Abstract
Cancer is an important cause of peripheral nervous system (PNS) dysfunction. In addition to toxic effects of chemotherapy and paraneoplastic disorders, one must also consider direct involvement of the PNS by metastatic disease. Neurologic dysfunction in PNS metastatic disease occurs from direct compression or infiltration by cancer. The location of involvement determines the patient’s presentation. Metastatic disease can affect motor neuron, sensory or autonomic ganglia, nerve roots, plexus, cranial and peripheral nerves, and muscle. PNS metastases often cause severe pain. Neurologic deficits are progressive if untreated. Treatment of metastases to the PNS is typically palliative and can include chemotherapy, radiation, surgery and pain management. The response to therapy is variable and may be temporary. Optimizing pain control and maximizing neurologic function and quality of life are important goals of treatment.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
- Peripheral nervous system
- Cancer
- Metastasis
- Peripheral nerve
- Plexus
- Muscle
- Radiation-induced plexopathy
- Neurolymphomatosis
Introduction
Clinically apparent involvement of the peripheral nervous system (PNS) is common in cancer and occurs in approximately 10% of cases. The interaction between cancer and the PNS is multifaceted and complex [1]. First, cancer affects the PNS by different mechanisms. These mechanisms include: (1) compression or infiltration by the tumor; (2) treatment effect (radiation or chemotherapy), which may be delayed; (3) nutritional and metabolic factors; (4) infectious complications related to immunosuppression; and (5) paraneoplastic disorders. Second, precise anatomic localization is important. Cancer can affect any portion of the PNS and can be multifocal. Motor neuron, sensory or autonomic ganglia, nerve roots, plexus, cranial and peripheral nerve, neuromuscular junction and muscle can all be affected. Third, different cellular structures can be damaged including neuronal cell body, axon, or myelin. Finally, different cancers have different mechanisms for producing PNS lesions. For example, hematologic malignancies infiltrate peripheral nerve more frequently than solid tumors [1]. With this framework in mind, this chapter will review metastases to the peripheral nervous system.
Metastases to Nerve Roots
Leptomeningeal metastasis occurs when malignant cells seed the leptomeninges (the pia, arachnoid and cerebrospinal fluid (CSF) within the subarachnoid space). Patients with involvement of plexus or nerve from lymphoma, leukemia and rarely small cell lung cancer may develop leptomeningeal disease and sometimes even cord compression when neoplastic cells track into the epidural space or meninges. In general, leptomeningeal disease is diagnosed in 1–5% of patients with solid tumors (carcinomatous meningitis), 5–15% of patients with leukemia (leukemic meningitis) and lymphoma (lymphomatous meningitis), and 1–2% of patients with primary brain tumors. Melanoma, breast, and lung cancer are the most common primary sites to metastasize to the leptomeninges. Adenocarcinoma is the most common histological subtype. Leptomeningeal disease can rarely be the first manifestation of cancer (5–10%) or present after a period of remission (20%). However, more typically, it presents in patients with widespread and progressive systemic cancer (>70%) [2].
Cancer cells can spread to the meninges by several paths: hematogenous spread, either through Batson’s plexus or arterial dissemination, direct extension from adjacent tumor deposits and through centripetal migration from systemic tumors along perineural or perivascular spaces. Once cancer cells arrive in the subarachnoid space, CSF flow disseminates them. This process results in potentially multifocal seeding of the leptomeninges. Conversely, impairment of CSF flow may also occur due to tumor-related adhesions [2].
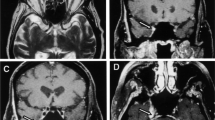
A 56 year old patient with a T1bN0MO melanoma, not on immunotherapy, presented with bilateral, asymmetric lower extremity and left upper extremity weakness with associated back pain and without significant sensory symptoms. EDX demonstrated polyradiculopathy. Three separate lumbar punctures showed normal cell count, elevated protein and negative cytology. Extensive infectious work-up was negative. MRI lumbar spine showed smooth linear enhancement of the cauda equine (a, b). Deficits and MRI abnormalities improved over 1 year with IVIG therapy (c, d). Diagnosis was an immune-mediated polyradiculopathy (Copyright by Amanda C. Guidon, MD)
A 54 year old patient with metastatic melanoma treated with nivolumab followed by ipilimumab, presented with diplopia, back pain, progressive sensory symptoms, weakness and gastroparesis. Lumbar puncture showed elevated protein, normal glucose, normal cell count and no evidence of malignancy. MRI brain showed abnormal enhancement along multiple cranial nerves and coating the dorsal and ventral brainstem. Leptomeningeal enhancement also involved the cervical and thoracic spinal cord, conus medularis and cauda equina. He subsequently developed a CIDP-like peripheral neuropathy. Symptoms and enhancement improved after stopping immunotherapy and treatment with high dose corticosteroids and intravenous immunoglobulin (IVIG) (Copyright by Amanda C. Guidon, MD)
Presentation
Spinal nerve roots and cranial nerves can be affected by leptomeningeal disease. The finding of disease at several points along the neuraxis in a patient with known malignancy suggests leptomeningeal disease. However, patients may also present with isolated findings such as a cranial mononeuropathy or cauda equina syndrome. Patients may appear clinically to have a polyradiculopathy or lower motor neuron disease. Differential diagnosis at presentation includes other forms of chronic meningitis such as fungal infection, sarcoid, and tuberculosis [2]. Inflammatory processes, which can be related to immunotherapy for the patient’s cancer, can also present with nerve root or leptomeningeal enhancement [3].
Imaging and Evaluation
Magnetic resonance imaging with gadolinium enhancement is the preferred imaging modality to evaluate patients with suspected leptomeningeal disease. When diffuse leptomeningeal disease is suspected, imaging of the brain and entire spine is required. A normal MRI does not exclude the diagnosis, as a 30% false negative rate exists [2]. Additionally, the diagnostic value of MRI may be less in patients with hematologic malignancies than with solid tumors [4]. This is likely attributable to the propensity of solid tumors to adhere to neural structures and form nodules [5]. In a patient with known malignancy and a typical presentation for leptomeningeal disease, however, an abnormal MRI alone can establish the diagnosis [2].
