Abstract
Human pluripotent stem cells (PSCs), including embryonic stem cells (ESCs) and induced PSCs (iPSCs), have the potential to differentiate into various cells types and may be used as cell sources for regenerative medicine in the context of various diseases, including severe heart failure. However, one of the biggest hurdles in the use of human PSCs for clinical applications is tumor formation due to contamination with residual tumor-forming cells, primarily undifferentiated PSCs. In addition, hundreds of millions of cardiomyocytes are required for heart repair. Two approaches have been developed for achievement of safer cardiac regenerative therapy using human PSCs: (1) selective elimination of residual tumor-forming cells before cell transplantation and (2) purification of PSC-derived cardiomyocytes. Many methodologies, including genetic and nongenetic modification, have been developed using these strategies. In this chapter, we focus on the current status of selective elimination of residual PSCs and purification of cardiomyocytes for safe stem cell therapy.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
8.1 Introduction
The prognosis of patients with severe heart failure is extremely poor, and heart transplantation is the only effective treatment (Lund et al. 2015). However, lack of donors is a major problem worldwide. Cardiac regenerative therapy using human pluripotent stem cells (PSCs) may represent an effective alternative treatment option for heart transplantation. Human induced PSCs (iPSCs) have the potential to differentiate into various types of cells, similar to human embryonic stem cells (ESCs) (Takahashi 2007; Thomson et al. 1998), and may have applications as a new cell source for regenerative medicine in the context of various diseases, including severe heart failure (Burridge et al. 2012; Passier et al. 2008).
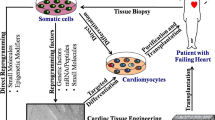
Although cardiac differentiation protocols have dramatically improved (Laflamme et al. 2007; Burridge et al. 2014; Lian 2012; Zhang et al. 2012; Willems et al. 2011; Minami et al. 2012), it may be impossible to stably differentiate into only target cells because many factors, including the specific cell lines used, affect differentiation efficiency (Kattman et al. 2011; Elliott et al. 2011; Osafune et al. 2008). Moreover, cardiac regenerative medicine using human PSCs will require hundreds of millions of cardiomyocytes. The use of this many cells increases the risk of contamination with residual PSCs or noncardiac proliferating cells, which is a major cause of tumor formation (Hentze et al. 2009; Miura et al. 2009; Kawamura et al. 2016; Zhang et al. 2014). Thus, many technologies have been developed to prevent tumor formation in cardiac regenerative medicine, including selective elimination of residual PSCs (Fig. 8.1a) and complete purification of cardiomyocytes (Fig. 8.1b).
In this chapter, we introduce these two strategies and discuss the use of these approaches for safe cardiac regeneration.
8.2 Elimination of Residual Pluripotent Stem Cells
Many studies have described methods for selective elimination of residual PSCs that have the capacity for teratoma formation (Fig. 8.1a). This strategy could theoretically have applications in all fields and is discussed in more detail in the following sections.
8.2.1 Cell Sorting by Stem Cell Markers
Separation strategies based on cell sorting using fluorescent-activated cell sorting (FACS) or magnetic-activated cell sorting (MACS) have been reported to eliminate residual undifferentiated PSCs. In such cell sorting methods, human PSC surface markers, such as TRA1–60, SSEA-4, and SSEA-5, are used (Fong et al. 2009; Tang 2011). In addition, claudin 6, a tight-junction protein specific for human PSCs, is also a useful surface marker for selective elimination of residual human PSCs through FACS (Ben-David et al. 2013). While these strategies are simple, they are not suitable for large-scale culture because they require single-cell dissociation, which would be a time-consuming process when sorting a large number of cells.
8.2.2 Small Molecules or Toxins
Many studies have reported the elimination of undifferentiated human PSCs by utilization of toxins or small molecules. Of the toxins commonly used for this purpose, podocalyxin-like protein 1, a primary cytotoxic antibody for human PSCs, can eliminate residual PSCs (Choo 2008; Tan et al. 2009). In addition, Clostridium perfringens enterotoxin, which binds to claudin 6, has been reported to eliminate undifferentiated PSCs (Ben-David et al. 2013). Recently, Tateno et al. identified a human PSC-specific lectin (rBC2LCN) by glycome analysis and created a recombinant lectin-toxin fusion protein (rBC2LCN) using the catalytic domain of Pseudomonas aeruginosa exotoxin A (Tateno et al. 2015). This fusion protein could be specifically taken up into human PSCs and could kill residual PSCs.
