Abstract
Cancer constitutes a significant public health burden in developing countries where more than 50% of new cases of cancers occurring worldwide are seen. In most of these less developed nations, sterling quality pathology services is either unavailable, or it is often sub-standard in quality. Where pathology services are available, the role of the pathologist may be poorly understood, and his services under-utilized. Inadequate pathology services can lead to a cycle of ineffective healthcare knowledge and practice. This chapter seeks to give an update on the efforts and the contributions of pathologists towards the diagnosis and the management of cancers. A detailed review of the available technical capacity in most countries, including pathologists and histo-technologists, the infrastructure, and the existing facilities for routine pathology tests and for ancillary investigations like immunohistochemistry and molecular diagnostics is presented. The various challenges mitigating against the provision of sterling quality pathology services in the region are enumerated. These include shortage of personnel, poor funding, lack of infrastructure, lack of standard operating procedures, and poor external and internal quality assurance schemes. Highlighted also are various recommendations for ensuring accurate and timely histopathology reports in the low income settings in SSA including the need for local and international collaborations amongst pathologists in order to establish regional training centers and develop clinical and translational research.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Cancer has become a major public health problem in developing countries with over half of the total number of cases seen worldwide occurring in this region. In 2012, there were 32.6 million people living with cancer with 14.1 million new cases and 8.2 million cancer deaths worldwide. Of the new cases, 57% occurred in the less developed regions of the world (IARC, GLOBOCAN 2012). In sub-Saharan Africa (SSA), reasons for the higher cancer mortality include ignorance about risk factors, late presentation and higher rate of default to follow-up, limited access to care, poverty and the fact that many of the population prefer traditional or religious care to orthodox medical care.
Pathology, the science at the core of medicine is the bedrock of effective cancer care because of its role in diagnosis, staging and grading, prognostication and clinical management of patients. Recent advances in molecular pathology have shown it to be a powerful tool for the screening and early detection of disease. Pathologists have a central role to play in bringing the understanding of disease and disease mechanisms to bear on patient management (Robboy et al. 2013). The widespread availability of pathology services and increased accessibility to such services are therefore essential to effective cancer care.
In SSA in particular, both the prevalence of cancer and the mortality rates are high while resources for diagnosis and management are inadequate. In the majority of countries in SSA, there is extreme shortage of pathology services and where available, they are often of sub-standard quality (Awadelkarim et al. 2010). The number of pathologists range from one pathologist per 84,133 people in Mauritius to one per 9,264,500 people in Niger (Yaziji et al. 2008). Countries such as Equatorial Guinea, Gambia, Guinea, Guinea-Bissau, Lesotho, Liberia and Swaziland have no public sector pathologist (African Pathologists’ Summit Working Groups 2015). This is not significantly different from an earlier informal survey carried out by Adesina et al. (2013); all countries (except South Africa and Botswana) have one pathologist to 500,000 people. This is a far cry compared to the USA where there are approximately 18,000 actively practicing pathologists (5.7 pathologists per 100,000 population), approximately 93% of whom are board certified (Robboy et al. 2013).
Pathology is poorly funded in many of these countries, and the national budgetary allocation is lower than the 15% of total which was agreed upon by the Heads of State of the countries in the African Union in 2001 (World Health Organisation 2011). Ten years after the agreement, only Rwanda and South Africa have met the target of allocating at least 15% of the annual budget to health.
The WHO Abuja Declaration came into existence in order to improve the health sector (Nelson et al. 2015). Eight goals were set for the purpose of tracking the progress made in adhering to the Abuja declaration (Millennium Declaration), three (3) of which were health-related; to reduce child mortality, to improve maternal mortality and to combat HIV/AIDS, malaria and other diseases. With respect to achievement of the Millennium Development Goals (MDG) for health, only 8 (17%) out of 46 countries are on track; the remaining 38 (83%) are off track (Nelson et al. 2015). As at 2013, most countries in SSA recorded an average total government expenditure on health as a percentage of the total government expenditure in most SSA that is far lower than that recorded by the developed countries of the world (World Health Organisation 2013, 2014), (Figs. 4.1 and 4.2). This inadequate health funding has resulted in inadequate human and material resources for pathology services. Where good clinical services exist, a patient may not receive appropriate management because the relevant information from pathological evaluation of patient specimens is not available (Rambau 2011). Inadequate pathology services can lead to a cycle of ineffective health-care knowledge and practice (Adesina et al. 2013).
