Abstract
Walking is considered symmetric in neurologically healthy individuals. However, asymmetry begins to occur with aging, and could be indicative of neurodegenerative diseases, such as Parkinson’s disease (PD). The aim of this chapter is to discuss gait asymmetry in people with PD. Specifically, we present a general idea about unilateral signs/symptoms of PD and its influence on asymmetry and review the literature about unobstructed gait asymmetry in people with PD. Finally, we show the effects of obstacle crossing and auditory cues on gait asymmetry in people with PD. Previous studies have indicated that people with PD presented greater asymmetry in the temporal parameters compared to neurologically healthy older subjects. For obstacle crossing during walking, both people with PD and neurologically healthy older individuals demonstrated a higher symmetric index for step duration during obstacle crossing while walking compared to unobstructed walking. Therefore, obstacle avoidance increases gait asymmetry of neurologically healthy older individuals and people with PD. Auditory cues decreased asymmetry for step length, duration and velocity, and cadence in individuals with PD compared to neurologically healthy older individuals. However, only neurologically healthy older individuals demosntrated greater asymmetry in the trials with auditory cues for step length, duration and velocity, and cadence. Therefore, auditory cues seem to have no effects on gait asymmetry for unobstructed and obstacle walking in individuals with PD, and impair gait asymmetry in neurologically healthy older individuals during obstacle avoidance.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
The term asymmetry can mean both structural and functional left–right dissimilarities [1]. Asymmetry can be considered as differences in performance and motor control between sides. The concepts of symmetry and asymmetry are closely tied to function of the two hemispheres of the human brain. Despite having a preferred limb during tasks, walking is considered symmetric in neurologically healthy individuals [2]. However, asymmetry begins to occur with aging [2], and could be indicative of neurodegenerative diseases [3, 4], such as Parkinson’s disease (PD). PD is characterized by a predominantly unilateral appearance of tremor, rigidity, and bradykinesia [5]. However, there is no obvious cause for the preferential unilateral neurodegenerative process [6].
The study of asymmetry in walking in people with PD may help in understanding the pathogenesis of the disease [6]. Clarifying what is responsible for motor asymmetry in PD is certainly important for understanding the cause of PD and its progression [7]. However, the motor control mechanisms of the limbs during walking are not well understood. The few studies that have analyzed asymmetry in gait evaluated tasks which presented little challenge, for example, walking without environmental demands (unobstructed walking). Challenging tasks, such as crossing an obstacle during walking, represent daily activities and can help in understanding the unilateral symptoms of PD. Therefore, in this chapter we discuss gait asymmetry in people with PD. Specifically, we present a general idea about unilateral signs/symptoms of PD and its influence on asymmetry and review the literature with regard to unobstructed gait asymmetry in people with PD. Finally, we show the effects of obstacle crossing and auditory cues on gait asymmetry in people with PD.
2 Parkinson’s Disease and Unilateral Motor Impairment
PD is characterized by the dysfunction or death of neurons that produce dopamine. PD affects 0.3 % of the general population, with the highest prevalence in individuals over 60 years old [8]. PD causes degeneration mainly in the substantia nigra of the basal ganglia that is located in the midbrain, causing several forms of motor impairment [9]. The reduced level of dopamine inhibits the thalamus cortical and brainstem motor systems, compromising other brain structures [10]. This degeneration affects the planning and execution of motor actions in people with PD, especially repetitive, simultaneous, and sequential movements [11].
The diagnosis of PD is generally detected through the characteristic signs and symptoms of the disease. The principal signs and symptoms of PD used for diagnosis are bradykinesia, akinesia, hypometry, resting tremors, and muscle stiffness [11]. In addition, the unilateral predominance of the disease is another important indicator for PD diagnosis [6, 7], which is related to asymmetry between the sides.
Sidedness is used to differentiate PD from other neurodegenerative diseases [6]. More than half of people with PD present sidedness in motor impairment [12– 16], commonly indicated by the Unified Parkinson’s Disease Rating Scale [12, 17, 18]. The unilateral predominance of signs and symptoms affects patients in the early stages of the disease and may persist throughout the progression period of the disease—20–30 years [6, 19]. Previous studies have reported that impairment in the lower and upper limbs of people with PD occurs in accordance with the extent of brain damage in regions containing dopamine in each hemisphere [20–22]. Individuals with unilateral PD present significant differences in striatal uptake between sides, in both the caudate and putamen nuclei [23, 24]. In addition, the dominant hemisphere has a widespread distribution in the thalamus circuitry [25–27].
