Abstract
Electrolytic production of metallic neodymium is carried out in fused fluoride salts containing neodymium oxide. Two major challenges pertaining to neodymium production are (a) low oxide solubility, (b) possibility of anodic fluorine gas evolution if the electrolysis rate exceeds feeding rate of neodymium oxide. In this study, a novel method is proposed in which iron fluoride (FeF3) is used as a fluorinating agent to convert neodymium oxide into neodymium fluoride. Electron Probe Micro Analysis (EPMA) results of as-converted salt show a complete conversion of neodymium oxide into neodymium fluoride. In the electrolysis process, iron is used as a reactive anode with electrochemical dissolution of iron into the melt, thus preventing fluorine gas evolution at the anode. Therefore, the fluorinating agent is constantly regenerated in situ which enables the continuous conversion of neodymium oxide feed. The cathodic product is a Nd–Fe alloy which can be directly used as a master alloy for the production of NdFeB permanent magnets.
Access provided by CONRICYT-eBooks. Download conference paper PDF
Similar content being viewed by others
Keywords
Introduction
The dominant industrial method for the extraction of rare earth metals from their oxides is the molten salt electrolysis process [1,2,3]. In comparison to hydrometallurgical processes, lower energy consumption, higher efficiency and higher purity of the deposits are advantages in molten salt routes compared to hydrometallurgy processes [4,5,6]. Solubility of the rare earth oxide (REO) in the fluoride electrolyte is an important factor, since it is the dissolved oxide that is subjected to electrolysis. However, the solubility of rare earth oxide in molten alkali fluoride does not exceed 4 wt% [7,8,9]. Moreover, neodymium oxide forms likely neodymium oxyfluoride in molten fluoride salts. Oxyfluoride formation has been reported to affect the neodymium electrodeposition adversely [10]. Another challenge in neodymium production is fluorine gas evolution on the carbon anode when the electrolysis rate exceeds the rare earth oxide feeding and dissolution rate [11].
Herein we adopt a two-fold approach to address the solubility issue of neodymium oxides in the fluoride melts. Initially, REOs are treated with strong fluorinating agents like FeF3 which converts REOs to REF3. To overcome halogen evolution on the anode, a reactive anode was employed. Iron was anodically dissolved re-generating the fluorinating agent FeF3 in situ in the electrochemical reactor. The rare earth fluoride thus formed can subsequently be processed through the electrolysis route in the same reactor to extract rare earth metal as the cathodic deposit. In this concept, the REO dissolution in the molten fluorides would become redundant due to the rapid and complete conversion of the oxide into the fluoride as REF3.
Experimental
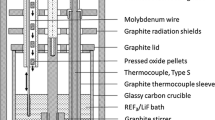
Lithium fluoride (98.5%-Alfa Aesar) was mixed with neodymium oxide (Rhodia) in a glove box. In the experiments for the conversion of neodymium oxide to neodymium fluoride, iron fluoride (97%-Alfa Aesar) was added to the mixture and the mixture was charged into a graphite crucible. For these experiments, the stoichiometry equivalent concentration of fluorinating agent was used in order to reach the complete conversion of Nd2O3 into NdF3. A molybdenum rod (2 mm diameter) was used as cathode. The immersion depth of the Mo rod was measured after each experiment. An iron rod (98%-Salomon metals, 2 mm diameter) was used as anode. All experiments were performed at 950 °C under a purified argon atmosphere. The argon gas was purified by passing through KOH flakes in order to remove traces of CO2 sulphur and also through silica gel and P2O5 to remove moisture. The gas was passed through a tube furnace containing Ti sponge which was held at 1123 K (850 °C) in order to remove the trace amount of O2. The samples were removed from the furnace and immediately quenched in liquid nitrogen after the experiment.
The samples were analyzed by Electron Probe Micro Analysis (EPMA), in order to derive the phases that are formed during the experiments from the local compositions. WDS point analyses or mappings were performed at 15 kV–15 nA and for the quantification, standard samples were used.
