Abstract
Heteropolyacids (HPAs) are a class of inorganic materials that have been widely used as additives to enhance the performance of fuel cell membranes, recently. This chapter covers the use of HPAs in the preparation of proton exchange membranes (PEM) for polymer electrolyte membrane fuel cells (PEMFCs). The fundamental aspects of HPAs and their corresponding salts in addition to various structural configurations such as Keggin, Wells–Dawson, and Lacunar are discussed. The use of HPAs for preparation of membranes for high-temperature PEMFC and direct methanol fuel cell (DMFC) based on the immobilization on various substrates including perfluorinated sulfonic acids (PFSAs), aromatic hydrocarbons, poly(vinyl alcohol) (PVA), and polybenzimidazole (PBI) are reviewed. The research challenges that need to be addressed to bring the new composite membranes to practical application are also discussed.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
The interest in developing highly stable and cost-effective new proton exchange membranes for polymer electrolyte fuel cells (PEMFCs) has been ever growing to promote the commercialization for this type of fuel cell [1–12]. This is to overcome the limitation of perfluorosulfonic acid (PFSA) membranes, which rely on water in proton conduction that prevent the operation of PEMFCs above 80 °C [13–15]. Higher fuel cell operation is desired to enhance reaction kinetics of electrochemical reactions, increase CO tolerance of electrodes and reduce or eliminate water management system [16–18].
Various approaches have been used to develop alternative membranes including modification of commercially available PFSA membranes, chemical synthesis of sulfonate hydrocarbon membranes, and formation of composite membranes [19]. Particularly, composite ionic membranes composed of organic–inorganic components have received great attention in the past decade because of their ability to be operated under wide ranges of temperatures including much higher ones than membranes made of the pure polymers [20]. Moreover, the incorporation of inorganic fillers provides a number of advantages ranging from improvement in the mechanical properties and water management of the membrane to inhibition of the fuel crossover by increasing the tortuosity of transport pathways. These composite membranes can be formed by: (1) introducing nanosized hygroscopic inorganic fillers such as SiO2, ZrO2, and TiO2, (2) doping of basic substrates with nonvolatile proton conducting solvents such as phosphoric acid, N-heterocycles, heteropolyacids (HPAs), and ionic liquids, and (3) incorporation of solid proton conductors such as phosphate salts of zirconium (ZrP) and HPAs. Various synthesis routes can be used to introduce one phase into the other one. A review on various methods of introducing each phase in organic/inorganic composite membrane is available [21].
Among composite membranes, those doped with or containing solid proton conductors such as HPAs are promising candidates for PEMFC [22, 23, 21]. This is because HPAs display the highest proton conductivity among inorganic solids near ambient temperatures. They also have distinct discrete ionic structures including heteropoly anions and counter cations (e.g., H+, H3O+, and H5O2 +) and therefore exhibit high-proton mobility. Moreover, the presence of bounded water molecule in HPAs makes them interesting for the development of moderate temperature PEMFC since proton conduction is independent of external humidification [2, 23].
The objective of this chapter is to review the progress of developments in regard to composite ionic membranes based on HPAs. A special attention is given to the types and structures of HPAs and their use in synthesis of nanocomposite polymeric materials with ion conducting functional groups for various types of PEMFC applications. The challenges remaining for further research to achieve important breakthroughs are also discussed.
2 Heteropolyacids Types and Structures
Polyoxometalates (POMs) are group of chemicals that have attracted an increasing attention due to the diversity and selectivity of their properties making them suitable for wide number of applications [24]. HPAs are complex proton acids incorporating polyoxometalate anions (heteropolyanions) having metal-oxygen octahedral as basic structural units [25, 26]. Keggin and Wells–Dawson are two important categories of the HPAs. In 1933, Keggin used X-ray diffraction technique to determinate the H3[PW12O40]5H2O structure and provided a clear description of the bonds between the WO6 octahedra of the molecule [27]. The Wells–Dawson structure was theoretically described by Wells in 1945 and experimentally confirmed in 1953 [28].
HPAs are subsets of metal oxides distinguished by central heteroatoms, which are surrounded by a number of metal-oxygen octahedra. There are strong bonds between the atoms supporting the polyhedra structures and connect them with the heteroatoms. The metal in HPAs is usually tungsten or molybdenum and less usually vanadium or uranium. The heteropoly anions having metal-oxygen octahedral as the basic structural units make up the primary structure of the HPA as shown in the Keggin anion (Fig. 1).
The Keggin HPAs, which is the most studied, represented by the formula \( {\text{X}}^{{\text{x}}+}{\text{M}}_{12}{\text{O}}{_{40}}{^{{\text{x}}-8}} \), contain 12 addenda atoms and one heteroatom, where X is the heteroatom (central atom, commonly P5+, Si4+, or B3+), x is its oxidation state, and M is the addenda atom (metal ion, commonly W and Mo). It is composed of a central tetrahedron (XO4), which is surrounded by 12 edge- and corner-sharing metal oxide octahedra (MO6). The octahedra are arranged in four M3O13 groups, and each group has three octahedra sharing edges. They are linked through strong oxygen bonds, which also connect the central tetrahedron [29]. A typical FTIR-ATR spectrum of Keggin-type phosphotungstic acid (PWA) showing four different W–O bonds and characteristic bands at 1080 (P–Oa), 983 (W = Od), 893 (W–Ob–W), and 797 (W–Oc–W) cm−1 is shown in Fig. 2.
Due to the complexity, other polyoxometalates including Wells–Dawson heteropolyanions \( {\text{X}}{_{2}}{^{{\text{x}}+}}{\text{M}}_{18}{\text{O}}{_{62}}{^{2{\text{x}}-16}} \), Keggin and Dawson lacunary anions, \( {\text{XM}}_{11}{\text{O}}{_{39}}{^{{\text{x}}-12}} \) and \( {\text{X}}_2{\text{M}}_{17}{\text{O}}{_{61}}{^{2{\text{x}}-20}} \), and transition metal complexes have not been fully examined.
In addition to metals, HPAs are interestingly having multiple active sites in their structure including protons and oxygens. Functionally, protons act as Brønsted acids to promote acid-catalyzed process including proton transports. Besides, some oxygen atoms on the surface of HPA (especially oxygens located on the lacunary sites of lacunary POM anions with a high negative charge) are basic enough to react with protons and hydronium ions (protonate) and can practically behave as active sites in base-catalyzed reactions. On the other hand, the metals of HPAs are active sites in all oxidative reactions. The details of various catalytic activities of HPAs were reviewed [30–32].
Although Dawson HPAs were used in various applications including proton transport materials [33], majority of the patents and investigations are based on the applications of the Keggin-type HPAs and their salts. This primarily includes H3PMo12O40 (PMA), H3PW12O40 (PWA), H4SiMo12O40 (SiMA), and H4SiW12O40 (SiWA). Solid HPAs commonly form ionic crystals composing of anions (which is heteropolyanions), cationic counterions (H+, H3O+, H5O2 +, etc.), and hydration water. The HPAs are noticeably crystallizing with a large number of water molecules as in PWA and PMA, both of which have 29 water molecules compared to 30 for SiWA [34, 35]. The status of water is important for assessing HPA role as a proton transfer material. Some HPAs loss water very easily even at room temperature where as others can retain water tightly even at high temperature.
In HPA crystals, the Keggin anions are quite mobile. Due to structural flexibility and mobility of HPAs, not only water but also a diversity of polar molecules can enter and leave HPA crystals that is important when using HPAs in fuel cell applications. It should be noted that proton conductivity of solids is generally related to their acid-based catalytic activities [36]. HPAs display strong acidity, good solubility in polar solvents such as water, lower alcohols, ketones, ethers and esters, in addition to good thermal stability and reversible redox behavior. One of the most important properties of HPAs is the ability to be chemically adjusted or tuned by simple modification of their structures. The acidity of the HPAs is dependent upon the total charge of the anion and the heteroatom in their structure [37–39]. The strong acidity level means fast proton mobility, which results in a high catalytic activity [29]. The acidity strength of crystalline HPAs decreases in the series PW > SiW ≥ PMo > SiMo, which is identical to that in solutions [40].
Due to their versatile properties, HPAs have been used in an extensive range of applications, including chemical analysis, biochemistry, ion selective membranes, sensors, and electrochemical energy devices [41, 42]. Particularly, HPAs have been useful constituent of polymer electrolyte fuel cells (PEFCs) and their corresponding fuel cells using liquid fuels such as methanol or ethanol. Intensive work has been reported on membranes incorporating HPAs for fuel cells, specifically, saturated HPAs and unsaturated (defective) or lacunary HPAs were evaluated for fuel cell applications [43]. Figure 3 compares the structure of saturated HPA of [SiW12O40]4− in a hydrated form of H4[SiW12O40]22H2O, which is also known as 12-HSiW and one of the corresponding lacunary structures [SiW11O39]8− in a hydrated form of H8[SiW11O39]26H2O known as 11-HSiW. As shown, the number of acidic hydrogens and water molecules is higher in lacunary HPAs. Interestingly, lacunary HPAs can be simply prepared by hydrolysis of the parent HPAs with a base under controlled conditions of temperature and ionic strength. Removal of one, two, or three metal oxide (MO) units from the parent HPAs results in a mono-, di-, or tri- lacunary POMs. A general reaction scheme for the preparation of various lacunary HPAs is shown Fig. 4.
Lacunary HPAs could be further modified by bonding with functional silanes that can subsequently crosslinked or polymerized to achieve more stable HPAs. Such HPAs offer high ionic conductivities prompting their use in fuel cells. For example, indium-substituted HPAs with Keggin H4[In(H2O)PW11O39]11H2O and H5[In(H2O)SiW11O39]8H2O, structures were reported to be solid high-proton conductors with a conductivity of 2.60 × 10−4 S cm−1 and 5.25 × 10−4 S cm−1 (at 18 °C and 80% relative humidity), respectively. The conductivity of these materials also increased with the rise of the temperature [44].
The conductivity performance of HPAs varies widely depending upon the type of the HPAs’ structure. Particularly, the change in the constitutional elements of polyanion can lead to the difference in the degree of hydration and proton mobility [45]. Typically, H5[SiW11VO40]15H2O shows an excellent conductivity of 7.93 × 10−3 S cm−1 at 15 °C and 50% RH [38]. In addition, the size of the HPAs influences considerably their performances specially when they are incorporated in the composite membranes [46].
Generally, HPAs have high thermal stability, in the order of PW > SiW > PMo > SiMo (Keggin types) that decompose at 465, 445, 375, and 350 °C, respectively. On the other hand, the influence of the heteroatom on the thermal stability is negligible [47]. The redox activity of HPAs promotes their application as redox bi-functional catalysts. With increasing electronegativity of the addenda atom, the reduction potential of the HPA decreases, while increases as the electronegativity of the counter cations or the heteroatoms increases, but it decreases as the electronegativity of the addenda atom increases [28]. The oxidation potential decreases in the Keggin structures as PMo > SiMo ≫ PW > SiW.
