Abstract
It is well known that the gold ores containing a high content of arsenic and antimony are very refractory. In this paper, the adverse effect of antimony on the extraction of gold by pretreatment-cyanide leaching was discussed in detail. Moreover, these processes of removing and recovering antimony from alkaline sodium sulfide or hydrochloric acid systems were introduced. Last but not the least, a novel cleaning technology for recovering antimony from refractory gold ores was proposed. Antimony was dissolved into the solution with sodium sulfide and sodium hydroxide as leaching agents. And then pressure oxidation technology was applied to prepare sodium pyroantimonate from sodium thioantimonite solutions. This process is more promising owing to its high removal ratio of antimony, low production cost and environment friendly, which provides a positive guidance for the extraction of antimony from antimonial refractory gold ores.
Access provided by CONRICYT-eBooks. Download conference paper PDF
Similar content being viewed by others
Keywords
Introduction
Gold is a strategic precious metal which can be widely used in gold ornaments, currency reserves and high-tech industries. According to United States Geological Survey (USGS), the amount of identified gold is 89,000 tons in the world, while a third of which is contained in the refractory ores [1]. With the gradual depletion of the high grade resources, refractory gold ores will become more and more important. China produces 450 tons gold annually, which ranks 1st in the world.
Refractory gold ore (RGO) stands for the minerals for which gold leaching ratio is less than 80% even after fine grinding. Especially for the arsenic containing refractory ores, with fine gold particles even in micron size encapsulated in pyrite or arsenopyrite, the gold leaching ratio can be as low as 50%. Therefore, refractory gold ore should be pretreated in order to improve gold leaching ratio.
There are many researches on the pretreatment methods of refractory gold ores. But the most widely applied methods in industry are two stages roasting, pressure oxidation and bacterial oxidation [2]. During the two stages roasting process, arsenic and sulfur are oxidized and volatilized in the form of As2O3 and SO2 while porous calcine is produced for leaching. This method has been widely applied due to its simple process and low cost. But Fe2O3 generated during the roasting process would cause a secondary encapsulation, resulting in gold leaching ratios vary between 75–92% and cyanide tailing with gold contents of 2.0–20.0 g/t. During pressure oxidation process, pyrite and arsenopyrite are decomposed in the acidic solution under high temperatures and oxygen pressures. This technology is efficient and less sensitive to other impurities, moreover, high gold recovery (95–97%) can be obtained. However, the large investment, high processing cost and large amount of waste water and residue constrained its wide industrial application. Bacterial oxidation is an advanced leaching technology. Pyrite and arsenopyrite can be oxidized with bacteria in an environment-friendly way. This method has a simple process, high leaching ratio of gold (92–95%) can be reached. The gold content of cyanide tailing is 2.0–5.0 g/t. The main disadvantages are long oxidation period and high investment, etc.
The influence of antimony on refractory gold ores pretreatment and following leaching process was summarized in this paper. Moreover, the differences on removing and recovering antimony between acidic and alkaline systems were introduced. Finally, an environment friendly process of removing and recovering antimony from antimonial refractory gold ores was proposed. This process provides a positive guidance for the extraction of antimony from antimonial refractory gold ores owing to high removal ratio of antimony and low production cost.
Effect of Antimony on Cyanide Leaching and Pretreatment
Effect of Antimony on Cyanide Leaching
The main antimony phase of antimonial refractory gold ores is stibnite (Sb2S3). In the process of alkaline cyanide leaching, stibnite increases the consumption of OH−, CN− and O2. The main reactions are listed as follows:
It can be seen from Eq. (3) that Sb2S3 is deposited on the surface of gold particles, forming a dense product layer and hindering the diffusion of CN− and O2 to the gold particles, leading to low leaching ratio [3, 4].
Effect of Antimony on Pretreatment Process
The melting point of antimony compounds, generated in the roasting process of antimonial refractory gold ores, is low(the melting point of Sb2S3 and Sb2O3 is 546 and 655 °C, respectively). It is easy to fuse with each other, which causes the gold particles wrapped again [5, 6], resulting in low gold leaching ratio [5, 6]. In the process of pressure oxidation pretreatment of refractory gold ores, the precipitates of antimony compounds were produced, covering with the surface of gold particles and hindering the further leaching reaction. In the process of biological oxidation pretreatment, the antimony minerals seriously affect the oxidation rate of gold bearing minerals [7].
