Abstract
The reciprocal induction and worsening relationship between hypertension (HTN) and chronic kidney disease (CKD) is well known and investigated for a long time. Increased arterial stiffness is a major nontraditional cardiovascular risk factor in CKD reflecting the difficulty of the large arteries to convert flow oscillations into continuous blood flow due to significant morphological changes induced by this pathological condition. Surrogate markers of cardiovascular disease used in CKD work-up include ankle-brachial index, carotid ultrasound (assessing carotid intima-media thickness and plaque), aortic pulse wave velocity, and the echocardiography quantification of the subclinical hypertensive heart disease (e.g., left ventricular mass, diastolic dysfunction). Current data support the idea that the integration of clinical characteristics (accurate measurement of blood pressure, 24-h ambulatory blood pressure monitoring, etc.) with information derived from arterial stiffness assessment may represent an accurate and cost-effective approach for individualizing CKD and HTN patients’ care and treatment.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
“First, the chicken or the egg” dilemma can be also identified in the relationship between hypertension (HTN) and chronic kidney disease (CKD), two growing worldwide health problems. In an epidemiological, cross-sectional, multicenter study (MULTIRISC) carried out in outpatient clinics belonging to cardiology, internal medicine, and endocrinology departments which defined CKD as an estimated glomerular filtration rate (eGFR) below 60 mL/min per 1.73 m2, from 2608 patients 62.7% did not have CKD, 18.9% had “established” CKD (in addition, the serum creatinine level was ≥1.3 mg/dL in men or ≥1.2 mg/dL in women), and 18.4% had “occult” CKD (the creatinine level was lower) [1]. When the eGFR decreased below 45 mL/min/1.73m2, mortality from cardiovascular disease increases more than threefold [2]. Within this binomial relationships has had to produce a significant change in mind-set for finding a solution to the problem how to motivate nephrologists to think more “cardiac” and cardiologists to think more “renal” this issue, making departmental barriers more permeable: the evaluation of renal function should be part of the work-up of patients with cardiovascular disease, and all patients with kidney disease should be assessed for cardiovascular disease [3].
Modern techniques to measure blood pressure (BP) were described more than 115 years ago starting with Scipione Riva Rocci mercury sphygmomanometer , but the features of the BP curve have highlighted other important goals, that is, the specific roles of pulse pressure (PP) , arterial stiffness, pulse wave velocity (PWV) , and wave reflections as potentially deleterious factors affecting the progression of HTN and CKD [4]. Furthermore, the level to which BP should be lowered is still controversial: below 125/7 5 mmHg among those with CKD and more than 1 g proteinuria (Joint National Commission-6 guidelines), below 130/80 mmHg among patients with CKD who are not on dialysis (Joint National Commission-7 guidelines), and a goal of less than 150/90 mmHg for hypertensive persons aged 60 years or older and for hypertensive persons 30–59 years of age to a diastolic goal of less than 90 mmHg with less evidence in hypertensive persons younger than 60 years for the systolic goal or in those younger than 30 years for the diastolic goal , a situation where the recommendation is BP of less than 140/90 mmHg (Joint National Commission-8 guidelines) [5, 6]. The same thresholds and goals are now recommended for hypertensive adults with diabetes or nondiabetic chronic kidney disease (CKD) as for the general hypertensive population younger than 60 years [6]. A full 60% of these recommendations were based on expert opinion, while just 10% were based on clinical trial evidence [7]. The available clinical trials targeted BP measured in the clinic but whose values are different from the real physiopathological changes : a meta-analysis using 24-h ambulatory BP monitoring shows that approximately 20% of patients with CKD have white-coat hypertension and about 5–10% have masked hypertension [8].
Surrogate markers of cardiovascular disease used in CKD work-up (mainly, for improvement of the risk stratification) include ankle–brachial index (clinical tool for gross estimation of obstruction in major-vessel lumen caliber), carotid ultrasound (assessing carotid intima-media thickness (IMT) and plaque – focal wall thickening by at least 50% of the surrounding IMT), aortic pulse wave velocity (reproducible evaluation of large-artery stiffness, using applanation tonometry, oscillometric pulse recognition algorithms, magnetic resonance imaging, or echo-tracking to measure diameter in end diastole and stroke change in diameter with a very high precision), and the echocardiography quantification of the subclinical hypertensive heart disease (e.g., left ventricular mass, diastolic dysfunction) [9].