There is extreme shortage of pathologists in SSA. The tertiary institutions with accreditation for providing training of specialist pathologists are few, training is highly variable, training time tends to be short because of the high cost and structured programs are lacking in many countries (Benediktsson et al. 2007). These trainees are often eager to leave the countries in SSA for one of the developed nations of the world. They leave because of a lack of infrastructure to practice, lack of conducive environment and good working conditions, poor remuneration and thus become part of the ‘brain drain.’
In Nigeria, only 6% of 3056 practicing specialist physicians are pathologists. According to the data from the Medical and Dental Council of Nigeria (MDCN), only 182 of 380 pathologists (58%) are practicing in Nigeria, the remaining 52% are lost to brain drain. When medical graduates or fellows travel for additional training in foreign countries, the difficulties with applying the new skills acquired to their practice on their return to the home country may encourage them to stay back in the developed nations where they acquired these skills. The pathologist’s role is not properly understood and this lack of recognition for pathology is not only seen among the general public but also among clinical colleagues, administrators and politicians who are at the forefront of developing policies for health in the various countries.
This deplorable situation of poor pathology services in SSA represents deterioration from the high standards that were in existence in many institutions in the early 1950s to the 1970s, during which period pathology services were comparable to the developed world (Adeyi 2011). Burkitt lymphoma, for example, was first described by Denis Burkitt, an Irish Surgeon based in Africa. Subsequent research on the pathogenesis of this disease was conducted on the Raji cell line produced from a health facility in Africa (Burkitt 1958).
The challenges to the provision of pathological services in SSA are enormous. They are not limited to poor funding and shortage of personnel alone; they also include inadequate infrastructure, laboratory facilities and equipment, facilities for data management and processing etc. Standard operating procedures, external and internal quality assurance schemes, facilities for shipping of specimens to reference pathology centres are important militating factors (Fitzgibbon and Wallis 2014).
There have been several efforts at addressing the myriad of problems confronting pathology practice in SSA particularly as it relates to cancer diagnosis and management. In 2013, the African Pathologists Summit under the auspices of African Organization for Research & Training in Cancer (AORTIC) in collaboration with several other organizations within and outside of Africa, held a meeting in Dakar, Senegal, with the main objective of identifying the constraints to pathology services and to find the avenues to addressing these constraints. It was noted that there is significant lack of professional and technical personnel, inadequate infrastructure, limited training opportunities, poor funding of pathology services and these have significant impact on patient care. Recommendations (discussed below) for urgent action were made in order to tackle these challenges (African Pathologists’ Summit Working Group 2015).
At the African Pathologists Summit, it was agreed that pathologists in SSA must come together to leverage upon the available resources by encouraging local and international collaborations for pathology. A consensus was reached to use specific strategies in tackling the issues affecting pathology practice in SSA including the improvement of diagnostic service, the establishment of regional training centers and the development of clinical and translational research that will provide information critical for policy making decisions.
The major pathology services provided in most SSA for cancer diagnosis and management are: surgical pathology and histochemistry, cytopathology (both gynaecological and non-gynaecological). Only a few centres have facilities for providing frozen sections, histochemistry, immunohistochemistry, and molecular pathology on a routine basis.