CSF examination with intracranial pressure measurement is the most useful laboratory test in the diagnosis of leptomeningeal metastasis. Cytology is considered the gold standard for diagnosis. The presence of malignant cells in CSF is diagnostic of leptomeningeal disease. However, cytologic analysis frequently cannot attribute the cells to a specific primary tumor. Abnormal CSF may be merely suggestive of but not diagnostic for leptomeningeal disease. The sensitivity for detecting malignancy when present is 65% on initial LP, which then increases to 80% after a second LP. Even after three lumbar punctures, false negative rate can be approximately 10% [2, 5]. Dissociation between cell count and cytology results may also exist [6]. Further complicating CSF analysis is the potential variability of CSF protein, glucose, and malignant cell at different levels of the neuraxis, even in the absence of CSF obstruction. Obtaining CSF from a site that is not symptomatically or radiographically affected, withdrawing small amounts of CSF (<10 ml), delayed processing, and analyzing only one sample are all associated with false negative results. Low sensitivity poses a challenge to diagnosis of leptomeningeal disease as well as assessing response to therapies [2]. CSF flow cytometry is useful in evaluating hematologic tumors and may be superior to cytology when evaluating for leptomeningeal disease [6]. MR imaging looking for enhancement should preferentially be obtained prior to LP; lumbar puncture itself can rarely cause a meningeal reaction leading to dural-arachnoid enhancement. In situations where there is no systemic manifestation of malignancy, CSF is inconclusive and the suspicion for leptomeningeal disease remains high, meningeal or nerve root biopsy from an enhancing region may be diagnostic [2].
Treatment
Early diagnosis of neoplastic leptomeningeal disease can afford a better prognosis. Treatment is intended primarily to stabilize neurologic function and prolong survival. Median survival with treatment still remains poor and for solid tumors is 2.3 months and for hematopoietic tumors is 4.7 months [5]. Response to treatment is difficult to assess, as there is a lack of standardized treatments and most studies treat all subtypes equivalently. Treatment may include a combination of surgery, radiation, systemic, and intrathecal chemotherapy, and supportive care. Radiation is often used for bulky disease or if CSF flow is obstructed [2].
Metastases to Plexus
Approximately one in a hundred patients with cancer will experience neoplastic plexopathy. Two large retrospective reviews from cancer hospitals showed a frequency of neoplastic brachial plexopathy at 0.43% and lumbosacral plexopathy 0.71%. However, the incidence is higher in certain cancer subtypes. For example, up to 5% of patients with breast cancer may experience neoplastic plexopathy within 5 years following treatment [7, 8]. Knowledge of plexus anatomy helps the clinician localize the area of abnormality. Differential diagnosis of plexopathy in cancer patients includes: radiation plexopathy (most common alternate consideration), epidural cord compression, neoplastic meningitis, primary plexus tumor, chemotoxicity (intraarterial therapy), (paraneoplastic) immune-mediated plexopathy, and postinfectious plexopathy [9].
Cervical Plexus
Cervical Plexus Anatomy
The cervical plexus is formed by the ventral rami of the first four spinal nerves (C1–C4), in a series of irregular loops. The plexus is situated close to the upper four vertebrae, between the deep anterior and lateral muscles of the neck, anterior to the middle scalene and deep to the sternocleidomastoid. Braches form cutaneous, muscular, and communicating nerves. The cutaneous branches (lesser occipital, great auricular, supraclavicular, and transverse cutaneous nerves of the neck) contain sensory information from the skin and soft tissues of the scalp and neck. The cervical plexus also provides motor innervation to several muscles including the diaphragm (via the phrenic nerve), sternocleidomastoid and trapezius (via the accessory nerve) and other deep cervical and hyoid muscles [9, 10]. There are also communicating branches to the accessory, hypoglossal, vagus, and sympathetic nerves [11].
Presentation of Cervical Plexopathy
Metastatic spread to cervical plexus typically occurs from neighboring tissue. Cancer may invade the plexus directly or indirectly via regional lymph nodes or bone (clavicle, first rib, or cervical vertebral bodies). Squamous cell carcinoma of the head and neck, lymphoma and adenocarcinoma of the lung and breast are the most commonly associated tumors [9].
Patients who develop metastatic disease in this area most commonly describe pain and stiffness in the neck, shoulder or throat. The pain is typically unrelenting and may worsen with coughing, swallowing and neck movement. General examination may be surprisingly normal or may demonstrate tender neck musculature unilaterally, palpable tumors or firm anterior or posterior cervical or supraclavicular lymph nodes. Patients often describe vague numbness, paresthesias, pressure, or burning; however, objective sensory loss may be difficult to identify. Additionally prior surgery in the area which can produce numbness in the skin of the anterior neck and submandibular area may complicate assessment of new sensory deficits. Clarifying the temporal relationship of symptom onset and surgery can help distinguish between these two etiologies [9].
Additional clinical manifestations depend on the area of the cervical plexus involved. Abnormality of the spinal accessory nerve or C3/4 roots may produce weakness of the trapezius. This typically manifests as shoulder weakness and scapular depression and winging, most notable with shoulder abduction. Phrenic involvement can present with an elevated and/or paralyzed hemi-diaphragm. Patients describe dyspnea, which is worse when supine. Unilateral weakness of the sternocleidomastoid, deep cervical, and hyoid muscles is typically asymptomatic. A history or exam suggestive of myelopathy raises the suspicion for epidural spread. Given the close proximity, manifestations of skull base, or brachial plexus involvement may also be present [9, 12].
Electrodiagnostic Findings in Cervical Plexopathy
EMG (Electromyography ) and NCS (nerve conduction studies ) are more limited in the assessment of involvement of the cervical plexus than the brachial or lumbosacral plexus but can add important information. In phrenic neuropathy, the phrenic CMAP (compound muscle action potential) may be abnormal and neurogenic changes may be seen on needle EMG of the diaphragm. In spinal accessory neuropathy, the CMAP of the spinal accessory nerve may be abnormal when recording from the trapezius and needle EMG of the trapezius and/or sternocleidomastoid may reveal neurogenic changes. These abnormalities in the phrenic nerve/diaphragm and spinal accessory nerve/trapezius can also be seen in a radiculopathy or segmental myelopathy affecting the C3/4/5 and C3/4 myotomes, respectively. Needle EMG of the upper and mid-cervical paraspinals can also reveal abnormal spontaneous activity suggestive of neurogenic abnormality. If metastatic disease is confined to the cervical plexus, routine median, ulnar and radial sensory, and motor responses should be normal [11].
