Abstract
Olive oil extraction and refining process generate large amounts of by-products that represent a huge environmental concern, especially for countries located within the Mediterranean region, because of their phytotoxicity against soil and aquatic environments. Their valorization is considered challenging due to their high organic content, complexity, and the presence of phenolic compounds that inhibit their biodegradation. In order to minimize their environmental impact, many research groups within the last decades have been focusing on exploring and suggesting strategies regarding their physicochemical and microbiological treatment. According to various reports, the potential of olive mill wastewater to be converted to sustainable resources of biofuels and bio-based products has been demonstrated. In the present chapter, the most significant advances concerning a variety of promising valorization scenarios have been reviewed.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keyword
1 Introduction
The popularity of the health benefits of olive oil, due to its high nutritional and antioxidative value, turned nontraditional consuming countries to important consumers and importers increasing global trade (Mateo and Maicas 2015). Almost 75% of the global olive oil production is taking place in Europe. Mediterranean countries have dominated the world olive oil production and consumption that increased significantly within the last decades. Around 99.5% of olive oil production occurs in Spain, Italy, Greece, and Portugal reaching up to 2.4 × 106 tons in 2012. In return, large amounts of olive mill wastewater (OMW) are produced, reaching up to 300 × 105 m3 in the short period of harvesting and olive oil extraction, which usually lasts 2 months (ElMekawy et al. 2014).
From an environmental point of view, disposal of OMW causes severe effects mostly because of its high organic load, acidic pH, and the high content of phytotoxic compounds such as phenols. OMW treatment and valorization can be approached as a strategy to biotransform it into valuable materials, while at the same time reduction of its organic load and toxicity will make its disposal to natural receiving bodies easier.
In the following sections, the different methods to extract oil from olives and the by-products occurring by each method are summarized. Emphasis has been given to the potential conversion of OMW to biofuels, extraction of antioxidant compounds, as well as the production of bio-based products by utilizing this complex type of wastewater.
2 Olive Oil Extraction Processes
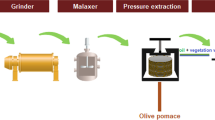
Extraction of olive oil may be achieved through discontinuous or continuous processes. Discontinuous process is based on using hydraulic press in order to squeeze out most of olive’s oil. The certain process is used in traditional mills and represents the oldest and most widespread method to produce olive oil (Dermeche et al. 2013). On the other hand, continuous process is based on phase separation by centrifugation. In particular, decanting systems are used in separating components (olive oil, water, and solid content) according to their density, which can be operated as three-phase or two-phase systems (Fig. 1). Three-phase systems are the most popular ones, especially in Greece, Italy, and Portugal, because they are smaller installations compared to two-phase systems and they are fully automated and result in higher quality oil, although energy requirements, water input, and OMW generated are higher. Typical physicochemical characteristics of OMW occurring during the three-phase olive oil extraction process are summarized in Table 1. Two-phase extraction systems represent a more environmentally friendly alternative because of the reduced volumes of OMW produced, and they are extremely used in Spain and Croatia. The utilization of such systems results in the generation of olive oil and wet pomace, which is a combination of olive husk and OMW, called two-phase olive mill waste, TPOMW. TPOMW is considered as a more concentrated OMW, thus is more difficult to handle (McNamara et al. 2008).
3 Biofuel Production
Untreated olive mill wastes can adversely affect natural ecosystems, especially in Mediterranean regions, which hold the lead in olive oil production worldwide. It is well known that these wastes have a negative effect on soil microbial communities, water bodies, and air quality; however, environmental threat can be overcome by employing different valorization strategies (Dermeche et al. 2013). It is a fact that high phenol, lipid, and organic acid concentrations make olive mill wastes phytotoxic; however, at the same time these wastes contain valuable compounds such as large proportions of organic substances and nutrients, which can be recycled (Roig et al. 2006). Concerning energy production, waste treatment technologies accompanied by energy recovery can reduce the environmental impact of olive oil production process while generating energy at the same time, to be used either on site, for process energy requirements, or for sale (Caputo et al. 2003).
Due to their chemical characteristics, olive mill wastes constitute effective substrates for biofuel production (Morillo et al. 2009), offering certain advantages compared to both fossil fuels and first-generation biofuels. Undoubtedly, depleting natural resources along with greenhouse gas (GHG) emissions make the use of fossil fuels unsustainable (Schenk et al. 2008). What is more, first-generation biofuels, mainly produced from food crops and oil seeds, are characterized by specific limitations, including competition for arable land and water used for agriculture and human consumption, as well as high production and processing cost, which prevent them from fulfilling global energy demand (Sims et al. 2010). In this context, second-generation biofuels, which are produced from lignocellulosic biomass and forest and non-food crop residues, appear to be highly promising renewable fuel sources. This also applies to third-generation biofuels derived from microbes and microalgae, despite the fact that more advanced technological development is still needed before these biofuels replace petroleum-based fuels (Antonopoulou et al. 2008; Venetsaneas et al. 2009; Nigam and Singh 2011; Kumar et al. 2016). Concerning bioenergy derived from OMW and TPOMW, much progress has been made in biohydrogen, methane, bioethanol, and biodiesel production.
3.1 Biohydrogen Production
Hydrogen is a clean energy source which can be produced either chemically, mainly by electrolysis of water, methane steam reforming, and coal or biomass gasification, or biologically, with the latter being rather advantageous. Due to the absence of CO2 emissions and an energy yield of 122 kJ/g, which is higher than that of fossil fuels, H2 is considered to be the energy source of the future (Momirlan and Veziroglu 2005; Venetsaneas et al. 2009). However, certain limitations, with respect to high energy requirements, have to be overcome in order to make biological hydrogen production sustainable. Biohydrogen can be produced by anaerobic and photosynthetic microorganisms and especially through direct biophotolysis, indirect biophotolysis, photo-fermentation, as well as dark fermentation (Sen et al. 2008; Barca et al. 2015; Urbaniec and Bakker 2015). More specifically, direct biophotolysis includes the photosynthetic production of hydrogen from water, a process performed by green microalgae. Under anaerobic conditions microalgae can produce H2 due to the fact that they possess the appropriate genetic, enzymatic, and metabolic machinery to do so (Levin et al. 2004; Ghimire et al. 2015). Cyanobacteria, having the appropriate enzymes, can also synthesize H2, indirectly through photosynthesis. In addition, photo-heterotrophic bacteria (purple non-sulfur bacteria) produce H2 under nitrogen deficiency. Concerning this mode, hydrogen production is higher when cells are immobilized on a solid matrix. As far as dark fermentation is concerned, biohydrogen can be formed by anaerobic bacteria which consume carbohydrates under dark conditions.
One of the major constraints in the fermentative biohydrogen production is the cost of raw materials used as substrates. Therefore, utilization of waste materials according to their availability, cost, carbohydrate content, and biodegradability makes biohydrogen production a highly promising alternative to conventional fuels (Kapdan and Kargi 2006; Arimi et al. 2015; Patel et al. 2015). OMW constitutes a substantial pollutant; thus, many studies focus both on its remediation and utilization for biohydrogen production (Table 2). Different concentrations of water-diluted OMW have been tested as the only substrate for H2 production by Rhodobacter sphaeroides (Eroglu et al. 2004). As it was demonstrated, H2 could be produced at an OMW content below 4%, with the highest production potential of 13.9 LH2/LOMW found for 2%, while at higher concentrations both high amount of inhibitory substances and the dark color of the wastewater probably hindered the photo-heterotrophic pathway of H2 production. Interestingly, nearly pure H2 was produced in all experiments, which makes its use with the existing electricity-producing systems feasible. Later on, a study on coupled biological systems, including a clay treatment step prior to photo-fermentation by Rhodobacter sphaeroides revealed that the efficiency of photobiological H2 production was substantially enhanced. This process resulted in a high hydrogen production of 35 LH2/LOMW, a light conversion efficiency of 0.42%, and a COD (chemical oxygen demand) conversion efficiency of 52% (Eroglu et al. 2006).
OMW can be used not only as a sole substrate for biohydrogen production but also as a co-substrate with other agro-wastes. That was the case in a study where a two-stage anaerobic digestion system was used to test the effect of hydraulic retention time (HRT) on biofuel production under mesophilic conditions (Dareioti and Kornaros 2014). The acidogenic reactor was started up with a waste mixture of 55% OMW, 40% cheese whey, and 5% (w/w) liquid cow manure. Afterward, different HRTs were tested in order to maximize biohydrogen production, with 0.75 d being the most effective, while the highest H2 production rate of 1.72 L/LR d and H2 yield of 0.54 mol H2/mol carbohydrates consumed were achieved at this HRT value. The same co-substrates were used in order to evaluate the effect of pH on biohydrogen production, and the highest hydrogen production yield (0.642 mol H2/mol equivalent glucose consumed) was achieved at pH 6 (Dareioti et al. 2014). Interestingly, it was observed that hydrogen productivity seemed to be primarily related to butyric acid production and lactic acid degradation.
Fermentative bio-H2 production is also feasible from olive pulp (TPOMW), used as substrate. The efficiency of hydrogen production which was found in a CSTR-type anaerobic digester at 35 °C was between 2.8 and 4.5 mmole H2 per g of carbohydrates consumed, depending on HRT, with the highest value observed at HRT of 30 h and the lowest at 7.5 h, respectively (Koutrouli et al. 2006). One factor which greatly affects H2 production is process temperature, with thermophilic mode (55 °C) being more effective than mesophilic one. In fact, it was found that when a hydrogenic digester was fed with diluted olive pulp, hydrogen yield was 0.32 mmole H2/g TS, compared to 0.19 mmole H2/g TS occurring under mesophilic conditions (Gavala et al. 2005; Koutrouli et al. 2009). Diluted OMW (1:4 v/v) has also been used in a two-stage system in order to produce H2 through anaerobic fermentation, and subsequently the derived effluent was used for biopolymers production (Ntaikou et al. 2009; Kourmentza et al. 2015). It was shown that not only hydrogen but also butyrate and acetate production were favored at HRT of 14.5 h.