2 Pathology Tissue Handling and the Challenges Faced in the Various Sub-sections
2.1 Histopathology/Surgical Samples
In SSA, this diagnostic procedure serves the purpose of providing a diagnosis, staging and grading and guiding clinical management including surgery, chemotherapy or radiotherapy. The use of the appropriate fixative for the preservation of tissues obtained at surgery as well as the cold ischaemia time is of importance as is the duration of fixation. The sample should be immersed in preservative as soon as it is removed, and not at the end of the surgical procedure. A container that is large enough to contain the sample as well as a volume of preservative that is at least 10 times the volume of the sample should be used. In breast cancer, the ratio of tissue/fixative of 1:20 was recommended and the sample must be placed in fixative within 1 h of removal. For breast specimens, the sample must remain in the fixative for no more than 8 h (Yaziji et al. 2008). Improper fixation and long cold ischaemia time have negative effects on the preservation of DNA.
The most commonly used fixative is 10% buffered formal saline (formalin) which is suitable for all tissues, it is cheap and easy to prepare. Paraffin-embedded tissue processing and staining using routine haematoxylin and eosin stains is provided by the pathology laboratories in most centres in SSA. Proper tissue processing can be done with manual, semi-automated or fully automated tissue processors. Manual tissue processing is the most prevalent in SSA due to a lack of availability of the tissue processing machine. This may result in the tissue becoming overly processed (‘fried’ or ‘cooked’ tissue). Sometimes poor quality paraffin wax is supplied for use at the laboratory since, in many countries; the procurement of laboratory consumables is done by non-technical personnel. This issue is further compounded by poor storage facilities of these consumables due to erratic power supply. Inappropriate tissue processing and poor facilities for storage and archival of tissue blocks and histopathology slides constitute major obstacles to conducting cancer research in many parts of SSA. In a molecular study investigating the incidence of K-RAS and BRAF mutations among patients with colorectal cancers in Nigeria, a high failure rate of 112 of the 200 cases (56%) of pyrosequencing was recorded and this was attributed to poor fixation of the tissues (Abdulkareem et al. 2012).
Pathology services in most parts of SSA are concentrated in major cities and therefore transportation of samples to these centers from remote areas remains a challenge, with these services being largely inaccessible to the rural areas. Poor road networks and difficulties with finding the funds to pay for transportation contribute to this problem. Where these samples are sent to the laboratories with pathologists, the Turn Around Time (TAT) is prolonged by the delay between obtaining the specimen and depositing it at the laboratory, the time lag being that required for transportation. Nelson et al. reported that the case loads of surgical samples processed each year ranged from less than 100 in Burundi to >20,000 in Nigeria, Kenya and >40,000 per year in South Africa while TAT ranged from 1–3 days to as long as 21–28 days in some countries (African Pathologists’ Working Groups 2015). They reported that there is statistically significant correlation between the number of pathologists and histotechnologists and the case load but TAT was inversely correlated and not significant.
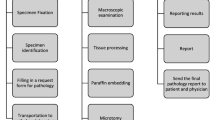
In order to standardize practice, The AORTIC Pathology Summit has therefore recommended step-by-step process of tissue handling from specimen acquisition, processing to final reporting as shown in the Table 4.1 below (Fitzgibbon and Wallis 2014).
It is hoped that, whether the budgetary allocation to pathology increases or not, the use of the above protocol would achieve the following: (1) It would reduce the TAT to 3 days (or less) for small biopsies and 5 days (or less) for large samples; with an emphasis being placed on excellent technical and diagnostic accuracy, (2) It would help develop useful collaboration between clinicians and pathologists with the establishment of Tumour Boards; (3) It would help define minimum standards for tissue processing to ensure good quality reports are provided in a timely manner.
2.2 Frozen Sections
This technique is used for rapid diagnosis from fresh surgical samples. In cancer management, it is also used for quick diagnosis for the presence of diagnostic material in tissues and the examination of tumour margins to guide surgical resections. This service is not provided in many laboratories in SSA. in some laboratories in Nigeria where the cryostat is available, it is non-functional, either because the scientist cannot operate or due to lack of the necessary parts or technical support for maintenance and repair.
