Abstract
N-acylethanolamines (NAEs) are bioactive lipids, structural analogues to the endocannabinoid arachidonoylethanolamide (anandamide), whose functions and properties are being elucidated in recent years. By activating their receptors, specifically peroxisome proliferator-activated receptors (PPARs), these molecules exert a variety of physiological effects via genomic and rapid non-genomic mechanisms. Regulation of lipid metabolism, energy homeostasis, and anti-inflammation are among the best-characterized effects of PPAR activation. NAEs are abundant in the CNS and their receptors are widely expressed both in neurons and in glial cells, where they modulate brain functions and are involved in the pathophysiology of neurological and psychiatric disorders. In the brain, they participate in the regulation of feeding behavior, cognitive functions, mood, reward, and sleep-wake cycles, and evidence suggests that they might be therapeutically exploited as neuroprotective agents, “anti-addictive” medications, anticonvulsant, and antidepressant.
In this chapter, we will review the state of the art on these neuromodulators and their receptors in the brain and will discuss new hypotheses on their physiological and pathophysiological roles.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
- Transient Receptor Potential Vanilloid
- Dopamine Neuron
- Ketogenic Diet
- Fatty Acid Amide Hydrolase
- nAChR Subunit
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
1 Introduction
Fatty acid ethanolamides, generally referred as N-acylethanolamines (NAEs), are a group of endogenous lipid molecules with long-chain fatty acids (Schmid et al. 1990; Hansen et al. 2000). It was long known that these molecules are ubiquitously found in animal tissues (Bachur et al. 1965), in both the periphery and the brain, and have attracted attention in recent years as they possess a variety of biological activities.
Among NAEs, the most studied are arachidonoylethanolamide (anandamide, AEA), palmitoylethanolamide (PEA), and oleoylethanolamide (OEA); other congeners are stearoylethanolamide (SEA) and linoleoylethanolamide (LEA) (Fig. 1) (Hansen 2010; Rahman et al. 2014). Anandamide is the first endocannabinoid to be discovered (Devane et al. 1992) and the only NAE to bind to cannabinoid type 1 (CB1) and type 2 (CB2) receptors. Quantitatively, anandamide is a minor component in most animal tissues when compared with other NAEs such as PEA, SEA, OEA, and LEA (Hansen and Diep 2009). NAEs, although belonging to the same extended endocannabinoid-like family as the cannabinoid agonist AEA, exert a variety of biological effects through several other receptors, in particular peroxisome proliferator-activated receptor-α (PPARα) (Hansen 2010; Lo Verme et al. 2005; Petrosino et al. 2010), but also G-protein-coupled receptors GPR55 (Baker et al. 2006) and GPR119 and transient receptor potential vanilloid type 1 (TRPV1) (Piomelli 2013).
Although these molecules were known from many years, the role of PEA and OEA, as well as of other NAEs, in the CNS has been elucidated only recently, when the discovery of AEA (Devane et al. 1992) fueled a renewed interest in these lipid messengers.
Specifically, functions and properties of PEA, a saturated fatty acid (palmitic acid) derivative, were discovered first in 1957, when this lipid (from soybeans, peanuts, and egg yolk) was found to exert anti-inflammatory activity in guinea pigs (Kuehl et al. 1957). High levels of PEA were measured in mammalian tissues, especially in the brain (Bachur et al. 1965). The finding that PEA is the most abundant NAE in the rat brain has been more recently substantiated by the use of lipidomics techniques (Kilaru et al. 2010).
The monounsaturated OEA is a potent anorectic lipid mediator (Fu et al. 2003, 2005; Rodriguez de Fonseca et al. 2001; Piomelli 2013) and shares this property with PEA, LEA (Hansen 2014; Hansen and Diep 2009), and SEA (Terrazzino et al. 2004). As anorexiant, however, PEA is significantly less potent than OEA in reducing food intake, and LEA is similar in potency to OEA (Rodriguez de Fonseca et al. 2001; Diep et al. 2011).
Besides their anti-inflammatory activity and their physiological functions as modulators of feeding behavior, NAEs, and specifically PEA and OEA, have been recently involved in pathophysiology of neurological and psychiatric disorders, ranging from addiction, neurodegenerative diseases, epilepsy, and mood disorders (Pistis and Melis 2010; Scherma et al. 2016; Melis and Pistis 2014). In this review, we will focus on physiology of non-cannabinoid NAEs in the CNS and on their relevance in neuropsychiatric disorders.
2 Synthesis and Catabolism
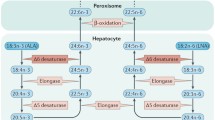
NAEs including AEA and the non-cannabinoid PEA and OEA are not stored in vesicles but produced “on demand”. Their endogenous levels are strictly regulated by enzymes responsible for their formation and degradation (Ueda et al. 2010a; Rahman et al. 2014). In fact, they are synthesized from glycerophospholipids via their corresponding N-acylphosphatidylethanolamines (NAPEs) (Okamoto et al. 2004; Rahman et al. 2014; Ueda et al. 2010b; Hansen 2010; Hansen et al. 2000). The classical pathway for NAE synthesis is a two-step process (Fig. 2). The first step is the generation of NAPE by a Ca2+-dependent N-acyltransferase (NAT), which transfers the Sn-1 fatty acid to phosphatidylethanolamine from a donor phospholipid (Hansen et al. 2000; Hansen and Diep 2009). N-Acyltransferase is not specific for any fatty acids, since it catalyzes the transfer of any acyl group from the Sn-1 position of donor phospholipids. NAPE is then hydrolyzed by NAPE-hydrolyzing PLD (NAPE-PLD) with the generation of NAEs (Rahman et al. 2014). Human, mouse, and rat NAPE-PLD cloning allowed the functional characterization of NAE biosynthetic pathways (Okamoto et al. 2004). NAPE-PLD−/− mice (Leung et al. 2006) show a highly reduced Ca2+-dependent conversion of NAPE to long-chain saturated and unsaturated NAEs in brain tissue. These mice display decreased levels of OEA and PEA, but similar levels of AEA compared with wild-type littermates (Leung et al. 2006). This indicates that AEA is formed by other parallel pathways (Hansen and Diep 2009) and strongly suggests the existence of other NAE-forming enzymes within NAPE-PLD-independent pathways (Tsuboi et al. 2011; Simon and Cravatt 2010; Leung et al. 2006; Rahman et al. 2014). No gross phenotype in NAPE-PLD-deficient mice has been reported, whereas in humans a polymorphism of NAPE-PLD was associated with obesity in a Norwegian population (Wangensteen et al. 2010).
Schematic diagram illustrating the canonical biosynthetic and catabolic pathways for NAE formation and their cellular mechanisms of actions through their receptor of PPARα. Phosphatidylcholine (Pcholine) donates a fatty acid moiety from the sn-1 position (R3) to a phosphatidylethanolamine (PEth). This reaction is catalyzed by N-acyltransferase (NAT). The resulting N-acylphosphatidylethanolamine (NAPE) is hydrolyzed by NAPE-PLD to the corresponding N-acylethanolamine (NAE). Activation of PPARα by NAEs results in genomic effects (gene transcription) and in non-genomic actions, such as activation of a tyrosine kinase and phosphorylation of β2*nAChRs (i.e., α4β2). Ca2+ entry mediated by α7-nAChRs activates NAS synthesis through the Ca2+-dependent NAT and NAPE-PLD. Fatty acid amide hydrolase (FAAH) and NAE-hydrolyzing acid amidase (NAAA) are the major inactivating enzymes for OEA, PEA, and AEA which convert them into ethanolamine and corresponding fatty acids (oleic, palmitic, and arachidonic acids, respectively)
NAPE-PLD is expressed in many tissues, including the brain (Egertova et al. 2008; Morishita et al. 2005; Cristino et al. 2008; Suarez et al. 2008). The cellular localization of NAPE-PLD in the brain is informative on the functional significance of NAEs in the CNS. NAPE-PLD mRNA and immunoreactivity are located both presynaptically and postsynaptically and are intense in axons of the vomeronasal nerve projecting to the accessory olfactory bulb (Egertova et al. 2008) and in the hippocampus (Cristino et al. 2008; Egertova et al. 2008; Nyilas et al. 2008). Other brain areas with NAPE-PLD immunoreactivity are the cortex, thalamus, hypothalamus (Reguero et al. 2014; Egertova et al. 2008), and cerebellum (Suarez et al. 2008). The postsynaptic localization of NAPE-PLD indicates that NAEs may act as an autocrine or paracrine signal at receptors expressed in the same or neighboring cells. On the other hand, the axonal localization suggests that synthesized NAEs may be released to target postsynaptic neurons and regulate synaptic signaling.
The generated NAEs are then degraded to their corresponding free fatty acids and ethanolamine (Cravatt et al. 1996; Deutsch et al. 2002) (Fig. 2). This hydrolysis is catalyzed mainly by fatty acid amide hydrolase (FAAH) (Cravatt et al. 1996) and NAE-hydrolyzing acid amidase (NAAA) (Tsuboi et al. 2005). FAAH hydrolyzes all NAEs with high efficiency, and it is expressed in many different tissues and cell types, including in the brain.
NAAA has no sequence homology with FAAH and is expressed in lysosomes. NAAA displays same specificity toward unsaturated NAEs such as PEA to much greater extent than AEA (Tsuboi et al. 2007a). In the rat, NAAA is highly expressed in the lung, spleen, thymus, and intestine (Tsuboi et al. 2007a), very high in alveolar macrophages (Tsuboi et al. 2007b), but low in the brain.
Alternatively, AEA and other polyunsaturated NAEs are oxygenated in their polyunsaturated fatty acyl moieties and converted to prostamides by cyclooxygenase-2 (COX-2) (Yu et al. 1997; Kozak et al. 2002) or to other oxygenated metabolites by lipoxygenases (Hampson et al. 1995; Ueda et al. 1995) or by cytochrome P-450 (Bornheim et al. 1993). In addition to these enzymes, more recent studies revealed the involvement of new players in NAE metabolism (see Rahman et al. 2014 for a comprehensive review).
3 Receptors for NAEs
3.1 Peroxisome Proliferator-Activated Receptor-α (PPARα)
PPARs belong to the large superfamily of transcription factors, accounting 48 members in the human genome (Germain et al. 2006).The PPAR subfamily, specifically, is composed of three isotypes: PPARα, PPARγ, and PPARβ/δ.
The denomination of these receptors dates back to the 1960s, when the lipid-lowering drug clofibrate was found to induce proliferation of peroxisomes in the liver and hepatomegaly in rodents (Thorp and Waring 1962; Hess et al. 1965). The receptor activated by clofibrate, other fibrates, and synthetic ligands was cloned and denominated peroxisome proliferator-activated receptor-α (Issemann and Green 1990). Although the other members of this subfamily (β/δ and γ) are structural homologs of PPARα, they do not induce peroxisome proliferation (Graves et al. 1992; Dreyer et al. 1992; Schmidt et al. 1992). Nevertheless, the name has remained.
PPARs, like other nuclear receptors, are composed of four domains (Fidaleo et al. 2014), among which the DNA-binding domain and the ligand-binding domain, which are the most conserved portions of the protein across the different isotypes (Desvergne and Wahli 1999; Escriva et al. 1998; Laudet et al. 1992). The ligand-binding domain, in particular, is strikingly large in comparison with other nuclear receptors (Desvergne and Wahli 1999). This explains why PPARs are activated by a large number of diverse endogenous ligands as well as by synthetic agonists: saturated and unsaturated fatty acids (palmitic acid, oleic acid, linoleic acid, and arachidonic acid), NAEs, and eicosanoids (Desvergne and Wahli 1999).
Considerable evidence suggests that NAEs display most of their CNS activity through PPARα. In HeLa cells stably expressing a luciferase reporter gene together with the ligand-binding domain of human PPARα, OEA activates PPARα with an EC50 of 120 nM, whereas PEA displays lower potency (EC50 of 3.1 μM) (Lo Verme et al. 2005; Fu et al. 2003). Under identical conditions, SEA was reported to be ineffective (Lo Verme et al. 2005).