Photobiological hydrogen production by photosynthetic microorganisms is currently of great interest as a highly promising renewable energy source, despite the fact that commercial exploitation is not yet feasible, as higher yields are still needed (Eroglu and Melis 2011). Recently, pretreated OMW at 50% dilution with a synthetic medium (TAP) was used as a substrate for H2 production by Chlamydomonas reinhardtii (Faraloni et al. 2011). It was shown that H2 production was 37% higher (150 ml H2 L−1 culture) in the TAP-OMW cultures, instead of 100 ml H2 L−1 culture, produced on TAP medium alone. A more concentrated OMW-containing medium has also been tested for biohydrogen production, after a pretreatment process (dephenolization). An OMW-based medium, including 30% of the liquid fraction of the pretreated OMW and 70% distilled water, was evaluated as an inexpensive feedstock for H2 production by Rhodopseudomonas palustris 42OL, a purple non-sulfur photosynthetic bacterium (Pintucci et al. 2013). Both the highest amount of hydrogen production and the average hydrogen evolution rate were achieved at an irradiance of 74 W/m2, while it was also found that by increasing the irradiance shorter, culture age was required.
3.2 Methane Production
Compared to methane and bioethanol, biohydrogen has a higher heating value which makes its use more promising, however, still not practical. Therefore, a higher demand for methane and bioethanol exists as they can be used directly through contemporary technology (Morillo et al. 2009). Methane, as a clean fuel, constitutes a highly advantageous renewable energy source which produces fewer atmospheric pollutants and less carbon dioxide per energy unit than other fossil fuels. Therefore, its use tends to increase in power generation, industrial applications, as well as in transportation sector (Chynoweth et al. 2001). Methane is produced through anaerobic digestion, a biological process that occurs when organic material decomposes by a microbial consortium in the absence of oxygen, or by thermal gasification of biomass, a process that is economic only at large scale (Kumar et al. 2014; Zhang et al. 2014; Pham et al. 2015). In turn, anaerobic digestion is an established waste treatment technology, the final products of which are digestates, residual mixtures rich in nutrients, and biogas which is mainly composed of methane (55–75%) and CO2 (25–45%), while H2S (0–1.5%) and NH3 (0–0.05%) might be present too.
Methane formation is a complex biochemical process that can be subdivided into four stages, each one characterized by the presence of different microbial consortia (Weiland 2010; Ali Shah et al. 2014). The first step is hydrolysis, which includes the conversion of complex biopolymers (proteins, carbohydrates, and fats) to soluble organic compounds, followed by acidogenesis, during which volatile fatty acids, alcohols, aldehydes, and gasses are formed by the conversion of soluble organic compounds. Subsequently, volatile fatty acids are converted to acetate, CO2, and H2, a step called acetogenesis, after which methanogenesis is finally taking place. This step includes the conversion of acetate, CO2, and H2 to methane. Biomass, irrespective of its origin, can be used for biogas production, as long as the appropriate components are present (Batstone and Virdis 2014).
Both OMW and TPOMW have been widely used as feedstock for methane production (Table 3), and much scientific research has focused on overcoming challenges derived from the chemical composition of olive mill wastes which hinder biogas production. Such problems stem mainly from the nutrient imbalance of these wastes due to their high C/N ratio, the low values of pH and alkalinity, as well as the presence of inhibitory substances, especially organic and phenolic compounds (Boubaker and CheikhRidha 2007). In order to avoid these constraints, several pretreatment methods can be applied to OMW before anaerobic digestion including aerobic biological pretreatment, chemical pretreatment, water dilution, and nitrogen addition. Concerning biological pretreatment, the use of fungi and yeasts has been proven effective in increasing biogas production. For instance, an aerobic detoxification step of OMW, carried out by Aspergillus niger, decreased wastewater toxicity through degradation of phenolic compounds and resulted in 60% COD removal and enhanced methane production (Hamdi 1991; Hamdi et al. 1992). Two different pretreatment methods, thermal pretreatment and pretreatment with the white rot fungus Pleurotus ostreatus P69, were employed prior to anaerobic digestion of OMW in a stirred tank reactor under mesophilic conditions (Blika et al. 2009). The use of fungus was proved to be more effective than sterilization, achieving process stability at HRT of 30 d. Apart from fungi, effective aerobic pretreatment can also be achieved with yeasts. Pretreatment of an OMW mixture with cheese whey by Candida tropicalis resulted in effective COD and phenol reduction. In addition, a high organic loading rate and a satisfactory biogas production rate of 1.25 Lbiogas Lreactor −1 day−1 were finally recorded (Martinez-Garcia et al. 2007). However, these practices currently applied for OMW pretreatment require inputs that increase cost-benefit ratio and decrease the organic load, thus the overall methanogenic potential of this feedstock (Sampaio et al. 2011).
As it concerns digesters configuration, an effective way to improve methane yield is through two-phase anaerobic digestion, compared to conventional one-phase systems. In two-phase systems physical separation of different microorganisms gives the opportunity to maximize their performance by separately achieving optimum conditions in each tank. As a matter of fact, through two-phase systems, the imbalance of acidogenesis and methanogenesis is successfully averted, resulting in excellent robustness, effective control, and optimization of AD process. Applying to anaerobic digestion of OMW, two-phase systems have been reported to enhance biogas production (Koutrouli et al. 2009). Usually, another technology of improving OMW bioconversion is concurrently applied: co-digestion with other substrates including diluted olive mill solid wastes (OMSW), poultry manure, cheese whey, and liquid cow manure (Gelegenis et al. 2007; Azbar et al. 2008; Dareioti et al. 2009; Fezzani and CheikhRidha 2010).
Co-digestion is an innovative waste treatment technology, where different organic substrates are combined and digested together in one anaerobic reactor. This practice offers a number of significant advantages, including improvement of plant profitability, increased methane yield, efficient use of plant facilities, and stable operation throughout a year which is often characterized by discontinuous production of specific waste streams such as OMW. Certain challenges encountered during OMW treatment derive mainly from inhibitory effect of polyphenols, lack of nitrogen, and low alkalinity of this waste stream. It has been demonstrated that such problems can be overcome via co-digestion of olive mill effluents with manure, resulting in approximately 40 L biogas/kg OMW, when 1:5 diluted OMW is used (Angelidaki and Ahring 1997). Also, a methane production rate of 0.91 L CH4 L−1 reactor d−1 was achieved when a mixture containing 20% OMW and 80% liquid cow manure was used. Digestion took place in two stages under mesophilic conditions with HRT of 19 days, proving this method sustainable and environmentally attractive for the valorization of such wastes (Dareioti et al. 2010). Lastly, stable methanogenesis with a high methane production rate of 0.33 L CH4/LR d was achieved when a two-stage anaerobic digestion system was fed with a co-mixture of OMW, cheese whey, and cow manure, operated at HRT of 25 days at 37 °C (Dareioti and Kornaros 2014).
3.3 Bioethanol Production
Depleting natural resources, industrialization, global warming, and climate change have shifted international interest into renewable energy sources. Among them, bioethanol is receiving increasing attention due to its potential as a valuable substitute of gasoline in the market of transport fuels (Sarkar et al. 2012). Bioethanol can be used as a modern biofuel, applied directly as a gasoline improver or subsistent, or in order to reduce exhaust gasses emissions. Ethanol production from traditional feedstocks, including sucrose- and starch-containing materials such as sugar substances, corn, wheat, and rice, is not desirable due to their high feed value, and alternative sources must be employed (Sarris and Papanikolaou 2016). Currently, the use of substrates such as crop residues and other biodegradable waste materials for low-cost bioethanol makes biofuel production sustainable (Li et al. 2007). However, in terms of economics, bioethanol production needs to become more cost-effective in order to outperform fossil fuels.
Ethanol can be produced from sugar-containing materials, through fermentation processes. The available raw materials can be categorized into three groups, with each one treated appropriately in order to produce ethanol (Lin and Tanaka 2006). The first group includes sugars mainly derived from sugarcane, molasses, or fruits, which can be directly converted to ethanol. Starches need first to be enzymatically hydrolyzed to fermentable sugars before producing ethanol. Likewise, lignocellulosic materials need to be converted into sugars, before microbial enzymes ferment them. Sugars from cellulose and hemicellulose can be converted to ethanol by either a simultaneous saccharification or fermentation process or by a separated enzymatic hydrolysis followed by fermentation process (Romero-García et al. 2014). However, bioconversion of this type of biomass to bioethanol is rather challenging. The reason is the resistance to breakdown, while a great content of sugars occurring from cellulose and hemicellulose polymers subsequently need suitable microorganisms to convert them and also due to the cost of both collection and storage of low-density lignocellulosic materials (Balat 2011). Concerning the recalcitrance of lignocellulosic biomass, physical, chemical, or biological pretreatment is always needed in order to make cellulose accessible to enzymes prior to hydrolysis (Zheng et al. 2009).
Among waste materials that can be converted to ethanol, olive mill wastes represent interesting substrates for bioethanol production, due to their high content of organic matter (Morillo et al. 2009). However, lowering the phenolic content prior to fermentation might be necessary in order to enhance process performance resulting in higher bioethanol yield (Zanichelli et al. 2007). The effect of such a pretreatment of OMW with the white rot fungus Pleurotus sajor-caju has been studied, subsequently evaluating ethanol production after anaerobic fermentation with the yeast Saccharomyces cerevisiae L-6 (Massadeh and Modallal 2008). An increase in ethanol production, which reached the maximum value of 14.2 g/L, was demonstrated after 48 h fermentation using 50% diluted and pretreated OMW. Enhanced glucose and xylose bioavailability was also observed after wet oxidation and enzymatic hydrolysis pretreatment of olive pulp, prior to fermentation by S. cerevisiae and Thermoanaerobacter mathranii (Haagensen et al. 2009). However, enzymatic pretreatment was proved to be more effective. OMW have been used also as co-substrates with molasses for bioethanol production by S. cerevisiae, as in large-scale processes OMW could replace water used for molasses dilution reducing in that way the cost of the process (Sarris et al. 2014). Effective decolorization and 28% of phenolic compound removal were observed, along with satisfactory ethanol production, despite the fact that yeast growth was performed under aerated conditions.
