Abstract
The assessment of potential health risks of engineered nanomaterials (ENMs) is a challenging task due to the high number and great variety of already existing and newly emerging ENMs. Reliable grouping or categorization of ENMs with respect to hazards could help to facilitate prioritization and decision making for regulatory purposes. The development of grouping criteria, however, requires a broad and comprehensive data basis. A promising platform addressing this challenge is the systems biology approach. The different areas of systems biology, most prominently transcriptomics, proteomics and metabolomics, each of which provide a wealth of data that can be used to reveal novel biomarkers and biological pathways involved in the mode-of-action of ENMs. Combining such data with classical toxicological data would enable a more comprehensive understanding and hence might lead to more powerful and reliable prediction models. Physico-chemical data provide crucial information on the ENMs and need to be integrated, too. Overall statistical analysis should reveal robust grouping and categorization criteria and may ultimately help to identify meaningful biomarkers and biological pathways that sufficiently characterize the corresponding ENM subgroups. This chapter aims to give an overview on the different systems biology technologies and their current applications in the field of nanotoxicology, as well as to identify the existing challenges.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
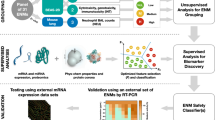
Engineered nanomaterials (ENMs) are becoming a mainstream technology in modern product design due to their unique physico-chemical properties. An ever growing number of ENMs are used worldwide in very diverse products and applications such as construction, food packaging, cosmetics, textiles or medicines. ENMs can enhance mechanical properties for instance in concrete, facilitate cleaning of surfaces in paints, enhance gas barrier capabilities in beverage packaging, block ultra-violet radiation from human skin in sunscreens, or produce self-healing surfaces. With the widespread use of ENMs possible health risks for humans must be addressed properly. One of the obvious challenges is how to assess the multitude of already existing and newly emerging ENMs in a reasonable time frame in a reliable and relevant manner. Thus, in nanotoxicology there is an urgent need of powerful prediction tools, which can ultimately support decision making with respect to prioritization and facilitate ENMs grouping or categorization. Systems biology in combination with predictive statistical tools may become a central piece of the future nanotoxicological toolbox as it will allow for in-depth understanding of affected pathways and at the same time support and facilitate grouping on the basis of the mode-of-action of ENMs (Fig. 6.1).
A thorough understanding of affected pathways, so called “toxicity pathways”, may then lead to the discovery of “adverse outcome pathways” (AOPs). The OECD [94] described the concept of AOPs in 2013, which integrate toxicological key events and describe in a cascade-like way the main steps from contact of the respective hazardous chemical to the ultimate adverse outcome. Importantly, AOPs do not only describe molecular and cellular events but also integrate data from tissues and whole organs [146]. Nowadays, an increasing number of toxicological endpoints can be described by AOPs as knowledge on underlying toxicity mechanisms is growing. Examples for well-defined AOPs are skin sensitization, cholestasis, liver fibrosis or liver steatosis. AOPs are not restricted to environmental chemicals, but may also be applied in nanotoxicology. However, so far examples in the field of nanotoxicology are still lacking. Thus, the use of systems biology and the description of AOPs for ENMs are of paramount interest in nanotoxicology. This would not only support the development of new and highly specific testing methods but also would allow grouping of ENMs. In a minimalistic view it would help to identify ENMs which require further testing, as well as ENMs where in-depth evaluation may not be needed [101]. Thus, the use of systems biology approaches in combination with modern statistical methods would also significantly reduce the number of animals for nanotoxicological testing and therefore implement the 3R paradigm (replacement, reduction and refinement) as first introduced in 1959 as a cornerstone of modern toxicology [116]. It also should be noted that the predictivity of animal models towards human adverse health effects is often limited [134]. It may be expected that knowledge on AOPs, however, will facilitate species-to-species extrapolation and thus the application of data obtained from animal models to humans, and also from animal models to in vitro approaches and vice versa [101].
The goal in animal based experiments is to determine the highest possible dose at which a given substance or ENM does not cause any adverse effect, also referred to as “no observed adverse effect level” (NOAEL) together with the lowest dose that would cause a pathological effects after a defined treatment period, which is referred to as “lowest observed adverse effect level” (LOAEL). By applying empirically derived assessment factors to take into account the uncertainty from inter- and intra-species extrapolation hazard reference values like the “derived no effect level” (DNEL) can be inferred, which are then employed for human health risk assessments. The use of NOAEL and DNEL values is applicable for toxicological effects with a threshold. In general, similar principles apply for ENMs despite the fact that some of the established guidelines may require additional adaptations for the assessment of ENMs.
Information about the molecular mechanisms of the observed adverse effects is currently considered a nice add-on and is no requirement for risk assessments. Traditionally, mechanistic insights are gained by hypothesis-driven research investigating perturbations after treatment with specific xenobiotics within one pathway at a time. Connections within and between pathways are inferred from additional experiments and from literature. This targeted approach has unravelled most of the vast molecular knowledge of biological systems available today.
Xenobiotics (and also ENMs) usually interact with multiple molecular targets in a biological system, often resulting in changes in cellular RNA molecules, proteins, lipids or metabolites. Often, affected proteins interact with other proteins and so on, creating networks of alterations that may even often overlap starting from different primary targets. The surface area of ENMs is large enough to provide the possibility for multiple interactions of a single nano-object with several biomolecules at the same time. The binding of molecules on the ENM surface may be used by the manufacturer to specifically alter the surface or may occur in a less specific and controlled way during application as soon as the ENM enters a biological environment [21], which results in the latter case in the formation of a biomolecule corona. Thus, different interactions are possible on a single nano-object. Due to interactions with ENMs biomolecules such as proteins might be denatured and/or presented out of context in the organism [37, 135]. In addition, on some ENMs surface reactions occur that generate secondary molecules that may interact with further undirected target molecules. For instance, reactive oxygen species may be generated due to surface catalysed reactions on ENMs that cause oxidative stress, which may lead to metabolite oxidation, DNA damage, and protein carbonylation [117]. Advances in life-sciences research in the fields of “omics” technologies provide tools to address the complexity of possible perturbations using descriptive approaches that record system-wide changes. The “omics” technologies collect very large data sets, often in a quantitative manner which mirror molecular responses in the genome, transcriptome, proteome and metabolome after exposure to environmental chemicals in living organisms. The four major approaches are genomics, transcriptomics, proteomics, and metabolomics. Examples of other more specialized fields are lipidomics and phosphoproteomics. Each of these technologies produces a static snapshot in one point of time, which represents only a small part of the whole organism even for the specific biomolecule class monitored. This limitation has to be considered in experimental design and data assessment such that for instance different time points are included. Genomics and transcriptomics are the predominantly used technologies in toxicological research. Both initiated the field of so-called toxicogenomics [90, 91]. Especially high-throughput micro-arrays are a valuable an often applied tool for the rapid screening and interpretation of toxicogenomic data, which subsequently can be used to define not only multiple modes of actions, but can ultimately in combination with other data be used to define adverse outcome pathways [4, 64, 109, 150]. Due to obvious similarities and common requirements for data analysis, data integration, and interpretation the use of “omics” technologies in toxicological research are summarised and commonly referred to as systems toxicology [148]. Systems toxicology usually applies methods of systems biology to toxicological problems and combines this knowledge with classical toxicological approaches. This allows for measurement of large networks of molecular and functional changes within one organism. The changes are recorded throughout multiple levels within a biological entity [122]. To this end, data from in vivo and in vitro experiments can be combined and also the combination of high-throughput data with “omics” proved useful [105]. Combining systems biology results with modern statistical approaches for data interpretation may support the elucidation of adverse outcome pathways. So far, systems biology is only beginning to be applied in nanotoxicology and currently only a few published studies are available. However, in particular in nanotoxicology the knowledge about affected pathways may help to design new test methods. Currently, there is high need of new test methods, which are suitable to screen many ENMs and which can support decision-making and prioritization. Furthermore, systems biology can substantially support the development of ENM grouping approaches based on the mode of action. In the next paragraphs we will give an overview of the different “omic” approaches and give examples how they have already been applied in nanotoxicology.
2 Transcriptomics
Gene-expression profiling, or transcriptomics, determines the changes in expression of mRNAs, rRNA, tRNA and other non-coding RNA molecules in a cell population [39], tissue/organ [14], or organism [136]. Transcriptomic analysis is arguably the best established and most widely used approach to investigate biological network responses [74]. Transcriptomics advanced with the development of oligonucleotide microarrays and the introduction of high-density array printing by Affymetrix. Limited by the known genomic sequence of the organism and using well-established bioinformatics tools to identify possible open reading frames, oligomer probe arrays can be designed covering the whole transcriptome. Clinical or environmental samples as well as samples derived from model systems can be investigated in high-throughput parallel analyses on these microarrays [82]. Current microarrays can cover each gene or its exons. Standard exon arrays are available for human, mouse, and rat. Especially the analysis of alternatively spliced RNA transcripts as well as the accuracy of the overall gene-expression has been greatly improved by the use of exon arrays [152]. However, with the establishment of next-generation sequencing (NGS) technologies it is now possible to achieve full cDNA sequencing and RNA sequencing of a cell or tissue in one analysis in a reasonable time frame. As NGS technologies are becoming more powerful and affordable they may replace microarray technologies in the near future [33, 97]. NGS approaches are expected to offer greater accuracy. In particular they provide transcript counts similar to quantitative PCR. Moreover, NGS methods are more flexible. They also allow for gene-expression studies in organisms for which microarrays are not available, such as many model systems used in environmental toxicology. NGS also allows for the assessment of the microbiome and its population responses to exposures [158].
3 Transcriptomics in Nanotoxicology
From all “omics” technologies transcriptomics is applied most often to study effects of ENMs due to the fact that approaches are well established in many laboratories. However, the amount of available transcriptome data for ENMs is still very low. Most available studies investigate only one ENM or a very small set of ENMs.
Transcriptomics has been applied in the nematode Caenorhabditis elegans to study the effects of gold nanoparticles (NPs), which were shown to induce transcriptional changes in the unfolded protein response [136]. Moreover, a comparison to previous data on silver NPs revealed diverse responses to the two ENMs [136].
Another study investigated carbon black Printex 90 NPs applied by instillation in mice. The authors found persistent elevated cytokine expression in dams and changes in liver mRNA of offspring at high doses [60]. A similar study revealed alteration of the hepatic cholesterol synthesis pathway in adult mice [14]. The latter study was combined with gene expression data of disease related studies for a subsequent bioinformatics analysis which revealed a similarity of carbon black induced effects and pulmonary injury and fibrosis [15].
Two types of multi-walled carbon nanotubes (MWCNTs) were instilled in mice showing overall similar transcriptional responses [107]. Larger MWCNTs exhibited an earlier stronger inflammatory response and stronger fibrosis [107].
Surface-modified TiO2 NPs induced elevated cytokine transcript levels and also of several miRNAs after short term inhalation of mice [51].
No effects were found for coated and aged TiO2 NPs on Caco-2 cells including in microarray analysis [36].
Comparing the effects of anatase and rutile NPs and bulk TiO2 NPs in Caenorhabditis elegans revealed different expression patterns for the different materials [114]. However, nano- and bulk form of the materials exhibited similar profiles [114]. It was observed that anatase particles exerted a greater effect on metabolic pathways, whereas rutile particles had a greater effect on developmental processes [114].
