Abstract
Interventional radiology (IR) plays an increasingly important role in the multidisciplinary management of post-operative complications following bariatric surgery. Rapid technological advances have resulted in a range of minimally invasive procedures, which are safe and effective for the treatment of both early and late complications. These include IR techniques for the management of post-operative anastomotic leaks, collections, haemorrhage, anastomotic strictures, prevention of pulmonary embolism, and choledocholithiasis. The chapter succinctly reviews the current role of interventional radiology in modern post-operative bariatric patient management and offers an insight into new trends and future directions.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
- Bariatric Surgical Procedures
- Roux-en-Y Gastric Bypass (RYGB)
- Self-expanding Plastic Stents (SEPS)
- Self-expanding Metal Stents (SEMS)
- Biliopancreatic Diversion Duodenal Switch (BPD/DS)
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
6.1 Introduction
Estimates suggest that 1.7 billion people worldwide are clinically overweight with a prevalence that increases year-on-year [1, 2]. The body mass index [BMI, kg m−2] which is widely used as the measure of obesity defines the term “clinically overweight” as an individual with a body mass index between 25 and 30 with the definition of obesity as a patient with a BMI of greater than 30. Currently two thirds of individuals living in the United States (USA) are overweight, and of those, almost half are obese [2]. In Europe, the proportion of adults who were considered to be overweight or obese varied in 2008 between 37.0 and 56.7% for women and between 51.0 and 69.3% for men [3].
Conservative management including both lifestyle and patient medication has historically offered the main choices for patient management although these do not necessarily achieve effective long-term weight reduction. Recent evidence has demonstrated the effectiveness of bariatric surgery in this group of patients by not only inducing weight reduction but also reducing the significant associated comorbidities and long-term mortality by up to 40% [4].
Currently approximately 350,000 bariatric surgical procedures are performed annually worldwide with 63% performed in the USA and Canada [5, 6].
In 2011, the global total number of bariatric surgical procedures was 340,768; the global total number of metabolic/bariatric surgeons was 6705. The most commonly performed procedures were Roux-en-Y gastric bypass (RYGB) 46.6%, sleeve gastrectomy (SG) 27.8%, adjustable gastric banding (AGB) 17.8%, and biliopancreatic diversion/duodenal switch (BPD/DS) 2.2%. The global trends from 2003 to 2008 to 2011 showed a decrease in RYGB, 65.1 to 49.0 to 46.6%; an increase, followed by a steep decline, in AGB, 24.4 to 42.3 to 17.8%; and a marked increase in SG, 0.0 to 5.3 to 27.89%. BPD/DS declined, 6.1 to 4.9 to 2.1% [7].
These surgical procedures can be broadly categorised into restrictive, malabsorptive, and combined types.
Restrictive procedures significantly reduce gastric volume in order to induce weight loss by restricting gastric capacity and thus promoting early satiety. Typical examples include laparoscopic adjustable gastric banding (LAGB) and laparoscopic sleeve gastrectomy (LSG) . Gastric banding which is currently in decline worldwide includes the implantation of a silicon band around the proximal stomach in order to create a small pouch. The band may be adjustable via a subcutaneous port in order to create a stomal communication to the distal stomach. LSG utilises a vertical transection of the stomach resulting in a reduction of functional gastric capacity of approximately 75%. Research suggests that the restrictive procedure also contributes to a decrease in appetite by the reduction of available ghrelin-producing cells in the gastric fundal region [8].
Malabsorptive procedures act by limiting food digestion and thus rate of absorption by reducing the length of effective small bowel. The group of procedures include jejunoileal bypass, biliopancreatic diversion, and the duodenal switch procedure. The procedures however are associated with significant complication rates including intermittent diarrhoea and steatorrhoea due to the malabsorption and metabolic effects. They are now rarely performed.
Mixed procedures however take advantage of both groups of operation. For example, the Roux-en-Y gastric bypass (RYGB) uses proximal gastric stapling in order to create a small gastric pouch (restrictive ) with a jejunal diversion to shorten the functional length of small bowel (malabsorptive ). Other rarer combinations include gastroplasty with gastric bypass, biliopancreatic diversion with gastric bypass, and gastric banding with gastric bypass. Today the three procedures of LSG, RYGB, and LAGB comprise the vast majority of bariatric surgical procedures performed worldwide.
Multiple publications have demonstrated that bariatric surgical procedures are safe with an overall low morbidity (Table 6.1).
However, a mortality rate of 29.4% has been described in 2007 from the Italian Society for Obesity Surgery during a 10-year analysis [9]. The mortality risk has been related to different factors including type of surgery, prolonged operative time, comorbidities, and volume of activity [9].