Apart from OMW, olive oil mill solid residue and olive pulp have been tested as potential feedstocks for ethanol production. In the first case, bioethanol production was investigated using the yeast Pachysolen tannophilus, and it was shown that carbohydrate biotransformation and higher ethanol yields were facilitated by thermochemical pretreatment of the substrate (Senkevich et al. 2012). Also, when enzymatic hydrolysis was employed for OP pretreatment, followed by fermentation with S. cerevisiae, the traditional baker’s yeast, a maximum ethanol production of 11.2 g/L was observed, without any nutrient addition or indication of yeast toxicity (Georgieva and Ahring 2007). However, it was suggested that incomplete conversion of olive pulp to ethanol constitutes this process not viable, and economic feasibility would improve only by simultaneous production of other added-value products, such as methane. Bioethanol production from olive mill solid wastes (OMSW) was also considered to be ineffective, even when endogenous yeasts grown on OMSW were used for fermentation, as it was found that xylitol was produced from xylose, instead of ethanol (Tayeh et al. 2014).
3.4 Biodiesel Production
Biodiesel is a highly attractive alternative diesel fuel that is considered to be one of the most important near-market biofuels, due to the fact that all industrial vehicles are diesel based (Schenk et al. 2008; Rico and Sauer 2015). Biodiesel derives from vegetable oils or animal fats, through a catalyzed chemical reaction between triglycerides present in the oil and fats with a monohydric alcohol resulting in monoalkylesters (Gerpen 2005). Biodiesel is a biodegradable, nontoxic, and clean biofuel that is traditionally obtained from different fuel crops, including soybean, rapeseed, canola, and palm, while terpenoid products from Copaifera species can also constitute an alternative biodiesel source (Yuan et al. 2008). Although the processing of biodiesel is rather simple, the produced fuel can greatly vary in quality.
The major challenge faced by first-generation biofuels is the competition for arable land, which is also the case for biodiesel production from vegetable oils. A great alternative to current biodiesel production is based on lipids produced by microalgae (Schenk et al. 2008). Microalgae have the ability not only to grow rapidly, as they double their biomass within 24 h, but also have high oil content, usually 20–50%, resulting in high oil productivities that are desired for biodiesel production (Chisti 2007). Especially, when microalgae cultivated for biodiesel production consume carbon dioxide as carbon source, either atmospheric or from power plants, the whole cycle generates zero carbon dioxide emissions to the atmosphere (Cheng and Timilsina 2011). Also, microalgae have the potential to grow in conditions where no freshwater input is required, for instance, in saline or brackish water and in wastewaters due to their ability to utilize abundant organic carbon and inorganic nitrogen and phosphorus present in effluents (Pittman et al. 2011).
Phenol-resistant microalgae have been used in order to evaluate phenol removal from OMW (Pinto et al. 2003). A limited reduction of 12% was demonstrated, suggesting that phenolic degradation could be enhanced by OMW co-treatment with suitable algae and fungi, or through a two-stage process where ligninolytic fungi would be employed prior to phenol-resistant microalgae. Additionally, OMW was used as a co-substrate with urban wastewater from secondary treatment (UWST) in order to evaluate Scenedesmus obliquus growth and its subsequent use for biofuels. Composition analysis of the fatty acids accumulated, when S. obliquus was cultivated in a mixture of UWST and OMW, showed that the obtained lipid fraction could result in a good quality biodiesel, as specified by the European Standard (Hodaifa et al. 2013).
Microbial lipid production generally offers great advantages in biodiesel technology, when oleaginous microorganisms that produce more than 20% of their weight lipids are used. Combining this technology with waste materials has been proved as an effective way to reduce cost and make such processes sustainable. In case of OMW, the presence of phenolics hinders microbial growth; however, several microorganisms have been able to grow on olive mill wastes (Lanciotti et al. 2005). The oleaginous yeast Lipomyces starkeyi has shown the ability to grow effectively on OMW reducing total organic carbon and phenolics present in olive mill wastewater (Yousuf et al. 2010). Most significantly, an increased lipid concentration of 28.6% was observed when L. starkeyi was cultivated in 50% diluted OMW. Among identified fatty acids, oleic acid was the most abundant (49.1%), which is rather satisfactory since oleic acid is considered ideal for biodiesel due to its better cold flow properties.
4 Bio-Based Products
4.1 Antioxidants
Phenolic compounds are molecules that have been reported to show antioxidant, anti-inflammatory, anti-allergic, antimutagenic, antiaging, as well as antibacterial activity (Lule and Xia 2005; Taguri et al. 2006; Larif et al. 2015). Due to their remarkable characteristics, they may find potential applications in the development of functional foods (Wildman and Kelley 2007); in pharmaceuticals, i.e., as active agents for cancer prevention and treatment (Huang et al. 2010); and in cosmetic formulations (Padilla et al. 2005). OMW is a waste by-product characterized by its high polyphenol content and therefore raises issues of environmental concern since, after disposal, high polyphenol concentrations result in toxic matter and environmental degradation (Kavvadias et al. 2015). Recovery of phenolic compounds from OMW, prior to its disposal, is considered advantageous since it reduces its phytotoxicity, while at the same time high added-value products are obtained (Barbera et al. 2014). The high content of phenolic compounds, with a wide range between low and high molecular weights (MW), is responsible for its black-brownish color and depends on the ratio between low and high MW polyphenols (Borja et al. 2006). For the recovery of polyphenols from OMW, such as hydroxytyrosol and tyrosol, different methodologies have been proposed and studied such as membrane separation, liquid-liquid extraction, solid-liquid extraction, cloud point extraction, and polymer incompatibility (Rahmanian et al. 2014), while some of them have been patented (López et al. 2008; Villanova et al. 2010; De Magalhães et al. 2011).
Membrane processes have been successfully used in the food and beverage field. Integrated membrane systems for phenolic compound recovery, including microfiltration (MF), ultrafiltration (UF), nanofiltration (NF), reverse osmosis (RF), as well as membrane bioreactors, have been tested by many researchers during the last three decades (Paraskeva et al. 2007; El-Abbassi et al. 2009, 2012; Garcia-Castello et al. 2010; Petrotos et al. 2014; Ochando-Pulido and Martinez-Ferez 2015). Several combinations of membrane processes for the fractionation of OMW to different by-products using UF, NF, and/or RO processes have been studied (Paraskeva et al. 2007). According to results, a fraction rich in phenols may be obtained after NF, whereas the residual effluent could be disposed in aquatic environments according to National and European regulations, or to be used for irrigation (around 75–80% of the initial volume). The application of MF to OMW, subsequent NF, of the MF, and concentration of the NF permeate by osmotic distillation (OD) was evaluated in a recent study (Garcia-Castello et al. 2010). MF allowed suspended solids and total organic carbon (TOC) removal up to 91 and 26%, respectively, and 78% polyphenol recovery from the raw material. After NF, of the MF permeate, almost all polyphenols were recovered, while TOC content was further reduced by 37%. OD resulted in a concentrated solution rich in high molecular weight polyphenols with hydroxytyrosol representing 56% of it. In another study, the application of direct contact membrane distillation (DCMD) using commercial polytetrafluoroethylene (PTFE) membranes of different pore sizes at different temperatures was examined, in order to treat and concentrate OMW (El-Abbassi et al. 2012). With their methodology, after 8 h, they were able to concentrate a permeate characterized by a concentration factor of 1.8. They also concluded that treatment at high temperatures (up to 80 °C) had no negative effect on the total phenolic fraction and its antioxidant activity, as a respective increase of 16 and 15% was achieved. HPLC analysis of the recovered monocyclic phenolic compounds of OMW showed that hydroxytyrosol was the dominant compound, by 70%, while gallic acid (11%), para-coumaric acid (10%), tyrosol (4%), hydroxytyrosol-4-β-glucoside (3%), and caffeic acid (1.7%) were also present.
Efforts have been made to recover phenolic compounds through liquid–liquid extraction from centrifuged OMW, and subsequent anaerobic digestion in order to minimize OMW toxicity (Khoufi et al. 2008). Ethyl acetate was used as solvent while phenol recovery reached up to 90%. Phenolic compounds present in the extract were identified to be hydroxytyrosol, tyrosol, homovanillic acid, caffeic acid, para-coumaric acid, and ferulic acid, by employing GC-MS analysis. Tyrosol and caffeic acid are characterized as natural antioxidants, whereas ferulic acid is considered to be nutraceutically positive. Another research team (Kalogerakis et al. 2013) studied the recovery of phenolic compounds from TPOMW. Due to the high solid content, the effluent was first filtered, through mess gauge filters, and subsequently centrifuged. After supernatant fat and solid removal, the aqueous phase was subjected again to filtration by a 0.45 μm filter. Liquid-liquid extraction was performed using a solvent to TPOMW ratio of 2:1 v/v, at ambient temperature, and continuous stirring at 120 rpm for 30 min. The solvents tested were ethyl acetate, diethyl ether, and a mixture of chloroform/isopropyl alcohol 7:3 v/v, which resulted in total phenols recovery of 57%, 47%, and 56%, respectively. In addition LCA methodology was applied in order to identify the environmental footprint of the process. It was shown that the use of ethyl acetate or diethyl ether has a similar environmental footprint concerning their impacts on ecosystem and fossil fuel resources, contribution to global warming, as well as on human health. However, ethyl acetate was considered advantageous, due to higher extraction efficiency. On the other hand, chloroform/isopropyl alcohol mixture was shown to pose detrimental environmental effects.
As it regards solid phase extraction, various adsorbent resins have been employed in order to achieve deodorizing and decolorization of OMW, polyphenol, and lactone recovery. A process in which the effluent is at first subjected to successive filtration steps to remove suspended solids, followed by adsorption using XAD16 and XAD7HP resins, subsequent thermal evaporation and recovery of the organic fraction, and finally, separation of the polyphenols through fast centrifuge partition chromatography has been proposed (Agalias et al. 2007). According to the results obtained, an odorless wastewater is characterized by 99.99 and 98% of polyphenols and COD removal, respectively, a rich in polyphenols and lactones extract, an extract which contained the coloring substances of the OMW and pure hydroxytyrosol. In another study, four resins, viz., XAD7, XAD16, IRA96, and ISOLUTE ENV+, were tested as solid adsorbing phases in two types of TPOMW obtained from different olive mills (Bertin et al. 2011). The solvents employed in desorption experiments were water, methanol, ethanol, and acidified ethanol (0.5 w/w HCl 37%). According to their results, ENV+ showed to be promising in terms of process productivity, adsorbing 84% of total phenols, hydroxytyrosol, and tyrosol, while with IRA96 the highest phenol adsorption ratios were achieved from the water phase. They concluded that in general nonpolar resins are more efficient. In more detail, the efficiency of the resins to adsorb phenols present in TPOMW decreased according to the following sequence: ENV+<XAD16<IRA96<XAD7.
