Abstract
The term “sepsis” was first described by Hippocrates (c.a. 460–370 BC) in reference to blood putrefaction (septicemia) and fever, and the connection between sepsis and bacteria was made by French chemist Louis Pasteur (1822–1895). No treatment has been shown to prevent the onset or hasten recovery of failed organ systems during sepsis, which often persists long after the infection has been eliminated and ultimately leads to the death of the patient. Mechanisms linking host-pathogen interactions to organ dysfunction remain poorly understood and related insights may provide the key to more effectively treating sepsis-induced organ failures. This chapter will discuss the current theories of sepsis-induced organ failure and potential future therapies that might be derived from new understanding of the pathophysiology of sepsis.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
Sepsis Overview
The term “sepsis” was first described by Hippocrates (c.a. 460–370 BC) in reference to blood putrefaction (septicemia) and fever, and the connection between sepsis and bacteria was made by French chemist Louis Pasteur (1822–1895). No treatment has been shown to prevent the onset or hasten recovery of failed organ systems during sepsis, which often persists long after the infection has been eliminated and ultimately leads to the death of the patient. Mechanisms linking host-pathogen interactions to organ dysfunction remain poorly understood and related insights may provide the key to more effectively treating sepsis-induced organ failures. This chapter will discuss the current theories of sepsis-induced organ failure and potential future therapies that might be derived from new understanding of the pathophysiology of sepsis.
Severe Sepsis and Organ Dysfunction
As discussed in preceding chapters, sepsis is historically defined as the presence of two of the four systemic inflammatory response syndrome (SIRS) criteria and a known or suspected infection. The definition of sepsis is limited by the nonspecific nature of the SIRS criteria which can be manifested in many diseases and even in healthy subjects undergoing physical or emotional stress. It is also limited by the difficulty in detecting or confirming infections.
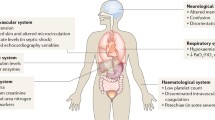
As displayed in Table 7.1, any vital organ can be adversely affected by sepsis. The extreme diversity of presentations both in terms of severity of organ dysfunction and heterogeneity of organ involvement has historically prompted investigators to look for systemic and unifying mechanisms of organ dysfunction, rather than considering organ-specific mechanisms. In this regard, circulating toxins released from infectious organisms and activated immune cells and/or alterations in the distribution of vital metabolic substrates are most commonly incriminated in the pathogenesis of sepsis-induced organ failures. However, these simplistic models of disease have not held up to scientific scrutiny, and new paradigms are emerging to explain the vital organ dysfunction in the context of sepsis.
The Spectrum of Organ Damage during Human Sepsis and Septic Shock
Before considering the likely mechanisms contributing to organ failures during human sepsis, which is most often gleaned from animal models, it is important to first establish what is observed to exist in humans. Beyond the physiological (e.g., fever, tachycardia, tachypnea, hypotension, lactic acidosis) and peripheral immune (e.g., leukocytosis or leukopenia) manifestations of sepsis, relatively little is known about the mechanisms contributing directly to organ failures in humans. Unfortunately, meaningful real-time analyses of the altered cells and tissues of failing organs are not feasible in living humans in the hospital setting, and mechanisms must be inferred from tissue specimens derived from nonvital organs (e.g., blood, skeletal muscle), imaging studies, or from post-mortem examinations.
Post-mortem examination of failed organs in the context of sepsis is logistically complicated and rarely feasible within a timeframe that would provide useful information. This is mainly due to the rapid degradation of the tissues and the need for immediate tissue harvesting. Several studies have overcome these obstacles and have provided critical insights into the likely mechanisms of sepsis-induced organ failures. An interesting series of investigations performed in the late 1970s sought to determine the ultrastructural changes in vital organs in the context of acute, overwhelming, and ultimately fatal septic shock [1, 2]. For example, one of the septic patients selected for these studies developed refractory shock with multiple organ failures and death within 24 h of acute bowel perforation. This scenario is uncommon in modern ICUs due to rapid resuscitation protocols. Nonetheless, ultrastructural analysis of the vital organs in those who died acutely of septic shock revealed widespread endoplasmic reticulum (ER) and mitochondrial swelling and disruption of mitochondrial structures with evidence of extensive cell necrosis. By contrast, those who survived the early phase of shock only to die of sustained multiple organ failures had evidence of extensive autophagocytosis, which is in keeping with attempts to remove damaged cellular components to avoid cell death. It was further noted that delayed deaths in septic shock were associated with a discrepancy between profound organ failure and the paucity of ultrastructural abnormalities. These early observations were substantiated in a more recent study by Takasu et al. wherein immediate autopsy was performed on patients who survived the early phases of severe sepsis or septic shock and had died thereafter during “chronic sepsis” (on average ~6 days after sepsis onset). Despite ongoing hemodynamic instability and renal dysfunction at the time of death, these patients were noted to have moderate degrees of mitochondrial swelling and autophagocytosis in the heart and kidneys with minimal cell death or signs of irreversible damage (e.g., fibrosis) [3]. In a comparable study, cardiac magnetic resonance imaging performed on severe sepsis survivors in a late stage of the disease (on average 6 days after sepsis onset) demonstrated a dramatic increase in mid-myocardial T2 signal intensity, in a non-ischemic pattern (i.e., sparing the endomyocardium), which implies altered metabolism or inflammation [4]. These findings are consistent with other observations in septic humans and animals, discussed in the following sections, challenging the notion that impaired tissue perfusion is primarily responsible for altered organ function in the setting of sepsis.
Host-Pathogen Interactions and Induction of Organ Failures
The observed nearly simultaneous or rapidly sequential failing of organs during sepsis implies a common and widespread process. Though systemic oxygen and other energy substrate deliveries (e.g., glucose, ketones) may be impaired under extreme circumstances with refractory septic shock, systemic blood flow is typically high and indices of tissue hypoxia are lacking during most cases of established sepsis-induced organ failures. It follows that circulating factors other than oxygen, glucose, or other metabolic substrates are contributing to organ damage and dysfunction during sepsis.