Brachial Plexus
Brachial Plexus Anatomy
The brachial plexus supplies the motor and sensory innervation of most of the upper limb. It is an arrangement of nerve fibers that runs from the spine (C5-T1 roots) through the neck, axilla, and into the arm. As the nerve fibers run proximally to distally, they are arranged into trunks, divisions, cords and individual nerve branches. The phrenic, dorsal scapular, and long thoracic nerve exit proximal to the plexus, off the nerve roots themselves and innervate the diaphragm, rhomboids and serratus anterior respectively. The ventral rami of the C5-T1 nerve roots then form three trunks: upper (C5/6), middle (C7) and lower (C8/T1). They are named with respect to their orientation to one another. They occupy a superficial position as they traverse the posterior cervical triangle. The lower trunk is adjacent to the lung apex and near the subclavian artery. Each trunk then divides into two divisions, anterior, and posterior. These divisions are retroclavicular and run between the middle third of the clavicle and the first rib. The three posterior divisions then form the posterior cord. The anterior divisions of the upper and middle trunk form the lateral cord and the anterior division of the lower trunk continues as the medial cord. The cords are named for their orientation to the axillary artery and are situated proximally in the axilla, next to the axillary lymph node chain. The posterior cord gives rise to the thoracodorsal nerve (latissimus dorsi), subscapular nerve (subscapularis), axillary nerve (deltoid), and radial nerve (triceps, brachioradialis, wrist, and finger extensors) and provides sensory supply to the posterior arm and forearm. The lateral cord divides into two branches: the musculocutaneous nerve and a branch, which joins a portion of the medial cord to form the median nerve. The rest of the medial cord forms the median and ulnar nerves and gives off the medial brachial cutaneous nerves of the arm and forearm, which supply sensation to the medial portion of the arm and forearm. The five terminal nerves of the upper extremity (musculocutaneous, axillary, median, ulnar, and radial) are distally situated in the axilla [11, 13].
Neoplastic Syndromes of the Brachial Plexus
Approximately 70% of tumors involving the brachial plexus come from either lung or breast. The remaining 30% percent arise from a combination of lymphoma, sarcoma, or others [8]. Most metastases to the brachial plexus involve the lower trunk or medial cord. Rarely, primary head or neck neoplasms grow inferiorly and invade the upper portions of the plexus. Sometimes plexus involvement is patchy. Neoplastic invasion of the plexus is rarely the earliest manifestation of cancer, except in Pancoast syndrome, which is typically caused by carcinoma at the apex of the lung invading the lower trunk/medical cord of the brachial plexus [11].
Pain is the most common presenting symptom of neoplastic brachial plexopathy. Typically, the pain originates in the shoulder or axilla and radiates along the medial arm and forearm and into the fourth and fifth digits and can be severe. In a large series, pain upon initial presentation was present in 75% of cases [8]. Sensory loss can follow the same distribution. Motor deficits most commonly (75%) affect the lower plexus. As such, patients will typically have weakness in intrinsic hand muscles, finger and wrist flexion and extension. Approximately one in five patients have an associated Horner’s syndrome due to involvement of the sympathetic trunk or ganglia near the first thoracic vertebrae. Given the proximity of this lesion to the spinal cord, a Horner’s syndrome should prompt a thorough evaluation for intraspinal disease [8].
Differential diagnosis of neoplastic brachial plexopathy depends on whether the patient has received radiation therapy (RT) . In patients who have received RT, the principal alternate diagnosis is radiation-induced brachial plexopathy. In patients who have received prior radiation and have a delayed brachial plexopathy, a radiation-induced nerve sheath tumor of the brachial plexus is also a consideration. This is much less common than radiation-induced brachial plexopathy and may develop 4–40 years after RT. Often presenting as a painful enlarging mass, these tumors are usually malignant. Radiation-induced arteritis may also be seen in patients with radiation-induced plexopathies. Chronic ischemic symptoms and signs can present in the arm and hand or they may have episodic discoloration in the fingers from emboli. This may appear similar to atherosclerotic disease on arteriography. In a patient with known malignancy who has not undergone radiation to the upper chest, the main alternate diagnoses are perioperative brachial plexus trauma or an unrelated episode of acute brachial plexus neuropathy. Primary tumors of the brachial plexus are rare. Most are benign peripheral nerve sheath tumors, including neurofibromas and benign schwannomas [11].
Lumbrosacral Plexus
Lumbosacral Plexus Anatomy
The lumbosacral plexus is derived from the ventral rami of the L1-S4 nerve roots and is made up of two sections. The upper portion is the lumbar plexus, which arises from the L1–L4 nerves roots with variable contribution from T12. The lower portion is the lumbosacral plexus, which arises from the L4-S4 nerve roots. The lumbosacral plexus lies within the psoas major muscle and exits from the lateral edge of the muscle. In general, the lumbosacral plexus supplies motor and sensory functions to the ipsilateral leg and pelvic girdle. The upper portion of the plexus gives rise to several major nerves: the iliohypogastric nerve (T12-L1), the ilioinguinal nerve (L1), the genitofemoral nerve (L1–2), the lateral femoral cutaneous nerve (L2–4), and the obturator nerve (L2–4). Major motor functions include hip flexion and adduction, and knee extension. The upper plexus supplies sensation to the groin, thigh (anterior, lateral, medial), and the medial portion of the leg. The lower portion of the plexus also gives rise to several major nerves: superior gluteal nerve (L4-S1), the inferior gluteal nerve (L5-S2), the sciatic nerve (L4-S3, consisting of the tibial and peroneal components), the posterior femoral cutaneous nerve (S1–S3), and the pudendal nerve. Major motor functions include hip abduction, hip extension, knee flexion, ankle movements, and control of the urinary and anal sphincters. It provides sensory function from the lower extremity distal to the knee (except the medial lower leg), posterior thigh, buttocks, and perineal region [14].
Neoplastic Syndromes of the Lumbrosacral Plexus
Metastatic lumbosacral plexopathy occurs most commonly from direct extension of abdominal and pelvic tumors. Occasionally, however, the plexus is affected by growth from metastases to regional lymph nodes or bony structures. Colorectal cancer is the most common primary tumor. Colorectal cancer along with retroperitoneal sarcoma and breast cancer account for almost half the cases. Lymphoma, cervical cancer, and other malignancies account for the remaining cases. In approximately 15% of patients, plexopathy is part of the initial presentation of cancer. Tumor can either directly invade the plexus or can track along connective tissue or epineurium. When tumor cells track, the presence of cancer can be difficult to demonstrate on imaging. Metastatic plexopathy is typically unilateral, but is bilateral in 25% of patients [9].