PPARα has been cloned and characterized in several species, including humans (Sher et al. 1993). PPARα expression is enriched in tissues with high fatty acid oxidation rates such as the liver, heart, skeletal muscle, brown adipose tissue, and kidney. It is also expressed in other tissues and cells including the intestine, vascular endothelium, smooth muscle, and immune cells such as monocytes, macrophages, and lymphocytes (Lefebvre et al. 2006; Chinetti et al. 1998) and in the CNS (Mandard et al. 2004; Galan-Rodriguez et al. 2009; Moreno et al. 2004; Braissant et al. 1996; Auboeuf et al. 1997). The highest PPARα expression was found in the basal ganglia; some thalamic, mesencephalic, and cranial motor nuclei; the reticular formation; and the large motoneurons of the spinal cord (Fidaleo et al. 2014; Moreno et al. 2004). Besides neuronal localization, a specific distribution of PPARα in ependymal and astroglial cells, but not in oligodendrocytes, was reported (Moreno et al. 2004).
Several structurally different classes of compounds bind to PPARα, including the hypolipidemic fibrates. Saturated or monounsaturated NAEs are high-affinity ligands for PPARα, particularly OEA which may be considered an endogenous ligand at concentrations normally achieved under physiological conditions (Fu et al. 2003).
3.2 GPR119 and GPR55
NAEs bind also to G-protein-coupled receptors 119 (GPR119) and 55 (GPR55). GPR119 is involved in insulin secretion, and, for this reason, it is emerging as a potential therapeutic target for type 2 diabetes, with beneficial effects on glucose homeostasis (Moran et al. 2014). There is no evidence of GPR119 expression in the CNS, therefore a detailed discussion of its functions is not within the aims of this chapter.
GPR55 is expressed in the CNS (Sawzdargo et al. 1999; Ryberg et al. 2007), as well as in peripheral tissues (intestine, bone marrow, immune and endothelial cells, spleen, and platelets) (Pietr et al. 2009; Balenga et al. 2011; Rowley et al. 2011; Cherif et al. 2015). GPR55 is a 319-amino acid protein that was cloned in 1999 and mapped to chromosome 2q37 in humans (Sawzdargo et al. 1999). GPR55 shows low amino acid homology with CB1 (13.5%) or CB2 (14.4%) (Ryberg et al. 2007; Kapur et al. 2009; Baker et al. 2006). Similarly to PPARα, GPR55 is a receptor for small lipid mediators, and it is also activated by some synthetic cannabinoids and related molecules. The lipid lysophosphatidylinositol (LPI), which activates GPR55 but has no affinity for CB1 or CB2 receptors, was the first endogenous ligand identified for this receptor (Oka et al. 2007). Among lipids and NAEs, PEA binds with high affinity at GPR55 (EC50 = 1–20 nM), whereas OEA is less potent (EC50 = 440 nM) (Baker et al. 2006; Ryberg et al. 2007). Recent evidence suggests that GPR55 is involved in the regulation of axonal growth during development (Cherif et al. 2015). GPR55 participates also in central processing of neuropathic and inflammatory pain (Deliu et al. 2015) as it has been reported to play a pronociceptive role in the periaqueductal gray. Consistently, GPR55−/− (knockout) mice have been shown to be protected in models of inflammatory and neuropathic pain. This suggests that GPR55 antagonists may have therapeutic potential as analgesics for both these pain types (Staton et al. 2008). It is not clear how the anti-inflammatory and anti-neuropathic effects of PEA can be reconciled with the functional role of GPR55 in pain and inflammation. As little is known on the physiological roles of GPR55, particularly in the CNS, further studies are needed to shed some lights into this receptor and its endogenous agonists.
4 Physiological Role of NAEs and PPAR
PPARα is a transcriptional regulator of genes involved in peroxisomal and mitochondrial β-oxidation, fatty acid transport, and, in rodents, hepatic glucose production (Xu et al. 2002). PPARα negatively regulates pro-inflammatory and acute phase response signaling pathways, as seen in rodent models of systemic inflammation (Gervois et al. 2004; Bensinger and Tontonoz 2008; Glass and Ogawa 2006). Consistently, PPARα−/− mice display longer inflammatory responses (Devchand et al. 1996), increased susceptibility to experimental colitis, and experimental autoimmune encephalitis (an experimental model of multiple sclerosis) (Straus and Glass 2007). PPARα agonists reduce peripheral inflammation in a PPARα-dependent manner (Sheu et al. 2002; Lo Verme et al. 2005).
The canonical mechanism of action downstream to PPARα activation is regulation of gene transcription (Ferre 2004; Berger and Moller 2002; Moreno et al. 2004). Specifically, ligand binding promotes dissociation of corepressor proteins, association of coactivators, and coactivator proteins and heterodimerization with the retinoid X receptor (RXR). This dimer is then translocated into the nucleus and binds to specific regions on DNA termed peroxisome proliferator response elements (PPRE). The result is either an increase or a decrease of gene transcription, depending on the target gene. Besides this typical transduction mechanism, non-transcriptional actions by PPARα have also been observed (Melis et al. 2008; Ropero et al. 2009; Gardner et al. 2005). These effects are rapid in onset (2–5 min). This short onset time rules out the possibility of a genomic mechanism of action and involves signaling pathways similar to those described for many other nuclear receptor ligands (Losel and Wehling 2003; Losel et al. 2003; Moraes et al. 2007; Ropero et al. 2009). Consistent with this scenario, PPARα activation induced production of cytosolic effectors, such as reactive oxygen species (Ropero et al. 2009; Melis et al. 2008).
These non-transcriptional effects take place also in the CNS and have the potential to regulate neuronal functions on a short timescale. In fact, our recent studies show that in ventral tegmental area (VTA), dopamine neurons OEA and PEA bind to PPARα within the cytosol and trigger a rapid non-genomic mechanism leading to increased endogenous hydrogen peroxide and consequent activation of tyrosine kinase(s) (Melis et al. 2008, 2010). In turn, these tyrosine kinases phosphorylate the β2 subunits of the nicotinic acetylcholine receptors (nAChRs) (Melis et al. 2013b). Phosphorylation of nAChRs is an efficient mechanism to control receptor functions and results in a faster desensitization rate or a downregulation (Huganir and Greengard 1990). As cholinergic afferents control firing rate and burst firing of midbrain dopamine cells, the functional regulation of β2-containing nAChRs (β2*nAChR, the asterisk indicates the presence of other subunits) alters dopaminergic activity. Thus, NAEs as PPARα ligands act as intrinsic modulators of cholinergic transmission and modify dopamine cell excitability, contributing to acetylcholine effects on dopamine system. Moreover, our studies revealed that PPARα-induced regulation of β2*nAChR abolished electrophysiological, neurochemical, and behavioral effects of nicotine (Scherma et al. 2008; Melis and Pistis 2014; Melis et al. 2013a, b; Panlilio et al. 2012). Therefore, enhancing brain levels of PPARα endogenous agonists or activating this receptor with synthetic ligands represents a novel strategy for anti-smoking medications (Melis and Pistis 2014) (see next section).
Noteworthy, this cellular mechanism of action explains why the effects of PPARα activation are specific to nicotine, as it does not attenuate cocaine or cannabinoid-induced electrophysiological or behavioral effects (Justinova et al. 2008, 2015; Luchicchi et al. 2010).
As mentioned above, PPARα activation can be achieved by preventing degradation of endogenous ligands or by promoting their synthesis (Melis et al. 2013a, b). NAE’s synthesis is increased by elevating intracellular Ca2+, as NAPE-PLD is a Ca2+-dependent enzyme (Ueda et al. 2001). In fact, activation of α7-nAChRs, a low-affinity Ca2+-permeable nAChR subunit combination, enhanced midbrain OEA and PEA levels and prevented nicotine-induced excitation of dopamine neurons both in vitro and in vivo (Melis et al. 2013b). Thus, these observations indicate that α7-nAChRs function as sensors in dopamine neurons for excessive cholinergic transmission, which is attenuated by subsequent PPARα activation by endogenous agonists and phosphorylation of β2*nAChRs.
5 Role of NAEs and PPARα in Neuropsychiatric Diseases
5.1 Addiction
In the past two decades, research advances have progressively sustained the idea of addiction as a brain disease (Volkow et al. 2016). In particular, addiction can be defined as a chronic, relapsing disorder of brain reward, motivation, cognition, affective functioning, memory, and related circuitry (American Society of Addiction Medicine, ASAM, 2011). Dysfunction in these pathways leads to a behavioral pathology characterized by compulsive drug seeking and use with progressive loss of control over consumption despite the emergence of significant negative or disadvantageous consequences (see Volkow et al. 2016 and references therein). In the fifth edition of the Diagnostic and Statistical Manual of Mental Disorders (DSM-5) (2013), the term addiction is synonymous with the most severe stage of substance use disorder (see Volkow et al. 2016). Drug addiction represents the most prevalent neuropsychiatric disorder affecting modern society (United Nations Office on Drugs and Crime, 2015). The global health burden attributable to alcohol and illicit drug use amounts to 5.4% of the total burden of diseases (WHO 2003).
A better knowledge of the neurobiological basis underlying addiction is certainly crucial for developing more effective pharmacological and behavioral interventions to counteract harmful consequences of drug use disorders. In this respect, the evidence that NAEs such as OEA and PEA block nicotine-induced excitation of midbrain dopamine neurons by acting on PPARα (Melis et al. 2008) pinpointed their key role in the modulation of the brain reward system and, therefore, in the pathophysiology of nicotine addiction ((Melis et al.2010); reviewed in (Pistis and Melis 2010)). In fact, since dopamine neurons projecting from the VTA to the nucleus accumbens (NAc) act as “reward sensors,” these results offered new perspectives in both understanding and treating tobacco dependence. Accordingly, a study by Scherma et al. (2008) unveiled that boosting brain levels of NAEs reverses abuse-related behavioral and neurochemical effects of nicotine in rats, including those predictive of relapse liability (Scherma et al. 2008). But how does URB597 exert its anti-addictive and anti-rewarding actions? Both the receptor and the mechanism responsible for the effect played by enhanced NAE levels against nicotine were identified soon after (Melis et al. 2008). In particular, it was demonstrated that URB597 counteracts nicotine-induced stimulation of the dopamine system by selectively enhancing intracellular levels of OEA and/or PEA within this circuit and by activating PPARα (Luchicchi et al. 2010; Melis et al. 2008; Mascia et al. 2011). However, even though PPARα was recognized as the receptor accounting for the “anti-nicotine” effect played by NAEs (Melis et al. 2008), a contribution of CB1 receptors activated by anandamide cannot be completely ruled out (Luchicchi et al. 2010; Melis et al. 2008; Mascia et al. 2011).
This wealth of experimental data revealed a novel property of endocannabinoid-like NAEs and suggested that increasing brain levels of OEA and PEA, or activating their receptors (PPARα) with synthetic ligands, might represent a novel and effective approach for achieving smoking cessation (Melis et al. 2013a, b; Melis and Pistis 2014; Scherma et al. 2008). Hence, a new frontier for the treatment of tobacco addiction, the number one preventable cause of death in the developed world, was opened. In keeping with these observations, synthetic PPARα ligands such as fibrates, long-standing clinically available hypolipidemic drugs, were found to suppress (i) nicotine-induced stimulation of dopamine neurons and increased extracellular dopamine levels in the shell of the NAc, which are key features of its acute rewarding properties (Panlilio et al. 2012), and (ii) nicotine intake and reinstatement of nicotine-seeking behavior after a period of prolonged abstinence (Panlilio et al. 2012). Thus, all this evidence has prompted the idea that targeting PPARα represents a promising therapeutic strategy in nicotine relapse prevention in humans and, in the end, for quitting smoking.
So far, anti-addictive actions of PPARα agonists seem to be almost exclusively limited to nicotine in laboratory animals (Justinova et al. 2008, 2015; Luchicchi et al. 2010). The fact that in dopamine neurons PPARα affects the phosphorylation status of β2*nAChRs by activating tyrosine kinase(s) (Melis et al. 2010, 2013b), therefore leading to a reduced excitation of dopamine neurons by nicotinic agonists, might provide a possible explanation for this specificity. Hence, it is not surprising that URB597 does not influence either self-administration of the main psychoactive ingredient of cannabis (i.e., Δ9-tetrahydrocannabinol, Δ9-THC) or cocaine in nonhuman primates (Justinova et al. 2008). Moreover, URB597 is unable to prevent dopamine neuronal responses to morphine or cocaine in rats (Luchicchi et al. 2010). Accordingly, in humans, it has been reported that a natural genetic variation in FAAH, +385 A/A (P129T), is associated with an increased risk of illicit, but not licit (i.e., nicotine, alcohol), drug use problems (Sipe et al. 2002; Tyndale et al. 2007). Notably, this FAAH variant displays a reduced expression and activity in humans, thereby supporting a potential link between enhanced NAE circulating levels and illicit drug abuse and dependence (Chiang et al. 2004).