Cloud point extraction (CPE) is a process of transferring a nonionic surfactant from one liquid phase to another by heating, taking advantage of the ability of surfactant molecules to form micelles. When temperature increases above the cloud point, micelles dehydrate and aggregate, which leads to macroscopic phase separation into two distinguished phases: solvent and surfactant-rich phase. CPE requires less time, labor cost, and equipment and represents a simpler and cleaner technology, compared to liquid-liquid and solid-liquid extraction. Therefore, the concentrated antioxidants obtained are suitable for food and pharmaceutical and cosmetic applications. Various low toxicity surfactants, such as Span 20, PEG 400, Tween 80, and Tween 20, have been examined for the separation of phenols from OMW (Katsoyannos et al. 2012). Among them, Tween 80 showed the highest recovery at a concentration of 5%, incubated at 55 °C for 30 min. One step CPE extraction resulted in 86.8% of phenols recovery, whereas when a double-step CPE extraction was performed, using 5 + 5% Tween 80, phenols recovery increased to 94.4%. In another study, CPE was performed to TPOMW, which was pretreated by ultrafiltration in order to remove the suspended solids present (El-Abbassi et al. 2014). Ultrafiltration resulted in color intensity reductions of 80 and 87%, estimated at 395 and 465 nm, respectively, while the COD and the dry residue were reduced by 31.4 and 27.6%. As it regards CPE, a range of concentrations, 0–10% of the surfactant Triton X-100 in OMW, incubated at different temperatures, 70 °C, 80 °C, and 90 °C, for 30 min were studied. After they examined the efficiency of the process, they observed that the highest yield of phenols extraction, 66.5%, was achieved using 10% of Triton X-100 at 90 °C.
Lately, polymer incompatibility has been suggested as a potential tool for polyphenol recovery from OMW (Hajji et al. 2014). The certain methodology is based on thermodynamic incompatibility between polymers and concerns the use of aqueous two-phase systems (ATPS). In their study, the ATPS consisted of solutions containing protein (6.33 or 7.45 wt.% caseinate, 18 wt.% ovalbumin) and a certain concentration of polysaccharide (0.75–2 wt.% alginate and methylcellulose) adjusted to neutral pH. In particular caseinate–alginate, caseinate–methylcellulose, and ovalbumin–methylcellulose systems were tested at ambient temperature. They concluded that caseinate–alginate systems, mixed with OMW, were more efficient in terms of separation, resulting to an upper polysaccharide-rich phase and a bottom protein-polyphenol-rich phase characterized by a polyphenol recovery yield of 85.8%.
In general, dephenolization and detoxification of OMW results in the recovery of high value-added compounds, while at the same time it presents an opportunity to reduce wastewater treatment cost. Furthermore, polyphenols are compounds that usually occur by chemical reactions, which is the main reason for their high cost. OMW and other types of wastewater, with high phenolic content, can be valorized toward the recovery of such remarkable compounds. Last, but not least, their recovery is considered advantageous because their presence in OMW inhibits its microbiological treatment.
4.2 Biosurfactants
Within the last years, OMW has been exploited for the production of biopolymers and fine chemicals, by employing pure as well as mixed microbial consortia, combining microbiological treatment with the production of high added-value products.
Biosurfactants are biologically derived surface-active agents, not associated with bacterial growth; therefore, they are characterized as secondary metabolites. They are amphiphilic compounds consisting of hydrophilic “heads” and hydrophobic “tails,” and they have the ability to decrease the surface tension of water and interfacial tension between water and hydrophobic substances. For those reasons, hydrophobic substrates are used in order to induce biosurfactant production, as bacteria secrete them in order to increase nutrient availability and grow on hydrophobic substrates. Studies have demonstrated the potential of OMW to be utilized as carbon source for biosurfactant production in the form of rhamnolipids, which are classified as glycolipid biosurfactants, as well as in the form of surfactin, a lipopeptide biosurfactant. This is due to the fact that residual oil and the polysaccharide content in OMW constitute the precursors of biosurfactant production.
The first attempt on investigating the production of rhamnolipids using this complex wastewater was performed by employing Pseudomonas sp. JAMM (Mercade et al. 1993). OMW was used as the sole carbon source whereas NaNO3 (2.5 g/L) was supplemented in the effluent to enhance rhamnolipids production. According to the results, rhamnolipids conversion yield reached up to 0.058 g/g of OMW, calculated on a COD basis, while at the same time a 50% reduction of OMW COD and a 55% reduction in the total phenol content was achieved after 3 days. A total bioconversion yield of 14 g of rhamnolipids per kg of OMW was estimated after 150 h of fermentation. Later on, OMW was used for the production of rhamnolipids from P. aeruginosa ATCC 10145 and its recombinant strain expressing Vitreoscilla hemoglobin gene vgb (Colak and Kahraman 2013). The maximum production of rhamnolipids reached up to 0.4 g/L, for both wild type and recombinant strain, when grown at 37 °C and 100 rpm after 3 days.
TPOMW was recently used as carbon source for the production of surfactin, from Bacillus subtilis DSM 3256 (Maass et al. 2015). After 36 h the maximum surfactin concentration was achieved, reaching up to 0.25 g/L, characterized by a productivity of 0.17 g/L/d, while the surface tension of the culture’s medium decreased to around 30 mN/m. Finally, in another study performed recently, the production of rhamnolipids and surfactin, by P. aeruginosa and B. subtilis, respectively, was investigated utilizing OMW (Ramírez et al. 2015). It was shown that rhamnolipids production ranged between 8.78 and 191.46 mg/L, with the highest concentration obtained by using 10% w/v OMW. On the other hand, surfactin production reached 3.12 mg/L using 2% w/v OMW, and it dropped to 0.57 mg/L when a more concentrated OMW solution of 10% w/v was used. Although OMW may be considered inhibitory for the production of biosurfactants, pretreatment regarding its dephenolization and detoxification may be proven beneficial and establish the appropriate conditions within the fermentation medium in order to enhance biosurfactant production.
4.3 Polyhydroxyalkanoates
Polyhydroxyalkanoates (PHAs) are polymers of hydroxyalkanoates produced by a wide variety of bacteria. They are produced as intracellular inclusions, called granules, which bear a diameter of 0.2–0.9 μm. Their monomeric structure may vary according to the carbon atoms present. The most common forms are polyhydroxybutyrate (PHB) and polyhydroxybutyrate-polyhydroxyvalerate (PHBV) copolymers. PHAs have attracted interest since they bear similar properties to polypropylene (PP) and low-density polyethylene (PE/LDPE) and represent an attractive alternative to replace those petrochemical plastics (Gao et al. 2011). It is worth mentioning that PP and PE/LDPE have gained a major position in the global plastic market that accounts for almost 50% of the total plastic demand in Europe (Plastics-The Facts 2015). Therefore, there is a wide application field for PHA biopolymers, and due to their biodegradability, their use can minimize the detrimental impact of persistent plastics on the environment (Kourmentza et al. 2015).
Within the last decade, researchers have been studying the valorization of OMW toward the production of PHAs. In particular, the feasibility of anaerobic fermented effluents, rich in volatile fatty acids (VFAs) serving as precursors for PHA production by enriched mixed cultures, has been investigated (Dionisi et al. 2005; Beccari et al. 2009; Kourmentza et al. 2009a; Ntaikou et al. 2009, 2014). Other studies concerning OMW utilization, as the sole carbon source, by pure cultures have also demonstrated the potential valorization of OMW for PHA production (Kourmentza et al. 2009b, 2015; Martinez et al. 2015).
VFAs are the most common carbon sources used for PHA production. Investigation on the anaerobic fermentation of OMW, with and without pretreatment steps, at different concentrations, and subsequent PHA production using the fermented effluent has been performed (Dionisi et al. 2005). It was shown that VFA concentration and yield were significantly increased after centrifugation, although centrifugation led to differences in acid distribution, which resulted in different hydroxyvalerate (HV) content in the PHBV copolymer. For PHA production, a mixed culture, enriched in an aerobic SBR under feast and famine conditions, was employed. After testing centrifuged fermented and not fermented OMW, they concluded that PHA production is feasible in both cases, although fermented OMW showed much higher potential, characterized by an initial specific rate of around 0.42 g COD/g COD/h, while the final PHA concentration reached up to 0.54 g PHA/g volatile suspended solids (VSS). The polymer obtained was a PHBV copolymer consisting 11% HV, on a molar basis.
The performance of a process consisting of three stages for PHA production from OMW has been also studied (Beccari et al. 2009). In the first stage, the OMW was anaerobically fermented, in a packed bed biofilm reactor, in order to produce an effluent rich in VFAs. In the second stage, the VFA-rich effluent was used as a feed for an aerobic SBR where mixed cultures were enriched to PHA-producing bacteria. Finally, in the third stage of the proposed process, PHA production was tested, by employing the enriched cultures, under aerobic batch conditions. They observed that during anaerobic fermentation of the wastewater, VFA content increased from 18 to 32%, in a COD basis, while during the second stage, an enriched culture with high PHA-storing capacity was formed, characterized by a maximum production rate and yield of 0.15 g COD/g COD/h and 0.36 COD/COD, respectively. In the final stage, they observed that PHA concentration increased almost linearly as a function of the organic loading rate (OLR), revealing the possibility to design a process operation using higher OLR. The highest PHA content in the biomass achieved was around 20% g COD/g COD, while storage yield was almost the same, 0.35 COD/COD. This scenario of microbiological treatment resulted in approximately 85% of COD removal; thus, it was considered effective for both OMW treatment and OMW valorization toward PHA production.