Structural, genetic, environmental, and toxic or metabolic mechanisms could explain this asymmetry in PD signs and symptoms [6]. However, two theories are most accepted to explain the unilateral signs and symptoms [6]. Before detailing the explanations, it is interesting to indicate that motor signs and symptoms emerge after the loss of about 50–70 % of the nigral neurons [9, 10]. The first theory indicates that there are normal inborn differences in the numbers of nigral dopaminergic neurons. Consequently, from birth, the number of nigral neurons on one side may be lower than that on the other side. The degenerative process of the disease affects both sides equally, but the side with the primarily reduced number of neurons reaches the critical point of vulnerability earlier. The second theoretical possibility is that, for some reason, one substantia nigra is more vulnerable than the other, and once the degenerative process starts, accelerated cell death occurs first on that side (see reference [6] for more details).
3 Gait Asymmetry in People with Parkinson’s Disease
Due to sidedness of motor impairments, people with PD present asymmetrical adjustments in walking . However, the mechanisms that cause gait asymmetry in people with PD are not well known. Gait asymmetries in PD reflect an asymmetrical depletion of dopamine in the substantia nigra [28, 29]. This results in asymmetrical deregulation of the striatum, leading to further asymmetrical dysfunction of multiple circuits involving the basal ganglia and cortical regions [30, 31].
Previous studies have indicated that people with PD presented greater asymmetry in the temporal parameters compared to neurologically healthy older subjects [4, 32–37]. Specifically, swing time and its variability are the most asymmetrical parameters in people with PD, which are related to freezing episodes, falls, and decreased executive function [4, 32–35]. However, step length [38] and ground reaction forces [32] seem to be symmetrical parameters in unobstructed walking for individuals with PD. Table 11.1 shows previous studies that investigated gait asymmetry in people with PD . It is important to indicate that the findings of these studies were from walking without environmental demand. The presence of an obstacle during walking could increase gait asymmetry due to the increased complexity of the task. In the next section we present our findings on the effects of obstacle avoidance on gait asymmetry in people with PD.
To mitigate the effects of the signs and symptoms, especially asymmetry, and to improve the quality of life of people with PD, some interventions are used. The most common is pharmacological treatment . Although pharmacological treatment is effective at improving motor performance in people with PD, it presents some side effects. Therefore, other interventions have also been used, such as the external cues paradigm [39, 40] to reduce asymmetry without side effects. Verbal external [41], visual [42], rhythmic [43, 44], and auditory [45–47] cues are conventionally used in PD treatment. Previous studies have indicated that auditory cues are most effective for people with PD [48, 49], which improve rhythmicity, walking speed, and step length [47, 50–52]. One possible explanation for the improvement in gait from auditory cues is that movement requires less effort and attention, which could facilitate walking [44]. However, the use of external cues, especially auditory cues, has not been sufficiently studied with respect to the effects on asymmetry in people with PD. It is possible, this intervention could decrease walking asymmetries in people with PD. The results of our study about the effects of auditory cues on gait asymmetry in unobstructed and with obstacle walking in people with PD are presented in the following sections.
4 Effects of Obstacle Avoidance on Gait Asymmetry in People with Parkinson’s Disease
Unobstructed walking of individuals with PD is asymmetric. However, spatial parameters [38] and ground reaction forces [32] seem to be symmetric in unobstructed walking. Despite important findings about gait asymmetry in previous studies, analysis of asymmetry during complex gait tasks, such as obstacle crossing during gait, which are performed daily by this population [53], is neglected. Obstacle crossing during walking is one of the main factors leading to falling in people with PD [53–55]. Bilateral asymmetry reflects medial–lateral imbalance and instability and, hence, a predisposition to falls [33]. Previous researches has indicated that obstacle crossing during walking increases gait impairment in people with PD, mainly the hypometry and bradykinesia [53, 56–60], decreasing the toe clearance during obstacle crossing and changing the foot position in relation to the obstacle [53, 54, 61, 62]. Therefore, due to the increase in difficulty, complexity, and motor requirements during obstacle crossing for people with PD, asymmetry during this type of gait could be exacerbated.