Of the small molecules commonly used for elimination of undifferentiated PSCs, Bieberich et al. showed that ceramide analogs induce apoptosis and eliminate residual PSCs; indeed, treatment with ceramide analogs prevents teratoma formation after transplantation (Bieberich et al. 2004). Lee et al. targeted the hPSC-specific anti-apoptotic factor survivin and demonstrated that inhibition of survivin could selectively eliminate pluripotent stem cells with teratoma potential (Lee 2013). Using chemical screening, Dabir et al. identified a small molecule that inhibits the translocation of redox-regulated proteins to the mitochondria and showed that this small molecule induces apoptosis in human ESCs but not in differentiated cells (Dabir Deepa et al. 2013). Furthermore, Ben-David et al. identified a small molecule that inhibits the biosynthesis of oleic acid and specifically kills human PSCs using a screening library of more than 50,000 small molecules (Ben-David 2013).
These strategies using small molecules or toxins have many advantages because they are simple, efficient, and applicable for large numbers of cells and do not require single-cell dissociation. However, the cost of these strategies may be high owing to the need for large amounts of antibodies or small molecules. In addition, the components of these systems may affect other PSC-derived differentiated cells.
8.2.3 Metabolism
Improving our understanding of metabolic processes in human PSCs is necessary in order to remove undifferentiated tumor-forming cells by exploiting the metabolic environment (Fig. 8.2). Many studies have examined glucose metabolism in mouse and human PSCs (Kondoh et al. 2007; Panopoulos et al. 2011; Folmes Clifford et al. 2011). Folmes et al. reported that human iPSCs exhibit characteristics of elevated glucose utilization compared with mouse embryonic fibroblasts (MEFs) and that inhibition of glucose metabolism reduces the reprogramming efficiency of the cells (Folmes Clifford et al. 2011). Our group also showed that mouse and human PSCs mainly depend on activated glycolysis for ATP and biomass production and that glucose deprivation efficiently removes residual PSCs (Tohyama 2013).
Metabolism in human PSCs. Human PSCs depend on glucose and glutamine metabolism. Glycolysis contributes to ATP and biomass (amino acids and nucleotides) production. Glutamine metabolism contributes to not only ATP generation via OXPHOS but also to the maintenance of pluripotency via reduced glutathione synthesis. Methionine metabolism plays a role to produce S-adenosyl-methionine (SAM) that leads to maintain pluripotency via histone methylation. G6P glucose-6-phosphate, 3PG glycerate 3-phosphate, Gln glutamine, Glu glutamate, αKG α-ketoglutarate
In contrast, few studies have examined the effects of amino acid metabolism on mouse or human PSCs. Shyh-Chang et al. reported that mouse ESCs are critically dependent on threonine catabolism, which is important for synthesis of S-adenosyl-methionine (SAM) and nucleotides (Shyh-Chang et al. 2013). Threonine starvation leads to decreased SAM levels, resulting in inhibition of histone H3K4 trimethylation and preventing mouse ESCs from maintaining pluripotency (Shyh-Chang et al. 2013; Wang et al. 2009). Additionally, Shiraki et al. evaluated the effects of essential amino acid deprivation on cell survival in human PSCs and found that methionine deprivation was the most effective inhibitor of human PSCs. They also reported that methionine is the main source of SAM production in human PSCs (Shiraki et al. 2014). Furthermore, Moussaieff et al. revealed that glucose-derived cytosolic acetyl-CoA contributes to the maintenance of pluripotency by induction of histone pan-acetylation (Moussaieff et al. 2015), and Carey et al. reported that naïve mouse ESCs utilize both glucose and glutamine catabolism to maintain a high level of intracellular α-ketoglutarate (αKG), which promotes histone and DNA demethylation and maintains pluripotency (Carey and Finley 2014). Recently, our group demonstrated that glutamine oxidation during the later steps of the tricarboxylic acid (TCA) cycle plays a key role in cell survival in human PSCs. In glucose-depleted conditions, glutaminolysis activation is increased, thereby promoting ATP production via oxidative phosphorylation (OXPHOS). Interestingly, human PSCs cannot utilize pyruvate efficiently because the expression levels of metabolic enzyme-related genes in the early steps of the TCA cycle are low, whereas those involved in the synthesis of cytosolic acetyl CoA are high. As a result, glucose deprivation and glutamine deprivation are most effective for elimination of residual human PSCs (Tohyama et al. 2016). Glutamine metabolism also contributes to synthesize reduced glutathione that plays a role in maintenance of pluripotency via prevention of OCT4 (Marsboom et al. 2016). Similar to approaches using small molecules and toxins, these approaches have many advantages, such as simplicity, efficiency, and suitability for large-scale culture, and do not require single-cell dissociation. Furthermore, these approaches are not expensive because they do not utilize antibodies or small molecules. However, these metabolic approaches also have the potential to cause damage to the other PSC-derived differentiated cells. Therefore, supplementation with alternative metabolites may be required to minimize the effects on other cells (Tohyama 2013).