Increased arterial stiffness is a major nontraditional cardiovascular risk factor in CKD reflecting the difficulty of the large arteries to convert flow oscillations into continuous blood flow due to fibroelastic intimal thickening, calcification of elastic lamellae, increased extracellular matrix, and extra collagen content [10]. Normally, by stretching, the arterial wall accumulates the elastic energy (aprox. 10% of the energy produced by the heart is stored in the large artery walls by their distension) that maintains the blood flow during diastole when the ejection phase is over (“Windkessel effect ”) [10].
Arteries become stiffer in physiological (aging) or pathological (hypertension, diabetes mellitus, and CKD) conditions. The “stiffness gradient” disappears, or a “stiffness mismatch ” occurs (increased central elastic artery stiffness combined with a decrease in peripheral muscular artery stiffness) leading to the reversal of the physiological stiffness gradient and promoting end-organ damages through increased forward pressure wave transmission into the microcirculation [11]. Renal dysfunction has been shown to increase arterial stiffness via several mechanisms , including vascular calcification, chronic volume overload, inflammation, endothelial dysfunction (maladapted endothelial phenotype characterized by reduced nitric oxide (NO) bioavailability, increased oxidative stress, elevated expression of proinflammatory and prothrombotic factors, and reduced endothelial-derived vasodilation), oxidative stress (inducing vascular wall remodeling, intrinsic changes in SMC stiffness, and aortic SMC apoptosis), and overproduction of uric acid [12]. Increased T helper secretion of cytokines, chemokines, and growth factors leads to an inflammatory process and may lead to fragmentation of elastic membranes and destruction of cell-protective matrix layers. Decreased turnover of collagen and elastin, increased advanced glycation end products (AGEs), and matrix metalloproteinase (MMP) (involved in the regulation of the structural integrity of the extracellular matrix – ECM) cross-links have been also demonstrated in vascular stiffening [12].
Noninvasive arterial testing for cardiovascular risk assessment providing a means for early detection of presymptomatic vascular disease that has been used to identify patients with subclinical atherosclerosis are arterial ultrasonography and measurements of arterial stiffness.
Flow-mediated dilatation (FMD) assessed by high-resolution ultrasonography of the brachial artery is considered a biomarker of endothelial function. Arterial vasodilatation in response to shear stress produced by increased flow is mediated predominantly by endothelium-derived nitric oxide. Impaired brachial artery dilatation to sublingually administered nitroglycerin is an “endothelium-independent” response that reflects arterial smooth muscle function. Relative disadvantages of this technique are that it is not easier to perform, requires a skilled operator with an appropriate training period, and these intrinsic difficulties make it more likely to be used in clinical research and not in individual evaluation [13].
Thickness of carotid artery intima and media (carotid IMT) can be measured optimally noninvasively by high-resolution ultrasonography with automated computerized edge-detection software and intravascular contrast agents that may decrease variability and improve precision [13].
Measurements of arterial stiffness include central pulse pressure/stroke volume index , pulse wave velocity (PWV) , total arterial compliance , pulse pressure amplification , and augmentation index [14]. Two measures of arterial stiffness have been studied: the velocity of arterial pulse wave transmission across an arterial segment and the analysis of the arterial waveforms to estimate augmentation of systolic pressure by peripheral wave reflection [13]. As suggested by the European Society of Hypertension (ESH) /European Society of Cardiology (ESC) guidelines for the management of arterial hypertension, the measurement that is most widely used among the direct or indirect methods proposed to quantify arterial stiffness (as a tool for the assessment of subclinical target organ damage) is the propagative model based on PWV measurement, introduced in physiology (the “elastic” properties of the arterial wall determine the velocity of pulse wave propagation ) by Bramwell and Hill (1922) [10, 12]. European Network for Non-invasive Investigation of Large Arteries position statement clarifies that arterial stiffness and central pressure measurements should be considered as recommended tests for the evaluation of cardiovascular risk, particularly in patients in whom target organ damage is not discovered by routine investigations [14]. Current methods for measuring arterial stiffness are carotid–femoral PWV (with predictive value for CV events and requires little technical expertise), central pulse wave analysis (with predictive value in patients with ESRD, hypertension, and CAD, provides additional information concerning wave reflections, and requires little technical expertise), and local arterial stiffness (with certain predictive value for CV events, is indicated for mechanistic analyses in research field, and requires a higher level of technical expertise) [14].