Metastatic lumbosacral plexopathy begins with leg pain in nearly all patients, which is followed by numbness and weakness. In a series of 85 patients, pain was so common during the course (98%) and at presentation (91%), that the authors indicated its absence should prompt consideration of alternate diagnoses [7]. Like with brachial plexopathy, the pain is typically constant, dull and aching. Sharp, radicular pain often coexists. Valsalva may exacerbate pain. Patients describe difficulty finding a comfortable position but lying supine may be particularly uncomfortable. Lying with hips flexed can relieve the pain if there is involvement of the iliopsoas [7].
Weakness and sensory disturbance develops in the majority of patients. Leg weakness (86%), sensory loss (73%), reflex loss (64%), and leg edema (47%) are the most common presenting features. A rectal mass may also be present. The distribution of numbness or weakness depends on which part of the plexus is affected. In this same series of 85 patients, the upper plexus (L1–4) was involved in 31% of patients, and the lower plexus (L5-S3) in 51%, while 18% had a pan-plexopathy [7]. In colorectal neoplasms and cervical carcinoma, the sacral plexus is most commonly involved. Accordingly, these patients typically have pain in the posterior thigh, leg or calf and weakness of ankle plantar flexion and knee flexion. Sensory disturbance may involve the posterior leg and sole of the foot [7]. Approximately 20–30% of patients describe a unilateral “hot dry foot.” The foot is objectively warm and has hypohydrosis. This is a manifestation of interruption of sympathetic ganglia or postganglionic fibers along the lumbosacral plexus or the peripheral nerves below the L3 segment. This can be an important localizing feature. Autonomic disturbances are not present with lesions of the nerve roots or cauda equina. These autonomic symptoms may precede the onset of weakness or numbness by several months or occur simultaneously [15]. When the upper plexus is involved, patients typically experience pain and sensory changes in the anterior thigh, groin and into the dorsum of the foot. Sarcoma is the most common neoplasm to present in this fashion. Loss of strength affects thigh muscles producing difficulty arising from a low-seated position or walking down stairs. Involvement of the lumbosacral trunk can produce foot drop, which is distinguished from a peroneal neuropathy by weakness of ankle inversion. Incontinence and impotence may be present, typically with bilateral plexus involvement. Pan-plexopathies, most commonly caused by genitourinary malignancies, can cause a combination of these findings [7, 9].
Imaging Evaluation of Brachial and Lumbosacral Plexus
CT, MR, and FDG-PET imaging are primary imaging modalities used to distinguish between malignant plexopathy, radiation-induced plexopathy, and radiation-induced tumor. MRI with and without contrast is generally the preferred method to confirm the diagnosis of suspected malignant plexopathy. The sensitivity of MRI for detecting malignant plexopathy is approximately 80% [9, 16]. Additionally, the presence of tumor recurrence either on imaging or clinical exam in the region of the plexus further supports the diagnosis of plexopathy. Therefore, for example, MRI of the lumbar spine and the pelvis complement plexus imaging in the case of suspected malignant lumbosacral plexopathy. Typically, a mass is seen in direct contact with the plexus or the expected course of a nerve that is clinically affected. Less commonly, thickening of the components of the plexus or abnormal signal in a nerve may be seen [17]. MR evidence of epidural lesions strongly suggests metastatic disease. However, malignant tissue may also infiltrate the plexus without distorting tissue planes and CT/MRI may not detect it. If MRI is normal or inconclusive, FDG-PET/CT is a reasonable next step. It may show increased uptake in the region of the plexus involved by the metastasis [18]. Additionally, combining diffusion weighted MR neurography with conventional MRI may improve detection of brachial plexopathy in symptomatic patients with known malignancy [19]. Plain radiographs or bone scan may support the diagnosis of malignant plexopathy if there is tumor in adjacent bones or lung. Finally, if there is a high suspicion for malignant plexopathy and the initial imaging is negative, repeating the MRI 4–6 weeks later may reveal a tumor that was not apparent on initial scans.
Radiation-induced plexopathy also produces variable appearance on MRI. Radiation fibrosis may appear as (1) diffuse thickening and enhancement of the brachial plexus without a focal mass or (2) soft tissue changes with low signal intensity (similar to muscle) on both T1- and T2-weighted images. T2-weighted images sometimes help distinguish radiation-induced fibrosis from tumor infiltration, as the former more frequently demonstrates low signal intensity and the later more frequently demonstrates a higher signal intensity. Routine administration of gadolinium is less helpful as both radiation-induced fibrosis and metastatic disease can show some degree of post-contrast enhancement [20]. Surgical exploration to obtain a biopsy should be considered in the event of persistent diagnostic uncertainty, however, even biopsy can be inconclusive [11].
Electrodiagnosis of Plexopathy
Electrodiagnostic studies (EDX) serve as an extension of the physical examination to help localize the lesion to plexus and exclude disorders of the nerve roots or peripheral nerve. In general, the sensory responses are spared in root lesions and affected in plexus lesions. EDX can also further delineate which root(s) and/or which part(s) of the plexus are affected. EDX also help delineate severity and chronicity of the process and exclude alternate peripheral neuropathic etiologies for symptoms.
The hallmark EDX abnormality which supports lower trunk/medial cord plexopathy is the combination of an abnormal or absent ulnar SNAP (sensory nerve action potential), ulnar CMAP, and median CMAP, with a normal median SNAP. Additionally, the radial SNAP and lateral antebrachial cutaneous responses are normal and the medial antebrachial cutaneous response is abnormal. EMG shows neurogenic changes in muscles with a C8/T1, medial cord, and lower trunk distribution.
In a lumbar plexopathy, nerve conduction study abnormalities may be limited to an abnormal femoral motor and saphenous sensory response. Needle EMG reveals abnormalities in the quadriceps, hip flexors, and adductors. In a low lumbosacral plexopathy, additional sensory and motor responses are abnormal and needle EMG of muscles with an L5/S1 innervation are abnormal [21]. In both cases, lumbar paraspinal muscles are expected to be spared; however, in reality, this can be variable. EDX for assessment of metastatic lumbosacral plexopathy can be limited or inconclusive for several reasons. First routine studies do not assess the inferior portion of the sacral plexus with contribution from the S2–S4 roots. Additionally, in this patient population, prior treatment with chemotherapy or age alone can affect the sensory responses in the lower extremities. Structural lumbar spine disease may coexist with cancer. All these factors can complicate interpretation of EDX for plexopathy. As such, EDX are used as one piece of the evaluation in combination with imaging [22].