The concept of “blood poisoning ” originally applied to microbial factors that are either directly or indirectly (through activation of immune cells) toxic to systemic organs. Among the first bacterial toxins to be carefully described in septic humans is a component of streptococcal bacteria that directly inhibits the action of coenzyme diphosphopyridine nucleotide (DPN) . This compound inhibits numerous metabolic pathways, including constituents of the citric acid cycle, which is vital for mitochondrial oxidative metabolism [5]. There have been many other bacterial toxins described, exhibiting an array of cytopathic effects and pathological manifestations. These include virulence factors introduced into eukaryotic cells by gram-negative organisms via a direct intercellular type III secretion mechanism. Exoenzymes-S and -T are potentially cytotoxic through inhibition of vital signaling pathways (e.g., RAS) and disruption of the cytoskeleton [6]. PA-I lectin/adhesin potently disrupts vital barrier functions to allow the translocation of bacteria across epithelial barriers (e.g., the gut) [7]. A comprehensive review of this topic is beyond the scope of this chapter; however, a summary of the putative mechanisms linking host-pathogen interactions to organ failures is provided in Fig. 7.1.
Bacteria, viruses, and other pathogens commonly promote cell and organ damage indirectly through the activation of an intense immune response. This leads to the destruction of the pathogen and simultaneously causes “collateral damage” to the host. Eukaryotic organisms have evolved a system by which common pathogen-associated molecular patterns (PAMPs) are identified to elicit a brisk counter-response by components of the immune system. The pattern recognizing receptors of the immune system, including Toll-like receptors (TLRs), nucleotide-binding oligomerization domain (NOD)-like receptors (NLRs), are somewhat specific in terms of the PAMPs to which they respond. The pattern of receptor activations leads to specialized immune responses designed to overcome the invasive features of each pathogen.
In the context of organ damage induced indirectly by the immune response to PAMPs, an important breakthrough in understanding these mechanisms was provided by Kevin Tracey and colleagues in the late 1980s. This study was the first to provide convincing evidence that tumor necrosis factor-alpha (TNFα) was produced by the host in response to bacterial infection and that release of this immune mediator was sufficient to produce a systemic inflammatory response and related organ injury equivalent to that induced by the infection itself [8]. This observation spawned a generation of research aimed to suppress the immune response during the early phases of sepsis. Despite numerous encouraging preclinical studies in animals wherein early inhibition of TNFα or other inflammatory mediators was shown to be protective, no single immune-modulating agent has proven effective in human sepsis clinical trials. The explanations for these failures are unclear, but presumably relates to the timing of therapy relative to the onset of sepsis in humans, which is often delayed by hours or days, and the complex nature of the host-immune response.
In addition to PAMPs and the robust immune response, tissue damage is capable of perpetuating the immune response through the release of host factors, referred to as “danger associated molecular patterns (DAMPs) .” DAMPs are mimics of bacterial PAMPs. For instance, mitochondria are derived from bacterial ancestors and retain many bacterial features, including N-formylated proteins, hypomethylated CpG DNA, cardiolipin, ATP, and mitochondrial transcription factor A (TFAM). All of these components are immunogenic in humans. Endogenous DAMPs are normally sequestered within intact host cells only to be released during cell damage or apoptosis. There are a number of DAMPs that have been reported in the context of trauma or tissue inflammation (e.g., arthritis), but only a few are implicated in the context of sepsis. Among these, mitochondrial factors have been detected systemically during severe sepsis in animals and mitochondrial DNA is shown to serve as a biomarker of mortality in septic humans [9, 10]. However, the contribution of mitochondrial and non-mitochondrial DAMPs to the pathogenesis of sepsis-induced organ failures remains to be more clearly defined.
While it appears evident from this discussion that early intervention to prevent excessive activation of the immune response in the setting of sepsis would help reduce organ damage, there is little evidence supporting this approach in the clinical setting. Despite numerous animal studies showing that pretreatment or early treatment with anti-TNFα agents or inhibitors of PAMPS or their receptors is protective against sepsis-induced organ failures, none of the subsequent human trials (>30 randomized controlled trials) have shown a benefit to these anti-inflammatory approaches. Indeed, most patients have passed through the “hyperimmune” phase of sepsis by the time they present to the healthcare providers, which explains why these strategies are ineffective. Thus, the focus has turned to the mechanisms responsible for organ failures in the later stages of sepsis during which most mortality is observed.
Theories of Sepsis-Induced Organ Dysfunction
Oxygen Debt Versus Altered Oxygen Utilization
Early models of ICU care were based upon the premise that organ failures were primarily caused by inadequate energy substrate delivery. An influential clinical observation published in the journal Science in 1964 and coauthored by Max H. Weil, one of the founding members of the Society of Critical Care Medicine, demonstrated a dramatic correlation between lactic acidosis severity and mortality in critically ill patients [11]. Since lactic acidosis often is associated with systemic hypotension and with the development of organ failures, it was logical to assume that the latter was a consequence of tissue hypoperfusion or “oxygen debt.” This paradigm strongly influenced patient management in the ICU setting for the past 50 years, such as the standardized use of monitoring parameters relating to tissue oxygen delivery (pulse oximetry, central venous oxygen probes), evolution of invasive (e.g., pulmonary artery catheters) and noninvasive measures of cardiac output, and efforts to monitor blood lactate levels. These parameters were used to guide therapies designed to optimize systemic oxygen delivery, based upon Eq. (7.1), wherein oxygen delivery (DO2) is directly related to cardiac output (CO; Eq. 7.1a) and the amount of oxygen present in the blood (VO2; Eq. 7.1b). However, it is becoming evident with time and further study that septic shock is distinct from other forms of shock and new research calls into the question the role of tissue hypoxia as a primary mechanism of organ failures in those with severe sepsis.