Most recently, a link of PPARα (and γ) with both alcohol consumption in rodents and withdrawal and dependence in humans emerged (Haile and Kosten 2017; Blednov et al. 2015). Specifically, Blednov et al. (2015) investigated the effects of different classes of PPAR agonists on chronic alcohol intake and preference in mice with a genetic predisposition for high alcohol consumption and then examined human genome-wide association data for polymorphisms in PPAR genes in alcohol-dependent subjects. According to the authors, the observed reduction of alcohol intake in mice and the genetic association between alcohol dependence and withdrawal (according to DSM-5 criteria) in humans underscore the potential for reconsidering clinically approved PPARα or PPARγ agonists for the treatment of alcohol use disorders and alcoholism (Blednov et al. 2015). Consistently, administration of either OEA or synthetic PPARα agonists was lately shown to block cue-induced reinstatement of alcohol-seeking behavior and reduce the severity of somatic withdrawal symptoms in alcohol-dependent rats (Bilbao et al. 2015; Haile and Kosten 2017; Blednov et al. 2016), thus supporting the intriguing possibility of using PPARα as novel therapeutic tool also for alcoholism.
5.2 Epilepsy and Other Neurological Disorders
Epilepsy is a common and prevalent neurological disorder affecting 1–2% of the population worldwide (Thurman et al. 2011). As of 2015, nearly 50 million of people suffer from this disease, characterized by recurrent spontaneous seizures which negatively impact quality of life and respond to medication in about 70% of cases (see for a recent review Varvel et al. 2015). In addition, currently available antiepileptic drugs are mainly symptomatic and have numerous side effects. Thus, an urgent need exists to identify and exploit new, effective therapies. Unraveling the neurobiological mechanisms that subserve the generation of epilepsy will help the development of drugs to modify the disease outcome and, potentially, to prevent epileptogenesis. Intriguingly, the interplay between PPARα and nAChRs shows relevance also for epilepsy and can be exploited as an innovative therapeutic target. Indeed, nAChRs are involved in the pathogenetic mechanisms underlying seizures and epilepsy. In particular, converging evidence from genetic studies in epileptic patients and animal models of seizures demonstrated that nAChR activity is increased in some types of epilepsy, including nocturnal frontal lobe epilepsy (De Fusco et al. 2000; Steinlein et al. 1997; Steinlein 2004; Sutor and Zolles 2001). From this standpoint, negative modulation of nAChRs exerted by endogenous NAEs, or by exogenous ligands of their receptors (PPARα), might represent a potential disease-modifying strategy for epilepsies where nAChRs contribute to neuronal excitation and synchronization. This is particularly relevant considering that, as above mentioned, current therapies, besides from being ineffective in the 30% of patients, are mainly symptomatic and do not necessarily affect the epileptogenic process or the disease progression.
Accordingly, several preclinical studies have shown that selective agonists of PPARα raise seizure thresholds, thus supporting PPARs as potential drug targets for seizure control (Auvin 2012). PEA, for example, proved to have antiepileptic effects in kindled rats (Sheerin et al. 2004) and showed anticonvulsant activity in mice (Lambert et al. 2001). Similarly, fenofibrate, a synthetic PPARα ligand long used as hypolipidemic agent in clinical practice, has been reported to be effective as anticonvulsant in pentylenetetrazole (PTZ)-induced seizures and on latencies to the onset of status epilepticus induced by lithium-pilocarpine in adult rats (Porta et al. 2009). Likewise, acute and chronic PPARα gonists (e.g., fenofibrate) are protective against nicotine-induced seizures and abolished nicotine-induced generation of ictal activity and synchronization in the frontal cortex (Puligheddu et al. 2013). Remarkably, these latter results were paralleled by an increased ratio of phosphorylated/dephosphorylated β2 nAChR subunits in the frontal cortex following acute PPARα ligand treatment, whereas the chronic regimen induced a threefold augment in OEA levels in the same brain region (Puligheddu et al., 2013).
Notably, these observations can also be applied to other models of epilepsy, since PEA was shown to display antiepileptic actions, though via direct PPARα and indirect CB1 activation, in a rat genetic model of absence epilepsy (Citraro et al. 2013) and in DBA/2 mice (Citraro et al. 2016). In addition, a recent study by Saha et al. (2014) reported the anti-kindling effect of the PPARα agonist bezafibrate in PTZ-induced kindling seizure model. Importantly, and not surprisingly, bezafibrate also reduced the neuronal damage and apoptosis in hippocampal areas of the rat brain (Saha et al. 2014).
In this scenario, it is worth to mention that ketogenic diet (KD) proved as efficacious as fenofibrate in exerting anticonvulsive properties in animal models of epilepsy (Porta et al. 2009). The KD is a high-fat, adequate-protein, and low-carbohydrate diet long used as beneficial therapy for refractory epilepsy, especially in children (Bough and Rho 2007). It has been proposed that KD, by increasing fast inhibition in the rat hippocampus dentate gyrus, might protect these neurons from excitotoxicity and, consequently, caused anticonvulsant and anti-epileptogenic effects (Bough et al. 2003). However, even though the KD mechanism of action is still unclear and under investigation (Porta et al. 2009; Bough and Rho 2007), it is tempting to hypothesize the existence of a direct, causal relationship between augmented levels of NAEs and activation of PPARα following KD. In fact, both brain and peripheral tissue levels of NAEs change with high-fat diets (see Pistis and Melis, 2010 and references therein), thus suggesting that these lipid molecules would behave like PPARα agonists (Cullingford 2008) and dampen hippocampal neuronal excitability (Bough et al. 2003). Moreover, circulating levels of different PPARα endogenous ligands (e.g., fatty acids, oxysterols, hormones) might increase following KD and exert themselves the anticonvulsive properties ascribed to this diet (Pistis and Melis 2010).
Whether or not NAEs, as well as KD, have anticonvulsants and anti-epileptogenic effects via activation of PPARα, it is undeniable the physiological role played by these receptors in neuroinflammation and protection from excitotoxicity and, therefore, a link between these phenomena (Pistis and Melis 2010; Melis and Pistis 2014). Hence, considering their multifaceted pharmacological properties (neurotrophic/neuroprotective and anti-inflammatory), PPAR pathways are under evaluation as potential therapeutic targets as well as vulnerability factors in Parkinson’s disease (PD), Alzheimer’s disease (AD), and multiple sclerosis (MS) (Pistis and Melis 2010; Fidaleo et al. 2014). For example, NAEs seem to be directly implicated in the pathogenesis of AD (Pazos et al. 2004), the most common cause of dementia and one of the leading sources of morbidity and mortality in the aging population (Reitz et al. 2011). Accordingly, OEA, PEA, and the NAE-enhancing FAAH inhibitor URB597 increase memory acquisition and consolidation in naïve rats in a PPARα-dependent manner (Mazzola et al. 2009; Campolongo et al. 2009), while PPARγ improve both learning and memory not only in an experimental model of AD (Heneka et al. 2005) but also in AD patients (Risner et al. 2006; Watson et al. 2005; Landreth et al. 2008; Jiang et al. 2008). Importantly, activation of PPARγ is also able to ameliorate AD-related signs by reducing both microglial activation and Aβ plaques (Heneka et al. 2005). In line with these observations, Scuderi et al. (2011, 2012) demonstrated that PEA anti-inflammatory properties are responsible for counteracting Aβ-induced astrogliosis and helped to identify the molecular apparatus by which PEA, via PPARα activation, contributes to downregulate astroglial reaction, pro-inflammatory signal overproduction, and neuronal loss. Next, an elegant in vivo study by D’Agostino et al. (2012) substantiated the fundamental role of PPARα for the neuroprotective and memory-rescuing effects of PEA in an AD animal model (see (Scuderi and Steardo 2013; Mattace Raso et al. 2014) for recent reviews).
On the other hand, MS subjects show reduced levels of NAEs (Di Filippo et al. 2008), thus suggesting the involvement of endocannabinoid system imbalances also in the pathogenesis of MS (Shohami and Mechoulam 2006), the most widespread disabling neurological condition of young adults around the world (see for a recent review Ascherio and Munger 2016). However, it should be pointed out that during MS clinical exacerbations, levels of OEA, PEA, and AEA increased, although being still lower than in controls. This evidence consolidates the hypothesis that NAEs can have a significant impact in reducing inflammation and, therefore, act as endogenous neuroprotective molecules (Di Filippo et al. 2008). In accordance with this, an increase of CNS PEA levels has been observed in a mouse chronic model of MS (Loria et al. 2008), which might be interpreted as an adaptive mechanism to preserve function and integrity during the development of neurodegenerative damage (Mattace Raso et al. 2014). Noteworthy, in the same MS chronic model, PEA administration induced, together with an anti-inflammatory effect, a reduction of motor disability (Loria et al. 2008). Concerning PD, which is among the most prevalent neurodegenerative conditions (Pringsheim et al. 2014), a protective/neurotrophic (and, therefore, potentially therapeutic) role of NAEs has also been demonstrated. In particular, it has been shown that OEA significantly decreased behavioral PD symptoms and exerted neuroprotective effects on the nigrostriatal circuit in an experimental model of the disease through a PPARα-dependent mechanism (Galan-Rodriguez et al. 2009; Gonzalez-Aparicio and Moratalla 2014; Avagliano et al. 2016). Similarly, systemic administration of PEA reduced 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-induced glial cell activation and protected against MPTP-related loss of tyrosine hydroxylase-positive neurons in the substantia nigra pars compacta, two effects that were blunted in PPARα−/− mice. Moreover, chronic administration of PEA reversed MPTP-associated motor deficits, as revealed by the analysis of forepaw step width and percentage of faults (Esposito et al. 2012). The fact that PEA proved to be neuroprotective even when administered after the insult is of particular importance, as the lack of PD biomarkers and the difficulties of early diagnosis make the pharmacotherapy of PD possible only when dopamine neuronal loss is advanced and the first symptoms have appeared (Esposito et al. 2012). Likewise, PPARγ ligands have been reported to arrest PD progression in preclinical settings (Randy and Guoying 2007; Carta 2013; Schintu et al. 2009; Pinto et al. 2016). However, it should be mentioned that a recent study published in The Lancet Neurology (NINDS Exploratory Trials in Parkinson Disease (NET-PD) FS-ZONE Investigators, 2015), which assessed the PPARγ agonist pioglitazone for its disease-modifying potential in early-stage PD, does not support initiation of further trials with this drug (see also Brundin and Wyse 2015).
