiol. 2004;43:405–9.
Nishimura RA, Otto CM, Bonow RO, Carabello BA, Erwin JP 3rd, Guyton RA, O'Gara PT, Ruiz CE, Skubas NJ, Sorajja P, Sundt TM 3rd, Thomas JD, American College of Cardiology/American Heart Association Task Force on Practice G. 2014 AHA/ACC guideline for the management of patients with valvular heart disease: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;63:2438–88.
Joint Task Force on the Management of Valvular Heart Disease of the European Society of C, European Association for Cardio-Thoracic S, Vahanian A, Alfieri O, Andreotti F, Antunes MJ, Baron-Esquivias G, Baumgartner H, Borger MA, Carrel TP, De Bonis M, Evangelista A, Falk V, Iung B, Lancellotti P, Pierard L, Price S, Schafers HJ, Schuler G, Stepinska J, Swedberg K, Takkenberg J, Von Oppell UO, Windecker S, Zamorano JL, Zembala M. Guidelines on the management of valvular heart disease (version 2012). Eur Heart J. 2012;33:2451–96.
Tribouilloy CM, Enriquez-Sarano M, Bailey KR, Tajik AJ, Seward JB. Quantification of tricuspid regurgitation by measuring the width of the vena contracta with Doppler color flow imaging: a clinical study. J Am Coll Cardiol. 2000;36:472–8.
Zoghbi WA, Enriquez-Sarano M, Foster E, Grayburn PA, Kraft CD, Levine RA, Nihoyannopoulos P, Otto CM, Quinones MA, Rakowski H, Stewart WJ, Waggoner A, Weissman NJ, American Society of E. Recommendations for evaluation of the severity of native valvular regurgitation with two-dimensional and Doppler echocardiography. J Am Soc Echocardiogr. 2003;16:777–802.
Chen TE, Kwon SH, Enriquez-Sarano M, Wong BF, Mankad SV. Three-dimensional color Doppler echocardiographic quantification of tricuspid regurgitation orifice area: comparison with conventional two-dimensional measures. J Am Soc Echocardiogr. 2013;26:1143–52.
Rodriguez L, Thomas JD, Monterroso V, Weyman AE, Harrigan P, Mueller LN, Levine RA. Validation of the proximal flow convergence method. Calculation of orifice area in patients with mitral stenosis. Circulation. 1993;88:1157–65.
Rodriguez L, Anconina J, Flachskampf FA, Weyman AE, Levine RA, Thomas JD. Impact of finite orifice size on proximal flow convergence. Implications for Doppler quantification of valvular regurgitation. Circ Res. 1992;70:923–30.
Sugeng L, Weinert L, Lang RM. Real-time 3-dimensional color Doppler flow of mitral and tricuspid regurgitation: feasibility and initial quantitative comparison with 2-dimensional methods. J Am Soc Echocardiogr. 2007;20:1050–7.
Mascherbauer J, Maurer G. The forgotten valve: lessons to be learned in tricuspid regurgitation. Eur Heart J. 2010;31:2841–3.
Topilsky Y, Tribouilloy C, Michelena HI, Pislaru S, Mahoney DW, Enriquez-Sarano M. Pathophysiology of tricuspid regurgitation: quantitative Doppler echocardiographic assessment of respiratory dependence. Circulation. 2010;122:1505–13.
de Agustin JA, Viliani D, Vieira C, Islas F, Marcos-Alberca P, Gomez de Diego JJ, Nunez-Gil IJ, Almeria C, Rodrigo JL, Luaces M, Garcia-Fernandez MA, Macaya C, Perez de Isla L. Proximal isovelocity surface area by single-beat three-dimensional color Doppler echocardiography applied for tricuspid regurgitation quantification. J Am Soc Echocardiogr. 2013;26:1063–72.
Quinones MA, Otto CM, Stoddard M, Waggoner A, Zoghbi WA, Doppler Quantification Task Force of the N, Standards Committee of the American Society of E. Recommendations for quantification of Doppler echocardiography: a report from the Doppler Quantification Task Force of the Nomenclature and Standards Committee of the American Society of Echocardiography. J Am Soc Echocardiogr. 2002;15:167–84.
Fadel BM, Almulla K, Husain A, Dahdouh Z, Di Salvo G, Mohty D. Spectral Doppler of the hepatic veins in tricuspid valve disease. Echocardiography. 2015;32:856–9.
Kim HK, Kim YJ, Park JS, Kim KH, Kim KB, Ahn H, Sohn DW, Oh BH, Park YB, Choi YS. Determinants of the severity of functional tricuspid regurgitation. Am J Cardiol. 2006;98:236–42.
