Abstract
A unique characteristic of the pelagic Antarctic silverfish Pleuragramma antarctica is the massive accumulation and storage of lipids in special oil sacs. The enormous lipid deposition beyond 50% of body dry mass functions primarily as buoyancy aid compensating for the missing swim bladder in these fishes, although the depot lipids could also serve as energy reserves. The lipid signature clearly reflects the life cycle of P. antarctica. Trophic marker fatty acids of the early larval and post-larval stages reveal feeding preferences on phyto- and zooplankton, mainly copepods, which these stages utilize for rapid somatic growth without special lipid storage. The juvenile stages tend to feed on calanoid copepods, while the adults shift to krill (Euphausia superba, E. crystallorophias) as major food items. The findings from fatty acid trophic markers are in accordance with gut content analyses. Juveniles to adults exhibit a pronounced lipid deposition, namely triacylglycerols, in the oil sacs. These triacylglycerols are composed of unmodified dietary fatty acids, but may also partially be synthesized de novo. This substantial lipid accumulation not only represents a key adaptation of P. antarctica to life in the pelagic realm. It is also of major importance as high-quality and high-energy food for other marine vertebrates such as seabirds and seals and ultimately ensures an efficient energy flow through the lipid-based high-Antarctic food web.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
The Antarctic fish fauna is unique, due to the overwhelming dominance of one perciform group, the Notothenioidei, with 97% of the species endemic to the Southern Ocean (Andriashev 1987). These species originate from a benthic ancestor without a swim bladder (Clarke and Johnston 1996; Eastman 2005), which may explain that only very few species permanently invaded the pelagic zone, a niche with plenty of lipid-rich food, e.g. copepods and krill, largely unoccupied by fishes (Eastman 2005). The Antarctic silverfish Pleuragramma antarctica is the key species among these pelagic fishes of the Antarctic ichthyofauna (Koubbi et al. 2009; La Mesa and Eastman 2012). The biomass of this circum-Antarctic shoaling species is estimated with 500,000 t (1 t km−2) in the Weddell Sea (Hubold 1992). The complete life cycle of the Antarctic silverfish has first been intensely studied by Hubold and colleagues (e.g. Hubold 1984, 1992, 2009) in the Atlantic sector of the Southern Ocean, especially the Weddell Sea. The ontogenetic development of P. antarctica relies strongly on lipids from the energy-rich eggs and yolk-sac larvae via post-larval and juvenile developmental stages to the adults (Wöhrmann et al. 1997). The incorporation of large amounts of triacylglycerols in the lipid sacs (Fig. 7.1), which are fully developed in the juveniles and adults, is an exclusive key adaptation of this pelagic species to counter negative buoyancy (Eastman and De Vries 1982; Friedrich and Hagen 1994; Hagen et al. 2000). Although not very flexible with regard to buoyancy regulation, these low-density lipid compounds compensate for the lacking up-thrust of a swim bladder and allow these sluggish fish to maintain their position in the water column without additional swimming effort. However, these lipid deposits may also be utilized for energetic requirements (Eastman and de Vries 1989).
Pleuragramma antarctica. Double row of subcutaneous lips sacs (sc) from a mid-ventral location in the trunk of a 90 mm length formalin-preserved specimen. Melanophores between sacs are in the mid-ventral line. Sacs are 0.7–0.9 mm in diameter (Figure and caption from La Mesa and Eastman (2012) (with kind permission of John Wiley and Sons Inc))
For the first time, ripe eggs of P. antarctica were detected in the stomachs of benthos-feeding fish (Trematomus spp.) at 450 m depth in the Weddell Sea in October 1986 (Hubold 1992, 2009). The embryonated eggs of P. antarctica have a diameter of about 2.0–2.5 mm and contain large amounts of lipid-rich yolk, which explains their initial positive buoyancy, but before hatching the eggs start to sink, as lipids are catabolized and converted to proteins (Evans et al. 2012). Lipids are also crucial for growth of the early larvae, which at least partially rely on the yolk sac for energy. Yolk-sac larvae of 8–10 mm length can survive more than 3 weeks of starvation (Hubold 1992), although if food is available in the field they may feed soon after hatching, as the mouth is already well developed (Bottaro et al. 2009). Hatching has never been observed in the Weddell Sea, but apparently it commences in early spring (November), with very small larvae also occurring in January and February. First yolk-sac larvae (9 mm mean length) were only collected (by Multinet) at depths below 500 m in mid-November. Within a few days very high concentrations of larvae occurred in the productive surface layer (<50 m depth) and in spite of near-freezing seawater temperatures the larvae showed surprisingly high growth rates comparable to boreal herring larvae (Hubold 1992). This early developmental phase of P. antarctica in the Weddell Sea appears to deviate from that described for the Antarctic silverfish in the Ross Sea, a phenomenon that may be due to different hydrographic conditions, but clearly requires further investigations. In Terra Nova Bay, Ross Sea, developing eggs and freshly hatched larvae of P. antarctica occur in very high concentrations near the surface among the platelet ice, directly under the congelation ice (Vacchi et al. 2004, 2012a), although the early larvae showed negative phototaxis and positive gravitaxis (Evans et al. 2012).