Instilled TiO2 NPs of different sizes and surface modifications in mice led to an overall similar inflammatory response by transcriptional analysis [52]. While this points to a common mechanism, closer analysis showed that the magnitude of the response was dependent on the ENM surface area [52].
Nephrotoxicity of nanoscale and microscale copper particles was addressed by a study in rats by gavage, demonstrating that a high dose of nanoscale but not microscale copper (by mass) induced strong transcriptional and necrotic responses [75]. A lower dose induced a smaller, partially overlapping, set of transcripts which were suggested as possible low dose indicators of toxicity.
Differential effects of silver ions, citrate-coated, and PVP-coated silver NPs were uncovered in an ecotoxicological study in Daphnia magna [108]. Silver ions exhibited a clearly different expression profile to the NPs, and PVP-coated NPs elicited a response in DNA damage repair genes and an overall stronger response than citrate-coated NPs [108].
Similarly, comparing the effects of silver ions to citrate-coated silver NPs in Oncorhynchus mykiss showed only a small number of specifically regulated transcripts [40]. However, linear discriminant analysis was able to separate both forms of silver [40].
The gender difference in rats exposed to silver NPs was investigated in kidneys, showing a higher expression of genes involved in xenobiotic metabolism in males and of cellular signalling in females [28].
Different types of polystyrene NPs and carbon nanotubes were compared in a human endothelial cell line [39]. Inflammation, oxidative stress, and DNA damage were the most regulated processes [39]. The more cytotoxic particles induced more transcriptional changes while the presence of serum decreased overall cytotoxicity but had little effect on the top regulated transcripts [39].
4 Proteomics
The proteome encompasses the full complement of proteins in a cell [89], biological liquids [67], tissue/organ [49], or organism [69]. Proteomics is the systematic approach to characterizing ideally all proteins. However, the broad spectrum of physico-chemical properties of proteins dictates that only a part, albeit a large part, of all proteins is detected with current technologies. Thus, for some purposes a subset of proteins first needs to be selectively enriched. In proteomics an additional complexity arises due to the fact that not only changes in overall protein amounts are of interest but also the measurement of posttranslational protein modifications. Proteins are both acceptors and mediators of altered biological responses as a consequence of exposure to substances. Changes in protein levels may correspond directly to mRNA expression or may be due to post-transcriptional regulation such as regulated translation or regulated proteolytic turnover. In addition, altered protein function may be a consequence of posttranslational modifications. For instance, protein phosphorylation can be addressed by high-throughput phosphoproteomics to characterize molecular events proximal to disease-related signalling mechanisms [32, 88]. Moreover, oxidation events due to oxidative stress in response to toxic exposures, the most discussed possible effect of ENMs, can be described by redox proteomic analysis for instance by assessing carbonylated proteins [143].
With its unmatched sensitivity and throughput, mass spectrometry (MS) is the key technology of modern proteomics. It allows for the detection of peptides in biological samples in the sub-femtomolar range with a mass accuracy of less than 10 ppm. Such a high degree of accuracy is requisite to comparisons between samples of proteins derived from different exposed and control samples. For non-targeted comparisons of samples usually isotope tagging for relative and absolute quantification (iTRAQ) is used. Even higher accuracy can be achieved by complementation with a targeted method. Typically in such a combination the untargeted assessment is used to unravel possible alterations, which are then followed up in a targeted, more precise measurement. Often selected reaction monitoring (SRM) is used for this purpose, which allows for the precise quantification of predetermined proteins. Peptides are generated by a controlled enzymatic digestion of the proteome and quantified by MS. The selection of proteins and peptides for precise quantification is done either by prior, non-targeted approaches (e.g. iTRAQ) or by a careful review of available data in the scientific literature. Bioinformatics tools are employed to predict the cleavage pattern of the selected protein. From this list at least two proteolytic peptides are selected for SRM. Those ideally should be highly specific for the protein and distinguish it from all other proteins. SRM transitions for each of those peptides are selected and unique identification and accurate quantification have to be verified through optimization and validation. Multiplexed approaches are possible where hundreds of proteins are quantified in a single MS run [72]. For example, the Nrf2-mediated stress response of macrophages to oxidised LDL was investigated demonstrating up-regulation of a group of antioxidant proteins [65].
Often proteomics approaches require the enrichment of a protein subset. Most often this is done by cellular sub-fractionation (e.g. by differential centrifugation) or by antibody-based pulldown approaches. In particular, antibody-based enrichment is used for assessing posttranslational modifications of proteins. Posttranslational modifications of proteins are important cellular mechanisms for regulating and diversifying the cellular proteome. Identification and characterization of this layer of cellular regulation can provide deeper insight into the cellular physiology and affected pathways in response to toxic insults. Examples of posttranslational modifications include phosphorylation, glycosylation, ubiquitination, nitrosylation, methylation, acetylation, lipidation and proteolysis [159]. For enrichment, phosphorylation motif specific antibodies [83], di-glycine-lysine-specific antibodies for ubiquitinated peptides [147], and other antibodies are employed. To enrich phosphorylated peptides after digestion of the protein lysates TiO2 resins are commonly used [131]. Another strategy is chemical labelling of proteins and immuno-detection after 2D gel electrophoresis. This approach is used in redox proteomics to detect protein carbonylation. In oxidative stress proteins become directly and indirectly oxidised generating various carbonyl groups. These protein carbonyl groups are conjugated to 2,4-dinitrophenylhydrazine and subsequently detected using anti-2,4-dinitrophenyl antibodies [153].
Proteomics and subsequent targeted proteomics yield important mechanistic details of toxicology pathways that were based on transcriptomic data.
5 Proteomics in Nanotoxicology
There are several studies using proteomics techniques to investigate toxicological effects of ENMs. However, similar to transcriptomics studies described above, most employ only one specific ENM and often they furthermore assess only a single concentration and/or one time point. While these studies provide important insights into ENM effects, the difference in ENM characteristics and in experimental settings makes it difficult or impossible to compare them in order to draw more general conclusions on ENM influences on the proteome and to understand how ENM properties influence toxicity.
Carbon nanotubes are one of the most investigated ENM. By comparing as-grown MWCNTs with thermally treated MWCNTs it was demonstrated that impurities were in large part responsible for the observed cytotoxicity [53]. However, stress response proteins were induced also by thermally treated MWCNTs [53]. A comparison of SWCNTs with graphene showed SWCNTs inducing proteins related to oxidative stress while graphene had little effect [156]. Lung tissue was investigated after a repeat-dose instillation of mice with SWCNTs, asbestos, and carbon black [130]. SWCNTs elicited the strongest response in regulated proteins with a similar profile to asbestos [130]. In a renal cell model, fullerenes, SWCNTs, and MWCNTs induced the most proteins in the lowest dose, suggesting that aggregation reduces the effect on cells [11]. Oxidized SWCNTs induced oxidative stress and interfered with intracellular metabolic routes, protein synthesis, and cytoskeletal systems in HepG2 cells [155]. Graphene oxide as a comparison had little effect on protein expression and was less cytotoxic [155]. Serum-free and surfactant-treated MWCNTs were compared for their effect on human aortic endothelial cells [144]. Different protein expression patterns were observed between the two suspensions with the eIF2 pathway as the only common pathway [144]. Lung tissue was investigated after repeat-dose instillation of rats with three different ENMs, Fe3O4, SiO2, and SWCNTs [77]. Seventeen commonly regulated proteins were identified and the authors suggest all three ENMs induce lung damage [77]. The secreted proteins by a macrophage model in response to MWCNTs and asbestos were investigated by proteomics [98]. Long rigid MWCNTs and asbestos showed similarities while tangled MWCNTs exhibited only limited overlap with rigid MWCNTs. All materials showed release of lysosomal proteins while only for rigid MWCNTs apoptosis-related proteins were secreted [98]. Levels of proteins in lung bronchoalveolar lavage fluid of mice treated by oropharyngeal aspiration with uncoated or aluminium oxide coated MWCNTs were determined to uncover their effect on lung tissue [54]. Uncoated MWCNTs elicited a stronger response but in similar pathways to coated MWCNTs [54].
Another heavily researched ENM is nanosilver. A comparison of PVP-coated silver NPs versus AgNO3 on plants revealed differing protein expression profiles, while redox regulation and sulphur metabolism were affected by both [142]. Similarly, silver NPs and AgNO3 were tested in mussels showing different protein expression profiles yet similar affected pathways [45]. The authors suggest that toxicity of NPs was mediated by oxidative stress-induced cell signalling cascades [45]. Citrate capped 20 and 100 nm silver NPs were compared on a colon cell line [143]. While overall the same pathways were affected (e.g. DNA damage repair), more proteins were affected by the 20 nm NPs [143]. Reanalysis of the proteomic data revealed that proteins involved in cell death and mitochondrial activity were more affected by 20 nm NPs than by 100 nm NPs, while proteins involved in cell growth were affected similarly by both particle sizes [84]. Carbonylated proteins as a marker for oxidative stress were investigated in Daphnia magna after treatment with silver NPs or AgNO3 [110]. Different profiles were found for the two treatments [110].
Recently, a large redoxproteomics study was published, which assessed protein carbonylation for a panel of 24 different ENMs [30]. The results reaffirmed that oxidative stress is a major affected pathway in response to cellular ENM exposure, and that protein carbonylation is a promising readout for this pathway.
6 Metabolomics
Metabolomics is the latest “omics” technology in the “omics” toolbox. Changes in metabolome are generally regarded to give an as-close-as-possible picture of the actual phenotype changes of the organism. The metabolome represents the ultimate change in the levels of chemical species usually resulting from molecular perturbations at the genomic and proteomic levels. Thereby, the metabolome ultimately represents the functional status of a cell.
This approach tries to quantify as many metabolites within the target organism as possible, e.g. sugars, lipids, steroids, amino acids, carnitines, nucleotides etc. To that end, hyphenated analytical techniques are applied, especially the combination of mass spectrometry with quantitative NMR is very common. The investigated metabolites encompass mostly products or substrates of enzyme-mediated processes [13]. It is also possible to detect and quantify internalized xenobiotics and their biotransformation products concomitant to the perturbed endogenous metabolome if the molecular size of the xenobiotic chemical is low enough. However, the latter requires some understanding of the kinetics of the xenobiotic toxicants and their metabolites as well as of related biomolecular adducts [111]. In a conventional approach, as many as possible metabolites are identified and changes in their abundance are quantified.
The biological matrix for metabolomics experiments can be very different and also highly complex. Generally a broad variety of different biological systems like cell cultures [12], 3D cell cultures, tissue samples [96] and whole organ cultures [76] including the emerging application area of microfluidic organ model systems [7] can be investigated for the assessment of the metabolome. Metabolomics experiments can also be performed with different body fluids like bronchoalveolar lavage fluid (BALF) [23] or serum. Even whole organisms can be assessed [112]. Due to the fact that the metabolome is very complex as it covers very different biomolecules it is not possible to use a single analytical technique to cover all metabolites within one biological system in one analysis. A variety of mass spectrometry methods (GC-MS, GCxGC-MS, LC-MS, Ion-mobility-MS) or quantitative NMR are used so far to assess quantitative changes in the metabolome after exposure to environmental chemicals. All these methods can also be used for the assessment of the metabolome in exposure assays to ENMs in nanotoxicology. Metabolomics has been also successfully used for the assessment of no observed adverse effect levels (NOAEL) in toxicology with similar or even higher sensitivity than common toxicological methods commonly used [140]. However, there are also pitfalls in the system, where metabolomics data show lower sensitivity than commonly used toxicological approaches. An overarching analysis found that 18 % of all investigated cases showed lower sensitivity than common toxicological approaches [140], indicating the need to combine not only results from other “omics” technologies, but also to combine “omics” approaches with established toxicological approaches in an integrated manner.