Based upon the common finding of lactic acidosis in the context of severe sepsis and septic shock, it has long been assumed and is presently taught in medical schools worldwide that lactic acidosis equates with inadequate tissue perfusion and portends ischemic organ injury and related organ failures. Many of the components of the Surviving Sepsis Management Bundle endorsed by the Society of Critical Care Medicine and many other institutions are focused on timely monitoring of blood lactate and correction of hemodynamic variables during the early phases of sepsis. Collectively, these measures to optimize hemodynamic parameters and to reverse lactic acidosis during sepsis are referred to as “Early Goal-Directed Therapy” (EGDT) . However, a number of studies conducted in patients with established severe sepsis (with organ failures) failed to show a benefit to enhancing or “optimizing” systemic oxygen delivery either through the use of ionotropic therapies or by increasing the oxygen carrying capacity of the blood [12, 13]. In fact, the use of ionotropic agents showed a trend toward increased sepsis mortality. Likewise, efforts to optimize systemic oxygen delivery using pulmonary artery catheters to guide therapy have not been shown to improve mortality [14]. Most recently, the efficacy of EGDT has been questioned by a large study published in the New England Journal of Medicine showing no clinical benefit relative to standard care [15, 16]. Finally, efforts to augment tissue perfusion by addressing potential mismatching of perfusion with energy substrate utilization at the microvascular level (i.e., microcirculatory dysfunction) with a potent vasodilator (nitric oxide) have shown no benefit in the setting of sepsis-induced organ failures and did not reduce lactate levels [17, 18]. These studies imply that impaired tissue perfusion at either macro- or micro-circulatory levels is not the primary cause of organ failures during sepsis.
The apparent disconnect between lactic acidosis and tissue perfusion is obviated when one considers the challenges of interpreting lactic acidosis in critically ill patients. In this regard, pyruvate is a critical metabolite of glucose metabolism that serves as a substrate for the efficient formation of high energy phosphates through aerobic respiration . The conversion of pyruvate to lactate is favored in the setting of impaired mitochondrial respiration, disruption of the citric acid cycle, or inhibition of pyruvate dehydrogenase (PDH) . In the context of tissue hypoxia, the ratio of lactate to pyruvate is relatively high, whereas there is a relative increase in pyruvate in the setting of cytopathic alterations, such as PDH inhibition or mitochondrial dysfunction. Research studies conducted in septic humans confirm that the lactate/pyruvate profile is not in keeping with the tissue hypoxia paradigm, and is more consistent with inhibition of PDH, and/or altered mitochondrial respiration [19]. Indeed, animal models indicate that both PDH inhibition and altered mitochondrial respiration, as well as inhibited lactate clearance by the liver and kidneys, may all contribute to elevated lactate levels during severe sepsis.
Given that the profile of lactic acidosis during sepsis is not adequately explained by tissue hypoxia and assuming that tissue hypoxia is deleterious to organ function, how do we interpret measures of tissue oxygen delivery in the clinical setting? Systemic oxygen during sepsis is commonly elevated and is characterized by increased cardiac output, low systemic vascular resistance, and enhanced tissue blood flow (e.g., warm, hyperemic skin). Adequate tissue oxygen delivery during septic shock is supported by measures of effluent venous blood (SvO2) and by indirect measures of tissue oxygenation in animal sepsis models [20]. Moreover, serum derived from patients with sepsis is capable of altering mitochondrial and cellular respiration in normal human cells, indicating the presence of circulating factors capable of altering oxygen metabolism at the cell and tissue level, and independent of blood flow or tissue energy substrate availability [21].
Additional clinical evidence favoring a cytopathic cause of organ failures during sepsis is provided by recent well-publicized clinical trials . As noted previously, a large study (1600 septic patients) performed in Australia demonstrated that early, aggressive resuscitation measures designed to optimize systemic oxygen delivery using EGDT in the early phase of sepsis had no effect on any patient outcome (e.g., mortality, duration of organ support) [15]. Other studies have indicated that the only interventions that significantly alter the clinical course of sepsis are early antibiotics and strategies to reduce ventilator-associated lung injury [22, 23].
Furthermore, human studies show that altered mitochondrial function, particularly Complex I-activity, and related changes in high energy phosphate levels are highly predictive of sepsis mortality [24, 25]. Based upon the existing evidence, it can be said that current sepsis protocols , which include intravascular volume resuscitation to optimize systemic blood flow and related oxygen delivery, provide adequate hemodynamic support during sepsis. Moreover, progress toward preventing and reversing organ failures in the context of sepsis should consider other disease mechanisms.
Systemic Inflammation, Organ Injury, and the “Cytokine Storm”
Another compelling explanation for the pathogenesis of sepsis-induced organ dysfunction is the concept of an “inflammatory storm,” often attributed to Dr. Lewis Thomas in his 1972 article [26]. The concept is that it is not the pathogen that results in organ-failure, but rather it is the host’s immune response to infection and resulting “collateral damage” that is responsible. This theory was strongly supported by investigations carried out by Kevin Tracey’s group in the 1980s showing for the first time that cytokines, such as TNFα, are capable of recapitulating the systemic inflammatory response as occurs in the setting or experimental sepsis or endotoxemia [8]. The theory was further supported by preclinical studies showing that suppression of TNFα prior to onset of sepsis, conferred potent protection in terms of mortality [27]. However, subsequent clinical trials have shown no benefit of anti-TNFα antibodies or other proinflammatory molecule inhibitors (e.g., activated protein C) in septic humans [28]. This is presumably because the recognition and treatment of sepsis in humans is often delayed such that the therapeutic window is missed.
With advances in the supportive care of critically ill patients within the past 20 years, many more septic patients survive the acute hyper-inflammatory phase of sepsis only to die days or weeks later. The cause of delayed deaths in septic patients is currently unknown. It is noted that as many as 50% of these patients develop secondary infections with organisms that are not typically pathogenic in immune competent hosts (e.g., candida species) [29, 30]. Additionally, reactivation of latent infections, such as CMV, has been shown to significantly increase mortality risk [31]. The cause of immune suppression is complex and discussed in another chapter. It is unclear if immune suppression and related secondary infections further delay or exacerbate organ failures. However, it is apparent that suppression of the immune response is not a viable treatment for patients during the late phases of sepsis, which represents a growing majority of hospitalized and institutionalized sepsis patients. Thus, other mechanisms to reverse organ failures need to be considered. If such therapies could be identified there is hope that septic patients will be liberated from life support devices and discharged from ICUs and hospitals, such that the associated risks of secondary infections would be minimized.