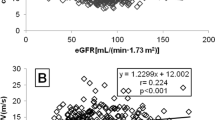
Typical values of PWV in the aorta range from approximately 5 to >15 m/s. A fixed threshold value (12 m/s) was proposed based on published epidemiological studies [15]. Aortic pulse wave velocity (PWV) is an estimate of the distance the pulse wave travels in the aorta and an estimate of the time that distance is traversed, the result (expressed in meters per second) being obtained by dividing the distance (usually expressed in millimeters) by the time (usually expressed in milliseconds) [9]. Three main arterial sites can be evaluated, mainly the aortic trunk (carotid–femoral) and the upper (carotid–brachial) and lower (femoral–dorsalis pedis) limbs. The “gold standard” method remains carotid–femoral PWV (cf-PWV) [14, 15]; brachial–ankle PWV (ba-PWV) , a related technique based on oscillations in cuffs placed on the brachial artery and calf, is popular in Asia because it avoids exposing the groin, but the pulse wave pathway is still being discussed and its validity is still contested [12]. Indirect techniques use aortic characteristic impedance (the minimal impedance for higher frequencies of pressure-and-flow harmonics at the aortic root that is proportional to PWV, but its reliability is reduced due to the difficulty of obtaining trustworthy noninvasive data for aortic flow and pressure) and the rigidity estimates derived from BP measurement (e.g., ABPM-derived arterial stiffness index or crude brachial PP) [12].
Aortic PWV is a research tool useful as a marker of vascular risk when measured once in a population that is followed-up longitudinally and as outcome predictor when measuring longitudinal changes after intervention, showing the degree of loss of kidney function (stiffness of the aorta increases with decreasing kidney function) [9].
Several factors in addition to age, diabetes, and hypertension affect aortic PWV, including decreasing kidney function (microalbuminuria and proteinuria), glucose concentration, heart rate, sex, vascular calcification, and left ventricular hypertrophy (LVH). It has been already demonstrated that there is an independent association between arterial stiffness indices, PWV and augmentation index (Aix – % of pulse pressure), and severely increased albuminuria in nondiabetic, hypertensive patients with CKD stages 1–2 treated with renin–angiotensin–aldosterone system blockers [16]. The aortic–brachial arterial stiffness mismatch was strongly and independently associated with increased mortality in dialysis population, proving that arterial stiffness is also the strongest risk factor for cardiovascular disease in end-stage renal patients [17, 18].
We must be aware that the pulsatile nature of the central hemodynamics may have a deleterious impact on vital organs and increased aortic pulse pressure causes renal microvascular damage through altered renal hemodynamics resulting from increased peripheral resistance and/or increased flow pulsation, as indicated by the result from a study on 133 patients with hypertension where pressure waveforms were recorded on the radial, carotid, femoral, and dorsalis pedis arteries with applanation tonometry to estimate the aortic pressures and aortic (carotid–femoral) and peripheral (carotid–radial and femoral–dorsalis pedis) pulse wave velocities [19]. The renal resistive index , defined as [1 – (end-diastolic velocity/peak systolic velocity)], was strongly correlated with the aortic pulse pressure, incident pressure wave, augmented pressure, and aortic pulse wave velocity, although not with the mean arterial pressure or peripheral pulse wave velocities. Moreover, each 0.1 increase in renal resistive index was associated with a 5.4-fold increase in the adjusted relative risk of albuminuria [19].
Non-dipping nocturnal feature at 24-h ambulatory blood pressure monitoring (ABPM) (defined as a fall in nocturnal BP of <10%) is typically found in CKD and is associated with disease progression, but also as glomerular filtration rate declines, reverse dipping (nighttime BP readings that are higher than those during the day) becomes more apparent [20]. For renal protection there is a need for newer treatments in CKD (e.g., selective ETA blocking drugs) that will not only lower BP beyond the levels achieved with standard therapies but also favorably affect the 24-h profile of BP and arterial stiffness. To increase reproducibility of the results, the circadian BP pattern by 48-h ABPM was assessed in 10,271 hypertensive patients with and without CKD (5506 men/4765 women), 58.0 ± 14.2 years old, enrolled in the Hygia Project. The largest difference between groups was in the prevalence of the riser BP pattern (i.e., asleep SBP mean greater than awake SBP mean) in patients with and without CKD, respectively (17.6% vs. 7.1%; p < 0.001), significantly and progressively increased from 8.1% among those with stage 1 CKD to a very high 34.9% of those with stage 5 CKD. Prevalence of the riser BP pattern , associated with highest CVD risk among all possible BP patterns, was 2.5-fold more prevalent in CKD and up to fivefold more prevalent in end-stage renal disease. A blunted sleep-time BP decline, a characteristic of the non-dipping pattern, is common in patients with CKD. These findings indicate that CKD should be included among the clinical conditions for which ABPM is mandatory for proper diagnosis, CVD risk assessment, and the therapeutic regimen evaluation [21].