Interventional radiology (IR) plays an important role in the multidisciplinary management of post-operative complications. A number of relevant IR techniques will be covered in more detail during the forthcoming chapter.
6.2 Post-operative Complications Following Bariatric Surgery
Each bariatric surgical procedure presents a different spectrum of complications. The most common procedures with their associated complications are as follows [11]:
6.3 Gastric Banding (GB)
GB includes procedures involving both adjustable and nonadjustable band placement.
Whilst the technique is in decline, many patients have implanted bands in situ.
Complications include:
-
Malpositioning of the gastric band: Commonly related to band slippage in the perigastric fat or distal stomach which can occur at various (early and late) periods of post-surgery.
-
Infection and gastric perforation (0.1–0.8% of patients): Band infection usually manifests in the early post-operative period with variable symptoms ranging from fever to severe abdominal pain and hypotension. Gastric perforation is uncommon and can lead to gastric obstruction/perforation.
-
Pouch dilatation occurs when there is overexpansion of the gastric pouch proximal to the band. This complication is usually due to band slippage/overinflation/overeating/stomal oedema; gastro-oesophageal reflux with vomiting may be the presenting symptoms.
-
Gastric band slippage and prolapse is defined as herniation of the distal stomach upward from below the band which may occur in an anterior or posterior direction. Slippage results in an abnormal band position with eccentric pouch dilatation and may lead to chronic stomal stenosis, which has been observed in 4–13% of patients. Slippage clinically presents with limited weight loss, severe gastro-oesophageal reflux, and nocturnal vomiting.
-
Intragastric erosions (occurring as 0.3–14% of patients in various series) are defined as partial or complete. Their aetiology may be secondary to small gastric wall injuries that can occur during band placement. Over-distension of the band can result in gastric wall ischaemia, band site infection, and inflammatory reactions. In addition, use of non-steroidal anti-inflammatory drugs may contribute to the degree of gastric erosions.
-
Oesophageal dysmotility and dilatation typically occur before oesophageal dilatation, but pre-existing insufficiency of the gastro-oesophageal sphincter may contribute. Other causes of oesophageal dilatation include insufficient change of dietary habit after the procedure, proximal pouch dilatation, and stomal narrowing.
-
Other delayed complications include disconnection of the band components, port site infection, and small bowel obstruction.
6.4 Sleeve Gastrectomy (SG)
- Gastric leak is the most common complication with an incidence of 1–10% of patients in published gastroplasty series [12]. The incidence can rise to 16–20% following repeat operative surgery [12]. Gastric leak has been defined by the UK Surgical Infection Study Group as “the leak of luminal contents from a surgical join between two hollow viscera” and is classified based on the timing of the leak during the post-operative period, namely, early (≤3 days after surgery), intermediate (≥4 and ≤7 days after surgery), and late (≤8 days after surgery) [13].
Classically leaks tend to appear between 5 and 6 days after surgery because of a lack of mural/anastomotic integrity. The typical location of gastric leak is the proximal third of the stomach, close to the gastro-oesophageal junction (85.7%), and less commonly occurs in the distal third (14.3%) [12]. Gastric leak management is still relatively empiric without accepted guidelines [13].
-
Collection/abscess is usually the result of gastric leak/fistulas and in many cases can be drained percutaneously under image guidance.
-
Haemorrhage and haematoma can be treated by percutaneous embolisation.
6.5 Roux-en-Y Gastric Bypass (RYGB)
RYBP includes both gastrojejunal anastomosis and enteroenteric anastomoses with both anastomoses susceptible to complications, which include:
-
Anastomotic leaks occur between 1.1 and 8.3% of patients during the early post-operative period and are managed in a similar fashion to leaks following the other main bariatric surgical procedures [12].
-
Gastrogastric fistulas : This rare complication, which may be the sequelae of a leak, results in a fistula between the proximal gastric pouch and excluded gastric remnant. The complication can lead to long-term problems of gastro-oesophageal reflux and stomal ulceration as well as patient weight gain.
-
Gastrojejunal anastomotic strictures are uncommon, and many are treated using endoscopy with balloon dilation.
-
Degradation of pouch restriction integrated often presents with a rapid passage of contrast material through a patulous anastomosis. The loss of the restrictive properties on the laparoscopic Roux-en-Y gastric bypass may cause the patient to feel insatiable and produce weight gain.