Fawzy H, Fukamachi K, Mazer CD, Harrington A, Latter D, Bonneau D, Errett L. Complete mapping of the tricuspid valve apparatus using three-dimensional sonomicrometry. J Thorac Cardiovasc Surg. 2011;141:1037–43.
Maffessanti F, Gripari P, Pontone G, Andreini D, Bertella E, Mushtaq S, Tamborini G, Fusini L, Pepi M, Caiani EG. Three-dimensional dynamic assessment of tricuspid and mitral annuli using cardiovascular magnetic resonance. Eur Heart J Cardiovasc Imaging. 2013;14:986–95.
Ton-Nu TT, Levine RA, Handschumacher MD, Dorer DJ, Yosefy C, Fan D, Hua L, Jiang L, Hung J. Geometric determinants of functional tricuspid regurgitation: insights from 3-dimensional echocardiography. Circulation. 2006;114:143–9.
Zoghbi WA, Adams D, Bonow RO, Enriquez-Sarano M, Foster E, Grayburn PA, Hahn RT, Han Y, Hung J, Lang RM, Little SH, Shah DJ, Shernan S, Thavendiranathan P, Thomas JD, Weissman NJ. Recommendations for Noninvasive Evaluation of Native Valvular Regurgitation. J Am Soc Echocardiogr. 2017;30(4):303–71.
Lawrence G. Rudski, Wyman W. Lai, Jonathan Afilalo, Lanqi Hua, Mark D. Handschumacher, Krishnaswamy Chandrasekaran, Scott D. Solomon, Eric K. Louie, Nelson B. Schiller, (2010) Guidelines for the Echocardiographic Assessment of the Right Heart in Adults: A Report from the American Society of Echocardiography. JJ Am Soc Echocardiogr. 23(7):685–713
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Electronic Supplementary Material
(MPG 814 kb)
(MPG 842 kb)
(MPG 928 kb)
(MPG 894 kb)
(MPG 1394 kb)
(MPG 1378 kb)
(MPG 962 kb)
(MPG 824 kb)
(MPG 948 kb)
(MPG 932 kb)
(MPG 874 kb)
(MPG 1464 kb)
(MPG 1496 kb)
(MPG 1480 kb)
(MPG 1358 kb)
(MPG 1204 kb)
(MPG 846 kb)
(MPG 396 kb)
(MPG 1502 kb)
(MPG 1404 kb)
(MPG 1502 kb)
(MPG 1078 kb)
(MPG 870 kb)
(MPG 974 kb)
(MPG 932 kb)
(MPG 948 kb)
(MPG 986 kb)
(MPG 868 kb)
(MPG 860 kb)
(MPG 796 kb)
(MPG 884 kb)
(MPG 890 kb)
(MPG 1394 kb)
(MPG 1402 kb)
(MPG 1202 kb)
(MPG 1182 kb)
(MPG 982 kb)
(MPG 1096 kb)
(MPG 974 kb)
(MPG 934 kb)
(MPG 1318 kb)
(MPG 1926 kb)
(MPG 1448 kb)
(MPG 1388 kb)
(MPG 1406 kb)
(MPG 1498 kb)
(MPG 1358 kb)
(MPG 1392 kb)
(MPG 828 kb)
(MPG 1926 kb)
(MPG 844 kb)
(MPG 864 kb)
(MPG 1774 kb)
(MPG 812 kb)
(MPG 1128 kb)
(MPG 1392 kb)
Review Questions
Review Questions
Select the Single Best Sentence
-
31.
Which of the following statements about normal tricuspid anatomy is true?
-
(a)
papillary muscles are smaller and widely spaced than in the left ventricle
-
(b)
tricuspid valve is less apical than mitral valve
-
(c)
right ventricle has two papillary muscles, which are larger than in left ventricle
-
(d)
posterior leaflet of the tricuspid valve is the largest of the three leaflets
-
(e)
anterior and posterior leaflets are exactly the same size
-
(a)
-
32.
Which of the following statements is correct about the TV leaflets in the apical 4-chamber view?
-
(a)
septal leaflet is often identified in 87% of cases
-
(b)
septal leaflet is identified in all subjects except in patients with dilated right ventricle
-
(c)
septal leaflet is identified in all subjects
-
(d)
septal leaflet is identified in all subjects only in the focused apical 4-chamber view
-
(e)
posterior leaflet is seen in all subjects in focused RV view
-
(a)
-
33.
Which of the following statements is correct about TV leaflets on 2D-TTE in the parasternal right ventricular inflow view with LV cavity displayed?
-
(a)
anterior leaflet is always seen in the near field and the septal leaflet in the far field
-
(b)
septal leaflet is identified in all subjects except in patients with dilated right ventricle
-
(c)
septal leaflet is identified in all subjects in the near filed
-
(d)
posterior leaflet is identified in all subjects in the far field
-
(e)
both anterior and posterior leaflets could be seen if transducer is posteriorly tilt to exclude the LV cavity
-
(a)
-
34.