Of particular interest in toxicology are the assessment of metabolomic changes in animals and humans. This is closely linked to the identification of biomarker sets correlated with certain diseases like diabetes [123] or kidney disease [31]. For biomarker identification it is a paramount requisite that the sampling should be as easy as possible. Therefore, either metabolic profiles from blood [19], urine samples or breath samples, e.g. for the assessment of bronchoalveolar infections, [38] are investigated. In addition, tissue samples [29] or organ biopsy samples [44] are studied for the assessment of quantitative metabolite changes associated with various diseases or disease states. In particular, this is used for cancer diagnosis to distinguish non-malignant from malignant tissue samples or for stage determination of cancer.
7 Metabolomics in Nanotoxicology
So far, only a limited number of metabolomics studies investigated the influence of ENMs. Again, similarly to the situation in transcriptome or proteome research in most of the studies only one ENM is investigated.
Many studies focused on the effects of TiO2 NPs. One study analysed metabolomics changes in human skin cells (HaCaT cells) after exposure to TiO2 NPs and metabolite changes could be associated with oxidative stress and influenced mitochondrial activity [137]. Another study also tested TiO2 NPs (anatase, 18 nm) in HGF cells [42], and subsequent metabolomics studies revealed an increase in prostaglandin levels within these cells after exposure together with an reduction of amino acid, urea cycle, polyamine, S- adenosylmethionine and glutathione biosynthesis. Metabolomics studies of mouse fibroblasts [12] showed a significant disturbance of the amino acid signature after exposure to colloidal nano-TiO2 solutions and that these disturbances could be correlated to the observed cytotoxicity of the ENM. Urine and serum were investigated by metabonomics in rats exposed orally to TiO2 NPs [18]. Disturbances in energy and amino acid metabolism and the gut microflora environment were found [18]. Intratracheally instilled TiO2 in rats induced metabolite changes in serum that indicated slight liver and kidney injury which was corroborated by clinical chemistry [124].
Other intensely studied ENMs are nanosilver and nanosilica. Metabolomics was already used to assess metabolic profile changes in rats after oral gavage of silver ions and silver NPs [50]. The results showed that nanosilver increased not only uric acid levels, but also allantoin levels in rat urine.
Metabonomics was highlighted as a potential and robust non-destructive tool for monitoring the temporal effect of NPs in cell culture media [59]. The metabonomic assay revealed pronounced effects of SiO2 NPs in lung alveolar A549 cells on glucose, lactate, histidine, phenylalanine, and tyrosine at early time points when cell viability was not impaired. Moreover, the data suggest that the different sizes of NPs induced different dose-dependent effects with different time courses [59]. The study also showed a dose-dependent increase of ROS formation. Different sizes of SiO2 particles were intravenously injected in mice and liver tissues and serum analysed by integrated metabonomics analysis [80]. Disturbances in energy metabolism, amino acid metabolism, lipid metabolism, and nucleotide metabolism were reported that may be attributable to the observed hepatotoxicity. No major differences were found by the different NP sizes among the metabolite profiles. Surface area had a greater effect than particle number on toxicity [80]. Metabolite perturbations after intranasal SiO2 NP application in rats implicated impairment in the tricarboxylic acid cycle and liver metabolism [99]. The authors suggested from their data that SiO2 NPs may have a potential to induce hepatotoxicity in rats [99].
Other ENMs are less often studied. Metabolic responses to MnO NPs in biofluids (plasma and urine) and tissues (liver, spleen, kidney, lung and brain) from rats could be divided into four classes: MnO biodistribution-dependent, time-dependent, dose-dependent and complicated metabolic variations [73]. Particle size and surface chemistry of NPs were correlated to changes in the metabolic profile [73]. Single-walled carbon nanotubes after intratracheal instillation in rats induced changes in blood plasma and liver tissues indicating liver injury [76]. Changes in lipids and lipid associated molecules suggested a mechanism involving oxidative stress [76]. Iron oxide NPs were intravenously injected in rats, and metabonomics analysis performed on urine and plasma [35]. Subtle metabolic changes in response to NPs were found in a number of metabolic pathways including energy, lipid, glucose and amino acid metabolism [35]. The authors followed up their investigation by analysing tissues, including kidney, liver and spleen [34]. The metabonomics analysis demonstrated correlations between biofluids and tissues in their response to NPs [34]. Size and surface chemistry of the NPs affected their biological effects [34, 35]. Another study investigated the metabolic changes caused by antimicrobial effects of carboxyl-capped bismuth NPs in Heliobacter pylori colonies [86]. The results showed an increased release of acetate, formic acid, glutamate, valine, glycine, and uracil into the culture medium after NP treatment, indicating perturbations of various metabolic pathways like the Krebs cycle and nucleotide and amino acid metabolism. Thus, metabolomics in combination with other “omics” technologies could also give insights for new ENM applications, e.g. by discovering antimicrobial effects.
A few studies only investigate several ENMs at the same time. One study describes metabolic changes within 265 cellular metabolites after exposure of liver HepG2 cells to four TiO2 and two CeO2 materials [66]. The results showed that five out of the six investigated ENMs significantly reduced glutathione concentrations and associated metabolite levels within HepG2 cells. The study showed that 8 nm CeO2 NPs significantly increased lipid levels including fatty acid concentrations within HepG2 cells, whilst all other investigated NPs did not show a similar effect. CeO2, but not TiO2, increased asymmetric dimethylarginine concentration and thus possible decreased iNOS activity and NO concentrations.
8 Lipidomics
The lipidome is an example of a specialized subset of the metabolome. It comprises the complete known lipid profile of a biological system [17]. Lipidomics is the systematic approach to characterize and quantify lipids in biological samples using analytical methods mostly based on MS.
Lipids are the fundamental constituents of all cellular membranes [47, 56], provide an important energy reserve [102], and exhibit intracellular as well as systemic signalling functions [141]. Exposure to environmental chemicals often induces considerable changes in the cellular and tissue lipid composition. Levels of specific lipids such as certain sphingolipids that are involved in lipid signalling can be indicative of a cells stress status [48]. Therefore, lipidomics as a powerful method to describe the overall lipid composition of biological matrices has great potential to identify and detect candidate biomarker signatures indicative of toxicity. Recent advances in MS-based techniques enable the identification and quantification of hundreds of molecular lipid species in a high throughput manner [145, 149]. It is possible to analyse large sample collections by automated methods in a 96-well format [61]. Multiple MS platforms can be employed to characterize the extracted lipid such as detecting the lipids by shotgun lipidomics or after separation by liquid chromatography to detect and quantify lipids of lower abundance.
Moreover, alterations of lipid homeostasis contribute to several pathophysiological conditions like diabetes, cardiovascular disease, Parkinson’s disease, Alzheimer’s disease or nonalcoholic fatty liver disease (NAFLD) to distinguish between the different disease states steatosis, nonalcoholic steatohepatitis (NASH), and cirrhosis [55, 106, 125]. Lipidomic studies, combined with other “omics” technologies in an integrated approach have the potential not only to get better understanding of the up- and downregulation of cellular signalling pathways [62] but may ultimately be one of the tools to assess adverse outcome pathways also for nanotoxicology.
9 Lipidomics in Nanotoxicology
Lipidomic studies were also already used to evaluate the nanotoxicity of single walled carbon nanotubes after inhalation and showed selective pulmonary peroxidation profiles [138]. Lipidomics in combination with proteomics was used to assess the influence of metal (silver) and metal oxide (CuO, TiO2 and ZnO) NPs to primary mouse hepatocytes [128], thereby revealing particle specific effects. While silver NPs increased triacylglycerol levels and decreased sphingomyelin levels, CuO NPs decreased phosphatidylethanolamines and phosphatidylinositols and caused down-regulation of electron transferring protein subunit beta. TiO2 caused the upregulation of ATP-synthase and electron transferring protein alpha. These investigations show the diversity of regulation mechanisms for a small subset of NPs and clearly indicate that a more general integrated “omics” approach is needed to fully assess nanotoxicity and possible involved adverse outcome pathways by combining resulting information from several “omics” technologies. Testing strategies for relevant “reference” subsets of nanoparticles are needed to establish and evaluate possible adverse outcome pathways and their subsequent “omics” perturbations not only when fully established but in a time-dependent manner [63]. That is how “omics” pattern gradually change from the time of exposure until adverse outcome effects like apoptosis are finally manifested. In that way, “omics” perturbations observed by exposure to newly emerging ENMs can not only be assessed against adverse outcome effects like apoptosis, but also against various intermitting time-dependent “omic” pattern changes. This would also ultimately give a tool to assess “positive” “omics” pattern changes after exposure to evaluate long-term effects caused by exposure to ENMs without acute manifestation of nanotoxicological effects.
10 High-Content Screening
Data of molecular changes gathered from “omics” technologies should ideally be corroborated by cellular or tissue-level observations measured under the same conditions. Histopathology is performed for the standard guidelines for regulatory assessment while “omics” technologies are employed to provide additional data. On the other hand, for small organisms and cells in culture, high-content screening (HCS) methods are available [85, 92]. These methods are based on the automated computer-aided visual detection of a panel of functional biomarkers in either a fixed specimen labelled with fluorescent reagents or directly on a living specimen during the time of the exposure. Mostly digital microscopy and flow cytometry are employed in HCS, which may provide precise temporal, spatial, and contextual information defining the biological status of the cells or organs and structure of small organisms. It should be noted that in terms of ENMs their possible interference with especially optical/visual techniques demands extra scrutiny for HCS methods [26]. A broad panel of biomarkers is available to quantify key cellular events such as apoptosis, autophagy, cell proliferation, cell viability, cytotoxicity, DNA damage, mitochondrial health, mitotic index, oxidative stress, nascent protein synthesis, and phospholipidosis and steatosis. Some of these biomarkers can be used in multiplexed approaches and allow quantitative measurements of the abundance and localization of proteins and/or changes in the morphology of the cell.
11 Combinatorial Omics and Integrated Data Analysis
A small number of studies concerning ENMs have integrated more than one omics technique.
Cunningham et al. [22] combined high throughput omics biotechnologies with systems biology to screen for toxicity of single walled carbon nanotubes (SWCNTs) compared to nanosize TiO2, quartzous SiO2, carbon black (Printex 90), and carbonyl iron on human primary epidermal keratinocytes and bronchial epithelial cells. Expression arrays for mRNA and microRNA were used together with 2D protein gel electrophoresis and mass spectrometry detection. Expression profile comparison revealed similar profiles of SWCNTs and carbonyl iron at non-cytotoxic doses and of SWCNTs and quartzous SiO2 at cytotoxic doses [22].
Silica-coated magnetic NPs containing Rhodamine B isothiocyanate MNPs@SiO2(RITC) were investigated for gene expression and metabolic changes in human embryo kidney 293 cells [120]. Based on microarray gene chip and gas chromatography mass spectrometry analysis, glutamic acid was increased and expression of genes related to the glutamic acid metabolic pathway as well as organic acids related to the Krebs cycle were disturbed at a high dose of particles. Furthermore, a decreased capacity of ATP synthesis, increases in ROS concentration, and mitochondrial damage were observed in functional assays [120].