2.3 Cytopathology
Cytopathology in the form of Fine Needle Aspiration Cytology (FNAC) and gynaecological cytopathology (Pap smear) which are commonly available in most pathology laboratories in SSA. FNAC is a safe, non-invasive, simple, convenient, rapid diagnostic procedure used to investigate superficial masses and lumps. In many centers, the sample is taken either by the pathologist or surgeon and in some advanced centers trained radiologist do it under ultrasound or CT Scan guidance. Some centers run FNAC clinics in pathology departments and are able to make results available promptly within 30 min. FNAC is commonly used for breast masses, neck masses such as lymph node and thyroid, other soft tissue and bone tumours as well as cases of suspected hepatic neoplasm. In one study aimed to determine the accuracy of this technique in the diagnosis of peripheral lymph node enlargement, the overall sensitivity of 79.6%, specificity of 95.9%, positive predictive value of 79.6% and negative predictive value of 95.5% (Akinde et al. 2011). In patients with chronic liver disease suspected to have hepatocellular carcinoma where needle core biopsy is contraindicated, ultrasound guided FNAC provides a safe method of approach and there are no major attendant complications (Mrzljak et al. 2010).
Gynaecologic cytopathology is routinely available in most centers to screen for presence of cervical intraepithelial (CIN) lesions. However, there is no organized screening program in most countries but opportunistic screening exists in several tertiary and secondary health facilities. Liquid based cytology is preferred to the conventional method but is not available in many centers in SSA. In few centers where available, irregular supply of the consumables make them abandon the method. Sample collection is done with the use of Ayre’s wooden spatula or endocervical brush. The brush is preferred because it is able to collect cells from the transformation zone and easier to make thin smear on the slide. Smear is best taken mid-cycle for pre-menopausal women (when the cells show optimal cytological details) and any time for menopausal women. Slide fixation is done using 95% ethanol or 80% isopropanol for 15–20 min after which the slide can be removed and transported to the laboratory for staining. When Liquid-based method is used, the commercially prepared bottles containing preservative are usually provided by the laboratory.
Human papilloma virus (HPV) DNA testing is only available in few centers. This when combined with Pap smear increases the sensitivity. In some cases triple-combined testing (Pap smear cytology, HPV DNA testing and cervicography) improved the high false negativity of cervical cytology and may be an effective tool in uterine cervical cancer screening (Kim et al. 2013). This may be cost effective in SSA where resources are limited and follow up is not feasible.
Cytology of fluid/aspirates-are also carried out in SSA to exclude or make diagnosis of cancer. Sample collection is however essential to making meaningful diagnosis. Sample should be collected in universal bottle (it does not have to be sterile bottle except if culture is required). It should be collected fresh during the day and taken to the laboratory immediately for processing. No preservative is needed; if there is any need to keep sample overnight, it should be kept in the refrigerator.
2.4 Immunohistochemistry and Molecular Diagnostics
It is useful for diagnostic, prognostic and therapeutic purposes. It plays important roles in determining the origin of unknown primary tumours, detecting micro-metastasis, detect micro-organisms and provide prognostic information for treatment of cancer. In developed centers, the technique is used routinely in surgical pathology practice with the traditional heamatoxylin and eosin stained histological sections. In the survey by Nelson et al. (2015), 16 (53%) of 30 SSA countries have IHC facilities and molecular diagnostics were available in only two (11%) of 18 countries. South Africa is the only country where both facilities are accessible routinely (African Pathologists’ Summit Working Groups 2015).
The role of the pathologist has evolved to providing specific information regarding tumour classification, prognosis and therapy. The latter requires IHC or molecular diagnostics. In breast cancer patients, hormone receptors, oestrogen (ER) and progesterone (PR) profiling is necessary for treatment planning as hormone receptor negative tumours do not respond hormone treatment. Likewise, human epidermal growth factor receptor 2 (HER2) test should be done routinely before treatment decisions are made. HER2 positive breast cancer respond to trastuzumab (Herceptin) a monoclonal antibody that interferes with HER2 receptor. Lack of this facility results in delay in initiating treatment as tissue blocks have to be sent abroad for IHC.