HPAs have been used in an extensive range of applications, including chemical analysis, biochemistry, ion selective membranes, sensors, and electrochemical energy devices [41, 42]. Most generally, they are used as inorganic catalysts. Depending on their composition, HPAs can be effective catalysts for both acid catalysis and redox catalysis [47]. One of the important specific characteristics of the HPAs is that they can be tuned by adding different elements to their structures, allowing for the design of HPAs for special reactions. The high selectivity, thermal stability as well as hydrolytic stability, long lifetimes in solution, high oxidation potential, and noncorrosive nature of many HPAs make them economically and environmentally attractive as a natural ‘green catalysts’ [48]. In addition, the list of proteins or enzymes that can interact with HPA is long which supports the use of HPAs in a number of biochemical and biomedical applications [49]. Moreover, due to antiviral and antitumor activity of HPAs, these find use in biomedicine applications [50, 51].
The appealing applications of HPAs as dopants and fillers in ionic composite membranes are indicated by the large number of patents and publications appeared recently [2, 52, 53, 37, 54, 55, 56]. This was motivated by their high ionic conductivity, thermal stability, very strong Brønsted acidity approaching the superacid region (more acidic than sulfuric acid and Nafion), and their capability to undergo redox processes under the mild conditions. Such membranes also found uses in ion selective electrodes, gas detection apparatuses, solid-state electrochromic devices, and solid or liquid electrolytes in electrolytic cells [57, 58, 59, 31, 32]. A great deal of work has been focused on the self-immobilization of POMs especially the Keggin type into the polymers matrices such as polypyrrole, polythiophene, and polyaniline to increase the performance of hybrid membranes.
3 HPAs and Proton Transport in Fuel Cells
Due to the dissociated protons combined with the anions having good mobility, HPAs are excellent proton conductors and promising solid electrolytes. The interest in HPAs began after reporting remarkably high conductivities of 2 × 10−1 S cm−1 at 25 °C, and low activation energies of 15.5 and 13.7 kJ/mol for crystals of H3[Mo12PO40]29H2O and H3[W12PO40]29H2O [60], respectively. However, upon application for fuel cell, keeping the samples in their fully hydrated form without decomposition is difficult since HPAs dissolve in the water that formed in a fuel cell during the operation [61]. Highly concentrated aqueous solution of HPA was reported for the use as a fuel cell electrolyte, which resulted in a comparable performance with PEMFC [62, 63]. Although stable cell performance for HPA electrolyte was reported for 300 h, however, the high crossover results in voltage loss of fuel cell.
HPAs with high stability in water have been obtained upon immobilization on clay [64], aluminum phosphate [53], and silica supports [65]. It was reported that the addition of aluminum phosphate in SPEEK/PWA remarkably increased water retention and effectively reduced leaching of PWA from the composite membrane [53]. On the other hand, the proton conductivity of mesoporous silica is as low as 10−6–10−4 S cm−1. However, its remarkable structural order, large surface area and pore volumes, availability and simple functionalization make it an ideal porous framework for HPA based proton conductors [47, 66, 67].
Typically, an inorganic glass composite membrane containing a mixture of PWA and PMAPWA/PMA–P2O5–SiO2 resulted in very highly conductivity values of 1.014 S cm−1 at 30 °C and 85% RH [68] and 1.01 × 10−1 S cm−1 at 85 °C under 85% RH for a mesoporous-structured PWA–P2O5–SiO2 glass [69]. The fuel cell performance of these inorganic materials was 35–42 mW cm−2 in H2/O2 at 30% RH and 30 °C [68, 69]. However, they cannot be used as electrolytes in fuel cells in their powdered form. Therefore, composite membranes with a polymer binder have been developed for fuel cell application.
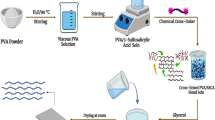
Several approaches to incorporate HPA into fuel cell membranes include fabricating unsupported HPA pellets [61], doping polymer electrolytes [70], and sol–gel methods [71]. Among these, HPAs have been mainly used as dopants in composite proton exchange membranes . It was suggested that specific interactions between HPAs and polymers could have a significant influence on the fuel cell performance at elevated temperatures and thus they have received considerable attention [71]. More details of various preparation methods can be found elsewhere [72–75]. Five different procedures were commonly used to produce such composite membranes as schematized in Fig. 5. They include mixing of HPA with a polymer in a solution form followed by casting [76, 77] or impregnation of HPA in a porous substrate [78, 70]. HPAs were supported on various substrates including silica. When preparing the membrane from a polymer solution, the incorporation of HPAs supported on silica within a polymeric membrane can be achieved by two different ways: (i) pre-formation of HPA–silica particles followed by direct mixing with a polymer solution [65, 79] and (ii) in situ preparation of the inorganic phase within the matrix during film formation, e.g., via the sol–gel process of an alkoxide precursor in the presence of PWA [80–82]. Particle size and distribution heterogeneity are two main concerns when using the first method. However, the sol–gel method is normally lead to better distribution, more stability and possible control for the size of the particles by variation of the preparation parameters such as pH, temperature, and concentration of starting components [83, 84].
HPA supported on the polymers and porous fillers are likely subjected to leaching out of HPA during the fuel cell operation, despite the improvement took place in other properties including proton conductivities [85]. Such limitation is due to low loading level, loose interaction with solid organic substrate and possible agglomeration leading to the formation of large particles or clusters in the polymer substrate during the casting process [86, 87]. To overcome this problem, a third effective method involving copolymerization of HPA containing monomers or covalent bonding of HPA to a polymer backbone was proposed [87, 88, 89, 43]. The covalent bonding of dopant to the polymer backbone minimizes leaching and enhances the immobilization level of HPAs. Nevertheless, this synthetic procedure is costly and, therefore, the application of this method was limited to few examples [52].
The forth method involves a self-immobilization of HPAs onto basic polymer backbones typically in a fiber form [2, 52] or adding another filler with high specific surface area such as reduced graphene oxide (rGO) [54] or rGO covalently modified with 3-aminopropyl-triethoxysilane (mGO) [90]. For example, amounts of up to 50 wt% of PWA could be simply immobilized onto nylon nanofibers by self-immobilization. The fifth proposed method for solving the leaching problem of HPAs was to use water-insoluble salt of HPAs such as Cs x H3−x PW12O40 [91]. In this regard, HPA salts such as CsH2PO4 have been intensively investigated because of their desirable high operating temperature (230–260 °C) [92, 93] and high-protonic conductivity (σ = 2.2 × 10−2 S cm−1 at 240 °C) [94]. Such advantages explain the reason for applying the compositions of CsH2PO4 with oxyanions [95], cations [96], and inorganic/organic scaffolds [97, 98] for improving proton conductivity, mechanical properties, and thermal stability at high temperatures in addition to reducing methanol permeability.
4 HPAs in PEM Fuel Cell
HPAs have been used extensively in the PEMFC as additive to the catalyst layer and membrane. As additive to the catalyst layer, HPAs have been used in both cathode and anode mainly to address the CO-poisoning by enhancing the tolerance. Particularly, it was reported that the addition of PMA and corresponding vanadium substituted analogs into Pt results in a remarkable performance improvements when the fuel cell was fed with reformed hydrogen containing 100 ppm CO [99]. It was concluded that PMA acts as an oxidizing agent for the reaction between CO and water in the presence of Pt, Pd, and Au [100, 22]. Besides, the addition of HPAs in the electrode is likely to improve the catalytic activity towards oxygen reduction reaction (ORR) and methanol oxidation reaction (MOR) [101, 55, 102]. However, HPA applications in the catalyst layers of electrodes are beyond the scope of this chapter and more details can be found elsewhere [22].
PFSAs are the choice materials for the membranes for PEMFC applications. However, these membranes are not gas tight enough especially at temperature above 80 °C [20, 5, 103]. Several low cost alternatives such as poly(vinyl alcohol) (PVA), sulfonated poly(ether ether ketone)s (SPEEK), and sulfonated polystyrene (SPS) have been evaluated for PEMFC applications. These membranes exhibited good chemical stability and low fuel crossover. However, their proton conductivity is significantly lower than PFSAs. Typically, the proton conductivity of PVA and SPEEK are in the ranges of 10−14–10−10 S cm−1 [22, 104] and 10−4–10−3 S cm−1 [83], respectively. HPAs have been introduced to various low cost PFSA alternatives to enhance the proton conductivity. This led to an increase in the conductivity up to the range of 10−3–10−1 S cm−1 [83, 105, 106]. Interestingly, such improvement in the conductivity was accompanied by an increase in the thermal stability of membranes.
5 HPAs in High-Temperature and Low-Humidity PEMFC
The main limitation of PFSA family of membranes is their dependence on level of hydration to achieve high conductivity, and this poses a problem when operating at temperatures higher than 80 °C as the proton conductivity of the membranes decreased significantly. The variation of hydration level undermines the permeability of H2 and O2 due to the involvement of the formed hydrated ionic clusters in the gas permeation mechanism [107, 108]. On the other hand, the retention and management of water within these types of membranes is challenging, costly and in most cases affect the PEMFC performance. Therefore, there is a strong demand for the PEMs to work at lower relative humidity and/or under anhydrous conditions. However, acceleration of electrochemical reactions and simplification of water management system of fuel cells under lower humidity and/or anhydrous conditions is challenged by dramatic losses of proton conductivity of PFSA membranes [109, 110, 5]. Typically, Nafion 112 losses an order of magnitude of its proton conductivity at around 60% RH, which increases two orders of magnitude with the reduction of RH to 25%.
Various materials and designing strategies were proposed to prepare alternative proton conductors for high-temperature fuel cell operation under dry conditions. The use of other media of proton conductions or less-volatile liquids such as phosphoric and phosphonic acids [1, 111] and amine heterocycles [112] have been widely considered. However, in most cases, the proton conductivity is not comparable to PFSAs and there is a risk of liquid electrolytes leaching. On the other hand, addition of an inorganic material with hydrophilic properties such as HPAs was proposed to maintain high water content levels at elevated temperatures or under dry conditions. There is a linear relationship between water content of the membrane and its conductivity at lower RH%.
A lot of work has been reported on incorporation of different HPAs in PFSA membranes [76, 113, 114] and other hydrocarbon membranes [83, 115, 105, 106, 116, 117] for PEMFCs application. It was shown that the activation energy for proton transport (E a) decreased significantly for PFSA membranes upon increasing the amount of HPAs under lower RH% [118]. For example, the addition of 5 wt% of PWA lowered E a by 40% at 60% RH compared to pristine membrane. Initial investigation on various HPAs such as PWA, SiWA, PMA, and SiMA indicated that the additives containing molybdenum were less stable in the membrane environment than those containing tungsten [76]. Upon operating in fuel cell for a few hours, the amount of molybdenum-based additive present in the cathode catalyst layer increased. It was suggested that molybdates migrated into the catalyst layers, where they had a detrimental effect on the performance by undergoing parasitic redox reactions on the surfaces of carbon and platinum, resulting in an increase in the activation overpotential. Table 1 summarizes some of the efforts to introduce HPAs to membranes used in the PEMFC.
