Abstract
The preparation of industrial ammonium molybdate from molybdenum concentrate was conducted according to the process of roasting—leaching—purification—precipitation—crystallization. The optimum technological conditions were determined: the roasting temperature was 680 °C, the roasting time was 2.5 h; in the leaching process the alkali excess coefficient was 1.2, the reaction temperature was 80 °C, the liquid-solid ratio was 3:1, the leaching time was 3 h; the amount of (NH4)2S added into the solution was 1.1 times of the theoretical amount, the temperature was 80–85 °C, and the terminal pH was 8.5–9 in the purification process; the density of the solution was 1.4–1.6 g/mL, the pH was 1.5–2 in the precipitation process; temperature of dissolution was controlled at 80–90 °C. The recovery of the preparation of ammonium molybdate can reach 85.18%.
Access provided by CONRICYT-eBooks. Download conference paper PDF
Similar content being viewed by others
Keywords
Introduction
One copper mine is rich in molybdenum resources while the molybdenum grade is low (0.020–0.035%) in Hubei province. So it’s hard to select molybdenum resources. Copper and molybdenum mixed concentrate was gained by flotation method in copper mine, then was separated into copper and low grade molybdenum concentrates (Mo content is about 20–30%) [1, 2]. Ammonium molybdate is produced by standard molybdenum concentrate (Mo ≥ 45%) in industry. A flowsheet of producing ammonium molybdate from low grade molybdenum concentrate was explored, which will improve the comprehensive utilization of resources in mining enterprises and economic benefits [3, 4].
Properties of Test Materials
According to certain standard, the molybdenum concentrate, taken from a copper mine in Hubei province, was sent to conduct multi-element analysis after a process of drying and sample preparation. The result was shown in Table 1. It can be known the molybdenum content was 23.80%, with a low content of copper and iron, which is non-standard molybdenum concentrate (molybdenum content of standard molybdenum concentrate is 45% or higher). Main gangue composition were SiO2, Al2O3, CaO, MgO, accounting for 43.63% of the total, so it’s a material of a high containing calcium and magnesium. The SEM image of the molybdenum concentrate was shown in Fig. 1.
The Method of Experiment
According to the composition and dissemination characteristics of molybdenum concentrate in the test (molybdenum mineral was symbiotic with gangue mineral closely. Ca and Mg content was higher while the content of Mo was about 20–30%), its grade was below the standard molybdenum concentrate (Mo ≥ 45%). Meanwhile, there’s a high cost in producing ammonium molybdate by hydrometallurgy, and a high requirement for equipment at the same time. While the oxidizing roasting method is simple, with a less consumption, lower cost, simpler equipment, etc. This study selected ammonia alkaline leaching process, and the flowchart was shown in Fig. 2. The main advantages of ammonia alkaline leaching process are as follows: (1) compared with molybdenum calcine pretreatment process, there’s no corrosion in equipment, and quantity of waste water and calcine is less, so the labor intensity is low. (2) Compared with ammonia leaching-acid precipitating process, the leaching rate of molybdenum is improved by translating traditional process into alliance leaching of ammonia and sodium carbonate.
Results and Analysis
The Roasting Process
As the material was low grade molybdenum concentrate , in addition, more talc and calcite were also contained. In order to make the molybdenum concentrate fully roasted and oxidized, MnO2 was chosen as the oxidant. The influences of roasting temperature, roasting time and the quantity of oxidant were studied in this experiment to explore the optimum roasting conditions. The orthogonal which was designed with three factors and three levels was used in this experiment. The three factors were followed: A-roasting time, B-roasting temperature, C-the quantity of oxidant (MnO2). The design of orthogonal test was shown in Table 2; the results were shown in Table 3.
It can be known from the results that the order of the influence of each factor on roasting was the temperature, the consumption of oxidant, the roasting time, consecutively; the appropriate process parameters of roasting were as follows: roasting temperature of 700 °C, the oxidant consumption of 10 g, the roasting time of 2.5 h.
