Abstract
Pancreatic cancer can have a deleterious effect on a patient’s nutritional status. There is a high prevalence of cachexia as well as malnutrition in many patients with pancreatic cancer. Exocrine pancreatic insufficiency (EPI) can contribute to cachexia secondary to steatorrhea-related symptoms and malabsorption both lending to limitations in food intake, absorption, and subsequently weight loss. EPI is diagnosed in one out of two patients preoperatively though this markedly increases postoperatively. It is unfortunately not assessed routinely or even at the time of diagnosis, and when diagnosed and treated, pancreatic enzyme replacement therapy is too often suboptimal. As new oncologic protocols present for patients with pancreatic cancer, it is imperative that nutritional status is optimized enabling patients to endure surgery, new (neo)adjuvant therapies, and palliative options in an effort to improve outcomes and quality of life. Future studies are direly needed to better understand the course of pancreatic insufficiency over time and optimal treatment for EPI in patients with pancreatic cancer.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
- Exocrine pancreatic insufficiency
- Pancreatic enzyme replacement therapy
- Malnutrition/undernutrition
- Cachexia
Case Study
A 52-year-old with history of pancreatic cancer who was well nourished preoperatively underwent Whipple resection and gastrojejunostomy. The patient reports to surgery clinic 4 weeks postoperatively and has a weight of 129 lbs, decreased appetite, dysgeusia, early satiety, and intolerance to higher fat foods; he has an obvious loss in lean body mass based on nutrition focused physical assessment. He describes his bowel movements as yellow, oily, and malodorous.
My Management
-
A.
Start on pancreatic enzyme replacement therapy after diagnosis of exocrine pancreatic insufficiency (EPI).
May not be necessary to dose via J-tube with semi-elemental or elemental enteral formulas vs. isotonic enteral formulas.
-
B.
There is a risk of clogging jejunostomy tube and/or decreasing efficacy of enzymes, but pancreatic enzyme capsules can be opened and emptied into either thickened acidic liquid suspension or thin food (apple sauce) or mixed with sodium bicarbonate to then infuse via J-tube.
-
C.
Dosing recommendations are 1000–2000 IU/kg lipase per meal or 25,000–50,000 IU lipase for main meal and 10,000–25,000 IU lipase for snacks, without exceeding 10,000 IU/kg lipase per day. Lipase per meal titrates up as the volume of food increases and/or signs/symptoms of EPI are apparent.
-
D.
Dose enzymes with first bite of food and throughout meal. This may make a difference for some patients though may also be dependent on transit time of food through the gut postoperatively.
Diagnosis and Assessment
Malnutrition is prevalent in pancreatic cancer and may have significant and adverse impact on quality of life and overall survival. It is estimated that more than 80% of patients with pancreatic adenocarcinoma will have weight loss at the time of presentation. Malnutrition “should be considered a significant independent risk factor in patients with pancreatic cancer and one of the primary goals of treatment should be to improve nutritional status.” Studies demonstrate that improvement in nutrition status is correlated with better survival and quality of life despite stage of disease [1,2,3,4,5,6].
Patients with pancreatic cancer also experience the highest incidence of cachexia estimated at 70–80% and is associated with poorer disease and surgical outcomes. The impact of cachexia on prognosis and outcome is significant including reduced treatment tolerance, worsened postoperative outcome, higher rates of metastatic disease, more progressive disease, reduced survival, and of course decreased quality of life. Malabsorption through EPI is an exacerbating factor of cachexia in pancreatic cancer [2].
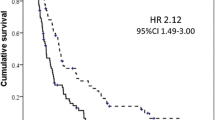
Weight loss in pancreatic cancer is associated with reduced survival. It was found that a weight loss of >5% and ≤10% of total body weight provided a 3.9-fold higher relative risk of death than those without weight loss, while a weight loss >10% of total body weight provided a sevenfold higher relative risk of death than those without weight loss [7].
In surgical patients, malnutrition and cachexia have been associated with infection, poor wound healing, increased postoperative complications, increased length of stay, and increased morbidity [8, 9]. Postoperative weight loss is an independent prognostic factor. Hashimoto et al. showed that severe weight loss is associated with poor prognosis and a trend toward shorter survival [10].
Exocrine pancreatic insufficiency in pancreatic cancer is very common with 25–45% having preoperative EPI and 50–80% of patients continuing to experience EPI post surgery at 3 months, 1 year, and 2 year postoperatively. One study reported steatorrhea worsening postoperatively, with return to baseline by 12 months [11,12,13].
Management
The deficiency in pancreatic enzymes results in inadequate absorption of fat, carbohydrates, and proteins, leading to steatorrhea, abdominal cramps, weight loss, and malnutrition. Exocrine enzyme insufficiency is common and progressive. Patients should be regularly screened for symptoms of enzyme insufficiency. Oral pancreatic exocrine enzyme replacement therapy is recommended for patients with pancreatic cancer who have symptoms of exocrine enzyme deficiency [14, 15].
Because pancreatic exocrine insufficiency occurs in up to 94% of patients undergoing pancreatic surgery and 50–89% of nonsurgical patients, therapy may be initiated based on symptomatology without diagnostic tests. Pancreatic enzyme replacement therapy helps maintain weight and quality of life in patients with unresectable pancreatic cancer [5, 14].
Outcome
Postoperatively, once EPI is identified and managed with pancreatic enzymes, the patient was able to eat more comfortably with improved digestion and begins to maintain or even gain weight and experience improvement in quality of life.