Initial studies reported the limiting changes in the stability of HPA in hydrated conditions and the strong influence of humidity on the proton conductivities and diffusional problems of the membranes. The high water solubility of HPAs triggers leaching problem and consequently high drop in a performance. Two different approaches of using alternative heteropolysalts instead of their acid and supported HPAs were widely used to avoid the leaching problem in high-temperature PEMs. More stable HPA containing membranes were achieved using heteropolysalts as fillers in membranes for PEMFCs. In this regard, larger cations such as Cs+, NH4 +, Rb+, and Tl+ [133] as well as organic cations such as 4,4′- and 2,2′-bipyridines [134] were combined with HPAs to produce heteropoly salts. Precipitation from aqueous solutions was normally used to achieve such partially substituted heteropolysalts [135, 136]. On the other hand, it was shown that a solid-state reaction involving mechano-chemical treatments using a high-energy ball mill is more efficient to prepare heteropolysalts and normally lead to more conductive heteropolysalts [137, 138], and subsequent composite membranes [115]. Additional investigation revealed that the reduction in the particle size facilitates the conductivity due to enhancement in the surface to volume ratio, which permits more efficient proton hopping and increase in conductivity [23]. For example, reducing a filler size from 1–2 mm to 30–50 nm resulted in 35% enhancement in proton conductivity [139].
In the second approach, HPAs or their corresponding salts were supported on different materials including SiO2 [86, 121, 84, 122, 77, 140, 123], ZrO2 [84, 120, 77], WO2 [77], and TiO2 [84, 77] followed by immobilization in polymeric membranes [86]. PWA was found to be the only heteropolyacid that has been found suitable for these hybrid membranes, and it was concluded that SiO2 is the best supporting material since there is a strong interaction between the H3PW12O40, Nafion®, and the SiO2 molecules [121]. The proton conductivity and the fuel cell performance at 100% RH of the composite membrane close to those of pristine membranes. However, the merits of HPA became evident upon increasing the temperature or reducing the RH%. Improved performance under dry conditions was also achieved for membranes containing CsxH3−x PWO40 immobilized on TiO2 [141] and CeO2 [142]. Nevertheless, immobilization of HPA salts and their stability on the inorganic supports were much better than pure HPAs [141]. Table 1 illustrates some of the results on HPA salts loading level and properties of the obtained composite membranes.
Commercially available polybenzimidazole (PBI) has been the most extensively studied and used for the PEMFC under high-temperature . Particularly, PBI membranes impregnated with phosphoric acid have been studied as polymer electrolytes in PEMFCs for two decades and were reasonably successful with excellent thermo-chemical stability and good conductivity [143, 144]. Several organic modifiers including ZrP [7] and PWA [145] have been incorporated into PBI to improve the performance of the PBI/H3PO4 membrane.
PWA and SiWA [128, 146], PMA [147], PWA/SiO2 [148], and SiWA/SiO2 [145] were initially used to fabricate PBI composite membranes. Initial results indicated that the conductivities of the composite membranes containing 20 and 30 wt% of PWA at 140 °C and that of SiWA at 200 °C were lower than that of pristine PBI membranes under the same conditions [128]. It was reported that the SiO2 support provided a stable structure and membranes were thermally stable up to 400 °C. The conductivity values of 1.5 × 10−3 S cm−1 at 150 °C for PWA/SiO2/PBI and 2.23 × 10−3 S cm−1 at 160 °C for SiWA/SiO2/PBI were reported. In a separate study, 37.5 wt% of SiWA-SiO2/mPBI showed excellent proton conductivity of 1.32 × 10−3 S cm−1 at room temperature [149]. Despite the good conductivity values of the composite membranes, there are few reports on fuel cell performance. Membranes composed of PWA/PBI or SiWA/PBI doped with phosphoric acid showed high conductivity compared to neat PBI membranes in high-temperature PEMFC [150]. At 150 °C, the conductivity of the 40% SiWA/PBI composite membrane was 0.1774 S cm−1. In a single cell test, for example, 40 wt% SiWA/PBI exhibits only around 10–15 mV loss in cell potential due to IR drop which is around 75 mV at 500 mA cm−2 for PBI. The best fuel cell performance was achieved with a 40 wt% SiWA/PBI composite membrane at 120 °C. However, at higher temperatures, contrary to the PBI membrane, the performance of the SiWA/PBI composite membrane declined in fuel cell. It has been suggested that the PWA/PBI composite membranes were suitable for the temperature limit of 120 °C.
CsHPA was used extensively for PEMFC due to its insolubility in water. Typically, PBI/Cs2.5H0.5PMo12O40/H3PO4 composite membrane exhibited conductivity as high as 0.15 × 10−2 S cm−1 and in a fuel cell gave a high power density of 0.7 W cm2 (at atmospheric pressure and 150 °C with H2/O2) [130]. Higher proton conductivities of 1.91 × 10−2 S cm−1 and 1.71 × 10−2 S cm−1 were achieved at 160 °C under anhydrous conditions for mechano chemically synthesized CsHPA composites of PBI-50H3PW12O40·50CsHSO4 with 87 wt% phosphoric acid doping level and PBI-50H4SiW12O40·50CsHSO4 with 82 wt% doping level, respectively [98]. Nevertheless, these composite membranes show better conductivity with comparable single cell results, despite the lower phosphoric acid doping level compared to PBI.
Comparative conductivity, stability and fuel cell performance of PBI composite membranes with four different cesium salts of CsPOMo, CsPOW, CsSiOMo, and CsSiOW were evaluated [131]. The composite membranes loaded with H3PO4 showed higher conductivities than that of the phosphoric acid loaded PBI membrane. The conductivity increased with an increase in the percentage of inorganic component in the composite up to 30%. CsPOMo/PBI/H3PO4 exhibited a conductivity of 0.12 S cm−1 under anhydrous conditions at 150 °C. The membrane having P form of the CsHPA demonstrated higher conductivity than those containing Si atom, although the mechanical strength was inferior. The performance of the fuel cell with these composite membranes was better than that with a phosphoric acid-doped PBI membrane under the same conditions. The CsPOMo gave the best power density of around 0.6 W cm2 with oxygen at atmospheric pressure and 150 °C.
6 HPAs in DMFC
DMFCs is an alternative cell to the hydrogen PEMFC, which avoids the limitations associated with the use of H2 as a fuel. In order to be competitive, the DMFC must be reasonably cheap and capable of delivering high power densities. However, the performance of DMFCs is limited by the catalyst and membranes. The slow kinetics of the anode reaction and poisoning of the platinum catalyst together with the high methanol crossover are among main concerns. Typically, PFSA membranes showed exceptional proton conductivity upon employing in DMFCs but excess methanol permeability causes fuel loss, which lowers energy efficiency and fuel cell performance. The use of diluted methanol fuel (<4 molar of methanol), which supposed to minimize excessive fuel permeability is likely to reduce the energy density of fuel cell significantly [151]. Particularly, these drawbacks reduce the energy density to as low as 2000 Wh kg−1 and yield low operating voltage compared to PEMFC [152]. The ultimate solution to high methanol crossover, which is a key barrier for the development of DMFC, is the utilization of membranes with low methanol permeability that have potential for operation at higher temperatures. Introducing nanoparticles into polymers substrates is an effective tool to suppress methanol crossover, but is often accompanied by the partial loss of proton conductivity because protons and methanol are transported through the same pathway. HPAs having very strong Brønsted acidity approaching to the level of the superacids (more acidic than 100% sulfuric acid and Nafion) and fast reversible redox transformations are interesting candidates for preparation of composite membranes for DMFC [153]. HPAs have been immobilized in various proton conducting membranes to improve methanol barrier property and/or proton conductivity [154, 155]. DMFC performance based on the PWA—meso-silica nanocomposite membrane and low Pt catalyst loading (1 mg cm2)—was found close to the advanced DMFCs (100–200 mW cm2) [156].
Majority of reported studies of HPAs based composite membranes were dedicated to PFSA based membranes including Nafion. Generally, various HPAs were used as dopants that are usually sonicated with liquid Nafion in a high boiling point solvent before casting. Typically, stable molybdophosphoric acid (PMA)-impregnated membranes were reported to be prepared either by exposing cast membranes to solvent vapor at room temperature for 24 h and annealing at 150 °C [157] or by imbedding in polymer films using mixed solvents [158]. The obtained membranes showed noticeable enhancement in the conductivity [157] and improvement in the methanol rejection [158] compared to commercial Nafion.
Operation at high temperatures significantly enhances the kinetics of methanol oxidation. It was reported that the addition of inorganic hygroscopic materials to Nafion extends the operating temperature range of DMFC [159, 160, 140]. Nafion–silica composite membranes doped with phosphotungstic and silicotungstic acids were investigated at 145 °C and improvements in the electrochemical characteristics at high current densities were reported [140]. For instance, a maximum power density of 400 mW cm−2 was obtained at 145 °C in the presence of oxygen feed, whereas the maximum power density in the presence of air feed was around 250 mW cm−2 [140]. In another study, the power densities of 33 mW cm−2 at 80 °C, 39 mW cm−2 at 160 °C, and 44 mW cm−2 at 200 °C were reported [160]. Such improved performance was correlated to the strong interaction of inorganic fillers with water at high temperature. Table 2 illustrates additional examples of HPAs composite membranes for DMFC applications at high temperature as well as normal operation temperature.
PWA in SPEEKs and poly(1,4-phenylene ether ether sulfone) stabilized by surface modification with polypyrrole (Ppy) using an in situ polymerization method showed a reduction in the methanol crossover without significant leaching in PWA [163, 179]. In addition, the water uptake, swelling ratio, and the tensile strength of the surface-coated membranes were improved with the increase in the thickness of Ppy layer. In a similar approach, PWA was microcapsulated into imidazole and incorporated into SPEEK membrane matrix prior to evaluation in DMFC and revealed improved DMFC performance [154].
Pore-filling of polymer substrates with HPAs was also reported to achieve an improved performance of DMFC [168, 167]. Typically, pores of PVDF substrates filled by sulfonated poly(aryl ether ketone sulfone) membranes blended with phosphotungstic acid resulted in a membrane with comparable conductivity and lower methanol permeability than Nafion [167]. The layer-by-layer (LbL) self-assembly is another method to introduce a thin multilayer film on a substrate by sequential electrostatic adsorption between the negatively and positively charged polyelectrolytes. A 50% reduction in the methanol permeability was reported for most of the LbL membranes. Typically, bilayers of chitosan and PWA onto sulfonated poly (aryl ether ketone) (SPAEK) substrate resulted in a membrane with high-proton conductivity values up to 0.24 S cm−1 at 80 °C and extremely low water swelling ratio and methanol permeability [166].
Inorganic PEM such as PWA immobilized into mesoporous silica matrix such as MCM-41 (Si-PWA) was assembled and used in DMFC [180, 181]. The PWA/MCM-41 electrolyte has a high-proton conductivity of 4.5 × 10−2 S cm−1 at 150 °C with an activation energy of ca. 8 kJ mol−1. Single fuel cell tests showed a peak power density of 95 mW cm−2 at 100 °C and 100% RH with H2/O2 system and 90 mW cm−2 in methanol/O2 at 150 °C and an extremely low RH of 0.67%. This sub-micron to nano-sized Si-PWA immobilized onto PVA crosslinked glutaraldehyde [170] and porous PVDF [168] were also reported. Lower methanol permeability than Nafion and a maximum power density of around 44 mW cm−2 were obtained at 60 °C.
An interesting class of materials for suppressing methanol permeability is the polymers containing basic groups including amine, amide, imine, and imidazole [182–184]. One of the key membranes of interest in DMFC is PBI based materials such as commercialized Celtec-V membrane, which is a blend of PBI and poly(vinylphosphonic acid) [185]. Although some encouraging results were reported [186], the conductivity was low and needed to be improved. PWA was self-immobilized on various substrates with basic groups to reduce methanol permeability [52, 10]. Since the HPA is immobilized, the leaching risk is minimized and the conductivity could be simply tuned by controlling the level of PWA. Upon using micro-pores chitosan as a substrate, comparable performance and excellent methanol-blocking performance of 60% lower than that of Nafion 212 membrane was achieved [10].