Larvae and post-larvae feed mainly on small cyclopoid copepods and early juveniles switch to calanoid copepods at a standard length of >60 mm (Hubold and Hagen 1997). During this developmental phase they start to store lipids via ingested food and/or de novo synthesis. In the juvenile stages enormous lipid depots, namely triacylglycerols, are accumulated. In the ice-covered regions the older stages of P. antarctica shift from copepods to the dominant neritic “ice krill”, Euphausia crystallorophias. Part of the P. antarctica population is transported by currents from the southern Weddell Sea towards the eastern side of the Antarctic Peninsula, where the fish mainly feed on the Antarctic krill, E. superba (Kellermann 1987). Pronounced lipid storage is a typical characteristic of the adults, with lipids reaching average levels of 50% of body dry mass (DM), but they may vary from 30% to 60%DM (Friedrich and Hagen 1994). With increasing age the adults descend to greater depths and return to the southern Weddell Sea shelf areas. The circle of this life cycle closes, when the adult P. antarctica migrate to their spawning grounds over the northeastern shelf areas of the high-Antarctic Weddell Sea (Hubold 1992).
Although P. antarctica is a key component of the high-Antarctic food web and an important and very lipid-rich prey for marine mammals and birds, e.g. toothed whales, seals and emperor penguins, there are few detailed reports on its lipid and fatty acid compositions as a unique characteristic of the Antarctic silverfish. We will summarize the lipid data and elucidate the role of these high-energy/low-density compounds in the life history of P. antarctica by tackling questions such as these: Is the strong lipid increase in the older stages based on de novo biosynthesis or is it accumulated from the diet? Is the lipid in the oil sacs rather inert or does it show an intense turnover, perhaps depending on a seasonally varying food supply? Is the huge amount of lipids necessary to maintain neutral buoyancy or is it also utilized as energy reserve? Has the sluggish mode of life of P. antarctica any influence on the lipid storage and demand?
2 Larval Stages: Rapid Growth and Low Lipid Levels
Pleuragramma larvae can be defined – besides their size – by their rather low total lipid content dominated by phospholipids. This group comprises larvae between 10 and 19 mm body length, with the largest specimens overlapping with the post-larval phase. They show rapid growth rates of 0.15–0.21 mm per day, which are similar to Atlantic herring larvae (Hubold 1985). Body dry mass extends over a very wide range from 0.3 to 2.8 mg per specimen. This also holds true for the total lipid amount, which increases accordingly from 0.06 mg in the youngest to 0.4 mg in the oldest larvae. The relative lipid levels (in % body dry mass, %DM) are rather low. Smallest larvae (ca. 10 mm) with yolk sac exhibit slightly elevated lipid levels with 19%DM, which decrease with further development to <14%DM in 15–16 mm specimens utilizing their yolk lipids. In larvae of 19 mm size lipid levels start to increase again to 23%DM (calculated from % of total lipid (%TL) in wet mass) (Table 7.1 and references therein). The respective lipid compositions of larval stages are characterized by phospholipids (ca. 70–80%TL) as the dominant component of biomembranes. These early stages contain only low but variable amounts of storage lipid, namely triacylglycerols (ca. 7–18%TL, Table 7.1). The lipid composition of the larvae is strongly determined by yolk lipids, which are essential to fuel initial somatic growth. During early larval development of the Atlantic herring for instance, these lipids are dominated by phospholipids, mainly phosphatidylcholine (PC) and phosphatidylethanolamine (PE) (Tocher et al. 1985). These lipid classes also prevail in the larvae of P. antarctica with PC and PE accounting for 34% and 20%TL, respectively (Hagen 1988), while Tavernier et al. (2012) reported 40% PC and 13% PE.
The fatty acid compositions of the larvae (Table 7.2) consist mainly of three principal components, 22:6(n-3), 20:5(n-3) and 16:0, which make up about 24%, 17% and 18% of total fatty acids (%TFA), respectively. This composition reflects the typical fatty acid pattern of marine biomembrane lipids. Other important but less abundant fatty acids (usually between 5 and 10%TFA) are 18:1(n-9), 18:1(n-7) and 16:1(n-7). The fatty acid compositions of the two major lipid classes, phospholipids and triacylglycerols, were analysed separately by Mayzaud et al. (2011) and Tavernier et al. (2012) for the larval size range of 17–19 mm.