Mechanisms of Altered Metabolism during Sepsis
The Early “Cytopathic” Phase of Sepsis
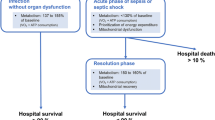
The early phase of sepsis is often associated with dramatic changes in the patient as they progress from localized infection to severe sepsis or septic shock (Table 7.2). In cases of acutely fatal septic shock, there is widespread tissue damage evident during histological and ultrastructural analyses in vital organs [1]. However, the severity and extent of vital organ injury is probably much less in most septic patients. As will be discussed in the following sections, those who survive the early phase of sepsis and go on to die days or weeks later have little evidence of cell death or irreversible cell or tissue damage. Despite the relatively favorable histology findings, none of the treatments currently available have demonstrable benefits in terms of reversing organ damage. Thus, a better understanding of the mechanisms contributing to organ failures during the late phase of sepsis will likely be required to identify novel treatments.
Metabolic Compensation During Established Sepsis
Mitochondria are vital for the function of cells and organs, and are vulnerable to injury during sepsis. In addition to their well-known functions relating to aerobic energy metabolism, mitochondria are the major source of potentially toxic reactive oxygen species (ROS) and are critical regulators of cell death. Mitochondria are dynamic in that new ones are produced to match the metabolic needs of the cell. Similarly, senescent or damaged mitochondria or unneeded mitochondria (e.g., during fasting) are efficiently removed during the lifespan of most cells. This mitochondrial turnover allows for the adaptation of cells to the environment. Changes in mitochondrial mass and increases in mitochondrial turnover are common during acute illnesses, such as the acute phase of sepsis. Autophagy selectively targets de-energized mitochondria and leads to reduced mitochondrial mass and net improvement in cellular efficiency [9].
Whereas many inflammatory mediators likely contribute to altered metabolism, TNFα is a prototypical mediator of the sepsis syndrome [27] and is capable of causing profound mitochondrial dysfunction. TNFα is released from activated mononuclear cells in response to various pathogen-associated mediators, such as lipopolysaccharide, and the release of TNFα is further regulated by the sympathetic and parasympathetic nervous systems [32]. TNFα binds to a number of TNF receptors, which promotes the intracellular release of ceramide and also the production of reactive oxygen species (ROS) formation, which are known to favor mitochondria dysfunction. TNF receptor activation promotes NF-kB pro-inflammatory pathways in various immune cells including PMNs and monocytes, which strongly induces ROS formation leading to mitochondrial DNA damage and inhibition of mitochondrial complex activities in exposed cells. Inhibition of mitochondrial complexes favors diversion of electrons through the Q-cycle to produce even more ROS. Ultimately, the formation of ROS exceeds the robust mitochondrial antioxidant mechanisms (manganese superoxide dismutase, glutathione reductase) which can trigger a catastrophic mitochondrial deenergization relating to the opening of mitochondrial “permeability transition pores” (PTP) [33]. The resulting mitochondrial permeability transition leads to dissolution of the electrochemical gradient required to form ATP and these deenergized mitochondria are targeted for removal via “self-ingestion” (i.e., autophagy) by lysosomes. Widespread induction of the mitochondrial PTP involving many mitochondria simultaneously can promote necrotic or programmed cell death (apoptosis). Finally, TNFα also inhibits mitochondrial biogenesis, in part through induction of HIF1a by inflammatory mechanisms [34, 35]. Thus, systemic TNFα release in the early phase of sepsis leads to a net reduction in mitochondrial mass and impaired mitochondrial function. It is also apparent that inflammatory mediators, such as TNFα, can alter the status of mitochondria during sepsis thereby influencing the viability and function of cells and vital organs through the induction of cell death, as noted above. In keeping with the notion that mitochondrial depletion contributes to organ failures, animal models of sepsis indicate that restoration of mitochondrial populations through biogenesis pathways is essential for recovery from sepsis [36].
Altered Function of Other Metabolic Pathways During Sepsis
In addition to changes in mitochondrial mass, qualitative changes in mitochondrial function and a shift toward glycolytic pathways are regulated by various hormones, enzymes, and regulatory pathways within the cells. Oxidant stress, particularly reactive derivatives of nitric oxide and superoxide anion (peroxynitrite), promote glycolysis by activating the rate-limiting step of the pentose pathway, glucose-6-phosphate dehydrogenase. The pentose pathway, in turn, leads to the formation of NADPH in favor of NADH. Whereas NADH is the substrate for high-energy phosphate formation mitochondrial oxidative phosphorylation, NADPH is vital for the formation and repair of proteins, DNA, and lipids. Consequently, by diverting glycolytic intermediates from the Kreb’s cycle to suppress aerobic mitochondrial respiration, the cells and tissues transition to a state of reduced oxygen consumption and lower ATP production. This is commonly referred to (e.g., in the context of cancer) as the “Warburg effect.” Under these circumstances, the cells and tissues are less reliant upon oxidative metabolism, thereby reducing oxidative stress and promoting the formation of reducing equivalents (e.g., Lactic acid, NADPH) that favor cell repair [37]. The Warburg effect and related mediators, such as HIF1α, are shown to be induced under conditions modeling sepsis [38] and confer cytoprotection in vital organs and inhibit inflammation under conditions of acute cell stress [39, 40]. However, HIF-1α activation in immune cells is shown to perpetuate pro-inflammatory pathway activation as well [41]. This serves as to remind us of the complex mechanisms at play during sepsis and why attempts to block any specific molecular target have failed to improve outcomes.
In addition to altered glucose metabolism, sepsis promotes the mobilization of free fatty acids (FFA) from fat stores and amino acids from skeletal muscle. FFA may be converted to ketones by the liver or directly metabolized by tissues to promote mitochondrial ATP formation. Amino acids released from muscle, particularly glutamine, are variably converted to acute phase proteins or deaminated to form urea by the liver [42]. Alternatively, amino acids can also serve as a mitochondrial energy substrate or can be recycled for the purpose of replacing damaged proteins in other vital organs. Thus, nonvital fat and skeletal muscle is scavenged to preserve vital organs (Fig. 7.2).