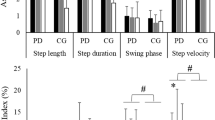
We investigated the influence of obstacle crossing on gait asymmetry in individuals with PD and neurologically healthy older individuals (control group) (Table 11.2). Participants performed five trials of unobstructed gait and ten trials of obstacle crossing during gait (five trials of crossing with each leg) at a self-selected speed. Both people with PD and neurologically healthy older individuals (control group) demonstrated a higher symmetric index [63–65] for step duration during obstacle crossing while walking compared to unobstructed walking. Obstacle crossing increased step duration of both crossing step limbs compared to step duration in unobstructed walking and increased step duration of the step with the most affected (people with PD)/non-dominant (control group) limb compared to the step with the least affected/dominant limb. Our findings confirmed the idea that obstacle crossing increased gait asymmetry compared to unobstructed walking.
Obstacle crossing requires that individuals perform adjustments during walking, necessitating rapid planning and execution of adaptive movements [66], which seems to exacerbate gait asymmetry. Obstacle avoidance involves complex circuitry, such as the parietal and motor cortex, the lateral cerebellum, and pontine gray nuclei [66], which appears to be more susceptible to sudden interference. For example, obstacle avoidance is characterized by reduced velocity, shortened step length, and larger step width [53, 55, 57, 67, 68], which needs a more conservative and cautious strategy. The combination of consciously controlled walking with deliberate obstacle avoidance may well outweigh the available resources, causing these adjustments and increasing gait asymmetry. Older people already present deficits in the circuitry indicated below, mainly in peripheral structures [69], which could cause gait asymmetry during complex locomotion. These structures are more impaired in PD, affecting the basal ganglia and requiring greater compensatory involvement from other parts of the brain during walking [70]. In addition, individuals with PD present a progressive difference in striatal uptake between the cerebral hemispheres in both the caudate and putamen nuclei and in the cortical–basal ganglia–thalamic circuitry [25–27], which cause gait asymmetry and seem to increase during challenging tasks. Therefore, obstacle avoidance increases gait asymmetry in neurologically healthy older individuals and people with PD.
5 Influence of Auditory Cues on Gait Asymmetry during Obstacle Avoidance
Previous studies have indicated that auditory cues are the most effective cues for people with PD [48, 49]. Auditory cues are external stimuli associated with the initiation and ongoing facilitation of a movement [40], which improves rhythmicity, walking speed, and step length [47, 50–52]. Due to auditory cues that influence the neural circuitry regulating gait, Brodie and collaborator [73] indicated that symmetry matched auditory cues compensated the unsteady gait in the majority people with PD, but did not interfere in gait asymmetry. Despite this, the authors suggest that during challenging walking task, such as turning or obstacle crossing, auditory cues could decrease gait asymmetry.
Our group investigated the influence of auditory cue on gait asymmetry during obstacle avoidance in people with PD and neurologically healthy older individuals. Participants performed five trials of unobstructed gait and ten trials of obstacle crossing during gait (five trials of crossing with each leg) with and without auditory cues. First, the individuals performed the trials without auditory cues. For the auditory cue trials, the cue (controlled by a metronome) was customized for each individual according to the cadence determined by three trials performed prior to starting the experiment.
People with PD and neurologically healthy older individuals presented different behaviors for gait asymmetry when auditory cues were provided (Table 11.3). Individuals with PD demonstrated lesser asymmetry for step length, duration and velocity, and cadence than neurological healthy older individuals when auditory cues were offered. For individuals with PD, auditory cues in unobstructed walking decreased step velocity asymmetry. Only neurologically healthy older individuals showed greater asymmetry in the trials with auditory cues for step length, duration and velocity, and cadence. In addition, the control group demonstrated greater asymmetry for stride velocity with auditory cues in obstacle crossing compared to unobstructed walking. Finally, neurologically healthy older individuals increased gait asymmetry (step length, velocity, and cadence) during obstacle crossing with auditory cues compared to without auditory cues.