8.3 Purification of Target Cells
Recently, both undifferentiated PSCs and other immature proliferating cells have been shown to have potential for tumor formation (Nori et al. 2015). In addition, contamination with noncardiomyocytes may induce arrhythmia after transplantation. Thus, complete purification of cardiomyocytes derived from human PSCs is necessary for safe realization of cardiac regenerative medicine (Fig. 8.1b).
8.3.1 Genetic Manipulation
Several studies have reported fluorescent protein expression-based purification of cardiomyocytes derived from mouse PSCs using various combinations of cardiomyocyte-specific promoters (e.g., αMHC, Mlc2v, Nkx2–5, and ANP) and reporters (e.g., green fluorescent protein [GFP]) (Gassanov et al. 2004; Anderson et al. 2007; Huber et al. 2007; van Laake et al. 2010). In humans, Elliott et al. introduced sequences encoding enhanced GFP (EGFP) into the NKX2–5 locus (NKX2–5-EGFP) by homologous recombination (Elliott et al. 2011). Furthermore, Ma et al. generated hiPSCs expressing a blasticidin-resistance gene under the control of the MYH6 promoter (MYH6-blasticidin) and obtain pure cardiomyocytes (Ma et al. 2011). While these methods are useful for basic research, they are not suitable for clinical application because they lack stability and safety. Therefore, it is necessary to establish nongenetic methods of purifying cardiomyocytes for clinical applications.
8.3.2 Nongenetic Cell Sorting
In nongenetic cell sorting strategies, some groups have attempted to obtain cardiac progenitor cells, whereas other groups, including ours, have attempted to isolate only cardiomyocytes. Yamashita et al. succeeded in obtaining mouse ESC-derived Flk-1-positive mesodermal cells, which could differentiate into several mesodermal lineages, including cardiomyocytes, smooth muscle cells, and endothelial cells (Yamashita et al. 2000). Hidaka et al. reported that prion protein and platelet-derived growth factor (PDGF) receptor α double-positive cells derived from mouse ESCs could differentiate into cardiomyocytes and smooth muscle cells (Hidaka et al. 2009).
On the other hand, it is difficult to isolate only cardiomyocytes because cardiomyocyte-specific surface markers have not yet been identified. Therefore, to isolate pure cardiomyocytes, we focused on the structural characteristics of cells rather than surface markers; using this approach, we succeeded in developing a nongenetic cardiomyocyte purification method (Hattori 2010). In short, because cardiomyocytes have many mature mitochondria and high mitochondrial membrane potential, we successfully purified cardiomyocytes (>99% purity) derived from mouse and human PSCs by a combination of FACS and the mitochondrial dye tetramethylrhodamine methyl ester perchlorate (TMRM). The fluorescence intensity of TMRM dye disappeared within 1 day, while that of other mitochondrial dyes was sustained over 5 days. Therefore, the effects of TMRM dye were suppressed. Other groups have also established nongenetic cardiomyocyte purification methods using FACS or MACS with antibodies against cell surface markers, including ALCAM (CD166) (Rust et al. 2009), signal-regulatory protein alpha (SIRPA) (Dubois et al. 2011), and vascular cell adhesion molecule 1 (VCAM1) (Uosaki 2011). Although cell sorting methods using antibodies or mitochondrial dyes are useful for the production of small numbers of cardiomyocytes, these methods are time consuming when using human PSC-derived mixed cell populations as the source cells (Fig. 8.3a). In addition, these methods require single-cell dissociation, which can damage the target cells, and transplantation of target cells with antibodies may result in immunogenicity. Therefore, further studies are needed to establish methods for scalable production of human PSC-derived pure cardiomyocytes for clinical applications.
8.3.3 Metabolic Selection
To establish an ideal method for scalable production of human cardiomyocytes for clinical applications, our group aimed to purify cardiomyocytes using specific metabolic culture conditions in which only cardiomyocytes and not residual PSCs can survive (Fig. 8.3b). To evaluate metabolic differences between PSCs and cardiomyocytes, we performed metabolome and transcriptome analyses. As mentioned above, we found that the PSCs mainly depended on activated glycolysis and that glucose deprivation could eliminate residual PSCs. However, because glucose-depleted conditions are also fatal for cardiomyocytes, supplementation with an alternative energy source is necessary for survival of cardiomyocytes. Interestingly, glucose and lactate are major energy substrates in fetal hearts, while fatty acids are major energy substrates in adult hearts based on the levels of energy substrates in the blood (Neely and Morgan 1974). Because PSC-derived cardiomyocytes show a fetal phenotype (Uosaki et al. 2015), we hypothesized that PSC-derived cardiomyocytes could efficiently utilize lactate for energy production and showed that mouse and human PSC-derived cardiomyocytes could survive under glucose-depleted and lactate-supplemented conditions. Moreover, because human PSC-derived noncardiac proliferating cells also depended on glycolysis like PSCs and cannot survive under these conditions, we were able to obtain pure cardiomyocytes (>95%) under these conditions (see Fig. 8.4).