Clinical Pearls/Pitfalls
-
Symptoms of EPI are often nonspecific, so a high index of clinical suspicion is needed to make a correct diagnosis, important to assess in virtually all pancreatic cancer patients.
-
Patients and caregivers should be instructed on recognizing signs and symptoms of EPI, and it is not uncommon to ask patients to keep a diary.
-
Patients should be reminded on taking their enzymes at first bite of eating, and sometimes there is improvement if dosed throughout the meal.
-
Too often patients under dose enzymes per meals do not find improvement in symptoms so they will discontinue completely.
-
Patients with a clinical suspicion of pancreatic exocrine insufficiency despite appropriate replacement should receive a more thorough nutritional evaluation by a registered dietitian nutritionist.
-
Some private insurers as well as Medicare and Medicaid may not provide coverage of enzyme replacement. Some pharmaceutical companies may offer patient vouchers for assistance or online patient assistant programs.
-
Excellent patient resources regarding EPI and pancreatic enzymes are available to patients and caregivers on websites such as Pancreatic Cancer Action Network (www.pancan.org) as well as National Pancreas Foundation (www.npf.org).
References
Bauer JD, Capra S. Nutrition intervention improves outcomes in patients with cancer cachexia receiving chemotherapy—a pilot study. Support Care Cancer. 2005;13(4):270–4.
Mueller TC, Burmeister MA, Bachmann J, Martignoni ME. Cachexia and pancreatic cancer: are there treatment options? World J Gastroenterol. 2014;20(28):9361–73.
Vashi P, Popiel B, Lammersfeld C, Gupta D. Outcomes of systematic nutritional assessment and medical nutrition therapy in pancreatic cancer. Pancreas. 2015;44(5):750–5.
Davidson W, Ash S, Capra S, Bauer J, Cancer Cachexia Study Group. Weight stabilization is associated with improved survival duration and quality of life in unresectable pancreatic cancer. Clin Nutr. 2004;23(2):239–47.
Pancreatic Section of the British Society of Gastroenterology, Pancreatic Society of Great Britain and Ireland, Association of Upper Gastrointestinal Surgeons of Great Britain and Ireland, Royal College of Pathologists, Special Interest Group for Gastro-Intestinal Radiology. Guidelines for the management of patients with pancreatic cancer periampullary and ampullary carcinomas. Gut. 2005;54(Suppl 5):v1–16.
Victorian Department of Health and Human Services. Optimal care pathway for people with pancreatic cancer. http://www.cancervic.org.au/downloads/health-professionals/optimal-carepathways/Optimal_care_pathway_for_people_with_pancreatic_cancer.pdf. Accessed Nov 2016.
Papadoniou N, Kosmas C, Gennatas K, Polyzos A, Mouratidou D, Skopelitis E, Tzivaras M, Sougioultzis S, Papastratis G, Karatzas G, Papalambros E, Tsavaris N. Prognostic factors in patients with locally advanced (unresectable) or metastatic pancreatic adenocarcinoma: a restrospective analysis. Anticancer Res. 2008;28(1B):543–9.
Kanda M, Fujji T, Kodera Y, Nagai S, Takeda S, Nakao A. Nutritional predictors of postoperative outcome in pancreatic cancer. Br J Surg. 2011;98(2):268–74.
LaTorre M, Ziparo V, Nigri G, Cavallini M, Balducci G, Ramacciato G. Malnutrition and pancreatic surgery: prevalence and outcomes. J Surg Oncol. 2013;107(7):702–8.
Hashimoto D, Chikamoto A, Ohmuraya M, et al. Impact of postoperative weight loss on survival after resection of pancreatic cancer. JPEN. 2015;39(5):598–603.
Sikkens EC, Cahen DL, de Wit J, Looman CW, van Eijck C, Bruno MJ. Prospective assessment of the influence of pancreatic cancer resection on exocrine pancreatic function. Br J Surg. 2014;101(2):109–13.
Matsumoto J, Traverso W. Exocrine function following the whipple operation as assessed by stool elastase. J Gastrointest Surg. 2006;10(9):1225–9.
Park JW, Jang JY, Kim EJ, Kang MJ, Kwon W, Chang YR, Han IW, Kim SW. Effect of pancreatectomy on nutritional state, pancreatic function and quality of life. Br J Surg. 2013;100(8):1064–70.
Bartel MJ, Asbun H, Stauffer J, Raimondo M. Pancreatic exocrine insufficiency in pancreatic cancer: a review of the literature. Dig Liver Dis. 2015;47(12):1013–20.
Tseng DS, Molenaar IQ, Besselink MG, van Eijck CH, Borel Rinkes IH, van Santvoort HC. Pancreatic exocrine insufficiency in patients with pancreatic or periampullary cancer: a systematic review. Pancreas. 2016;45(3):325–30.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 Springer International Publishing AG
About this chapter
Cite this chapter
Mills, J.B. (2017). Optimizing Nutrition for the Patient after Pancreaticoduodenectomy: Pancreatic Insufficiency. In: Gardner, T., Smith, K. (eds) Pancreatology. Springer, Cham. https://doi.org/10.1007/978-3-319-53091-8_12
Download citation
DOI: https://doi.org/10.1007/978-3-319-53091-8_12
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-53090-1
Online ISBN: 978-3-319-53091-8
eBook Packages: MedicineMedicine (R0)