Radiation-Induced Plexopathy
In patients previously treated with radiation, clinicians often must distinguish between radiation-induced brachial or lumbosacral plexopathy and recurrent tumor. At times, these conditions coexist. Radiation-induced plexopathy sometimes occurs as a mild reversible syndrome. Much more commonly it presents after a latent period ranging from several months to many years, with onset of neurologic symptoms most commonly 2–4 years after radiation. The precise pathophysiology of delayed radiation-induced plexopathy remains unclear. RT can injure the plexus both by direct toxic effects on axons and the vasa nervorum and also secondary microinfarction. Widespread fibrosis within and surrounding nerve, with demyelination and axonal loss, are consistently found at surgery and/or autopsy [23].
Presentation
Radiation plexopathy is more common than neoplastic plexopathy. However, reliably estimating the incidence is challenging for several reasons. (1) Reviews may have limited follow-up periods. Studies with longer follow-up report a higher incidence due to delayed onset, (2) The possible connection of presenting symptoms with prior RT may be overlooked and (3) Many different RT protocols have been used. As an example, in breast cancer, the incidence of radiation-induced brachial plexopathy varies in accordance to radiation technique. Incidence ranges from approximately 66% with 60 Gy in 5 Gy fractions as was used in the 1960s to approximately 1% with the regimen of 50 Gy in 2 Gy fractions used in contemporary treatment [24]. The risk of radiation-induced brachial plexopathy may be increased with the use of large RT fields, two separate courses of “subthreshold” RT and possibly when patients receive concurrent chemotherapy with RT [9, 23]. The majority of patients with radiation-induced brachial plexopathy will worsen gradually over the course of several years to severe neurologic disability whereas others will stabilize after 1–3 years. Recovery of function is unusual.
Radiation-induced injury of the lumbosacral plexus most frequently occurs after treatment of pelvic or testicular tumors and tumors involving para-aortic lymph nodes. Patients may present after external bean photon therapy, interstitial or intracavitary radiation implants, or combined photon and proton beam RT. Bowel or bladder symptoms are unusual and more often attributed to RT induced proctitis or bladder fibrosis. RT lumbosacral plexopathy also tends to progress slowly over months to years, though a few patients may progress more rapidly or demonstrate spontaneous stabilization or improvement [23].
Electrodiagnostic Evaluation
Electrodiagnostic studies can lend support for a diagnosis of radiation-induced brachial plexopathy or lumbosacral plexopathy. Most characteristics of radiation-induced brachial plexopathy look identical to neoplastic plexopathy. Nerve conduction studies are abnormal in the vast majority (90%) of both neoplastic brachial plexopathy and radiation-induced brachial plexopathy. On needle EMG, abnormal spontaneous activity (fibrillations/positive sharp waves and fasciculations) are seen in both entities, as are reinnervation changes in motor unit potential morphology. Myokymic discharges are the only electrodiagnostic feature, which can be useful in distinguishing radiation-induced brachial or lumbosacral plexopathy. In one series, this finding was present in approximately 60% of patients, compared to <5% of patients with neoplastic brachial plexopathy [25]. Myokymic discharges are spontaneous, grouped repetitive discharges of the same motor unit seen on needle EMG. Clinically, these discharges can sometimes be seen as involuntary rippling or quivering of muscle on physical examination. Additionally, these myokymic discharges are present in a larger percentage of muscles (approximately 25%) in the radiation plexopathy group, most commonly in the pronator teres and abductor pollicis brevis. Interestingly, in this cohort, the patients with neoplastic plexopathy who had myokymic discharges had received prior radiation therapy. Fibrillation potentials can also be seen in paraspinal muscles in a larger percentage of patients (approximately 20%) in the radiation plexopathy group, possibly due to inclusion of the posterior rami, nerve roots or the paraspinal muscles themselves in the field of radiation. Conduction block with proximal stimulation for CMAPs may sometimes be observed; however, these proximal responses can be technically challenging [25].
Treatment for Malignant and Radiation-Induced Plexopathies
Treatment for malignant plexopathy due to metastatic cancer is mainly symptom-based and aimed at eliminating or shrinking the tumor, if possible, with chemotherapy and/or radiation. Typically, pain remains prominent and pharmacologic and interventional strategies for pain management are required. Patients may benefit from being seen in a pain clinic. Treatment for radiation-induced brachial and lumbosacral plexopathy has generally been studied only in case reports or case series. Treatments have included surgical intervention and hyperbaric oxygen therapy, both of which showed no improvement and patients may actually worsen with surgery [23]. Anticoagulation has provided anecdotal improvement in symptoms and conduction block on EDX but is not standard of care [26]. Physical therapy can be important to help prevent secondary musculoskeletal complications in brachial plexopathy and aide in mobility with lumbosacral plexopathy. Primary prevention using the lowest dose and most targeted radiotherapy is paramount [9].
Metastases to Peripheral Nerve
As secondary malignant tumors, metastases must be distinguished from other mass lesions arising from peripheral nerve, including primary malignant peripheral nerve tumors. In general, these lesions fall into several categories: benign non-neoplastic nerve tumors (e.g., neuroma, lipoma), benign neoplasms of non-neural sheath origin (e.g., hemangioma), benign nerve sheath neoplasms (e.g., neurofibroma, schwannoma), and malignant peripheral nerve sheath tumors (e.g., MPNSTs).
Presentation
In general, benign lesions tend to be slowly progressive whereas malignant tumors tend to grow rapidly with progressive pain and/or neurologic deficit. Taking a careful family history to assess for the possibility of an underlying neurogenic disorder such as neurofibromatosis or schwannomatosis is important, particularly in patients without a known primary malignancy [27].