5.3 Mood Disorders and Schizophrenia
The magnitude, suffering, and burden of mental health disorders in terms of disability and costs for individuals, families, and societies are astonishing. According to WHO’s Global Burden of Disease reports, four of the six primary causes of years lived with disability are due to neuropsychiatric disorders (depression, alcohol use disorders, schizophrenia, and bipolar disorder). In particular, unipolar depression alone leads to 12.15% of years lived with disability and ranks as the third most important contributor to the global burden of diseases (Whiteford et al. 2013 and references therein). Depression is indeed a very common illness worldwide, with an estimated 350 million people affected, and is one of the priority conditions covered by WHO’s Mental Health Gap Action Programme (Reed et al. 2015). Current therapies for this disorder are ineffective in many patients or cause intolerable side effects, thus highlighting the importance of novel, improved therapeutic options. Mesolimbic dopamine system dysfunctions have been implicated in numerous brain disorders, including depression (reviewed in Nestler and Carlezon 2006; Russo and Nestler 2013). Apart from specific anti-inflammatory and neuroprotective mechanisms, it is has been proposed that PPARs may affect mood by interfering with its neurobiological bases (Rolland et al. 2013). Thus, NAEs, as endogenous PPARα ligands and modulators of midbrain dopamine neuronal activity, are well suited to regulate several dopamine-dependent brain physiological and pathological processes. Specifically, NAEs regulate cholinergic input onto dopamine cells and dampen hypercholinergic drive (Fig. 2) and the consequent aberrant excitation of dopamine neurons. This intrinsic negative feedback mechanism might be exploited as a therapeutic strategy in depression, which is characterized also by an unbalance between dopamine and acetylcholine systems (Melis et al. 2013b). Accordingly, experimental evidence suggests that abnormal nicotinic signaling may contribute to depressive symptoms (Mineur and Picciotto 2010; Tizabi et al. 2000) and raises the possibility that β2*nAChR availability could be a biomarker of depression, correlated with severity of the symptoms (see for a review Picciotto et al. 2015). Consistently, a recent human imaging study has revealed that ACh levels are elevated in patients who are actively depressed, as measured by occupancy of nAChRs throughout the brain, and remain high in patients who have a history of depression (Saricicek et al. 2012). In this context, the PPARα agonist fenofibrate (Jiang et al. 2017; Scheggi et al. 2016) or WY-14643 (Yang et al. 2017), PEA (Yu et al. 2011), and FAAH inhibitors show antidepressant-like activity in rodents (Gobbi et al. 2005; Bortolato et al. 2007; Adamczyk et al. 2008; Umathe et al. 2011). In addition, OEA was also lately proven to be effective in ameliorating animal depression-like behaviors, even though through complex mechanisms (Yu et al. 2015; Jin et al. 2015). Similarly, PPARγ agonists (i.e., pioglitazone, rosiglitazone) have displayed some antidepressant-like properties in preclinical settings (Eissa Ahmed and Al-Rasheed 2009; Sadaghiani et al. 2011). Even though it is still unclear whether the PPARγ beneficial effect lays on the NO pathways (Sadaghiani et al. 2011) or whether it is NMDAR-related (Salehi-Sadaghiani et al. 2012), these results encouraged clinical research. In humans, few studies have been carried out so far, the first of which reporting a moderate improvement in two different depression scales in depressed (and insulin-resistant) patients treated with rosiglitazone (Rasgon et al. 2010). Subsequently, randomized controlled trials tested pioglitazone as adjunctive therapy (Sepanjnia et al. 2012) or as monotherapy (Colle et al. 2017) on subjects with a major depressive disorder and demonstrated a significant amelioration in early response, remission, and symptom attenuation for the pioglitazone-treated group. More recently, open-label administration of this PPARγ agonist was found to be associated with improvement of depressive symptoms in bipolar disorder patients (Kemp et al. 2014). Remarkably, a positive correlation has been observed between depressive symptomatology score and change in interleukin (IL)-6, thus indicating that reduction in inflammation might contribute to the mechanism by which pioglitazone modulates mood (Kemp et al. 2014).
Noteworthy, NAEs can also play a role in the etiopathogenesis of schizophrenia (see for a review Rolland et al. 2013), in which substantial dopamine transmission dysfunctions are strongly involved. Briefly, schizophrenia is a severe mental disorder afflicting approximately 1% of the population around the world (which corresponds to more than 21 million people) (see for a recent review Owen et al. 2016). Clinically, schizophrenia is characterized by profound disruptions in thinking that affect language, perception, and the sense of self. The so-called psychotic experiences (or positive symptoms), such as hearing voices or delusions, are the main target of current antipsychotic drugs which all block dopamine D2 receptors (Leucht et al. 2009). In line with this, and with the above discussed observations, PPARα may exert antipsychotic effects by dampening dopamine neuron activity. Data from preclinical and clinical studies seem to support this hypothesis. For example, the PPARα agonist fenofibrate reduces prepulse inhibition disruption in a neurodevelopmental model of schizophrenia, an effect that might be explained by a direct action of fenofibrate on dopaminergic transmission (Rolland et al. 2012). Consistently, PPARα or α7-nAChRs agonists have been demonstrated to beneficial in animal models of schizophrenia (Zanaletti et al. 2012; Rolland et al. 2012; Kucinski et al. 2012; Pichat et al. 2007; Thomsen et al. 2012). Moreover, since the idea is emerging that schizophrenia displays a pro-inflammatory phenotype (e.g., pro-inflammatory cytokines IL-6 and TNF-α are upregulated in obesity, inflammation, and schizophrenia), a recent human genetic study measured expression of prototypic obesogenic and immunogenic genes in peripheral blood cells from controls and schizophrenic patients (Chase et al. 2015). Intriguingly, Chase et al. reported a profound dysregulation of genes relating to metabolic inflammation, including a significant decrease in PPARα mRNA levels and increases in IL-6 and TNF-α, with additional BMI interactions. Finally, a recent (Croatian) population study aimed at determining whether a functional L162V polymorphism in PPARα gene, extensively investigated in the etiology of abnormal lipid and glucose metabolism, was also linked to schizophrenia risk (Nadalin et al. 2014). While the PPARα-L162V polymorphism did not show an association with schizophrenia risk, it impacts clinical expression of the illness and plasma lipid concentrations in female patients (Nadalin et al. 2014). According to the authors, these polymorphisms could have a possible protective effect toward negative symptom severity in female schizophrenic patients.
6 Concluding Remarks
The explosion of research in the field of the endocannabinoid system in the last two decades has fueled an increased interest in lipid messengers. NAEs have many similarities with endocannabinoids: they share synthetic and catabolic pathways but also the logic in signaling mechanism, as they are produced “on demand” and their diffusion and targets are not restricted to the prototypical anterograde signaling that characterizes classical neurotransmitters. Yet, peculiarities are emerging that might render NAEs and their receptors a new Pandora’s box for pharmacologists. NAE signaling in the CNS is engaged under physiological conditions but, more importantly, under several pathological circumstances. Thus, exploiting these targets with novel pharmacological tools will help us to better understand pathophysiological mechanisms in neuropsychiatric disorders and will pave new roads to therapeutic intervention.
References
Adamczyk P, Golda A, McCreary AC, Filip M, Przegalinski E (2008) Activation of endocannabinoid transmission induces antidepressant-like effects in rats. J Physiol Pharmacol 59(2):217–228
American Psychiatric Association (2013) Diagnostic and statistical manual of mental disorders (DSM-5®). American Psychiatric Pub
Ascherio A, Munger KL (2016) Epidemiology of multiple sclerosis: from risk factors to prevention-an update. Semin Neurol 36(2):103–114. doi:10.1055/s-0036-1579693
Auboeuf D, Rieusset J, Fajas L, Vallier P, Frering V, Riou JP, Staels B, Auwerx J, Laville M, Vidal H (1997) Tissue distribution and quantification of the expression of mRNAs of peroxisome proliferator-activated receptors and liver X receptor-alpha in humans: no alteration in adipose tissue of obese and NIDDM patients. Diabetes 46(8):1319–1327
Auvin S (2012) Fatty acid oxidation and epilepsy. Epilepsy Res 100(3):224–228. doi:10.1016/j.eplepsyres.2011.05.022
Avagliano C, Russo R, De Caro C, Cristiano C, La Rana G, Piegari G, Paciello O, Citraro R, Russo E, De Sarro G, Meli R, Mattace Raso G, Calignano A (2016) Palmitoylethanolamide protects mice against 6-OHDA-induced neurotoxicity and endoplasmic reticulum stress: In vivo and in vitro evidence. Pharmacol Res 113(Pt A):276–289. doi:10.1016/j.phrs.2016.09.004
Bachur NR, Masek K, Melmon KL, Udenfriend S (1965) Fatty acid amides of ethanolamine in mammalian tissues. J Biol Chem 240:1019–1024
Baker D, Pryce G, Davies WL, Hiley CR (2006) In silico patent searching reveals a new cannabinoid receptor. Trends Pharmacol Sci 27(1):1–4. doi:10.1016/j.tips.2005.11.003
Balenga NA, Aflaki E, Kargl J, Platzer W, Schroder R, Blattermann S, Kostenis E, Brown AJ, Heinemann A, Waldhoer M (2011) GPR55 regulates cannabinoid 2 receptor-mediated responses in human neutrophils. Cell Res 21(10):1452–1469. doi:10.1038/cr.2011.60
Bensinger SJ, Tontonoz P (2008) Integration of metabolism and inflammation by lipid-activated nuclear receptors. Nature 454(7203):470–477. doi:10.1038/nature07202
Berger J, Moller DE (2002) The mechanisms of action of PPARs. Annu Rev Med 53:409–435
Bilbao A, Serrano A, Cippitelli A, Pavon FJ, Giuffrida A, Suarez J, Garcia-Marchena N, Baixeras E, Gomez de Heras R, Orio L, Alen F, Ciccocioppo R, Cravatt BF, Parsons LH, Piomelli D, Rodriguez de Fonseca F (2015) Role of the satiety factor oleoylethanolamide in alcoholism. Addict Biol. doi:10.1111/adb.12276