Recently, it was reported that self-immobilization of high level of PWA on electrospun nylon nanofiberous results in a highly selective methanol barrier with undetectable leaching of PWA [52]. Upon assembling in a sandwiched proton conducting membranes (Fig. 6) with Nafion outer layers, superior methanol barrier properties (viz. P = 3.59 × 10−8 cm2 s−1) and selectivity mounting to more than 20 times higher than Nafion 115 coupled with proton conductivities reaching 58.6 × 10−3 mS cm−1 at 30 °C were achieved. When tested in DMFC single cell, the performance of hybrid membrane was far better than Nafion 115 especially at higher methanol concentrations. For example, at 2 M methanol feed, the composite membrane had an improvement in power peak by 49.6% over Nafion 115. An increase in the power peak by 113.3% was obtained with 5 M methanol feed and the maximum power density of 127.1 mW cm−2 that was observed for 3L membrane compared to 59.6 mW cm−2 for Nafion 115. In addition, the OCV, which is directly related to the methanol crossover, was improved to 693 and 675 mV giving about 41 and 78 mV higher than that of Nafion 115 in 2 and 5 M methanol, respectively. Self-immobilization of PWA on PES/PVP-PWA also resulted in comparable performance of 132 mW cm−2 to that of Nafion 212 at 80 °C [187, 11].
Three-layered membranes composed of self-immobilized PWA on electrospun nylon and two outer Nafion layers [52]
Cesium phosphotungstate salt enhances the proton conductivity and reduces methanol permeability of various host substrates including SPEEK [174] chitosan-hydroxy ethyl cellulose (CS-HEC) [188], chitosan [173], PVA [169] and PVA/PAA [171], etc. Cesium phosphotungstate salt has excellent conductive capability and increased stability in aqueous media. PVA/PAA membranes with various Cs salt of heteropolyacids were assembled for DMFC [171]. The blend of PVA and PAA was crosslinked with glutaraldehyde, and the Cs salts of different HPAs, including PMA, PWA, and silicotungstic acid (SiWA), were incorporated into the polymer network to form PVA/PAA–CsPMA, PVA/PAA–CsPWA, and PVA/PAA–CsSiWA. A dense network formation was achieved through crosslinking with glutaraldehyde, which led to an order of magnitude decrease in the methanol permeability compared to Nafion 115 membrane. The hybrid membrane containing CsSiWA exhibited a very low methanol permeability of 1.4 × 10−8 cm2 s−1) compared to other membranes. Better performance was achieved when sulfosuccinic acid used as a crosslinker possibly due to the presence of sulfonic acid in the structure of crosslinker [189]. Ultralow methanol permeability resulted to a power density of 150 mW cm−2 at a current density of 500 mA cm−2.
To avoid any heterogeneity in the membrane preparation and consequent decline in both proton conductivity and mechanical properties of composite membranes, which may cause by the relatively large particle sizes, an in situ synthesis of proton-conductive nanoparticles within a polymer matrix was reported [172]. Particularly, nanoparticles of Cs hydrogen salts of phosphotungstic acid (CsPW) synthesized in situ in Nafion (CsPW–Nafion) resulted in a 101.3% increase of maximum power density relative to pristine Nafion in DMFC [172].
7 Concluding Remarks and Future Perspectives
Various types of HPAs in a pure or modified forms and as an additive have been incorporated in composite membranes in various types of PEMFC. In composite proton exchange membranes , HPAs and theirs corresponding salts have been immobilized into various inert and ionomeric substrates to reduce fuel crossover and enhance the proton transport and fuel cell performance especially at high temperatures and in dry conditions. In addition, HPAs were evaluated in DMFC to suppress methanol crossover. Among various types of HPAs, phosphotungstic acid and corresponding cesium salts have been used widely due to their long-term stability in fuel cell.
Effective immobilization of HPAs onto polymeric substrates is still challenging due to the highly solubility of such fillers in water. Using of corresponding salts of HPAs, covalent attachments to the polymer backbones and immobilization on the porous silica or fibrous structured amid-type backbones have been proposed to overcome the HPAs leaching during PEMFC or DMFC operation. Although some preliminarily reports indicate a stable immobilization of HPAs in the composite membranes, the long-term stability under dynamic fuel cell conditions has been not provided in many cases. Particularly, the PEMFC performance under higher temperature and low relative humidity is lacking and this aspect is needed to be investigated comprehensively.
Abbreviations
- CS-HEC:
-
Chitosan-hydroxy ethyl cellulose
- CsPW:
-
Cs hydrogen salts of phosphotungstic acid
- HPA:
-
Heteropolyacid
- LbL:
-
Layer by layer
- mGO:
-
Graphene oxide modified with 3-aminopropyl-triethoxysilane
- MMT:
-
K10 montmorillonite
- MO:
-
Metal oxide
- MOR:
-
Methanol oxidation reaction
- ORR:
-
Oxygen reduction reaction
- PBI:
-
Polybenzimidazole
- PDDA:
-
Poly(diallyl dimethyl ammonium chloride)
- PEFC:
-
Polymer electrolyte fuel cell
- PEMFC:
-
Polymer electrolyte fuel cell
- PFSA:
-
Perfluorosulfonic acid
- PMA:
-
Molybdophosphoric acid or phosphomolybdic acid, H3PMo12O40
- POM:
-
Polyoxometalate
- Ppy:
-
Polypyrrole
- PVA:
-
Poly(vinyl alcohol)
- PWA:
-
Phosphotungstic acid, H3PW12O40
- QDPSU:
-
Quaternary diazabicyclo-octane polysulfone
- rGO:
-
Reduced graphene oxide
- SiMA:
-
Silicomolybdic acid, H4SiMo12O40
- SiWA:
-
Silicotungestico acid H4SiW12O40
- SPAEK:
-
Sulfonated poly(aryl ether ketone)
- SPBN:
-
Sulfonated polynorbornene
- SPEEK:
-
Sulfonated poly(ether ether ketone)s
- SPS:
-
Sulfonated polystyrene
- ZrP:
-
Zirconium phosphate
References
Abouzari-Lotf E, Ghassemi H, Mehdipour-Ataei S, Shockravi A (2016) Phosphonated polyimides: enhancement of proton conductivity at high temperatures and low humidity. J Membr Sci 516:74–82. doi:10.1016/j.memsci.2016.06.009
Abouzari-lotf E, Jacob MV, Ghassemi H, Ahmad A, Nasef MM, Zakeri M, Mehdipour-Ataei S (2016) Enhancement of fuel cell performance with less-water dependent composite membranes having polyoxometalate anchored nanofibrous interlayer. J Power Sources 326:482–489. doi:10.1016/j.jpowsour.2016.07.027
Aili D, Zhang J, Dalsgaard Jakobsen MT, Zhu H, Yang T, Liu J, Forsyth M, Pan C, Jensen JO, Cleemann LN, Jiang SP, Li Q (2016) Exceptional durability enhancement of PA/PBI based polymer electrolyte membrane fuel cells for high temperature operation at 200 °C. J Mater Chem A 4(11):4019–4024. doi:10.1039/C6TA01562J
He C, Han KF, Yu JH, Zhu H, Wang ZM (2016) Novel anti-oxidative membranes based on sulfide-containing polybenzimidazole for high temperature proton exchange membrane fuel cells. Eur Polymer J 74:168–179. doi:10.1016/j.eurpolymj.2015.11.024
Nasef MM (2014) Radiation-grafted membranes for polymer electrolyte fuel cells: current trends and future directions. Chem Rev 114(24):12278–12329. doi:10.1021/cr4005499
Nasef MM, Fujigaya T, Abouzari-Lotf E, Nakashima N (2016a) Electrospinning of poly(vinylpyrrodine) template for formation of ZrO2 nanoclusters for enhancing properties of composite proton conducting membranes. Int J Polym Mater. doi:10.1080/00914037.2016.1201829
Nasef MM, Fujigaya T, Abouzari-Lotf E, Nakashima N, Yang Z (2016) Enhancement of performance of pyridine modified polybenzimidazole fuel cell membranes using zirconium oxide nanoclusters and optimized phosphoric acid doping level. Int J Hydrogen Energy 41(16):6842–6854. doi:10.1016/j.ijhydene.2016.03.022
Tanaka M (2016) Development of ion conductive nanofibers for polymer electrolyte fuel cells. Polym J 48(1):51–58. doi:10.1038/pj.2015.76
Wu H-W (2016) A review of recent development: transport and performance modeling of PEM fuel cells. Appl Energy 165:81–106. doi:10.1016/j.apenergy.2015.12.075
Wu Q, Wang H, Lu S, Xu X, Liang D, Xiang Y (2016) Novel methanol-blocking proton exchange membrane achieved via self-anchoring phosphotungstic acid into chitosan membrane with submicro-pores. J Membr Sci 500:203–210. doi:10.1016/j.memsci.2015.11.019
Xu X, Wang H, Lu S, Peng S, Xiang Y (2016) A phosphotungstic acid self-anchored hybrid proton exchange membrane for direct methanol fuel cells. RSC Adv 6(49):43049–43055. doi:10.1039/C6RA07318B
Ye Y, Wu X, Yao Z, Wu L, Cai Z, Wang L, Ma X, Chen Q-H, Zhang Z, Xiang S (2016) Metal-organic frameworks with a large breathing effect to host hydroxyl compounds for high anhydrous proton conductivity over a wide temperature range from subzero to 125 [degree]C. J Mater Chem A 4(11):4062–4070. doi:10.1039/C5TA10765B
Hickner MA, Ghassemi H, Kim YS, Einsla BR, McGrath JE (2004) Alternative polymer systems for proton exchange membranes (PEMs). Chem Rev 104(10):4587–4612
Kim YS, Lee K-S (2015) Fuel cell membrane characterizations. Polym Rev 55(2):330–370. doi:10.1080/15583724.2015.1011275
Zhang L, Chae S-R, Hendren Z, Park J-S, Wiesner MR (2012) Recent advances in proton exchange membranes for fuel cell applications. Chem Eng J 204–206:87–97. doi:10.1016/j.cej.2012.07.103
Li J, Wang Z, Li J, Pan M, Tang H (2014) Nanostructure-based proton exchange membrane for fuel cell applications at high temperature. J Nanosci Nanotechnol 14(2):1181–1193. doi:10.1166/jnn.2014.8744
Subianto S (2014) Recent advances in polybenzimidazole/phosphoric acid membranes for high-temperature fuel cells. Polym Int 63(7):1134–1144. doi:10.1002/pi.4708