Cancer infrequently affects peripheral nerve; however, it can do so in several ways. The first is through direct compression of the nerve from nearby metastasis. Metastases to the skull base, which occur most commonly in prostate, breast, and lung cancer as well as lymphoma, can cause compression of surrounding nerves [28]. This is most commonly a late stage of cancer where patients already have widespread bone metastases. Craniofacial pain with accompanying cranial nerve palsies alerts the clinician to this complication. The symptoms correlate with the location of the metastasis and which adjacent nerve is compressed. Syndromes include orbital syndrome (frontal headache, proptosis, ophthamoplegia, decreased vision), cavernous sinus syndrome (ophthalmoplegia, facial numbness, and periorbital swelling), middle fossa syndrome/gasserian ganglion syndrome (atypical facial pain and paresthesias), jugular foramen syndrome (occipital pain, hoarseness, dysphagia), and occipital condyle syndrome (occipital pain, dysarthria due to CN XII palsy). Metastases to the mandible or skull base can cause compression of the inferior alveolar nerve. This causes “numb chin” syndrome characterized by oral and facial numbness in the distribution of the mental nerve over the chin and lower lip [28].
In addition to compression of nearby nerves, metastases can also spread via local extension to cranial or peripheral nerves with resulting neurologic dysfunction. One example of this is a vocal cord paralysis from lung cancer through invasion of the recurrent laryngeal nerve from invasion of mediastinal lymph nodes [29]. Other intraneural metastases are rare, presumably due to a protective blood-nerve barrier; however, case reports exist [30,31,32].
Presentation of Neurolymphomatosis
Neurolymphomatosis (NL) is infiltration of the peripheral nervous system by lymphoma. Malignant lymphocytic infiltrates can occur in root, plexus, or peripheral nerve. NL is distinct from other disorders affecting peripheral nerve associated with lymphoma such as irradiation, chemotherapy or paraneoplastic phenomenon [33]. The vast majority of cases are diffuse large B cell lymphoma. T cell lymphomas and acute leukemias have been reported rarely [34]. NL is rare but precise incidence is unknown. One series estimated an annual incidence of 3 cases per 100 new cases of intermediate/high-grade B cell NHL [35]. Clinical presentation is variable but generally follows 4 typical patterns: (1) Painful involvement of multiple peripheral nerves or nerve roots; (2) cranial neuropathy with or without pain; (3) painless involvement of multiple peripheral nerves; (4) painful or painless involvement of a single peripheral nerve [33]. Additionally, NL affects more than one anatomic structure in approximately 60% of patients and involvement of plexus may also occur. In approximately 25% of patients, NL is the initial manifestation of malignancy. Alternatively, NL can present as a relapse or progression of previously treated disease. When neuropathy is present, a mixed sensory and motor neuropathy is most common. Pure motor neuropathies have been reported and a pure sensory neuropathy has been described in one patient [34]. Some patients may have demyelinating features on EDX, which, at times, can fulfill criteria for diagnosis of chronic inflammatory demyelinating polyradiculoneuropathy (CIDP). As such, diagnosis can be challenging. Some patients even respond temporarily to treatment for CIDP [steroids and/or intravenous immunoglobulin (IVIG)], further clouding diagnosis [36]. In older series, diagnosis was established only at autopsy in almost half of patients [33]. This rate of postmortem diagnosis has reduced substantially to less than 10% in more recent series likely due to increased awareness of NL and advances in imaging. Despite these advances, diagnosis is often delayed and remains challenging [34].
Diagnosis of Neurolymphomatosis
Diagnostic evaluation includes electrodiagnostic studies for localization and characterization, imaging (MRI and sometimes FDG-PET-CT scan), CSF analysis typically including cytology and flow cytometry and nerve biopsy if the rest of the evaluation is inconclusive. Diagnostic yield of MRI and PET-CT is high, with abnormal findings observed in 77 and 84%, respectively, in one series [34]. MRI abnormalities include abnormal enhancement of the affected neural structure. Nerve thickening is observed in approximately 50% of patients and can be diffuse (17%) or nodular (30%). These MRI findings, however, are non-specific and can sometimes be seen in acute or chronic polyradiculoneuropathies, neurofibromatosis, and malignant peripheral nerve sheath tumors [34].
CSF findings in NL have also been reported in detail. In one series, protein was elevated in 61%, glucose low in 11%, elevated cell count (>5 cells/mm3) in 44%. Cytology was malignant in 40% or suspicious in 13%. Some patients have abnormal or suspicious cytology in the face of a normal cell count. Additionally, CSF cell count may be elevated in patients who have no abnormality on imaging studies [34]. In this series, nerve biopsy was performed in just over 50% of patients and was abnormal, demonstrating NL, in 88% of patients. Nerve biopsy must be performed of a clinically affected structure, which can be challenging based on which areas are commonly affected; common and less invasive biopsy sites, including the sural nerve, may be unaffected [34]. These insights highlight the importance of multi-modal evaluation to arrive at the correct diagnosis of NL, particularly in patients who do not have an established diagnosis of hematologic malignancy.
See Figs. 7.3a–c, 7.4a, b, and 7.5a, b [37].
A previously healthy 57 year old patient with progressive left leg weakness, numbness and pain over 3 months. The pelvis MRI showed abnormal thickening and enhancement at left lumbosacral nerve roots, plexus and sciatic nerve (a, b) with resolution after treatment (c) (images reprinted with permission of BMJ Publishing Group from Tsai et al. [37], August 2015)
The brain MRI study revealed abnormal enhancement at left trigeminal nerve (a) which resolved after chemotherapy (b) (images reprinted with permission of BMJ Publishing Group from Tsai et al. [37], August 2015)
The whole body positron emission tomography (PET) showed increased metabolism at bilateral brachial plexuses, left lumbosacral nerve roots and left sciatic nerve (a) which resolved after chemotherapy (b). The cytology of repeated cerebrospinal fluid studies proved large B cell lymphoma. The bone marrow study did not show evidence of hematologic malignancy (images reprinted with permission of BMJ Publishing Group from Tsai et al. [37], August 2015)
Treatment of Neurolymphomatosis
Treatment of NL consists of chemotherapy or chemotherapy combined with radiation. Defined standards are lacking and optimal management is unknown. Intra-CSF chemotherapy and standard craniospinal radiation fields will not treat all involved areas as NL often involves nerve roots within and beyond the subarachnoid space, and multiple sites are often involved. Chemotherapy protocols are often based on those to treat CNS involvement by lymphoma. Limited field radiotherapy can be effective in treating refractory pain from a particular area of nerve, plexus, or root involvement. Clinical improvement (reduction of pain and/or functional recovery) and radiographic resolution (improvement of nerve root enlargement/enhancement or normalization of FDG-PET uptake) has been observed in 50–70% of treated patients. Limited data exist about overall survival in this group and this may depend on whether the NL was part of the primary presentation or secondary. In general, in one series, median survival from diagnosis of NL was 10 months with 24% surviving at least 36 months [34]. Some patients may relapse solely with NL despite ongoing complete remission in sites outside the nervous system [35].