Blednov YA, Benavidez JM, Black M, Ferguson LB, Schoenhard GL, Goate AM, Edenberg HJ, Wetherill L, Hesselbrock V, Foroud T, Harris RA (2015) Peroxisome proliferator-activated receptors alpha and gamma are linked with alcohol consumption in mice and withdrawal and dependence in humans. Alcohol Clin Exp Res 39(1):136–145. doi:10.1111/acer.12610
Blednov YA, Black M, Benavidez JM, Stamatakis EE, Harris RA (2016) PPAR Agonists: II. Fenofibrate and Tesaglitazar Alter behaviors related to voluntary alcohol consumption. Alcohol Clin Exp Res 40(3):563–571. doi:10.1111/acer.12972
Bornheim LM, Kim KY, Chen B, Correia MA (1993) The effect of cannabidiol on mouse hepatic microsomal cytochrome P450-dependent anandamide metabolism. Biochem Biophys Res Commun 197(2):740–746
Bortolato M, Mangieri RA, Fu J, Kim JH, Arguello O, Duranti A, Tontini A, Mor M, Tarzia G, Piomelli D (2007) Antidepressant-like activity of the fatty acid amide hydrolase inhibitor URB597 in a rat model of chronic mild stress. Biol Psychiatry 62(10):1103–1110. doi:10.1016/j.biopsych.2006.12.001
Bough KJ, Rho JM (2007) Anticonvulsant mechanisms of the ketogenic diet. Epilepsia 48(1):43–58. doi:10.1111/j.1528-1167.2007.00915.x
Bough KJ, Schwartzkroin PA, Rho JM (2003) Calorie restriction and ketogenic diet diminish neuronal excitability in rat dentate gyrus in vivo. Epilepsia 44(6):752–760. doi:55502 [pii]
Braissant O, Foufelle F, Scotto C, Dauca M, Wahli W (1996) Differential expression of peroxisome proliferator-activated receptors (PPARs): tissue distribution of PPAR-alpha, -beta, and -gamma in the adult rat. Endocrinology 137(1):354–366
Brundin P, Wyse R (2015) Parkinson disease: laying the foundations for disease-modifying therapies in PD. Nat Rev Neurol 11(10):553–555. doi:10.1038/nrneurol.2015.150
Campolongo P, Roozendaal B, Trezza V, Cuomo V, Astarita G, Fu J, McGaugh JL, Piomelli D (2009) Fat-induced satiety factor oleoylethanolamide enhances memory consolidation. Proc Natl Acad Sci U S A 106(19):8027–8031. doi:10.1073/pnas.0903038106
Carta AR (2013) PPAR-gamma: therapeutic prospects in Parkinson’s disease. Curr Drug Targets 14(7):743–751
Chase KA, Rosen C, Gin H, Bjorkquist O, Feiner B, Marvin R, Conrin S, Sharma RP (2015) Metabolic and inflammatory genes in schizophrenia. Psychiatry Res 225(1–2):208–211. doi:10.1016/j.psychres.2014.11.007
Cherif H, Argaw A, Cecyre B, Bouchard A, Gagnon J, Javadi P, Desgent S, Mackie K, Bouchard JF (2015) Role of GPR55 during Axon growth and target innervation(1,2,3). eNeuro 2(5). doi:10.1523/ENEURO.0011-15.2015
Chiang KP, Gerber AL, Sipe JC, Cravatt BF (2004) Reduced cellular expression and activity of the P129T mutant of human fatty acid amide hydrolase: evidence for a link between defects in the endocannabinoid system and problem drug use. Hum Mol Genet 13(18):2113–2119. doi:10.1093/hmg/ddh216
Chinetti G, Griglio S, Antonucci M, Torra IP, Delerive P, Majd Z, Fruchart J-C, Chapman J, Najib J, Staels B (1998) Activation of proliferator-activated receptors alpha and gamma induces apoptosis of human monocyte-derived macrophages. J Biol Chem 273(40):25573–25580. doi:10.1074/jbc.273.40.25573
Citraro R, Russo E, Scicchitano F, van Rijn CM, Cosco D, Avagliano C, Russo R, D’Agostino G, Petrosino S, Guida F, Gatta L, van Luijtelaar G, Maione S, Di Marzo V, Calignano A, De Sarro G (2013) Antiepileptic action of N-palmitoylethanolamine through CB1 and PPAR-alpha receptor activation in a genetic model of absence epilepsy. Neuropharmacology 69:115–126. doi:10.1016/j.neuropharm.2012.11.017
Citraro R, Russo E, Leo A, Russo R, Avagliano C, Navarra M, Calignano A, De Sarro G (2016) Pharmacokinetic-pharmacodynamic influence of N-palmitoylethanolamine, arachidonyl-2’-chloroethylamide and WIN 55,212-2 on the anticonvulsant activity of antiepileptic drugs against audiogenic seizures in DBA/2 mice. Eur J Pharmacol 791:523–534. doi:10.1016/j.ejphar.2016.09.029
Colle R, de Larminat D, Rotenberg S, Hozer F, Hardy P, Verstuyft C, Feve B, Corruble E (2017) Pioglitazone could induce remission in major depression: a meta-analysis. Neuropsychiatr Dis Treat 13:9–16. doi:10.2147/ndt.s121149
Cravatt BF, Giang DK, Mayfield SP, Boger DL, Lerner RA, Gilula NB (1996) Molecular characterization of an enzyme that degrades neuromodulatory fatty-acid amides. Nature 384(6604):83–87
Cristino L, Starowicz K, De Petrocellis L, Morishita J, Ueda N, Guglielmotti V, Di Marzo V (2008) Immunohistochemical localization of anabolic and catabolic enzymes for anandamide and other putative endovanilloids in the hippocampus and cerebellar cortex of the mouse brain. Neuroscience 151(4):955–968. doi:10.1016/j.neuroscience.2007.11.047
Cullingford T (2008) Peroxisome proliferator-activated receptor alpha and the ketogenic diet. Epilepsia 49(Suppl 8):70–72. doi:10.1111/j.1528-1167.2008.01840.x
D’Agostino G, Russo R, Avagliano C, Cristiano C, Meli R, Calignano A (2012) Palmitoylethanolamide protects against the amyloid-beta25-35-induced learning and memory impairment in mice, an experimental model of Alzheimer disease. Neuropsychopharmacology 37(7):1784–1792. doi:10.1038/npp.2012.25
De Fusco M, Becchetti A, Patrignani A, Annesi G, Gambardella A, Quattrone A, Ballabio A, Wanke E, Casari G (2000) The nicotinic receptor beta 2 subunit is mutant in nocturnal frontal lobe epilepsy. Nat Genet 26 (3):275-276. doi:10.1038/81566
Deliu E, Sperow M, Console-Bram L, Carter RL, Tilley DG, Kalamarides DJ, Kirby LG, Brailoiu GC, Brailoiu E, Benamar K, Abood ME (2015) The lysophosphatidylinositol receptor GPR55 modulates pain perception in the periaqueductal Gray. Mol Pharmacol 88(2):265–272. doi:10.1124/mol.115.099333
Desvergne B, Wahli W (1999) Peroxisome proliferator-activated receptors: nuclear control of metabolism. Endocr Rev 20(5):649–688. doi:10.1210/er.20.5.649
Deutsch DG, Ueda N, Yamamoto S (2002) The fatty acid amide hydrolase (FAAH). Prostaglandins Leukot Essent Fatty Acids 66(2-3):201–210. doi:10.1054/plef.2001.0358
Devane WA, Hanus L, Breuer A, Pertwee RG, Stevenson LA, Griffin G, Gibson D, Mandelbaum A, Etinger A, Mechoulam R (1992) Isolation and structure of a brain constituent that binds to the cannabinoid receptor. Science 258(5090):1946–1949
Devchand PR, Keller H, Peters JM, Vazquez M, Gonzalez FJ, Wahli W (1996) The PPARalpha-leukotriene B4 pathway to inflammation control. Nature 384(6604):39–43. doi:10.1038/384039a0
Di Filippo M, Pini LA, Pelliccioli GP, Calabresi P, Sarchielli P (2008) Abnormalities in the cerebrospinal fluid levels of endocannabinoids in multiple sclerosis. J Neurol Neurosurg Psychiatry 79(11):1224–1229. doi:10.1136/jnnp.2007.139071
Diep TA, Madsen AN, Holst B, Kristiansen MM, Wellner N, Hansen SH, Hansen HS (2011) Dietary fat decreases intestinal levels of the anorectic lipids through a fat sensor. FASEB J 25(2):765–774. doi:10.1096/fj.10-166595
Dreyer C, Krey G, Keller H, Givel F, Helftenbein G, Wahli W (1992) Control of the peroxisomal beta-oxidation pathway by a novel family of nuclear hormone receptors. Cell 68(5):879–887. 0092-8674(92)90031-7 [pii]
Egertova M, Simon GM, Cravatt BF, Elphick MR (2008) Localization of N-acyl phosphatidylethanolamine phospholipase D (NAPE-PLD) expression in mouse brain: a new perspective on N-acylethanolamines as neural signaling molecules. J Comp Neurol 506(4):604–615. doi:10.1002/cne.21568
Eissa Ahmed AA, Al-Rasheed NM (2009) Antidepressant-like effects of rosiglitazone, a PPARgamma agonist, in the rat forced swim and mouse tail suspension tests. Behav Pharmacol 20(7):635–642. doi:10.1097/FBP.0b013e328331b9bf
Escriva H, Langlois MC, Mendonca RL, Pierce R, Laudet V (1998) Evolution and diversification of the nuclear receptor superfamily. Ann N Y Acad Sci 839:143–146
Esposito E, Impellizzeri D, Mazzon E, Paterniti I, Cuzzocrea S (2012) Neuroprotective activities of palmitoylethanolamide in an animal model of Parkinson’s disease. PloS One 7(8):e41880. doi:10.1371/journal.pone.0041880
Ferre P (2004) The biology of peroxisome proliferator-activated receptors: relationship with lipid metabolism and insulin sensitivity. Diabetes 53(Suppl 1):S43–S50
Fidaleo M, Fanelli F, Ceru MP, Moreno S (2014) Neuroprotective properties of peroxisome proliferator-activated receptor alpha (PPARalpha) and its lipid ligands. Curr Med Chem 21(24):2803–2821
Fu J, Gaetani S, Oveisi F, Lo Verme J, Serrano A, Rodriguez De Fonseca F, Rosengarth A, Luecke H, Di Giacomo B, Tarzia G, Piomelli D (2003) Oleylethanolamide regulates feeding and body weight through activation of the nuclear receptor PPAR-alpha. Nature 425(6953):90–93
Fu J, Oveisi F, Gaetani S, Lin E, Piomelli D (2005) Oleoylethanolamide, an endogenous PPAR-alpha agonist, lowers body weight and hyperlipidemia in obese rats. Neuropharmacology 48(8):1147–1153
Galan-Rodriguez B, Suarez J, Gonzalez-Aparicio R, Bermudez-Silva FJ, Maldonado R, Robledo P, Rodriguez de Fonseca F, Fernandez-Espejo E (2009) Oleoylethanolamide exerts partial and dose-dependent neuroprotection of substantia nigra dopamine neurons. Neuropharmacology 56(3):653–664
Gardner OS, Dewar BJ, Graves LM (2005) Activation of mitogen-activated protein kinases by peroxisome proliferator-activated receptor ligands: an example of nongenomic signaling. Mol Pharmacol 68(4):933–941
Germain P, Staels B, Dacquet C, Spedding M, Laudet V (2006) Overview of nomenclature of nuclear receptors. Pharmacol Rev 58(4):685–704. doi:10.1124/pr.58.4.2
Gervois P, Kleemann R, Pilon A, Percevault F, Koenig W, Staels B, Kooistra T (2004) Global suppression of IL-6-induced acute phase response gene expression after chronic in vivo treatment with the peroxisome proliferator-activated receptor-alpha activator fenofibrate. J Biol Chem 279(16):16154–16160. doi:10.1074/jbc.M400346200
Glass CK, Ogawa S (2006) Combinatorial roles of nuclear receptors in inflammation and immunity. Nat Rev Immunol 6(1):44–55. doi:10.1038/nri1748
Gobbi G, Bambico FR, Mangieri R, Bortolato M, Campolongo P, Solinas M, Cassano T, Morgese MG, Debonnel G, Duranti A, Tontini A, Tarzia G, Mor M, Trezza V, Goldberg SR, Cuomo V, Piomelli D (2005) Antidepressant-like activity and modulation of brain monoaminergic transmission by blockade of anandamide hydrolysis. PNAS 102(51):18620–18625
Gonzalez-Aparicio R, Moratalla R (2014) Oleoylethanolamide reduces L-DOPA-induced dyskinesia via TRPV1 receptor in a mouse model of Parkinson s disease. Neurobiol Dis 62:416–425. doi:10.1016/j.nbd.2013.10.008
Graves RA, Tontonoz P, Spiegelman BM (1992) Analysis of a tissue-specific enhancer: ARF6 regulates adipogenic gene expression. Mol Cell Biol 12(3):1202–1208