The notion of “multiple organ failure” versus “multiple organ success” refers to the benefits of adopting a lower resting metabolic state (impaired organ function) and associated alterations of the metabolic profile to promote tissue repair following the early phases of sepsis [43]. In this regard, and in contrast to current practice patterns wherein adrenergic agonists are commonly used to provide hemodynamic support in the setting of severe sepsis and septic shock, a recent clinical trial provides evidence that beta-adrenergic inhibition reduces metabolic demand (VO2), reduces fluid resuscitation requirements, protects against organ damage , and appears to reduce mortality in these patients [44, 45]. Moreover, measures to aggressively correct hyperglycemia resulting in transient hypoglycemia or efforts to fully correct protein catabolism by providing enteral protein-rich diets appear to be associated with increased mortality [46].
Organ-Specific Mechanisms
In addition to the global changes in cellular metabolism incriminated in the pathogenesis of organ failure, the mechanisms specifically contributing to altered organ function may vary from one organ to the next. Moreover, host factors, including age and comorbid disease, contribute significantly to organ failures during sepsis. In this regard, it is interesting to note that genetic variations associated with enhanced mitochondrial function are the only known genetic predictors of sepsis mortality [47]. Hence, it is likely that global metabolic alterations combined with organ-specific mechanisms conspire to promote organ failures. Other chapters address the organ-specific mechanisms that lead to impaired function, but the lung is special inthat damage to the vascular endothelium is sufficient to cause impaired lung function due to altered vascular permeability and increased lung fluid (non-cardiogenic pulmonary edema). Support for the role of vascular injury (e.g., tissue edema or impaired blood flow) in other vital organs is not as convincing. Recent studies of the heart provide convincing evidence in support of the concept that altered energy substrate delivery, the accepted paradigm , does not primarily promote organ failure or damage in the context of sepsis.
Cardiac Dysfunction
Severe sepsis is commonly associated with reduced LVEF and troponin release. Troponin elevation predicts mortality even in the absence of atherosclerotic coronary disease and irrespective of hemodynamic status (e.g., shock) [48, 49]. This indicates that myocardial damage may be a manifestation of a more global process affecting multiple organs. In this regard, the concept of “demand ischemia” has been applied to the condition of myocardial injury in the setting of severe sepsis. “Demand ischemia” is based on the premise that increased oxygen demands relating to the septic response (i.e., tachycardia, increased cardiac output) lead to ischemic injury. However, recent post-mortem analyses of the heart in the setting of fatal sepsis show no evidence of myocardial ischemia [3]. Histologic analysis of cardiac tissues shows no significant myocardial cell death by necrosis or apoptosis compared to matching ICU controls. Ultrastructural evaluation (electron microscopy) of the heart demonstrates moderate mitochondrial swelling and damage but the appearance of the contractile units and all other cardiomyocyte structures largely appeared normal [3]. In keeping with the post-mortem findings, in vitro cardiac magnetic resonance imaging performed within days after the onset of sepsis revealed a non-ischemic injury pattern characterized by epicardial and mid-myocardial T2 elevation. This pattern of T2 elevation is in contrast to ischemic heart disease which is associated with an endomyocardial T2 pattern [4]. Thus, the mechanism of cardiac injury in the context of severe sepsis is unlikely to relate to irreversible ischemic damage (e.g., necrosis), and other explanations need to be considered.
The experimental evidence provided from animal models of sepsis points to multiple potential mechanisms contributing to non-ischemic myocardial injury and dysfunction (Fig. 7.3). A recent review by Antonucci et al. nicely summarizes many of these mechanisms, and further suggests that a single therapeutic treatment is unlikely to normalize myocardial function or prevent myocardial injury [50]. Given the extensive research relating to cardiac dysfunction during sepsis, a full review of all proposed mechanisms of myocardial injury and dysfunction is beyond the scope of this chapter. Suffice it to say that more research is needed to explain the early damage and adaptations of the heart to the stress of sepsis, a condition that is associated with a nearly twofold higher risk of all-cause mortality [51, 52].
Mechanisms of cardiac dysfunction in severe sepsis. Figure previously published by Antonucci et al. [50]
Evidence that Metabolic Alterations Contribute to Dysfunction of Other Vital Organs
Other chapters will address organ-specific mechanisms of injury and altered function during sepsis. However, this chapter focuses on critical metabolic pathways that are common to all vital organs and are likely to contribute independently to altered organ function.
The kidneys are particularly sensitive to injury during sepsis, and some degree of renal dysfunction is detected in a majority of patients with sepsis. Multiple mechanisms including inflammation, oxidative stress, mitochondrial injury, and hypoperfusion all likely contribute to AKI during sepsis [53]. AKI in setting of experimental sepsis is characterized by acute alterations of parenchymal mitochondrial function and inactivation of endogenous mitochondrial antioxidant functions (e.g., manganese superoxide dismutase) [54]. Protection is conferred through induction of mitochondrial biogenesis or by targeting antioxidants to mitochondria [54, 55]. Given that delivery of mitochondrial targeted antioxidants and treatments designed to promote mitochondrial biogenesis are feasible, it is reasonable to expect that such therapies could prove to be protective against AKI during sepsis.
Altered mentation is the most common central nervous system clinical manifestation of sepsis. However, little data exists regarding the mechanisms causing encephalopathy during sepsis. There is evidence of neuronal apoptosis in specific regions of the brain in fatal cases of septic shock [56]. Other studies incriminate alterations of the blood-brain barrier relating to endothelial damage as a mechanism contributing to inflammatory cytokine release into the brain [57]. Additionally, regional disruption of vasoregulation and induction of coagulation pathways may occasionally lead to the formation of hemorrhagic lesions [57]. Animal models also support the notion that oxidative stress leads to metabolic reprogramming of neurons to favor cellular repair (e.g., of DNA damage) while suppressing ATP formation via mitochondrial oxidative phosphorylation [58]. This altered metabolic profile is equivalent to the “Warburg effect ” that occurs in the context of other pathological conditions.
The mechanisms of gastrointestinal damage during sepsis are complex, and include oxidative damage to the contractile units and epithelium [59]. The source of reactive oxidant species includes activated immune cells, damaged mitochondria, and the intracellular release of highly reactive free iron. The ensuing damage to the gut epithelium, with attendant release of “danger signals,” can promote the conversion of non-pathogenic colonizing bacteria into invasive and pathogenic organisms [59]. As such, preservation of intestinal barrier function may be of primary importance during the progression and recovery from multiple organ failure during sepsis.