In a clinical trial of Nigerian patients with gastrointestinal stromal tumour (GIST) who had their c-Kit (encodes for a receptor tyrosine kinase protein) gene status tested benefitted from free treatment from a clinical trial of the drug Imatinib mesylate, a tyrosine-kinase inhibitor. Twenty two (81%) of twenty seven patients had Imatinib as the primary therapy with overall survival rate of 71.9% at 2 years (Durosinmi et al. 2013). IHC service in the few centers where available, is fraught with numerous challenges ranging from bureaucracy associated with supply of reagents and consumables, improper storage due to power outage to, quality assurance and quality control issues among others.
Infections such as human papillomavirus, helicobacter pylori, hepatitis B and C viruses and Epstein Barr virus are responsible for about one quarter of cancers in developing countries. Molecular diagnostics can utilize the pathogen genome as a tumour marker to promoting diagnosis, monitoring, and targeted therapy (Gulley and Morgan 2014). Development in this area promises to address the gaps in health care through rapid, user friendly and cost effective devices reflecting clinical priorities in resource poor areas (Gulley and Morgan 2014).
3 Cancer Registries
The knowledge about cancer incidence and mortality is gained through properly organized cancer registries which is supposed to be population based backed by adequate pathology services. Data from SSA are not representative of the actual burden because most tumour registries in these countries do not meet the required standard. Some institutions such as University College Hospital, Ibadan was the first to be established in Nigeria and Uganda Cancer registry contributed data for the publication of Cancer in five continents by International agency on cancer research. Underfunding of these registries has adversely affected their performance. There is the need to improve on the standard of the existing cancer registries in SSA to give accurate information and data because this is crucial for planning, implementation and evaluation of cancer control programs (Stefan et al. 2015).
4 Autopsy Services
Autopsies are not widely practiced in developing countries because of cultural barriers, lack of facilities and trained personnel (Adesina et al. 2013). The rates have declined significantly in SSA in the last decade. In cancer patients in whom diagnosis was made prior to death, relatives, and sometimes attending health workers do not appreciate the need for autopsy in such patients. In most SSA, inadequate technical manpower and infrastructure as well as quality of cancer data systems all contribute to inaccurate data on cancer burden.
5 Conclusion
The prevalence of cancer and the mortality rates in SSA are high while pathology services are inadequate. This inadequate provision of pathological services in SSA constitutes a major barrier to effective cancer care. The shortage of pathology personnel, inadequate infrastructure and other necessary facilities are the result of poor national budgetary to health services in general and also the poor understanding of the role of pathology in cancer care. Despite all the challenges however, there is need for pathologists in SSA to come together to leverage upon the available resources and encourage local and international collaborations for pathology. In order for pathology in SSA to play its pivotal role in effective cancer care, the recommendation of the AORTIC Pathology Summit to use specific strategies to improve diagnostic service, establish regional training centers and develop clinical and translational research should be adopted and implemented by all concerned.
References
Abdulkareem FB, Sanni LA, Richman SD, Chambers P, Hemmings G, Grabsch H, et al. KRAS and BRAF mutations in Nigerian colorectal cancers. West Afr J Med. 2012;31(3):198–203.
Adesina A, Chumba D, Nelson AN, Orem J, Roberts D, Wabinga H, Wislon M, Rebbeck T. Cancer control in Africa 2. Improvement of pathology in sub-Saharan Africa. Lancet Oncol. 2013;14:E152–e157.
Adeyi OA. Pathology services in developing countries-the West African experience. Arch Pathol Lab Med. 2011;135:183–6.
African Pathologists’ Summit Working Groups. Proceedings of the African Pathologists Summit; March 22–23, 2013; Dakar, Senegal: a summary. Arch Pathol Lab Med. 2015; 139(1):126–32. Accessed 11 Feb 2016.
Akinde OR, Abudu EK, Anunobi CC, Daramola AO, Banjo AAF, Abdulkareem FB, Osunkalu VO. Accuracy of fine needle aspiration in the diagnosis of peripheral lymph node enlargements in Lagos University teaching hospital, Lagos, Nigeria. Nig Q J Hosp Med. 2011;21(1):59–63.