Proteomics and miRNA sequencing technologies were utilized to investigate effects of silver NPs on human dermal fibroblasts [58]. Of the 57 pathways found regulated in response to the ENM, four pathways were concurrently affected by differentially expressed miRNA, target mRNAs and target proteins: “Regulation of actin cytoskeleton”, “Signalling of hepatocyte growth factor receptor”, “Insulin signalling”, and “MAPK signalling pathway”. The results indicated that silver NPs might induce toxicity by affecting the cytoskeleton, ATP synthesis and apoptosis [58].
Exposure of three human cell lines to two high aspect ratio ENM types, TiO2 nanobelts and multiwalled carbon nanotubes (MWCNT) was investigated by global transcriptome and proteome analyses [132]. Macrophage-like THP-1 cells, small airway epithelial HT29, and intestinal Caco-2 cells exhibited unique patterns of gene and protein expressions, with no differentially expressed genes or proteins overlapping across all three cell types. Exposure of 1 h induced similar expression patterns in response to both TiO2 and MWCNT while being different for each cell type. This apparent general response to insult stood in contrast to the response after 24 h, which was unique to each ENM. In THP-1 cells TiO2 exposure affected regulation of pathways associated with inflammation, apoptosis, cell cycle arrest, DNA replication stress and genomic instability, whereas MWCNTs elicited increased cell proliferation, DNA repair and anti-apoptotic pathways. The authors suggest that the differential regulation of the biological pathways might represent cellular responses to high (TiO2) and low (MWCNT) ENM toxicity, respectively [132].
The mode of action of TiO2 in the dark on Escherichia coli was investigated using transcriptomic and proteomic analysis [121]. Pathway enrichment was observed for the lipid A synthesis pathway, gluconeogenesis, the fatty acid β-oxidation pathway, and importantly for trehalose biosynthesis and several specific membrane transporters indicating osmotic stress. The study revealed that the bactericidal mechanism of TiO2 in the dark comprises depolarization and loss of membrane integrity, resulting in cellular ion imbalance and depletion of the intracellular ATP content. At the molecular level it manifests as an osmotic stress response [121].
PVP-coated CeO2 NPs were investigated in the alga Chlamydomonas reinhardtii [127]. While growth was unaffected, metabolomic and transcriptomic analysis revealed down-regulation of photosynthesis associated pathways at high concentrations. This response was ENM-specific as neither CeNO3 nor PVP showed such an effect [127].
Overall, system toxicology attempts to combine all available data to reveal AOPs. AOPs are defined as a sequence of key events starting with a molecular initiating point and culminating in an adverse outcome of interest to risk assessment [6]. This provides a framework, which is different from the toxicant and species-specific mode of action concept. An AOP knowledge base (https://aopkb.org/) is made available by the OECD together with the US-EPA, the European commission, and the ERDC. The platform provides public access to a peer-reviewed wiki-based tool to develop AOPs (https://aopkb.org/aopwiki/). The OECD has also developed a handbook to guide in the development of AOPs (https://aopkb.org/common/AOP_Handbook.pdf). Not every technique is used for every toxicant, and it is believed that the wealth of data provided by “omics” technology allows for some extrapolation. However, the massive amounts of data also pose major challenges. Many of the techniques are in early development which means that data generation has the potential to still increase in large part because costs are decreasing.
Genomics, transcriptomics, proteomics, and metabolomics are involved in different ways in the definition of the phenotype. While the genome is rather static, epigenetics is a recent research field that involves regulation by DNA modifications as well as post-translational protein modification that has yet to acquire AOP relevant information. The transcriptome is much more dynamic and largely responsible for the regulation [74]. Proteomics and metabolomics have an even higher variability and therefore more directly participate in an observed change in phenotype. More immediate responses and rapid regulation of signalling pathways are for example mediated by post-translational modifications such as phosphorylation [95]. In addition, there are numerous examples for the regulatory influence of endogenous metabolites [2, 154]. The integration of data from the different “omics” techniques still represents a challenge as the techniques based on the measurement principles and molecular classes have different scales in terms of abundance, data accuracy and variance [5]. Methods and tools that manage, integrate, and process data are being developed [151]. Software tools are being developed that store and manage data and also provide details about the experimental setup, such as EMMA [27], and MIMAS [43]. A standard based on minimum information about microarray experiments (MIAME) [16], MAGE-TAB [113], has been adopted by public databases such as ArrayExpress (http://www.ebi.ac.uk/arrayexpress/) and Gene Expression Omnibus (http://www.ncbi.nlm.nih.gov/geo/). Similarly, for proteomics minimum information about proteomics experiments (MIAPE) has been developed as a standard [126]. Ideally, data would also be standardised so that exchange between platforms and techniques is facilitated. To this end, scoring methods are available that allow the direct combination of data, e.g. from proteomics and metabolomics [93]. With the development of genome-wide visualization and modelling platforms such as Cytoscape the situation has improved, and commercial vendors now provide the built-in inspection and analysis of data from different omics techniques, e.g. the Ingenuity Pathway Analysis (IPA) software [41]. The IPA software also provides analysis with respect to known molecular toxicological reactions [41]. Analysis starts with the identification of significantly modified individual genes, proteins or metabolites, and enrichments in certain pathways leads to the identification of affected signal transduction or other biosynthetic and metabolic pathways. The biologically relevant integration of many different marker molecules of multiple “omics” techniques in this higher level analysis makes it less susceptible to fluctuations in individual genes / proteins / metabolites. An increasing number of transcriptomics (e.g. [1, 3, 107], proteomics [70] and metabolomics studies [119] have been performed. Key to successful classification/grouping strategies is the identification of adverse outcome pathways, which needs to be as detailed and accurate as possible by integrating various omics data. These can then add to the definition of AOPs [146]. Definition of AOPs for ENMs is seen as an important step towards the classification of their effects and grouping of ENMs.
Different algorithms are required for the bioinformatics data analysis of signalling pathways [24, 139]. The various algorithms allow for different perspectives on the data for their evaluation. Statistical analyses are highly susceptible to the quality of the underlying data and this still presents a challenge for increasing the reliability of the conclusions reached [104]. Data integration over the different platforms still represents a formidable challenge [46]. The more so as for ENMs additionally physico-chemical parameters must be brought together with classical toxicity data, transcriptome, proteome, metabolome and possibly heterogeneous data from other sources (publications, other projects) of which the structure varies widely. Tools for data integration are being developed, but even more work is needed for heterogeneous data sets [151]. Once a data matrix is created, the data can be examined for correlations by means of principal component analysis (principal component analysis, PCA), hierarchical cluster analysis (Hierarchical Cluster Analysis, HCA) and other statistical analysis methods such as partial-least-squares (PLS), and orthogonal projection to latent structures discriminant analysis (OPLS-DA), or Random Forest.
For instance, it was possible to correlate oxidative stress to the conduction band energy levels of metal oxide NPs in a large data set of physico-chemical conditions and in vitro experiments [157]. Twenty four metal oxide NPs were investigated by different in vitro cytotoxicity assays not addressing specific mechanisms, and in addition using an automated multi-parametric HTS assay. Changes in ROS production (DCF and MitoSox red fluorescence), intracellular calcium flux (Fluo-4 fluorescence), mitochondrial membrane potential (JC-1 fluorescence), and surface membrane permeability (PI uptake) were quantitatively assesses in two cell lines cells. A selection of materials was also tested for acute pro-inflammatory effects by oropharyngeally instillation in mice.
Induction of ROS production and pro-inflammatory effects were strongly correlated to overlap conduction band energy levels with the cellular redox potential. Both cellular assays exhibited good correlation with the generation of acute neutrophilic inflammation and cytokine responses in vivo. This analysis is based primarily on the use of high-throughput methods and the interpretation of the resulting large amounts of data [87].
Another type of data can be obtained from reporter gene library expression data and select panel quantitative PCR. A comparative study investigated the genotoxic effects of anatase TiO2, carbon black, single wall carbon nanotube (SWCNT) and fullerene in Escherichia coli, Saccharomyces cerevisiae, and human A549 cells [71]. Through integration of data from the different assays, it was demonstrated that anatase TiO2 and carbon black induce oxidative stress which contributes to DNA damage in eukaryotic cells [71]. On the other hand, single wall carbon nanotube (SWCNT) and fullerene appear to induce DNA double strand breaks in a different way [71]. Gene expression profiles also indicate different types of DNA repair mechanisms involved for the different materials [71].
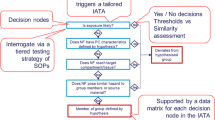
Supervised machine learning can also be used, as demonstrated by a decision tree developed on the toxicity of cobalt ferrite NPs [57]. In addition to the grouping based on the aforementioned band gap of metal oxide NPs, the size of the particle surface has been associated with oxidative stress responses to ENM [115]. Due to the huge variety of possible nano-objects it may be necessary to additionally perform an expert-assisted weight of evidence analysis in most cases [78, 160].
Direct interpretation of results obtained from in vitro studies in the context of potential in vivo exposures is not possible in most cases. To date, most in vitro models do not yield information on pharmacokinetics, i.e. the processes regarding absorption, distribution, metabolism and excretion. However, these processes govern the exposure of the target tissue in the intact organism, making it a crucial difference between the situation in vitro and in vivo. Moreover, this issue is not limited to the in vivo-in vitro comparison, many differences in toxicity from test animals to humans originate in differences in pharmacokinetics [59]. For this reason, data on the mechanisms of action as well as data on pharmacokinetic behaviour are required for a comprehensive prediction of the biological activity of compounds [8, 9].
Quantitative in vitro to in vivo extrapolation (QIVIVE) models the environmental exposures to a chemical that could produce target tissue exposures in humans equivalent to those associated with effects in an in vitro toxicity test. Typically, in vitro toxicity tests yield an EC50, a Benchmark concentration, or an interaction threshold identified by a biologically based dose-response model for the toxicity pathway of concern that can be used in such calculations. Cellular assays can reveal specific molecular and cellular perturbations, and can be used to characterize dose-dependent transitions that may result in organ/system insult. Using these data together with in vitro and in silico approaches including quantitative structure activity relationship (QSAR) modelling, physiologically based pharmacokinetic (PBPK) modelling, and information on metabolism, transport, binding, and other model parameters from cell- and/or cell derived material-based assays, QIVIVE can provide an estimate of the likelihood of harmful effects in vivo from expected environmental exposures. Blaauboer et al. recommended a scheme for the incorporation of in vitro assay data, QSAR and QSPR information, in vitro metabolism data, and pharmacokinetic modelling in the estimation of human toxicity [10]. In this scheme, a chemical-specific pharmacokinetic model is parameterised using the available in vitro data on the absorption, tissue distribution, metabolism, and excretion of a chemical. While this scheme holds true also for ENMs, much less data are available and novel parameters concerning physico-chemical properties have to be taken into account. For chemicals, currently available quantitative structure-property relationship (QSPR) techniques can be used in many cases to estimate chemical properties and kinetics when the specific data for that chemical are lacking. For example, tissue partitioning of a chemical can be estimated using simple empirical correlations from its water solubility, vapour pressure, and octanol/water partitioning co-efficient [25, 100, 118]. QSPR techniques are currently being developed for ENMs, and require the input of systematic and high quality data [20, 81, 133]. The complexity of the possible changes to ENMs in the body, such as (partial) solubility, protein corona formation and evolution, and aggregation has to be reflected in a pharmacokinetic model. Pharmacokinetic models are not only useful in estimating expected equivalent doses associated with toxicity by in vivo exposure from concentrations at which toxicity is observed in an in vitro toxicity assay. Modelling of the in vitro toxicity assay can also provide important information on the temporal profile of cellular exposure to free chemical that can be used in the design of the most appropriate in vitro experimental protocol [129].