Zhang J, Xie Z, Zhang J, Tang Y, Song C, Navessin T, Shi Z, Song D, Wang H, Wilkinson DP, Liu ZS, Holdcroft S (2006) High temperature PEM fuel cells. J Power Sources 160(2):872–891. doi:10.1016/j.jpowsour.2006.05.034
Nasef H, Saidi H, Uthman H, Sithambaranathan P (2013) Advances in membranes for high temperature polymer electrolyte membrane fuel cells. In: Inamuddin (ed) Advanced functional polymers and composites: materials devices and allied applications, vol 1. Nova Science Publishers Inc., USA, pp 1–44
Alberti G, Casciola M (2003) Composite membranes for medium-temperature PEM fuel cells. Annu Rev Mater Res 33(1):129–154. doi:10.1146/annurev.matsci.33.022702.154702
Tripathi BP, Shahi VK (2011) Organic–inorganic nanocomposite polymer electrolyte membranes for fuel cell applications. Prog Polym Sci 36(7):945–979. doi:10.1016/j.progpolymsci.2010.12.005
Kourasi M, Wills RGA, Shah AA, Walsh FC (2014) Heteropolyacids for fuel cell applications. Electrochim Acta 127:454–466. doi:10.1016/j.electacta.2014.02.006
Sachdeva S, Turner JA, Horan JL, Herring AM (2011) The use of heteropoly acids in proton exchange fuel cells. Struct Bond 141:115–168
Katsoulis DE (1998) A Survey of applications of polyoxometalates. Chem Rev 98(1):359–388. doi:10.1021/cr960398a
Maksimov GM (1995) Advances in the synthesis of polyoxometalates and in the study of heteropolyacids. Russ Chem Rev 64(5):445
Pope MT, Müller A (1991) Polyoxometalate chemistry: an old field with new dimensions in several disciplines. Angew Chem Int Ed Engl 30(1):34–48. doi:10.1002/anie.199100341
Calligaris M (1985) Structural inorganic chemistry by A.F. Wells. Acta Crystallogr Sect B 41(3):208. doi:10.1107/S0108768185001987
Baker LCW, Glick DC (1998) Present general status of understanding of heteropoly electrolytes and a tracing of some major highlights in the history of their elucidation. Chem Rev 98(1):3–50. doi:10.1021/cr960392l
Kozhevnikov IV (1998) Catalysis by heteropoly acids and multicomponent polyoxometalates in liquid-phase reactions. Chem Rev 98(1):171–198
Song Y-F, Tsunashima R (2012) Recent advances on polyoxometalate-based molecular and composite materials. Chem Soc Rev 41(22):7384–7402. doi:10.1039/C2CS35143A
Walsh JJ, Bond AM, Forster RJ, Keyes TE (2016) Hybrid polyoxometalate materials for photo(electro-) chemical applications. Coord Chem Rev 306(Part 1):217–234. doi:10.1016/j.ccr.2015.06.016
Wang S-S, Yang G-Y (2015) Recent advances in polyoxometalate-catalyzed reactions. Chem Rev 115(11):4893–4962. doi:10.1021/cr500390v
Wu X, Wu W, Qian X, Wu Q, Yan W (2015) Proton-conducting materials based on heteropoly acid and matrixes. J Non-Cryst Solids 426:88–91. doi:10.1016/j.jnoncrysol.2015.06.010
Pope MT, Müller A (2001) Polyoxometalate chemistry from topology via self-assembly to applications. Kluwer Academic Publishers, New York
Singh RP, Khatri RP, Dubois J, Gaur SS, Abe M (1990) Synthesis of dodecamolybdoantimonate(V) salts containing the Keggin structure. J Chem Soc Dalton Trans (3):947–951. doi:10.1039/DT9900000947
Pandey G (1994) Zeolite, clay, and heteropoly acid in organic reactions. Von Y. Izumi, K. Urabe und M. Onaka. VCH verlagsgesellschaft, weinheim, 1992. 166 S., geb. 128.00 DM.—ISBN 3-527-29011-7. Angewandte Chemie 106(21):2316–2317. doi:10.1002/ange.19941062133
Cai H, Huang T, Wu Q, Yan W (2016) Synthesis and conduction mechanism of high proton conductor H6SiW10V2O40·14H2O. Funct Mater Lett 09(03):1650048. doi:10.1142/S179360471650048X
Huang T, Tian N, Wu Q, Yan Y, Yan W (2015) Synthesis, crystal structure and conductive mechanism of solid high-proton conductor tungstovanadosilicic heteropoly acid. Mater Chem Phys 165:34–38. doi:10.1016/j.matchemphys.2015.08.026
Jansen RJJ, van Veldhuizen HM, Schwegler MA, van Bekkum H (1994) Recent (1987-1993) developments in heteropolyacid catalysts in acid catalyzed reactions and oxidation catalysis. Recl Trav Chim Pays-Bas 113(3):115–135. doi:10.1002/recl.19941130302
Misono M (1987) Heterogeneous catalysis by heteropoly compounds of molybdenum and tungsten. Catal Rev 29(2–3):269–321. doi:10.1080/01614948708078072
Coronado E, Gómez-García CJ (1998) Polyoxometalate-based molecular materials. Chem Rev 98(1):273–296. doi:10.1021/cr970471c
Cronin L, Muller A (2012) From serendipity to design of polyoxometalates at the nanoscale, aesthetic beauty and applications. Chem Soc Rev 41(22):7333–7334. doi:10.1039/C2CS90087D
Vernon DR, Meng F, Dec SF, Williamson DL, Turner JA, Herring AM (2005) Synthesis, characterization, and conductivity measurements of hybrid membranes containing a mono-lacunary heteropolyacid for PEM fuel cell applications. J Power Sources 139(1–2):141–151. doi:10.1016/j.jpowsour.2004.07.027
Wu X, Huang T, Wu Q, Xu L (2015) Synthesis and conductive performance of indium-substituted ternary heteropoly acids with Keggin structures. Dalton Trans 45(1):271–275. doi:10.1039/c5dt02541a
Tong X, Tian N, Wu W, Zhu W, Wu Q, Cao F, Yan W, Yaroslavtsev AB (2013) Preparation and electrochemical performance of tungstovanadophosphoric heteropoly acid and its hybrid materials. J Phys Chem C 117(7):3258–3263. doi:10.1021/jp3102223
Ramani V, Kunz HR, Fenton JM (2005) Effect of particle size reduction on the conductivity of Nafion®/phosphotungstic acid composite membranes. J Membr Sci 266(1–2):110–114. doi:10.1016/j.memsci.2005.05.019
Athens GL, Ein-Eli Y, Chmelka BF (2007) Acid-functionalized mesostructured aluminosilica for hydrophilic proton conduction membranes. Adv Mater 19(18):2580–2587. doi:10.1002/adma.200602781
Misono M (2009) Recent progress in the practical applications of heteropolyacid and perovskite catalysts: catalytic technology for the sustainable society. Catal Today 144(3–4):285–291. doi:10.1016/j.cattod.2008.10.054
Jelikić-Stankov M, Uskoković-Marković S, Holclajtner-Antunović I, Todorović M, Djurdjević P (2007) Compounds of Mo, V and W in biochemistry and their biomedical activity. J Trace Elem Med Biol 21(1):8–16. doi:10.1016/j.jtemb.2006.11.004
Crans DC (1994) Enzyme interactions with labile oxovanadates and other polyoxometalates. Comments Inorg Chem 16(1–2):35–76. doi:10.1080/02603599408035851
Rhule JT, Hill CL, Judd DA, Schinazi RF (1998) Polyoxometalates in Medicine. Chem Rev 98(1):327–358. doi:10.1021/cr960396q
Abouzari-Lotf E, Nasef MM, Ghassemi H, Zakeri M, Ahmad A, Abdollahi Y (2015) Improved methanol barrier property of nafion hybrid membrane by incorporating nanofibrous interlayer self-immobilized with high level of phosphotungstic acid. ACS Appl Mater Interfaces 7(31):17008–17015. doi:10.1021/acsami.5b02268
Banerjee S, Kar KK (2016) Synergistic effect of aluminium phosphate and tungstophosphoric acid on the physicochemical properties of sulfonated poly ether ether ketone nanocomposite membrane. J Appl Polym Sci 133(5). doi:10.1002/app.42952
Cai H, Lian X, Wu Q, Cao F, Yan W (2016) A novel SPEEK/PW11V/rGO hybrid film for proton conduction. J Non-Cryst Solids 447:202–206. doi:10.1016/j.jnoncrysol.2016.04.034
Renzi M, Mignini P, Giuli G, Marassi R, Nobili F (2016) Rotating disk electrode study of Pt/Cs3HPMo11VO40 composite catalysts for performing and durable PEM fuel cells. Int J Hydrogen Energy 41(26):11163–11173. doi:10.1016/j.ijhydene.2016.04.194
Santamaria M, Pecoraro CM, Di Franco F, Di Quarto F, Gatto I, Saccà A (2016) Improvement in the performance of low temperature H2–O2 fuel cell with chitosan–phosphotungstic acid composite membranes. Int J Hydrogen Energy 41(11):5389–5395. doi:10.1016/j.ijhydene.2016.01.133
Bijelic A, Rompel A (2015) The use of polyoxometalates in protein crystallography—an attempt to widen a well-known bottleneck. Coord Chem Rev 299:22–38. doi:10.1016/j.ccr.2015.03.018
Herrmann S, Ritchie C, Streb C (2015) Polyoxometalate—conductive polymer composites for energy conversion, energy storage and nanostructured sensors. Dalton Trans 44(16):7092–7104. doi:10.1039/C4DT03763D
Jameel U, Zhu M, Chen X, Tong Z (2016) Recent progress of synthesis and applications in polyoxometalate and nanogold hybrid materials. J Mater Sci 51(5):2181–2198. doi:10.1007/s10853-015-9503-1
Nakamura O, Kodama T, Ogino I, Miyake Y (1979) High-conductivity solid proton conductors: dodecamolybdophosphoric acid and dodecatungstophosphoric acid crystals. Chem Lett 8(1):17–18. doi:10.1246/cl.1979.17
Nakamura O, Ogino I (1982) Electrical conductivities of some hydrates of dodecamolybdophosphoric acid and dodecatungstophosphoric acid and their mixed crystals. Mater Res Bull 17(2):231–234. doi:10.1016/0025-5408(82)90150-7
Creeth A (2013) PEM fuel cells can now rival diesel engines on performance. Fuel Cells Bull 2013(7):12–14. doi:10.1016/S1464-2859(13)70270-0
Giordano N, Staiti P, Hocevar S, Aricò AS (1996) High performance fuel cell based on phosphotungstic acid as proton conducting electrolyte. Electrochim Acta 41(3):397–403. doi:10.1016/0013-4686(95)00316-9
Mohtar SS, Ismail AF, Matsuura T (2011) Preparation and characterization of SPEEK/MMT-STA composite membrane for DMFC application. J Membr Sci 371(1–2):10–19. doi:10.1016/j.memsci.2011.01.009
Staiti P, Freni S, Hocevar S (1999) Synthesis and characterization of proton-conducting materials containing dodecatungstophosphoric and dodecatungstosilic acid supported on silica. J Power Sources 79(2):250–255. doi:10.1016/S0378-7753(99)00177-9
Colomer MT (2006) Nanoporous anatase thin films as fast proton-conducting materials. Adv Mater 18(3):371–374. doi:10.1002/adma.200500689