Because of the adverse effects of antimony on pretreatment cyanidation process, it is very difficult for metallurgical enterprises to make full use of antimonial refractory gold ores. Therefore, it is necessary to remove antimony from antimonial refractory gold ores before conducting conventional pretreatment process. In general, removal antimony process can be divided into alkaline sodium sulfide system and hydrochloric acid system.
Recovering of Antimony from Sodium Sulfide Solution
Removing of Antimony from Antimonial Refractory Gold Ores
The main antimony phase of antimonial refractory gold ores is stibnite (Sb2S3). Sodium sulfide is proposed as leaching agent to remove antimony from antimonial refractory gold ores [8]. Meanwhile, sodium hydroxide is also used to prevent the hydrolysis of sodium sulfide. The main equations are expressed as follows:
Leaching of antimony in antimonial refractory gold ores with sodium sulfide alkaline solution has some advantages, such as high leaching ratio of antimony and limited dissolution of arsenic and gold.
Recovering of Antimony from Sodium Sulfide Solution
There are many researches on recovering antimony from sodium thioantimonite solution. The major methods are as below:
-
(1)
Neutralization precipitation
The sodium thioantimonite solutions can be treated by neutralization precipitation method. The main neutralization agents are sulphuric acid, carbon dioxide or sulfur dioxide. And then antimony can be recovered in the form of antimony sulfide. The corresponding reaction equation can be expressed as follows:
Compared with other methods, although neutralization precipitation has the characteristics of short flow and low production cost, some shortcomings also are inevitable, including low-quality products and bad operation conditions.
-
(2)
Displacement
The antimony in the sodium thioantimonite solutions can be displaced by metals powder as displacement agent, such as aluminium, zinc, copper and sodium amalgam. The main chemical reactions can be listed as follows:
Although antimony can be recovered by displacement methods, the products are impure.
-
(3)
Pressure hydrogen reduction
The antimony can be produced by pressure hydrogen reduction method with a high temperature. The main chemical reaction can be expressed as follows:
Sodium sulfide can be recovered from the process, but slow reaction rate and extremely high production cost restricts its wide application.
-
(4)
Electrodeposition
Electrodeposition is one of the most important methods to produce antimony. Moreover, sodium sulfide is also recovered [9]. The technology can be divided into diaphragm electrolysis and non-diaphragm electrolysis. For diaphragm electrolysis, sodium hydroxide and sodium thioantimonite are used as anode and cathode electrolytes, respectively. Sodium thioantimonite solution is regarded as electrolyte for non-diaphragm electrolysis. The main chemical reaction during electro-winning process is as follows:
The electro-winning process can obtain a qualified product, but heavy energy consumption and terrible working environment are the main problems.
-
(5)
Air-oxidation
Sodium pyroantimonate and sodium thiosulfate can be recovered simultaneously by air-oxidation technology [10]. The main chemical reactions are described as follows:
The precipitation ratio of antimony is up to 98.0% during air-oxidation process. This method has been successfully used on recovering antimony from jamesonite. High quality of products and low production cost can be obtained, but the oxidation rate is very slow.
The Comparison of Different Methods
When antimonial refractory gold ores are dealt in alkaline solution of sodium sulfide, the removing ratio of antimony is high. All these methods mentioned have their advantages and disadvantages for recovering antimony from sodium thioantimonite solutions. Therefore a satisfactory process must fully consider many factors such as the open circuit of sulfur, working environment, treatment cost and the stability of process.
Recovering Antimony from Hydrochloric Acid System
Leaching Antimony in Hydrochloric Acid System
Antimony is dissolved into the solution in the form of SbCl3 in hydrochloric acid system with oxidizing agent added. The oxidizing agents include Cl2, FeCl3, SbCl5, H2O2, ect. The mainly chemical reaction is as follows:
The high Cl− concentration and acidity are required to prevent the hydrolysis of Sb3+. This process has the advantage of high leaching ratio of antimony. But its disadvantages are also existent, such as high consumption of reagent, high loss of gold, serious corrosion to devices, and difficulty to recycle the leaching solution.