Haile CN, Kosten TA (2017) The peroxisome proliferator-activated receptor alpha agonist fenofibrate attenuates alcohol self-administration in rats. Neuropharmacology 116:364–370. doi:10.1016/j.neuropharm.2017.01.007
Hampson AJ, Hill WA, Zan-Phillips M, Makriyannis A, Leung E, Eglen RM, Bornheim LM (1995) Anandamide hydroxylation by brain lipoxygenase: metabolite structures and potencies at the cannabinoid receptor. Biochim Biophys Acta 1259(2):173–179
Hansen HS (2010) Palmitoylethanolamide and other anandamide congeners. Proposed role in the diseased brain. Exp Neurol 224(1):48–55. doi:10.1016/j.expneurol.2010.03.022
Hansen HS (2014) Role of anorectic N-acylethanolamines in intestinal physiology and satiety control with respect to dietary fat. Pharmacol Res 86:18–25. doi:10.1016/j.phrs.2014.03.006
Hansen HS, Diep TA (2009) N-acylethanolamines, anandamide and food intake. Biochem Pharmacol. doi:10.1016/j.bcp.2009.04.024
Hansen HS, Moesgaard B, Hansen HH, Petersen G (2000) N-Acylethanolamines and precursor phospholipids – relation to cell injury. Chem Phys Lipids 108(1–2):135–150. S0009308400001924 [pii]
Heneka MT, Sastre M, Dumitrescu-Ozimek L, Hanke A, Dewachter I, Kuiperi C, O'Banion K, Klockgether T, Van Leuven F, Landreth GE (2005) Acute treatment with the PPARgamma agonist pioglitazone and ibuprofen reduces glial inflammation and Abeta1-42 levels in APPV717I transgenic mice. Brain 128(Pt 6):1442–1453. doi:10.1093/brain/awh452
Hess R, Staubli W, Riess W (1965) Nature of the hepatomegalic effect produced by ethyl-chlorophenoxy-isobutyrate in the rat. Nature 208(5013):856–858
Huganir RL, Greengard P (1990) Regulation of neurotransmitter receptor desensitization by protein phosphorylation. Neuron 5(5):555–567
Issemann I, Green S (1990) Activation of a member of the steroid hormone receptor superfamily by peroxisome proliferators. Nature 347(6294):645–650. doi:10.1038/347645a0
Jiang Q, Heneka M, Landreth GE (2008) The role of peroxisome proliferator-activated receptor-gamma (PPARgamma) in Alzheimer’s disease: therapeutic implications. CNS Drugs 22(1):1–14
Jiang B, Wang YJ, Wang H, Song L, Huang C, Zhu Q, Wu F, Zhang W (2017) Antidepressant-like effects of fenofibrate in mice via the hippocampal brain-derived neurotrophic factor signalling pathway. Br J Pharmacol 174(2):177–194. doi:10.1111/bph.13668
Jin P, HL Y, Tian L, Zhang F, Quan ZS (2015) Antidepressant-like effects of oleoylethanolamide in a mouse model of chronic unpredictable mild stress. Pharmacol Biochem Behav 133:146–154. doi:10.1016/j.pbb.2015.04.001
Justinova Z, Mangieri RA, Bortolato M, Chefer SI, Mukhin AG, Clapper JR, King AR, Redhi GH, Yasar S, Piomelli D, Goldberg SR (2008) Fatty acid amide hydrolase inhibition heightens anandamide signaling without producing reinforcing effects in primates. Biol Psychiatry 64(11):930–937. doi:10.1016/j.biopsych.2008.08.008
Justinova Z, Panlilio LV, Moreno-Sanz G, Redhi GH, Auber A, Secci ME, Mascia P, Bandiera T, Armirotti A, Bertorelli R, Chefer SI, Barnes C, Yasar S, Piomelli D, Goldberg SR (2015) Effects of fatty acid amide hydrolase (FAAH) inhibitors in non-human primate models of nicotine reward and relapse. Neuropsychopharmacology 40(9):2185–2197. doi:10.1038/npp.2015.62
Kapur A, Zhao P, Sharir H, Bai Y, Caron MG, Barak LS, Abood ME (2009) Atypical responsiveness of the orphan receptor GPR55 to cannabinoid ligands. J Biol Chem 284(43):29817–29827. doi:10.1074/jbc.M109.050187
Kemp DE, Schinagle M, Gao K, Conroy C, Ganocy SJ, Ismail-Beigi F, Calabrese JR (2014) PPAR-gamma agonism as a modulator of mood: proof-of-concept for pioglitazone in bipolar depression. CNS Drugs 28(6):571–581. doi:10.1007/s40263-014-0158-2
Kilaru A, Isaac G, Tamura P, Baxter D, Duncan SR, Venables BJ, Welti R, Koulen P, Chapman KD (2010) Lipid profiling reveals tissue-specific differences for ethanolamide lipids in mice lacking fatty acid amide hydrolase. Lipids 45(9):863–875. doi:10.1007/s11745-010-3457-5
Kozak KR, Crews BC, Morrow JD, Wang LH, Ma YH, Weinander R, Jakobsson PJ, Marnett LJ (2002) Metabolism of the endocannabinoids, 2-arachidonylglycerol and anandamide, into prostaglandin, thromboxane, and prostacyclin glycerol esters and ethanolamides. J Biol Chem 277(47):44877–44885. doi:10.1074/jbc.M206788200
Kucinski A, Syposs C, Wersinger S, Bencherif M, Stachowiak MK, Stachowiak EK (2012) Alpha7 neuronal nicotinic receptor agonist (TC-7020) reverses increased striatal dopamine release during acoustic PPI testing in a transgenic mouse model of schizophrenia. Schizophr Res 136(1–3):82–87
Kuehl FA, Jacob TA, Ganley OH, Ormond RE, Meisinger MAP (1957) The identification of N-(2-hydroxyethyl)-palmitamide as a naturally occurring anti-inflammatory agent. J Am Chem Soc 79(20):5577–5578. doi:10.1021/ja01577a066
Lambert DM, Vandevoorde S, Diependaele G, Govaerts SJ, Robert AR (2001) Anticonvulsant activity of N-palmitoylethanolamide, a putative endocannabinoid, in mice. Epilepsia 42(3):321–327
Landreth G, Jiang Q, Mandrekar S, Heneka M (2008) PPARgamma agonists as therapeutics for the treatment of Alzheimer’s disease. Neurotherapeutics 5(3):481–489. doi:10.1016/j.nurt.2008.05.003. S1933-7213(08)00092-5 [pii]
Laudet V, Hanni C, Coll J, Catzeflis F, Stehelin D (1992) Evolution of the nuclear receptor gene superfamily. EMBO J 11(3):1003–1013
Lefebvre P, Chinetti G, Fruchart JC, Staels B (2006) Sorting out the roles of PPAR alpha in energy metabolism and vascular homeostasis. J Clin Invest 116(3):571–580. doi:10.1172/JCI27989
Leucht S, Corves C, Arbter D, Engel RR, Li C, Davis JM (2009) Second-generation versus first-generation antipsychotic drugs for schizophrenia: a meta-analysis. Lancet 373(9657):31–41. doi:10.1016/S0140-6736(08)61764-X
Leung D, Saghatelian A, Simon GM, Cravatt BF (2006) Inactivation of N-acyl phosphatidylethanolamine phospholipase D reveals multiple mechanisms for the biosynthesis of endocannabinoids. Biochemistry (Mosc) 45(15):4720–4726. doi:10.1021/bi060163l
Lo Verme J, Fu J, Astarita G, La Rana G, Russo R, Calignano A, Piomelli D (2005) The nuclear receptor peroxisome proliferator-activated receptor-alpha mediates the anti-inflammatory actions of palmitoylethanolamide. Mol Pharmacol 67(1):15–19. doi:10.1124/mol.104.006353
Loria F, Petrosino S, Mestre L, Spagnolo A, Correa F, Hernangomez M, Guaza C, Di Marzo V, Docagne F (2008) Study of the regulation of the endocannabinoid system in a virus model of multiple sclerosis reveals a therapeutic effect of palmitoylethanolamide. Eur J Neurosci 28(4):633–641. doi:10.1111/j.1460-9568.2008.06377.x
Losel R, Wehling M (2003) Nongenomic actions of steroid hormones. Nat Rev Mol Cell Biol 4(1):46–56
Losel RM, Falkenstein E, Feuring M, Schultz A, Tillmann HC, Rossol-Haseroth K, Wehling M (2003) Nongenomic steroid action: controversies, questions, and answers. Physiol Rev 83(3):965–1016
Luchicchi A, Lecca S, Carta S, Pillolla G, Muntoni AL, Yasar S, Goldberg SR, Pistis M (2010) Effects of fatty acid amide hydrolase inhibition on neuronal responses to nicotine, cocaine and morphine in the nucleus accumbens shell and ventral tegmental area: involvement of PPAR-alpha nuclear receptors. Addict Biol 15(3):277–288. doi:10.1111/j.1369-1600.2010.00222.x
Mandard S, Muller M, Kersten S (2004) Peroxisome proliferator-activated receptor alpha target genes. Cell Mol Life Sci 61(4):393–416. doi:10.1007/s00018-003-3216-3
Mascia P, Pistis M, Justinova Z, Panlilio LV, Luchicchi A, Lecca S, Scherma M, Fratta W, Fadda P, Barnes C, Redhi GH, Yasar S, Le Foll B, Tanda G, Piomelli D, Goldberg SR (2011) Blockade of nicotine reward and reinstatement by activation of alpha-type peroxisome proliferator-activated receptors. Biol Psychiatry 69(7):633–641. doi:10.1016/j.biopsych.2010.07.009
Mattace Raso G, Russo R, Calignano A, Meli R (2014) Palmitoylethanolamide in CNS health and disease. Pharmacol Res 86:32–41. doi:10.1016/j.phrs.2014.05.006
Mazzola C, Medalie J, Scherma M, Panlilio LV, Solinas M, Tanda G, Drago F, Cadet JL, Goldberg SR, Yasar S (2009) Fatty acid amide hydrolase (FAAH) inhibition enhances memory acquisition through activation of PPAR-alpha nuclear receptors. Learn Mem 16 (5):332-337. doi:16/5/332 [pii] 10.1101/lm.1145209
Melis M, Pistis M (2014) Targeting the interaction between fatty acid ethanolamides and nicotinic receptors: therapeutic perspectives. Pharmacol Res 86:42–49. doi:10.1016/j.phrs.2014.03.009
Melis M, Pillolla G, Luchicchi A, Muntoni AL, Yasar S, Goldberg SR, Pistis M (2008) Endogenous fatty acid ethanolamides suppress nicotine-induced activation of mesolimbic dopamine neurons through nuclear receptors. J Neurosci 28(51):13985–13994
Melis M, Carta S, Fattore L, Tolu S, Yasar S, Goldberg SR, Fratta W, Maskos U, Pistis M (2010) Peroxisome proliferator-activated receptors-alpha modulate dopamine cell activity through nicotinic receptors. Biol Psychiatry 68(3):256–264. doi:10.1016/j.biopsych.2010.04.016
Melis M, Carta G, Pistis M, Banni S (2013a) Physiological role of peroxisome proliferator-activated receptors type alpha on dopamine systems. CNS Neurol Disord Drug Targets 12(1):70–77
Melis M, Scheggi S, Carta G, Madeddu C, Lecca S, Luchicchi A, Cadeddu F, Frau R, Fattore L, Fadda P, Ennas MG, Castelli MP, Fratta W, Schilstrom B, Banni S, De Montis MG, Pistis M (2013b) PPARalpha regulates cholinergic-driven activity of midbrain dopamine neurons via a novel mechanism involving alpha7 nicotinic acetylcholine receptors. J Neurosci 33(14):6203–6211. doi:10.1523/JNEUROSCI.4647-12.2013
Mineur YS, Picciotto MR (2010) Nicotine receptors and depression: revisiting and revising the cholinergic hypothesis. Trends Pharmacol Sci 31(12):580–586. doi:10.1016/j.tips.2010.09.004
Moraes LA, Swales KE, Wray JA, Damazo A, Gibbins JM, Warner TD, Bishop-Bailey D (2007) Nongenomic signaling of the retinoid X receptor through binding and inhibiting Gq in human platelets. Blood 109(9):3741–3744
Moran BM, Abdel-Wahab YH, Flatt PR, McKillop AM (2014) Activation of GPR119 by fatty acid agonists augments insulin release from clonal beta-cells and isolated pancreatic islets and improves glucose tolerance in mice. Biol Chem 395(4):453–464. doi:10.1515/hsz-2013-0255
Moreno S, Farioli-Vecchioli S, Ceru MP (2004) Immunolocalization of peroxisome proliferator-activated receptors and retinoid X receptors in the adult rat CNS. Neuroscience 123(1):131–145
Morishita J, Okamoto Y, Tsuboi K, Ueno M, Sakamoto H, Maekawa N, Ueda N (2005) Regional distribution and age-dependent expression of N-acylphosphatidylethanolamine-hydrolyzing phospholipase D in rat brain. J Neurochem 94(3):753–762. doi:10.1111/j.1471-4159.2005.03234.x