-
Small bowel obstruction is more common after laparoscopic gastric bypass than after open procedures with an incidence of up to approximately 8% [14]. The aetiology of small bowel obstruction may be due to a variety of causes including anastomotic leaks and narrowing, mural and mesenteric haematomas, post-operative adhesions, and internal hernias. Internal hernias may occur through defects in the small bowel mesentery and transverse mesocolon or through a potential space posterior to the Roux limb termed the Peterson space. Various simplified classification systems have been proposed to stratify the varied aetiologies.
-
Haemorrhage and haematoma commonly occur from the staple line. Endoscopic management using clips, adrenaline injection, and electrocautery can be used to manage bleeding from the proximal pouch, but haemorrhage from the distal pouch is more difficult to treat. Percutaneous embolisation techniques play a role when managing this complication.
-
Abscess is usually the result of intestinal perforation. These can be drained percutaneously under image guidance.
6.6 The Role of Interventional Radiology in the Management of Post-operative Surgical Complications
Interventional radiology (IR) plays an important role in the minimally invasive management of various post-operative bariatric surgical complications particularly when further surgical re-intervention increases the complication risk to the patient.
Since the advent of IR techniques, diagnostic imaging technology along with IR equipment has undergone rapid technological advances. In particular, developments in IR techniques and equipment have led to various safe and effective procedures. For example, manufacturing advances have resulted in a variety of catheters and guide wires with characteristics such as torsional strength, diameter, hydrophilic properties, and specific shape to the type of procedure undertaken.
In parallel, equipment used to guide interventional procedures has advanced with in particular the cross-sectional techniques of ultrasound (US) and computed tomography (CT) now widely available allowing the precise placement of interventional equipment. Multimodality imaging is also now routinely used during interventional procedures with more recent developments facilitating image fusion such as the overlay of 3D cross-sectional datasets with fluoroscopy. These advances have also been associated with the significant reduction in radiation exposure to both patients and staff.
The type of interventional procedure performed will depend on the specific post-operative complication, with IR techniques most commonly used to aspirate and drain collections, embolise/stent bleeding vessels, dilate anastomotic strictures, and more recently facilitate GI tract stent placement following leaks.
In addition, IR can play an important role in delayed complications including choledocholithiasis formation in patients following RYGB (Table 6.2).
6.7 Aspiration/Drainage of Collections
Percutaneous drainage (PD) of post-operative collections is the first-line therapy for patients who do not have other indications for immediate surgery. This is particularly true for the post-operative bariatric patient with a well-contained collection.
Primary SG may have a leak rate of up to 9% with an increase in incidence of up to 13% following revision surgery.
Whilst authors have recommended immediate surgical re-intervention in order to close the anastomotic defect, other minimally invasive techniques may be used with Corona et al. reporting that PD has been the stand-alone procedure in 58% of patients in their unit with gastric leak after SG [15].
Whilst PD is generally safe and effective, procedures require careful planning in order to determine the optimum access pathway to a collection. In particular, a pathway should be direct and straightforward and avoid inadvertent injury to adjacent structures and organs. Pre-procedure planning and guidance are in most cases performed using either US or CT. The choice of modality depends on various parameters including collection characteristics, operator choice, and imaging availability.
US offers a number of advantages such as real-time imaging, no ionising radiation, accurate assessment of collection contents due to high-contrast resolution, and equipment mobility allowing procedures to be performed in the IR suite or at the patient’s bedside. The modality however is very operator dependent particularly in obese patients, and collections containing gas may be poorly visualised. In addition, enteric leaks that occur from anastomoses following gastric bypass procedures commonly present in anatomical locations adjacent/deep to gas-filled organs including the stomach, duodenum, small bowel, and colon making ultrasound guidance challenging or impossible. Therefore, whilst US can be used in large and superficial collections, CT plays an important role in this group of patients.
Despite the disadvantage of requiring ionising radiation, CT is widely used when feasible in the bariatric patient in order to plan the access pathway to a collection. The modality allows accurate assessment of the collection position, depth, and adjacent structures thus facilitating the calculation of the optimal angle/direction for the access pathway. The standard CT scanner however has a maximum allowed patient weight of around 160 kgs with a bore of 70 cms, thus restricting the use of the modality in this group of patients. In response to this drawback, a number of manufacturers have developed dedicated bariatric machines allowing up to 300 kgs bodyweight with an enlarged bore of 80 cms.
6.8 Equipment Choice
Needles: The majority of abdominal collections can be aspirated through an 18 gauge needle which offers approximately 1/20th the resistance to flow when compared to a 21/22 gauge needle. In addition, an 18 gauge needle is easier to visualise and control using both US and CT guidance as well as accepting a 0.038 inch guide wire.
The majority of 21/22 gauge needle systems however require insertion of an initial 0.018 inch guide wire followed by a coaxial dilator thus adding to the complexity of the procedure.