Ambulatory arterial stiffness index (AASI) is a parameter derived from the regression slope of the diastolic on systolic blood pressure, using all of the readings during ambulatory blood pressure monitoring (ABPM) . AASI was significantly higher in CKD group, positively correlated to age and pulse pressure, and negatively correlated to nocturnal BP fall [22].
In hypertensive CKD patients, seric uric acid was correlated with the two indices of arterial stiffness, PWV and Aix (augmentation index adjusted for heart rate), with sex-specific variations. However, seric uric acid was associated independently with only Aix, but not with PWV, in the entire patient population and only in men [23].
Work-up for hypertension and CKD patient (Fig. 9.1) starts by identifying the concomitant conditions (age, diabetes mellitus, obesity) often associated with resistant hypertension. Older patients and patients with chronic kidney disease are particularly susceptible to salt intake; in diabetes the insulin resistance increases sympathetic nervous activity, vascular smooth muscle cell proliferation, and sodium retention; obesity is associated with an increased sympathetic activity, higher cardiac output, and a rise in peripheral vascular resistance due to reduced endothelium-dependent vasodilation; plasma aldosterone and endothelin are also increased, while excessive surrounding adipose tissue results in increased intrarenal pressures and changes in renal architecture [24]. We continue with the clinical evaluation and classification of each of these associate diseases: for hypertension based on ESH/ESC classification (blood pressure level and risk factors, asymptomatic organ damage or disease) and for CKD on KDIGO (Kidney Disease: Improving Global Outcomes) classification based on estimated glomerular filtration rate (eGFR) and urine albumin/creatinine ratio (ACR) categories. These two simple tests allow asserting the diagnosis of CKD irrespective of the etiology: urinary albumin/creatinine ratio (ACR) more than 30 mg/g and eGFR, as measured by the Modification of Diet in Renal Diseases (MDRD) Study equation, less than 60 mL/min/1.73m2 on at least two different occasions over 3 or more months. An accurate BP measurement is necessary and mandatory to avoid, for example, a “pseudoresistant hypertension” diagnosis : technical faults are related to not letting the patient rest at minimum of 5 min before measurement and using a small cuff (the cuff’s air bladder must encircle at least 80% of the arm circumference); the average of two readings taken a minute apart represents the patient’s blood pressure [24]. The correlation between BP level and target-organ damage, cardiovascular disease (CVD) risk, and long-term prognosis is greater for ambulatory than clinic BP. In addition to determining the usual mean BP values (awake, asleep, or 24 h), employed to diagnose hypertension based on ambulatory BP monitoring (ABPM) , some specific features of the 24-h BP pattern have been assessed, among these is a blunted sleep-time BP decline, a characteristic of the non-dipping pattern, being common in patients with CKD [21]. Certainly, the target organs of hypertension are the three, well-known musketeers, the heart, brain, and kidneys, but we often forget the fourth musketeer, missing, by the way, from Dumas’s book title too: the arteries. Identification of alterations in arterial function and structure may help refine cardiovascular risk assessment and labeling candidates for an aggressive therapy [13]. Ultrasound-derived carotid intima-media thickness (IMT) is considered a surrogate for systemic atherosclerotic disease burden, and carotid–femoral PWV (cf-PWV) is considered as the “gold standard” measurement of arterial stiffness, independently associated with glomerular filtration rate .
Work-up for hypertension and CKD patient . HTN hypertension, CKD chronic kidney disease, BP blood pressure, eGFR estimated glomerular flow rate, ACR albumin/creatinine ratio, ABPM ambulatory blood pressure monitoring, US ultrasonography, IMT intima-media thickness, PWV pulse wave velocity, CV cardiovascular
Further clinical trials are required for assessing the value of “destiffening ” the aorta distinct from blood pressure reduction and to confirm the predictive value of arterial stiffness and wave reflection for the reduction in CV events in the long-term intervention studies [9].