The Ammonia-Alkali Leaching
MoO3 is easily dissolved in alkaline solution and could produce sodium molybdate and ammonium molybdate solution, so molybdenum can be recovered. The orthogonal test of four factors and three levels was conducted in order to study the effect of temperatures, reaction time, excess coefficient of alkali, liquid to solid ratio on the roasting sample by ammonia-alkali leaching . The four factors were as follows: A-temperature, B-reaction time, C-the excess coefficient of Na2CO3, D-liquid-solid ratio. The orthogonal test was shown in Table 4; the results were shown in Table 5.
It showed that the order of the influence of each factors on the molybdenum leaching rate was: temperature, liquid-solid ratio, reaction time, alkali excess coefficient. The optimal conditions of the ammonia-alkali leaching process were as follows: the alkali excess coefficient of 1.2–1.4, the reaction temperature of 70–80 °C, liquid to solid ratio of 3:1, the reaction time of 2.5–3 h.
The Purification of the Leaching Solution
The Influence of the Terminal pH on the Purification
The purpose of the purifying process of the leaching solution was to remove the metal ions impurities such as Cu, Fe, etc. The density of leaching solution was 1.14–1.16 g/ml and would be injected slowly according to the amount that was 1.05 times of the theoretical amount of the (NH4)2S solution under the condition of stirring. The container was placed in the thermostat water bath for 20–30 min. By this way, the metal ions could react with the sulfide ions. The influence of terminal pH on purification was studied and the results were shown in Table 6.
As was shown in Table 6, with the terminal pH increasing, the content of Cu2+ and Fe2+ in the solution reducing; while the color of the solution turned to yellow after the terminal pH was 10, which showed the fact that the content of (NH4)2S was excessive. So the purifying terminal pH should be controlled in the range of 8.5–9, so it would meet the requirement of removing the impurities. Besides, excessive (NH4)2S is harmful of the subsequent operation, which would make the existence of impurity S2− in the filtrate.
Test of the Condition of the Addition Contents of (NH4)2S Solution
The purpose of adding (NH4)2S solution was to remove the metal impurities such as Cu and Fe effectively, excessive (NH4)2S was not convenient for subsequent operation because of the S2− [5]. Therefore, the control of adding (NH4)2S is very important. The results of test were shown in Table 7. The actual addition of (NH4)2S was a fluctuation value; some adjustments must be made according to the pH value and the change of the color in practice.
From Table 7, the color of the solution become shallow and the content of Cu and Fe in the filtrate were decreased while (NH4)2S content increased. When the addition of the (NH4)2S reached 1.2 times of the theoretical value, the color of the solution started to mix, then was yellow as the amount of (NH4)2S increased. Observing the color change of the solution is also an effective way to judge whether the addition of (NH4)2S can remove the impurity or not. The color of (NH4)2S solution is yellow, so the color of the solution is yellow when (NH4)2S was excessive, therefore, the amount of (NH4)2S added into the solution controlled at 1.1–1.2 times of the theoretical value was appropriate.
Effect of the Temperature on Purification
100 ml leaching solution was taken, the density was 1.14 g/ml, the terminal pH was at 8.5–9, after the arrival of the terminal pH value, holding the temperature for 20–30 min. To investigate the effect of temperature on the purification, the results of the test were shown in Table 8.
The results showed that, with the increase of the temperature of the water, the content of Cu and Fe ions in filtrate decreased, the color of the solution become shallow. At a low temperature, the contents of metal ions such as Fe and Cu were higher, the speed of the reaction of Fe, Cu and other metal ions with S2− was slower, and there were some metal ions failed to precipitate, and remaining in the solution. It was suitable to control the purification temperature at 80–85 °C after comprehensively considered.
The Effect of pH on Precipitation Process
Precipitated by acid is a process of ammonium molybdate crystallization. Ammonium molybdate solution was heated to boiled, the partial water in the solution evaporated and ammonia volatilized, so the volume of the solution was reduced, the concentration of molybdenum in the solution was increased, and the alkalinity of the solution was reduced so the yield of acid precipitation was increased. Under the condition of stirring, the temperature of acid precipitation was control at 50 °C. The shape of the deposit changed as the pH of the solution decreased.