Auditory cues had no effects on gait asymmetry for unobstructed and obstacle walking in individuals with PD. Only step velocity in unobstructed walking improved gait asymmetry with auditory cues. The findings corroborate with Brodie and collaborators [73] who did not find an influence of auditory cues on gait asymmetry. This finding could be interpreted as asymmetry in morphology, musculature, and joints related to aging than directly through mechanisms related to the diagnosis of PD [73]. However, although auditory cues influence the neural circuitry regulating gait [43, 46], improving automatic movement [71, 72] and decreasing asymmetry, when individuals with PD cross an obstacle, auditory cues did not improve, but did not impair, gait asymmetry. The findings seem to indicate that people with PD do not use auditory cues, or that obstacle avoidance impairs the benefit of auditory stimuli in gait asymmetry. An important aspect to analyze in future studies is if people with PD are able to synchronize their steps (heel contact) with auditory cues. It is possible that the higher demands of this task prevent adequate the use adequately of the auditory information.
More surprising were the results for neurologically healthy older individuals. The combination of obstacle avoidance and auditory cues increased gait asymmetry. Auditory cues seem to disrupt the gait pattern of neurologically healthy older individuals, which increases gait variability [39]. Possibly the combination of auditory cues and obstacle crossing increases the robustness of the task, increasing gait pattern disruption. Auditory cues seem to interfere with well-learned movement patterns in people without basal ganglia deficits, which perturbs the functioning of internal mechanisms [73]. Therefore, the use of auditory cues for neurologically healthy older individuals during obstacle avoidance seems not to be recommended.
6 Final Remarks
Neurologically healthy older individuals seem to start presenting asymmetry in gait, probably caused by aging effects. However, gait asymmetry is exacerbated in people with PD due to the effects of the disease that generate unilateral motor degradation. Several studies have found greater unobstructed gait asymmetry in individuals with PD, especially for temporal gait parameters. However, little is known about gait asymmetry during obstacle avoidance in both people with PD and neurologically healthy older individuals.
Obstacle avoidance seems to increase gait asymmetry in both people with PD and neurologically healthy older individuals . Differently from unobstructed gait, the effects of aging seem to increase gait asymmetry in complex walking, independent of the effects of PD. Older people already present deficits in brain circuitry, which are more impaired in people with PD, increasing gait asymmetry during obstacle avoidance. Older individuals, and particularly people with PD, are especially impaired to switching in motor tasks, requiring subjects to make corrective steps instead of walking straight [66], predominantly during challenging walking tasks. It is possible that the basal ganglia are important for set-dependent adaptation of movement patterns for changes in condition, indicating that individuals have inflexible control [74], which is aggravated by sensory and perceptual deficits such as in people with PD [75].
Auditory cues seem to have no effects on gait asymmetry for unobstructed and obstacle walking in individuals with PD, but they impair gait asymmetry in neurologically healthy older individuals during obstacle avoidance. Possibly the combination of auditory cues and obstacle crossing disrupted the gait pattern in neurologically health older individuals, increasing gait asymmetry. In addition, individuals with PD were not able to use auditory cues to improve gait asymmetry during obstacle avoidance. This task seems to be a complex movement for people with PD that demands more movement coordination and attention, impairing the use of auditory stimulus.
References
Hugdahl K. Symmetry and asymmetry in the human brain. Eur Rev. 2005;13:119–33.
Sadeghi H, Allard P, Prince F, Labelle H. Symmetry and limb dominance in able-bodied gait: a review. Gait Posture. 2000;12(1):34–45.
Wall JC, Turnbull GI. Gait asymmetries in residual hemiplegia. Arch Phys Med Rehabil. 1986;67(8):550–3.
Plotnik M, Giladi N, Balash Y, Peretz C, Hausdorff JM. Is freezing of gait in Parkinson’s disease related to asymmetric motor function? Ann Neurol. 2005;57(5):656–63.
Hughes AJ, Daniel SE, Kilford L, Lees AJ. Accuracy of clinical diagnosis of idiopathic Parkinson’s disease: a clinico-pathological study of 100 cases. J Neurol Neurosurg Psychiatry. 1992;55(3):181–4.