Nadalin S, Giacometti J, Buretic-Tomljanovic A (2014) PPARalpha-L162V polymorphism is not associated with schizophrenia risk in a Croatian population. Prostaglandins Leukot Essent Fatty Acids 91(5):221–225. doi:10.1016/j.plefa.2014.07.003
Nestler EJ, Carlezon WA Jr (2006) The mesolimbic dopamine reward circuit in depression. Biol Psychiatry 59(12):1151–1159
Nyilas R, Dudok B, Urban GM, Mackie K, Watanabe M, Cravatt BF, Freund TF, Katona I (2008) Enzymatic machinery for endocannabinoid biosynthesis associated with calcium stores in glutamatergic axon terminals. J Neurosci 28(5):1058–1063. doi:10.1523/JNEUROSCI.5102-07.2008
Oka S, Nakajima K, Yamashita A, Kishimoto S, Sugiura T (2007) Identification of GPR55 as a lysophosphatidylinositol receptor. Biochem Biophys Res Commun 362(4):928–934. doi:10.1016/j.bbrc.2007.08.078
Okamoto Y, Morishita J, Tsuboi K, Tonai T, Ueda N (2004) Molecular characterization of a phospholipase D generating anandamide and its congeners. J Biol Chem 279(7):5298–5305
Owen MJ, Sawa A, Mortensen PB (2016) Schizophrenia. Lancet doi:10.1016/S0140-6736(15)01121-6
Panlilio LV, Justinova Z, Mascia P, Pistis M, Luchicchi A, Lecca S, Barnes C, Redhi GH, Adair J, Heishman SJ, Yasar S, Aliczki M, Haller J, Goldberg SR (2012) Novel use of a lipid-lowering fibrate medication to prevent nicotine reward and relapse: preclinical findings. Neuropsychopharmacology 37(8):1838–1847. doi:10.1038/npp.2012.31
Pazos MR, Nunez E, Benito C, Tolon RM, Romero J (2004) Role of the endocannabinoid system in Alzheimer’s disease: new perspectives. Life Sci 75(16):1907–1915. doi:10.1016/j.lfs.2004.03.026. S0024-3205(04)00560-0 [pii]
Petrosino S, Iuvone T, Di Marzo V (2010) N-palmitoyl-ethanolamine: Biochemistry and new therapeutic opportunities. Biochimie 92(6):724–727. doi:10.1016/j.biochi.2010.01.006
Picciotto MR, Lewis AS, van Schalkwyk GI, Mineur YS (2015) Mood and anxiety regulation by nicotinic acetylcholine receptors: a potential pathway to modulate aggression and related behavioral states. Neuropharmacology 96(Pt B):235–243. doi:10.1016/j.neuropharm.2014.12.028
Pichat P, Bergis OE, Terranova JP, Urani A, Duarte C, Santucci V, Gueudet C, Voltz C, Steinberg R, Stemmelin J, Oury-Donat F, Avenet P, Griebel G, Scatton B (2007) SSR180711, a novel selective alpha7 nicotinic receptor partial agonist: (II) efficacy in experimental models predictive of activity against cognitive symptoms of schizophrenia. Neuropsychopharmacology 32(1):17–34. doi:10.1038/sj.npp.1301188
Pietr M, Kozela E, Levy R, Rimmerman N, Lin YH, Stella N, Vogel Z, Juknat A (2009) Differential changes in GPR55 during microglial cell activation. FEBS Lett 583(12):2071–2076. doi:10.1016/j.febslet.2009.05.028
Pinto M, Nissanka N, Peralta S, Brambilla R, Diaz F, Moraes CT (2016) Pioglitazone ameliorates the phenotype of a novel Parkinson’s disease mouse model by reducing neuroinflammation. Mol Neurodegener 11:25. doi:10.1186/s13024-016-0090-7
Piomelli D (2013) A fatty gut feeling. Trends Endocrinol Metab 24(7):332–341. doi:10.1016/j.tem.2013.03.001
Pistis M, Melis M (2010) From surface to nuclear receptors: the endocannabinoid family extends its assets. Curr Med Chem 17(14):1450–1467
Porta N, Vallee L, Lecointe C, Bouchaert E, Staels B, Bordet R, Auvin S (2009) Fenofibrate, a peroxisome proliferator-activated receptor-alpha agonist, exerts anticonvulsive properties. Epilepsia 50(4):943–948. doi:10.1111/j.1528-1167.2008.01901.x
Pringsheim T, Jette N, Frolkis A, Steeves TD (2014) The prevalence of Parkinson’s disease: a systematic review and meta-analysis. Mov Disord 29(13):1583–1590. doi:10.1002/mds.25945
Puligheddu M, Pillolla G, Melis M, Lecca S, Marrosu F, De Montis MG, Scheggi S, Carta G, Murru E, Aroni S, Muntoni AL, Pistis M (2013) PPAR-alpha agonists as novel antiepileptic drugs: preclinical findings. PloS One 8(5):e64541. doi:10.1371/journal.pone.0064541
Rahman IA, Tsuboi K, Uyama T, Ueda N (2014) New players in the fatty acyl ethanolamide metabolism. Pharmacol Res 86:1–10. doi:10.1016/j.phrs.2014.04.001
Randy LH, Guoying B (2007) Agonism of peroxisome proliferator receptor-gamma may have therapeutic potential for neuroinflammation and Parkinson’s disease. Curr Neuropharmacol 5(1):35–46
Rasgon NL, Kenna HA, Williams KE, Powers B, Wroolie T, Schatzberg AF (2010) Rosiglitazone add-on in treatment of depressed patients with insulin resistance: a pilot study. Sci World J 10:321–328. doi:10.1100/tsw.2010.32
Reed GM, Rebello TJ, Pike KM, Medina-Mora ME, Gureje O, Zhao M, Dai Y, Roberts MC, Maruta T, Matsumoto C, Krasnov VN, Kulygina M, Lovell AM, Stona AC, Sharan P, Robles R, Gaebel W, Zielasek J, Khoury B, de Jesus Mari J, Luis Ayuso-Mateos J, Evans SC, Kogan CS, Saxena S (2015) WHO’s global clinical practice network for mental health. Lancet Psychiatry 2(5):379–380. doi:10.1016/S2215-0366(15)00183-2
Reguero L, Puente N, Elezgarai I, Ramos-Uriarte A, Gerrikagoitia I, Bueno-Lopez JL, Donate F, Grandes P (2014) Subcellular localization of NAPE-PLD and DAGL-alpha in the ventromedial nucleus of the hypothalamus by a preembedding immunogold method. Histochem Cell Biol 141(5):543–550. doi:10.1007/s00418-013-1174-x
Reitz C, Brayne C, Mayeux R (2011) Epidemiology of Alzheimer disease. Nat Rev Neurol 7(3):137–152. doi:10.1038/nrneurol.2011.2
Risner ME, Saunders AM, Altman JF, Ormandy GC, Craft S, Foley IM, Zvartau-Hind ME, Hosford DA, Roses AD (2006) Efficacy of rosiglitazone in a genetically defined population with mild-to-moderate Alzheimer’s disease. Pharmacogenomics J 6(4):246–254. doi:10.1038/sj.tpj.6500369
Rodriguez de Fonseca F, Navarro M, Gomez R, Escuredo L, Nava F, Fu J, Murillo-Rodriguez E, Giuffrida A, LoVerme J, Gaetani S, Kathuria S, Gall C, Piomelli D (2001) An anorexic lipid mediator regulated by feeding. Nature 414(6860):209–212
Rolland B, Marche K, Cottencin O, Bordet R (2012) The PPARalpha agonist fenofibrate reduces prepulse inhibition disruption in a neurodevelopmental model of Schizophrenia. Schizophr Res Treat 2012:839853. doi:10.1155/2012/839853
Rolland B, Deguil J, Jardri R, Cottencin O, Thomas P, Bordet R (2013) Therapeutic prospects of PPARs in psychiatric disorders: a comprehensive review. Curr Drug Targets 14(7):724–732
Ropero AB, Juan-Pico P, Rafacho A, Fuentes E, Bermudez-Silva FJ, Roche E, Quesada I, de Fonseca FR, Nadal A (2009) Rapid non-genomic regulation of Ca2+ signals and insulin secretion by PPAR alpha ligands in mouse pancreatic islets of Langerhans. J Endocrinol 200(2):127–138
Rowley JW, Oler AJ, Tolley ND, Hunter BN, Low EN, Nix DA, Yost CC, Zimmerman GA, Weyrich AS (2011) Genome-wide RNA-seq analysis of human and mouse platelet transcriptomes. Blood 118(14):e101–e111. doi:10.1182/blood-2011-03-339705
Russo SJ, Nestler EJ (2013) The brain reward circuitry in mood disorders. Nat Rev Neurosci 14(9):609–625. doi:10.1038/nrn3381
Ryberg E, Larsson N, Sjogren S, Hjorth S, Hermansson NO, Leonova J, Elebring T, Nilsson K, Drmota T, Greasley PJ (2007) The orphan receptor GPR55 is a novel cannabinoid receptor. Br J Pharmacol 152(7):1092–1101. doi:10.1038/sj.bjp.0707460
Sadaghiani MS, Javadi-Paydar M, Gharedaghi MH, Fard YY, Dehpour AR (2011) Antidepressant-like effect of pioglitazone in the forced swimming test in mice: the role of PPAR-gamma receptor and nitric oxide pathway. Behav Brain Res 224(2):336–343. doi:10.1016/j.bbr.2011.06.011
Saha L, Bhandari S, Bhatia A, Banerjee D, Chakrabarti A (2014) Anti-kindling effect of bezafibrate, a peroxisome proliferator-activated receptors alpha agonist, in pentylenetetrazole induced kindling seizure model. J Epilepsy Res 4(2):45–54
Salehi-Sadaghiani M, Javadi-Paydar M, Gharedaghi MH, Zandieh A, Heydarpour P, Yousefzadeh-Fard Y, Dehpour AR (2012) NMDA receptor involvement in antidepressant-like effect of pioglitazone in the forced swimming test in mice. Psychopharmacology (Berl) 223(3):345–355. doi:10.1007/s00213-012-2722-0
Saricicek A, Esterlis I, Maloney KH, Mineur YS, Ruf BM, Muralidharan A, Chen JI, Cosgrove KP, Kerestes R, Ghose S, Tamminga CA, Pittman B, Bois F, Tamagnan G, Seibyl J, Picciotto MR, Staley JK, Bhagwagar Z (2012) Persistent beta2*-nicotinic acetylcholinergic receptor dysfunction in major depressive disorder. Am J Psychiatry 169(8):851–859. doi:10.1176/appi.ajp.2012.11101546
Sawzdargo M, Nguyen T, Lee DK, Lynch KR, Cheng R, Heng HH, George SR, O’Dowd BF (1999) Identification and cloning of three novel human G protein-coupled receptor genes GPR52, PsiGPR53 and GPR55: GPR55 is extensively expressed in human brain. Brain Res Mol Brain Res 64(2):193–198
Scheggi S, Melis M, De Felice M, Aroni S, Muntoni AL, Pelliccia T, Gambarana C, De Montis MG, Pistis M (2016) PPARalpha modulation of mesolimbic dopamine transmission rescues depression-related behaviors. Neuropharmacology 110(Pt A):251–259. doi:10.1016/j.neuropharm.2016.07.024
Scherma M, Panlilio LV, Fadda P, Fattore L, Gamaleddin I, Le Foll B, Justinova Z, Mikics E, Haller J, Medalie J, Stroik J, Barnes C, Yasar S, Tanda G, Piomelli D, Fratta W, Goldberg SR (2008) Inhibition of anandamide hydrolysis by URB597 reverses abuse-related behavioral and neurochemical effects of nicotine in rats. J Pharmacol Exp Ther. doi:10.1124/jpet.108.142224
Scherma M, Muntoni AL, Melis M, Fattore L, Fadda P, Fratta W, Pistis M (2016) Interactions between the endocannabinoid and nicotinic cholinergic systems: preclinical evidence and therapeutic perspectives. Psychopharmacology (Berl). doi:10.1007/s00213-015-4196-3
Schintu N, Frau L, Ibba M, Caboni P, Garau A, Carboni E, Carta AR (2009) PPAR-gamma-mediated neuroprotection in a chronic mouse model of Parkinson’s disease. Eur J Neurosci 29(5):954–963
Schmid HH, Schmid PC, Natarajan V (1990) N-acylated glycerophospholipids and their derivatives. Prog Lipid Res 29(1):1–43
Schmidt A, Endo N, Rutledge SJ, Vogel R, Shinar D, Rodan GA (1992) Identification of a new member of the steroid hormone receptor superfamily that is activated by a peroxisome proliferator and fatty acids. Mol Endocrinol 6(10):1634–1641
Scuderi C, Steardo L (2013) Neuroglial roots of neurodegenerative diseases: therapeutic potential of palmitoylethanolamide in models of Alzheimer’s disease. CNS Neurol Disord Drug Targets 12(1):62–69
Scuderi C, Esposito G, Blasio A, Valenza M, Arietti P, Steardo L, Jr., Carnuccio R, De Filippis D, Petrosino S, Iuvone T, Di Marzo V, Steardo L (2011) Palmitoylethanolamide counteracts reactive astrogliosis induced by beta-amyloid peptide. J Cell Mol Med 15 (12):2664-2674. doi:10.1111/j.1582-4934.2011.01267.x