Differences between these lipid classes mainly concern the fatty acids 22:6(n-3) and 16:0, which show twice as high percentages in the phospholipids than in the triacylglycerols (Table 7.3). In addition, 16:1(n-7), 18:4(n-3) and 18:1(n-9) were clearly higher in the triacylglycerols as compared to the phospholipids. Typically, the fatty acids of triacylglycerols reflect dietary preferences, whereas phospholipids have a more conservative and homogeneous composition less influenced by dietary interactions (Dalsgaard et al. 2003). A trophic signature is partially reflected in the fatty acid compositions of the larvae, although it is unknown to what extent the fatty acid compositions of eggs and yolk influence the overall composition. The fatty acids 16:1(n-7) and 18:4(n-3) in the larvae may originate from feeding on diatoms and flagellates, respectively. However, it cannot be excluded that these algae were ingested by e.g. herbivorous copepods and that this prey together with the phytoplankton markers was incorporated by P. antarctica. Gut content analyses by Koubbi et al. (2007) showed that the larvae (15–30 mm) from Dumont d’Urville Sea, East Antarctica, are omnivorous, feeding mainly on diatoms and copepods. Vallet et al. (2011) and Tavernier et al. (2012) reported that about 70% of the larvae feed on a mixture of phytoplankton and zooplankton, while the rest ingests exclusively phytoplankton, mainly diatoms. In contrast, diatoms represented only a negligible food item in the guts of larvae from the southern Weddell Sea (von Dorrien 1989; Hubold and Hagen 1997). In both Antarctic regions the ingestion of diatoms by the larvae is reflected by the 16:1(n-7) marker fatty acid, although the signal is not as high as expected from the gut content analyses. In addition, polyunsaturated fatty acids with 16 carbon atoms, another typical diatom marker, are almost missing. These findings do not suggest an intense incorporation of diatom fatty acids by P. antarctica. Vice versa, fragile flagellates are often damaged and thus very difficult to detect in the guts of the larvae. This may explain why higher portions of the 18:4(n-3) flagellate marker are not corroborated by gut content analyses. It should also be kept in mind that gut contents provide only a snapshot impression of the ingested food items, whereas fatty acid trophic markers integrate dietary signals over several weeks (Graeve et al. 1994). This emphasizes an advantage of trophic marker fatty acid studies over conventional gut content analyses (which may provide higher taxonomic resolution). On the other hand, the rapid growth of the P. antarctica larvae may limit the applicability of trophic marker fatty acids, due to their immediate conversion and intense utilization for growth and energetic requirements.
3 Post-Larval Stages: Slower Growth and Initial Lipid Accumulation
With the development from larvae to post-larvae (ca. 30–50 mm length) there is a clear increase in total lipid levels, indicating the onset of lipid accumulation in special subcutaneous and intermuscular depots, the oil sacs typical of Pleuragramma (Eastman and De Vries 1989). Dry mass and lipid mass increase clearly with length to 50 mg DM and 14 mg TL, respectively (Table 7.1). This results in a near doubling of the lipid content from about 19%DM in the smaller to 30%DM in the larger post-larvae. This initial lipid increase in the post-larvae is clearly due to an accumulation of triacylglycerols (Table 7.1). These compounds comprise the only neutral lipid component stored by P. antarctica and reach about 40% of total lipids in these post-larvae.
Apparently, during this critical phase of early development the post-larvae need to invest energy into somatic growth, but also channel already substantial amounts of energy, namely triacylglycerols, towards lipid deposition. It is a matter of conjecture, whether this lipid storage is primarily functioning as buoyancy adaptation, since there are no density data available for the post-larval phase. This lipid deposition may explain that – in contrast to the rapid growth of the larvae – growth of post-larvae slows down drastically to 0.06–0.08 mm per day (La Mesa and Eastman 2012). These post-larvae use the ingested energy partially for a distinct lipid deposition, which is quite unique among early developmental stages. It is in contrast to other species, e.g. herring or hake post-larvae, which suffer from high predation pressure and tend to rapidly outgrow this critical early developmental phase (Grote et al. 2012).
In accordance with the increasing triacylglycerol portions, the fatty acid compositions of the post-larvae reflect more clearly their dietary preferences. Unfortunately, for the post-larval stages only fatty acid data of total lipids are available, but not of triacylglycerols, which would provide stronger dietary signals. This signal is indicated by higher concentrations of long-chain monounsaturated fatty acids, in particular 20:1(n-9), in the most advanced post-larvae. Other principal fatty acids are similar to those of the larval stages. The 20:1(n-9) fatty acid supports the intense ingestion of calanid copepods, namely the older lipid-rich copepodite stages of Calanoides acutus. This trophic marker comprises about a quarter of total fatty acids in C. acutus (Kattner et al. 1994). It is the only Antarctic copepod species characterized by high amounts of wax esters with long-chain monounsaturated fatty acids and alcohols, both moieties dominated by 20:1(n-9). These wax esters make up 90% of total lipids in this copepod species. The other principal prey items are cyclopoid copepods of the genus Oncaea (Hubold and Hagen 1997). They are similarly rich in wax esters as C. acutus, but its fatty acids are strongly dominated by 18:1(n-9) (33–79%TFA), while 14:0 and 16:0 prevail in the fatty alcohol moieties (Kattner et al. 2003). This is partially reflected in the most advanced post-larvae. P. antarctica hardly contains any wax esters. The trace amounts detected may still originate from undigested food in the guts, although according to Giraldo et al. (2013) the gut content has no significant influence on the lipid analyses. Thus, wax esters have to be cleaved into fatty acids and alcohols, probably in the liver. Both moieties may be used for metabolic demands and for the production of triacylglycerols, with fatty alcohols following conversion into fatty acids. Alternatively, the alcohols may not be absorbed and egested unutilized, making no use of this high-energy compound. However, Sargent et al. (1979) suggested that fatty alcohols are efficiently assimilated and converted to fatty acids, usually as triacylglycerol moieties, by marine fish, e.g. Atlantic herring. The suggested feeding preferences of P. antarctica based on its fatty acid markers is supported by conventional gut content analysis, since the wax ester-rich older C. acutus have been reported as the dominant prey item in P. antarctica specimens <50 mm comprising more than 40% of prey biomass (Hubold and Hagen 1997). Small cyclopoid copepods of the genus Oncaea were the second most important prey and accounted for 25% of the ingested biomass and 60% in terms of prey abundance.