Estimation of the metabolic clearance is arguably the greatest challenge in parameterizing even the simplest pharmacokinetic model. Currently, the most extensive data in this respect are on drug pharmacokinetics. ENMs pose an extra challenge in that they are often composed of more than one material, might release chemicals depending on the different compartments, or even dissolve and reform in the body [103]. For soluble chemicals, e.g. released by an ENM, it would be necessary to perform in vitro assays of the dose-response (capacity and affinity) for metabolic clearance [79]. A qualitative classification system has been developed based on physico-chemical properties to predict whether a chemical was likely to be cleared by metabolism (including the CYP isozyme involved) or by urinary excretion [68]. As data accumulates for a greater number of chemicals across a wider range of chemical classes, it may be possible to predict both qualitative and quantitative clearance using QSAR approaches over a broader domain of applicability.
12 Conclusion
Systems biology is increasingly used in toxicology as we currently observe a paradigm change and there is increasing interest in understanding underlying toxicity mechanisms and defining AOPs. It is advisable to combine “omics” technology with classical toxicological endpoints. If possible, different “omics” techniques should be used to assess the full complexity of changes and also to derive more reliable information on affected pathways. However, data integration over the different platforms still represents a formidable challenge as the techniques are based on different measurement principles and different molecular classes have different scales in terms of abundance, data accuracy and variance. The more so this holds true for ENMs, where additional factors account for an even larger variability. Currently, knowledge is only beginning to emerge how different physico-chemical parameters truly affect toxicity and which influence batch-to-batch variations play. Thus, the material characterization and the sample preparation (e.g. preparation of ENM dispersions or also mode of ENM presentation to the cells) deserve much more attention when assessing ENMs.
Additionally one should take into account different possible uptake routes for ENMs (ingestion, dermal, inhalation or injection). Another important issue is the choice of the cell model for in vitro studies or the strain & species for animal studies. Large differences in responses may be expected in different cell lines as well as in different strains of a given species.
Ultimately only the combination of “omics” technologies with high power statistical integrative data interpretation methodologies will unravel important and relevant information with respect to toxicity. In part, concepts already exist how omics data can be used for risk assessment, e.g. for quantitative assessment of the metabolome. Thus systems biology is getting more and more established. It may be expected that current limitations, e.g. in data integration and data analysis, might be overcome soon. Systems biology, by providing very large data sets offers the unique advantage of getting information on underlying molecular mechanisms and identifying affected signalling pathways, often referred to as toxicity pathways. This in turn may allow the development of AOPs. For ENMs such mechanistic based knowledge is highly needed in order to develop grouping approaches. It is well accepted that traditional risk assessment paradigms, e.g. assessing each ENM variant in a case-by-case basis, will not be sufficient to deal with the large amount of ENMs in a reasonable time frame. Systems biology can support the development of grouping approaches. However, prerequisite is the development of better standardized approaches starting for instance with the definition of benchmark materials which allow for comparison between different studies. The largest bottleneck is that currently most studies assess only one ENM at a time or a very limited number of ENMs only. This renders it very difficult to compare outcomes of different studies.
However, intensive research efforts are ongoing. Many large currently funded European projects focus on the use of systems biology for a larger set of ENMs. First possible grouping approaches for ENMs are already discussed in scientific literature. By integrating omics based data one may expect a huge progress.
References
Adam N, Vergauwen L, Blust R, Knapen D (2015) Gene transcription patterns and energy reserves in Daphnia magna show no nanoparticle specific toxicity when exposed to ZnO and CuO nanoparticles. Environ Res 138C:82–92
Alisi A, Leoni S, Piacentani A, Conti Devirgiliis L (2003) Retinoic acid modulates the cell-cycle in fetal rat hepatocytes and HepG2 cells by regulating cyclin-cdk activities. Liver Int Off J Int Assoc Study Liver 23:179–186
Bajak E, Fabbri M, Ponti J, Gioria S, Ojea-Jimenez I, Collotta A, Mariani V, Gilliland D, Rossi F, Gribaldo L (2015) Changes in Caco-2 cells transcriptome profiles upon exposure to gold nanoparticles. Toxicol Lett 233:187–199
Bandara LR, Kennedy S (2002) Toxicoproteomics – a new preclinical tool. Drug Discov Today 7:411–418
Baumann S, Kalkhof S, Hackermuller J, Otto W, Tomm JM, Wissenbach DK, RK U, von Bergen M (2013) Requirements and perspectives for integrating metabolomics with other omics data. Curr Metabolomics 1:15–27
Becker RA, Ankley GT, Edwards SW, Kennedy SW, Linkov I, Meek B, Sachana M, Segner H, Van Der Burg B, Villeneuve DL, Watanabe H, Barton-Maclaren TS (2015) Increasing Scientific Confidence in Adverse Outcome Pathways: Application of Tailored Bradford-Hill Considerations for Evaluating Weight of Evidence. Regul Toxicol Pharmacol 72:514–537
Bhatia SN, Ingber DE (2014) Microfluidic organs-on-chips. Nat Biotechnol 32:760–772
Blaauboer BJ (2002) The necessity of biokinetic information in the interpretation of in vitro toxicity data. Altern Lab Anim ATLA 30(Suppl 2):85–91
Blaauboer BJ (2003) The integration of data on physico-chemical properties, in vitro-derived toxicity data and physiologically based kinetic and dynamic as modelling a tool in hazard and risk assessment. A commentary. Toxicol Lett 138:161–171
Blaauboer BJ, Clewell HJ, Clothier R, Crespi C, Gerson B, Hawksworth G, Kedderis GL, Rozman K, Willhite C (2001) In vitro methods for assessing acute toxicity: biokinetic determinations. In: Report of the International Workshop on in vitro methods for assessing acute systemic toxicity: results of an International Workshop Organized by ICCVAM and NICEATM (NIH Publication 01–4499). NIEHS, Research Triangle Park. pp 47–60
Blazer-Yost BL, Banga A, Amos A, Chernoff E, Lai X, Li C, Mitra S, Witzmann FA (2011) Effect of carbon nanoparticles on renal epithelial cell structure, barrier function, and protein expression. Nanotoxicology 5:354–371
Bo Y, Jin C, Liu Y, Yu W, Kang H (2014) Metabolomic analysis on the toxicological effects of TiO(2) nanoparticles in mouse fibroblast cells: from the perspective of perturbations in amino acid metabolism. Toxicol Mech Methods 24:461–469
Bouhifd M, Hartung T, Hogberg HT, Kleensang A, Zhao L (2013) Review: toxicometabolomics. J Appl Toxicol JAT 33:1365–1383
Bourdon JA, Halappanavar S, Saber AT, Jacobsen NR, Williams A, Wallin H, Vogel U, Yauk CL (2012) Hepatic and pulmonary toxicogenomic profiles in mice intratracheally instilled with carbon black nanoparticles reveal pulmonary inflammation, acute phase response, and alterations in lipid homeostasis. Toxicol Sci Off J Soc Toxicol 127:474–484
Bourdon JA, Williams A, Kuo B, Moffat I, White PA, Halappanavar S, Vogel U, Wallin H, Yauk CL (2013) Gene expression profiling to identify potentially relevant disease outcomes and support human health risk assessment for carbon black nanoparticle exposure. Toxicology 303:83–93
Brazma A, Hingamp P, Quackenbush J, Sherlock G, Spellman P, Stoeckert C, Aach J, Ansorge W, Ball CA, Causton HC, Gaasterland T, Glenisson P, Holstege FC, Kim IF, Markowitz V, Matese JC, Parkinson H, Robinson A, Sarkans U, Schulze-Kremer S, Stewart J, Taylor R, Vilo J, Vingron M (2001) Minimum information about a microarray experiment (MIAME)-toward standards for microarray data. Nat Genet 29:365–371
Brugger B (2014) Lipidomics: analysis of the lipid composition of cells and subcellular organelles by electrospray ionization mass spectrometry. Annu Rev Biochem 83:79–98
Bu Q, Yan G, Deng P, Peng F, Lin H, Xu Y, Cao Z, Zhou T, Xue A, Wang Y, Cen X, Zhao YL (2010) NMR-based metabonomic study of the sub-acute toxicity of titanium dioxide nanoparticles in rats after oral administration. Nanotechnology 21:125105
Buesen R, Landsiedel R, Sauer UG, Wohlleben W, Groeters S, Strauss V, Kamp H, van Ravenzwaay B (2014) Effects of SiO(2), ZrO(2), and BaSO(4) nanomaterials with or without surface functionalization upon 28-day oral exposure to rats. Arch Toxicol 88:1881–1906
Carbo-Dorca R, Besalu E (2011) Construction of coherent nano quantitative structure-properties relationships (nano-QSPR) models and catastrophe theory. SAR QSAR Environ Res 22:661–665
Cheng LC, Jiang X, Wang J, Chen C, Liu RS (2013) Nano-bio effects: interaction of nanomaterials with cells. Nanoscale 5:3547–3569
Cunningham MJ, Shah M, Lema C, Magnuson SR, Falduto MT, Balzano L, Resasco DE (2007) An OMICs approach for assessing the safety of single-walled carbon nanotubes in human skin and lung cells. In: 2007 NSTI nanotechnology conference and trade show – NSTI Nanotech 2007, Technical Proceedings, vol 2. pp 651–654
Dailey LA, Hernandez-Prieto R, Casas-Ferreira AM, Jones MC, Riffo-Vasquez Y, Rodriguez-Gonzalo E, Spina D, Jones SA, Smith NW, Forbes B, Page C, Legido-Quigley C (2014) Adenosine monophosphate is elevated in the bronchoalveolar lavage fluid of mice with acute respiratory toxicity induced by nanoparticles with high surface hydrophobicity. Nanotoxicology 9:106–115
de Ridder D, de Ridder J, Reinders MJ (2013) Pattern recognition in bioinformatics. Brief Bioinform 14:633–647
DeJongh J, Verhaar HJ, Hermens JL (1997) A quantitative property-property relationship (QPPR) approach to estimate in vitro tissue-blood partition coefficients of organic chemicals in rats and humans. Arch Toxicol 72:17–25
Dhawan A, Sharma V (2010) Toxicity assessment of nanomaterials: methods and challenges. Anal Bioanal Chem 398:589–605
Dondrup M, Albaum SP, Griebel T, Henckel K, Junemann S, Kahlke T, Kleindt CK, Kuster H, Linke B, Mertens D, Mittard-Runte V, Neuweger H, Runte KJ, Tauch A, Tille F, Puhler A, Goesmann A (2009) EMMA 2 – a MAGE-compliant system for the collaborative analysis and integration of microarray data. BMC Bioinformatics 10:50