Djaldetti R, Ziv I, Melamed E. The mystery of motor asymmetry in Parkinson’s disease. Lancet Neurol. 2006;5(9):796–802.
Melamed E, Poewe W. Taking sides: is handedness involved in motor asymmetry of Parkinson’s disease? Mov Disord. 2012;27(2):171–3.
de Lau LM, Breteler MM. Epidemiology of Parkinson’s disease. Lancet Neurol. 2006;5(6):525–35.
Takakusaki K, Saitoh K, Harada H, Kashiwayanagi M. Role of basal ganglia-brainstem pathways in the control of motor behaviors. Neurosci Res. 2004;50(2):137–51.
Obeso JA, Rodriguez-Oroz MC, Rodriguez M, Lanciego JL, Artieda J, Gonzalo N, Olanow CW. Pathophysiology of the basal ganglia in Parkinson’s disease. Trends Neurosci. 2000;23(10 Suppl):S8–19.
Mazzoni P, Shabbott B, Cortés JC. Motor Control Abnormalities in Parkinson’s Disease. Cold Spring Harb Perspect Med. 2012;2(6):a009282.
Uitti RJ, Baba Y, Whaley NR, Wszolek ZK, Putzke JD. Parkinson disease: handedness predicts asymmetry. Neurology. 2005;64(11):1925–30.
Yust-Katz S, Tesler D, Treves TA, Melamed E, Djaldetti R. Handedness as a predictor of side of onset of Parkinson’s disease. Parkinsonism Relat Disord. 2008;14(8):633–5.
Stewart KC, Fernandez HH, Okun MS, Rodriguez RL, Jacobson CE, Hass CJ. Side onset influences motor impairments in Parkinson disease. Parkinsonism Relat Disord. 2009;15(10):781–3.
Haaxma CA, Helmich RC, Borm GF, Kappelle AC, Horstink MW, Bloem BR. Side of symptom onset affects motor dysfunction in Parkinson’s disease. Neuroscience. 2010;170(4):1282–5.
van der Hoorn A, Bartels AL, Leenders KL, de Jong BM. Handedness and dominant side of symptoms in Parkinson’s disease. Parkinsonism Relat Disord. 2011;17(1):58–60.
Elbaz A, McDonnell SK, Maraganore DM, Strain KJ, Schaid DJ, Bower JH, et al. Validity of family history data on PD: evidence for a family information bias. Neurology. 2003;61(1):11–7.
Toth C, Rajput M, Rajput AH. Anomalies of asymmetry of clinical signs in parkinsonism. Mov Disord. 2004;19(2):151–7.
Verreyt N, Nys GM, Santens P, Vingerhoets G. Cognitive differences between patients with left-sided and right-sided Parkinson’s disease. A review. Neuropsychol Rev. 2011;21(4):405–24.
Kumar A, Mann S, Sossi V, Ruth TJ, Stoessl AJ, Schulzer M, Lee CS. [11c]dtbz-pet correlates of levodopa responses in asymmetric Parkinson’s disease. Brain. 2003;126:2648–55.
Foster ER, Black KJ, Antenor-Dorsey JA, Perlmutter JS, Hershey T. Motor asymmetry and substantia nigra volume are related to spatial delayed response performance in Parkinson disease. Brain Cogn. 2008;67(1):1–10.
Lewis MM, Smith AB, Styner M, Gu H, Poole R, Zhu H, et al. Asymmetrical lateral ventricular enlargement in Parkinson’s disease. Eur J Neurol. 2009;16(4):475–81.
Knable MB, Jones DW, Coppola R, Hyde TM, Lee KS, Gorey J, Weinberger DR. Lateralized differences in iodine-123-ibzm uptake in the basal ganglia in asymmetric Parkinson’s disease. J Nucl Med. 1995;36(7):1216–25.
Tatsch K, Schwarz J, Mozley PD, Linke R, Pogarell O, Oertel WH, Kung HF. Relationship between clinical features of Parkinson’s disease and presynaptic dopamine transporter binding assessed with [123i]IPT and single-photon emission tomography. Eur J Nucl Med. 1997;24(4):415–21.