Metastases to Muscle
Overall, the prevalence of skeletal muscle metastasis is low and depending on the series of patients and modality used to detect them, ranges from 0.03 to 17.5%. Because they are rare, trauma, hematoma and abscess should be considered on the differential. Several benign and malignant entities including muscle hemangioma, intramuscular ganglion, myxoma and ischiogluteal bursitis, and sarcoma can mimic intramuscular metastases [38].
Several mechanisms for intramuscular metastatic spread have been proposed. Arterial hematologic spread, including through arterial emboli of tumor cells, is the most likely primary method. Malignant tumors can also metastasize through venous circulation, especially through paravertebral venous plexus. Spread from intramuscular lymph nodes and perineural spread are also possible though less likely. Although muscle can accounts for approximately 50% of total body mass and has an abundant blood supply, skeletal muscle metastases are rare, occurring in less than 5% of patients with any malignancy subtype. As such, muscle may be resistant both to primary and to metastatic cancer. Several mechanisms of resistance have been hypothesized. These mechanisms include secretion of factors, which inhibit proliferation of tumor cells, variable blood flow, and involvement in lactic acid metabolism [38, 39].
Presentation
Metastatic disease to muscle should be considered in patients with cancer who develop a soft tissue mass within muscle. These masses are frequently asymptomatic and discovered incidentally on staging imaging. Conversely, they can be painful and associated with swelling, erythema of the overlying skin, and restricted movement. Those that are painful typically may be larger and associated with massive muscle infiltration or destruction. Skeletal muscle metastases can occur in upper extremity, lower extremity, abdominal or thoracic wall and paravertebral muscles. Certain locations are more common. In one large series, the most common location was the iliopsoas, followed by paravertebral and then gluteal muscles, together accounting for over 60% of the metastases. The most common tumor types were genital, gastrointestinal, urological, and melanoma. Most commonly, skeletal muscle metastasis exist in the context of other metastatic lesions; however, rarely they can be the only manifestation of metastatic disease. Muscle metastases tend to present in five different patterns: solitary or multiple round/oval masses, multiple intramuscular calcifications, abscess-like lesions, diffuse infiltration with muscle swelling and intramuscular hemorrhage. The first two types tend to be painless and found incidentally on imaging and the later three clinically symptomatic and painful. Secondary abscesses can develop [38].
Diagnosis
MRI is thought to be superior to CT in detection of muscle metastases. Imaging findings, however, are non-specific and most often a complete histological examination is needed. CT guidance can be useful in obtaining biopsy tissue for analysis [40].
Treatment
Therapy includes surgical resection if metastases are isolated. Extensive disease or residual tumor after incomplete resection warrants radiation or chemotherapy. Prognosis in patients with clinically evident metastases to muscle is poor despite treatment, with a median survival of less than 12 months [40].
Radiation-Induced Myopathy
Radiation itself can also adversely affect muscle. Radiation exposure can cause a delayed onset radiation-induced myopathy manifesting 2 to over 40 years after therapy. This typically occurs after neck and/or upper torso radiation and presents with head drop and periscapular muscle weakness. Neuromuscular respiratory failure rarely occurs. CKs are typically normal to only mildly elevated. The presentation is quite distinct from metastatic disease to muscle [41].
Conclusion
Peripheral nervous system metastatic disease is diverse and complex. Two major challenges face the clinician [1]. The first is suspecting an underlying malignancy in a patient with a PNS disorder without an established diagnosis of cancer. The patient’s presentation depends on the anatomic location of involvement. However, in general, severe pain and/or relentless progression of deficits typically are hallmarks of underlying PNS metastatic disease. The second is identifying a PNS disorder in patients with malignancy and determining whether symptoms are due to the malignancy itself or to treatment associated factors. This diagnostic process can be challenging since investigations for tumor involvement can be initially negative, even with tumor is present. Clinicians must implement a multi-modal evaluation including several complementary imaging exams, lab studies and repeated evaluation as needed. Early diagnosis and treatment of the cancer hold the most promise for alleviating pain, eliminating the cancer, when possible, improving symptoms and preventing further neurologic deficit.
References
Antoine J-C, Camdessanché J-P. Peripheral nervous system involvement in patients with cancer. Lancet Neurol. 2007;6(1):75–86.
Chamberlain MC. The role of chemotherapy and targeted therapy in the treatment of intracranial meningioma. Curr Opin Oncol. 2012;24(6):666–71.
Ali S, Lee S-K. Ipilimumab therapy for melanoma: a mimic of leptomeningeal metastases. AJNR Am J Neuroradiol. 2015;36(12):E69–70.
Pauls S, Fischer A-C, Brambs H-J, Fetscher S, Höche W, Bommer M. Use of magnetic resonance imaging to detect neoplastic meningitis: Limited use in leukemia and lymphoma but convincing results in solid tumors. Eur J Radiol. 2012 May 1;81(5):974–8.
Clarke JL, Perez HR, Jacks LM, Panageas KS, Deangelis LM. Leptomeningeal metastases in the MRI era. Neurology. 2010;74(18):1449–54.
Ahluwalia MS, Wallace PK, Peereboom DM. Flow cytometry as a diagnostic tool in lymphomatous or leukemic meningitis. Cancer. 2011;118(7):1747–53.
Jaeckle KA, Young DF, Foley KM. The natural history of lumbosacral plexopathy in cancer. Neurology. 1985;35(1):8–15.
Kori SH, Foley KM, Posner JB. Brachial plexus lesions in patients with cancer: 100 cases. Neurology. 1981;31(1):45–50.
Jaeckle KA. Neurological manifestations of neoplastic and radiation-induced plexopathies. Semin Neurol. 2004;24(4):385–93.
Ramchandren S, Dalmau J. Metastases to the peripheral nervous system. J Neurooncol. 2005;75(1):101–10.
Stewart JD. Focal peripheral neuropathies. 4th ed. West Vancouver: JBJ Publishing; 2010. p. 1.