Dong MS, Choi JY, Sung JH, Kim JS, Song KS, Ryu HR, Lee JH, Bang IS, An K, Park HM, Song NW, Yu IJ (2013) Gene expression profiling of kidneys from Sprague-Dawley rats following 12-week inhalation exposure to silver nanoparticles. Toxicol Mech Methods 23:437–448
Dowling P, Hughes DJ, Larkin AM, Meiller J, Henry M, Meleady P, Lynch V, Pardini B, Naccarati A, Levy M, Vodicka P, Neary P, Clynes M (2015) Elevated levels of 14-3-3 proteins, serotonin, gamma enolase and pyruvate kinase identified in clinical samples from patients diagnosed with colorectal cancer. Clin Chim Acta 441:133–141
Driessen MD, Mues S, Vennemann A, Hellack B, Bannuscher A, Vimalakanthan V, Riebeling C, Ossig R, Wiemann M, Schnekenburger J, Kuhlbusch TA, Renard B, Luch A, Haase A (2015) Proteomic analysis of protein carbonylation: a useful tool to unravel nanoparticle toxicity mechanisms. Part Fibre Toxicol 12:36
Duranton F, Lundin U, Gayrard N, Mischak H, Aparicio M, Mourad G, Daures JP, Weinberger KM, Argiles A (2014) Plasma and urinary amino acid metabolomic profiling in patients with different levels of kidney function. Clin J Am Soc Nephrol 9:37–45
Edelmann MJ, Shack LA, Naske CD, Walters KB, Nanduri B (2014) SILAC-based quantitative proteomic analysis of human lung cell response to copper oxide nanoparticles. PLoS One 9:e114390
Feliu N, Kohonen P, Ji J, Zhang Y, Karlsson HL, Palmberg L, Nystrom A, Fadeel B (2015) Next-generation sequencing reveals low-dose effects of cationic dendrimers in primary human bronchial epithelial cells. ACS Nano 9:146–163
Feng J, Liu H, Bhakoo KK, Lu L, Chen Z (2011) A metabonomic analysis of organ specific response to USPIO administration. Biomaterials 32:6558–6569
Feng J, Liu H, Zhang L, Bhakoo K, Lu L (2010) An insight into the metabolic responses of ultra-small superparamagnetic particles of iron oxide using metabonomic analysis of biofluids. Nanotechnology 21:395101
Fisichella M, Berenguer F, Steinmetz G, Auffan M, Rose J, Prat O (2012) Intestinal toxicity evaluation of TiO2 degraded surface-treated nanoparticles: a combined physico-chemical and toxicogenomics approach in caco-2 cells. Part Fibre Toxicol 9:18
Fleischer CC, Payne CK (2014) Secondary structure of corona proteins determines the cell surface receptors used by nanoparticles. J Phys Chem B 118:14017–14026
Fowler SJ, Basanta-Sanchez M, Xu Y, Goodacre R, Dark PM (2015) Surveillance for lower airway pathogens in mechanically ventilated patients by metabolomic analysis of exhaled breath: a case-control study. Thorax 70:320–325
Frohlich E, Meindl C, Wagner K, Leitinger G, Roblegg E (2014) Use of whole genome expression analysis in the toxicity screening of nanoparticles. Toxicol Appl Pharmacol 280:272–284
Gagne F, Andre C, Skirrow R, Gelinas M, Auclair J, van Aggelen G, Turcotte P, Gagnon C (2012) Toxicity of silver nanoparticles to rainbow trout: a toxicogenomic approach. Chemosphere 89:615–622
Ganter B, Zidek N, Hewitt PR, Muller D, Vladimirova A (2008) Pathway analysis tools and toxicogenomics reference databases for risk assessment. Pharmacogenomics 9:35–54
Garcia-Contreras R, Sugimoto M, Umemura N, Kaneko M, Hatakeyama Y, Soga T, Tomita M, Scougall-Vilchis RJ, Contreras-Bulnes R, Nakajima H, Sakagami H (2015) Alteration of metabolomic profiles by titanium dioxide nanoparticles in human gingivitis model. Biomaterials 57:33–40
Gattiker A, Hermida L, Liechti R, Xenarios I, Collin O, Rougemont J, Primig M (2009) MIMAS 3.0 is a Multiomics Information Management and Annotation System. BMC Bioinformatics 10:151
Giskeodegard GF, Bertilsson H, Selnaes KM, Wright AJ, Bathen TF, Viset T, Halgunset J, Angelsen A, Gribbestad IS, Tessem MB (2013) Spermine and citrate as metabolic biomarkers for assessing prostate cancer aggressiveness. PLoS One 8:e62375
Gomes T, Pereira CG, Cardoso C, Bebianno MJ (2013) Differential protein expression in mussels Mytilus galloprovincialis exposed to nano and ionic Ag. Aquat Toxicol 136:79–90
Gomez-Cabrero D, Abugessaisa I, Maier D, Teschendorff A, Merkenschlager M, Gisel A, Ballestar E, Bongcam-Rudloff E, Conesa A, Tegner J (2014) Data integration in the era of omics: current and future challenges. BMC Syst Biol 8(Suppl 2):I1
Goni FM (2014) The basic structure and dynamics of cell membranes: an update of the Singer-Nicolson model. Biochim Biophys Acta 1838:1467–1476
Grosch S, Schiffmann S, Geisslinger G (2012) Chain length-specific properties of ceramides. Prog Lipid Res 51:50–62
Guo L, Panderi I, Yan DD, Szulak K, Li Y, Chen YT, Ma H, Niesen DB, Seeram N, Ahmed A, Yan B, Pantazatos D, Lu W (2013) A comparative study of hollow copper sulfide nanoparticles and hollow gold nanospheres on degradability and toxicity. ACS Nano 7:8780–8793
Hadrup N, Lam HR, Loeschner K, Mortensen A, Larsen EH, Frandsen H (2012) Nanoparticulate silver increases uric acid and allantoin excretion in rats, as identified by metabolomics. J Appl Toxicol JAT 32:929–933
Halappanavar S, Jackson P, Williams A, Jensen KA, Hougaard KS, Vogel U, Yauk CL, Wallin H (2011) Pulmonary response to surface-coated nanotitanium dioxide particles includes induction of acute phase response genes, inflammatory cascades, and changes in microRNAs: a toxicogenomic study. Environ Mol Mutagen 52:425–439
Halappanavar S, Saber AT, Decan N, Jensen KA, Wu D, Jacobsen NR, Guo C, Rogowski J, Koponen IK, Levin M, Madsen AM, Atluri R, Snitka V, Birkedal RK, Rickerby D, Williams A, Wallin H, Yauk CL, Vogel U (2015) Transcriptional profiling identifies physicochemical properties of nanomaterials that are determinants of the in vivo pulmonary response. Environ Mol Mutagen 56:245–264
Haniu H, Matsuda Y, Takeuchi K, Kim YA, Hayashi T, Endo M (2010) Proteomics-based safety evaluation of multi-walled carbon nanotubes. Toxicol Appl Pharmacol 242:256–262
Hilton GM, Taylor AJ, McClure CD, Parsons GN, Bonner JC, Bereman MS (2015) Toxicoproteomic analysis of pulmonary carbon nanotube exposure using LC-MS/MS. Toxicology 329:80–87
Hla T, Dannenberg AJ (2012) Sphingolipid signaling in metabolic disorders. Cell Metab 16:420–434
Holthuis JC, Menon AK (2014) Lipid landscapes and pipelines in membrane homeostasis. Nature 510:48–57
Horev-Azaria L, Baldi G, Beno D, Bonacchi D, Golla-Schindler U, Kirkpatrick JC, Kolle S, Landsiedel R, Maimon O, Marche PN, Ponti J, Romano R, Rossi F, Sommer D, Uboldi C, Unger RE, Villiers C, Korenstein R (2013) Predictive toxicology of cobalt ferrite nanoparticles: comparative in-vitro study of different cellular models using methods of knowledge discovery from data. Part Fibre Toxicol 10:32
Huang Y, Lü X, Ma J (2014) Toxicity of silver nanoparticles to human dermal fibroblasts on MicroRNA level. J Biomed Nanotechnol 10:3304–3317
Irfan A, Cauchi M, Edmands W, Gooderham NJ, Njuguna J, Zhu H (2014) Assessment of temporal dose-toxicity relationship of fumed silica nanoparticle in human lung A549 cells by conventional cytotoxicity and (1)H-NMR-based extracellular metabonomic assays. Toxicol Sci Off J Soc Toxicol 138:354–364
Jackson P, Hougaard KS, Vogel U, Wu D, Casavant L, Williams A, Wade M, Yauk CL, Wallin H, Halappanavar S (2012) Exposure of pregnant mice to carbon black by intratracheal instillation: toxicogenomic effects in dams and offspring. Mutat Res 745:73–83
Jung HR, Sylvanne T, Koistinen KM, Tarasov K, Kauhanen D, Ekroos K (2011) High throughput quantitative molecular lipidomics. Biochim Biophys Acta 1811:925–934
Jungnickel H, Potratz S, Baumann S, Tarnow P, von Bergen M, Luch A (2014) Identification of lipidomic biomarkers for coexposure to subtoxic doses of benzo[a]pyrene and cadmium: the toxicological cascade biomarker approach. Environ Sci Technol 48:10423–10431
Kalkhof S, Dautel F, Loguercio S, Baumann S, Trump S, Jungnickel H, Otto W, Rudzok S, Potratz S, Luch A, Lehmann I, Beyer A, von Bergen M (2015) Pathway and time-resolved benzo[a]pyrene toxicity on Hepa1c1c7 cells at toxic and subtoxic exposure. J Proteome Res 14:164–182
Kim KB, Um SY, Chung MW, Jung SC, Oh JS, Kim SH, Na HS, Lee BM, Choi KH (2010) Toxicometabolomics approach to urinary biomarkers for mercuric chloride (HgCl(2))-induced nephrotoxicity using proton nuclear magnetic resonance ((1)H NMR) in rats. Toxicol Appl Pharmacol 249:114–126
Kinter CS, Lundie JM, Patel H, Rindler PM, Szweda LI, Kinter M (2012) A quantitative proteomic profile of the Nrf2-mediated antioxidant response of macrophages to oxidized LDL determined by multiplexed selected reaction monitoring. PLoS One 7:e50016
Kitchin KT, Grulke E, Robinette BL, Castellon BT (2014) Metabolomic effects in HepG2 cells exposed to four TiO2 and two CeO2 nanomaterials. Environ Sci Nano 1:466–477
Kreyling WG, Fertsch-Gapp S, Schaffler M, Johnston BD, Haberl N, Pfeiffer C, Diendorf J, Schleh C, Hirn S, Semmler-Behnke M, Epple M, Parak WJ (2014) In vitro and in vivo interactions of selected nanoparticles with rodent serum proteins and their consequences in biokinetics. Beilstein J Nanotechnol 5:1699–1711
Kusama M, Toshimoto K, Maeda K, Hirai Y, Imai S, Chiba K, Akiyama Y, Sugiyama Y (2010) In silico classification of major clearance pathways of drugs with their physiochemical parameters. Drug Metab Dispos Biol Fate Chem 38:1362–1370
Kuznetsova GP, Larina OV, Petushkova NA, Kisrieva YS, Samenkova NF, Trifonova OP, Karuzina II, Ipatova OM, Zolotaryov KV, Romashova YA, Lisitsa AV (2014) Effects of fullerene C60 on proteomic profile of Danio rerio fish embryos. Bull Exp Biol Med 156:694–698
Lai ZW, Yan Y, Caruso F, Nice EC (2012) Emerging techniques in proteomics for probing nano-bio interactions. ACS Nano 6:10438–10448
Lan J, Gou N, Gao C, He M, Gu AZ (2014) Comparative and mechanistic genotoxicity assessment of nanomaterials via a quantitative toxicogenomics approach across multiple species. Environ Sci Technol 48:12937–12945
Lange V, Picotti P, Domon B, Aebersold R (2008) Selected reaction monitoring for quantitative proteomics: a tutorial. Mol Syst Biol 4:222
Li J, Zhao Z, Feng J, Gao J, Chen Z (2013) Understanding the metabolic fate and assessing the biosafety of MnO nanoparticles by metabonomic analysis. Nanotechnology 24:455102
Li JJ, Biggin MD (2015) Gene expression. Statistics requantitates the central dogma. Science 347:1066–1067
Liao M, Liu H (2012) Gene expression profiling of nephrotoxicity from copper nanoparticles in rats after repeated oral administration. Environ Toxicol Pharmacol 34:67–80
Lin BC, Zhang HS, Lin ZQ, Fang YJ, Tian L, Yang HL, Yan J, Liu HL, Zhang W, Xi ZG (2013) Studies of single-walled carbon nanotubes-induced hepatotoxicity by NMR-based metabonomics of rat blood plasma and liver extracts. Nanoscale Res Lett 8:236
Lin ZQ, Ma L, X ZG, Zhang, HS, Lin BC (2013) A comparative study of lung toxicity in rats induced by three types of nanomaterials. Nanoscale Res Lett 8:521.