4 Juveniles and Adults: Slow Growth and Pronounced Lipid Storage
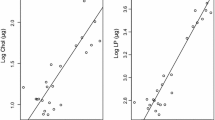
The strong lipid accumulation already noted in the post-larvae continues exponentially in the juveniles, which comprise several age classes. First-year juveniles had a total lipid mass of about 35 mg and a lipid content of 37% DM (calculated from wet mass by Mayzaud et al. 2011). The exponential increase in dry and lipid mass from the younger to the older juveniles (size range 54–98 mm) is shown in Figs. 7.2 and 7.3, reaching about 1.2 and 0.6 g, respectively (Table 7.1). Second and third-year juveniles exhibit slightly higher mean lipid levels between 30% and 47%DM. Adult P. antarctica (size range 120–190 mm) reach highest mean lipid levels around 47%DM with a maximum of 58%DM (Friedrich and Hagen 1994). These total lipids are clearly dominated by the storage lipid triacylglycerol, increasing from 60%TL in first-year juveniles to 80%TL in the adults (Fig. 7.4). The remaining lipid compounds comprise primarily phospholipids, but also low levels of cholesterol (Table 7.1).
The fatty acid compositions reveal a change in trophic preferences from larvae to juveniles and adults. As already indicated by the older post-larvae, portions of 20:1(n-9) reached up to 17%TFA in juveniles, but decreased again towards the adults to 6%TFA. A similar trend occurred for 22:1(n-11) and 22:1(n-9) with maxima of 12% and 10%, respectively, in juveniles decreasing to about 3–4%TFA in the adults (Table 7.2). These 22:1 isomers suggest an increasing importance of the larger calanoid copepod, Calanus propinquus, in the diet of juvenile P. antarctica. C. propinquus deposits triacylglycerols instead of wax esters and it is the only dominant Antarctic copepod, which biosynthesizes very high amounts of the fatty acid 22:1(n-9) with up to 26%TFA (Kattner et al. 1994). This intriguing shift from C. acutus to C. propinquus by larger P. antarctica (60–100 mm) is clearly supported by gut content analyses (Hubold and Hagen 1997). The 22:1(n-11) isomer shows even higher percentages in C. propinquus, but it is also typical of Calanoides acutus, as discussed above. Wax esters may contribute 90% of the total lipids in C. acutus, hence the percentage of the 22:1(n-11) alcohol is comparable to that of the corresponding fatty acid in C. propinquus. Although the 22:1(n-11) trophic marker is a powerful tool to identify these two dominant calanid copepods as food items, it is limited with regard to the differentiation between these species. Apart from their dominance, it is probably also energetically advantageous for P. antarctica to feed on these two calanid species, since long-chain moieties have a higher energy content than shorter ones (Albers et al. 1996). The preference for C. propinquus as prey suggests that it is easier for P. antarctica to utilize the large amounts of triacylglycerols in comparison to wax esters.
The decreasing importance of the long-chain monounsaturated fatty acids with increasing size of P. antarctica may be compensated by higher lipid deposits. It also emphasizes the change in dietary preferences of the older specimens supported by the corresponding change in fatty acid compositions. 18:1(n-9) is by far the dominant fatty acid in the adults (25%TFA), followed by similar amounts of 16:0 and 14:0 (ca. 15%TFA each, Table 7.2). These fatty acids are not as specific trophic indicators as the long-chain monounsaturates, since they are rather common end-products of the fatty acid biosynthesis. The fatty acid 18:1(n-9) usually originates from the elongation and desaturation of 14:0 and 16:0 dietary precursors, but may also derive from de novo biosynthesis. This may reflect a considerable biosynthetic production of lipids in adult P. antarctica. Despite its limitation as a trophic marker, 18:1(n-9) can provide dietary information, as it is a major fatty acid component of the ice krill Euphausia crystallorophias (Bottino 1975, Kattner and Hagen 1998), especially in juveniles and adults with up to 75% in the wax ester fraction, which may comprise 50% of total lipids. In addition, the 14:0 fatty alcohol, the predominant wax ester moiety in ice krill (75% of total alcohols), may be converted to the corresponding 14:0 fatty acid by P. antarctica. Hence, these fatty acid components provide supportive evidence that older P. antarctica rely on ice krill as major prey item. In addition to calanid copepods, various authors report the importance of euphausiids, especially ice krill in high-Antarctic waters, in the diet of P. antarctica (Hubold 1985; Hubold and Ekau 1990). In more northerly regions of the Southern Ocean, e.g. off the Antarctic Peninsula, older silverfish specimens shift to the Antarctic krill (La Mesa and Eastman 2012 and references therein). However, the lipid and fatty acid compositions of E. superba do not exhibit specific trophic markers (Hagen et al. 2001) and thus do not provide evidence for the ingestion of this krill species. It should be noted that Hubold (1991) reported a strong seasonal shift for juvenile P. antarctica from a krill-based diet in summer to a copepod-dominated diet in late winter. This diet was mainly composed of deep-living copepods (Spinocalanidae, etc.), which the juveniles apparently encountered at depth. The fatty acid composition of these copepods is not known, however, due to their carnivorous feeding mode, they are very unlikely to biosynthesize long-chain monounsaturated fatty acids typical of herbivorous calanids.