Which of the following statements about TV leaflets on 2D-TTE in the standard parasternal short-axis view are not correct (check all that apply)?
-
(a)
the leaflet to the next to aorta is the anterior leaflet in 50% of cases
-
(b)
the leaflet next to aorta is the septal leaflet in 50% of cases
-
(c)
the leaflet next to aorta is the septal leaflet in 100% of cases
-
(d)
the leaflet next attached to RV free is the anterior leaflet in almost 100% of cases
-
(e)
the leaflet next attached to RV free is the posterior leaflet in almost 100% of cases
-
(a)
-
35.
Which of the following parameters/modalities is adequate for the assessment of tricuspid valve regurgitation severity grade?
-
(a)
Color Doppler of tricuspid regurgitation jet
-
(b)
Continuous-wave Doppler of tricuspid regurgitation jet
-
(c)
Pulsed-wave Doppler of tricuspid regurgitation jet
-
(d)
2D-TEE of annulus enlargement and chamber dilatation
-
(e)
All of the above must be combined together for a reliable assessment of TR severity
-
(a)
-
36.
Which of the following statements are correct regarding frame rate?
-
(a)
2D-TTE has a higher frame rate than M-mode
-
(b)
The biplane simultaneous multiplane imaging has a similar frame rate to 2D-TTE
-
(c)
The simultaneous multiplane imaging has a similar frame rate to the iRotate mode
-
(d)
The biplane simultaneous multiplane imaging has a higher frame rate to 2D-TTE
-
(e)
The iRotate mode has a similar frame rate to 2D-TTE
-
(a)
-
37.
Doppler estimation of tricuspid valve cross-sectional area is based on the concept of:
-
(a)
conservation of energy theory
-
(b)
conservation of mass theory
-
(c)
flow augmentation theory
-
(d)
Simpson’s formula
-
(e)
Cubic formula
-
(a)
-
38.
Which of the following is not a sign of a hemodynamically significant tricuspid stenosis?
-
(a)
Mean pressure gradient of 5 or more mmHg
-
(b)
Right ventricular inflow velocity time integral of 60 or more cm
-
(c)
Right ventricular inflow pressure half time of 190 or more
-
(d)
tricuspid valve area of less than 1 cm2
-
(e)
dilated right ventricular, and smaller right atrial volume
-
(a)
-
39.
Which of the following is not a sign of a severe tricuspid regurgitation?
-
(a)
Right ventricular eccentricity index of 1.4
-
(b)
TR jet color area more than 10 cm2 or >30% of RA area
-
(c)
TR jet vena contracta width more than 7 mm
-
(d)
TR jet PISA radium of more than 9 mm
-
(e)
TR effective regurgitant orifice area of 40 or more mm2
-
(a)
-
40.
Which of the following statements is false regarding tricuspid leaflets coaptation?
-
(a)
Normal coaptation height is seen at the level of the annulus or just below it.
-
(b)
Is considered normal if the coaptation length of 5–10 mm.
-
(c)
Leaflet tethering is considered present if the tenting distance >8 mm.
-
(d)
Leaflet tethering is considered present if the tenting area > 1.6 cm2.
-
(e)
The coaptation height is often larger than coaptation length.
-
(a)
-
41.
Regarding functional tricuspid regurgitation, which of the following statements is false?
-
(a)
Is the most common form of TR, which occur in almost 80% of all TR
-
(b)
Annuloplasty is enough to treat patients with tricuspid annulus of more than 40 mm and absent leaflet coaptation
-
(c)
Some patients with mild TR could be surgically treated if tricuspid annulus is more than 40 mm
-
(d)
Patients with tricuspid annulus of more than 40 mm and edge-to-edge leaflet coaptation are treated with Annuloplasty
-
(e)
Patients with tricuspid annulus of more than 21 mm/m2 and edge-to-edge leaflet coaptation are treated with Annuloplasty
-
(a)
Rights and permissions
Copyright information
© 2018 Springer International Publishing AG
About this chapter
Cite this chapter
Soliman, O.I., McGhie, J., Anwar, A.M., Strachinaru, M., Geleijnse, M.L., ten Cate, F.J. (2018). Tricuspid Valve Disease: Imaging Using Transthoracic Echocardiography. In: Soliman, O.I., ten Cate, F.J. (eds) Practical Manual of Tricuspid Valve Diseases. Springer, Cham. https://doi.org/10.1007/978-3-319-58229-0_5
Download citation
DOI: https://doi.org/10.1007/978-3-319-58229-0_5
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-58228-3
Online ISBN: 978-3-319-58229-0
eBook Packages: MedicineMedicine (R0)