Xiong L, Nogami M (2006) Proton-conducting ordered mesostructured silica monoliths. Chem Lett 35(8):972–973. doi:10.1246/cl.2006.972
Celik SU, Akbey U, Graf R, Bozkurt A, Spiess HW (2008) Anhydrous proton-conducting properties of triazole-phosphonic acid copolymers: a combined study with MAS NMR. Phys Chem Chem Phys 10(39):6058–6066. doi:10.1039/B807659F
Uma T, Nogami M (2007) Structural and transport properties of mixed phosphotungstic acid/phosphomolybdic acid/SiO2 glass membranes for H2/O2 fuel cells. Chem Mater 19(15):3604–3610. doi:10.1021/cm070567f
Malhotra S, Datta R (1997) Membrane-supported nonvolatile acidic electrolytes allow higher temperature operation of proton-exchange membrane fuel cells. J Electrochem Soc 144(2):L23–L26
Honma I, Nakajima H, Nishikawa O, Sugimoto T, Nomura S (2003) Family of high-temperature polymer-electrolyte membranes synthesized from amphiphilic nanostructured macromolecules. J Electrochem Soc 150(5):A616–A619. doi:10.1149/1.1566018
Honma I, Nakajima H, Nishikawa O, Sugimoto T, Nomura S (2003) Organic/inorganic nano-composites for high temperature proton conducting polymer electrolytes. Solid State Ionics 162–163:237–245. doi:10.1016/S0167-2738(03)00260-1
Honma I, Nakajima H, Nomura S (2002) High temperature proton conducting hybrid polymer electrolyte membranes. Solid State Ionics 154–155:707–712. doi:10.1016/S0167-2738(02)00431-9
Honma I, Takeda Y, Bae JM (1999) Protonic conducting properties of sol–gel derived organic/inorganic nanocomposite membranes doped with acidic functional molecules. Solid State Ionics 120(1–4):255–264. doi:10.1016/S0167-2738(98)00562-1
Lavrenčič Štangar U, Grošelj N, Orel B, Schmitz A, Colomban P (2001) Proton-conducting sol–gel hybrids containing heteropoly acids. Solid State Ionics 145(1–4):109–118. doi:10.1016/S0167-2738(01)00920-1
Ramani V, Kunz HR, Fenton JM (2004) Investigation of Nafion®/HPA composite membranes for high temperature/low relative humidity PEMFC operation. J Membrane Sci 232(1–2):31–44. doi:10.1016/j.memsci.2003.11.016
Shao Z-G, Xu H, Li M, Hsing IM (2006) Hybrid nafion–inorganic oxides membrane doped with heteropolyacids for high temperature operation of proton exchange membrane fuel cell. Solid State Ionics 177(7–8):779–785. doi:10.1016/j.ssi.2005.12.035
Lu JL, Fang QH, Li SL, Jiang SP (2013) A novel phosphotungstic acid impregnated meso-nafion multilayer membrane for proton exchange membrane fuel cells. J Membrane Sci 427:101–107. doi:10.1016/j.memsci.2012.09.041
Zaidi SMJ, Ahmad MI (2006) Novel SPEEK/heteropolyacids loaded MCM-41 composite membranes for fuel cell applications. J Membr Sci 279(1–2):548–557. doi:10.1016/j.memsci.2005.12.048
Aparicio M, Mosa J, Etienne M, Durán A (2005) Proton-conducting methacrylate-silica sol–gel membranes containing tungstophosphoric acid. J Power Sources 145(2):231–236. doi:10.1016/j.jpowsour.2005.01.071
Lavrenčič Štangar U, Grošelj N, Orel B, Schmitz A, Colomban P (2001) Proton-conducting sol–gel hybrids containing heteropoly acids. Solid State Ionics 145(1–4):109–118. doi:10.1016/S0167-2738(01)00920-1
Nakajima H, Nomura S, Sugimoto T, Nishikawa S, Honma I (2002) High temperature proton conducting organic/inorganic nanohybrids for polymer electrolyte membrane. J Electrochem Soc 149(8):A953–A959. doi:10.1149/1.1485080
Colicchio I, Wen F, Keul H, Simon U, Moeller M (2009) Sulfonated poly(ether ether ketone)-silica membranes doped with phosphotungstic acid. Morphology and proton conductivity. J Membr Sci 326(1):45–57. doi:10.1016/j.memsci.2008.09.008
Ramani V, Kunz HR, Fenton JM (2006) Metal dioxide supported heteropolyacid/Nafion® composite membranes for elevated temperature/low relative humidity PEFC operation. J Membr Sci 279(1–2):506–512. doi:10.1016/j.memsci.2005.12.044
Herring AM (2006) Inorganic-polymer composite membranes for proton exchange membrane fuel cells. J Macromol Sci C 46(3):245–296. doi:10.1080/00222340600796322
Bose AB, Gopu S, Li W (2014) Enhancement of proton exchange membrane fuel cells performance at elevated temperatures and lower humidities by incorporating immobilized phosphotungstic acid in electrodes. J Power Sources 263:217–222. doi:10.1016/j.jpowsour.2014.04.043
Horan JL, Genupur A, Ren H, Sikora BJ, Kuo M-C, Meng F, Dec SF, Haugen GM, Yandrasits MA, Hamrock SJ, Frey MH, Herring AM (2009) Copolymerization of divinylsilyl-11-silicotungstic acid with butyl acrylate and hexanediol diacrylate: synthesis of a highly proton-conductive membrane for fuel-cell applications. ChemSusChem 2(3):226–229. doi:10.1002/cssc.200800237
Horan JL, Lingutla A, Ren H, Kuo M-C, Sachdeva S, Yang Y, Seifert S, Greenlee LF, Yandrasits MA, Hamrock SJ, Frey MH, Herring AM (2013) Fast proton conduction facilitated by minimum water in a series of divinylsilyl-11-silicotungstic acid-co-butyl acrylate-co-hexanediol diacrylate polymers. J Phys Chem C 118(1):135–144. doi:10.1021/jp4089657
Motz AR, Horan JL, Kuo M-C, Herring AM (2015) Water uptake in novel, water-stable, heteropoly acid films. ECS Trans 69(17):587–590. doi:10.1149/06917.0587ecst
Kim Y, Ketpang K, Jaritphun S, Park JS, Shanmugam S (2015) A polyoxometalate coupled graphene oxide-Nafion composite membrane for fuel cells operating at low relative humidity. J Mater Chem A 3(15):8148–8155. doi:10.1039/c5ta00182j
Dsoke S, Kolary-Zurowska A, Zurowski A, Mignini P, Kulesza PJ, Marassi R (2011) Rotating disk electrode study of Cs 2.5H 0.5PW 12O 40 as mesoporous support for Pt nanoparticles for PEM fuel cells electrodes. J Power Sources 196(24):10591–10600. doi:10.1016/j.jpowsour.2011.09.010
Haile SM, Liu H, Secco RA (2003) High-temperature behavior of CsH2PO4 under both ambient and high pressure conditions. Chem Mater 15(3):727–736. doi:10.1021/cm020138b
Kim G, Griffin JM, Blanc F, Haile SM, Grey CP (2015) Characterization of the dynamics in the protonic conductor CsH2PO4 by 17O solid-state NMR spectroscopy and first-principles calculations: correlating phosphate and protonic motion. J Am Chem Soc 137(11):3867–3876. doi:10.1021/jacs.5b00280
Haile SM, Chisholm CRI, Sasaki K, Boysen DA, Uda T (2007) Solid acid proton conductors: from laboratory curiosities to fuel cell electrolytes. Faraday Discuss 134:17–39. doi:10.1039/B604311A
Chisholm CRI, Haile SM (2000) Superprotonic behavior of Cs2(HSO4)(H2PO4)—a new solid acid in the CsHSO4–CsH2PO4 system. Solid State Ionics 136–137:229–241. doi:10.1016/S0167-2738(00)00315-5
Ikeda A, Kitchaev DA, Haile SM (2014) Phase behavior and superprotonic conductivity in the Cs1-xRbxH2PO4 and Cs1-xKxH2PO4 systems. J Mater Chem A 2(1):204–214. doi:10.1039/C3TA13889E
Oh S-Y, Kawamura G, Muto H, Matsuda A (2012) Mechanochemical synthesis of proton conductive composites derived from cesium dihydrogen phosphate and guanine. Solid State Ionics 225:223–227. doi:10.1016/j.ssi.2012.02.058
Oh S-Y, Yoshida T, Kawamura G, Muto H, Sakai M, Matsuda A (2010) Inorganic-organic composite electrolytes consisting of polybenzimidazole and Cs-substituted heteropoly acids and their application for medium temperature fuel cells. J Mater Chem 20(30):6359–6366. doi:10.1039/C0JM00318B
Stanis RJ, Kuo MC, Turner JA, Herring AM (2008) Use of W, Mo, and v substituted heteropolyacids for CO mitigation in PEMFCs. J Electrochem Soc 155(2):B155–B162. doi:10.1149/1.2815917
Kim WB, Voitl T, Rodriguez-Rivera GJ, Evans ST, Dumesic JA (2005) Preferential oxidation of CO in H2 by aqueous polyoxometalates over metal catalysts. Angew Chem Int Ed 44(5):778–782. doi:10.1002/anie.200461601
Baker PS, Bonville LJ, Kunz HR (2014) Performance of silicotungstic acid modified platinum cathodes at high temperature and low relative humidity for PEMFCs. J Electrochem Soc 161(12):F1307–F1313. doi:10.1149/2.1101412jes
Stanis RJ, Kuo M-C, Rickett AJ, Turner JA, Herring AM (2008) Investigation into the activity of heteropolyacids towards the oxygen reduction reaction on PEMFC cathodes. Electrochim Acta 53(28):8277–8286. doi:10.1016/j.electacta.2008.06.052
Rhee HW, Ghil LJ (2012) Polymer nanocomposites in fuel cells. In: Advances in polymer nanocomposites: types and applications. Elsevier Inc., pp 433–471
Thanganathan U (2010) Proton-conducting PVA/PMA hybrid membranes for fuel cell applications. World Acad Sci Eng Technol 71:403–406
Shanmugam S, Viswanathan B, Varadarajan TK (2006) Synthesis and characterization of silicotungstic acid based organic-inorganic nanocomposite membrane. J Membr Sci 275(1–2):105–109. doi:10.1016/j.memsci.2005.09.009
Thanganathan U, Kumar S, Kishimoto A, Kimura K (2012) Synthesis of organic/inorganic hybrid composite membranes and their structural and conductivity properties. Mater Lett 72:81–87. doi:10.1016/j.matlet.2011.12.066
Broka K, Ekdunge P (1997) Oxygen and hydrogen permeation properties and water uptake of Nafion® 117 membrane and recast film for PEM fuel cell. J Appl Electrochem 27(2):117–124
Yılmaztürk S, Ercan N, Deligöz H (2012) Influence of LbL surface modification on oxygen cross-over in self-assembled thin composite membranes. Appl Surf Sci 258(7):3139–3146. doi:10.1016/j.apsusc.2011.11.051
Ketpang K, Shanmugam S, Suwanboon C, Chanunpanich N, Lee D (2015) Efficient water management of composite membranes operated in polymer electrolyte membrane fuel cells under low relative humidity. J Membr Sci 493:285–298. doi:10.1016/j.memsci.2015.06.055
Li Q, He R, Jensen JO, Bjerrum NJ (2003) Approaches and recent development of polymer electrolyte membranes for fuel cells operating above 100 °C. Chem Mater 15(26):4896–4915. doi:10.1021/cm0310519