Other relevant food items include the calanoid copepods Rhincalanus gigas and Euchaeta spp. (Hubold and Hagen 1997). These species also accumulate large amounts of wax esters and have similar fatty acid and alcohol compositions as the ice krill, although 18:1(n-9) is less dominant in the omnivorous R. gigas than in carnivorous Euchaeta spp. (Kattner et al. 1994; Hagen et al. 1995; Albers et al. 1996). Hence, these lipid components provide no distinguishing dietary resolution. Nevertheless, the high percentage of 16:1(n-7) in those copepod species (up to 25%TFA in Euchaeta) seems to be reflected in older Pleuragramma specimens (ca. 10%TFA). This high percentage is intriguing, since 16:1(n-7) is a typical marker of diatoms (Graeve et al. 1994), but it may be incorporated unmodified via the ingestion of these copepods and retained by P. antarctica.
An overview of the fatty acid data of the various P. antarctica stages from larvae to adults sorted by Principal Component Analysis (PCA) is given in Fig. 7.5, which highlights the differences and similarities. The first two principal components explain 86% of the variance. The first axis (PC1) discriminates mainly between developmental stages with the early larvae arranged towards the left hand side and the juveniles and adults towards the right hand side. Post-larval data are not as tightly sorted due to their higher variability and comprise a larger area, partially overlapping with larvae and juveniles. PC1 is negatively correlated with the fatty acids 22:6(n-3), 20:5(n-3), 16:0, 18:1(n-7) and 18:0 (in decreasing order of explanatory power) typical of larval stages, especially from summer. The positive values of PC1 are associated with the isomers 22:1(n-11) and (n-9), the isomers 20:1(n-9) and (n-7) as well as 14:0 and 16:1(n-7) characteristic of the juveniles. Within the stages the data are also discriminated according to season, but this influence is less pronounced. The few data of the adults and some juveniles, both from summer, are separated from the younger stages and mostly represented by positive correlations with 18:1(n-9), 14:0, 18:1(n-7), 16:1(n-7), 16:3(n-4), and negative values of 22:6(n-3), 22:1(n-11, n-9) and 20:5(n-3) (PC2). The figure emphasizes the close correlation of the ontogenetic development and lipid accumulation, reflected by the changing fatty acid compositions as one of the most important features of P. antarctica.
Pleuragramma antarctica. Principal Component Analysis (PCA) of larvae to adults, sorted based on their fatty acid compositions. Light colours: spring data, dark colours: summer data, data in circle from Tavernier et al. (2012). PCA was conducted based on the composition of the most abundant fatty acids using the Primer v6 software. Prior to PCA, proportions of the fatty acids were normalized with arcsine-square-root transformation to correct deficiencies in normality and homogeneity of variance
5 Importance of Lipids as Buoyancy Aid and Energy Reserve
Two key aspects are discussed with regard to the function of lipids in P. antarctica, buoyancy and energetics (Eastman 1988). The Antarctic silverfish is one of the very few fully pelagic Antarctic fish species, which originates from a bottom-dwelling notothenioid ancestor without a swim bladder (La Mesa and Eastman 2012). Hence, P. antarctica has no efficient buoyancy aid to regulate its density in the water column, an obvious disadvantage for a pelagic life style. However, due to different adaptive mechanisms, the species was able to strongly reduce its density and thus achieved almost neutral buoyancy in seawater (Eastman 1985). P. antarctica shows various adaptations to increase its sinking resistance and to reduce its density, e.g. enlargement of pectoral and pelvic fins, reduced ossification and calcification of the skeleton, replacement of bones by cartilage, small otoliths, and a persistent notochord. The species is also retaining larval features, which delays the formation of scales (Albertson et al. 2010; La Mesa and Eastman 2012).
The other prominent feature of P. antarctica contributing to neutral buoyancy are the intermuscular and subcutaneous oil sacs, which apparently serve to compensate for the lack of a swim bladder. Eastman and de Vries (1989) suggested that the lipid sacs are primarily used as buoyancy aid and doubted their utilization as energy reserves, due to the limited cell membrane surface area. To fulfil this buoyancy function, it would be much more effective for P. antarctica to store these deposits as wax esters (specific gravity at 5 °C: 0.90 g cm3) instead of triacylglycerols (specific gravity at 5 °C: 0.96 g cm3), because wax esters provide one third more up-thrust than triacylglycerols (Lee and Patton 1989). Many marine copepod species follow this strategy of wax ester deposition and they are able to biosynthesize enormous amounts of these lipids. Ingesting wax ester-rich copepods could enable P. antarctica to transfer and incorporate huge amounts of these low-density lipids in their oil sacs. However, P. antarctica and apparently the whole group of Notothenioidei do not accumulate wax esters (Phleger et al. 1999b; Hagen et al. 2000; Mayzaud et al. 2011), which also indicates their inability to biosynthesize these lipids. (The high wax ester contents in flesh and lipid sacs of adult P. antarctica as well as the very high hydrocarbon levels in the larvae reported by Reinhardt and Van Vleet (1986) must be erroneous results.) During digestion wax esters are cleaved into fatty acids and fatty alcohols, the latter are obviously converted into the corresponding fatty acids. This is a common biochemical pathway known of many triacylglycerol-storing fish species, e.g. the well-investigated Atlantic herring (Sargent et al. 1979), and apparently it is also used by P. antarctica. In contrast, Antarctic myctophids (Phleger et al. 1999a) and many other marine fish species, e.g. capelin, as well as meso- and bathypelagic fish, e.g. deep-sea cod, biosynthesize wax esters, as already reviewed by Nevenzel (1970).