Clinical Implications of Sepsis-Related Metabolism Alterations
Accepting that sepsis induces alterations of metabolism favoring cell and tissue repair at the cost of impaired organ function, it follows that certain patient populations would be predisposed to organ failures during sepsis based upon the concept of a metabolic “threshold” below which vital organs begin to fail. Direct support for this concept is provided by compelling population-based genetic data, showing that mitochondrial DNA (mtDNA) genetic variants promoting altered mitochondrial function are highly predictive of death due to sepsis. In this regard, mtDNA haplotype associated with enhanced mitochondrial respiration, including haplotype H in Europeans and haplotype R in Chinese populations, are highly protective (OR ~ 0.5) [60, 61]. In contrast, the mtDNA allele 4216C is associated with sepsis of greater severity in the setting of burn injury [62]. In addition to these genetic mtDNA variants, it is well recognized that the humans acquire mtDNA damage and associated loss of mitochondrial function with age, and the elderly are at greatly increased risk of severe sepsis compared to younger adults and children [63]. Together with data showing that altered mitochondrial function is predictive of sepsis mortality, these data strongly suggest that conditions associated with altered metabolic reserve predispose to organ failure and death in the context of sepsis.
Altered glucose metabolism also has important implications for the management of sepsis. Glycolysis is promoted in the setting of sepsis and even mild hypoglycemia in septic patients portends higher mortality and increased risk for ICU complications [64, 65]. Indeed, the severity of hypoglycemia correlates strongly with mortality risk and with adverse neurological outcome [66]. Since mitochondrial function is reduced during sepsis, inferring increased reliance on glycolysis to maintain adequate ATP production, it follows that the combination of sepsis-induced inhibition of mitochondrial ATP formation together with the lack of glucose as a substrate for glycolytic ATP production would result in critically reduced tissue ATP levels. Tissue ATP depletion, in turn, is shown to be associated with increased sepsis mortality [24].
There are many other clinical implications of altered metabolism during sepsis as relates to patient management. These include the potentially important effects of commonly used medications that influence metabolic pathways (e.g., antimicrobial agents, hypoglycemic medications, lipid altering drugs), the implications relating to changes in dietary requirements, and controversies relating to early mobilization of critically patients, all of which have unclear implications in terms of metabolic adaptation during sepsis. Finally, it is further unclear how the early management of septic patients influences organ recovery and quality-of-life in sepsis survivors . Extensive research is needed to resolve these controversies and to better understand the mechanisms of organ failure, repair, and recovery in the context of severe sepsis.
References
Cowley RA, Mergner WJ, Fisher RS, Jones RT, Trump BF. The subcellular pathology of shock in trauma patients: studies using the immediate autopsy. Am Surg. 1979;45(4):255–69.
Sato T, Kamiyama Y, Jones RT, Cowley RA, Trump BF. Ultrastructural study on kidney cell injury following various types of shock in 26 immediate autopsy patients. Adv Shock Res. 1978;1:55–69.
Takasu O, Gaut JP, Watanabe E, To K, Fagley RE, Sato B, et al. Mechanisms of cardiac and renal dysfunction in patients dying of sepsis. Am J Respir Crit Care Med. 2013;187(5):509–17.
Siddiqui Y, Crouser ED, Raman SV. Nonischemic myocardial changes detected by cardiac magnetic resonance in critical care patients with sepsis. Am J Respir Crit Care Med. 2013;188(8):1037–9.
Carlson AS, Kellner A, Bernheimer AW, Freeman EB. A streptococcal enzyme that acts specifically upon diphosphopyridine nucleotide: characterization of the enzyme and its separation from streptolysin O. J Exp Med. 1957;106(1):15–26.
Sundin C, Henriksson ML, Hallberg B, Forsberg A, Frithz-Lindsten E. Exoenzyme T of Pseudomonas aeruginosa elicits cytotoxicity without interfering with Ras signal transduction. Cell Microbiol. 2001;3(4):237–46.
Patel NJ, Zaborina O, Wu L, Wang Y, Wolfgeher DJ, Valuckaite V, et al. Recognition of intestinal epithelial HIF-1alpha activation by Pseudomonas aeruginosa. Am J Physiol Gastrointest Liver Physiol. 2007;292(1):G134–42.
Tracey KJ, Beutler B, Lowry SF, Merryweather J, Wolpe S, Milsark IW, et al. Shock and tissue injury induced by recombinant human cachectin. Science. 1986;234(4775):470–4.
Crouser ED, Julian MW, Huff JE, Struck J, Cook CH. Carbamoyl phosphate synthase-1: a marker of mitochondrial damage and depletion in the liver during sepsis. Crit Care Med. 2006;34(9):2439–46.
Nakahira K, Kyung SY, Rogers AJ, Gazourian L, Youn S, Massaro AF, et al.. Circulating mitochondrial DNA in patients in the ICU as a marker of mortality: derivation and validation. PLoS Med. 2013;10(12):e1001577; discussion e1001577.
Broder G, Weil MH. Excess lactate: an index of reversibility of shock in human patients. Science. 1964;143(3613):1457–9.
Gattinoni L, Brazzi L, Pelosi P, Latini R, Tognoni G, Pesenti A, et al. A trial of goal-oriented hemodynamic therapy in critically ill patients. SvO2 Collaborative Group. N Engl J Med. 1995;333(16):1025–32.
Hayes MA, Timmins AC, Yau EH, Palazzo M, Hinds CJ, Watson D. Elevation of systemic oxygen delivery in the treatment of critically ill patients. N Engl J Med. 1994;330(24):1717–22.
Shah MR, Hasselblad V, Stevenson LW, Binanay C, O'Connor CM, Sopko G, et al. Impact of the pulmonary artery catheter in critically ill patients: meta-analysis of randomized clinical trials. JAMA. 2005;294(13):1664–70.
Peake SL, Delaney A, Bailey M, Bellomo R, Cameron PA, Cooper DJ, et al. Goal-directed resuscitation for patients with early septic shock. N Engl J Med. 2014;371(16):1496–506.
Yealy DM, Kellum JA, Huang DT, Barnato AE, Weissfeld LA, Pike F, et al. A randomized trial of protocol-based care for early septic shock. N Engl J Med. 2014;370(18):1683–93.