Current data support the idea that the integration of demographic and clinical characteristics with information derived from arterial stiffness assessment may represent an accurate and cost-effective approach for individualizing CKD and HTN patients’ care and treatment [25].
Agents that modulate mineral metabolism abnormalities (a noncalcium-containing phosphate binder – sevelamer, cinacalcet) and lipid-lowering agents (atorvastatin) may positively affect arterial stiffness [25].
Pharmacological strategies to date have included:
-
Progressive withdrawal of alpha-blocking agents
-
Efficacy of beta-blockers for coronary prevention
-
The use of angiotensin blockade in HTN with glomerular injury, using angiotensin-converting enzyme inhibition or receptor blockade (first-line therapeutic intervention), as mono- but never double-blockade, to avoid major complications [7]
-
Development of combination therapies with diuretics and/or calcium channel blockers [4]
Specific interventions, such as renin–angiotensin-system blockade, the use of statins, and decrease of calcium–phosphate product, may delay the progression of degeneration process in CKD patients.
Postural hypotension should be monitored closely, particularly in elderly, diabetics, and patients with arterial stiffness.
The level of albuminuria/proteinuria has become the principal criterion on which to stratify target blood pressure, irrespective of CKD stage.
Perspectives
Aortic stiffness is independently and significantly associated with progressive renal impairment in hypertensive patients with CKD irrespective of the stage, as a measure of arterial damage, and after the standardization of the measurement protocols and quality control procedures and risk-defining threshold values were established, this should be regarded as part of clinical cardiovascular risk stratification algorithms and target of future intervention studies.
References
Amenos AC, Gonzalez-Juanatey JR, Gutierrez PC, Gilarranz AM, Costae CG. Prevalence of chronic kidney disease in patients with or at a high risk of cardiovascular disease. Rev Esp Cardiol. 2010;63(2):225–8.
Hallan SI, Dahl K, Oien CM, et al. Screening strategies for chronic kidney disease in the general population: follow-up of cross sectional health survey. BMJ. 2006;333(7577):1047.
Kes P, Milicić D, Basić-Jukić N. How to motivate nephrologists to think more “cardiac” and cardiologists to think more “renal”? Acta Med Croatica. 2011;65(Suppl 3):85–9.
Kheder-Elfekih R, Yannoutsos A, Blacher J, London GM, Safar ME. Hypertension and chronic kidney disease: respective contribution of mean and pulse pressure and arterial stiffness. J Hypertens. 2015;31:[Epub ahead of print].
Agarwal R, Martinez-Castelao A, Wiecek A, Massy Z, Suleymanlar G, Ortiz A, Blankestijn PJ, Covic A, Dekker FW, Jager KJ, Lindholm B, Goldsmith D, Fliser D, London G, Zoccali C. EUropean REnal and CArdiovascular medicine working group of the European renal association–European dialysis and transplant association (ERA–EDTA). The lingering dilemma of arterial pressure in CKD: what do we know, where do we go? Kidney Int Suppl. 2011;1(1):17–20.
James PA, Oparil S, Carter BL, Cushman WC, Dennison-Himmelfarb C, Handler J, Lackland DT, LeFevre ML, MacKenzie TD, Ogedegbe O, Smith SC Jr, Svetkey LP, Taler SJ, Townsend RR, Wright JT Jr, Narva AS, Ortiz E, MD. 2014 evidence-based guideline for the management of high blood pressure in adults report from the panel members appointed to the eighth Joint National Committee (JNC 8). JAMA. 2014;311(5):507–20.
Covic A, Goldsmith D, Donciu M-D, Siriopol D, Popa R, Kanbay M, London G. From profusion to confusion: the Saga of managing hypertension in chronic kidney disease! J Clin Hypertens. 2015;17(6):421–7.
Bangash F, Agarwal R. Masked hypertension and white-coat hypertension in chronic kidney disease: a meta-analysis. Clin J Am Soc Nephrol. 2009;4(3):656–64.
Rubin MF, Rosas SE, Chirinos JA, Townsend RR. Surrogate markers of cardiovascular disease in CKD: what’s under the hood? Am J Kidney Dis. 2011;57(3):488–97.
Covic A, Siriopol D. Pulse wave velocity ratio: the new “gold standard” for measuring arterial stiffness. Hypertension. 2015;65(2):289–90.