The fat reserves in the lipid sacs of P. antarctica would be quite useful during periods of food shortage, e.g. in winter, or in times of higher energy demand such as gonad maturation and egg formation (vitellogenesis). The few seasonal data from early spring and from summer show no clear differences in total lipid contents between seasons for the juveniles (Hubold and Hagen 1997). Surprisingly, lipid levels of these juveniles were lower in summer than in early spring (Fig. 7.3), but the few data do not allow a sound explanation. It is a matter of conjecture, if this difference indicates poorer feeding conditions in summer than in spring. The development of P. antarctica demands maximum amounts of lipid during the formation of the oil sacs, which requires plenty of (lipid-rich) food. Once they have reached adulthood and filled their oil sacs, there is less need for further energy investment to maintain the buoyancy function of the sacs. This is in accordance with the rather sluggish and thus energy-saving mode of life suggested for P. antarctica (Zimmermann and Hubold 1998). If lipids were crucial for neutral buoyancy, utilization of the lipid sacs by P. antarctica would result in negative buoyancy. The species would be forced to increase its swimming activity to maintain its position in the water column, further depleting its lipid depots, a vicious circle. Accordingly, a model applied by Maes et al. (2006) suggests maximum fitness of P. antarctica, if the oil sacs function as metabolically inert buoyancy aids and are not utilized as energy stores. However, the variability of lipid levels in P. antarctica, which range from 30% to almost 60%DM in the adults (Friedrich and Hagen 1994) indicates that lipids are not only maintained for optimum buoyancy, but may suggest utilization, depending on the available food supply. We know from feeding experiments of copepods with labelled phytoplankton that the turnover in their oil sacs is considerable and lipids are exchanged within 2–3 weeks during good feeding conditions (Graeve et al. 2005). Unfortunately, information about the fatty acid composition of adult P. antarctica during ageing is still missing.
The pronounced lipid accumulation of P. antarctica represents not only an impressive adaptive mechanism to occupy the productive pelagic realm, this species also represents a crucial component within the Antarctic food web. Trophodynamics in the Southern Ocean are largely based on the efficient transfer of high-energy and high-quality (rich in (n-3) fatty acids also known as omega-3 fatty acids) lipids from one trophic level to the next, from extremely lipid-rich calanoid copepods and euphausiids via oily P. antarctica to the top predators, warm-blooded vertebrates with a high-performance metabolism. This fragile and well-balanced Antarctic system is quite vulnerable to climate change and may result in the displacement of P. antarctica populations for instance in the Antarctic Peninsula region, which already shows dramatic changes due to warming (Mintenbeck et al. 2011). Investigations in the Ross Sea emphasize the close association of Pleuragramma’s life cycle, especially spawning and larval development, with the cryopelagic habitat and sea-ice cover (La Mesa and Eastman 2012; Vacchi et al. 2012b). The reduction and eventually disappearance of P. antarctica stocks in more northerly Antarctic regions may result in a dramatic shift from a lipid-based to a less energy-rich food web with drastic effects for the whole community. In this respect, the Antarctic silverfish may prove to be a keystone predator, an essential component of the Antarctic Ocean.