Recovering Antimony from Hydrochloric Acid System
The methods on the recovery of antimony from SbCl3 acidic solutions are as below [11]:
-
(1)
Hydrolysis
Sb3+ can be precipitated in the form of SbCl3, SbOCl and Sb2O3 by adjusting pH of SbCl3 solution by adding H2O or NaOH. The reaction is as follows:
Hydrolysis process is a purify process in essence. During the formation process of Sb4O5Cl2 precipitates, the acidity is about 0.5–0.6 mol/L. Main impurities are still present in the leaching solutions. Therefore, hydrolysis method for the recovery of antimony is widely used in industry.
-
(2)
Distillation method
The main principle for distillation method is that with a temperature above SbCl3 boiling point, SbCl3, AsCl3 and SnCl4 can be separated from CuCl2, PbCl2 and ZnCl2 by distillation method due to the diffidence of boiling point. Then high purity SbCl3 can be obtained by gradually cooling process. Chlorination-distillation process has been researched in depth. High energy consumption is the main problem for this method.
-
(3)
Replacement method
Antimony can be recycled from leaching solutions with some metal powder added by replacement reactions. The most common reagent is iron power due to its low price. The reaction is as follows:
Although antimony can be recycled effectively from leaching solution, it is difficult to recycle leaching solutions due to the presence of Fe2+.
-
(4)
Electrodeposition
Electrodeposition refers to a method that metal antimony is produced from leaching solutions by electrolysis. After completing the process, the residual electrolytes are returned to leaching process. The cathode and anode reactions are listed as follows:
High purity products can be obtained by electrodeposition, but high energy consumption and bad operation conditions constrain its widely application.
Slurry Electrolysis
Slurry electrolysis method is a novel hydrometallurgical technology. During this process, leaching and electrodeposition are conducted simultaneously in an electrolytic cell [12]. Metal is dissolved in anode by oxidation reaction and objective metal is deposited in cathode by reduction reaction. HCl–NaCl, HCl–CaCl2 and HCl–NH4Cl solutions are widely used in slurry electrolysis process. The reactions are as follows:
Slurry electrolysis method has some disadvantages, such as high failure rate of devices, serious abrasion of anode plate, accumulation of ferrous iron and poor operating environment.
The Comparison of Different Acid Systems
The used recovery methods include hydrolysis, distillation and electrodeposition. Although these methods can extract antimony effectively, these problems such as high failure rate of device, poor operating environment and high production cost are inevitable. Moreover, Fe and As also are dissolved into solution, causing a high burden for following purification and electro-winning process. All these shortcomings restrict their widely application.
A Clean Process for Extracting Antimony
Process Flow
Based on the advantages and disadvantages of every process to extract antimony from antimonial refractory gold concentration, a clean process is proposed, and the process flow is presented in Fig. 1. Firstly, antimony will dissolve into the solution as sodium thioantimonite by leaching of antimonial refractory gold concentration with sodium sulfide. Then air oxidation technology is used to prepare sodium pyroantimonate from sodium thioantimonite solution, the oxidated solution are neutralized, purified, and concentrated to recover sodium thiosulfate. After pretreatment, the leaching residue can be raw material to extract gold by cyaniding technology.
Technics Characteristic
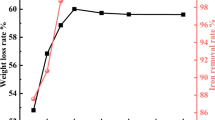
The antimonial refractory gold concentration (Sb 6.30%, As 5.50%, S 17.68%, Fe 15.74%, Au 58.8 g/L) is leaching in the solution containing sodium sulfide and caustic soda, the leaching efficiency of Sb can reach 97.08% under optimal conditions, only 0.20% Sb remains in the residue. The pressure oxidation technology is applied to prepare sodium pyroantimonate from sodium thioantimonite solution, and the Sb precipitation ratio can reach 99.80%. The product is composed of Sb2O3 (58.98%, by mass), Na2O (12.55%, by mass) and Sb3+ (≤0.01%, by mass). The oxidated solution is neutralized with sulphuric acid, As (III) and Sb (III) in the solution will precipitate as As2S3 and Sb2S3 at pH 6.5, respectively. After liquid-solid separation, when the solution is concentrated till its density reaches more than 1.5 g/cm3, sodium sulfite and sodium sulfate will crystallize together at 90–95 °C, then the heat solution is filtered again. Sodium thiosulfate will crystallize from the filtrate when it cools down to room temperature. The purity of sodium thiosulfate product can reach more than 95.0%.