Scuderi C, Valenza M, Stecca C, Esposito G, Carratu MR, Steardo L (2012) Palmitoylethanolamide exerts neuroprotective effects in mixed neuroglial cultures and organotypic hippocampal slices via peroxisome proliferator-activated receptor-alpha. J Neuroinflamm 9:49. doi:10.1186/1742-2094-9-21
Sepanjnia K, Modabbernia A, Ashrafi M, Modabbernia MJ, Akhondzadeh S (2012) Pioglitazone adjunctive therapy for moderate-to-severe major depressive disorder: randomized double-blind placebo-controlled trial. Neuropsychopharmacology 37(9):2093–2100. doi:10.1038/npp.2012.58
Sheerin AH, Zhang X, Saucier DM, Corcoran ME (2004) Selective antiepileptic effects of N-palmitoylethanolamide, a putative endocannabinoid. Epilepsia 45(10):1184–1188. doi:10.1111/j.0013-9580.2004.16604.x
Sher T, Yi HF, McBride OW, Gonzalez FJ (1993) cDNA cloning, chromosomal mapping, and functional characterization of the human peroxisome proliferator activated receptor. Biochemistry (Mosc) 32(21):5598–5604
Sheu MY, Fowler AJ, Kao J, Schmuth M, Schoonjans K, Auwerx J, Fluhr JW, Man MQ, Elias PM, Feingold KR (2002) Topical peroxisome proliferator activated receptor-alpha activators reduce inflammation in irritant and allergic contact dermatitis models. J Invest Dermatol 118(1):94–101. doi:10.1046/j.0022-202x.2001.01626.x
Shohami E, Mechoulam R (2006) Multiple sclerosis may disrupt endocannabinoid brain protection mechanism. Proc Natl Acad Sci U S A 103(16):6087–6088
Simon GM, Cravatt BF (2010) Characterization of mice lacking candidate N-acyl ethanolamine biosynthetic enzymes provides evidence for multiple pathways that contribute to endocannabinoid production in vivo. Mol Biosyst 6(8):1411–1418. doi:10.1039/c000237b
Sipe JC, Chiang K, Gerber AL, Beutler E, Cravatt BF (2002) A missense mutation in human fatty acid amide hydrolase associated with problem drug use. Proc Natl Acad Sci U S A 99(12):8394–8399. doi:10.1073/pnas.08223579999/12/8394. [pii]
Staton PC, Hatcher JP, Walker DJ, Morrison AD, Shapland EM, Hughes JP, Chong E, Mander PK, Green PJ, Billinton A, Fulleylove M, Lancaster HC, Smith JC, Bailey LT, Wise A, Brown AJ, Richardson JC, Chessell IP (2008) The putative cannabinoid receptor GPR55 plays a role in mechanical hyperalgesia associated with inflammatory and neuropathic pain. Pain 139(1):225–236. doi:10.1016/j.pain.2008.04.006
Steinlein OK (2004) Genetic mechanisms that underlie epilepsy. Nat Rev Neurosci 5 (5):400-408. doi:10.1038/nrn1388 nrn1388 [pii]
Steinlein OK, Magnusson A, Stoodt J, Bertrand S, Weiland S, Berkovic SF, Nakken KO, Propping P, Bertrand D (1997) An insertion mutation of the CHRNA4 gene in a family with autosomal dominant nocturnal frontal lobe epilepsy. Hum Mol Genet 6(6):943–947
Straus DS, Glass CK (2007) Anti-inflammatory actions of PPAR ligands: new insights on cellular and molecular mechanisms. Trends Immunol 28(12):551–558. doi:10.1016/j.it.2007.09.003
Suarez J, Bermudez-Silva FJ, Mackie K, Ledent C, Zimmer A, Cravatt BF, de Fonseca FR (2008) Immunohistochemical description of the endogenous cannabinoid system in the rat cerebellum and functionally related nuclei. J Comp Neurol 509(4):400–421. doi:10.1002/cne.21774
Sutor B, Zolles G (2001) Neuronal nicotinic acetylcholine receptors and autosomal dominant nocturnal frontal lobe epilepsy: a critical review. Pflugers Arch 442(5):642–651
Terrazzino S, Berto F, Dalle Carbonare M, Fabris M, Guiotto A, Bernardini D, Leon A (2004) Stearoylethanolamide exerts anorexic effects in mice via down-regulation of liver stearoyl-coenzyme A desaturase-1 mRNA expression. FASEB J 18(13):1580–1582. doi:10.1096/fj.03-1080fje
Thomsen MS, Hansen HH, Timmerman DB, Mikkelsen JD (2012) Cognitive improvement by activation of alpha7 nicotinic acetylcholine receptors: from animal models to human pathophysiology. Curr Pharm Des 16(3):323–343
Thorp JM, Waring WS (1962) Modification of metabolism and distribution of lipids by ethyl chlorophenoxyisobutyrate. Nature 194:948–949
Thurman DJ, Beghi E, Begley CE, Berg AT, Buchhalter JR, Ding D, Hesdorffer DC, Hauser WA, Kazis L, Kobau R, Kroner B, Labiner D, Liow K, Logroscino G, Medina MT, Newton CR, Parko K, Paschal A, Preux PM, Sander JW, Selassie A, Theodore W, Tomson T, Wiebe S (2011) Standards for epidemiologic studies and surveillance of epilepsy. Epilepsia 52(Suppl 7):2–26. doi:10.1111/j.1528-1167.2011.03121.x
Tizabi Y, Rezvani AH, Russell LT, Tyler KY, Overstreet DH (2000) Depressive characteristics of FSL rats: involvement of central nicotinic receptors. Pharmacol Biochem Behav 66(1):73–77
Tsuboi K, Sun YX, Okamoto Y, Araki N, Tonai T, Ueda N (2005) Molecular characterization of N-acylethanolamine-hydrolyzing acid amidase, a novel member of the choloylglycine hydrolase family with structural and functional similarity to acid ceramidase. J Biol Chem 280(12):11082–11092. doi:10.1074/jbc.M413473200
Tsuboi K, Takezaki N, Ueda N (2007a) The N-acylethanolamine-hydrolyzing acid amidase (NAAA). Chem Biodivers 4(8):1914–1925. doi:10.1002/cbdv.200790159
Tsuboi K, Zhao LY, Okamoto Y, Araki N, Ueno M, Sakamoto H, Ueda N (2007b) Predominant expression of lysosomal N-acylethanolamine-hydrolyzing acid amidase in macrophages revealed by immunochemical studies. Biochim Biophys Acta 1771(5):623–632. doi:10.1016/j.bbalip.2007.03.005
Tsuboi K, Okamoto Y, Ikematsu N, Inoue M, Shimizu Y, Uyama T, Wang J, Deutsch DG, Burns MP, Ulloa NM, Tokumura A, Ueda N (2011) Enzymatic formation of N-acylethanolamines from N-acylethanolamine plasmalogen through N-acylphosphatidylethanolamine-hydrolyzing phospholipase D-dependent and -independent pathways. Biochim Biophys Acta 1811(10):565–577. doi:10.1016/j.bbalip.2011.07.009
Tyndale RF, Payne JI, Gerber AL, Sipe JC (2007) The fatty acid amide hydrolase C385A (P129T) missense variant in cannabis users: studies of drug use and dependence in Caucasians. Am J Med Genet B Neuropsychiatr Genet 144B(5):660–666. doi:10.1002/ajmg.b.30491
Ueda N, Yamamoto K, Yamamoto S, Tokunaga T, Shirakawa E, Shinkai H, Ogawa M, Sato T, Kudo I, Inoue K et al (1995) Lipoxygenase-catalyzed oxygenation of arachidonylethanolamide, a cannabinoid receptor agonist. Biochim Biophys Acta 1254(2):127–134
Ueda N, Liu Q, Yamanaka K (2001) Marked activation of the N-acylphosphatidylethanolamine-hydrolyzing phosphodiesterase by divalent cations. Biochim Biophys Acta 1532(1-2):121–127
Ueda N, Tsuboi K, Uyama T (2010a) Enzymological studies on the biosynthesis of N-acylethanolamines. Biochim Biophys Acta (BBA) – Mol Cell Biol Lipids 1801(12):1274–1285. doi:10.1016/j.bbalip.2010.08.010
Ueda N, Tsuboi K, Uyama T (2010b) N-acylethanolamine metabolism with special reference to N-acylethanolamine-hydrolyzing acid amidase (NAAA). Prog Lipid Res 49(4):299–315. doi:10.1016/j.plipres.2010.02.003
Umathe SN, Manna SS, Jain NS (2011) Involvement of endocannabinoids in antidepressant and anti-compulsive effect of fluoxetine in mice. Behav Brain Res 223(1):125–134. doi:10.1016/j.bbr.2011.04.031
Varvel NH, Jiang J, Dingledine R (2015) Candidate drug targets for prevention or modification of epilepsy. Annu Rev Pharmacol Toxicol 55:229–247. doi:10.1146/annurev-pharmtox-010814-124607
Volkow ND, Koob GF, McLellan AT (2016) Neurobiologic advances from the brain disease model of addiction. N Engl J Med 374(4):363–371. doi:10.1056/NEJMra1511480
Wangensteen T, Akselsen H, Holmen J, Undlien D, Retterstøl L (2010) A common haplotype in NAPEPLD is associated with severe obesity in a norwegian population-based cohort (the HUNT study). Obesity 19(3):612–617. doi:10.1038/oby.2010.219
Watson GS, Cholerton BA, Reger MA, Baker LD, Plymate SR, Asthana S, Fishel MA, Kulstad JJ, Green PS, Cook DG, Kahn SE, Keeling ML, Craft S (2005) Preserved cognition in patients with early Alzheimer disease and amnestic mild cognitive impairment during treatment with rosiglitazone: a preliminary study. Am J Geriatr Psychiatry 13(11):950–958. doi:10.1176/appi.ajgp.13.11.950
Whiteford HA, Ferrari AJ, Baxter AJ, Charlson FJ, Degenhardt L (2013) How did we arrive at burden of disease estimates for mental and illicit drug use disorders in the Global Burden of Disease Study 2010? Curr Opin Psychiatry 26(4):376–383. doi:10.1097/YCO.0b013e328361e60f
World Health Organization (2003) Investing in mental health
Xu J, Xiao G, Trujillo C, Chang V, Blanco L, Joseph SB, Bassilian S, Saad MF, Tontonoz P, Lee WN, Kurland IJ (2002) Peroxisome proliferator-activated receptor alpha (PPARalpha) influences substrate utilization for hepatic glucose production. J Biol Chem 277(52):50237–50244. doi:10.1074/jbc.M201208200
Yang R, Wang P, Chen Z, Hu W, Gong Y, Zhang W, Huang C (2017) WY-14643, a selective agonist of peroxisome proliferator-activated receptor-alpha, ameliorates lipopolysaccharide-induced depressive-like behaviors by preventing neuroinflammation and oxido-nitrosative stress in mice. Pharmacol Biochem Behav 153:97–104. doi:10.1016/j.pbb.2016.12.010
Yu M, Ives D, Ramesha CS (1997) Synthesis of prostaglandin E2 ethanolamide from anandamide by cyclooxygenase-2. J Biol Chem 272(34):21181–21186
Yu HL, Deng XQ, Li YJ, Li YC, Quan ZS, Sun XY (2011) N-palmitoylethanolamide, an endocannabinoid, exhibits antidepressant effects in the forced swim test and the tail suspension test in mice. Pharmacol Rep 63(3):834–839
Yu HL, Sun LP, Li MM, Quan ZS (2015) Involvement of norepinephrine and serotonin system in antidepressant-like effects of oleoylethanolamide in the mice models of behavior despair. Neurosci Lett 593:24–28. doi:10.1016/j.neulet.2015.03.019
Zanaletti R, Bettinetti L, Castaldo C, Cocconcelli G, Comery T, Dunlop J, Gaviraghi G, Ghiron C, Haydar SN, Jow F, Maccari L, Micco I, Nencini A, Scali C, Turlizzi E, Valacchi M (2012) Discovery of a novel alpha-7 nicotinic acetylcholine receptor agonist series and characterization of the potent, selective, and orally efficacious agonist 5-(4-acetyl[1,4]diazepan-1-yl)pentanoic acid [5-(4-methoxyphenyl)-1H-pyrazol-3-yl] amide (SEN15924, WAY-361789). J Med Chem 55(10):4806–4823
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 Springer International Publishing AG
About this chapter
Cite this chapter
Pistis, M., Muntoni, A.L. (2017). Roles of N-Acylethanolamines in Brain Functions and Neuropsychiatric Diseases. In: Melis, M. (eds) Endocannabinoids and Lipid Mediators in Brain Functions. Springer, Cham. https://doi.org/10.1007/978-3-319-57371-7_11
Download citation
DOI: https://doi.org/10.1007/978-3-319-57371-7_11
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-57369-4
Online ISBN: 978-3-319-57371-7
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)