Abstract
The thermodynamic analysis on chromium ore pellets in solid reduction chromium process is carried out, the result of which is validated by pre-reduction experiment of cold briquetted chromium ore. Polarization Microscope and SEM are employed to observe microstructure and element composition. The thermodynamic analyses indicate that the Cr2O3 will be reduced to metallic chromium in solid reaction with the ratio of CO and CO2 pressure reaching 2017 at 1200 °C. The experimental results agree well with the thermodynamic analyses.
Access provided by CONRICYT-eBooks. Download conference paper PDF
Similar content being viewed by others
Keywords
Introduction
Nowadays mining yield of chromium ore for smelting ferrochrome is about 20 million tons per year. The fine chromium ores account for about 80%. The poor permeability of burden, irregular furnace operation and slag boil are caused by the use of fine chromium ore in the process of smelting ferrochrome. Therefore, the fine chromium ore must be handled before they are into submerged arc furnace. The pre-process to fine chromium ore has been an important topic for ferroalloy worker all the time.
Solid reduction chromium (SRC) was developed by Showa Denko and its industrial production first began in 1970. This process was also called preheating pellets and process flow diagram is shown in Fig. 1.
The pre-reduction pellets were manufactured as follows:
-
1.
The size of ground fine chromium ore below 200 meshes account for 70–80%.
-
2.
The pellets were prepared from ground fine chromium ore and binder in the cylinder ball making machine.
-
3.
The pellets were dried in grate and the strength of them should be improved.
-
4.
The pellets were roasted in rotary kiln under reducing atmosphere. There is a layer of hard shell formed, which is beneficial in the strength improvement. The pellets from kiln could be hot charged into submerged arc furnace.
As for the fundamental research about the SRC process, only NEUSCHUTZ has made a kinetic analysis. In this paper, the pre-reduction mechanism of chromium pellets in the SRC process was studied.
Thermodynamics Analysis of Reduction Reaction in SRC Process
It is obvious that the success of SRC process depends on the degree of pre-reduction of chromium pellets in kiln, which concerns the effect of whole process. The reaction equations of chromium pellets are shown in (1) to (4).
It could be concluded from Eq. (1) to (4) that the initial temperature of \( {\text{Cr}}_{ 2} {\text{O}}_{ 3} \) reduction reaction is about 1523 K. If the reduction product is \( {\text{Cr}}_{ 3} {\text{C}}_{ 2} \), initial temperature is about 1373 K. All these reactions are between pure substances. While the existing form of Cr in chromium ore is magnesia-chrome spinel and its molecular formula is \( \left( {{\text{Mg}},{\text{Fe}}} \right)\left( {{\text{Cr}},{\text{Al}}} \right)_{ 2} {\text{O}}_{ 4} \). In these conditions, the initial temperature of \( {\text{Cr}}_{ 2} {\text{O}}_{ 3} \) reduction reaction is higher than that illustrated in Eq. (1)–(4).
There are some iron oxides in magnesite chrome ore, which also could react with C and it was illustrated in Eq. (5).
The CO was also generated when the iron oxides were reduced to Fe. There are a mount of chromium ores in pellets after the reduction reaction. The CO gas could not be oxidized to \( {\text{CO}}_{ 2} \) with the excess reducing agent. Iron oxide and \( {\text{Cr}}_{ 2} {\text{O}}_{ 3} \) could coexist in magnesia-chrome spinel and the high density CO would form around or in the magnesia-chrome spinel crystal.
\( {\text{Cr}}_{ 2} {\text{O}}_{ 3} \) was reduced by CO could illustrated in Eq. (6)–(8).
Equation (10) could be obtained from above Equations.
According to the gibbs free energy of ideal gas Equation, Eq. (10) could be expressed as follows.
Another formula could be concluded from Eq. (11) when reduction reaction begin.
If reduction reaction happens, the relationship of temperature and \( {\text{P}}_{{{\text{CO}}}} /{\text{P}}_{{{\text{CO}}_{{\text{2}}} }} \) is illustrated in Table 1 and Fig. 2.
It could be concluded that \( {\text{P}}_{{{\text{CO}}}} /{\text{P}}_{{{\text{CO}}_{{\text{2}}} }} \) reduce gradually with the increase of temperature. This value is 2017 when the temperature is 1473 K. The Cr2O3 could be reduced at this temperature with the excess carbon, which could meet the thermaldynamic requirement in and around the magnesia-chrome spinel crystal. The reducing condition would improve with the increase of temperature, which would be improved obviously when the temperature is 1673 K.
Experiment Analysis
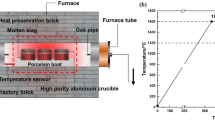
Cold briquetted samples containing carbon were made from South Africa chromite concentrates to investigate the solid pre-reduction at 1000 and 1200 °C in which graphite carbon acted as the reducing agent. Lithofacies samples were made after pre-reduction, and their microstructures and element compositions were analyzed through Leica large polarizing microscope and scanning electron microscopy (SEM).