As can be seen in Table 9, when the pH was 1, the deposit was not formed and the color of the product was yellow, therefore, it was appropriate to control pH at 1.5–2 in practice. When the solid and liquid separate after the acid precipitation , adding ammonia of which the density is 0.9 g/cm3 and an appropriate amount of deionized water in the deposit, then stirring and gradually heat up to 90–80 °C, when the solution density was 1.5–1.6 g/cm3, the solution was saturated, the ammonium molybdate can be obtained after cooling and crystallization.
Optimal Process Conditions and Product Inspection
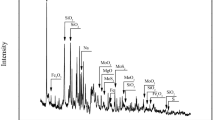
According to the experimental study, the optimum technological conditions were determined: the roasting temperature of 680 °C, the roasting time of 2.5 h; in the leaching process the alkali excess coefficient of 1.2, the reaction temperature of 80 °C, the liquid-solid ratio of 3:1, the leaching time of 3 h; the amount of (NH4)2S added into the solution was controlled at 1.1 times of the theoretical value, the temperature was 80–85 °C, and the terminal pH was 9–8.5 in the purification process; the density of the solution was 1.4–1.6 g/mL, the pH was 2–1.5 in the acid precipitation process; temperature of ammonia dissolution was controlled at 80–90 °C, with ammonium molybdate dissolved in ammonia water to saturation. The results of test were shown in Table 10, under the optimized conditions, the ammonium molybdate recovery could reach 85.18% after the leaching of low grade molybdenum. The analysis of ammonium molybdate was carried out, and the results were as follows: it contained 53.56% molybdenum, impurity content 0.0023% copper, and iron of 0.0032%. The SEM image and XRD pattern of the product were shown in Figs. 3 and 4.
Conclusion
Through the analysis of low grade molybdenum ore, the process of roasting , leaching, purification and precipitation was determined, the feasible process of the preparation of ammonium molybdate was recommended.
The optimum technological conditions were determined: the roasting temperature was 680 °C, the roasting time was 2.5 h; in the leaching process the alkali excess coefficient was 1.2, the reaction temperature was 80 °C, the liquid-solid ratio was 3:1, the leaching time was 3 h; the amount of (NH4)2S added into the solution was controlled at 1.1 times of the theoretical value, the temperature was 80–85 °C, and the terminal pH was 9–8.5 in the purification process; the density of the solution was 1.4–1.6 g/mL, the pH was 2–1.5 in the acid precipitation process; temperature of ammonia dissolution was controlled at 80–90 °C. Under the optimized conditions, the recovery of industrial ammonium molybdate could reach 85.18%. It contained 53.56% molybdenum, 0.0023% copper, and 0.0032% iron. The product should be purified further to meet customers’ different requirements.
References
P. Huiqing, H. Wei, Experimental study and production practice of copper molybdenum separation in a copper mine. Nonferrous Met. (Min. Process. Sect.) 1, 23–26 (2007)
H. Wei, W. Daijun, Improve the selecting of copper recovery of Tongshankou Copper Mine. Min. Metall. 2, 17–19 (2006)
Z. Dechang, Development and application of four ammonium molybdate. Hydrometall. China 1, 9–12 (1994)
L. Guojian, Z. Hong et al., Experimental study on producing ammonium molybdate molybdenum concentrate. Hydrometall. China 3, 19–23 (2005)
X. Tiegen, Extractive Metallurgy of Molybdenum (Central South University Press, Changsha, 2002), pp. 35–127
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 The Minerals, Metals & Materials Society
About this paper
Cite this paper
Qin, Q., Liu, Z., Chen, T., Huang, Z., Yang, J., Han, W. (2017). Study for Preparation of Industrial Ammonium Molybdate from Low Grade Molybdenum Concentrate. In: Kim, H., Alam, S., Neelameggham, N., Oosterhof, H., Ouchi, T., Guan, X. (eds) Rare Metal Technology 2017. The Minerals, Metals & Materials Series. Springer, Cham. https://doi.org/10.1007/978-3-319-51085-9_28
Download citation
DOI: https://doi.org/10.1007/978-3-319-51085-9_28
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-51084-2
Online ISBN: 978-3-319-51085-9
eBook Packages: Chemistry and Materials ScienceChemistry and Material Science (R0)