Metabolic differences in human PSCs and PSC-derived cardiomyocytes. Under glucose- and glutamine-depleted conditions with pyruvate or lactate supplementation, human PSCs cannot utilize pyruvate efficiently because of low gene expression during the early steps in the TCA cycle. In contrast, human PSC-derived cardiomyocytes can efficiently use lactate-derived pyruvate because of high gene expression during the early steps in the TCA cycle
As mentioned above, our group has recently reported that human PSCs depend on glycolysis and glutamine oxidation for ATP generation. Glucose and glutamine deprivation enabled complete removal of human PSCs in a much shorter period (Tohyama et al. 2016). Surprisingly, lactate supplementation could rescue only human PSC-derived cardiomyocytes because cardiomyocytes efficiently utilize lactate not only for ATP generation via OXPHOS but also for glutamate synthesis under glucose- and glutamine-depleted conditions. In short, lactate can compensate for the lack of intermediate metabolites and overcome the problem of cell damage in human PSC-derived cardiomyocytes, whereas residual human PSCs cannot utilize lactate-derived pyruvate, as mentioned above (Tohyama et al. 2016). In addition, most of the obtained pure cardiomyocytes were myosin light chain 2v (MLC2v)-positive ventricular cells. This metabolism-based method has the following advantages: (1) suitability for large-scale production of pure cardiomyocytes (Fonoudi et al. 2015; Hemmi et al. 2014), (2) simple procedure without specialized instrumentation, (3) low cost of culture medium, and (4) high yield of target cells (Aalto-Setala et al. 2015).
8.3.4 Other Nongenetic Methods
Xu succeeded in enriching human PSC-derived cardiomyocytes using a Percoll density gradient procedure (Xu et al. 2006), yielding cultures containing 35–66% cardiomyocytes. Nguyen et al. reported that the formation of cardiospheres derived from human PSCs enabled the enrichment of cardiomyocytes to over 80% (Nguyen Doan et al. 2014).
Ban et al. reported the purification of cardiomyocytes from mouse and human PSCs by a combination of FACS and molecular probes consisting of 15–30-bp dual-labeled oligonucleotides with a fluorophore and a quencher. In short, molecular probes could be used to identify and visualize cardiomyocyte-specific mRNA in live cells (Ban 2013). Recently, Miki et al. succeeded in establishing an efficient method for purifying cardiomyocytes based on endogenous microRNA (miRNA) activity (Miki et al. 2015). They utilized synthetic mRNAs encoding a fluorescent protein with sequences targeted by cardiomyocyte-specific miRNAs and purify cardiomyocytes with or without FACS.
Conclusions
To realize safe cardiac regenerative medicine using human PSCs, it is important to provide systems for producing target cells with high quality and sufficient quantity. Based on this requirement, metabolic selection systems may be an ideal method to efficiently obtain large numbers of cardiomyocytes derived from human PSCs (Tohyama 2013; Tohyama et al. 2016). This method using glucose-depleted media is also applicable to drug screening and elucidation of pathogenesis using patient-specific iPSCs (Burridge et al. 2016; Kodo et al. 2016; Matsa et al. 2016; Hinson et al. 2015; Dudek et al. 2015). At the same time, methods are needed to detect tumor-forming PSCs with higher sensitivity. Several studies have reported the detection of PSCs at a ratio of 0.001–0.01% (Tano et al. 2014; Kuroda et al. 2012). Further studies are needed to determine whether this sensitivity is sufficient for evaluation of the safety of techniques for regenerative medicine. Recent studies showed that human PSC-derived transplanted cardiomyocytes could electrically integrate with the host heart (Shiba et al. 2012; Gerbin et al. 2015) and mature over time (Hattori 2010; Chong et al. 2014; Funakoshi et al. 2016). Although the effectiveness of transplantation of human PSC-derived cardiomyocytes has been demonstrated in large animals (Chong et al. 2014; Ye et al. 2014; Kawamura et al. 2012), there is a risk of ventricular arrhythmia (Chong et al. 2014; Shiba et al. 2014). While the mechanism is unknown, there are two major possibilities: contamination with noncardiac cells derived from human PSCs and immaturity of PSC-derived cardiomyocytes. Large-scale purification methods for cardiomyocytes may yield solutions for overcoming both of these challenges in order to realize safe cardiac regenerative medicine.