Boutouyrie P, Fliser D, Goldsmith D, Covic A, Wiecek A, Ortiz A, et al. Assessment of arterial stiffness for clinical and epidemiological studies: methodological considerations for validation and entry into the European renal and cardiovascular medicine registry. Nephrol Dial Transplant. 2014;29(2):232–9.
Jia G, Aroor AR, Sowers JR. Arterial stiffness: a nexus between cardiac and renal disease. Cardiorenal Med. 2014;4:60–71.
Kullo IJ, Malik AR. Arterial ultrasonography and tonometry as adjuncts to cardiovascular risk stratification. J Am Coll Cardiol. 2007;49(13):1413–26.
Laurent S, Cockcroft J, Van Bortel L, Boutouyrie P, Giannattasio C, Hayoz D, Pannier B, Vlachopoulos C, Wilkinson I, Struijker-Boudier H, on behalf of the European Network for Non-invasive Investigation of Large Arteries. Expert consensus document on arterial stiffness: methodological issues and clinical applications. Eur Heart J. 2006;27(21):2588–605.
Reference Values for Arterial Stiffness’ Collaboration. Determinants of pulse wave velocity in healthy people and in the presence of cardiovascular risk factors: ‘establishing normal and reference values’. Eur Heart J. 2010;31(19):2338–50.
Kalaitzidis RG, Karasavvidou DP, Tatsioni A, Pappas K, Katatsis G, Liontos A, Elisaf MS. Albuminuria as a marker of arterial stiffness in chronic kidney disease patients. World J Nephrol. 2015;4(3):406–14.
Fortier C, Mac-Way F, Desmeules S, Marquis K, De Serres SA, Lebel M, Boutouyrie P, Agharazii M. Aortic-brachial stiffness mismatch and mortality in dialysis population. Hypertension. 2015;65(2):378–84.
Ma Y, Zhou L, Dong J, Zhang X, Yan S. Arterial stiffness and increased cardiovascular risk in chronic kidney disease. Int Urol Nephrol. 2015;47(7):1157–64.
Hashimoto J, Ito S. Central pulse pressure and aortic stiffness determine renal hemodynamics. Pathophysiological implication for microalbuminuria in hypertension. Hypertension. 2011;58(5):839–46.
Dhaun N, Moorhouse R, MacIntyre IM, Melville V, Oosthuyzen W, Kimmitt RA, Brown KE, Kennedy ED, Goddard J, Webb DJ. Diurnal variation in blood pressure and arterial stiffness in chronic kidney disease. The role of endothelin-1. Hypertension. 2014;64(2):296–304.
Mojón A, Ayala D, Piñeiro L, Otero A, Crespo JJ, Moyá A, Bóveda J, de Lis JP, Fernández JR, Hermida RC, on behalf of the Hygia Project Investigators. Comparison of ambulatory blood pressure parameters of hypertensive patients with and without chronic kidney disease. Chronobiol Int. 2012;30(1–2):145–58.
Gismondi RA, Neves MF, Oigman W, Bregman R. Ambulatory arterial stiffness index is higher in hypertensive patients with chronic kidney disease. Int J Hypertens. 2012;2012:178078. doi:10.1155/2012/178078.
Elsurer R, Afsar B. Serum uric acid and arterial stiffness in hypertensive chronic kidney disease patients: sex-specific variations. Blood Press Monit. 2014;19(5):271–9.
Makris A, Seferou M, Papadopoulos DP. Resistant hypertension workup and approach to treatment. Int J Hypertens. 2011;2011:598694. doi:10.4061/2011/598694.
Bellasi A, Ferramosca E, Ratti C. Arterial stiffness in chronic kidney disease: the usefulness of a marker of vascular damage. Int J Nephrol. 2011;2011:734832. doi:10.4061/2011/734832.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 Springer International Publishing AG
About this chapter
Cite this chapter
Petriş, A.O. (2017). Secondary Causes: Work-Up and Its Specificities in CKD: Influence of Arterial Stiffening. In: Covic, A., Kanbay, M., Lerma, E. (eds) Resistant Hypertension in Chronic Kidney Disease. Springer, Cham. https://doi.org/10.1007/978-3-319-56827-0_9
Download citation
DOI: https://doi.org/10.1007/978-3-319-56827-0_9
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-56825-6
Online ISBN: 978-3-319-56827-0
eBook Packages: MedicineMedicine (R0)