The typical manufacturing needle length is 15–20 cm, which may be inadequate to reach a deep collection in the bariatric patient. The use of a 55 cm Colapinto needle (Cook Incorporated, Bloomington, IN) will however allow access to most collections.
Catheters: Drainage catheters vary in size and design but invariably involve a locking pigtail design. Catheter effectiveness is based on the degree of kink resistance and internal diameter as well as choice of coating facilitating ease of placement.
Pigtail catheters are preferred because the design allows a reduced risk of accidental displacement. The choice of catheter size can be determined by the needle aspiration test, which dictates that if fluid can be easily aspirated through a 10 mL syringe (1 mL in 1 s) using an 18 gauge needle, then an 8.5 F catheter diameter will be effective.
Complex collections however may require a catheter size of up to 16 F.
6.9 Technique
The two standard techniques for percutaneous collection drainage are the trocar or “one-step” and Seldinger or “two-step” procedures.
Trocar technique : This uses a catheter mounted on a central trocar and stylet.
Following subcutaneous infiltration of local anaesthetic, a direct puncture with the mounted catheter is used to access the collection. The central stylet is then removed, and aspiration is performed to confirm correct catheter tip location. The catheter is then advanced over the trocar into the collection. The technique is only suitable for large and superficial collections.
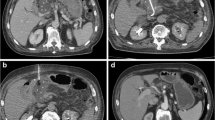
Seldinger technique : The technique is more appropriate and in most cases much safer for use in bariatric patients. Following subcutaneous infiltration of local anaesthetic, an appropriate needle (usually 18 gauge) is introduced into the collection under image guidance. After successful aspiration, a guide wire is then inserted through the needle into the collection, and following needle removal, the tract is dilated over the wire to the required diameter prior to wire-guided placement of the catheter. If a smaller needle diameter is initially used to puncture the collection, then either further guide wire exchanges can be used to upsize the tract or a second 18 gauge needle can be introduced adjacent to the initial needle (Fig. 6.1).
CT-guided percutaneous drain placement using the Seldinger technique : (a) Non-contrast CT scan with oral contrast (Gastrografin), confirming a leak and perigastric collection. (b) CT-guided puncture of the collection using an 18 gauge coaxial needle. (c) Subsequent fluoroscopic image confirming position of a 12 French Malecot (Cook Medical) drain within the collection
Both techniques require appropriate final catheter fixation to the skin with a range of devices available. Importantly an adequate specimen must be sent to microbiology and free drainage of collection contents confirmed into the catheter bag.
Collections containing viscid contents require regular flushing using 10–20 mL of saline once or twice daily in order to maintain catheter patency.
6.10 Role of Covered Stents in the Management of Leaks
Whilst the percutaneous drainage of post-operative collections is very effective, further minimally invasive techniques may be required to manage anastomotic or staple line leaks, which carry a mortality rate of up to 6.5%.
The placement of covered self-expanding metallic stents has been recommended. Stents provide a barrier between endoluminal bacteria/acidic bowel contents and the disrupted anastomosis [16]. The use of covered stents is effective allowing enteral nutrition and earlier patient discharge thus avoiding surgical re-intervention.
Stents can be placed under endoscopic guidance, fluoroscopic guidance, or using a combination of the techniques. If endoscopic placement is unsuccessful or contraindicated, multidisciplinary team management using interventional radiology can offer an alternative technique for stent insertion using fluoroscopic guidance.
6.11 Equipment Choice
Stents: Common stents used today are listed in Table 6.3. They can be divided into uncovered self-expanding metal stents (SEMS), partially covered self-expanding metal stents, and covered self-expanding plastic stents (SEPS). Uncovered stents are not commonly used due to the difficulty of stent retrieval with covered stents allowing ease of removal.
Stent placement is successful in 80–94% of patients with acute post-operative anastomotic leaks, and most patients resume an oral liquid diet within 1–3 days.
Stents are normally left in situ over varied times ranging from a mean of 41 days to 3.2 months depending on the stent characteristics [13].
The most common post-procedure side effects include early satiety, nausea, epigastric pain, and hypersialosis [17, 18].
Stent placement is associated with a number of recognised complications [19]:
-
Stent migration has been reported in 15–60% of cases. Whilst covered stents have an increased risk of migration when compared to uncovered stents, the indications for their use are a likely confounding factor [19]. Partially covered SEMS appear to have the least potential for migration [12].