References
Aalto-Setala K, Fuerstenau-Sharp M, Zimmermann ME, Stark K, Jentsch N, Klingenstein M et al (2015) Generation of highly purified human cardiomyocytes from peripheral blood mononuclear cell-derived induced pluripotent stem cells. PLoS One 10(5):e0126596
Anderson D, Self T, Mellor IR, Goh G, Hill SJ, Denning C (2007) Transgenic enrichment of Cardiomyocytes from human embryonic stem cells. Mol Ther 15(11):2027–2036
Ban K (2013) Purification of cardiomyocytes from differentiating pluripotent stem cells using molecular beacons that target cardiomyocyte-specific mRNA. Circulation 128:1897–1909. doi:10.1161/CIRCULATIONAHA.113.004228
Ben-David U (2013) Selective elimination of human pluripotent stem cells by an oleate synthesis inhibitor discovered in a high-throughput screen. Cell Stem Cell 12:162–179
Ben-David U, Nudel N, Benvenisty N (2013) Immunologic and chemical targeting of the tight-junction protein Claudin-6 eliminates tumorigenic human pluripotent stem cells. Nat Commun 4:1992
Bieberich E, Silva J, Wang G, Krishnamurthy K, Condie BG (2004) Selective apoptosis of pluripotent mouse and human stem cells by novel ceramide analogues prevents teratoma formation and enriches for neural precursors in ES cell-derived neural transplants. J Cell Biol 167:723–734. doi:10.1083/jcb.200405144
Burridge PW, Keller G, Gold JD, Wu JC (2012) Production of de novo cardiomyocytes: human pluripotent stem cell differentiation and direct reprogramming. Cell Stem Cell 10(1):16–28
Burridge PW, Matsa E, Shukla P, Lin ZC, Churko JM, Ebert AD et al (2014) Chemically defined generation of human cardiomyocytes. Nat Methods 11(8):855–860
Burridge PW, Li YF, Matsa E, Wu H, Ong SG, Sharma A et al (2016) Human induced pluripotent stem cell-derived cardiomyocytes recapitulate the predilection of breast cancer patients to doxorubicin-induced cardiotoxicity. Nat Med 22(5):547–556
Carey BW, Finley LW, Cross JR, Allis CD, Thompson CB (2014) Intracellular alpha-ketoglutarate maintains the pluripotency of embryonic stem cells. Nature 518(7539):413–416
Chong JJ, Yang X, Don CW, Minami E, Liu YW, Weyers JJ et al (2014) Human embryonic-stem-cell-derived cardiomyocytes regenerate non-human primate hearts. Nature 510(7504):273–277
Choo AB (2008) Selection against undifferentiated human embryonic stem cells by a cytotoxic antibody recognizing podocalyxin-like protein-1. Stem Cells 26:1454–1463. doi:10.1634/stemcells.2007-0576
Dabir Deepa V, Hasson Samuel A, Setoguchi K, Johnson Meghan E, Wongkongkathep P, Douglas Colin J et al (2013) A small molecule inhibitor of redox-regulated protein translocation into mitochondria. Dev Cell 25(1):81–92
Dubois NC, Craft AM, Sharma P, Elliott DA, Stanley EG, Elefanty AG et al (2011) SIRPA is a specific cell-surface marker for isolating cardiomyocytes derived from human pluripotent stem cells. Nat Biotechnol 29(11):1011–1018
Dudek J, Cheng IF, Chowdhury A, Wozny K, Balleininger M, Reinhold R et al (2015) Cardiac-specific succinate dehydrogenase deficiency in Barth syndrome. EMBO Mol Med 8(2):139–154
Elliott DA, Braam SR, Koutsis K, Ng ES, Jenny R, Lagerqvist EL et al (2011) NKX2-5eGFP/w hESCs for isolation of human cardiac progenitors and cardiomyocytes. Nat Methods 8(12):1037–1040
Folmes Clifford DL, Nelson Timothy J, Martinez-Fernandez A, Arrell DK, Lindor Jelena Z, Dzeja Petras P et al (2011) Somatic oxidative bioenergetics transitions into pluripotency-dependent glycolysis to facilitate nuclear reprogramming. Cell Metab 14(2):264–271
Fong CY, Peh GS, Gauthaman K, Bongso A (2009) Separation of SSEA-4 and TRA-1-60 labelled undifferentiated human embryonic stem cells from a heterogeneous cell population using magnetic-activated cell sorting (MACS) and fluorescence-activated cell sorting (FACS). Stem Cell Rev 5:72–80. doi:10.1007/s12015-009-9054-4
Fonoudi H, Ansari H, Abbasalizadeh S, Larijani MR, Kiani S, Hashemizadeh S et al (2015) A universal and robust integrated platform for the scalable production of human cardiomyocytes from pluripotent stem cells. Stem Cells Transl Med 4(12):1482–1494