Conclusion and Expectation
Considering antimony plays a negative role for extraction of gold from antimonial refractory gold ores , it is necessary to remove antimony before conducting pretreatment-leaching process to improve gold recovery. Antimony can be removed successfully from antimonial refractory gold ores with Na2S–NaOH as leaching agents. However, many factors on the recovery of antimony from sodium thioantimonite solutions, including the open circuit of sulfur, working environment, treatment cost and the stability of process, must be considered adequately. Antimony also can be removed successfully from antimonial refractory gold ores in hydrochloric acid system with oxidizing agent added. However, some problems must be considered, such as high failure rate of devices, poor operating environment and high production cost.
In this paper, a clean process for extracting antimony from antimonial refractory gold ores is proposed. Antimony is dissolved into the solution with sodium sulfide as leaching agents. And then pressure oxidation technology is applied to prepare sodium pyroantimonate from sodium thioantimonite solution. Sodium thiosulfate is produced from oxidation residual solution by neutralization, purification and concentrated crystallization. The leaching residues obtained can be used to extract gold by pretreatment-cyanide leaching process.
References
George MW et al (2011) U.S. geological survey mineral yearbook. U.S. Geol Survey 5:1–18
Yang TZ et al (2005) Metallurgy and chemicals of Piecious metals. Central South University Press, Changsha, NY, pp 210–215
Jiang T et al (1998) Chemical of extracting gold. Hunan Science and Technology Press, Changsha, NY, pp 8–15
Celep O, Alp I, Deveci H (2011) Improved gold and sliver extraction from a refractory antimony ore by pretreatment with alkaline sulphide leach. Hydrometallurgy 105:234–239
Jin SB et al (2009) Influence of antimony on refractory gold ore roasting and cyanide leaching. Gold 30(2):121–128
Yuan CX, Wang Y (2003) Roasting and cyanide leaching process study on As, Sb, and C content refractory gold concentrate. Nonferrous Metall 3:32–34
Cui RC et al (2011) Biooxidation-cyanidation leaching of gold concentrates with different arsenic types. Chin J Nonferrous Metals 21(3):694–699
Ubadini S et al (2000) Process of flow-sheet for gold and antimony recovery from stibnite. Hydrometallurgy 57(5–6):187–199
Sun LG et al (2015) Status and development of gold extraction from refractory gold ore. Nonferrous Mteals (Metallurgy part) 4:38–43
Yang TZ, Liu WF Jiang MX (2006) Production and processes of sodium pyroantimonate in China. In: Sohn international symposium advanced processing of metals and materials held during TMS 2006 Annual Meeting, vol 8, San Diego, CA, pp 27–31, pp 411–420
Zhao TC (1987) Metallurgy of antimony. Central South University of Technology Press, Changsha, NY, pp 358–462
Zhang YL et al (2014) Pant practice of slurry electrolysis of high arsenic gold-bearing stibnite concentrate. Nonferrous Metals (Metallurgy Part) 11:16–20
Acknowledgements
The financial support from Young Scientists Fund of National Natural Science Foundation of China (Grant No. 51404296) is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 The Minerals, Metals & Materials Society
About this paper
Cite this paper
Liu, W., Fu, X., Rao, S., Yang, T., Zhang, D., Chen, L. (2017). Selection on the Process for Removing and Recovering Antimony from Antimonial Refractory Gold Ores. In: Ikhmayies, S., et al. Characterization of Minerals, Metals, and Materials 2017. The Minerals, Metals & Materials Series. Springer, Cham. https://doi.org/10.1007/978-3-319-51382-9_53
Download citation
DOI: https://doi.org/10.1007/978-3-319-51382-9_53
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-51381-2
Online ISBN: 978-3-319-51382-9
eBook Packages: Chemistry and Materials ScienceChemistry and Material Science (R0)