Boerma EC, Koopmans M, Konijn A, Kaiferova K, Bakker AJ, van Roon EN, et al. Effects of nitroglycerin on sublingual microcirculatory blood flow in patients with severe sepsis/septic shock after a strict resuscitation protocol: a double-blind randomized placebo controlled trial. Crit Care Med. 2010;38(1):93–100.
Trzeciak S, Glaspey LJ, Dellinger RP, Durflinger P, Anderson K, Dezfulian C, et al. Randomized controlled trial of inhaled nitric oxide for the treatment of microcirculatory dysfunction in patients with sepsis. Crit Care Med. 2014;42(12):2482–92.
Gore DC, Jahoor F, Hibbert JM, DeMaria EJ. Lactic acidosis during sepsis is related to increased pyruvate production, not deficits in tissue oxygen availability. Ann Surg. 1996;224(1):97–102.
Hotchkiss RS, Karl IE. Reevaluation of the role of cellular hypoxia and bioenergetic failure in sepsis. JAMA. 1992;267(11):1503–10.
Trentadue R, Fiore F, Massaro F, Papa F, Iuso A, Scacco S, et al. Induction of mitochondrial dysfunction and oxidative stress in human fibroblast cultures exposed to serum from septic patients. Life Sci. 2012;91(7–8):237–43.
Prabhu S. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. The Acute Respiratory Distress Syndrome Network. N Engl J Med. 2000;342(18):1301–8.
Gaieski DF, Mikkelsen ME, Band RA, Pines JM, Massone R, Furia FF, et al. Impact of time to antibiotics on survival in patients with severe sepsis or septic shock in whom early goal-directed therapy was initiated in the emergency department. Crit Care Med. 2010;38(4):1045–53.
Brealey D, Brand M, Hargreaves I, Heales S, Land J, Smolenski R, et al. Association between mitochondrial dysfunction and severity and outcome of septic shock. Lancet. 2002;360(9328):219–23.
Svistunenko DA, Davies N, Brealey D, Singer M, Cooper CE. Mitochondrial dysfunction in patients with severe sepsis: an EPR interrogation of individual respiratory chain components. Biochim Biophys Acta. 2006;1757(4):262–72.
Thomas L. Germs. N Engl J Med. 1972;287(11):553–5.
Tracey KJ, Fong Y, Hesse DG, Manogue KR, Lee AT, Kuo GC, et al. Anti-cachectin/TNF monoclonal antibodies prevent septic shock during lethal bacteraemia. Nature. 1987;330(6149):662–4.
Ranieri VM, Thompson BT, Barie PS, Dhainaut JF, Douglas IS, Finfer S, et al. Drotrecogin alfa (activated) in adults with septic shock. N Engl J Med. 2012;366(22):2055–64.
Hotchkiss RS, Monneret G, Payen D. Sepsis-induced immunosuppression: from cellular dysfunctions to immunotherapy. Nat Rev Immunol. 2013;13(12):862–74.
Hotchkiss RS, Opal S. Immunotherapy for sepsis—a new approach against an ancient foe. N Engl J Med. 2010;363(1):87–9.
Heininger A, Haeberle H, Fischer I, Beck R, Riessen R, Rohde F, et al. Cytomegalovirus reactivation and associated outcome of critically ill patients with severe sepsis. Crit Care. 2011;15(2):R77.
Rosas-Ballina M, Ochani M, Parrish WR, Ochani K, Harris YT, Huston JM, et al. Slenic nerve is required for cholinergic intiinflammatory pathway control of TNF in endotoxemia. Proc Natl Acad Sci U S A. 2008;105(31):11008–13.
Exline MC, Crouser ED. Mitochondrial mechanisms of sepsis-induced organ failure. Front Biosci. 2008;13:5030–41.
Remels AH, Gosker HR, Schrauwen P, Hommelberg PP, Sliwinski P, Polkey M, et al. TNF-alpha impairs regulation of muscle oxidative phenotype: implications for cachexia? FASEB J. 2010;24(12):5052–62.
Scharte M, Han X, Uchiyama T, Tawadrous Z, Delude RL, Fink MP. LPS increases hepatic HIF-1alpha protein and expression of the HIF-1-dependent gene aldolase A in rats. J Surg Res. 2006;135(2):262–7.
MacGarvey NC, Suliman HB, Bartz RR, Fu P, Withers CM, Welty-Wolf KE, et al. Activation of mitochondrial biogenesis by heme oxygenase-1-mediated NF-E2-related factor-2 induction rescues mice from lethal Staphylococcus aureus sepsis. Am J Respir Crit Care Med. 2012;185(8):851–61.
Meloche J, Pflieger A, Vaillancourt M, Paulin R, Potus F, Zervopoulos S, et al. Role for DNA damage signaling in pulmonary arterial hypertension. Circulation. 2014;129(7):786–97.
Cheng SC, Quintin J, Cramer RA, Shepardson KM, Saeed S, Kumar V, et al. mTOR- and HIF-1alpha-mediated aerobic glycolysis as metabolic basis for trained immunity. Science. 2014;345(6204):1250684.
Chin BY, Jiang G, Wegiel B, Wang HJ, Macdonald T, Zhang XC, et al. Hypoxia-inducible factor 1alpha stabilization by carbon monoxide results in cytoprotective preconditioning. Proc Natl Acad Sci U S A. 2007;104(12):5109–14.
Cicchillitti L, Di Stefano V, Isaia E, Crimaldi L, Fasanaro P, Ambrosino V, et al. Hypoxia-inducible factor 1-alpha induces miR-210 in normoxic differentiating myoblasts. J Biol Chem. 2012;287(53):44761–71.
Tannahill GM, Curtis AM, Adamik J, Palsson-McDermott EM, McGettrick AF, Goel G, et al. Succinate is an inflammatory signal that induces IL-1beta through HIF-1alpha. Nature. 2013;496(7444):238–42.
Druml W, Heinzel G, Kleinberger G. Amino acid kinetics in patients with sepsis. Am J Clin Nutr. 2001;73(5):908–13.
Singer M, De Santis V, Vitale D, Jeffcoate W. Multiorgan failure is an adaptive, endocrine-mediated, metabolic response to overwhelming systemic inflammation. Lancet. 2004;364(9433):545–8.