Funakoshi S, Miki K, Takaki T, Okubo C, Hatani T, Chonabayashi K et al (2016) Enhanced engraftment, proliferation, and therapeutic potential in heart using optimized human iPSC-derived cardiomyocytes. Sci Rep 6:19111
Gassanov N, Er F, Zagidullin N, Hoppe UC (2004) Endothelin induces differentiation of ANP-EGFP expressing embryonic stem cells towards a pacemaker phenotype. FASEB J 18(14):1710–1712
Gerbin KA, Yang X, Murry CE, Coulombe KL (2015) Enhanced electrical integration of engineered human myocardium via intramyocardial versus epicardial delivery in infarcted rat hearts. PLoS One 10(7):e0131446
Hattori F (2010) Nongenetic method for purifying stem cell-derived cardiomyocytes. Nat Methods 7:61–66. doi:10.1038/nmeth.1403
Hemmi N, Tohyama S, Nakajima K, Kanazawa H, Suzuki T, Hattori F et al (2014) A massive suspension culture system with metabolic purification for human pluripotent stem cell-derived cardiomyocytes. Stem Cells Transl Med 3(12):1473–1483
Hentze H, Soong PL, Wang ST, Phillips BW, Putti TC, Dunn NR (2009) Teratoma formation by human embryonic stem cells: evaluation of essential parameters for future safety studies. Stem Cell Res 2(3):198–210
Hidaka K, Shirai M, Lee JK, Wakayama T, Kodama I, Schneider MD et al (2009) The cellular prion protein identifies bipotential cardiomyogenic progenitors. Circ Res 106(1):111–119
Hinson JT, Chopra A, Nafissi N, Polacheck WJ, Benson CC, Swist S et al (2015) Titin mutations in iPS cells define sarcomere insufficiency as a cause of dilated cardiomyopathy. Science 349(6251):982–986
Huber I, Itzhaki I, Caspi O, Arbel G, Tzukerman M, Gepstein A et al (2007) Identification and selection of cardiomyocytes during human embryonic stem cell differentiation. FASEB J 21(10):2551–2563
Kattman SJ, Witty AD, Gagliardi M, Dubois NC, Niapour M, Hotta A et al (2011) Stage-specific optimization of activin/nodal and BMP signaling promotes cardiac differentiation of mouse and human pluripotent stem cell lines. Cell Stem Cell 8(2):228–240
Kawamura M, Miyagawa S, Miki K, Saito A, Fukushima S, Higuchi T et al (2012) Feasibility, safety, and therapeutic efficacy of human induced pluripotent stem cell-derived cardiomyocyte sheets in a porcine ischemic cardiomyopathy model. Circulation 126(11 Suppl 1):S29–S37
Kawamura A, Miyagawa S, Fukushima S, Kawamura T, Kashiyama N, Ito E et al (2016) Teratocarcinomas arising from allogeneic induced pluripotent stem cell-derived cardiac tissue constructs provoked host immune rejection in mice. Sci Rep 6:19464
Kodo K, Ong SG, Jahanbani F, Termglinchan V, Hirono K, InanlooRahatloo K et al (2016) iPSC-derived cardiomyocytes reveal abnormal TGF-beta signalling in left ventricular non-compaction cardiomyopathy. Nat Cell Biol 18(10):1031–1042
Kondoh H, Lleonart ME, Nakashima Y, Yokode M, Tanaka M, Bernard D et al (2007) A high glycolytic flux supports the proliferative potential of murine embryonic stem cells. Antioxid Redox Signal 9(3):293–299
Kuroda T, Yasuda S, Kusakawa S, Hirata N, Kanda Y, Suzuki K et al (2012) Highly sensitive in vitro methods for detection of residual undifferentiated cells in retinal pigment epithelial cells derived from human iPS cells. PLoS One 7(5):e37342
van Laake LW, Qian L, Cheng P, Huang Y, Hsiao EC, Conklin BR et al (2010) Reporter-based isolation of induced pluripotent stem cell- and embryonic stem cell-derived cardiac progenitors reveals limited Gene expression variance. Circ Res 107(3):340–347
Laflamme MA, Chen KY, Naumova AV, Muskheli V, Fugate JA, Dupras SK et al (2007) Cardiomyocytes derived from human embryonic stem cells in pro-survival factors enhance function of infarcted rat hearts. Nat Biotechnol 25(9):1015–1024
Lee MO (2013) Inhibition of pluripotent stem cell-derived teratoma formation by small molecules. Proc Natl Acad Sci U S A 110:E3281–E3290. doi:10.1073/pnas.1303669110
Lian X (2012) Robust cardiomyocyte differentiation from human pluripotent stem cells via temporal modulation of canonical Wnt signaling. Proc Natl Acad Sci 109:E1848–E1857. doi:10.1073/pnas.1200250109