Stewart JD. Focal peripheral neuropathies. 4th ed. West Vancouver: JBJ Publishing; 2010. p. 225–333.
Ferrante MA. Brachial plexopathies. Continuum (Minneap Minn). 2014 Oct;20(5 Peripheral Nervous System Disorders):1323–42.
Dyck PJB, Thaisetthawatkul P. Lumbosacral plexopathy. Continuum (Minneap Minn). 2014 Oct;20(5 Peripheral Nervous System Disorders):1343–58.
Dalmau J, Graus F, Marco M. “Hot and dry foot” as initial manifestation of neoplastic lumbosacral plexopathy. Neurology. 1989;39(6):871–2.
Thyagarajan D, Cascino T, Harms G. Magnetic resonance imaging in brachial plexopathy of cancer. Neurology. 1995;45(3 Pt 1):421–7.
Brejt N, Berry J, Nisbet A, Bloomfield D, Burkill G. Pelvic radiculopathies, lumbosacral plexopathies, and neuropathies in oncologic disease: a multidisciplinary approach to a diagnostic challenge. Cancer Imaging. 2013;13(4):591–601.
Ahmad A, Barrington S, Maisey M, Rubens RD. Use of positron emission tomography in evaluation of brachial plexopathy in breast cancer patients. Br J Cancer. 1999;79(3–4):478–82.
Andreou A, Sohaib A, Collins DJ, Takahara T, Kwee TC, Leach MO, et al. Diffusion-weighted MR neurography for the assessment of brachial plexopathy in oncological practice. Cancer Imaging. 2015;15(1):1023.
Wittenberg KH, Adkins MC. MR imaging of nontraumatic brachial plexopathies: frequency and spectrum of findings. Radiographics. 2000;20(4):1023–32.
Krarup C, Crone C. Neurophysiological studies in malignant disease with particular reference to involvement of peripheral nerves. J Neurol. 2002;249(6):651–61.
Tavee J, Mays M, Wilbourn AJ. Pitfalls in the electrodiagnostic studies of sacral plexopathies. Muscle Nerve. 2007;35(6):725–9.
Dropcho EJ. Neurotoxicity of radiation therapy. Neurol Clin NA. 2010 Feb 1;28(1):217–34.
Delanian S, Lefaix J-L, Pradat P-F. Radiation-induced neuropathy in cancer survivors. Radiother Oncol. 2012 Dec 1;105(3):273–82.
Harper CM, Thomas JE, Cascino TL, Litchy WJ. Distinction between neoplastic and radiation-induced brachial plexopathy, with emphasis on the role of EMG. Neurology. 1989;39(4):502–6.
Soto O. Radiation-induced conduction block: resolution following anticoagulant therapy. Muscle Nerve. 2005;31(5):642–5.
Bhattacharyya AK, Perrin R, Guha A. Peripheral nerve tumors: management strategies and molecular insights. J Neurooncol. 2004;69(1–3):335–49.
Laigle-Donadey F, Taillibert S, Martin-Duverneuil N, Hildebrand J, Delattre J-Y. Skull-base metastases. J Neurooncol. 2005;75(1):63–9.
Paquette CM, Manos DC, Psooy BJ. Unilateral vocal cord paralysis: a review of CT findings, mediastinal causes, and the course of the recurrent laryngeal nerves. Radiographics. 2012;32(3):721–40.
Hansen JM, Rasti Z, Smith T, Lassen LH. Sciatic neuropathy as first sign of metastasising prostate cancer. Case Rep. 2010 Oct 11;2010 (Oct 08 2):bcr1220092529–9.
Matsumine A, Kusuzaki K, Hirata H. Intraneural metastasis of a synovial sarcoma to a peripheral nerve. Bone Joint J. 2005;87(11):1553–5.
Cantone G, Rath SA, Richter HP. Intraneural metastasis in a peripheral nerve. Acta Neurochir (Wien). 2000;142(6):719–20.
Baehring JM, Damek D, Martin EC, Betensky RA, Hochberg FH. Neurolymphomatosis. Neuro-Oncol. 2003;5(2):104–15.
Grisariu S, Avni B, Batchelor TT, van den Bent MJ, Bokstein F, Schiff D, et al. Neurolymphomatosis: an International Primary CNS Lymphoma Collaborative Group report. Blood. 2010 Jun 17;115(24):5005–11.
Gan HK, Azad A, Cher L, Mitchell PLR. Neurolymphomatosis: diagnosis, management, and outcomes in patients treated with rituximab. Neuro-Oncol. 2010;12(2):212–5.
Tomita M, Koike H, Kawagashira Y, Iijima M, Adachi H, Taguchi J, et al. Clinicopathological features of neuropathy associated with lymphoma. Brain. 2013;136(8):2563–78.
Tsai H-H, Chen Y-F, Hsieh S-T, Chao C-C. Neurolymphomatosis as the primary presentation of non-Hodgkin’s Lymphoma. J Neurol Neurosurg Psychiatry. 2015;86(8):929–30.
Surov A, Hainz M, Holzhausen H-J, Arnold D, Katzer M, Schmidt J, et al. Skeletal muscle metastases: primary tumours, prevalence, and radiological features. Eur Radiol. 2009;20(3):649–58.
Seely S. Possible reasons for the high resistance of muscle to cancer. Med Hypotheses. 1980;6(2):133–7.
Tuoheti Y, Okada K, Osanai T, Nishida J, Ehara S, Hashimoto M, et al. Skeletal muscle metastases of carcinoma: a clinicopathological study of 12 cases. Jpn J Clin Oncol. 2004;34(4):210–4.
Ghosh PS, Milone M. Clinical and laboratory findings of 21 patients with radiation-induced myopathy. J Neurol Neurosurg Psychiatry. 2015;1(86):152–8.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 Springer International Publishing AG
About this chapter
Cite this chapter
Guidon, A.C. (2018). Peripheral Nervous System Metastases as Complications of Systemic Cancer. In: Schiff, D., Arrillaga, I., Wen, P. (eds) Cancer Neurology in Clinical Practice. Springer, Cham. https://doi.org/10.1007/978-3-319-57901-6_7
Download citation
DOI: https://doi.org/10.1007/978-3-319-57901-6_7
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-57899-6
Online ISBN: 978-3-319-57901-6
eBook Packages: MedicineMedicine (R0)