Awadelkarim KD, Muhammedani AA, Barberis M. Role of pathology in sub-Saharan Africa: an example from Sudan. Pathol Lab Med Int. 2010;2:49–57.
Benediktsson H, Whitelaw J, Roy I. Pathology services in developing countries-a challenge. Arch Pathol Lab Med. 2007;131:1636–9.
Burkitt D. A sarcoma involving the jaws in African children. Br J Surg. 1958;46:218–23.
Durosinmi MA, Salawu L, Lawal OO, Ojo OS, Alatishe OI, Oyekunle AA, et al. Imatinib (Glivec) and gastrointestinal stromal tumours in Nigerians. Afr J Med Med Sci. 2013;42(4):325–32.
Fitzgibbon JE, Wallis CL. Laboratory challenges conducting international clinical research in resource-limited settings. J Acquir Immune Def Syndr. 2014;65(01):S36–9.
Gulley ML, Morgan DR. Molecular oncology testing in resource-limited settings. J Mol Diagn. 2014;16(6):601–11.
IARC, GLOBOCAN. Estimated cancer incidence mortality and prevalence worldwide in 2012. 2012. http://globocan.iarc.fr/Pages/fact_sheets_cancer.aspx. Accessed 11 Feb 2016.
Kim JH, Kim IW, Kim YW, Park DC, Kim YW, Lee KH, Ahn TG, et al. Comparison of single-, double- and triple-combined testing, including pap test, HPV DNA test and cervicography, as screening methods for the detection of uterine cervical cancer. Oncol Rep. 2013;29(4):1645–51. doi:10.3892/or.2013.2257. Epub 2013 Jan 29.
Mrzljak A, Kardum-Skelin I, Cvrlje VC, Filipec-Kanizaj T, Sustercić D, Skegro D. Role of fine needle aspiration cytology in management of hepatocellular carcinoma: a single centre experience. Coll Antropol. 2010;34(2):381–5.
Nelson AM, Milner DA, Rebbeck TR, Iliyasu Y. Oncological care and pathology resources in Africa: survey and recommendations. J Clin Oncol. 2015;33:1–7.
Rambau PF. Pathology practice in a resource-poor setting: Mwanza, Tanzania. Arch Pathol Lab Med. 2011;135(2):191–3.
Robboy SJ, Weintraub S, Horvath AE, Jensen BW, Alexander CB, Fody EP, et al. Pathologist workforce in the United States: I. Development of a predictive model to examine factors influencing supply. Arch Pathol Lab Med. 2013;137(12):1723–32. doi:10.5858/arpa.2013-0200-OA. Epub 2013 Jun 5.
Stefan DC, Masalu N, Ngendahayo L, Amaon D, Botteghi M, Mendy M, et al. Pathology and oncology in Africa: education and training for the future in cancer research – East African regional meeting. Infect Agents Cancer. 2015;10:48.
World Health Organization. Abuja declaration-ten years on. 2014. http://www.who.int/healthsystems/publications/abuja_report_aug_2011.pdf. Accessed 11 Feb 2016.
World Health Organization. Total government expenditure on health as percentage of total government expenditure 2013. 2013. http://www.who.int/gho/health_financing/government_expenditure/en/. Accessed 11 Feb 2016
Yaziji H, Tay MA, Goldstein NS, Dabbs DJ, Hammond EH, Hewlett B, et al. Consensus recommendations on estrogen receptor testing on breast cancer by immunohistochemistry. Appl Immunohistochem Mol Morphol. 2008;16(6):513–20.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 Springer International Publishing AG
About this chapter
Cite this chapter
Abdulkareem, F.B., Odubanjo, O.M., Awolola, A.N. (2017). Pathological Services in Sub-Saharan Africa, a Barrier to Effective Cancer Care. In: Adedeji, O. (eds) Cancer in Sub-Saharan Africa. Springer, Cham. https://doi.org/10.1007/978-3-319-52554-9_4
Download citation
DOI: https://doi.org/10.1007/978-3-319-52554-9_4
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-52553-2
Online ISBN: 978-3-319-52554-9
eBook Packages: MedicineMedicine (R0)