Lund LH, Edwards LB, Kucheryavaya AY, Benden C, Dipchand AI, Goldfarb S et al (2015) The registry of the international society for heart and lung transplantation: thirty-second official adult heart transplantation report–2015; focus theme: early graft failure. J Heart Lung Transplant 34(10):1244–1254
Ma J, Guo L, Fiene SJ, Anson BD, Thomson JA, Kamp TJ et al (2011) High purity human-induced pluripotent stem cell-derived cardiomyocytes: electrophysiological properties of action potentials and ionic currents. Am J Physiol Heart Circ Physiol 301(5):H2006–H2017
Marsboom G, Zhang G-F, Pohl-Avila N, Zhang Y, Yuan Y, Kang H et al (2016) Glutamine metabolism regulates the Pluripotency transcription factor OCT4. Cell Rep 16(2):323–332
Matsa E, Burridge PW, Yu KH, Ahrens JH, Termglinchan V, Wu H et al (2016) Transcriptome profiling of patient-specific human iPSC-cardiomyocytes predicts individual drug safety and efficacy responses in vitro. Cell Stem Cell 19(3):311–325
Miki K, Endo K, Takahashi S, Funakoshi S, Takei I, Katayama S et al (2015) Efficient detection and purification of cell populations using synthetic microRNA switches. Cell Stem Cell 16(6):699–711
Minami I, Yamada K, Otsuji TG, Yamamoto T, Shen Y, Otsuka S et al (2012) A small molecule that promotes cardiac differentiation of human pluripotent stem cells under defined, cytokine- and xeno-free conditions. Cell Rep 2(5):1448–1460
Miura K, Okada Y, Aoi T, Okada A, Takahashi K, Okita K et al (2009) Variation in the safety of induced pluripotent stem cell lines. Nat Biotechnol 27(8):743–745
Moussaieff A, Rouleau M, Kitsberg D, Cohen M, Levy G, Barasch D et al (2015) Glycolysis-mediated changes in acetyl-CoA and histone acetylation control the early differentiation of embryonic stem cells. Cell Metab 21(3):392–402
Neely JR, Morgan HE (1974) Relationship between carbohydrate and lipid metabolism and the energy balance of heart muscle. Annu Rev Physiol 36:413–459
Nguyen Doan C, Hookway Tracy A, Wu Q, Jha R, Preininger Marcela K, Chen X et al (2014) Microscale generation of cardiospheres promotes robust enrichment of cardiomyocytes derived from human pluripotent stem cells. Stem Cell Reports 3(2):260–268
Nori S, Okada Y, Nishimura S, Sasaki T, Itakura G, Kobayashi Y et al (2015) Long-term safety issues of iPSC-based cell therapy in a spinal cord injury model: oncogenic transformation with epithelial-mesenchymal transition. Stem Cell Reports. 4(3):360–373
Osafune K, Caron L, Borowiak M, Martinez RJ, Fitz-Gerald CS, Sato Y et al (2008) Marked differences in differentiation propensity among human embryonic stem cell lines. Nat Biotechnol 26(3):313–315
Panopoulos AD, Yanes O, Ruiz S, Kida YS, Diep D, Tautenhahn R et al (2011) The metabolome of induced pluripotent stem cells reveals metabolic changes occurring in somatic cell reprogramming. Cell Res 22(1):168–177
Passier R, van Laake LW, Mummery CL (2008) Stem-cell-based therapy and lessons from the heart. Nature 453(7193):322–329
Rust W, Balakrishnan T, Zweigerdt R (2009) Cardiomyocyte enrichment from human embryonic stem cell cultures by selection of ALCAM surface expression. Regen Med 4(2):225–237
Shiba Y, Fernandes S, Zhu WZ, Filice D, Muskheli V, Kim J et al (2012) Human ES-cell-derived cardiomyocytes electrically couple and suppress arrhythmias in injured hearts. Nature 489(7415):322–325
Shiba Y, Filice D, Fernandes S, Minami E, Dupras SK, Biber BV et al (2014) Electrical integration of human embryonic stem cell-derived cardiomyocytes in a guinea pig chronic infarct model. J Cardiovasc Pharmacol Ther 19(4):368–381
Shiraki N, Shiraki Y, Tsuyama T, Obata F, Miura M, Nagae G et al (2014) Methionine metabolism regulates maintenance and differentiation of human pluripotent stem cells. Cell Metab 19(5):p780–p794
Shyh-Chang N, Locasale JW, Lyssiotis CA, Zheng Y, Teo RY, Ratanasirintrawoot S et al (2013) Influence of threonine metabolism on S-adenosylmethionine and histone methylation. Science 339(6116):222–226
Takahashi K (2007) Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 131:861–872. doi:10.1016/j.cell.2007.11.019
Tan HL, Fong WJ, Lee EH, Yap M, Choo A (2009) mAb 84, a cytotoxic antibody that kills undifferentiated human embryonic stem cells via oncosis. Stem Cells 27:1792–1801. doi:10.1002/stem.109