References
Albers CS, Kattner G, Hagen W (1996) The compositions of wax esters, triacylglycerols and phospholipids in Arctic and Antarctic copepods: evidence of energetic adaptations. Mar Chem 55:347–358
Albertson RC, Yan YL, Titus TA et al (2010) Molecular pedomorphism underlies craniofacial skeletal evolution in Antarctic notothenioid fishes. BMC Evol Biol 10:4. doi:10.1186/1471-2148-10-4
Andriashev AP (1987) A general review of the Antarctic bottom fish fauna. In: Kullander SO, Fernholm B (eds), Proceedings of fifth congress of European ichthyologists, Stockholm 1985, pp 357–372
Bottaro M, Oliveri D, Ghigliotti L et al (2009) Born among the ice: first morphological observations on two developmental stages of the Antarctic silverfish Pleuragramma antarcticum, a key species of the Southern Ocean. Rev Fish Biol Fish 19:249–259
Bottino NR (1975) Lipid composition of two species of Antarctic krill; Euphausia superba and E. crystallorophias. Comp Biochem Physiol 50B:479–484
Clarke A, Johnston IA (1996) Evolution and adaptive radiation of Antarctic fishes. Trends Ecol Evol 11:212–218
Dalsgaard J, St John M, Kattner G et al (2003) Fatty acid trophic markers in the pelagic marine environment. Adv Mar Biol 46:225–340
Eastman JT (1985) The evolution of neutrally buoyant notothenioid fishes: their specializations and potential interactions in the Antarctic marine food web. In: Siegfried WR, Condy PR, Laws RM (eds) Antarctic nutrient cycles and food webs. Springer, Berlin, pp 430–436
Eastman JT (1988) Lipid storage systems and the biology of two neutrally buoyant Antarctic notothenioid fishes. Comp Biochem Physiol 90B:529–537
Eastman JT (2005) The nature of the diversity of Antarctic fishes. Polar Biol 28:93–107
Eastman JT, De Vries AL (1982) Buoyancy studies of notothenioid fishes in McMurdo Sound, Antarctica. Copeia 1982:385–393
Eastman JT, De Vries AL (1989) Ultrastructure of the lipid sac wall in the Antarctic notothenioid fish Pleuragramma antarcticum. Polar Biol 9:333–335
Evans CW, Williams DE, Vacchi M et al (2012) Metabolic and behavioural adaptations during early development of the Antarctic silverfish, Pleuragramma antarcticum. Polar Biol 35:891–898
Friedrich C, Hagen W (1994) Lipid contents of five species of notothenioid fish from high-Antarctic waters and ecological implications. Polar Biol 14:359–369
Giraldo C, Mayzaud P, Tavernier E et al (2013) Lipid components as a measure of nutritional condition in fish larvae (Pleuragramma antarcticum) in East Antarctica. Mar Biol 160:877–887
Graeve M, Kattner G, Hagen W (1994) Diet-induced changes in the fatty acid composition of Arctic herbivorous copepods: experimental evidence of trophic markers. J Exp Mar Biol Ecol 182:97–110
Graeve M, Albers C, Kattner G (2005) Assimilation and biosynthesis of lipids in Arctic Calanus species based on feeding experiments with a 13C labelled diatom. J Exp Mar Biol Ecol 317:109–125
Grote B, Ekau W, Stenevik EK et al (2012) Characteristics of survivors – growth and nutritional condition of early life stages of the hake species Merluccius paradoxus and M. capensis in the southern Benguela ecosystem. ICES J Mar Sci 69:553–562
Hagen W (1988) On the significance of lipids in Antarctic zooplankton. Rep Polar Res 49:1–129 English version: Can Transl Fish Aquatic Sciences 5458, 1989, 1–149
Hagen W, Kattner G, Graeve M (1995) On the lipid biochemistry of polar copepods: compositional differences in the Antarctic calanoids Euchaeta antarctica and Euchirella rostromagna. Mar Biol 123:451–457
Hagen W, Kattner G, Friedrich C (2000) The lipid compositions of high-Antarctic notothenioid fish species with different life strategies. Polar Biol 23:785–791
Hagen W, Kattner G, Terbrüggen A et al (2001) Lipid metabolism of the Antarctic krill Euphausia superba and its ecological implications. Mar Biol 139:95–104
Hubold G (1984) Spatial distribution of Pleuragrarnma antarcticum (Pisces: Nototheniidae) near the Filchner- and Larsen ice shelves (Weddell Sea/Antarctica). Polar Biol 3:231–236
Hubold G (1985) Stomach contents of the Antarctic silverfish Pleuragramma antarcticum from the southern and eastern Weddell Sea (Antarctica). Polar Biol 5:43–48
Hubold G (1991) Ecology of notothenioid fishes in the Weddell Sea. In: di Prisco G, Maresca B, Tota B (eds) Biology of Antarctic fish. Springer, Berlin, pp 3–22
Hubold G (1992) Zur Ökologie der Fische im Weddellmeer. Rep Polar Res 103:1–157
Hubold G (2009) The Weddell Sea and the Pleuragramma story. In: Hempel G, Hempel I (eds) Biology studies in polar oceans – exploration of life in icy waters. Wirtschaftsverlag NW, Bremerhaven, pp 165–170
Hubold G, Ekau W (1990) Feeding patterns of post-larval and juvenile notothenioids in the southern Weddell Sea (Antarctica). Polar Biol 10:255–260
Hubold G, Hagen W (1997) Seasonality of feeding and lipid content in juvenile Pleuragramma antarcticum (Pisces: Nototheniidae) in the southern Weddell Sea. Proc Sixth SCAR Symp on Antarctic Biology in Venice, Italy, pp 277–283
Kattner G, Hagen W (1998) Lipid metabolism of the Antarctic euphausiid Euphausia crystallorophias and its ecological implications. Mar Ecol Prog Ser 170:203–213