Combination of OMW anaerobic fermentation, employed for the biological production of hydrogen, and subsequent feeding of an SBR reactor, used for PHAs production, with the obtained effluent has been recently proposed (Ntaikou et al. 2009). In that way the VFAs present in the acidogenic reactor’s effluent serve as the precursors for PHA production while at the same time COD reduction occurs. For the anaerobic fermentation, a continuous stirred tank reactor (CSTR) was used at different HRT, ranging from 7.5 to 60 h. HRTs were examined in terms of biohydrogen and VFA production. It was shown that at an HRT of 27–33 h propionate production was favored while at lower HRT acetate and butyrate were dominant. Biohydrogen productivity rates and yields were severely affected in lower HRT with the most effective ones achieved at 24 h calculated at 165 mL/d and 330 mL/L of OMW (diluted 1:4), respectively. The effluent of the anaerobic fermenter was forwarded to an SBR used for the enrichment of activated sludge to PHA-forming bacteria and also the production of PHAs. During the growth phase acetate, propionate and butyrate were fully consumed, whereas during the PHA accumulation phase butyrate was preferably consumed, followed by propionate and acetate. Substrate preference of the specific culture was identical when fed with a mixture of synthetic VFAs (Kourmentza et al. 2009a, b). This conclusion is of great importance since during anaerobic fermentation, manipulation of operating conditions may lead to a desirable VFA profile that will eventually favor PHA production and can also result to certain HV content in the PHBV copolymer, due to the presence of propionate. In this study PHA production capacity reached up to 9% g PHA/g cell dry weight (CDW) and a PHBV copolymer was obtained.
Recently, scaling up of the abovementioned proposed process in a 20 L reactor has been investigated (Ntaikou et al. 2014). The highest PHAs capacity achieved was increased from 9%, in lab scale, to 24.6% g PHAs/g VSS. Previous studies performed indicated that the enriched PHA-forming bacteria culture mainly consisted of strains that belong to Pseudomonas sp., in particular P. putida (Kourmentza et al. 2009a, b, 2015). As a result a copolymer consisting of both PHB and polyhydroxyoctanoate (PHO) was produced characterized by a weight average molecular weight of 490 kDa and a polydispersity index (PI) of 5.15. The PI of the polymer is considered high for a biological polymer (usually ~1) indicating its heterogenicity probably due to the diversity of the carbon sources consumed and/or the microbial consortium eventually formed.
As it regards the production of PHAs by pure cultures lately, the potential of fermented OMW for PHA production from an enriched culture and strains that consisted this mixed consortium was studied (Kourmentza et al. 2015). The isolated strains were identified to belong to Pseudomonas genus. Batch experiments were conducted, under nitrogen-limiting conditions, which revealed that the specific strains had the ability to accumulate 0.9–6.2% g PHAs/g VSS. Under conditions of dual nitrogen-oxygen limitation, PHA production ranged from 0.6 to 11.5% g PHAs/g VSS.
Finally, different concentrations of OMW solutions (25, 50, 75 and 100% v/v) have been exploited for the production of PHAs by Cupriavidus necator DSM 545 (Martinez et al. 2015). It was shown that the maximum PHA capacity, 60% g PHAs/g CDW, was obtained by using 75% diluted OMW, with a conversion yield of 0.26 g PHAs/g VFAs resulting in the formation of a PHBV copolymer consisting of 20% mol HV. Although, they concluded that the presence of phenols significantly contributes to inhibitory effects.
4.4 Polysaccharides
Exopolysaccharides (EPS) are extracellular polymeric substances produced by numerous bacterial species. EPS are a structurally diverse class of biological macromolecules with a wide broad of applications in pharmaceuticals, cosmetics, and bioremediation (Liang and Wang 2015).
Xanthan gum is an exopolysaccharide that is mainly secreted by Xanthomonas campestris and is considered one of the most commercially important microbial polysaccharides due to its remarkable physical properties. Xanthan gum is composed by repeated pentasaccharide units, consisting of glucose, mannose, and glucuronic acid at a molar ratio of 2:2:1. It has been extensively used as a food supplement and rheology modifier, as an emulsion stabilizer in cosmetic formulations, and as a thickening agent in salad dressings (Petri 2015). Production of xanthan gum, using OMW as the sole source of nutrients, was firstly described by X. campestris NRRL B1459-S4L41 (López and Ramos-Cormenzana 1996). Results showed that biomass and xanthan production were inhibited at OMW concentrations above 60%. Although, maximum xanthan production reached up to 4 g/L when X. campestris was fed with 30% v/v OMW solution. Xanthan production was increased when phosphate buffer was added in the medium, resulting to a final xanthan concentration of 6.3 g/L. In a further study, four strains of X. campestris using different % v/v OMW solutions were tested, and according to the results obtained, the highest xanthan production was achieved using 30% v/v OMW (López et al. 2001a). Xanthan concentrations ranged from 3.48 to 7.01 g/L while the viscosity of the broth varied from 3890 to 4710 mPa s. In another study, xanthan production was achieved when nitrogen was supplemented in a TPOMW resulting to the production of 3.5 g/L of xanthan (López et al. 2001b).
The production of another type of EPS produced by Paenibacillus strains has also been reported when using OMW. Paenibacillus jamilae sp., isolated from corn compost treated with OMW, was able to grow on a 100% v/v OMW solution, at 30 °C and pH 7, and produce EPS (Aguilera et al. 2001). The heteropolysaccharide produced consisted of fucose, xylose, rhamnose, arabinose, mannose, galactose, and glucose. Production of EPS from the bacteria P. jamilae CP-7 when grown on a medium containing 80% v/v OMW has also been reported (Ruiz-Bravo et al. 2001). After 72 h of incubation at 30 °C, a water-soluble EPS in the form of white powder was obtained with a yield of 5.5 g/L. In another study TPOMW was investigated as the substrate of EPS production by P. jamilae CECT 5266 (Morillo et al. 2006). They observed that maximum EPS yield of 2 g/L was obtained using 20% v/v TPOMW, whereas nutrient supplementation, in the form of nitrate, phosphate, and other inorganic nutrients, did not favor EPS production. In another study performed by the same research group (Morillo et al. 2007), 5.1 g/L EPS were obtained by P. jamilae CECT 5266 using 80% v/v OMW, while inhibition on growth and EPS production was observed for increased OMW concentration of 100% v/v OMW, resulting in 2.7 g/L of EPS. Characterization of the EPS showed that it was composed of two EPS fractions of different molecular weights, above 2000 kDa and 500 kDa. Both fractions consisted mainly of the carbohydrates glucose, galactose, mannose, arabinose, rhamnose, as well as hexosamines and uronic acid. Later on, researchers isolated 60 different strains from compost treated with OMW (Aguilera et al. 2008). From those, ten strains were selected due to their ability to produce EPS-utilizing OMW as the sole carbon source. Initial experiments performed in shake flasks showed that P. jamilae CP-38 was characterized by the maximum yield, as it was able to produce 4.2 g/L EPS, within 48 h, using 80% v/v OMW. Further tests in a 2 L bioreactor showed that the yield of EPS was increased to 5.2 g/L after 72 h of incubation. Although the most interesting conclusion was that the toxicity of OMW was decreased by 75% within the first 24 h, with a subsequent decrease until the end of the fermentation. High phenolic degrading activity is a characteristic of Paenibacillus strains (Raj et al. 2007) and so is the production of extracellular enzymes able to degrade polysaccharides (Ko et al. 2007). Taking those into account, it was assumed that the production of EPS plays an important role on OMW detoxification.
5 Perspectives
Olive oil processing constitutes one basic economic activity, especially in Mediterranean regions, which offers significant advantages along with serious environmental issues that have to be handled. Millions of tons of olive mill wastewaters and by-products which are produced annually may serve as renewable sources for green biomaterials and energy production, toward a more sustainable and environmentally friendly economy. Undoubtedly, challenges of our times demand immediate action in terms of depleting energy sources, energy insecurity, pollution and climate change, and wastes including those produced by olive mill industry can contribute to this direction. Effective exploitation of OMW and TPOMW can substantially reduce the environmental impact of olive oil production, while concurrently biopolymers, and other valuable natural components, as well as gas and liquid biofuel production can successfully materialize. Additionally, it is of high significance that nothing is wasted and all residues can be recycled. Despite the fact that further research and technological development are needed in order to establish such an economical industrial scale production, great effort and progress has been done so far, and it is only a matter of time for this venture to be widely established.