Results and Discussion
EPMA mapping of the sample from the LiF-Nd2O3-FeF3 salt bath that was quenched in liquid nitrogen after 3 h at 950 °C, is shown in Fig. 1. It can be seen that neodymium and oxygen do not occur in the same regions, whereas iron and oxygen are clearly distributed in the same area, confirming the iron oxide formation. Neodymium has a high concentration around the FeO boundaries. The light-grey lamellar phase is NdF3 within the dark LiF, indicating LiF-NdF3 eutectic microstructure. In the EPMA mapping, in which Li cannot be detected, complete conversion of neodymium oxide to neodymium fluoride is exhibited.
The EPMA quantitative results are presented in Table 1. The composition in the column NdF3 was measured in the Nd-regions of Fig. 1 and the FeOx composition in the Fe- and O-rich regions. In this table, the presence of NdF3 (26 at.% Nd and 72 at.% F) and FeOx (45 at.% Fe and 54 at.% O) are confirmed. The phase indicated as iron oxide can be a mixture of FeO and Fe2O3, since the concentrations can be related to magnetite phase (Fe3O4). Based on the calculated standard Gibbs energy (FactSage 7.0) for the reaction (1),
conversion of neodymium oxide to neodymium fluoride using iron fluoride is feasible. According to this reaction, Fe2O3 formation is expected, but as EPMA results show, the oxide of iron which is formed can be wüstite or non-stoichiometric FeOx. In the argon ambient and low oxygen pressure atmosphere, hematite (Fe2O3) will not be formed or depending upon the oxygen pressure prevailing, the formed Fe2O3 is decomposed to Fe3O4 and FeO. At high temperatures (more than ca. 560 °C), stable oxide is wüstite (FeO).
NdF3, formed as the result of the reaction between Nd2O3 and FeF3, will go through the electrolysis process. In this process, the cathodic reaction would be:
Whereas in the anodic part of the reaction, fluorine gas is generated if an inert anode such as graphite is used:
In order to avoid fluorine gas formation on the anode, the novel idea in the present work is to use iron as reactive anode . In this process, iron in the anode is dissolved in the salt as the result of an anodic reaction which leads to in situ formation of iron fluoride. Besides avoiding the fluorine gas evolution, another advantage of this method is that iron fluoride is generated and used in the system, which leads to less material consumption, meaning that fluorinating agent is constantly generated and consumed in the system. In this case the anodic reaction will be iron dissolution:
A series of experiments was carried out in order to examine the electrochemical dissolution of iron in the molten salt. In these experiments iron served as anode and Mo as cathode, which were immersed in LiF-Nd2O3 mixture. The experiment was performed in constant current mode at 1.5 A for 3 h at 950 °C. Based on Faraday’s law, considering 3 exchanged electrons in the cathodic reduction of neodymium metal, mass of the dissolved iron can be calculated:
where I is the electrolysis current in A, t is the time of the electrolysis in s, n is the number of exchanged electrons, F is the Faraday’s constant (F = 96,500 °C), m is the mass of the element in gram and M is the atomic mass of the element.
The cathodic product on the molybdenum electrode was analysed by EPMA. The result is shown in Fig. 2.
From EPMA analysis result, it can be seen that a layer of iron is formed on the molybdenum cathode and neodymium is reduced onto this layer. Fe might be deposited from the iron fluoride, which is formed from dissolution of the iron anode, since iron fluoride has a less positive decomposition voltage compared to neodymium fluoride. Part of the iron fluoride which reacts with neodymium oxide based on reaction (1), forms iron oxide. The dissolved iron oxide can also participate in the electro-decomposition process, and co-deposition of Fe occurs at the cathode. Nd–Fe alloy production is desirable since it can be used as master alloy for magnet production. The challenge would be as to how to remove the undissolved iron oxide in order to minimize their side effect on cathodic deposition of Nd. A new design of a two-chamber furnace system is one way for removing the undissolved oxide, which is presently under study. A possible alternative may be to take advantage of the density difference between the formed oxide and the fluoride bath in order to separate the oxides from the molten salt.