Johnson-Frey SH. The neural bases of complex tool use in humans. Trends Cogn Sci. 2004;8(2):71–8.
Binkofski F, Buccino G, Zilles K, Fink GR. Supramodal representation of objects and actions in the human inferior temporal and ventral premotor cortex. Cortex. 2004;40(1):159–61.
Potgieser AR, de Jong BM. Different distal-proximal movement balances in right- and left-hand writing may hint at differential premotor cortex involvement. Hum Mov Sci. 2011;30(6):1072–8.
Booij J, Tissingh G, Boer GJ, Speelman JD, Stoof JC, Janssen AGM, Wolters EC, van Royen EA. [123I] FP-CIT SPECT shows a pronounced decline of striatal dopamine transporter labelling in early and advanced Parkinson's disease. J Neurol Neurosurg Psychiatry. 1997;62:133–40.
Kaasinen V, Nurmi E, Bergman J, Eskola O, Solin O, Sonninen P, Rinne JO. Personality traits and brain dopaminergic function in Parkinson’s disease. Proc Natl Acad Sci U S A. 2001;98(23):13272–7.
Clower DM, Dum RP, Strick PL. Basal ganglia and cerebellar inputs to ‘AIP’. Cereb Cortex. 2005;15(7):913–20.
Middleton FA, Strick PL. Basal ganglia and cerebellar loops: motor and cognitive circuits. Brain Res Brain Res Rev. 2000;31(2-3):236–50.
Baltadjieva R, Giladi N, Gruendlinger L, Peretz C, Hausdorff JM. Marked alterations in the gait timing and rhythmicity of patients with de novo Parkinson’s disease. Eur J Neurosci. 2006;24(6):1815–20.
Yogev G, Plotnik M, Peretz C, Giladi N, Hausdorff JM. Gait asymmetry in patients with Parkinson’s disease and elderly fallers: when does the bilateral coordination of gait require attention? Exp Brain Res. 2007;177(3):336–46.
Plotnik M, Hausdorff JM. The role of gait rhythmicity and bilateral coordination of stepping in the pathophysiology of freezing of gait in Parkinson’s disease. Mov Disord. 2008;23(2):444–50.
Plotnik M, Giladi N, Hausdorff JM. Bilateral coordination of gait and Parkinson’s disease: the effects of dual tasking. J Neurol Neurosurg Psychiatry. 2009;80(3):347–50.
Frazzitta G, Pezzoli G, Bertotti G, Maestri R. Asymmetry and freezing of gait in parkinsonian patients. J Neurol. 2013;260(1):71–6.
Park K, Roemmich RT, Elrod JM, Hass CJ, Hsiao-Wecksler ET. Effects of aging and Parkinson’s disease on joint coupling, symmetry, complexity and variability of lower limb movements during gait. Clin Biomech. 2016;33:92–7.
Nanhoe-Mahabier W, Snijders AH, Delval A, Weerdesteyn V, Duysens J, Overeem S, Bloem BR. Walking patterns in Parkinson’s disease with and without freezing of gait. Neuroscience. 2011;182:217–24.
Baker K, Rochester L, Nieuwboer A. The effect of cues on gait variability-reducing the attentional cost of walking in people with Parkinson’s disease. Parkinsonism Relat Disord. 2008;14(4):314–20.
Rochester L, Burn DJ, Woods G, Godwin J, Nieuwboer A. Does auditory rhythmical cueing improve gait in people with Parkinson’s disease and cognitive impairment? A feasibility study. Mov Disord. 2009;24(6):839–45.
Behrman AL, Teitelbaum P, Cauraugh JH. Verbal instructional sets to normalise the temporal and spatial gait variables in Parkinson’s disease. J Neurol Neurosurg Psychiatry. 1998;65(4):580–2.
Azulay JP, Mesure S, Amblard B, Blin O, Sangla I, Pouget J. Visual control of locomotion in Parkinson’s disease. Brain. 1999;122:111–20.