References
Agalias A, Magiatis P, Skaltsounis AL, Mikros E, Tsarbopoulos A, Gikas E, Spanos I, Manios T (2007) A new process for the management of olive oil mill wastewater and recovery of natural antioxidants. J Agric Food Chem 55:2671–2676. doi:10.1021/jf063091d
Aguilera M, Monteoliva-Sanchez M, Suarez A, Guerra V, Lizama C, Bennasar A, Ramos-Cormenzana A (2001) Paenibacillus jamilae sp. nov., an exopolysaccharide-producing bacterium able to grow in olive-mill wastewater. Int J Syst Evol Microbiol 51:1687–1692. doi:10.1099/00207713-51-5-1687
Aguilera M, Quesada MT, del Aguila VG, Morillo JA, Rivadeneyra MA, Ramos-Cormenzana A, Monteoliva-Sanchez M (2008) Characterisation of Paenibacillus jamilae strains that produce exopolysaccharide during growth on and detoxification of olive mill wastewaters. Bioresour Technol 99:5640–5644. doi:10.1016/j.biortech.2007.10.032
Ali Shah F, Mahmood Q, Maroof Shah M, Pervez A, Ahmad Asad S (2014) Microbial ecology of anaerobic digesters: the key players of anaerobiosis. Sci World J 2014:1–21. doi:10.1155/2014/183752
Angelidaki I, Ahring BK (1997) Codigestion of olive oil mill wastewaters with manure, household waste or sewage sludge. Biodegradation 8:221–226. doi:10.1023/A:1008284527096
Antonopoulou G, Stamatelatou K, Venetsaneas N, Kornaros M, Lyberatos G (2008) Biohydrogen and methane production from cheese whey in a two-stage anaerobic process. Ind Eng Chem Res 47:5227–5233. doi:10.1021/ie071622x
Arimi MM, Knodel J, Kiprop A, Namango SS, Zhang Y, Geiben SU (2015) Strategies for improvement of biohydrogen production from organic-rich wastewater: a review. Biomass Bioenergy 75:101–118. doi:10.1016/j.biombioe.2015.02.011
Azbar N, Keskin T, Yuruyen A (2008) Enhancement of biogas production from olive mill effluent (OME) by co-digestion. Biomass Bioenergy 32:1195–1201. doi:10.1016/j.biombioe.2008.03.002
Balat M (2011) Production of bioethanol from lignocellulosic materials via the biochemical pathway: a review. Energy Convers Manage 52:858–875. doi:10.1016/j.enconman.2010.08.013
Barbera AC, Maucieri C, Ioppolo A, Milani M, Cavallaro V (2014) Effects of olive mill wastewater physico-chemical treatments on polyphenol abatement and Italian ryegrass (Lolium miltiflorum Lam.) germinability. Water Res 52:275–281. doi:10.1016/j.watres.2013.11.004
Barca C, Soric A, Ranava D, Giudici-Orticoni MT, Ferrasse JH (2015) Anaerobic biofilm reactors for dark fermentative hydrogen production from wastewater—a review. Bioresour Technol 185:386–398. doi:10.1016/j.biortech.2015.02.063
Batstone DJ, Virdis B (2014) The role of anaerobic digestion in the emerging energy economy. Curr Opin Biotechnol 27:142–149. doi:10.1016/j.copbio.2014.01.013
Beccari M, Bertin L, Dionisi D, Fava F, Lampis S, Majone M, Valentino F, Vallini G, Villano M (2009) Exploiting olive oil mill effluents as a renewable resource for production of biodegradable polymers through a combined anaerobic-aerobic process. J Chem Technol Biotechnol 84:901–908. doi:10.1002/jctb.2173
Bertin L, Ferri F, Scoma A, Marchetti L, Faba F (2011) Recovery of high added value natural polyphenols from actual olive mill wastewater through solid phase extraction. Chem Eng J 171:1287–1293. doi:10.1016/j.cej.2011.05.056
Blika PS, Stamatelatou K, Kornaros M, Lyberatos G (2009) Anaerobic digestion of olive mill wastewater. Global Nest J 11:364–372
Borja R, Rincon B, Raposo F (2006) Anaerobic biodegradation of two-phase olive mill solid wastes and liquid effluents: kinetic studies and process performance. J Chem Technol Biotechnol 81:1450–1462. doi:10.1016/j.biortech.2013.03.155
Boubaker F, Cheikh Ridha B (2007) Anaerobic co-digestion of olive mill wastewater with olive mill solid waste in a tubular digester at mesophilic temperature. Bioresour Technol 98:769–774. doi:10.1016/j.biortech.2006.04.020
Caputo AC, Scacchia F, Pelagagge PM (2003) Disposal of by-products in olive oil industry: waste-to-energy solutions. Appl Therm Eng 23:197–214. doi:10.1016/S1359-4311(02)00173-4
Cheng JJ, Timilsina GR (2011) Status and barriers of advanced biofuel technologies: a review. Renew Energy 36:3541–3549. doi:10.1016/j.renene.2011.04.031
Chisti Y (2007) Biodiesel from microalgae. Biotechnol Adv 25:294–306. doi:10.1016/j.biotechadv.2007.02.001
Chynoweth DP, Owens JM, Legrand R (2001) Renewable methane from anaerobic digestion of biomass. Renew Energ 22:1–8. doi:10.1016/S0960-1481(00)00019-7
Colak AK, Kahraman H (2013) The use of raw cheese whey and olive oil mill wastewater for rhamnolipid production by recombinant Pseudomonas aeruginosa. Environ Exp Biol 11:125–130
Dareioti MA, Kornaros M (2014) Effect of hydraulic retention time (HRT) on the anaerobic co-digestion of agro-industrial wastes in a two-stage CSTR system. Bioresour Technol 167:407–415. doi:10.1016/j.biortech.2014.06.045
Dareioti MA, Dokianakis SN, Stamatelatou K, Zafiri C, Kornaros M (2009) Biogas production from anaerobic co-digestion of agroindustrial wastewaters under mesophilic conditions in a two-stage process. Desalination 248:891–906. doi:10.1016/j.desal.2008.10.010
Dareioti MA, Dokianakis SN, Stamatelatou K, Zafiri C, Kornaros M (2010) Exploitation of olive mill wastewater and liquid cow manure for biogas production. Waste Manage 30:1841–1848. doi:10.1016/j.wasman.2010.02.035
Dareioti MA, Vavouraki AI, Kornaros M (2014) Effect of pH on the anaerobic acidogenesis of agroindustrial wastewaters for maximization of bio-hydrogen production: a lab-scale evaluation using batch tests. Bioresour Technol 162:218–227. doi:10.1016/j.biortech.2014.03.149
De Magalhães ML, Cardador SJL, Figueiredo AA, Marques NAV, Duarte CM, Goulão CJP (2011) Method of obtaining a natural hydroxytyrosol-rich concentrate from olive tree residues and subproducts using clean technologies. Patent: US8066881
Dermeche S, Nadour M, Larroche C, Moulti-Mati F, Michaud P (2013) Olive mill wastes: biochemical characterizations and valorization strategies. Process Biochem 48:1532–1552. doi:10.1016/j.procbio.2013.07.010
Dionisi D, Carucci G, Papini MP, Riccardi C, Majone M, Carrasco F (2005) Olive oil mill effluents as a feedstock for production of biodegradable polymers. Water Res 39:2076–2084. doi:10.1016/j.watres.2005.03.011
El-Abbassi A, Hafidi A, García-Payo MC, Khayet M (2009) Concentration of olive mill wastewater by membrane distillation for polyphenols recovery. Desalination 245:670–674. doi:10.1016/j.desal.2009.02.035
El-Abbassi A, Kiai H, Hafidi A, Garcia-payo MC, Khayet M (2012) Treatment of olive mill wastewater by membrane distillation using polytetrafluoroethylene membranes. Sep Purif Technol 98:55–61. doi:10.1016/j.seppur.2012.06.026
El-Abbassi A, Kiai H, Raiti J, Hafidi A (2014) Cloud point extraction of phenolic compounds from pretreated olive mill wastewater. J Environ Chem Eng 2:1480–1486. doi:10.1016/j.jece.2014.06.024
ElMekawy A, Diels L, Bertin L, De Wever H, Pant D (2014) Potential biovalorization techniques for olive mill biorefinery wastewater. Biofuel Bioprod Bioref 8:283–293. doi:10.1002/bbb.1450
Eroglu E, Melis A (2011) Photobiological hydrogen production: recent advances and state of the art. Bioresour Technol 102:8403–8413. doi:10.1016/j.biortech.2011.03.026
Eroglu E, Gündüz U, Yücel M, Turker L, Eroglu I (2004) Photobiological hydrogen production by using olive mill wastewater as a sole substrate source. Int J Hydrogen Energy 29:163–171. doi:10.1016/S0360-3199(03)00110-1
Eroglu E, Eroglu I, Gündüz U, Turker L, Yücel M (2006) Biological hydrogen production from olive mill wastewater with two-stage processes. Int J Hydrogen Energy 31:1527–1535. doi:10.1016/j.ijhydene.2006.06.020
Faraloni C, Ena A, Pintucci C, Torzillo G (2011) Enhanced hydrogen production by means of sulfur-deprived Chlamydomonas reinhardtii cultures grown in pretreated olive mill wastewater. Int J Hydrogen Energy 36:5920–5931. doi:10.1016/j.ijhydene.2011.02.007
Fezzani B, Cheikh Ridha B (2010) Two-phase anaerobic co-digestion of olive mill wastes in semi-continuous digesters at mesophilic temperature. Bioresour Technol 101:1628–1634. doi:10.1016/j.biortech.2009.09.067
Gao X, Chen JC, Wu Q, Chen GQ (2011) Polyhydroxyalkanoates as a source of chemicals, polymers, and biofuels. Curr Opin Biotechnol 22:768–774. doi:10.1016/j.copbio.2011.06.005
Garcia-Castello E, Cassano A, Criscuoli A, Conidi C, Drioli E (2010) Recovery and concentration of polyphenols from olive mill wastewaters by integrated membrane system. Water Res 44:3883–3892. doi:10.1016/j.watres.2010.05.005
Gavala HN, Skiadas IV, Ahring BK, Lyberatos G (2005) Potential for biohydrogen and methane production from olive pulp. Water Sci Technol 52:209–215
Gelegenis J, Georgakakis D, Angelidaki I, Christopoulou N, Goumenaki M (2007) Optimization of biogas production from olive-oil mill wastewater, by codigesting with diluted poultry-manure. Appl Energy 84:646–663. doi:10.1016/j.apenergy.2006.12.001
Georgieva TI, Ahring BK (2007) Potential of agroindustrial waste from olive oil industry for fuel ethanol production. Biotechnol J 2:1547–1555. doi:10.1002/biot.200700128