Abouzari-Lotf E, Ghassemi H, Shockravi A, Zawodzinski T, Schiraldi D (2011) Phosphonated Poly(arylene ether)s as potential high temperature proton conducting materials. Polymer 52(21):4709–4717. doi:10.1016/j.polymer.2011.08.020
Amiinu IS, Li W, Wang G, Tu Z, Tang H, Pan M, Zhang H (2015) Toward anhydrous proton conductivity based on imidazole functionalized mesoporous silica/nafion composite membranes. Electrochim Acta 160:185–194. doi:10.1016/j.electacta.2015.02.070
Tazi B, Savadogo O (2000) Parameters of PEM fuel-cells based on new membranes fabricated from Nafion, silicotungstic acid and thiophene. Electrochim Acta 45(25–26):4329–4339. doi:10.1016/S0013-4686(00)00536-3
Tian H, Savadogo O (2006) Silicotungstic acid Nafion composite membrane for proton-exchange membrane fuel cell operation at high temperature. J New Mater Electrochem Syst 9(1):61–71
Oh SY, Yoshida T, Kawamura G, Muto H, Sakai M, Matsuda A (2010) Proton conductivity and fuel cell property of composite electrolyte consisting of Cs-substituted heteropoly acids and sulfonated poly(ether-ether ketone). J Power Sources 195(18):5822–5828. doi:10.1016/j.jpowsour.2010.01.063
Tian N, Wu X, Yang B, Wu Q, Cao F, Yan W, Yaroslavtsev AB (2015) Proton-conductive membranes based on vanadium substituted heteropoly acids with Keggin structure and polymers. J Appl Polym Sci 132(27). doi:10.1002/app.42204
Zaidi SMJ, Mikhailenko SD, Robertson GP, Guiver MD, Kaliaguine S (2000) Proton conducting composite membranes from polyether ether ketone and heteropolyacids for fuel cell applications. J Membr Sci 173(1):17–34. doi:10.1016/S0376-7388(00)00345-8
Meng F, Aieta NV, Dec SF, Horan JL, Williamson D, Frey MH, Pham P, Turner JA, Yandrasits MA, Hamrock SJ, Herring AM (2007) Structural and transport effects of doping perfluorosulfonic acid polymers with the heteropoly acids, H3PW12O40 or H4SiW12O40. Electrochim Acta 53(3):1372–1378. doi:10.1016/j.electacta.2007.06.047
Tazi B, Savadogo O (2001) Effect of various heteropolyacids (HPAs) on the characteristics of Nafion®—HPAS membranes and their H2/O2 polymer electrolyte fuel cell parameters. J New Mater Electrochem Syst 4(3):187–196
Saccà A, Carbone A, Pedicini R, Marrony M, Barrera R, Elomaa M, Passalacqua E (2008) Phosphotungstic acid supported on a nanopowdered ZrO2 as a filler in nafion-based membranes for polymer electrolyte fuel cells. Fuel Cells 8(3–4):225–235. doi:10.1002/fuce.200800009
Mahreni A, Mohamad AB, Kadhum AAH, Daud WRW, Iyuke SE (2009) Nafion/silicon oxide/phosphotungstic acid nanocomposite membrane with enhanced proton conductivity. J Membr Sci 327(1–2):32–40. doi:10.1016/j.memsci.2008.10.048
Shao Z-G, Joghee P, Hsing IM (2004) Preparation and characterization of hybrid Nafion–silica membrane doped with phosphotungstic acid for high temperature operation of proton exchange membrane fuel cells. J Membr Sci 229(1–2):43–51. doi:10.1016/j.memsci.2003.09.014
Yan X-M, Mei P, Mi Y, Gao L, Qin S (2009) Proton exchange membrane with hydrophilic capillaries for elevated temperature PEM fuel cells. Electrochem Commun 11(1):71–74. doi:10.1016/j.elecom.2008.10.040
Pecoraro CM, Santamaria M, Bocchetta P, Di Quarto F (2015) Influence of synthesis conditions on the performance of chitosan–heteropolyacid complexes as membranes for low temperature H2–O2 fuel cell. Int J Hydrogen Energy 40(42):14616–14626. doi:10.1016/j.ijhydene.2015.06.083
Santamaria M, Pecoraro CM, Di Quarto F, Bocchetta P (2015) Chitosan-phosphotungstic acid complex as membranes for low temperature H2-O2 fuel cell. J Power Sources 276:189–194. doi:10.1016/j.jpowsour.2014.11.147
Martínez-Morlanes MJ, Martos AM, Várez A, Levenfeld B (2015) Synthesis and characterization of novel hybrid polysulfone/silica membranes doped with phosphomolybdic acid for fuel cell applications. J Membr Sci 492:371–379. doi:10.1016/j.memsci.2015.05.031
Anis A, Banthia AK, Mondal S, Thakur AK (2006) Synthesis and characterization of hybrid proton conducting membranes of poly(vinyl alcohol) and phosphomolybdic acid. Chin J Polym Sci 24(5):449–456. doi:10.1142/S0256767906001515 (English Edition)
He R, Li Q, Xiao G, Bjerrum NJ (2003) Proton conductivity of phosphoric acid doped polybenzimidazole and its composites with inorganic proton conductors. J Membr Sci 226(1–2):169–184. doi:10.1016/j.memsci.2003.09.002
Lee JW, Kim K, Khan SB, Han P, Seo J, Jang W, Han H (2014) Synthesis, characterization, and thermal and proton conductivity evaluation of 2,5-polybenzimidazole composite membranes. J Nanomater 2014. doi:10.1155/2014/460232
Li M-Q, Shao Z-G, Scott K (2008) A high conductivity Cs2.5H0.5PMo12O40/polybenzimidazole (PBI)/H3PO4 composite membrane for proton-exchange membrane fuel cells operating at high temperature. J Power Sources 183(1):69–75. doi:10.1016/j.jpowsour.2008.04.093
Xu C, Wu X, Wang X, Mamlouk M, Scott K (2011) Composite membranes of polybenzimidazole and caesium-salts-of- heteropolyacids for intermediate temperature fuel cells. J Mater Chem 21(16):6014–6019. doi:10.1039/c1jm10093a
Xu C, Wang X, Wu X, Cao Y, Scott K (2012) A composite membrane of caesium salt of heteropolyacids/quaternary diazabicyclo-octane polysulfone with poly (tetrafluoroethylene) for intermediate temperature fuel cells. Membranes 2(3):384
Safronova EY, Osipov AK, Baranchikov AE, Yaroslavtsev AB (2015) Proton conductivity of MxH3-xPX12O40 and MxH4-xSiX12O40 (M = Rb, Cs; X = W, Mo) acid salts of heteropolyacids. Inorg Mater 51(11):1157–1162. doi:10.1134/S0020168515110102
Dey C, Kundu T, Aiyappa HB, Banerjee R (2015) Phosphate enriched polyoxometalate based ionic salts for proton conduction. RSC Adv 5(3):2333–2337. doi:10.1039/C4RA07598F
Narasimharao K, Brown DR, Lee AF, Newman AD, Siril PF, Tavener SJ, Wilson K (2007) Structure-activity relations in Cs-doped heteropolyacid catalysts for biodiesel production. J Catal 248(2):226–234. doi:10.1016/j.jcat.2007.02.016
Okuhara T, Watanabe H, Nishimura T, Inumaru K, Misono M (2000) Microstructure of cesium hydrogen salts of 12-tungstophosphoric acid relevant to novel acid catalysis. Chem Mater 12(8):2230–2238. doi:10.1021/cm9907561
Daiko Y, Takagi H, Katagiri K, Muto H, Sakai M, Matsuda A (2008) Mechanochemically synthesized cesium-ion-substituted phosphotungstic acid using several types of cesium-containing salts. Solid State Ionics 179(21–26):1174–1177. doi:10.1016/j.ssi.2008.02.004
Matsuda A, Kikuchi T, Katagiri K, Daiko Y, Muto H, Sakai M (2007) Mechanochemical synthesis of proton conductive cesium hydrogen salts of 12-tungstophosphoric acid and their composites. Solid State Ionics 178(7–10):723–727. doi:10.1016/j.ssi.2007.02.033
Ramani V, Kunz HR, Fenton JM (2005) Stabilized composite membranes and membrane electrode assemblies for elevated temperature/low relative humidity PEFC operation. J Power Sources 152:182–188. doi:10.1016/j.jpowsour.2005.03.135
Staiti P, Aricò AS, Baglio V, Lufrano F, Passalacqua E, Antonucci V (2001) Hybrid Nafion–silica membranes doped with heteropolyacids for application in direct methanol fuel cells. Solid State Ionics 145(1–4):101–107. doi:10.1016/S0167-2738(01)00919-5
Wang L, Yi BL, Zhang HM, Xing DM (2007) Cs2.5H0.5PWO40/SiO2 as addition self-humidifying composite membrane for proton exchange membrane fuel cells. Electrochim Acta 52(17):5479–5483. doi:10.1016/j.electacta.2007.03.007
Zhao D, Yi BL, Zhang HM, Yu HM, Wang L, Ma YW, Xing DM (2009) Cesium substituted 12-tungstophosphoric (CsxH3–xPW12O40) loaded on ceria-degradation mitigation in polymer electrolyte membranes. J Power Sources 190(2):301–306. doi:10.1016/j.jpowsour.2008.12.133
Carollo A, Quartarone E, Tomasi C, Mustarelli P, Belotti F, Magistris A, Maestroni F, Parachini M, Garlaschelli L, Righetti PP (2006) Developments of new proton conducting membranes based on different polybenzimidazole structures for fuel cells applications. J Power Sources 160(1):175–180. doi:10.1016/j.jpowsour.2006.01.081
Li Q, Jensen JO, Savinell RF, Bjerrum NJ (2009) High temperature proton exchange membranes based on polybenzimidazoles for fuel cells. Prog Polym Sci 34(5):449–477. doi:10.1016/j.progpolymsci.2008.12.003
Staiti P, Minutoli M, Hocevar S (2000) Membranes based on phosphotungstic acid and polybenzimidazole for fuel cell application. J Power Sources 90(2):231–235. doi:10.1016/S0378-7753(00)00401-8
Verma A, Scott K (2008) Development of high-temperature PEMFC based on heteropolyacids and polybenzimidazole. J Solid State Electrochem 14(2):213–219. doi:10.1007/s10008-008-0678-0
Asensio JA, Borrós S, Gómez-Romero P (2003) Enhanced conductivity in polyanion-containing polybenzimidazoles. Improved materials for proton-exchange membranes and PEM fuel cells. Electrochem Commun 5(11):967–972. doi:10.1016/j.elecom.2003.09.007
Staiti P (2001) Proton conductive membranes based on silicotungstic acid/silica and polybenzimidazole. Mater Lett 47(4–5):241–246. doi:10.1016/S0167-577X(00)00241-X
Xie X, Mao Z, Xu J, Zhen Y (2002) A hybrid membrane of modified polybenzimidazole and heteropoly acid for direct methanol fuel cell. In: PowerCon 2002–2002 international conference on power system technology, Proceedings, pp 169–172
Zhu X, Liu Y, Zhu L (2009) Polymer composites for high-temperature proton-exchange membrane fuel cells. Polymer membranes for fuel cells. Springer, US, pp 159–184
Li X, Faghri A (2013) Review and advances of direct methanol fuel cells (DMFCs) Part I: design, fabrication, and testing with high concentration methanol solutions. J Power Sources 226:223–240. doi:10.1016/j.jpowsour.2012.10.061