-
Granulation tissue formation (0–13%) leading to perforation/fistula (0–7% cases) and haemorrhage (0–19%). Emergency surgical procedures for stent erosion through the gastrointestinal wall resulting in blood vessel laceration have also been described, and IR can contribute to the management of patients in this area [12].
6.12 Technique
The procedure for stent placement is most commonly performed under general anaesthesia with endotracheal intubation.
If following multidisciplinary discussion a combination technique is performed, an endoscope is used to identify the location of the dehisced anastomosis and mark the location with radio-opaque clips. Following removal of the scope and under fluoroscopic guidance, a 0.035′ hydrophilic wire and catheter (100–120 cm) are used to access the appropriate lumen and cross the marker clips. The site of the leak is demonstrated by injecting contrast media via the catheter.
A 0.035′ stiff or super stiff Amplatz guide wire is then placed across the anastomosis under fluoroscopic guidance. The stent delivery system is advanced over the wire and positioned across the leak prior to deployment of the stent (Fig. 6.2).
Enteral stent placement : (a) Fluoroscopic image following fluoroscopy-guided stent placement demonstrating no evidence of a residual anastomotic leak. Note tip of percutaneous drain within adjacent collection. (b) Subsequent fluoroscopic image post-stent removal shows complete resolution of the leak
Overall length of procedure can range from 23 to 47 min [17, 18].
If endoscopy is contraindicated, a fluoroscopic technique can be performed. This requires initial placement of a catheter at the level of leak followed by contrast injection in order to define the position and severity of the leak. A 0.035′ stiff or super stiff Amplatz guide wire is then placed across the anastomosis and the stent deployed to cover the leak.
Subsequent removal of the stent is normally performed using endoscopy under light sedation.
Stent extraction has been demonstrated to be most straightforward with fully covered SEMS or SEPS because the stent can be grasped with large toothed graspers and withdrawn using firm but steady pressure. Partially covered SEMS may have tissue ingrowth at either end resulting in more complex endoscopic extraction.
6.13 Balloon Dilatation of Strictures
Anastomotic strictures are late complication of RYGB (7%) and SG (<1%). Stomal obstruction can also occur following gastric banding due to post-operative oedema and is normally managed conservatively using nasogastric tube decompression with removal of band reserved for refractory cases. Balloon dilation has been reported to be effective especially if repeated with varying different balloon calibres [19].
Endoscopic balloon dilation is performed for the vast majority of patients. However, in cases that prove refractory due to difficult access or poor procedure tolerance, fluoroscopic-guided balloon dilatation by the IR team is usually successful.
The technique of fluoroscopic-guided balloon dilatation is in many ways similar to stent insertion with initial manipulation of a 0.035′ hydrophilic wire and catheter (100–120 cm) across the anastomotic stricture followed by exchange to a 0.035′ stiff or super stiff Amplatz guide wire in order to facilitate safe positioning of the balloon prior to dilatation.
Whilst the use of cutting balloons has been described for the dilatation of strictures secondary to neoplasia, the evidence base for their role in anastomotic strictures post-bariatric surgery is currently insufficient to recommend their use.
6.14 Postsurgical Haemorrhage
The post-operative risk of bleeding after bariatric surgical procedures has been estimated to be between 1 and 4%. Bleeding can occur either intra- or extraluminally.
In many cases, endoscopic management of haemorrhage using adrenaline or procoagulant materials remains the first-line treatment following surgery, as anastomotic suture lines are dependent on good blood supply and are prone to dehisce if rendered ischaemic.
Nevertheless, percutaneous embolisation techniques offer a safe and quick method of controlling haemorrhage in inaccessible locations. In addition, procedures avoid the need for general anaesthetic in patients who have significant comorbid disease. With careful pre-procedure planning and the pragmatic use of IR techniques, effective trans-arterial control can be achieved in the vast majority of patients. In addition, IR plays an important role in the subsequent percutaneous drainage of haematomas, which are prone to infection.
6.15 Equipment Choice
A range of embolisation materials are commercially available for use in this group of patients, including:
Coils: These are available in different sizes and are made of stainless steel or platinum with or without Dacron fibres. Coils use mechanical obstruction with platelet activation to fully and permanently occlude the bleeding vessel.
Gelfoam (Upjohn Company, Kalamazoo, MI): This is a water-insoluble haemostatic agent that induces haemostasis. The effect is temporary with vessels recanalising over 1–2 weeks.
Microparticles: These include polyvinyl alcohol (PVA, Cook, Bloomington, IN; Contour Boston Scientific, Natick, MA, USA) and spherical embolics (microsphere) (Embosphere BioSphere Medical, Rockland, MA, USA). These produce semi-permanent mechanical occlusion of vessels. Microspheres are more predictable as occlusion agent.