Del Olmo MF, Ariasa P, Furiob MC, Pozoc MA, Cudeiro J. Evaluation of the effect of training using auditory stimulation on rhythmic movement in parkinsonian patients - a combined motor and [18F]-FDG PET study. Parkinsonism Relat Disord. 2006;12(3):155–64.
Rochester L, Hetherington V, Jones D, Nieuwboer A, Willems AM, Kwakkel G, Van Wegen E. The effect of external rhythmic cues (auditory and visual) on walking during a functional task in homes of people with Parkinson’s disease. Arch Phys Med Rehabil. 2005;86(5):999–1006.
Almeida QJ, Frank JS, Roy EA, Patla AE, Jog MS. Dopaminergic modulation of timing control and variability in the gait of Parkinson’s disease. Mov Disord. 2007;22(12):1735–42.
Hausdorff JM, Lowenthal J, Herman T, Gruendlinger L, Peretz C, Giladi N. Rhythmic auditory stimulation modulates gait variability in Parkinson’s disease. Eur J Neurosci. 2007;26(8):2369–75.
Picelli A, Camin M, Tinazzi M, Vangelista A, Cosentino A, Fiaschi A, Smania N. Three-dimensional motion analysis of the effects of auditory cueing on gait pattern in patients with Parkinson’s disease: a preliminary investigation. Neurol Sci. 2010;31(4):423–30.
Rochester L, Nieuwboer A, Baker K, Hetherington V, Willems AM, Chavret F, Kwakkel G, Van Wegen E, Lim I, Jones D. The attentional cost of external rhythmical cues and their impact on gait in Parkinson’s disease: effect of cue modality and task complexity. J Neural Transm. 2007;114(10):1243–8.
Nieuwboer A, Baker K, Willems AM, Jones D, Spildooren J, Lim I, Rochester L. The short-term effects of different cueing modalities on turn speed in people with Parkinson’s disease. Neurorehabil Neural Repair. 2009;23(8):831–6.
Willems AM, Nieuwboer A, Chavret F, Desloovere K, Dom R, Rochester L, Jones D, Kwakkel G, Van Wegen E. The use of rhythmic auditory cues to influence gait in patients with Parkinson’s disease, the differential effect for freezers and non-freezers, an explorative study. Disabil Rehabil. 2006;28(11):721–8.
van Iersel MB, Munneke M, Esselink RA, Benraad CE, Olde Rikkert MG. Gait velocity and the timed-up-and-go test were sensitive to changes in mobility in frail elderly patients. J Clin Epidemiol. 2008;61(2):186–91.
Hausdorff JM. Gait dynamics in Parkinson’s disease: common and distinct behavior among stride length, gait variability, and fractal-like scaling. Chaos. 2009;19(2):026113.
Vitorio R, Pieruccini-Faria F, Stella F, Gobbi S, Gobbi LT. Effects of obstacle height on obstacle crossing in mild Parkinson’s disease. Gait Posture. 2010;31(1):143–6.
Galna B, Peters A, Murphy AT, Morris ME. Obstacle crossing deficits in older adults: a systematic review. Gait Posture. 2009;30(3):270–5.
Galna B, Murphy AT, Morris ME. Obstacle crossing in people with Parkinson’s disease: foot clearance and spatiotemporal deficits. Hum Mov Sci. 2010;29(5):843–52.
Sofuwa O, Nieuwboer A, Desloovere K, Willems AM, Chavret F, Jonkers I. Quantitative gait analysis in Parkinson’s disease: comparison with a healthy control group. Arch Phys Med Rehabil. 2005;86(5):1007–13.
Pieruccini-Faria F, Menuchi MRTP, Vitório R, Gobbi LTB, Stella F, Gobbi S. Kinematic parameters for gait with obstacles among elderly patients with Parkinson’s disease, with and without levodopa: a pilot study. Braz J Phys Ther. 2006;10(2):233–9.
Yang YR, Lee YY, Cheng SJ, Lin PY, Wang RY. Relationships between gait and dynamic balance in early Parkinson’s disease. Gait Posture. 2008;27(4):611–5.
Vitorio R, Teixeira-Arroyo C, Lirani-Silva E, Barbieri FA, Caetano MJ, Gobbi S, et al. Effects of 6-month, multimodal exercise program on clinical and gait parameters of patients with idiopathic Parkinson’s disease: a pilot study. ISRN Neurol. 2011;2011:714947.