Tang C (2011) An antibody against SSEA-5 glycan on human pluripotent stem cells enables removal of teratoma-forming cells. Nat Biotechnol 29:829–834. doi:10.1038/nbt.1947
Tano K, Yasuda S, Kuroda T, Saito H, Umezawa A, Sato Y (2014) A novel in vitro method for detecting undifferentiated human pluripotent stem cells as impurities in cell therapy products using a highly efficient culture system. PLoS One 9(10):e110496
Tateno H, Onuma Y, Ito Y, Minoshima F, Saito S, Shimizu M et al (2015) Elimination of tumorigenic human pluripotent stem cells by a recombinant lectin-toxin fusion protein. Stem Cell Rep 4(5):811–820
Thomson JA, Itskovitz-Eldor J, Shapiro SS, Waknitz MA, Swiergiel JJ, Marshall VS et al (1998) Embryonic stem cell lines derived from human blastocysts. Science 282(5391):1145–1147
Tohyama S (2013) Distinct metabolic flow enables large-scale purification of mouse and human pluripotent stem cell-derived cardiomyocytes. Cell Stem Cell 12:127–137. doi:10.1016/j.stem.2012.09.013
Tohyama S, Fujita J, Hishiki T, Matsuura T, Hattori F, Ohno R et al (2016) Glutamine oxidation is indispensable for survival of human pluripotent stem cells. Cell Metab 23(4):663–674
Uosaki H (2011) Efficient and scalable purification of cardiomyocytes from human embryonic and induced pluripotent stem cells by VCAM1 surface expression. PLoS One 6:e23657. doi:10.1371/journal.pone.0023657
Uosaki H, Cahan P, Lee Dong I, Wang S, Miyamoto M, Fernandez L et al (2015) Transcriptional landscape of cardiomyocyte maturation. Cell Rep 13(8):1705–1716
Wang J, Alexander P, Wu L, Hammer R, Cleaver O, McKnight SL (2009) Dependence of mouse embryonic stem cells on threonine catabolism. Science 325(5939):435–439
Willems E, Spiering S, Davidovics H, Lanier M, Xia Z, Dawson M et al (2011) Small-molecule inhibitors of the Wnt pathway potently promote cardiomyocytes from human embryonic stem cell-derived mesoderm. Circ Res 109(4):360–364
Xu C, Police S, Hassanipour M, Gold JD (2006) Cardiac bodies: a novel culture method for enrichment of cardiomyocytes derived from human embryonic stem cells. Stem Cells Dev 15(5):631–639
Yamashita J, Itoh H, Hirashima M, Ogawa M, Nishikawa S, Yurugi T et al (2000) Flk1-positive cells derived from embryonic stem cells serve as vascular progenitors. Nature 408(6808):92–96
Ye L, Chang Y-H, Xiong Q, Zhang P, Zhang L, Somasundaram P et al (2014) Cardiac repair in a porcine model of acute myocardial infarction with human induced pluripotent stem cell-derived cardiovascular cells. Cell Stem Cell 15(6):750–761
Zhang J, Klos M, Wilson GF, Herman AM, Lian X, Raval KK et al (2012) Extracellular matrix promotes highly efficient cardiac differentiation of human pluripotent stem cells: the matrix sandwich method. Circ Res 111(9):1125–1136
Zhang L, Pan Y, Qin G, Chen L, Chatterjee T, Weintraub N et al (2014) Inhibition of stearoyl-coA desaturase selectively eliminates tumorigenic Nanog-positive cells: improving the safety of iPS cell transplantation to myocardium. Cell Cycle 13(5):762–771
Acknowledgments
The present work was supported by the Highway Program for Realization of Regenerative Medicine from Japan Science and Technology Agency (to K.F.) and SENSHIN Medical Research Foundation (to S.T.).
Compliance with Ethical Standards
Conflict of Interest
The Shugo Tohyama declare that they have no conflict of interest. Keiichi Fukuda is a cofounder of Heartseed Inc.
Ethical approval
This article does not contain any studies with human participants performed by any of the authors.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 Springer International Publishing AG
About this chapter
Cite this chapter
Tohyama, S., Fukuda, K. (2017). Purification of Pluripotent Stem Cell-Derived Cardiomyocytes for Safe Cardiac Regeneration. In: Ieda, M., Zimmermann, WH. (eds) Cardiac Regeneration. Cardiac and Vascular Biology, vol 4. Springer, Cham. https://doi.org/10.1007/978-3-319-56106-6_8
Download citation
DOI: https://doi.org/10.1007/978-3-319-56106-6_8
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-56104-2
Online ISBN: 978-3-319-56106-6
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)