Morelli A, Ertmer C, Westphal M, Rehberg S, Kampmeier T, Ligges S, et al. Effect of heart rate control with esmolol on hemodynamic and clinical outcomes in patients with septic shock: a randomized clinical trial. JAMA. 2013;310(16):1683–91.
Tang C, Yang J, Wu LL, Dong LW, Liu MS. Phosphorylation of beta-adrenergic receptor leads to its redistribution in rat heart during sepsis. Am J Physiol. 1998;274(4 Pt 2):R1078–86.
Investigators N-SS, Finfer S, Chittock DR, Su SY, Blair D, Foster D, et al. Intensive versus conventional glucose control in critically ill patients. N Engl J Med. 2009;360(13):1283–97.
Lorente L, Iceta R, Martin MM, Lopez-Gallardo E, Sole-Violan J, Blanquer J, et al. Survival and mitochondrial function in septic patients according to mitochondrial DNA haplogroup. Crit Care. 2012;16(1):R10.
John J, Woodward DB, Wang Y, Yan SB, Fisher D, Kinasewitz GT, et al. Troponin-I as a prognosticator of mortality in severe sepsis patients. J Crit Care. 2010;25(2):270–5.
Kang EW, Na HJ, Hong SM, Shin SK, Kang SW, Choi KH, et al. Prognostic value of elevated cardiac troponin I in ESRD patients with sepsis. Nephrol Dial Transplant. 2009;24(5):1568–73.
Antonucci E, Fiaccadori E, Donadello K, Taccone FS, Franchi F, Scolletta S. Myocardial depression in sepsis: from pathogenesis to clinical manifestations and treatment. J Crit Care. 2014;29(4):500–11.
Bessiere F, Khenifer S, Dubourg J, Durieu I, Lega JC. Prognostic value of troponins in sepsis: a meta-analysis. Intensive Care Med. 2013;39(7):1181–9.
Sheyin O, Davies O, Duan W, Perez X. The prognostic significance of troponin elevation in patients with sepsis: a meta-analysis. Heart Lung. 2015;44(1):75–81.
Gomez H, Ince C, De Backer D, Pickkers P, Payen D, Hotchkiss J, et al. A unified theory of sepsis-induced acute kidney injury: inflammation, microcirculatory dysfunction, bioenergetics, and the tubular cell adaptation to injury. Shock. 2014;41(1):3–11.
Patil NK, Parajuli N, MacMillan-Crow LA, Mayeux PR. Inactivation of renal mitochondrial respiratory complexes and manganese superoxide dismutase during sepsis: mitochondria-targeted antioxidant mitigates injury. Am J Physiol Renal Physiol. 2014;306(7):F734–43.
Tran M, Tam D, Bardia A, Bhasin M, Rowe GC, Kher A, et al. PGC-1alpha promotes recovery after acute kidney injury during systemic inflammation in mice. J Clin Invest. 2011;121(10):4003–14.
Sharshar T, Gray F, Lorin de la Grandmaison G, Hopkinson NS, Ross E, Dorandeu A, et al. Apoptosis of neurons in cardiovascular autonomic centres triggered by inducible nitric oxide synthase after death from septic shock. Lancet. 2003;362(9398):1799–805.
Sharshar T, Annane D, de la Grandmaison GL, Brouland JP, Hopkinson NS, Francoise G. The neuropathology of septic shock. Brain Pathol. 2004;14(1):21–33.
Bozza FA, D'Avila JC, Ritter C, Sonneville R, Sharshar T, Dal-Pizzol F. Bioenergetics, mitochondrial dysfunction, and oxidative stress in the pathophysiology of septic encephalopathy. Shock. 2013;39(Suppl 1):10–6.
Mittal R, Coopersmith CM. Redefining the gut as the motor of critical illness. Trends Mol Med. 2014;20(4):214–23.
Baudouin SV, Saunders D, Tiangyou W, Elson JL, Poynter J, Pyle A, et al. Mitochondrial DNA and survival after sepsis: a prospective study. Lancet. 2005;366(9503):2118–21.
Yang Y, Shou Z, Zhang P, He Q, Xiao H, Xu Y, et al. Mitochondrial DNA haplogroup R predicts survival advantage in severe sepsis in the Han population. Genet Med. 2008;10(3):187–92.
Huebinger RM, Gomez R, McGee D, Chang LY, Bender JE, O'Keeffe T, et al. Association of mitochondrial allele 4216C with increased risk for sepsis-related organ dysfunction and shock after burn injury. Shock. 2010;33(1):19–23.
Martin GS, Mannino DM, Moss M. The effect of age on the development and outcome of adult sepsis. Crit Care Med. 2006;34(1):15–21.
Ebata T, Hirata K, Denno R, Gotoh Y, Azuma K, Ishida K, et al. Hepatic glycolytic intermediates and glucoregulatory enzymes in septic shock due to peritonitis: experimental study in rats. Nihon Geka Gakkai Zasshi. 1984;85(1):1–5.
Park S, Kim DG, Suh GY, Kang JG, Ju YS, Lee YJ, et al. Mild hypoglycemia is independently associated with increased risk of mortality in patients with sepsis: a 3-year retrospective observational study. Crit Care. 2012;16(5):R189.
Bagshaw SM, Bellomo R, Jacka MJ, Egi M, Hart GK, George C, et al. The impact of early hypoglycemia and blood glucose variability on outcome in critical illness. Crit Care. 2009;13(3):R91.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 Springer International Publishing AG
About this chapter
Cite this chapter
Closser, D.R., Exline, M.C., Crouser, E.D. (2017). Mechanisms of Organ Dysfunction and Altered Metabolism in Sepsis. In: Ward, N., Levy, M. (eds) Sepsis. Respiratory Medicine. Humana Press, Cham. https://doi.org/10.1007/978-3-319-48470-9_7
Download citation
DOI: https://doi.org/10.1007/978-3-319-48470-9_7
Published:
Publisher Name: Humana Press, Cham
Print ISBN: 978-3-319-48468-6
Online ISBN: 978-3-319-48470-9
eBook Packages: MedicineMedicine (R0)