Linkov I, Massey O, Keisler J, Rusyn I, Hartung T (2015) From “weight of evidence” to quantitative data integration using multicriteria decision analysis and Bayesian methods. ALTEX 32:3–8
Lipscomb JC, Meek ME, Krishnan K, Kedderis GL, Clewell H, Haber L (2004) Incorporation of pharmacokinetic and pharmacodynamic data into risk assessments. Toxicol Mech Methods 14:145–158
Lu X, Tian Y, Zhao Q, Jin T, Xiao S, Fan X (2011) Integrated metabonomics analysis of the size-response relationship of silica nanoparticles-induced toxicity in mice. Nanotechnology 22:055101
Lubinski L, Urbaszek P, Gajewicz A, Cronin MT, Enoch SJ, Madden JC, Leszczynska D, Leszczynski J, Puzyn T (2013) Evaluation criteria for the quality of published experimental data on nanomaterials and their usefulness for QSAR modelling. SAR QSAR Environ Res 24:995–1008
Malone JH, Oliver B (2011) Microarrays, deep sequencing and the true measure of the transcriptome. BMC Biol 9:34
Mandell JW (2003) Phosphorylation state-specific antibodies: applications in investigative and diagnostic pathology. Am J Pathol 163:1687–1698
Miethling-Graff R, Rumpker R, Richter M, Verano-Braga T, Kjeldsen F, Brewer J, Hoyland J, Rubahn HG, Erdmann H (2014) Exposure to silver nanoparticles induces size- and dose-dependent oxidative stress and cytotoxicity in human colon carcinoma cells. Toxicol In Vitro Int J Publ Assoc BIBRA 28:1280–1289
Mikut R, Dickmeis T, Driever W, Geurts P, Hamprecht FA, Kausler BX, Ledesma-Carbayo MJ, Maree R, Mikula K, Pantazis P, Ronneberger O, Santos A, Stotzka R, Strahle U, Peyrieras N (2013) Automated processing of zebrafish imaging data: a survey. Zebrafish 10:401–421
Nazari P, Dowlatabadi-Bazaz R, Mofid MR, Pourmand MR, Daryani NE, Faramarzi MA, Sepehrizadeh Z, Shahverdi AR (2014) The antimicrobial effects and metabolomic footprinting of carboxyl-capped bismuth nanoparticles against Helicobacter pylori. Appl Biochem Biotechnol 172:570–579
Nel AE (2013) Implementation of alternative test strategies for the safety assessment of engineered nanomaterials. J Intern Med 274:561–577
Newman RH, Zhang J, Zhu H (2014) Toward a systems-level view of dynamic phosphorylation networks. Front Genet 5:263
Ng CT, Yung LY, Swa HL, Poh RW, Gunaratne J, Bay BH (2015) Altered protein expression profile associated with phenotypic changes in lung fibroblasts co-cultured with gold nanoparticle-treated small airway epithelial cells. Biomaterials 39:31–38
NRC (2007) Applications of toxicogenomic technologies to predictive toxicology and risk assessment. Washington, DC
Nuwaysir EF, Bittner M, Trent J, Barrett JC, Afshari CA (1999) Microarrays and toxicology: the advent of toxicogenomics. Mol Carcinog 24:153–159
O’Brien PJ (2014) High-content analysis in toxicology: screening substances for human toxicity potential, elucidating subcellular mechanisms and in vivo use as translational safety biomarkers. Basic Clin Pharmacol Toxicol 115:4–17
Oberbach A, Bluher M, Wirth H, Till H, Kovacs P, Kullnick Y, Schlichting N, Tomm JM, Rolle-Kampczyk U, Murugaiyan J, Binder H, Dietrich A, von Bergen M (2011) Combined proteomic and metabolomic profiling of serum reveals association of the complement system with obesity and identifies novel markers of body fat mass changes. J Proteome Res 10:4769–4788
OECD (2013) Guidance document on developing and assessing adverse outcome pathways. In: Series on testing and assessment. Environment Directorate of the OECD, Paris
Olsen JV, Blagoev B, Gnad F, Macek B, Kumar C, Mortensen P, Mann M (2006) Global, in vivo, and site-specific phosphorylation dynamics in signaling networks. Cell 127:635–648
Overmyer KA, Thonusin C, Qi NR, Burant CF, Evans CR (2015) Impact of anesthesia and euthanasia on metabolomics of mammalian tissues: studies in a C57BL/6 J mouse model. PLoS One 10:e0117232
Ozsolak F, Milos PM (2011) RNA sequencing: advances, challenges and opportunities. Nat Rev Genet 12:87–98
Palomaki J, Sund J, Vippola M, Kinaret P, Greco D, Savolainen K, Puustinen A, Alenius H (2015) A secretomics analysis reveals major differences in the macrophage responses towards different types of carbon nanotubes. Nanotoxicology 9:719–728
Parveen A, Rizvi SH, Gupta A, Singh R, Ahmad I, Mahdi F, Mahdi AA (2012) NMR-based metabonomics study of sub-acute hepatotoxicity induced by silica nanoparticles in rats after intranasal exposure. Cell Mol Biol 58:196–203
Paterson S, Mackay D (1989) Correlation of tissue, blood, and air partition coefficients of volatile organic chemicals. Br J Ind Med 46:321–328
Patlewicz G, Simon TW, Rowlands JC, Budinsky RA, Becker RA (2015) Proposing a scientific confidence framework to help support the application of adverse outcome pathways for regulatory purposes. Regul Toxicol Pharmacol 71:463–477
Peirce V, Carobbio S, Vidal-Puig A (2014) The different shades of fat. Nature 510:76–83
Peters R, Kramer E, Oomen AG, Rivera ZE, Oegema G, Tromp PC, Fokkink R, Rietveld A, Marvin HJ, Weigel S, Peijnenburg AA, Bouwmeester H (2012) Presence of nano-sized silica during in vitro digestion of foods containing silica as a food additive. ACS Nano 6:2441–2451
Pettit S, des Etages SA, Mylecraine L, Snyder R, Fostel J, Dunn RT 2nd, Haymes K, Duval M, Stevens J, Afshari C, Vickers A (2010) Current and future applications of toxicogenomics: results summary of a survey from the HESI Genomics State of Science Subcommittee. Environ Health Perspect 118:992–997
Plant NJ (2015) An introduction to systems toxicology. Toxicol Res Uk 4:9–22
Platt FM (2014) Sphingolipid lysosomal storage disorders. Nature 510:68–75
Poulsen SS, Saber AT, Williams A, Andersen O, Kobler C, Atluri R, Pozzebon ME, Mucelli SP, Simion M, Rickerby D, Mortensen A, Jackson P, Kyjovska ZO, Molhave K, Jacobsen NR, Jensen KA, Yauk CL, Wallin H, Halappanavar S, Vogel U (2014) MWCNTs of different physicochemical properties cause similar inflammatory responses, but differences in transcriptional and histological markers of fibrosis in mouse lungs. Toxicol Appl Pharmacol 284:16–32
Poynton HC, Lazorchak JM, Impellitteri CA, Blalock BJ, Rogers K, Allen HJ, Loguinov A, Heckman JL, Govindasmawy S (2012) Toxicogenomic responses of nanotoxicity in Daphnia magna exposed to silver nitrate and coated silver nanoparticles. Environ Sci Technol 46:6288–6296
Rabilloud T, Lescuyer P (2015) Proteomics in mechanistic toxicology: History, concepts, achievements, caveats, and potential. Proteomics 15:1051–1074
Rainville LC, Carolan D, Varela AC, Doyle H, Sheehan D (2014) Proteomic evaluation of citrate-coated silver nanoparticles toxicity in Daphnia magna. Analyst 139:1678–1686
Rappaport SM, Li H, Grigoryan H, Funk WE, Williams ER (2012) Adductomics: characterizing exposures to reactive electrophiles. Toxicol Lett 213:83–90
Ratnasekhar C, Sonane M, Satish A, Mudiam MK (2015) Metabolomics reveals the perturbations in the metabolome of Caenorhabditis elegans exposed to titanium dioxide nanoparticles. Nanotoxicology 9:1–11
Rayner TF, Rocca-Serra P, Spellman PT, Causton HC, Farne A, Holloway E, Irizarry RA, Liu J, Maier DS, Miller M, Petersen K, Quackenbush J, Sherlock G, Stoeckert CJ Jr, White J, Whetzel PL, Wymore F, Parkinson H, Sarkans U, Ball CA, Brazma A (2006) A simple spreadsheet-based, MIAME-supportive format for microarray data: MAGE-TAB. BMC Bioinformatics 7:489
Rocheleau S, Arbour M, Elias M, Sunahara GI, Masson L (2014) Toxicogenomic effects of nano- and bulk-TiO particles in the soil nematode Caenorhabditis elegans. Nanotoxicology 9(4):502–512
Rushton EK, Jiang J, Leonard SS, Eberly S, Castranova V, Biswas P, Elder A, Han X, Gelein R, Finkelstein J, Oberdorster G (2010) Concept of assessing nanoparticle hazards considering nanoparticle dosemetric and chemical/biological response metrics. J Toxicol Environ Health Part A 73:445–461
Russell WMS, Burch RL (1959) The principles of humane experimental technique. Methuen, London, 238 pp
Sarkar A, Ghosh M, Sil PC (2014) Nanotoxicity: oxidative stress mediated toxicity of metal and metal oxide nanoparticles. J Nanosci Nanotechnol 14:730–743
Schmitt W (2008) General approach for the calculation of tissue to plasma partition coefficients. Toxicol In Vitro Int J Publ Assoc BIBRA 22:457–467
Schnackenberg LK, Sun J, Beger RD (2012) Metabolomics techniques in nanotoxicology studies. Methods Mol Biol 926:141–156
Shim W, Paik MJ, Nguyen DT, Lee JK, Lee Y, Kim JH, Shin EH, Kang JS, Jung HS, Choi S, Park S, Shim JS, Lee G (2012) Analysis of changes in gene expression and metabolic profiles induced by silica-coated magnetic nanoparticles. ACS Nano 6:7665–7680
Sohm B, Immel F, Bauda P, Pagnout C (2015) Insight into the primary mode of action of TiO2 nanoparticles on Escherichia coli in the dark. Proteomics 15:98–113
Sturla SJ, Boobis AR, FitzGerald RE, Hoeng J, Kavlock RJ, Schirmer K, Whelan M, Wilks MF, Peitsch MC (2014) Systems toxicology: from basic research to risk assessment. Chem Res Toxicol 27:314–329