Maiyalagan T (2009) Silicotungstic acid stabilized Pt-Ru nanoparticles supported on carbon nanofibers electrodes for methanol oxidation. Int J Hydrogen Energy 34(7):2874–2879. doi:10.1016/j.ijhydene.2009.01.069
Cavani F (1998) Heteropolycompound-based catalysts: a blend of acid and oxidizing properties. Catal Today 41(1–3):73–86. doi:10.1016/S0920-5861(98)00039-X
Wu H, Shen X, Cao Y, Li Z, Jiang Z (2014) Composite proton conductive membranes composed of sulfonated poly(ether ether ketone) and phosphotungstic acid-loaded Imidazole microcapsules as acid reservoirs. J Membrane Sci 451:74–84. doi:10.1016/j.memsci.2013.09.058
Xiang Y, Yang M, Zhang J, Lan F, Lu S (2011) Phosphotungstic acid (HPW) molecules anchored in the bulk of nafion as methanol-blocking membrane for direct methanol fuel cells. J Membrane Sci 368(1–2):241–245. doi:10.1016/j.memsci.2010.11.049
Thomas SC, Ren X, Gottesfeld S, Zelenay P (2002) Direct methanol fuel cells: progress in cell performance and cathode research. Electrochim Acta 47(22–23):3741–3748. doi:10.1016/S0013-4686(02)00344-4
Dimitrova P, Friedrich K, Stimming U, Vogt B (2002) Modified Nafion®-based membranes for use in direct methanol fuel cells. Solid State Ionics 150(1):115–122
Sauk J, Byun J, Kim H (2005) Composite Nafion/polyphenylene oxide (PPO) membranes with phosphomolybdic acid (PMA) for direct methanol fuel cells. J Power Sources 143(1–2):136–141. doi:10.1016/j.jpowsour.2004.11.030
Aricò AS, Baglio V, Di Blasi A, Creti P, Antonucci PL, Antonucci V (2003) Influence of the acid–base characteristics of inorganic fillers on the high temperature performance of composite membranes in direct methanol fuel cells. Solid State Ionics 161(3–4):251–265. doi:10.1016/S0167-2738(03)00283-2
Kim H-J, Shul Y-G, Han H (2006) Sulfonic-functionalized heteropolyacid–silica nanoparticles for high temperature operation of a direct methanol fuel cell. J Power Sources 158(1):137–142. doi:10.1016/j.jpowsour.2005.10.001
He XH, Zheng Y, Yao HL, Chen YW, Chen DF (2014) Hybrid network sulfonated polynorbornene/silica membranes with enhanced proton conductivity by doped phosphotungstic acid. Fuel Cells 14(1):26–34. doi:10.1002/fuce.201300142
Ponce ML, Prado L, Ruffmann B, Richau K, Mohr R, Nunes SP (2003) Reduction of methanol permeability in polyetherketone–heteropolyacid membranes. J Membr Sci 217(1–2):5–15. doi:10.1016/S0376-7388(02)00309-5
Neelakandan S, Kanagaraj P, Sabarathinam RM, Nagendran A (2015) Polypyrrole layered SPEES/TPA proton exchange membrane for direct methanol fuel cells. Appl Surf Sci 359:272–279. doi:10.1016/j.apsusc.2015.10.122
Xu W, Lu T, Liu C, Xing W (2005) Low methanol permeable composite Nafion/silica/PWA membranes for low temperature direct methanol fuel cells. Electrochim Acta 50(16–17):3280–3285. doi:10.1016/j.electacta.2004.12.014
Yang M, Lu S, Lu J, Jiang SP, Xiang Y (2010) Layer-by-layer self-assembly of PDDA/PWA-Nafion composite membranes for direct methanol fuel cells. Chem Commun 46(9):1434–1436. doi:10.1039/b912779h
Zhao C, Lin H, Cui Z, Li X, Na H, Xing W (2009) Highly conductive, methanol resistant fuel cell membranes fabricated by layer-by-layer self-assembly of inorganic heteropolyacid. J Power Sources 194(1):168–174. doi:10.1016/j.jpowsour.2009.05.021
Xu L, Xu J, Liu M, Han H, Ni H, Ma L, Wang Z (2015) Fabrication of sulfonated poly(aryl ether ketone sulfone) membranes blended with phosphotungstic acid and microporous poly(vinylidene fluoride) as a depository for direct-methanol fuel cells. Int J Hydrogen Energy 40(22):7182–7191. doi:10.1016/j.ijhydene.2015.02.139
Pandey J, Shukla A (2014) PVDF supported silica immobilized phosphotungstic acid membrane for DMFC application. Solid State Ionics 262:811–814. doi:10.1016/j.ssi.2013.10.029
Helen M, Viswanathan B, Srinivasa Murthy S (2006) Fabrication and properties of hybrid membranes based on salts of heteropolyacid, zirconium phosphate and polyvinyl alcohol. J Power Sources 163(1):433–439. doi:10.1016/j.jpowsour.2006.09.041
Pandey J, Mir FQ, Shukla A (2014) Synthesis of silica immobilized phosphotungstic acid (Si-PWA)-poly(vinyl alcohol) (PVA) composite ion-exchange membrane for direct methanol fuel cell. Int J Hydrogen Energy 39(17):9473–9481. doi:10.1016/j.ijhydene.2014.03.237
Helen M, Viswanathan B, Murthy SS (2010) Poly(vinyl alcohol)–polyacrylamide blends with cesium salts of heteropolyacid as a polymer electrolyte for direct methanol fuel cell applications. J Appl Polym Sci 116(6):3437–3447. doi:10.1002/app.31940
Rao S, Xiu R, Si J, Lu S, Yang M, Xiang Y (2014) In situ synthesis of nanocomposite membranes: comprehensive improvement strategy for direct methanol fuel cells. ChemSusChem 7(3):822–828. doi:10.1002/cssc.201301060
Xiao Y, Xiang Y, Xiu R, Lu S (2013) Development of cesium phosphotungstate salt and chitosan composite membrane for direct methanol fuel cells. Carbohydr Polym 98(1):233–240. doi:10.1016/j.carbpol.2013.06.017
Doğan H, Inan TY, Unveren E, Kaya M (2010) Effect of cesium salt of tungstophosphoric acid (Cs-TPA) on the properties of sulfonated polyether ether ketone (SPEEK) composite membranes for fuel cell applications. Int J Hydrogen Energy 35(15):7784–7795. doi:10.1016/j.ijhydene.2010.05.045
Helen M, Viswanathan B, Murthy SS (2007) Synthesis and characterization of composite membranes based on α-zirconium phosphate and silicotungstic acid. J Membr Sci 292(1–2):98–105. doi:10.1016/j.memsci.2007.01.018
Xu W, Liu C, Xue X, Su Y, Lv Y, Xing W, Lu T (2004) New proton exchange membranes based on poly (vinyl alcohol) for DMFCs. Solid State Ionics 171(1–2):121–127. doi:10.1016/j.ssi.2004.04.009
Anis A, Banthia AK, Bandyopadhyay S (2008) Synthesis and characterization of polyvinyl alcohol copolymer/phosphomolybdic acid-based crosslinked composite polymer electrolyte membranes. J Power Sources 179(1):69–80. doi:10.1016/j.jpowsour.2007.12.041
Lin CW, Thangamuthu R, Chang PH (2005) PWA-doped PEG/SiO2 proton-conducting hybrid membranes for fuel cell applications. J Membr Sci 254(1–2):197–205. doi:10.1016/j.memsci.2005.01.007
Xu D, Zhang G, Zhang N, Li H, Zhang Y, Shao K, Han M, Lew CM, Na H (2010) Surface modification of heteropoly acid/SPEEK membranes by polypyrrole with a sandwich structure for direct methanol fuel cells. J Mater Chem 20(41):9239–9245. doi:10.1039/C0JM02167A
Lu S, Wang D, Jiang SP, Xiang Y, Lu J, Zeng J (2010) HPW/MCM-41 phosphotungstic acid/mesoporous silica composites as novel proton-exchange membranes for elevated-temperature fuel cells. Adv Mater 22(9):971–976. doi:10.1002/adma.200903091
Zeng J, Shen PK, Lu S, Xiang Y, Li L, De Marco R, Jiang SP (2012) Correlation between proton conductivity, thermal stability and structural symmetries in novel HPW-meso-silica nanocomposite membranes and their performance in direct methanol fuel cells. J Membr Sci 397–398:92–101. doi:10.1016/j.memsci.2012.01.018
Argun AA, Ashcraft JN, Hammond PT (2008) Highly conductive, methanol resistant polyelectrolyte multilayers. Adv Mater 20(8):1539–1543. doi:10.1002/adma.200703205
Ni HJ, Pei Y, Zhang QT, Zhao J, Wang XX, Huang MY (2015) Preparation and performances of SPEEK/PANI/PWA composite proton exchange membranes. Xiandai Huagong/Mod Chem Ind 35(11):72–76. doi:10.16606/j.cnki.issn0253-4320.2015.11.017
Roziere J, Jones DJ (2003) Non-fluorinated polymer materials for proton exchange membrane fuel cells. Ann Rev Mater Res 33:503–555. doi:10.1146/annurev.matsci.33.022702.154657
Lobato J, Cañizares P, Rodrigo MA, Linares JJ, López-Vizcaíno R (2008) Performance of a vapor-fed polybenzimidazole (PBI)-based direct methanol fuel cell. Energy Fuels 22(5):3335–3345. doi:10.1021/ef8001839
Gubler L, Kramer D, Belack J, Ünsal Ö, Schmidt TJ, Scherer GG (2007) Celtec-V: a Polybenzimidazole-based membrane for the direct methanol fuel cell. J Electrochem Soc 154(9):B981–B987. doi:10.1149/1.2754078
Lu S, Xu X, Zhang J, Peng S, Liang D, Wang H, Xiang Y (2014) A self-anchored phosphotungstic acid hybrid proton exchange membrane achieved via one-step synthesis. Adv Energy Mater 4(17). doi:10.1002/aenm.201400842
Mohanapriya S, Bhat SD, Sahu AK, Pitchumani S, Sridhar P, Shukla AK (2009) A new mixed-matrix membrane for DMFCs. Energy Environ Sci 2(11):1210–1216. doi:10.1039/b909451b
Bhat SD, Sahu AK, Jalajakshi A, Pitchumani S, Sridhar P, George C, Banerjee A, Chandrakumar N, Shukla AK (2010) PVA–SSA–HPA Mixed-matrix-membrane electrolytes for DMFCs. J Electrochem Soc 157(10):B1403–B1412. doi:10.1149/1.3465653
Acknowledgements
Authors wish to acknowledge the financial support from the Malaysian Ministry of Higher Education under the LRGS program (vote no. #4L817) and Universiti Teknologi Malaysia (vote no. #00D20).
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 Springer International Publishing AG
About this chapter
Cite this chapter
Abouzari-lotf, E., Nasef, M.M., Zakeri, M., Ahmad, A., Ripin, A. (2017). Composite Membranes Based on Heteropolyacids and Their Applications in Fuel Cells. In: Inamuddin, D., Mohammad, A., Asiri, A. (eds) Organic-Inorganic Composite Polymer Electrolyte Membranes. Springer, Cham. https://doi.org/10.1007/978-3-319-52739-0_5
Download citation
DOI: https://doi.org/10.1007/978-3-319-52739-0_5
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-52738-3
Online ISBN: 978-3-319-52739-0
eBook Packages: Chemistry and Materials ScienceChemistry and Material Science (R0)