Onyx (ev3, Inc., Plymouth, MN, USA): This is an ethylene vinyl alcohol copolymer dissolved in various concentrations of dimethyl sulfoxide (DMSO) and opacified with tantalum powder. The material forms a soft elastic embolic agent when in contact with blood.
The choice of embolic material varies depending on target vessel size, potential risks of nontarget embolisation, and vascular hyper−/hypo-dynamism.
6.16 Technique
Pre-procedure planning is essential in the nonemergency setting with a haemodynamically stable patient. Procedures should commence with dedicated CT angiography (CTA) because of the increased sensitivity of CTA in detecting haemorrhage when compared to conventional angiography. In addition, the CTA provides an anatomical road map of vessel anatomy in order to facilitate subsequent catheter placement. Careful evaluation of anticoagulation history, renal insufficiency, and any contrast allergy must be carried out prior to angiography.
Access is typically via the common femoral artery (CFA). Exceptions include patients with known iliac obstruction.
Diagnostic angiography should be undertaken following selective cannulation of the celiac axis and superior mesenteric artery (SMA). A microcatheter system is then commonly used to select smaller vessels such as the superior or inferior gastroepiploic plexus or gastroduodenal artery (Fig. 6.3).
Endovascular management of a patient with post-operative pseudoaneurysm formation following sleeve gastrectomy : (a) Digital subtraction angiogram with catheter placed in coeliac axis demonstrating splenic (SA) and gastroepiploic (GE) artery pseudoaneurysms (b) Post-embolisation image demonstrating embolisation coils (Balt Extrusion, Montmorency, France) within GE and coil/Onyx 34 (EV3, Micro Therapeutics, Inc., Irvine, CA) embolisation of SA
Once the haemorrhaging vessel is identified, this is superselectively catheterised prior to embolisation. As discussed, the choice of embolic material will depend on a number of factors.
Following the procedure, careful review of the patient’s vital signs as well as measurement of serial haemoglobin and haematocrit levels is mandatory to establish continued haemostasis.
6.17 Venous Filter Placement
In addition to other post-operative complications, patients with severe obesity have a 0.34% risk of deep venous thrombosis and embolism after bariatric surgery according to a 2011 database containing 73,921 patients [20]. Anticoagulation is contraindicated during the post-operative period following surgery, and interventional radiology plays an important role in the placement of filters within the inferior vena cava (IVC) in order to reduce the risk of pulmonary emboli (PE). Due to wide heterogeneity in patient populations and indications and diagnostic criteria on the role of IVC filters, defining appropriate guidelines is challenging; hence at the time of writing, there are no widely agreed consensus guidelines published. For example, a study by Vizaki et al. which evaluated a small group of high-risk bariatric patients concluded that 44 patients had an acceptably low incidence of DVT (5%) and no clinically evident PE [21].
The use of venous filters to prevent PE was first described in 1967 [22]. Rapid technological advances soon led to percutaneous device placement with more recent developments which have heralded the use of removable devices increasing the acceptance of the technique.
The aetiology and natural history of venous thromboembolism (VTE) have been well described. Sapala et al. identified four comorbid factors associated with a risk of PE: severe venous stasis, body mass index (BMI) >60, truncal obesity, and obesity hypoventilation syndrome/sleep apnea [20]. Additional risk factors include a previous documented history of DVT/PE, hypercoagulable state, strong family history of DVT, use of oral contraceptives, age >60 years, and expected prolonged immobilisation.
In addition, an accepted indication for IVC filter placement includes bariatric patients who receive post-operative epidural analgesia for pain control. The resulting lower limb immobility increases the risk of DVT with anticoagulation contraindicated due to the high risk of an epidural haematoma [23].
6.18 Equipment Choice
Vena cava filters : Retrievable filters are now preferred to permanent filters because the long-term placement of a permanent filter is associated with an increased lifetime risk of PE. This risk is magnified in younger bariatric patients.
Manufacturer guidelines vary with regard to recommendations for the timing of filter removal, typically in the range of 6 weeks. Retrieval failure rates are described between 5 and 50%. The incidence of retrieval failure increases with time, due to the risk of anchor penetration into the IVC wall and associated fibrotic reaction. The technique for filter retrieval varies between devices, and thus individual manufacturer recommendations should be carefully considered (Table 6.4).
6.19 Technique
Whilst bariatric patients present a number of technical challenges for successful IVC filter placement, these challenges can be safely overcome with meticulous pre-procedure planning.
Initially the femoral and iliac venous systems should be assessed using US for existing thrombus.