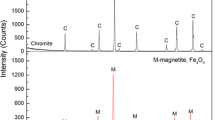
Results of lithofacies identification and SEM analysis showed that
-
(1)
Solid reductions were taken place in different degrees for samples at 1000 and 1200 °C. The reduction of Fe was taken place along the edges and cleavage fissures. Magnesiochromites were reduced into chromites within the cleavage fissures. Magnesium and aluminium contents decreased and iron content increased.
-
(2)
Magnesiochromite grain edges were broken after calcinations at 1000 °C. Few of chromite grains were intergrown. And there was some metallic iron reduced from chromite particles or at magnesiochromite grain edge.
-
(3)
Samples after solid reduction at 1200 °C were similar to that at 1000 °C. The reduction of Fe took place along the edges and cleavage fissures, and magnesiochromites were reduced into chromites. Iron content increased and magnesium and aluminium contents decreased.
-
(4)
Much more aluminium chromium slag and some molten slag phase were formed after solid reduction at 1200 °C, and some magnesiochromite were bonded by the molten slag phase.
-
(5)
There were chromium oxide particles wrapped by metallic chromium in the samples after solid reduction at 1200 °C.
Conclusions of Reduction Mechanism of SRC Process
Thermodynamic calculation results show that the reduction of Fe was taken place along the edges and cleavage fissures after calcinations at 1000 °C. Few of chromite grains were intergrown, and there was some metallic iron reduced from chromite particles or at magnesiochromite grain edge. While at 1200 °C with the CO and CO2 ratio up to 2017, the reduction of Fe took place along the edges and cleavage fissures, and magnesiochromites were reduced into chromites. Magnesium and aluminium contents decreased and iron content increased. There were chromium oxide particles wrapped by metallic chromium in the samples. It was implied that the solid reduction of Cr2O3 happened and metallic Cr was generated. Experimental verification was achieved about the generation of metallic Cr under the pre-reduction of chromite concentrates cold briquetted pellets at high temperature. In practice, temperature is mostly controlled above 1400 °C. SRC solid pre-reduction is thermodynamically feasible according to the thermodynamic calculation and reducing roasting experiments. The roasting temperature can be lowered depending on the working condition.
Smelting operations show that comparing with the traditional lump ore or sintering method, cold charge of SRC pre-reduction pellets could save more than 30% of electricity, reduce the amounts of coke and fume and improve the productivity of electric furnace and the recovery of chromium. Additionally, it is helpful for the total enclosing of electric furnace, the recovery of waste heat and the decrease of pollution.
SRC pellets pre-reduction process is advanced ferrochrome production process technology. And it will be the development direction of ferrochrome production technology under the present domestic and foreign raw materials and product markets.
References
N. Ralph, A review of the deposits and beneficiation of lower-grade chromite. J. S. Afr. Inst. Min. Metall. 8, 205–226 (1982)
Q.X. Zhang, Ferro-Alloys. 2, 33–39 (2000)
W. Dai, L. Shu, Ferroalloys Metallurgical Engineering (Metallurgical Industry Press, Beijing, 1999), p. 195
J.H. Zhou, Ferroalloys Production Technology (Science Press, Beijing, 1991), p. 77
Y. Otani,K. Ichikawa, Manufacture and use of prereduced chromium-ore pellets. Paper presented at the 1st international ferro-alloys congress, Johannesburg, pp. 31–37, 22 Apr 1974
C.T. Bi, Ferro-Alloys. 2, 38–43 (1992)
X.G. Zhao, Ferro-Alloys. 5, 6–10 (1998)
R.T. Li, J.S. Chen, Zhejiang Metallurgy. 3, 1–6 (1987)
D. Neuschutz,Kinetic aspects of chromite ore reduction with coal at 1200 to 1550 °C. Paper presented at the 6th international Ferro-Alloys Congress, Cape Town, pp. 65–70, 8 Mar 1992
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 The Minerals, Metals & Materials Society
About this paper
Cite this paper
Liu, Px., Li, Yx., Guo, Hj. (2017). Study on Pre-reduction Mechanisms of Chromium Ore Pellets in SRC Process. In: Kim, H., Alam, S., Neelameggham, N., Oosterhof, H., Ouchi, T., Guan, X. (eds) Rare Metal Technology 2017. The Minerals, Metals & Materials Series. Springer, Cham. https://doi.org/10.1007/978-3-319-51085-9_18
Download citation
DOI: https://doi.org/10.1007/978-3-319-51085-9_18
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-51084-2
Online ISBN: 978-3-319-51085-9
eBook Packages: Chemistry and Materials ScienceChemistry and Material Science (R0)