Gerpen JV (2005) Biodiesel processing and production. Fuel Process Technol 86:1097–1107. doi:10.1016/j.fuproc.2004.11.005
Ghimire A, Frunzo L, Pirozzi F, Trably E, Escudie R, Lens PNL, Esposito G (2015) A review on dark fermentative biohydrogen production from organic biomass: process parameters and use of by-products. Appl Energy 144:73–95. doi:10.1016/j.apenergy.2015.01.045
Haagensen F, Skiadas IV, Gavala HN, Ahring BK (2009) Pre-treatment and ethanol fermentation potential of olive pulp at different dry matter concentrations. Biomass Bioenergy 33:1643–1651. doi:10.1016/j.biombioe.2009.08.006
Hajji F, Kunz B, Weissbrodt J (2014) Polymer incompatibility as a potential tool for polyphenol recovery from olive mill wastewater. Food Chem 156:23–28. doi:10.1016/j.foodchem.2014.01.068
Hamdi M (1991) Effects of agitation and pretreatment on the batch anaerobic digestion of olive mill wastewater. Bioresour Technol 36:173–178. doi:10.1016/0960-8524(91)90176-K
Hamdi M, Garcia JL, Ellouz R (1992) Integrated biological process for olive mill wastewater treatment. Bioprocess Eng 8:79–84. doi:10.1007/BF00369268
Hodaifa G, Sanchez S, Martinez ME, Orpez R (2013) Biomass production of Scenedesmus obliquus from mixtures of urban and olive-oil mill wastewaters used as culture medium. Appl Energy 104:345–352. doi:10.1016/j.apenergy.2012.11.005
Huang WY, Cai YZ, Zhang Y (2010) Natural phenolic compounds from medicinal herbs and dietary plants: potential use for cancer prevention. Nutr Cancer 62:1–20. doi:10.1080/01635580903191585
Kalogerakis N, Politi M, Foteinis S, Chatzisymeon E, Mantzavinos D (2013) Recovery of antioxidants from olive mill wastewaters: a viable solution that promotes their overall sustainable management. J Environ Manage 128:749–758. doi:10.1016/j.jenvman.2013.06.027
Kapdan IK, Kargi F (2006) Bio-hydrogen production from waste materials. Enzyme Microb Technol 38:569–582. doi:10.1016/j.enzmictec.2005.09.015
Katsoyannos E, Gortzi O, Chatzilazarou A, Athanasiadis V, Tsaknis J, Lalas S (2012) Evaluation of the sustainability of low hazard surfactants for the separation of phenols and carotenoids from red-flesh orange juice and olive mill wastewater using cloud point extraction. J Sep Sci 35:2665–2670. doi:10.1002/jssc.201200356
Kavvadias V, Doula M, Papadopoulou M, Theocharopoulos S (2015) Long-term application of olive-mill wastewater affects soil chemical and microbial properties. Soil Res 53:461–473. doi:10.1071/SR13325
Khoufi S, Aloui F, Sayadi S (2008) Extraction of antioxidants from olive mill wastewater and electro-coagulation of exhausted fraction to reduce its toxicity on anaerobic digestion. J Hazard Mater 151:531–539. doi:10.1016/j.jhazmat.2007.06.017
Ko CH, Chen WL, Tsai CH, Jane WN, Liu CC, Tu J (2007) Paenibacillus campinasensis BL11: a wood material-utilizing bacterial strain isolated from black liquor. Bioresour Technol 98:2727–2733. doi:10.1016/j.biortech.2006.09.034
Kourmentza C, Ntaikou I, Kornaros M, Lyberatos G (2009a) Production of PHAs from mixed and pure cultures of Pseudomonas sp. using short-chain fatty acids as carbon source under nitrogen limitation. Desalination 248:723–732. doi:10.1016/j.desal.2009.01.010
Kourmentza C, Mitova E, Stoyanova N, Ntaikou I, Kornaros M (2009b) Investigation of PHAs production from acidified olive oil mill wastewater (OOMW) by pure cultures of Pseudomonas spp. strains. New Biotechnol 25:S269. doi:10.1016/j.nbt.2009.06.604
Kourmentza C, Ntaikou I, Lyberatos G, Kornaros M (2015) Polyhydroxyalkanoates from Pseudomonas sp. using synthetic and olive mill wastewater under limiting conditions. Int J Biol Macromol 74:202–210. doi:10.1016/j.ijbiomac.2014.12.032
Koutrouli EC, Gavala HN, Skiadas IV, Lyberatos G (2006) Mesophilic biohydrogen production from olive pulp. Process Saf Environ Prot 84:285–289. doi:10.1205/psep.05165
Koutrouli EC, Kalfas H, Gavala HN, Skiadas IV, Stamatelatou K, Lyberatos G (2009) Hydrogen and methane production through two-stage mesophilic anaerobic digestion of olive pulp. Bioresour Technol 100:3718–3723. doi:10.1016/j.biortech.2009.01.037
Kumar P, Pant DC, Mehariya S, Sharma R, Kansal A, Kalia VC (2014) Ecobiotechnological strategy to enhance efficiency of bioconversion of wastes into hydrogen and methane. Indian J Microbiol 54:262–267. doi:10.1007/s12088-014-0467-7
Kumar P, Mehariya S, Ray S, Mishra A, Kalia VC (2016) Biotechnology in aid of biodiesel industry effluent (glycerol): biofuels and bioplastics. In: Kalia VC (ed) Microbial cell factories. Springer, New Delhi, pp 105–119. isbn: 978-81-322-2597-3. doi: 10.1007/978-81-322-2598-0_7
Lanciotti R, Gianotti A, Baldi D, Angrisani R, Suzzi G, Mastrocola D, Guerzoni ME (2005) Use of Yarrowia lipolitica strains for the treatment of olive mill wastewaters. Bioresour Technol 96:317–322. doi:10.1016/j.biortech.2004.04.009
Larif M, Ouhssine M, Soulaymani A, Elmidaoui A (2015) Potential effluent oil mills and antibacterial activity polyphenols against some pathogenic strains. Res Chem Intermed 41:1213–1225. doi:10.1007/s11164-013-1267-0
Levin DB, Pitt L, Love M (2004) Biohydrogen production: prospects and limitations to practical application. Int J Hydrogen Energy 29:173–185. doi:10.1016/S0360-3199(03)00094-6
Li A, Antizar-Ladislao B, Khraisheh MAM (2007) Bioconversion of municipal solid waste to glucose for bio-ethanol production. Bioproc Biosyst Eng 30:189–196. doi:10.1007/s00449-007-0114-3
Liang TW, Wang SL (2015) Recent advances in exopolysaccharides from Paenibacillus spp.: production, isolation structure, and bioactivities. Mar Drugs 13:1847–1863. doi:10.3390/md13041847
Lin Y, Tanaka S (2006) Ethanol fermentation from biomass resources: current state and prospects. Appl Microbiol Biotechnol 69:627–642. doi:10.1007/s00253-005-0229-x
López MJ, Ramos-Cormenzana A (1996) Xanthan production from olive-mill wastewaters. Int Biodeterior Biodegrad 38:263–270. doi:10.1016/S0964-8305(96)00059-5
López MJ, Moreno J, Ramos-Cormenzana A (2001a) Xanthomonas campestris strain selection for xanthan production from olive mill wastewaters. Water Res 35:1828–1830. doi:10.1016/S0043-1354(00)00430-9
López MJ, Moreno J, Ramos-Cormenzana A (2001b) The effect of olive mill wastewaters variability on xanthan production. J Appl Microbiol 90:829–835. doi:10.1046/j.1365-2672.2001.01326.x
López MJA, Streitenberger SA, Mellado MP, Ortiz PM (2008) Process and apparatus for the production of hydroxytyrosol containing extract from olives and solids containing residues of olive oil extraction. Patent: WO20008090460 A1
Lule SU, Xia W (2005) Food phenolics, pros and cons: a review. Food Rev Int 21:367–388. doi:10.1080/87559120500222862
Maass D, Ramirez IM, Roman MG, Alameda EJ, de Souza AAU, Valle JAB, Vaz DA (2015) Two-phase olive mill waste (alpeorujo) as carbon source for biosurfactant production. J Chem Technol Biotechnol 91(7):1990–1997. doi:10.1002/jctb.4790
Martinez GA, Bertin L, Scoma A, Rebecchi S, Braunegg G, Fava F (2015) Production of polyhydroxyalkanoates from dephenolised and fermented olive mill wastewaters by employing a pure culture of Cupriavidus necator. Biochem Eng J 97:92–100. doi:10.1016/j.bej.2015.02.015
Martinez-Garcia G, Johnson AC, Bachmann RT, Williams CJ, Burgoyne A, Edyvean RGJ (2007) Two-stage biological treatment of olive mill wastewater with whey as co-substrate. Int Biodeterior Biodegrad 59:273–282. doi:10.1016/j.ibiod.2007.03.008
Massadeh MI, Modallal N (2008) Ethanol production from olive mill wastewater (OMW) pretreated with Pleurotus sajor-caju. Energy Fuel 22:150–154. doi:10.1021/ef7004145
Mateo JJ, Maicas S (2015) Valorization of winery and oil mill wastes by microbial technologies. Food Res Int 73:13–25. doi:10.1016/j.foodres.2015.03.007
McNamara CJ, Anastasiou CC, O’Flaherty V, Mitchell R (2008) Bioremediation of olive mill wastewater. Int Biodeterior Biodegrad 61:127–134. doi:10.1016/j.ibiod.2007.11.003
Mercade ME, Manresa MA, Robert M, Espuny MJ, de Andres C, Guinea J (1993) Olive oil mill effluent (OOME). New substrate for biosurfactant production. Bioresour Technol 43:1–6. doi:10.1016/0960-8524(93)90074-L
Momirlan M, Veziroglu TN (2005) The properties of hydrogen as fuel tomorrow in sustainable energy system for a cleaner planet. Int J Hydrogen Energy 30:795–802. doi:10.1016/j.ijhydene.2004.10.011
Morillo JA, Aguilera M, Ramos-Cormenzana A, Monteoliva-Sanchez M (2006) Production of a metal-binding exopolysaccharide by Paenibacillus jamilae using two-phase olive-mill waste as fermentation substrate. Curr Microbiol 53:189–193. doi:10.1007/s00284-005-0438-7
Morillo JA, del Aguila VG, Aguilera M, Ramos-Cormenzana A, Monteoliva-Sanchez M (2007) Production and characterization of the exopolysaccharide produced by Paenibacillus jamilae grown on olive mill-waste waters. World J Microbiol Biotechnol 23:1705–1710. doi:10.1007/s11274-007-9418-3
Morillo JA, Antizar-Ladislao B, Monteoliva-Sánchez M, Ramos-Cormenzana A, Russell NJ (2009) Bioremediation and biovalorisation of olive-mill wastes. Appl Microbiol Biotechnol 82:25–39. doi:10.1007/s00253-008-1801-y