Venous access is often the most challenging step in bariatric patients, and any puncture should be ultrasound guided in order to reduce the risk of complications. Filters can be placed via the internal jugular or superficial femoral veins. In addition, a brachial venous approach has also been described.
A standard Seldinger approach using an 18G needle is typically used, although some reports advocate initial use of a small-gauge (22G) seeker needle in order to prevent “tenting” of the vein which can result in an increased risk of contralateral sidewall puncture. Gentle rotation of the needle can however reduce the degree of tenting.
A 0.035 inch guide wire is then manipulated into the IVC prior to placement of an appropriately sized sheath (typically 6 French) to allow insertion of the filter carrier system.
Venography is then performed via a catheter placed at the L2–L3 in order to assess the presence of thrombus in the IVC, diameter of the IVC, position of the renal veins, and anatomical variants. The filter is typically placed with its tip at or just below the level of the renal veins.
Final fluoroscopy is undertaken to confirm a centrally placed filters without angulation within the IVC lumen. This reduces the risk of subsequent complicated filter retrieval.
6.20 Transhepatic Percutaneous Management of Choledocholithiasis
Cholelithiasis and choledocholithiasis are late complications following bariatric surgery and in particular are seen following bypass procedures.
Stone formation is thought to be the sequelae in alterations to bile salt concentration and circulation. Intraoperative cholecystectomy has been suggested but is considered by many centres to represent an unnecessary additional risk to the patient.
In addition, the management of gallstones following bariatric restrictive and bypass procedures can be problematic as the postsurgical anatomy prevents ERCP. There are also significant risks associated with obese patients, which limit subsequent laparoscopic or open cholecystectomy.
Interventional radiology and in particular modern percutaneous trans-hepatic access techniques can therefore play an important role in the multidisciplinary management of patients with choledocholithiasis. A detailed review of the various procedures is beyond the scope of the chapter although trans-hepatic access for the management of obstructing biliary calculi is commonly performed worldwide. Various case reports also describe the use of trans-catheter balloons for the mobilisation of obstructing calculi. These minimally invasive procedures can avoid the significant risks associated with more traditional open procedures [25].
6.21 Summary
Bariatric surgical procedures offer the obese patient an effective solution for weight loss as well as a significant reduction in associated long-term morbidity and mortality. The role of the multidisciplinary team is central to achieving optimal patient outcomes, and IR services continue to expand and evolve worldwide in order to meet the ever-increasing demand for minimal access procedures.
IR services are thus increasingly being incorporated into surgical pathways as the benefits of multimodality image-guided procedures are recognised. In addition, an expanding evidence base and increasing IR service profile are helping to consolidate these techniques as a core part of modern patient management. Familiarity with the discipline of IR is essential for all members of the clinical team in order to achieve the best outcomes for their patients, and as the prevalence of obesity increases worldwide, pioneering technological advances will continue to offer exciting new avenues in this important field.
Key Point
-
1.
Multimodality imaging is routinely used during interventional procedures with more recent developments facilitating image fusion such as the overlay of 3D cross-sectional datasets with fluoroscopy.
References
Deitel M (2003) Overweight and obesity worldwide now estimated to involve 1.7 billion people. Obes Surg 13:329–330
National Center for Health Statistics NHANES IV Report. http://www.cdc.gov/nchs/product/pubs/pubd/hestats/obes/obese99.htm2012
Eurostat. http://ec.europa.eu/eurostat/statistics-explained/index.php/Overweight_and_obesity_-_BMI_statistics
Adams TD, Gress RE, Smith SC, Halverson RC, Simper SC, Rosamond WD, Lamonte MJ, Stroup AM, Hunt SC (2007) Long-term mortality after gastric bypass surgery. N Engl J Med 357(8):753–761
World Health Organization. World Health Report. http://www.iotf.org