Suhre K, Meisinger C, Doring A, Altmaier E, Belcredi P, Gieger C, Chang D, Milburn MV, Gall WE, Weinberger KM, Mewes HW, Hrabe de Angelis M, Wichmann HE, Kronenberg F, Adamski J, Illig T (2010) Metabolic footprint of diabetes: a multiplatform metabolomics study in an epidemiological setting. PLoS One 5:e13953
Tang M, Zhang T, Xue Y, Wang S, Huang M, Yang Y, Lu M, Lei H, Kong L, Wang Y, Pu Y (2011) Metabonomic studies of biochemical changes in the serum of rats by intratracheally instilled TiO2 nanoparticles. J Nanosci Nanotechnol 11:3065–3074
Taskinen MR, Boren J (2015) New insights into the pathophysiology of dyslipidemia in type 2 diabetes. Atherosclerosis 239:483–495
Taylor CF, Paton NW, Lilley KS, Binz PA, Julian RK Jr, Jones AR, Zhu W, Apweiler R, Aebersold R, Deutsch EW, Dunn MJ, Heck AJ, Leitner A, Macht M, Mann M, Martens L, Neubert TA, Patterson SD, Ping P, Seymour SL, Souda P, Tsugita A, Vandekerckhove J, Vondriska TM, Whitelegge JP, Wilkins MR, Xenarios I, Yates JR 3rd, Hermjakob H (2007) The minimum information about a proteomics experiment (MIAPE). Nat Biotechnol 25:887–893
Taylor NS, Merrifield R, Williams TD, Chipman JK, Lead JR, Viant MR (2015) Molecular toxicity of cerium oxide nanoparticles to the freshwater alga Chlamydomonas reinhardtii is associated with supra-environmental exposure concentrations. Nanotoxicology 1–10. doi: 10.3109/17435390.2014.948941
Tedesco S, Bayat N, Danielsson G, Buque X, Aspichueta P, Fresnedo O, Cristobal S (2015) Proteomic and lipidomic analysis of primary mouse hepatocytes exposed to metal and metal oxide nanoparticles. JIOMICS 5:44–57
Teeguarden JG, Barton HA (2004) Computational modeling of serum-binding proteins and clearance in extrapolations across life stages and species for endocrine active compounds. Risk Anal Off Publ Soc Risk Anal 24:751–770
Teeguarden JG, Webb-Robertson BJ, Waters KM, Murray AR, Kisin ER, Varnum SM, Jacobs JM, Pounds JG, Zanger RC, Shvedova AA (2011) Comparative proteomics and pulmonary toxicity of instilled single-walled carbon nanotubes, crocidolite asbestos, and ultrafine carbon black in mice. Toxicol Sci Off J Soc Toxicol 120:123–135
Thingholm TE, Jensen ON, Larsen MR (2009) Analytical strategies for phosphoproteomics. Proteomics 9:1451–1468
Tilton SC, Karin NJ, Tolic A, Xie Y, Lai X, Hamilton RF Jr, Waters KM, Holian A, Witzmann FA, Orr G (2014) Three human cell types respond to multi-walled carbon nanotubes and titanium dioxide nanobelts with cell-specific transcriptomic and proteomic expression patterns. Nanotoxicology 8:533–548
Toropova AP, Toropov AA, Benfenati E, Korenstein R, Leszczynska D, Leszczynski J (2015) Optimal nano-descriptors as translators of eclectic data into prediction of the cell membrane damage by means of nano metal-oxides. Environ Sci Pollut Res Int 22:745–757
Tralau T, Riebeling C, Pirow R, Oelgeschlager M, Seiler A, Liebsch M, Luch A (2012) Wind of change challenges toxicological regulators. Environ Health Perspect 120:1489–1494
Treuel L, Jiang X, Nienhaus GU (2013) New views on cellular uptake and trafficking of manufactured nanoparticles. J R Soc Interface R Soc 10:20120939
Tsyusko OV, Unrine JM, Spurgeon D, Blalock E, Starnes D, Tseng M, Joice G, Bertsch PM (2012) Toxicogenomic responses of the model organism Caenorhabditis elegans to gold nanoparticles. Environ Sci Technol 46:4115–4124
Tucci P, Porta G, Agostini M, Dinsdale D, Iavicoli I, Cain K, Finazzi-Agro A, Melino G, Willis A (2013) Metabolic effects of TiO2 nanoparticles, a common component of sunscreens and cosmetics, on human keratinocytes. Cell Death Dis 4:e549
Tyurina YY, Kisin ER, Murray A, Tyurin VA, Kapralova VI, Sparvero LJ, Amoscato AA, Samhan-Arias AK, Swedin L, Lahesmaa R, Fadeel B, Shvedova AA, Kagan VE (2011) Global phospholipidomics analysis reveals selective pulmonary peroxidation profiles upon inhalation of single-walled carbon nanotubes. ACS Nano 5:7342–7353
Valerio LG Jr, Choudhuri S (2012) Chemoinformatics and chemical genomics: potential utility of in silico methods. J Appl Toxicol JAT 32:880–889
van Ravenzwaay B, Montoya GA, Fabian E, Herold M, Krennrich G, Looser R, Mellert W, Peter E, Strauss V, Walk T, Kamp H (2014) The sensitivity of metabolomics versus classical regulatory toxicology from a NOAEL perspective. Toxicol Lett 227:20–28
Vance DE (2008) In: Vance DE, editor. Biochemistry of lipids, lipoproteins and membranes Elektronische Ressource, 5th edn. Elsevier, Amsterdam. 1st ed
Vannini C, Domingo G, Onelli E, Prinsi B, Marsoni M, Espen L, Bracale M (2013) Morphological and proteomic responses of Eruca sativa exposed to silver nanoparticles or silver nitrate. PLoS One 8:e68752
Verano-Braga T, Miethling-Graff R, Wojdyla K, Rogowska-Wrzesinska A, Brewer JR, Erdmann H, Kjeldsen F (2014) Insights into the cellular response triggered by silver nanoparticles using quantitative proteomics. ACS Nano 8:2161–2175
Vidanapathirana AK, Lai X, Hilderbrand SC, Pitzer JE, Podila R, Sumner SJ, Fennell TR, Wingard CJ, Witzmann FA, Brown JM (2012) Multi-walled carbon nanotube directed gene and protein expression in cultured human aortic endothelial cells is influenced by suspension medium. Toxicology 302:114–122
Vihervaara T, Suoniemi M, Laaksonen R (2014) Lipidomics in drug discovery. Drug Discov Today 19:164–170
Vinken M (2013) The adverse outcome pathway concept: a pragmatic tool in toxicology. Toxicology 312:158–165
Wagner SA, Beli P, Weinert BT, Nielsen ML, Cox J, Mann M, Choudhary C (2011) A proteome-wide, quantitative survey of in vivo ubiquitylation sites reveals widespread regulatory roles. Mol Cell Proteomics 10(M111):013284
Waters M, Boorman G, Bushel P, Cunningham M, Irwin R, Merrick A, Olden K, Paules R, Selkirk J, Stasiewicz S, Weis B, Van Houten B, Walker N, Tennant R (2003) Systems toxicology and the Chemical Effects in Biological Systems (CEBS) knowledge base. EHP Toxicogenomics J Natl Inst Environ Health Sci 111:15–28
Wenk MR (2010) Lipidomics: new tools and applications. Cell 143:888–895
Wilson VS, Keshava N, Hester S, Segal D, Chiu W, Thompson CM, Euling SY (2013) Utilizing toxicogenomic data to understand chemical mechanism of action in risk assessment. Toxicol Appl Pharmacol 271:299–308
Wruck W, Peuker M, Regenbrecht CR (2014) Data management strategies for multinational large-scale systems biology projects. Brief Bioinform 15:65–78
Xiao X, Lee JH (2010) Systems analysis of alternative splicing and its regulation. Wiley Interdiscip Rev Syst Biol Med 2:550–565
Yan LJ, Forster MJ (2011) Chemical probes for analysis of carbonylated proteins: a review. J Chromatogr B Analyt Technol Biomed Life Sci 879:1308–1315
Yang M, Soga T, Pollard PJ (2013) Oncometabolites: linking altered metabolism with cancer. J Clin Invest 123:3652–3658
Yuan J, Gao H, Sui J, Duan H, Chen WN, Ching CB (2012) Cytotoxicity evaluation of oxidized single-walled carbon nanotubes and graphene oxide on human hepatoma HepG2 cells: an iTRAQ-coupled 2D LC-MS/MS proteome analysis. Toxicol Sci Off J Soc Toxicol 126:149–161
Yuan JF, Gao HC, Ching CB (2011) Comparative protein profile of human hepatoma HepG2 cells treated with graphene and single-walled carbon nanotubes: An iTRAQ-coupled 2D LC-MS/MS proteome analysis. Toxicol Lett 207:213–221
Zhang H, Ji Z, Xia T, Meng H, Low-Kam C, Liu R, Pokhrel S, Lin S, Wang X, Liao YP, Wang M, Li L, Rallo R, Damoiseaux R, Telesca D, Madler L, Cohen Y, Zink JI, Nel AE (2012) Use of metal oxide nanoparticle band gap to develop a predictive paradigm for oxidative stress and acute pulmonary inflammation. ACS Nano 6:4349–4368
Zhang Y, Zhao F, Deng Y, Zhao Y, Ren H (2015) Metagenomic and metabolomic analysis of the toxic effects of trichloroacetamide-induced gut microbiome and urine metabolome perturbations in mice. J Proteome Res 14:1752–1761
Zhao Y, Jensen ON (2009) Modification-specific proteomics: strategies for characterization of post-translational modifications using enrichment techniques. Proteomics 9:4632–4641
Zuin S, Micheletti C, Critto A, Pojana G, Johnston H, Stone V, Tran L, Marcomini A (2011) Weight of evidence approach for the relative hazard ranking of nanomaterials. Nanotoxicology 5:445–458
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 Springer International Publishing AG
About this chapter
Cite this chapter
Riebeling, C., Jungnickel, H., Luch, A., Haase, A. (2017). Systems Biology to Support Nanomaterial Grouping. In: Tran, L., Bañares, M., Rallo, R. (eds) Modelling the Toxicity of Nanoparticles. Advances in Experimental Medicine and Biology, vol 947. Springer, Cham. https://doi.org/10.1007/978-3-319-47754-1_6
Download citation
DOI: https://doi.org/10.1007/978-3-319-47754-1_6
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-47752-7
Online ISBN: 978-3-319-47754-1
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)