Vitório R, Lirani-Silva E, Barbieri FA, Raile V, Batistela RA, Stella F, Gobbi LT. The role of vision in Parkinson’s disease locomotion control: free walking task. Gait Posture. 2012;35(2):175–9.
van Hedel HJ, Waldvogel D, Dietz V. Learning a high-precision locomotor task in patients with Parkinson’s disease. Mov Disord. 2006;21(3):406–11.
Dietz V, Michel J. Locomotion in Parkinson’s disease: neuronal coupling of upper and lower limbs. Brain. 2008;131(Pt 12):3421–31.
Herzog W, Nigg BM, Read LJ, Olsson E. Asymmetries in ground reaction force patterns in normal human gait. Med Sci Sports Exerc. 1989;21:110–4.
Beretta VS, Gobbi LTB, Lirani-Silva E, Simieli L, Orcioli-Silva D, Barbieri FA. Challenging postural tasks increase asymmetry in patients with Parkinson’s disease. PLoS One. 2015;10(9), e0137722.
Barbieri FA, Polastri PF, Baptista AM, Lirani-Silva E, Simieli L, Orcioli-Silva D, Beretta VS, Gobbi LTB. Effects of disease severity and medication state on postural control asymmetry during challenging postural tasks in individuals with Parkinson’s disease. Hum Mov Sci. 2016;46:96–103.
Snijders AH, Weerdesteyn V, Hagen YJ, Duysens J, Giladi N, Bloem BR. Obstacle avoidance to elicit freezing of gait during treadmill walking. Mov Disord. 2010;25(1):57–63.
Patla AE, Prentice SD, Robinson C, Neufeld J. Visual control of locomotion: strategies for changing direction and for going over obstacles. J Exp Psychol Hum Percept Perform. 1991;17(3):603–34.
Patla AE. Strategies for dynamic stability during adaptive human locomotion. IEEE Eng Med Biol Mag. 2003;22:48–52.
Seidler RD, Bernard JA, Burutolu TB, Fling BW, Gordon MT, Gwin JT, Kwak Y, Lipps DB. Motor control and aging: links to age-related brain structural, functional, and biochemical effects. Neurosci Biobehav Rev. 2010;34(5):721–33.
Berardelli A, Rothwell JC, Thompson PD, Hallett M. Pathophysiology of bradykinesia in Parkinson’s disease. Brain. 2001;124(Pt 11):2131–46.
Morris ME, Iansek R, Matyas TA, Summers JJ. Stride length regulation in Parkinson’s disease. Normalization strategies and underlying mechanisms. Brain. 1996;119(2):551–68.
Cunnington R, Iansek R, Bradshaw JL. Movement-related potentials in Parkinson’s disease: external cues and attentional strategies. Mov Disord. 1999;14(1):63–8.
Brodie MA, Dean RT, Beijer TR, Canning CG, Smith ST, Menant JC, Lord SR. Symmetry matched auditory cues improve gait steadiness in most people with Parkinson’s disease but not in healthy older people. J Parkinsons Dis. 2015;5(1):105–16.
Cools R, Barker R, Sahakian B, Robbins T. L-dopa medication remediates cognitive inflexibility, but increases impulsivity in patients with Parkinson’s disease. Neuropsychologia. 2003;41:1431–41.
Almeida QJ, Frank JS, Roy EA, Jenkins ME, Spaulding S, Patla AE, Jog MS. An evaluation of sensorimotor integration during locomotion toward a target in Parkinson’s disease. Neuroscience. 2005;134:283–93.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 Springer International Publishing AG
About this chapter
Cite this chapter
Barbieri, F.A. et al. (2017). Parkinson’s Disease and Gait Asymmetry. In: Barbieri, F., Vitório, R. (eds) Locomotion and Posture in Older Adults. Springer, Cham. https://doi.org/10.1007/978-3-319-48980-3_11
Download citation
DOI: https://doi.org/10.1007/978-3-319-48980-3_11
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-48979-7
Online ISBN: 978-3-319-48980-3
eBook Packages: MedicineMedicine (R0)