Kattner G, Graeve M, Hagen W (1994) Ontogenetic and seasonal changes in lipid and fatty acid/alcohol compositions of the dominant Antarctic copepods Calanus propinquus, Calanoides acutus and Rhincalanus gigas. Mar Biol 118:637–644
Kattner G, Albers C, Graeve M et al (2003) Fatty acid and alcohol composition of the small polar copepods, Oithona and Oncaea: indication on feeding modes. Polar Biol 26:666–671
Kellermann A (1987) Food and feeding ecology of postlarval and juvenile Pleuragramma antarcticum (Pisces; Notothenioidei) in the seasonal pack ice zone off the Antarctic Peninsula. Polar Biol 7:307–315
Koubbi P, Vallet C, Razouls S et al (2007) Condition and diet of larval Pleuragramma antarcticum from Terre Adélie (Antarctica) during summer. Cybium 31:67–76
Koubbi P, Duhamel G, Hecq J-H et al (2009) Ichthyoplankton in the neritic and coastal zone of Antarctica and Subantarctic islands: a review. J Mar Syst 78:547–556
La Mesa M, Eastman JT (2012) Antarctic silverfish: life strategies of a key species in the high-Antarctic ecosystem. Fish Fish 13:241–266
Lee RF, Patton JS (1989) Alcohol and waxes. In: Ackman RG (ed) Marine biogenic lipids, fats and oils. CRC Press, Boca Raton, pp 73–102
Maes J, Van de Putte A, Hecq J-H et al (2006) State-dependent energy allocation in the pelagic Antarctic silverfish Pleuragramma antarcticum: trade-off between winter reserves and buoyancy. Mar Ecol Prog Ser 326:269–282
Mayzaud P, Chevallier J, Tavernier E et al (2011) Lipid composition of the Antarctic fish Pleuragramma antarcticum. Influence of age class. Polar Sci 5:264–271
Mintenbeck K, Barrera-Oro ER, Brey T et al (2011) Impact of climate change on fishes in complex Antarctic ecosystems. Adv Ecol Res 46:351–426
Nevenzel JC (1970) Occurrence, function and biosynthesis of wax esters in marine organisms. Lipids 5:308–319
Phleger CF, Nelson MM, Mooney BD et al (1999a) Wax esters versus triacylglycerols in myctophid fishes from the Southern Ocean. Antarct Sci 11:436–444
Phleger CF, Nichols PD, Erb E et al (1999b) Lipids of the notothenioid fishes Trematomus spp. and Pagothenia borchgrevinki from East Antarctica. Polar Biol 22:241–247
Reinhardt SB, Van Vleet ES (1986) Lipid composition of twenty-two species of Antarctic midwater zooplankton and fish. Mar Biol 91:149–159
Sargent JR, McIntosh R, Bauermeister A et al (1979) Assimilation of the wax esters of marine zooplankton by herring (Clupea harengus) and rainbow trout (Salmo gairdneri). Mar Biol 51:203–207
Tavernier E, Mayzaud P, Boutoute M et al (2012) Lipid characterization of Pleuragramma antarcticum (Nothoteniidae) larvae off East Antarctica (139°E-145.10°E) during summer. Polar Biol 35:829–840
Tocher DR, Fraser AJ, Sargent JR et al (1985) Lipid class composition during embryonic and early larval development in Atlantic herring (Clupea harengus L.) Lipids 20:84–89
Vacchi M, La Mesa M, Dalu M et al (2004) Early life stages in the life cycle of Antarctic silverfish, Pleuragramma antarcticum in Terra Nova Bay, Ross Sea. Antarct Sci 162:99–305
Vacchi M, DeVries AL, Evans CW et al (2012a) A nursery area for the Antarctic silverfish Pleuragramma antarcticum at Terra Nova Bay (Ross Sea): first estimate of distribution and abundance of eggs and larvae under the seasonal sea-ice. Polar Biol 35:1573–1585
Vacchi M, Koubbi P, Ghigliotti L et al (2012b) Sea-ice interactions with polar fish - focus on the Antarctic silverfish life history. In: Verde C, di Prisco G (eds) Adaptation and evolution in marine environments. “From pole to pole” series. Springer, Berlin/Heidelberg, pp 51–73
Vallet C, Beans C, Koubbi P et al (2011) Food preferences of larvae of Antarctic silverfish Pleuragramma antarcticum Boulenger, 1902 from Terre Adélie coastal waters during summer 2004. Polar Sci 5:239–251
Von Dorrien C (1989) Ichthyoplankton in Abhängigkeit von Hydrographie und Zooplankton im Weddellmeer. MSc Thesis, Univ Kiel, 67pp
Wöhrmann APA, Hagen W, Kunzmann A (1997) Adaptations of the Antarctic silverfish Pleuragramma antarcticum (Pisces: Nototheniidae) to pelagic life in high-Antarctic waters. Mar Ecol Prog Ser 151:205–218
Zimmermann C, Hubold G (1998) Respiration and activity of Arctic and Antarctic fish with different modes of life: a multivariate analysis of experimental data. In: di Prisco G, Pisano E, Clarke A (eds) Fishes of Antarctica: a biological overview. Springer, Milano, pp 163–174
Acknowledgements
We thank Petra Wencke for excellent analytical support and Maya Bode for conducting the PCA analyses. We are grateful to Gerd Hubold for fruitful discussions concerning the early life history of P. antarctica in the Weddell Sea. We thank the anonymous reviewers for their constructive comments.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 Springer International Publishing AG
About this chapter
Cite this chapter
Hagen, W., Kattner, G. (2017). The Role of Lipids in the Life History of the Antarctic Silverfish Pleuragramma antarctica . In: Vacchi, M., Pisano, E., Ghigliotti, L. (eds) The Antarctic Silverfish: a Keystone Species in a Changing Ecosystem. Advances in Polar Ecology, vol 3. Springer, Cham. https://doi.org/10.1007/978-3-319-55893-6_7
Download citation
DOI: https://doi.org/10.1007/978-3-319-55893-6_7
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-55891-2
Online ISBN: 978-3-319-55893-6
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)