Conclusion
The problem of low solubility of the neodymium oxide in molten fluorides is solved to a large extent by converting neodymium oxide to neodymium fluoride in situ. Once neodymium fluoride is formed, it can subsequently be electrolyzed and extracted on the cathode. In this study, a novel method is studied in which iron is used as anode, promoting electrochemical dissolution of iron into the melt, thus preventing fluorine gas evolution at the anode. Therefore, the fluorinating agent is constantly regenerated in situ which enables the continuous conversion of neodymium oxide feed. The cathodic product is Nd–Fe alloy which can be directly used as a master alloy for the production of NdFeB magnets.
References
S. Seetharaman, O. Grinder, US patent application no. 12/991128, ref. no.: 12057 (2010)
A. Abbasalizadeh et al., Highlights of the salt extraction process. J. Mater. 65(11), 1552–1558 (2013)
A. Abbasalizadeh et al., Neodymium extraction using salt extraction process. Miner. Process. Extr. Metall. 124(4), 191–198 (2015)
B. Mishra, D.L. Olson, Molten salt applications in materials processing. J. Phys. Chem. Solids 66(2–4), 396–401 (2005)
W. Han et al., Electrodeposition of Mg-Li-Al-La alloys on inert cathode in molten LiCl-KCl eutectic salt. Metall. Mater. Trans. B 42(6), 1367–1375 (2011)
Y. Castrillejo et al., Electrochemical behaviour of praseodymium (III) in molten chlorides. J. Electroanal. Chem. 575(1), 61–74 (2005)
E. Morrice, T.A. Henrie, Electrowinning high-purity neodymium, praseodymium, and didymium metals from their oxides (Washington D.C., U.S. Department of the Interior, Bureau of Mines, 1967)
J.E. Murphy, D.K. Dysinger, M.F. Chambers, Electrowinning neodymium metal from chloride and oxide-fluoride electrolytes, in Light Metals 1995, Warrendale, Pennsylvania, 1995
B. Porter, E.A. Brown, Determination of oxide solubility in molten fluorides (Washington, D.C., U.S. Department of the Interior, Bureau of Mines, 1961), 3139
E. Stefanidaki, C. Hasiotis, C. Kontoyannis, Electrodeposition of neodymium from LiF-NdF3-Nd2O3 melts. Electrochim. Acta 46(17), 2665–2670 (2001)
A. Kaneko, Y. Yamamoto, C. Okada, Electrochemistry of rare earth fluoride molten salts. J. Alloy. Compd. 193, 44–46 (1993)
Acknowledgements
This research has received funding from the European Community’s Seventh Framework Programme ([FP7/2007-2013]) under grant agreement no. 607411 (MC-ITN EREAN: European Rare Earth Magnet Recycling Network, Project website: www.erean.eu). This publication reflects only the authors’ view, exempting the Community from any liability.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 The Minerals, Metals & Materials Society
About this paper
Cite this paper
Abbasalizadeh, A., Seetharaman, S., Venkatesan, P., Sietsma, J., Yang, Y. (2017). Novel Reactive Anode for Electrochemical Extraction of Rare Earth Metals from Rare Earth Oxides. In: Kim, H., Alam, S., Neelameggham, N., Oosterhof, H., Ouchi, T., Guan, X. (eds) Rare Metal Technology 2017. The Minerals, Metals & Materials Series. Springer, Cham. https://doi.org/10.1007/978-3-319-51085-9_9
Download citation
DOI: https://doi.org/10.1007/978-3-319-51085-9_9
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-51084-2
Online ISBN: 978-3-319-51085-9
eBook Packages: Chemistry and Materials ScienceChemistry and Material Science (R0)