Nigam P, Singh A (2011) Production of liquid biofuels from renewable resources. Prog Energy Combust Sci 37:52–68. doi:10.1016/j.pecs.2010.01.003
Ntaikou I, Kourmentza C, Koutrouli EC, Stamatelatou K, Zampraka A, Kornaros M, Lyberatos G (2009) Exploitation of olive oil mill wastewater for combined biohydrogen and biopolymers production. Bioresour Technol 100:3724–3730. doi:10.1016/j.biortech.2008.12.001
Ntaikou I, Valencia Peroni C, Kourmentza C, Ilieva VI, Morelli A, Chiellini E, Lyberatos G (2014) Microbial bio-based plastics from olive-mill wastewater: generation and properties of polyhydroxyalkanoates from mixed cultures in a two-stage pilot scale system. J Biotechnol 188:138–147. doi:10.1016/j.jbiotec.2014.08.015
Ochando-Pulido JM, Martinez-Ferez A (2015) On the recent use of membrane technology for olive mill wastewater purification. Membranes 5:513–531. doi:10.3390/membranes5040513
Padilla M, Palma M, Barroso CG (2005) Determination of phenolics in cosmetic creams and similar emulsions. J Chromatogr A 1091:83–88. doi:10.1016/j.chroma.2005.07.041
Paraskeva CA, Papadakis VG, Tsarouchi E, Kanellopoulou DG, Koutsoukos PG (2007) Membrane processing for olive mill wastewater fractionation. Desalination 213:218–229. doi:10.1016/j.desal.2006.04.087
Patel SKS, Kumar P, Singh M, Lee JK, Kalia VC (2015) Integrative approach to produce hydrogen and polyhydroxybutyrate from biowaste using defined bacterial cultures. Bioresour Technol 176:136–141. doi:10.1016/j.biortech.2014.11.029
Petri DFS (2015) Xanthan gum: a versatile biopolymer for biomedical and technological applications. J Appl Polym Sci 132:42035. doi:10.1002/app.42035
Petrotos KB, Lellis T, Kokkora MI, Gkoutsidis PE (2014) Purification of olive mill wastewater using microfiltration membrane technology. J Membr Sep Technol 3:50–55
Pham TPT, Kaushik R, Parshetti GK, Mahmood R, Balasubramanian R (2015) Food waste-to-energy conversion technologies: current status and future directions. Waste Manage 38:399–408. doi:10.1016/j.wasman.2014.12.004
Pinto G, Pollio A, Previtera L, Stanzione M, Temussi F (2003) Removal of low molecular weight phenols from olive oil mill wastewater using microalgae. Biotechnol Lett 25:1657–1659. doi:10.1023/A:1025667429222
Pintucci C, Giovannelli A, Traversi ML, Ena A, Padovani G, Carlozzi P (2013) Fresh olive mill waste deprived of polyphenols as feedstock for hydrogen photo-production by means of Rhodopseudomonas palustris 42OL. Renew Energy 51:358–363. doi:10.1016/j.renene.2012.09.037
Pittman JK, Dean AP, Osundeko O (2011) The potential of sustainable algal biofuel production using wastewater resources. Bioresour Technol 102:17–25. doi:10.1016/j.biortech.2010.06.035
Plastics-The Facts 2015 An analysis of European latest plastics production, demand and waste data. Plastics Europe: Association of Plastics Manufacturers. http://www.plasticseurope.org/Document/plastics---the-facts-2015.aspx?FolID=2
Rahmanian N, Jafari SM, Galanakis CM (2014) Recovery and removal of phenolic compounds from olive mill wastewater. J Am Oil Chem Soc 91:1–18. doi:10.1007/s11746-013-2350-9
Raj A, Reddy MMK, Chandra R (2007) Decolourisation and treatment of pulp and paper mill effluent by lignin-degrading Bacillus sp. J Chem Technol Biotechnol 82:399–406. doi:10.1002/jctb.1683
Ramírez IM, Tsaousi K, Rudden M, Marchant R, Alameda EJ, Roman MG, Banat IM (2015) Rhamnolipid and surfactin production from olive oil mill waste as sole carbon source. Bioresour Technol 198:231–236. doi:10.1016/j.biortech.2015.09.012
Rico JAP, Sauer IL (2015) A review of Brazilian biodiesel experiences. Renew Sust Energy Rev 45:513–529. doi:10.1016/j.rser.2015.01.028
Roig A, Cayuela ML, Sanchez-Monedero MA (2006) An overview on olive mill wastes and their valorisation methods. Waste Manage 26:960–969. doi:10.1016/j.wasman.2005.07.024
Romero-García JM, Niño L, Martínez-Patiño C, Álvarez C, Castro E, Negro MJ (2014) Biorefinery based on olive biomass. State of the art and future trends. Bioresour Technol 159:421–432. doi:10.1016/j.biortech.2014.03.062
Ruiz-Bravo A, Jimenez-Valera M, Moreno E, Guerra V, Ramos-Cormenzana A (2001) Biological response modifier activity of an exopolysaccharide from Paenibacillus jamilae CP-7. Clin Diagn Lab Immunol 8:706–710. doi:10.1128/CDLI.8.4.2001
Sampaio MA, Goncalves MR, Marques IP (2011) Anaerobic digestion challenge of raw olive mill wastewater. Bioresour Technol 102:10810–10818. doi:10.1016/j.biortech.2011.09.001
Sarkar N, Kumar Ghosh S, Bannerjee S, Aikat A (2012) Bioethanol production from agricultural wastes: an overview. Renew Energy 37:19–27. doi:10.1016/j.renene.2011.06.045
Sarris D, Papanikolaou S (2016) Biotechnological production of ethanol: biochemistry, processes and technologies. Eng Life Sci 16:307–329. doi:10.1002/elsc.201400199
Sarris D, Matsakas L, Aggelis G, Koutinas AA, Papanikolaou S (2014) Aerated vs non-aerated conversions of molasses and olive mill wastewaters blends into bioethanol by Saccharomyces cerevisiae under non-aseptic conditions. Ind Crops Prod 56:83–93. doi:10.1016/j.indcrop.2014.02.040
Schenk PM, Thomas-Hall SR, Stephens E, Marx UC, Mussgnug JH, Posten C, Kruse O, Hankamer B (2008) Second generation biofuels: high-efficiency microalgae for biodiesel production. Bioenergy Res 1:20–43. doi:10.1007/s12155-008-9008-8
Sen U, Shakdwipee M, Banerjee R (2008) Status of biological hydrogen production. J Sci Ind Res 67:980–993
Senkevich S, Ntaikou I, Lyberatos G (2012) Bioethanol production from thermochemically pre-treated olive mill solid residues using the yeast Pachysolen tannophilus. Global Nest J 14:118–124
Sims REH, Mabee W, Saddler JN, Taylor M (2010) An overview of second generation biofuel technologies. Bioresour Technol 101:1570–1580. doi:10.1016/j.biortech.2009.11.046
Taguri T, Tanaka T, Kound I (2006) Antibacterial spectrum of plants polyphenols and extracts depending upon hydroxyphenyl structure. Biol Pharm Bull 29:2226–2235. doi:10.1248/bpb.29.2226
Tayeh HA, Najami N, Dosoretz C, Tafesh A, Azaizeh H (2014) Potential of bioethanol production from olive mill solid wastes. Bioresour Technol 152:24–30. doi:10.1016/j.biortech.2013.10.102
Urbaniec K, Bakker RR (2015) Biomass residues as raw materials for dark hydrogen fermentation—a review. Int J Hydrogen Energy 40:3648–3658. doi:10.1016/j.ijhydene.2015.01.073
Venetsaneas N, Antonopoulou G, Stamatelatou K, Kornaros M, Lyberatos G (2009) Using cheese whey for hydrogen and methane generation in a two-stage continuous process with alternative pH controlling. Bioresour Technol 100:3713–3717. doi:10.1016/j.biortech.2009.01.025
Villanova L, Villanova L, Fasiello G, Merendino A (2010) Process for the recovery of tyrosol and hydroxytyrosol from oil mill wastewaters and catalytic oxidation method in order to convert tyrosol in hydroxytyrosol. Patent: EP2147898 A1
Weiland P (2010) Biogas production: current state and perspectives. Appl Microbiol Biotechnol 85:849–860. doi:10.1007/s00253-009-2246-7
Wildman REC, Kelley M (2007) Nutraceuticals and functional foods. In: REC W (ed) Handbook of nutraceuticals and functional foods, 2nd edn. CRC Press, Taylor & Francis Group, London, New York, pp 1–22
Yousuf A, Sannino F, Addorisio V, Pirozzi D (2010) Microbial conversion of olive oil mill wastewaters into lipids suitable for biodiesel production. J Agric Food Chem 58:8630–8635. doi:10.1021/jf101282t
Yuan JS, Tiller KH, Al-Ahmad H, Stewart NR, Stewart CN Jr (2008) Plants to power: bioenergy to fuel the future. Trends Plant Sci 13:421–429. doi:10.1016/j.tplants.2008.06.001
Zanichelli D, Carloni F, Hasanaj E, D’Andrea N, Filippini A, Setti L (2007) Production of ethanol by an integrated valorization of olive oil byproducts. The role of phenolic inhibition. Environ Sci Pollut Res 14:5–6. doi:10.1065/espr2006.06.316
Zhang C, Su H, Baeyens J, Tan T (2014) Reviewing the anaerobic digestion of food waste for biogas production. Renew Sust Energy Rev 38:383–392. doi:10.1016/j.rser.2014.05.038
Zheng Y, Pan Z, Zhang R (2009) Overview of biomass pretreatment for cellulosic ethanol production. Int J Agric Biol Eng 2:51–68. doi:10.3965/j.issn.1934-6344.2009.03.051-068
Acknowledgements
Dr. Constantina Kourmentza would like to thank the European Commission for providing support through the FP7-PEOPLE-2013-IEF-Marie-Curie Action: Intra-European Fellowships for Career Development (grant no. 625774).
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 Springer International Publishing AG
About this chapter
Cite this chapter
Kourmentza, C., Koutra, E., Venetsaneas, N., Kornaros, M. (2017). Integrated Biorefinery Approach for the Valorization of Olive Mill Waste Streams Towards Sustainable Biofuels and Bio-Based Products. In: Kalia, V., Kumar, P. (eds) Microbial Applications Vol.1. Springer, Cham. https://doi.org/10.1007/978-3-319-52666-9_10
Download citation
DOI: https://doi.org/10.1007/978-3-319-52666-9_10
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-52665-2
Online ISBN: 978-3-319-52666-9
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)