Buchwald H, Avidor Y, Braunwald E, Jensen MD, Bariatric Surgery A (2004) Systematic review. JAMA 292:1724–1738
H B, Oien DM (2013) Metabolic/bariatric surgery worldwide 2011. Obes Surg 23(4):427–436
Triantafyllidis G, Lazoura O, Sioka E, Tzovaras G, Antoniou A, Vassiou K, Zacharoulis D (2011) Anatomy and complications following laparoscopic sleeve gastrectomy: radiological evaluation and imaging pitfalls. Obes Surg 21(4):473–478
Morino M, Toppino M, Forestieri P, Angrisani L, Allaix ME, Scopinaro N (2007) Mortality after bariatric surgery: analysis of 13,871 morbidly obese patients from a national registry. Ann Surg 246(6):1002–1007. discussion 1007-9
Bray GA, Frühbeck G, Ryan DH, Wilding JP (2016) Management of obesity. Lancet 387(10031):1947–1956. doi:10.1016/S0140-6736(16)00271-3. Feb 8. pii: S0140–6736(16)00271–3
Riaz RM, Myers DT, Williams TR, Multidetector CT (2016) Imaging of bariatric surgical complications: a pictorial review. Abdom Radiol (NY) 41(1):174–188
Walsh C, Karmali S (2015) Endoscopic management of bariatric complications: a review and update. World J Gastrointest Endosc 7(5):518–523
Rosenthal RJ, Diaz AA, Arvidsson D, Baker RS, Basso N, Bellanger D, Boza C, El Mourad H, France M, Gagner M et al (2012) International sleeve Gastrectomy expert panel consensus statement: best practice guidelines based on experience of >12,000 cases. Surg Obes Relat Dis 8:8–19
Jones KB Jr, Afram JD, Benotti PN, Capella RF, Cooper CG, Flanagan L, Hendrick S, Howell LM, Jaroch MT, Kole K, Lirio OC, Sapala JA, Schuhknecht MP, Shapiro RP, Sweet WA, Wood MH (2006) Open versus laparoscopic Roux-en-Y gastric bypass: a comparative study of over 25,000 open cases and the major laparoscopic bariatric reported series. Obes Surg 16(6):721–727
Corona M, Zini C, Allegritti M, Boatta E, Lucatelli P, Cannavale A, Wlderk A, Cirelli C, Fiocca F, Salvatori FM, Fanelli F (2013) Minimally invasive treatment of gastric leak after sleeve gastrectomy. Radiol Med 118(6):962–970
Serra C, Baltasar A, Andreo L, Pérez N, Bou R, Bengochea M, Chisbert JJ (2007) Treatment of gastric leaks with coated self-expanding stents after sleeve gastrectomy. Obes Surg 17(7):866–872
Salinas A, Baptista A, Santiago E, Antor M, Salinas H (2006) Self-expandable metal stents to treat gastric leaks. Surg Obes Relat Dis 2:570–572
Puli SR, Spofford IS, Thompson CC (2012) Use of self-expandable stents in the treatment of bariatric surgery leaks: a systematic review and meta-analysis. Gastrointest Endosc 75:287–293
Eubanks S, Edwards CA, Fearing NM, Ramaswamy A, de la Torre RA, Thaler KJ, Miedema BW, Scott JS (2008) Use of endoscopic stents to treat anastomotic complications after bariatric surgery. J Am Coll Surg 206(5):935–938
Sapala JA, Wood MH, Schuhknecht MP et al (2003) Fatal pulmonary embolism after bariatric operations for morbid obesity: a 24-year retrospective analysis. Obes Surg 13:819–825
Vaziri K, Devin Watson J, Harper AP, Lee J, Brody FJ, Sarin S, Ignacio EA, Chun A, Venbrux AC, Lin PP (2011) Prophylactic inferior vena cava filters in high-risk bariatric surgery. Obes Surg 21(10):1580–1584
Mobin-Uddin K, Smith PE, Martinez LO, Lombardo CR, Jude JR (1967) A vena caval filter for the prevention of pulmonary embolus. Surg Forum 18:209–211
Winegar DA, Sherif B, Pate V, DeMaria EJ (2011) Venous thromboembolism after bariatric surgery performed by bariatric surgery Center of Excellence Participants: analysis of the bariatric outcomes longitudinal database. Surg Obes Relat Dis 7(2):181–188
Passman MA, Dattilo JB, Guzman RJ, Naslund TC (2005) Bedside placement of inferior vena cava filters by using transabdominal duplex ultrasonography and intravascular ultrasound imaging. J Vasc Surg 42(5):1027–1032
Marialessia M, Alfa-Wali M, Leuratti L, McCall J, Bonanomi G (2014) Percutaneous transhepatic cholangiography for choledocholithiasis after laparoscopic gastric bypass surgery. Int J Surg Case Rep 5(5):249–252
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 Springer International Publishing AG
About this chapter
Cite this chapter
Zini, C., Llewelyn, R., Corona, M., Jackson, S. (2018). The Role of Interventional Radiology in the Management of Post-Operative Complications. In: Laghi, A., Rengo, M. (eds) Imaging in Bariatric Surgery. Springer, Cham. https://doi.org/10.1007/978-3-319-49299-5_6
Download citation
DOI: https://doi.org/10.1007/978-3-319-49299-5_6
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-49297-1
Online ISBN: 978-3-319-49299-5
eBook Packages: MedicineMedicine (R0)